Abstract
The pathogenesis of dermatophytic infections involves the interplay of three major factors: the dermatophyte, the inherent host defense, and the adaptive host immune response. The fungal virulence factors determine the adhesion and invasion of the skin while the immune response depends on an interaction of the pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMP) with pattern recognition receptors (PRRs) of the host, which lead to a differential Th (T helper) 1, Th2, Th17, and Treg response. While anthropophilic dermatophytes Trichophyton rubrum and now increasingly by T. interdigitale subvert the immune response via mannans, zoophilic species are eliminated due to a brisk immune response. Notably, delayed-type hypersensitivity (Th1) response of T lymphocytes causes the elimination of fungal infection, while chronic disease caused by anthropophilic species corresponds to toll-like receptor 2 mediated IL (interleukin)-10 release and generation of T-regulatory cells with immunosuppressive potential. Major steps that determine the ultimate clinical course and chronicity include genetic susceptibility factors, impaired epidermal and immunological barriers, variations in the composition of sebum and sweat, carbon dioxide tension, skin pH, and topical steroid abuse. It is important to understand these multifarious aspects to surmount the problem of recalcitrant dermatophytosis when the disorder fails conventional therapeutic agents.
Keywords: Anthropophilic species, epidermal barrier, fungal virulence factors, immune responses, recalcitrant dermatophytosis, skin pH, steroid abuse, Th1 and Th 17 cells
Introduction
The pathogenesis of dermatophytic infections involves three major factors, and it is important to understand the interplay between them, in the era of recalcitrant dermatophyte infections.[1,2] These factors include the fungi, the inherent host factors including the skin barrier function, and the immune response mounted against the fungus. Among these, the type of fungi and immune response of the host play a major role, and these are crucial in causing recalcitrance and relapses. Dermatophytes belong to 3 genera -Trichophyton, Epidermophyton, and Microsporum. These are divided into anthropophilic, zoophilic, and geophilic according to their primary habitat. While possibly in other parts of the world, anthropophilic dermatophyte Trichophyton rubrum is a common cause, but this is now being increasingly replaced by T. interdigitale in some geographical locations.[1,3,4] T. interdigitale is currently the prevalent strain in India and causes mild inflammation and chronic infections. There are known variations in the fungal virulence factors among different species, and it is likely to be playing a role in the current scenario the country is facing. For example, protease and lipase enzymes vary significantly between T. tonsurans and Trichophyton equinum.[5] This has been reinforced by a study where comparative proteomic analysis of T. rubrum and Trichophyton violaceum revealed differences in the amount and specificity of secreted proteins between them.[6]
Experimental models are required to transcend speciation and to assess the virulence factors and the immuno-pathogenetic pathways of diverse dermatophytes species. The immune responses elicited by zoophilic dermatophytes are studied using guinea pigs and mice. In contrast, in anthropophilic species, studies have been performed in ex-vivo models using epidermal tissues and keratinocyte co-cultures, which could have direct applicability to clinical situations.[7] In spite of this methodological issue of translation of research, we have attempted in this review to distill the vast amount of data to help understand the complex pathogenesis.
Dermatophyte specific factors
The immune response stimulated by Dermatophytes vary depending on the species stimulate an immune response. Trichophyton rubrum causes chronic infections, possibly by the release of glycopeptides that can inhibit the proliferation of T lymphocytes in vitro, thereby suppressing host immunity.[8]
The dermatophytes' armamentaria mainly comprises of the surface molecules for adhesion, secreted enzymes to degrade and metabolise host molecules for nutrition, thermo-tolerance, and dimorphism (i.e., converting between mycelial and conidial forms depending on ambient conditions). It is important to emphasize that though certain factors related to the potential host predispose to dermatophytoses, it is the dermatophyte virulence factors which are primarily implicated for causing infection, regardless of the patient's immune status.[9]
The major steps involved in the establishment and perpetuation of dermatophyte infection are detailed below:
Adhesion
The initial step is the interaction of dermatophytes with the host tissues, and adhesion to the epidermis occurs within 1hour. Adhesins present on the cell wall of fungi are crucial to the initial attachment. The molecules implicated include Sub3 secreted protease of the subtilisin family in M.canis, and sowgp gene and dipeptidyl-peptidase IV (DppIV) of the Trichophyton spp.[10,11] This is followed by germination, where arthroconidia germinate, and the hyphae quickly enter the stratum corneum to prevent elimination with cell shedding, which occurs within 3-4 hours.[12] Between 24 hours and 3 days, the hyphae spread through the skin. Fibril like structures have been demonstrated in the case of Trichophyton mentagrophytes, facilitating adhesion and thereby preventing elimination from the host tissue. The arthrocidium becomes flat, and the fibril-like structures become short and fine when it invades the deeper layers of the epidermis, leading to increased contact surface with the host tissue and thereby better adhesion and more nutrient acquisition.[13]
On the 4th day, the hyphae reach the granular layer, which coincides with a loss of the integrity of the epidermal barrier.[14] Consequently, the keratinocytes release antimicrobial peptides (AMP) and pro-inflammatory cytokines, resulting in control and, ultimately, the resolution of infection via the immune system's stimulation.[15] Further, there may be a role of biofilm formation by making connections between adjacent arthroconidia.[16]
Enzymatic activity and virulence factors
A critical virulence factor of dermatophytes is best distinguished by its enzymatic activity. Dermatophytes produce several enzymes, including proteases, lipases, elastases, collagenases, phosphatases, and esterases, which have been implicated in host invasion and assimilation of nutrition.[16] The proteolytic enzymes, notably the keratinolytic proteases (keratinases), have a well-established role in the pathogenicity of dermatophytoses helping in invasion into tissues as well as providing oligopeptides and amino acids on keratin breakdown, which are important nutrients for the fungi. It was found that the Trichophyton spp. had the highest keratinase activity in a study that compared the keratolytic action of T. rubrum, T. interdigitale, M. canis, and M. gypseum using spectrophotometry.[17] This high keratinase activity of Trichophyton spp. has been implicated for adaptation of these dermatophytes to human skin and is referred to as “anthropization.”[17]
Lipids are also degraded and utilized by the dermatophytes using relevant enzymes. Probably because of the destructive effects and the resulting alteration in epidermal differentiation by dermatophytes, the epidermal barrier of the glabrous skin is distinctly impaired in tinea.[18] However, in nails, before the action of the keratinolytic proteases can commence, the abundant disulphide bonds present within the hard keratin must be broken. This is achieved by a sulfite efflux pump, encoded by TruSsu1 gene in T .rubrum. Cysteine dioxygenase (CDO) activity is also essential for the same, and hence arthroderma benhamiae cdo1 and ssu1 knockout mutants were found unable to grow on hair and nails.[19]
The role of ambient pH
The initial environment encountered by the dermatophytes on skin and nail is acidic. The acidic pH is maintained by a complex balance between acidifying (products from glands and their breakdown by resident flora, filaggrin–histidine–urocanic acid pathway-related breakdown products) and alkalinizing (ammonia, carbon-dioxide, and bicarbonates) factors.[1]
Proteases that function optimally at an acidic pH are unsuppressed, and keratin utilization begins. The use of keratin as the source of carbon generates metabolites which shift the ambient pH to alkaline. This is associated with the upregulation of proteases with optimal function at an alkaline pH. Thus, the fungus can obtain nutrition from proteins over a wide pH range.
This adaptive pH regulatory mechanism is an important virulence factor and depends on the PacC/Pal signal transduction pathway, which is highly conserved on pathogenic fungi, including dermatophytes. PacC is a transcriptional regulator regulating the pH-dependent gene expression.[20] The proteolytic cleavage of PacC is triggered by the signaling cascade which is composed of the six pal genes (palA, palB, palC, palF, palH, and palI) that senses the alkaline pH.[21,22,23,24,25,26] While the initiation of dermatophytic infection is promoted by the genes upregulated at acidic pH, those that function at alkaline pH are crucial for its maintenance. Also, the growth in keratin cultures containing hsp90 inhibitor lowered the pacC transcripts concentration, indicating an association between pacC and hsp90 that may affect fungal virulence.
The role of heat shock proteins (HSP)
Various HSPs have been found to be over-expressed during dermatophyte invasion of host tissues. T. rubrum grown on human nails in vitro resulted in an increased expression of hsp60, hsp70, and hsp78 genes, while hsp70, hsp90, hsp related gene hsf1 and hspSSc1 are overexpressed when it is grown on skin.[20] Fungal virulence on the human nail was diminished when 17-allylamino-17-demethoxygeldanamycin (17-AAG, Hsp90 inhibiter) was used in an inhibitor concentration-dependent manner, suggesting the involvement of this HSP in pathogenicity.[27] Chemical inhibition of hsp 90 results in increased susceptibility of T. rubrum to itraconazole and micafungin, which can be used clinically as this is suggestive of a potential for combination therapy with existing antifungals.
Toxin secretion
Toxin production has also been implicated, and the salient compounds are xanthomegnin released by T. megninii, T. rubrum, and T. violaceum, hemolysins released by T. rubrum and T. interdigitale and [28,29] lipophilic toxins, such as xanthomegnin and aflatoxin-like substances all of which are known to have immunosuppressive effects.[30]
Role of mannan
Mannan, a glycoprotein component of the fungal cell wall, facilitates infection by inhibiting the proliferation of keratinocytes, thus preventing shedding, and suppression of the inflammatory response. T.rubrum produces mannan in larger amounts than M.canis.[16] Furthermore, mannan produced by T.rubrum inhibits cell proliferation and lympho-proliferation more effectively.
Biofilm formation
Although many dermatophyte strains have been shown to form biofilms under experimental conditions and on ex-vivo nail tissue, their existence on the skin in tinea corporis/cruris has not yet been demonstrated.[31]
Host Immune Response to Dermatophytes
There are several host defense mechanisms that prevent the establishment of tinea infections, including the physical and chemical composition of skin, UV light exposure, lack of humidity and temperature, action of phagocytic cells, and commensal microbiota. The rapid proliferation of keratinocytes also plays an important role in defense against dermatophyte infections by continual renewal and epithelial shedding. While there is a suggestion that commensal skin fungi may play a role in host-dependent immunity and the various fungi isolated including Malassezia, Penicillium and Aspergillus amongst others (Alternaria, Candida, Rhodotorula, Cladosporium and Mucor) — their interaction with dermatophyte is as yet unknown though they may influence the Th17 cell and its pathway.[32] The various host defense mechanisms are listed in Table 1.
Table 1.
Summary of the various host defense mechanisms against Dermatophyte Infection
| 1. An increase in cell proliferation rate |
| 2. An increase in anti-microbial peptides (AMP) including beta defensins 2 and 3, RNAse7 and psoriasin |
| 3. Fungal phagocytosis by neutrophils and macrophages |
| 4. Complement mediated mechanisms |
| 5. A complex immune response which helps to eliminate the infection |
Dermatophyte- keratinocyte immune interaction
Keratinocytes are the first cellular elements that come into contact with dermatophytes during infection and are capable of modulating the host immune response.[33] Upon exposure to dermatophytes or their antigenic determinants, the keratinocytes produce a wide range of cytokines, including interleukin (IL)-8 (a potent neutrophil chemotactic factor), the pro-inflammatory cytokine TNF (tumor necrosis factor)-α, IFN (interferon)- γ, IL-1ß, IL-22 and IL-16 among others, to destroy dermatophytes [Figure 1].[34]
Figure 1.

A depiction of the immune responses to dermatophyte infection. While Th1 and Th17 response leads to clearance of the dermatophytes, Th2 response inhibits fungal clearance and persistent Treg activation leads to chronic persistent infections. The immune response elicited also varies with the dermatophyte species involved. While zoophilic dermatophytes such as Arthroderma benhamiae induce a wide range of cytokines, anthropophilic dermatophytes such as Trichophyton rubrum induce the production of a limited array of mediators (highlighted in bold)
The ability to prompt the secretion of pro-inflammatory cytokines in the keratinocytes varies among the dermatophytes species. Zoophilic species like Arthroderma benhamiae, can lead to the expression of various cytokines by the keratinocytes, which lead to enhanced inflammation. On the contrary, T. tonsurans, an anthropophilic dermatophyte, has a limited ability to induce cytokines, thus leading to less inflammation.[35,36]
A study comparing the cytokine profile of T. tonsurans and A. benhamiae found that T. tonsurans triggered eotaxin-2, IL-16, and IL-8 secretion from the infected keratinocytes, whereas A. benhamiae produced a wide variety of pro-inflammatory and immunomodulatory cytokines/chemokines.[36] Complementary DNA microarray analysis shows that the genes encoding IL-1ß, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-16, IL-17, and IFN- γ were upregulated by A. benhamiae, while T. tonsurans only upregulated genes encoding for IL-1ß and IL-6. Also, both dermatophytes enhanced the IL-8 mRNA expression in keratinocytes. It was also found that T. mentagrophytes induces higher expression of IL-1α by keratinocytes than T.rubrum.[37] Another recent study conducted to distinguish the change in the gene expression of HaCaT (Cultured human keratinocyte) cell line following dermatophytic infection found that pro-inflammatory cytokines and chemokines triggered the infiltration of neutrophils and other immune cell to the infection site following zoophilic M gypseum infection due to the upregulated expression of genes related to immune response, while with T rubrum, metabolic pathway genes were upregulated instead immune response-related genes.[38] This corroborates with the clinical observations that in acute infections provoked by the zoophilic fungi T. mentagrophytes and M. gypseum, marked neutrophils are seen in the epidermis, whereas T. rubrum lead to chronic infections and are characterized by a mononuclear infiltrate.[11] Trichophyton-induced inflammation causes the proliferation of peripheral blood mononuclear cells (PBMC) and the production of IFN- γ and IFN- γ -positive CD4+ cells leading to a Th1 response[39,40] [Figure 1].
In addition to the vigorous immune response, antimicrobial peptides (AMP) such as cathelicidins and defensins are secreted by the human keratinocytes with likely antifungal activity as well.[41] In vitro fungistatic and fungicidal activity of human-defensins and cathelicidin (LL-37), respectively against T. rubrum, has been shown by several authors, and also there is increased expression of AMP in vivo in tinea corporis caused by this trichophyte.[18,42]
Innate immune response
Epidermal dendritic cells (DC), especially Langerhans cells (LC), are crucial in initiating and modulating the adaptive immune responses against dermatophytes. A recent study has shown that a reduced number of LC in the epidermis increases the risk of dermatophyte infections.[43] These cells contain receptors for pathogen-associated molecular patterns (PAMPs) called pattern recognition receptors (PRRs), such as, Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and the galectin family proteins, that sense the PAMPs and the damage-associated molecular patterns (DAMPs) that are present on fungi. [Figure 2] The major PAMPs for dermatophytes are the cell wall components, mainly glucans and mannans. Apart from TLRs, CLRs form the major receptors involved in recognition of the PAMPs of dermatophytes. These include Dectin-1, Dectin-2, Dectin-3, MR, Mincle, and dendritic cell-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing non-integrin (DC-SIGN) [Figure 2].
Figure 2.
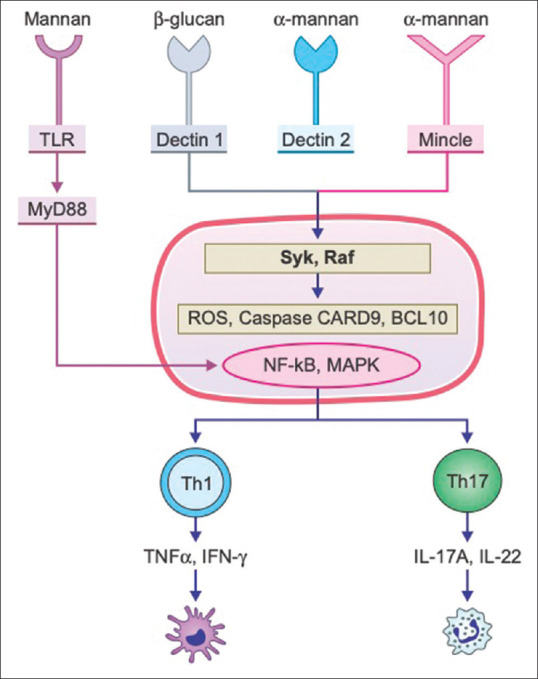
A depiction of the interaction of the pathogen-associated molecular patterns (PAMPS) and the pattern recognition receptors (PRR) for dermatophytes. The major PRRs for dermatophytes included the Toll-like receptors (TLR), dectin-1, minicle and dectin-2 which recognize the mannans and glucans on surface of dermatophytes. Their activation initiate a cascade of signals that induce the nuclear factor (NF)-kB and mitogen-activated protein kinase (MAPK) pathways, which in turn stimulate the T-helper (Th) 17 and Th1 cells which play important roles in the host immune response
DC-SIGN (CD209) is an important PRR and is structurally a type II transmembrane protein belonging to the CLR family and recognizes ß-glucan on fungal cell walls.[44,45] Dectin-2, a CLR expressed in most DC, such as LC, recognizes and binds to M. canis and T. rubrum hyphae, causing the production of pro-inflammatory cytokines like IL-12, IL-10, and TNFα and help present the antigens to CD4+ T cells, promoting their proliferation and release of IL-4, IL-10, and IFN- γ.[46]
Dectin-1 is another CLR that functions as a transmembrane PRR for fungal pathogens through its ability to bind ß-glucan carbohydrates.[47] [Figure 2] Macrophages act as an intermediary of the keratinocyte and dendritic cell-mediated immune responses, and they either kill the fungus or undergo destruction. Toll-like receptor-2 located on the surface of monocytes mediates phagocytosis of conidia as well as promotes the a pro-inflammatory response with the secretion of cytokines such as TNF-α.[48] Downstream signaling and the resultant immune effects are depicted in Figure 1.
Besides keratinocytes and DC, neutrophils also play a vital role in innate immunity against dermatophytes that accumulate soon after the corneocyte adherence to the conidia during germination. Neutrophils, along with macrophages, are thought to be the final effector cells in eliminating dermatophytosis and the mediator of intra and extracellular lysis of dermatophytes both via the oxidative pathway and release of TNF-α.[40,49] A fungal hydrophobin, released following induction of the expression of hypA gene following interaction of A. benhamiae with keratinocytes, renders them less susceptible to neutrophil action.[50]
The contribution of innate immunity in clearing fungal infections was recently corroborated by a paper which showed that Rag2-/- mice, which lack T and B cells, can ultimately clear the dermatophyte infection.[51] Also, a study in immunocompetent wild-type mice showed that even there was only a modest reduction in the fungal load in a secondary infection versus a primary one, suggesting that innate immunity plays the major role in the immune responses against fungi.
Expression of IL–17 as early as three days after a dermatophyte infection means that there is a rapid activation of IL–17 is reminiscent of the innate Th-17 responses against fungal infections rather than adaptive immune response.[52]
Acquired immune response
The comparative role of specific humoral and cellular immune responses against fungal infections has been a debatable conundrum in mycology. While cell-mediated immunity (CMI) is protective against many fungi, including dermatophytes, certain types of antibody responses may also provide protection. CMI also increases epidermal proliferation facilitating dermatophyte elimination.[1] The type of CMI response is critical in determining resistance or vulnerability to fungal infection even though the cytotoxic activity against dermatophytes is seen with both CD4 and CD8 T cells.[1]
Overall, the elimination of a fungal infection is mediated by Th1-type CMI, while Th2 response predisposes to infection or leads to allergic responses. Th1 cells produce cytokines such as IFN- γ and trigger phagocyte stimulation.[1,40,53,54,55] In contrast, a Th2- response results in enhanced production of antibodies and IL-4, IL-5, and IL-13.[1,56] IL- 4 mediates IgE production by B cells while IL-5 helps in eosinophil recruitment via vascular cell adhesion molecule (VCAM)-very late antigen (VLA)-4 adhesion molecule pathway and enhances eosinophil production from the bone marrow.[1]
It has been shown that initially, IFN- γ level is low along with high levels of IL-10, which inhibit Th1 response. Over time, IL-10 production decreases, while the levels of IFN- γ increase. Thus, apart from inducing a Th2-type response, IL-10 plays a significant role in innate immunity and immune response regulation. IL-10 prevents a damaging inflammatory response by blocking excessive production of TNF-α and other cytotoxic metabolites, enabling the development of a specific immune response.[57] Interestingly, in a murine model study, the levels of IL-10 were notably higher during the early stage of the infection.[58]
Recently, the focus has shifted to the Th17 cell pathway, which promotes the Th1-type immune responses and inhibits Th2-type responses. IL-17A has been shown to mobilize neutrophils and stimulate defensins' secretion, contributing to the rapid and effective control of infection as an innate response.[59,60]
The earlier studies on cytokine profile at the dermatophyte infection site reveals TGF-ß, IL-1ß, and IL-6, which are involved in the initiation and perpetuation of the Th17 pathway. Further, an increase in IL-22 mRNA was also observed in experimental models, both findings suggesting that the Th17 cell pathway may be involved in the immunopathogenesis of dermatophytic infections.[61]
In more recent studies, these hypotheses have been tested and validated, and the protective role of the Th17 pathway has now clearly emerged. Heinen et al. recently reported that with dermatophytic infections, the adaptive immunity is polarized to both Th1 and Th17 responses, with dermatophyte clearance mediated by the Th17 antifungal response and the Th1 response being involved in fungal clearance as well as the down-modulation of Th17 induced inflammation. Also, a study revealed that it may exert antifungal activity by enhancing the epidermal barrier function.[62] In fact, cytokines IFN-γ and IL-17A, signature cytokines of Th1 and Th17 lineages, respectively, are needed for optimal protection to the disease. Moreover, the authors found that IL–17 and IFN-γ show complementary immunological effects during the resolution of T. benhamiae infection.[63] However, in contrast to these findings, Rai et al. demonstrated high Th17 and T-reg expression in peripheral blood of patients with chronic skin dermatophytoses, suggesting a complex interplay of T cell lineages in causing disease persistence.[64] A summary of the role of Th cells is listed in Table 2. Here it is pertinent to point out that T Reg cells may effect a variety of sequels ranging from protective tolerance (defined as a host response that safeguards host's survival through a trade-off between sterilizing immune responses and their negative regulation, which limits pathogen elimination) to overt immunosuppression.[65]
Table 2.
Role of various T cells subtypes
| T Cells | Putative role |
|---|---|
| Th1 cells | -Th 1 cell response correlates with protective immunity |
| - Determined by DC response to the combination of TLR and CLR signals provided by fungi | |
| - Leads to increase in IFN γ , activates phagocytosis | |
| - Th 1 cell predicts asymptomatic and mild forms of the infection | |
| - Estradiol increases Th1 response | |
| Th2 cells | -IL-4 and IL-13 reduce the protective Th 1 cell response |
| - Favours fungal infections, fungus-associated allergic responses and disease relapse | |
| - Limiting IL-4 production restores antifungal resistance | |
| - Leads to Elevated levels of IgE, IgA and IgG | |
| Th17 cells | -Activation via the SYK-CARD9, MYD88 and mannose receptor signaling pathways in DCs and macrophages |
| - Promote Th 1-type immune responses and restrain Th 2-type responses | |
| - Enables neutrophil action | |
| - chronic infection is due to failure to restrain inflammation following IL-17A-dependent neutrophil recruitment, thereby preventing optimal protection and favoring fungal persistence. | |
| T reg Cells | - inverse relationship between IFN γ and IL-10 production in patients with fungal infections |
| - High levels of IL-10, which negatively affect IFN γ production, are detected in chronic candidal diseases | |
| - IL-10 acts as a homeostatic host-driven response to keep inflammation under control | |
| - T Reg cell response is to reduce damage & also lead to fungal persistence and immunosuppression |
DC: Dendritic cell; TLR: Toll like receptor; CLR: C-type lectin receptors; IFN γ: Interferon gamma
Interestingly, the severity of the inflammatory reaction depends on the depth of skin invasion, which is largely determined by proteases. It has been proposed that dermatophytes that are weakly invasive elude soluble or cellular components of the immune system by residing in the superficial non-living skin layers.[66] Specific immunogenic properties of the secreted proteases may also effect immune defenses. Notably, proteases 'subtilisin' from T. rubrum (Tri r2) and 'dipeptidyl-peptidase V' from Trichophyton tonsurans (Tri t 4) can modulate the immune responses.[1,67] Also, the surface antigen subtilisin Sub1 of T. rubrum has also been proposed to play a role in immune-modulation. Moreover, Trichophyton rubrum cell wall mannans may be a cause of dose-dependent immunosuppression and inhibit the in vitro lymphoproliferative response and stratum corneum turnover either directly or by altering lymphocyte function.
Acute dermatophytosis is associated with a delayed-type hypersensitivity (DTH) response to trichophyton and blastogenic response of T lymphocytes with progression to healing, while persistent disease corresponds to inadequate cellular immune responses with immediate hypersensitivity (IH) responses, high levels of IgE and IgG4 antibodies, and of Th2 cytokines released by mononuclear leukocytes.[68,69] Furthermore, chronic dermatophytosis, due to association with IH and Th2 cytokines, may underlie the pathogenesis of allergic diseases, mainly asthma.[70]
Though it is largely believed that the immune response to dermatophytes is DTH-dependent, there are contrarian findings. In AIDS, the incidence of invasive dermatophytoses is expected to be higher due to reduced CD4 + T lymphocyte counts. However, this is not seen in practice while it is more frequent in non-AIDS immunocompromised patients, suggesting different immunological mechanisms play a role.[1,71,72] Furthermore, patients with chronic dermatophytosis may have DTH against trichophyton.[73]
Host Dependent Factors
Susceptibility to dermatophytosis is variable.[74] The risk factors favoring dermatophytosis include defective epidermal and immunological barriers.[18,67] Individual susceptibility factors are still unclear but include alteration in sebum fatty acids, presence of moisture or transferrin, and other inhibitors for the dermatophyte growth in sweat or serum and skin, including carbon dioxide concentration.[1,75]
An impaired epidermal barrier aggravated by scratching, atopy, ichthyosis, low level of sebum secretion before puberty, and possibly elevated environmental humidity, may promote dermatophytosis.[1,18,67] Impaired peripheral blood circulation with concomitant diminished nutrient availability, reduced oxygenation, and delay in the relocation of immune-competent cells or release of antimicrobial peptides (AMP) at infection site may also increase the susceptibility for infections. Diabetes predisposes to an almost three-fold increased chance of dermatophytosis, especially foot and nail tinea, due to altered peripheral blood circulation and nerve endings.[76] Further, extensive and invasive infections have been reported in various underlying conditions such as patients with atopic dermatitis, leukemia or lymphoma, diabetes, hepatitis B and C related cirrhosis, on haemodialysis for renal failure, alcoholic liver disease, congenital adrenal hyperplasia, Cushing disease, patients on immunosuppressants/modulators for systemic lupus erythematosus, psoriasis, rheumatoid arthritis, Behcet's disease, autoimmune hepatitis and myasthenia gravis.[71,72,77]
It is important to note here that topical steroid use disturbs the local immune function and severely impairs the clearing of the fungi from the skin. The crucial effects on local immunity are detailed in Table 3.[78,79]
Table 3.
The effect of corticosteroids on various steps involved in pathogenesis of dermatophytosis
| Immune mediators | Action |
|---|---|
| Effect on the APCs, neutrophils | 1.Glucocorticoids (GCs) act directly on APCs via cytoplasmic/nuclear receptors to suppress the production ofIL-12. |
| The inhibition of IL-12 production may represent a major mechanism by which GCs affect the Th1/Th2 balance since IL-12 is the main inducer of Th1 responses, enhancing production Th1 cytokines such as IFN-γ by antigen-primed CD4+ T cells and inhibiting the synthesis of Th2 cytokines such as IL-4 by T cells. | |
| 2. Additionally, exposure of DC to GC induces the expression of TLR2 on their surface and stimulation of these cells with a TLR2 ligand initiates the secretion of IL-10, IL-6, and TNFα, which inhibit Th1 cell activation. | |
| 3. GC have also been shown to decrease the expression of IL-23 in DC, prohibiting Th17 polarization. | |
| 4.GCs may decrease antigen presentation by APCs by decreasing MHC II expression | |
| 5 GCs have been shown to reduce macrophage and neutrophil chemotaxis and decreases the IL-1 and IFN-γ release by macrophages with a small effect on the respiratory burst. | |
| Effect on adaptive immunity (T cells, cytokines) | 1.GCs suppress the Th1-cellular immunity axis and mediate a shift towards Th2 immune responses. This results from both a direct effect on T cells by downregulating the expression of IL-12 receptors on T and NK cells leading to a loss of IL-12 responsiveness and indirectly via inhibition of IL-12 production. |
| 2.GCs have a direct effect on Th2 cells by upregulating the production of IL-4, IL-10, and IL-13 | |
| 3.The effects of GC on Th17 cells are unclear; the existing data suggests that both Th17 differentiation and function may be affected by GC. GC administration has been shown to decrease the expression of IL-23 in DC andIL-6, TGFβ as well as IL-17 in the joints of arthritic mice. | |
| 4 In addition to affecting differentiation of specific T-cell subsets, GC directly or indirectly suppress the activation of proinflammatory cytokine genes such as TNFα and IL-1β. |
APC: Antigen presenting cell; DC: Dendritic cell; TLR: Toll like receptor; IFN γ: Interferon gamma; NK cell: Natural killer cell; TGFβ: Transforming growth factor beta; TNFα: Tumour necrosis factor alpha
Lastly, various genetic predisposition factors have been identified, and certain HLA haplotypes predict increased risk of dermatophyte infections, particularly HLA-DR8 has been shown to increase susceptibility for onychomycosis.[80] Mutations in gene CLEC7A, which encodes protein dectin-1 that binds to fungal ß-glucans, as well as mutations in the genes of signaling pathways such as CARD9 and STAT3, engaged in the antifungal immune response, are associated with an increased incidence of dermatophytosis.[81] Low copy numbers of gene DEFB4, encoding the antimicrobial peptide (AMP) ß-defensin-2, also increase vulnerability to dermatophytosis.[82]
Clinical Implications
It is obvious that the immunological interaction of the host immune response and the fungi determine the clinical sequelae. Thus, understanding of the complex immune responses to Trichophyton spp is essential for developing adequate therapeutic strategies for treating chronic tinea infections.[54] It has been estimated that about a fifth of all T. rubrum infections are chronic. T.mentagrophyte/interdigitale complex, which is currently the prevalent strain causing dermatophytoses in India, also seems to subvert host immunity, though the precise mechanisms are not known at present. It is known that chronic infections with T. rubrum can invade the deeper levels of the epidermis, yet suppressing an immune response, and prolonging symptoms such as mild to intense itch, scaling, and development of plaques. These chronic infections often do not respond to treatment, having severe implications for the patients.
-
Chronic or relapsing dermatophytic infections in immunocompetent individuals are related to the prevalence of immediate hypersensitivity mediated by IgE (immunoglobulin E) to the fungus, as well as high serum levels of IgE and IgG4 (immunoglobulin G4).[83] T. rubrum produces substances, e.g., the mannans associated with glycoproteins that decrease the immune response, thus preventing complete eradication of the fungus.[84] Anthropophilic species induce immune-suppression through toll-like receptor 2 (TLR2) mediated IL-10 release, with consequent generation of CD4+ CD25+ T-regulatory cells with immunosuppressive potential.[85] Consequently, there is increased Th2-type responses that is inadequate to fight fungal infections leading to chronic and extensive infection.[86] Moreover, a study showed that macrophages and neutrophils have reduced T rubrum phagocytosis and downstream cytokine release in patients with chronic dermatophytoses.
Also, the release of H2O2 and NO and T rubrum killing activity of these cells was reduced in these patients and was normal in patients with acute tinea pedis infections suggesting Trichophyton-specific functional defects in patients with chronic dermatophytoses.[87] The fungal cell wall mannans can inhibit DC-SIGN-dependent cell adhesion to ICAM-3 of wild-type T cells, thereby decreasing the initial interactions between DC and wild-type T cells, thus blocking antigen presentation and activation of T cells, favoring the development of invasive or disseminated infections caused by dermatophytes.[32] The clinical correlate of this is the leathery, lichenified lesions seen in some patients, and this is a surrogate clinical sign of chronicity.[1]
The use of immunosuppression via oral agents or topical steroid abuse can subvert almost all aspects of the host immune response. [Table 3] Dermatophytes can invade the dermal tissue, particularly after local trauma in patients with chronic infections.[88] In immune-deprived patients, dermatophytosis may involve the subcutaneous tissues and even deep organs, possibly becoming a life-threatening disease in the absence of appropriate treatment.[89]
A combination of impaired peripheral blood circulation, reduced oxygenation, and delay in migration of immune-competent cells or production of antimicrobial peptides (AMP) at the site of infection may favor the infectious process, which explains the 3X increased risk of dermatophytosis, especially foot and nail tinea in diabetes.[90]
A deranged barrier can lead to recurrences, hence epidermal barrier integrity is a crucial aspect that can determine a chronic infection.[1,18,67]
The pH of skin is important, and an alkaline pH can potentiate the virulence of the dermatophyte species.[21,22,23,24,25,26] Thus, the aggressive use of soaps can prove to be detrimental to the host defense, and thus the use of these products has no scientific rationale.[1] Moreover, providing sub-optimal concentrations of antifungals at the infection site can promote further resistance.[91] The acidic pH of skin is important to maintain the stratum corneum barrier function and thus acts as a resistance to invasion by dermatophytes.[92] It has been demonstrated that the skin pH at the site of infection in tinea pedis is higher than the normal skin pH.[93] A higher pH has also been demonstrated in the intertriginous regions of diabetic patients, providing a possible explanation for a higher incidence of candidal infections involving these sites.[94] The use of a syndet bar would help in maintaining the barrier function and a protective pH to withstand the infection with dermatophytes, and may thus be recommended.
The spread between family members, suggests (though it has not been scientifically proven) that the prevalent strain is transmitted through fomites. It is prudent to advise washing clothes at high temperatures (=60°C) as it is likely to be beneficial based on the results of in vitro experiments on various dermatophytes.[95]
A study on T. rubrum showed that the SLC11A1, RNASE7, and CSF2 genes were stimulated, and their products play a role in signaling and migration of immune cells and have potential antimicrobial activity. RNASE7 encodes for ribonuclease 7, which is part of the keratinocyte innate defense mechanisms. CSF2 encodes for granulocyte-macrophage colony-stimulating factor (GM-CSF), which is involved in recruiting immune cells to the site of infection and is part of the host innate immune response. SLC11A1 gene encodes an integral membrane protein involved in actuating macrophages and causing several effects on the signaling of the innate immune system such as TNFα, IL1-ß, among others. Genes that are involved in the maintenance of epithelial barrier integrity such as FLG and KRT1 were inhibited. This study revealed that modulation of the genes involved in T. rubrum-host interaction could signify possible targets for the management of dermatophytoses.[96] Also, products that target Heat shock protein 90 (Hsp90) can also be used to treat T. rubrum infections.[27]
Conclusions
Studies on antifungal resistance in dermatophytes lag far behind those available for systemic fungi.[86] With increasing reports of resistance to terbinafine, the use of azoles (specifically itraconazole) for dermatophytoses is on an upward trend.[1,3] Even though Indian dermatologists have found that the western recommendations on the use of itraconazole for tinea corporis/cruris are proving inadequate to eradicate infections and thus higher doses and longer durations of treatment are being suggested, the MIC data and existing data on skin pK of antifungal drugs, reliably predicts efficacy to azoles.[1,97,98]
Almost all studies show sensitivity to itraconazole, implying that the solution to recalcitrant infection might need a synergism of antifungal drugs with the host immune response to clear the fungi.[3,4,91,99] Discovering the means by which the species evade the innate host defense and bypass the cellular immune response is the key to emerging effectual antifungal therapeutic modalities. More efficacious treatment for chronic dermatophytoses would therefore include aiming at the mechanisms by which the species restricts the immune response, such as the mechanism by which it inhibits TLRs, and finding a way to reestablish the cell-mediated immune response, such as reviving the macrophages phagocytic activity. Also, drugs that target the various virulence factors can help in treating dermatophytosis.
Here it is important to emphasize that although there is some experimental reasoning on the ability of T.rubrum to cause chronic minimally inflammatory disease, the presently prevalent species (T.interdigitale) barely has any such data to its credit. Thus, there is an emergent need to study the virulence factors of the prevalent strain. In essence, understanding the interaction of the immune response with the dermatophyte, can help supplant and supplement the existing pharmacological remedies that fail in recalcitrant dermatophytoses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the Squalene epoxidase (SQLE) gene. Mycoses. 2018;61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 2.Sardana K, Khurana A. Overview of Causes and Treatment of Recalcitrant Dermatophytoses. In: Sardana K, Khurana A, Garg S, Poojary S, editors. IADVL Manual on Management of Dermatophytoses. 1st ed. n. Delhi: CBS; 2018. pp. 80–105. [Google Scholar]
- 3.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, et al. Mutation in the Squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522–17. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabas Y, Xess I, Singh G, Pandey M, Meena S. Molecular identification and antifungal susceptibility patterns of clinical dermatophytes following CLSI and EUCAST guidelines. J Fungi. 2017;3:E17. doi: 10.3390/jof3020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preuett BL, Schuenemann E, Brown JT, Kovac ME, Krishnan SK, Abdel-Rahman SM. Comparative analysis of secreted enzymes between the anthropophilic- zoophilic sister species Trichophyton tonsurans and Trichophyton equinum. Fungal Bio l. 2010;114:429–37. doi: 10.1016/j.funbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Giddey K, Monod M, Barblan J, Potts A, Waridel P, Zaugg C, et al. Comprehensive analysis of proteins secreted by Trichophyton rubrum and Trichophyton violaceum under in vitro conditions. J Proteome Res. 2007;6:3081–92. doi: 10.1021/pr070153m. [DOI] [PubMed] [Google Scholar]
- 7.Corzo-León DE, Munro CA, MacCallum DM. An ex vivo Human Skin Model to Study Superficial Fungal Infections. Front Microbiol. 2019;10:1172. doi: 10.3389/fmicb.2019.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacGregor JM, Hamilton A, Hay RJ. Possible mechanisms of immune modulation in chronic dermatophytosis-an in vitro study. Br J Dermatol. 1992;127:233–8. doi: 10.1111/j.1365-2133.1992.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 9.Hay RJ. Dermatophytosis and other superficial mycosis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. n. New York: Churchill-Livingstone; 1995. pp. 2375–86. [Google Scholar]
- 10.Baldo A, Mathy A, Tabart J, Camponova P, Vermout S, Massart L, et al. Secreted subtilisin Sub3 from Microsporumcanis is required for adherence to but not for invasion of the epidermis. Br J Dermatol. 2010;162:990–7. doi: 10.1111/j.1365-2133.2009.09608.x. [DOI] [PubMed] [Google Scholar]
- 11.Monod M, Lechenne B, Jousson O, Grand D, Zaugg C, Stöcklin R, et al. Aminopeptidases and dipeptidyl-peptidases secreted by the dermatophyte Trichophyton rubrum. Microbiology. 2005;15:145–55. doi: 10.1099/mic.0.27484-0. [DOI] [PubMed] [Google Scholar]
- 12.Duek L, Kaufman G, Ulman Y, Berdicevsky I. The pathogenesis of dermatophyte infections in human skin sections. J Infect. 2004;48:175–80. doi: 10.1016/j.jinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman G, Horwitz BA, Duek L, Ullman Y, Berdicevsky I. Infection stages of the dermatophyte pathogen Trichophyton: Microscopic characterization and proteolytic enzymes. Med Mycol. 2007;45:149–55. doi: 10.1080/13693780601113618. [DOI] [PubMed] [Google Scholar]
- 14.Faway É, Cambier L, Mignon B, Poumay Y, Lambert de Rouvroit C. Modeling dermatophytosis in reconstructed human epidermis: A new tool to study infection mechanisms and to test antifungal agents. Med Mycol. 2017;55:485–494. doi: 10.1093/mmy/myw111. [DOI] [PubMed] [Google Scholar]
- 15.Faway É, Lambert de Rouvroit C, Poumay Y. In vitro models of dermatophyte infection to investigate epidermal barrier alterations. Exp Dermatol. 2018;27:915–22. doi: 10.1111/exd.13726. [DOI] [PubMed] [Google Scholar]
- 16.Peres NT, Maranhão FC, Rossi A, Martinez-Rossi NM. Dermatophytes: Host-pathogen interaction and antifungal resistance. An Bras Dermatol. 2010;85:657–67. doi: 10.1590/s0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Chandra S, Sharma M. Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses. 2012;55:410–5. doi: 10.1111/j.1439-0507.2011.02133.x. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JM, Pfeiffer S, Akaki T, Schröder JM, Kleine M, Neumann C, et al. Barrier function, epidermal differentiation and human b-defensin 2expression in tinea corporis. J Invest Dermatol. 2007;127:1720–7. doi: 10.1038/sj.jid.5700788. [DOI] [PubMed] [Google Scholar]
- 19.Grumbt M, Monod M, Yamada T, Hertweck C, Kunert J, Staib P. Keratin degradation by dermatophytes relies on cysteine dioxygenase and a sulfite efflux pump. J Invest Dermatol. 2013;133:1550–5. doi: 10.1038/jid.2013.41. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Rossi NM, Peres NT, Rossi A. Pathogenesis of Dermatophytosis: Sensing the Host Tissue. Mycopathologia. 2017;182:215–227. doi: 10.1007/s11046-016-0057-9. [DOI] [PubMed] [Google Scholar]
- 21.Orejas M, Espeso EA, Tilburn J, Sarkar S, Arst HN, Penalva MA. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 1995;9:1622–32. doi: 10.1101/gad.9.13.1622. [DOI] [PubMed] [Google Scholar]
- 22.Maccheroni W, Jr, May GS, Martinez-Rossi NM, Rossi A. The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene. 1997;194:163–7. doi: 10.1016/s0378-1119(97)00095-4. [DOI] [PubMed] [Google Scholar]
- 23.Calcagno-Pizarelli AM, Negrete-Urtasun S, Denison SH, Rudnicka JD, Bussink HJ, Múnera-Huertas T, et al. Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot Cell. 2007;6:2365–75. doi: 10.1128/EC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galindo A, Hervas-Aguilar A, Rodriguez-Galan O, Vincent O, Arst HN, Jr, Tilburn J, et al. PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32. Traffic. 2007;8:1346–64. doi: 10.1111/j.1600-0854.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hervas-Aguilar A, Rodriguez JM, Tilburn J, Arst HN, Jr, Pen˜alva MA. Evidence for the direct involvement of theproteasome in the proteolytic processing of the Aspergillusnidulans zinc finger transcription factor PacC. J BiolChem. 2007;282:34735–47. doi: 10.1074/jbc.M706723200. [DOI] [PubMed] [Google Scholar]
- 26.Galindo A, Calcagno-Pizarelli AM, Arst HN, Jr, Penalva MA. An ordered pathway for the assembly of fungalESCRT-containing ambient pH signalling complexes atthe plasma membrane. J Cell Sci. 2012;125:1784–95. doi: 10.1242/jcs.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob TR, Peres NT, Martins MP, Lang EA, Sanches PR, Rossi A, et al. Heat shock protein 90 (Hsp90) as a molecular target for the development of novel drugs against the dermatophyte Trichophyton rubrum. Front Microbiol. 2015;6:1241. doi: 10.3389/fmicb.2015.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta AK, Ahmad I, Borst I, Summerbell RC. Detection of xanthomegnin in epidermal materials infected with Tricho-phyton rubrum. J Invest Dermato. 2000;115:901–5. doi: 10.1046/j.1523-1747.2000.00150.x. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Martinez R, Manzano-Gayosso P, Mier T, Mendez-Tovar LJ, Hernandez-Hernandez F. Exoenzymes of dermatophytes isolated from acute and chronic tinea. Rev Latinoam Microbiol. 1994;36:17–20. [PubMed] [Google Scholar]
- 30.Almeida SR. Immunology of dermatophytosis. Mycopathologia. 2008;166:277–83. doi: 10.1007/s11046-008-9103-6. [DOI] [PubMed] [Google Scholar]
- 31.Brilhante RS, Correia EE, Guedes GM, de Oliveira JS, Castelo-Branco DD, Cordeiro RD, et al. In vitro activity of azole derivatives and griseofulvin against planktonic and biofilm growth of clinical isolates of dermatophytes. Mycoses. 2018;61:449–454. doi: 10.1111/myc.12763. [DOI] [PubMed] [Google Scholar]
- 32.Underhill DM, Iliev ID. The mycobiota: Interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147:176–83. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura Y, Kano R, Hasegawa A, Watanabe S. Interleukin-8 and tumor necrosis factor alpha production in human epidermal keratinocytes induced by Trichophyton mentagrophytes. ClinDiagn Lab Immunol. 2002;9:935–7. doi: 10.1128/CDLI.9.4.935-937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiraki Y, Ishibashi Y, Hiruma M, Nishikawa A, Ikeda S. Cytokine secretion profiles of human keratinocytes during Trichophyton tonsurans and Arthroderma benhamiae infections. J Med Microbiol. 2006;55:1175–1185. doi: 10.1099/jmm.0.46632-0. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa H, Summerbell RC, Clemons KV, Koga T, Ran YP, Rashid A, et al. Dermatophytes and host defence in cutaneous mycoses. Med Mycol. 1998;36:166–73. [PubMed] [Google Scholar]
- 38.Deng W, Liang P, Zheng Y, Su Z, Gong Z, Chen J, et al. Differential gene expression in HaCaT cells may account for the various clinical presentation caused by anthropophilic and geophilic dermatophytes infections. Mycoses. 2020;63(1):21–29. doi: 10.1111/myc.13021. [DOI] [PubMed] [Google Scholar]
- 39.Koga T, Ishizaki H, Matsumoto T, Hori Y. In vitro release of interferon-gamma by peripheral blood mononuclear cells of patients with dermatophytosis in response to stimulation with trichophytin. Br J Dermatol. 1993;128:703–704. doi: 10.1111/j.1365-2133.1993.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 40.Bressani VO, Santi TN, Domingues-Ferreira M, Almeida A, Duarte AJ, Moraes-Vasconcelos D. Characterization of the cellular immunity in patients presenting extensive dermatophytoses due to Trichophyton rubrum. Mycoses. 2013;56:281–288. doi: 10.1111/myc.12018. [DOI] [PubMed] [Google Scholar]
- 41.Mignon B, Tabart J, Baldo A, Mathy A, Losson B, Vermout S. Immunization and dermatophytes. CurrOpin Infect Dis. 2008;21:134–40. doi: 10.1097/QCO.0b013e3282f55de6. [DOI] [PubMed] [Google Scholar]
- 42.Tani K, Adachi M, Nakamura Y, Kano R, Makimura K, Hasegawa A, et al. The effect of dermatophytes on cytokine production by human keratinocytes. Arch Dermatol Res. 2007;299:381–7. doi: 10.1007/s00403-007-0780-7. [DOI] [PubMed] [Google Scholar]
- 43.Reis APC, Correia FF, Jesus TM, Pagliari C, Sakai-Valente NY, Belda Júnior W, et al. In situ immune response in human dermatophytosis: Possible role of Langerhans cells (CD1a+) as a risk factor for dermatophyte infection. Rev Inst Med Trop Sao Paulo. 2019;61:e56. doi: 10.1590/S1678-9946201961056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 45.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Santiago K, Bomfim GF, Criado PR, Almeida SR. Monocyte-derived dendritic cells from patients with dermatophytosisrestrict the growth of Trichophyton rubrumand induce CD4-T cell activation. PLoS One. 2014;9(11):e110879. doi: 10.1371/journal.pone.0110879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown G. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 48.Celestrino GA, Reis APC, Criado PR, Benard G, Sousa MGT. Trichophyton rubrum Elicits Phagocytic and Pro-inflammatory Responses in Human Monocytes Through Toll-Like Receptor 2. Front Microbiol. 2019;10:2589. doi: 10.3389/fmicb.2019.02589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brasch J. Current knowledge of host response in human tinea. Mycoses. 2009;52(4):304–12. doi: 10.1111/j.1439-0507.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 50.Heddergott C, Bruns S, Nietzsche S, Leonhardt I, Kurzai O, Kniemeyer O, et al. The Arthrodermabenhamiae hydrophobin HypA mediates hydrophobicity and influences recognition by human immune effector cells. Eukaryot Cell. 2012;11:673–82. doi: 10.1128/EC.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinen MP, Cambier L, Antoine N, Gabriel A, Gillet L, Bureau F, et al. Th1 and Th17 Immune Responses Act Complementarily to Optimally Control Superficial Dermatophytosis. J Invest Dermatol. 2019;139(3):626–637. doi: 10.1016/j.jid.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 52.Verma AH, Gaffen SL. Dermatophyte Immune Memory Is Only Skin-Deep. J Invest Dermatol. 2019;139(3):517–519. doi: 10.1016/j.jid.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodfolk JA, Platts-Mills TA. The immune response to dermatophytes. Res Immunol. 1998;149:436–45. doi: 10.1016/s0923-2494(98)80767-0. [DOI] [PubMed] [Google Scholar]
- 54.Waldman A, Segal R, Berdicevsky I, Gilhar A. CD4+ and CD8+T cells mediated direct cytotoxic effect against Trichophyton rubrum and Trichophyton mentagrophytes. Int J Dermatol. 2010;49:149–57. doi: 10.1111/j.1365-4632.2009.04222.x. [DOI] [PubMed] [Google Scholar]
- 55.Traynor TR, Huffnagle GB. Role of chemokines in fungal infections. Med Mycol. 2001;39:41–50. doi: 10.1080/mmy.39.1.41.50. [DOI] [PubMed] [Google Scholar]
- 56.Al HM, Fitzgerald SM, Saoudian M, Krishnaswamy G. Dermatology for the practicing allergist, Tinea pedis and its complications. ClinMol Allergy. 2004;2:5. doi: 10.1186/1476-7961-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saraiva M, O'garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 58.Venturini J, Alvares AM, Camargo MR, Marchetti CM, de Campos Fraga-Silva TF, Luchini AC, et al. Dermatophyte-host relationship of a murine model of experimental invasive dermatophytosis. Microbes Infect. 2012;14:1144–51. doi: 10.1016/j.micinf.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Gaffen SL, Hernandez-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huppler AR, Conti HR, Hernandez-Santos N, Darville T, Biswas PS, Gaffen SL. Role of neutrophils in IL-17 dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cambier L, Weatherspoon A, Defaweux V, Bagut ET, Heinen MP, Antoine N, et al. Assessment of the cutaneous immune response during Arthrodermabenhamiae and A. vanbreuseghemii infection using an experimental mouse model. Br J Dermatol. 2014;170:625–33. doi: 10.1111/bjd.12673. [DOI] [PubMed] [Google Scholar]
- 62.Burstein VL, Guasconi L, Beccacece I, Theumer MG, Mena C, Prinz I, et al. IL-17–Mediated Immunity Controls Skin Infection and T Helper 1 Response during Experimental Microsporum canis Dermatophytosis. J Invest Dermatol. 2018;138(8):1744–53. doi: 10.1016/j.jid.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 63.Heinen MP, Cambier L, Antoine N, Gabriel A, Gillet L, Bureau F, et al. Th1 and Th17 Immune Responses Act Complementarily to Optimally Control Superficial Dermatophytosis. J Invest Dermatol. 2019;139(3):626–637. doi: 10.1016/j.jid.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 64.Rai G, Das S, Ansari MA, Singh PK, Pandhi D, Tigga RA, et al. The interplay among Th17 and T regulatory cells in the immune dysregulation of chronic dermatophytic infection. Microb Pathog. 2020;139:103921. doi: 10.1016/j.micpath.2019.103921. [DOI] [PubMed] [Google Scholar]
- 65.Heinen MP, Cambier L, Fievez L, Mignon B. Are Th17 Cells Playing a Role in Immunity to Dermatophytosis. Mycopathologia. 2017;182(1-2):251–261. doi: 10.1007/s11046-016-0093-5. [DOI] [PubMed] [Google Scholar]
- 66.Dahl MV, Grando SA. Chronic dermatophytosis: What is special about Trichophyton rubrum? AdvDermatol. 1994;9:97–109. [PubMed] [Google Scholar]
- 67.Beauvais A, Monod M, Wyniger J, Debeaupuis JP, Grouzmann E, Brakch N, et al. Dipeptidyl-peptidase IV secreted by Aspergillus fumigatus, a fungus pathogenic to humans. Infect Immun. 1997;65:3042–7. doi: 10.1128/iai.65.8.3042-3047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodfolk JA, Sung SS, Benjamin DC, Lee JK, Platts-Mills TA. Distinct human T cell repertoires mediate immediate and delayed-type hypersensitivity to the Trichophyton antigen, Tri r 2. J Immunol. 2000;165:4379–87. doi: 10.4049/jimmunol.165.8.4379. [DOI] [PubMed] [Google Scholar]
- 69.Woodfolk JA, Wheatley LM, Piyasena RV, Benjamin DC, Platts-Mills TA. Trichophyton antigens associated with IgE antibodies and delayed type hypersensitivity. Sequence homology to two families of serine proteinases. J Biol Chem. 1998;273:29489–96. doi: 10.1074/jbc.273.45.29489. [DOI] [PubMed] [Google Scholar]
- 70.Woodfolk JA. Allergy and dermatophytes. Clin Microbiol Rev. 2005;18:30–43. doi: 10.1128/CMR.18.1.30-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marconi VC, Kradin R, Marty FM, Hospenthal DR, Kotton CN. Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: Case report and review of the literature. Med Mycol. 2010;48:518–27. doi: 10.3109/13693780903213512. [DOI] [PubMed] [Google Scholar]
- 72.Hay RJ, Baran R. Deep dermatophytosis: Rare infections or common, but unrecognised, complications of lymphatic spread? Curr Opin Infect Dis. 2004;17:77–9. doi: 10.1097/00001432-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Koga T. Immune surveillance against dermatophyte infection. In: Fidel PL, Huffnagle GB, editors. Fungal Immunology: From an Organ Perspective. 1st edn. New York: Springer; 2005. pp. 443–452. [Google Scholar]
- 74.Calderon RA. Immunoregulation in dermatophytosis. Crit RevMicrobiol. 1989;16:339–68. doi: 10.3109/10408418909104472. [DOI] [PubMed] [Google Scholar]
- 75.Gupta AK, Konnikov N, MacDonald P, Rich P, Rodger NW, Edmonds MW, et al. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: A multicentre survey. Br J Dermatol. 1998;139:665–71. doi: 10.1046/j.1365-2133.1998.02464.x. [DOI] [PubMed] [Google Scholar]
- 76.King RD, Khan HA, Foye JC, Greenberg JH, Jones HE. Transferrin, iron, and dermatophytes. I. Serum dermatophyte inhibitory component definitively identified as unsaturated transferrin. J Lab Clin Med. 1975;86:204–12. [PubMed] [Google Scholar]
- 77.Rouzaud C, Hay R, Chosidow O, Dupin N, Puel A, Lortholary O, et al. Severe dermatophytosis and acquired or innate immunodeficiency: A review. J Fungi. 2015;2:4. doi: 10.3390/jof2010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flammer JR, Rogatsky I. Minireview: Glucocorticoids in autoimmunity: Unexpected targets and mechanisms. Mol Endocrinol. 2011;25:1075–86. doi: 10.1210/me.2011-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–46. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 80.García-Romero MT, Granados J, Vega-Memije ME, Arenas R. Analysis of genetic polymorphism of the HLA-B and HLA-DR loci in patients with dermatophytic onychomycosis and in their first-degree relatives. Actas Dermosifiliogr (English Edition) 2012;103(1):59–62. doi: 10.1016/j.adengl.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 81.Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaradat SW, Cubillos S, Krieg N, Lehmann K, Issa B, Piehler S, et al. Low DEFB4 copy number and high systemic hBD-2 and IL-22 levels are associated with dermatophytosis. J Invest Dermatol. 2015;135:750–758. doi: 10.1038/jid.2014.369. [DOI] [PubMed] [Google Scholar]
- 83.Hay RJ, Reid S, Talwet E, Macnamara K. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581–9. doi: 10.1111/j.1365-2133.1983.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 84.Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am AcadDermatol. 1993;28:S19–S23. doi: 10.1016/s0190-9622(09)80303-4. [DOI] [PubMed] [Google Scholar]
- 85.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, Van Krieken JH, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 86.Sousa MD, Santana GB, Criado PR, Benard G. Chronic widespread dermatophytosis due to Trichophyton rubrum: A syndrome associated with a Trichophyton-specific functional defect of phagocytes. Front Microbiol. 2015;6:801. doi: 10.3389/fmicb.2015.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Criado PR, Oliveira CB, Dantas KC, Takiguti FA, Benini LV, Vasconcellos C. Superficial mycosis and the immune response elements. An Bras Dermatol. 2011;86:726–31. doi: 10.1590/s0365-05962011000400015. [DOI] [PubMed] [Google Scholar]
- 88.Smith KJ, Neafie RC, Skelton HG, III, Barrett TL, Graham JH, Lupton GP. Majocchi's granuloma. J Cutan Pathol. 1991;18:28–35. doi: 10.1111/j.1600-0560.1991.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 89.Squeo RF, Beer R, Silvers D, Weitzman I, Grossman M. Invasive Trichophyton rubrum resembling blastomycosis infection in the immunocompromised host. J Am Acad Dermatol. 1998;39:379–80. doi: 10.1016/s0190-9622(98)70396-2. [DOI] [PubMed] [Google Scholar]
- 90.Gupta AK, Konnikov N, MacDonald P, Rich P, Rodger NW, Edmonds MW, et al. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: A multicentre survey. Br J Dermatol. 1998;139:665–71. doi: 10.1046/j.1365-2133.1998.02464.x. [DOI] [PubMed] [Google Scholar]
- 91.Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med. 2014;5:a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93:261–7. doi: 10.2340/00015555-1531. [DOI] [PubMed] [Google Scholar]
- 93.Chikakane K, Takahashi H. Measurement of skin pH and its significance in cutaneous diseases. Clin Dermatol. 1995;13:299–306. doi: 10.1016/0738-081x(95)00076-r. [DOI] [PubMed] [Google Scholar]
- 94.Yosipovitch G, Tur E, Cohen O. Skin surface pH in intertriginous areas in NIDDM patients. Possible correlation to candidal intertrigo. Diabetes Care. 1993;16:560–563. doi: 10.2337/diacare.16.4.560. [DOI] [PubMed] [Google Scholar]
- 95.Essien J, Jonah I, Umoh A, Eduok S, Akpan E, Umoiyoho A. Heat resistance of dermatophyte's conidiospores from athletes kits stored in Nigerian University Sport's Center. Acta Microbiol Immunol Hung. 2009;56:71–9. doi: 10.1556/AMicr.56.2009.1.5. [DOI] [PubMed] [Google Scholar]
- 96.Petrucelli M, Peronni K, Sanches P, Komoto TT, Matsuda JB, Silva WA, et al. Dual RNA-Seq Analysis of Trichophyton rubrum and HaCat Keratinocyte Co-Culture Highlights Important Genes for Fungal-Host Interaction. Genes. 2018;9:362. doi: 10.3390/genes9070362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rajagopalan M, Inamadar A, Mittal A, Miskeen AK, Srinivas CR, Sardana K, et al. Expert Consensus on The Management of Dermatophytosis in India (ECTODERM India) BMC Dermatol. 2018;18:6. doi: 10.1186/s12895-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sardana K, Arora P, Mahajan K. Intracutaneous pharmacokinetics of oral antifungals and their relevance in recalcitrant cutaneous dermatophytosis: Time to revisit basics. Indian J Dermatol Venereol Leprol. 2017;83:730–732. doi: 10.4103/ijdvl.IJDVL_1012_16. [DOI] [PubMed] [Google Scholar]
- 99.Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is antifungal resistance a cause for treatment failure in dermatophytosis: A study focused on tinea corporis and cruris from a tertiary centre. Indian Dermatology Online J. 2018;9:90–95. doi: 10.4103/idoj.IDOJ_137_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


