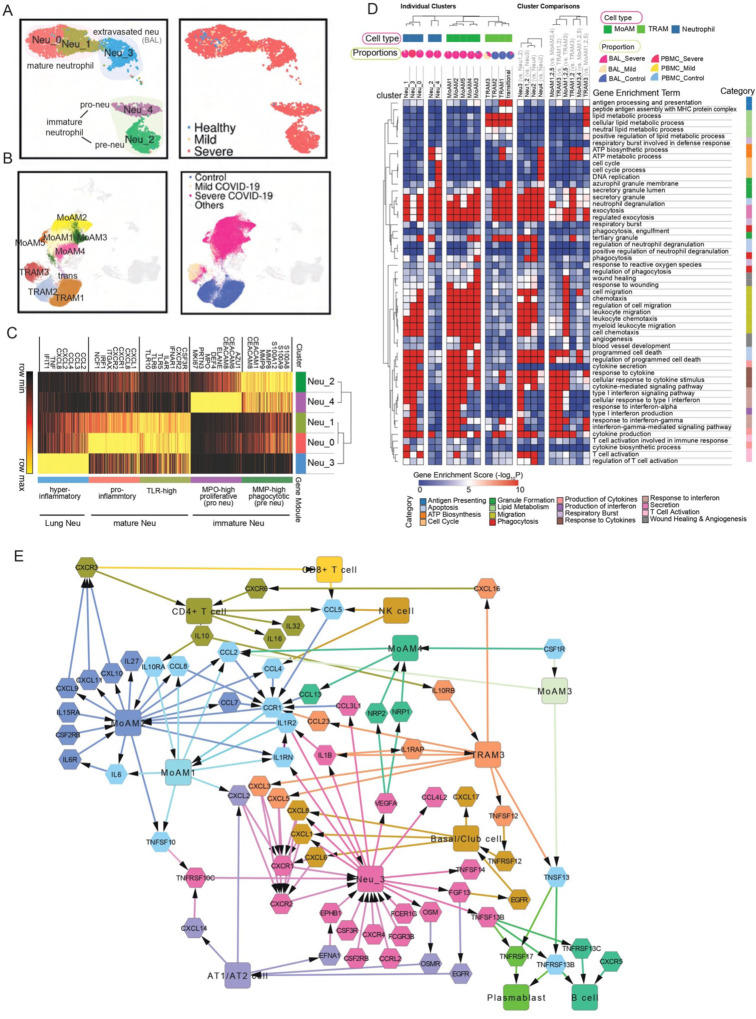
Fig. 3. Functional analysis of compartment-specific immature and subtype-differentiated neutrophils and monocytic macrophages in COVID-19 patients.
(A) Five sub-clusters and three cell groups were identified after the integration of neutrophils in peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage (BAL) (Left). The distribution of compartments is shown on the right. (B) Sub-clusters (Left) and COVID-19 conditions (Right) of monocyte-derived macrophages and tissue-resident macrophages were identified after integration of BAL datasets. (C) Heatmap of gene modules from ToppCell with top 200 upregulated genes for each neutrophil sub-cluster. Important neutrophil-associated genes and inferred roles of sub-clusters were shown on two sides. (D) Heatmap of associations between subclusters of neutrophils and macrophages and myeloid-cell-associated pathways (Gene Ontology). Gene modules with 200 upregulated genes for sub-clusters were used for enrichment in ToppCluster. Additionally, enrichment of top 200 differentially expressed genes (DEGs) for comparisons in fig. S5D and fig. S6B were appended on the right. Gene enrichment scores, defined as −log10(adjusted p-value), were calculated as the strength of associations. Pie charts showed the proportions of COVID-19 conditions in each cluster. (E) Gene interaction network in the BAL of severe patients. Highly expressed ligands and receptors of each cell type were drawn based on fig. S8. Interaction was inferred using both CellChat database and embedded cell interaction database in ToppCell.