Abstract
The prevalence of deoxynivalenol (DON) is a concern for swine producers, and although there has been extensive research into the effects of DON in pigs, focus has been in young pigs and/or in short-term studies. The objective of the study was to determine the effect of long-term exposure to DON-contaminated diets in finisher pigs. A total of 200 pigs (76.6 ± 3.9 kg initial weight) were group housed (five pigs per pen; n = 10 pens/treatment) in a 6-wk trial. Pigs were fed a wheat-barley-soybean meal-based control (CONT) diet with no DON or the basal diet in which clean wheat was replaced by DON-contaminated wheat and wheat screenings to provide DON content of 1, 3, or 5 ppm (DON1, DON3, and DON5, respectively). Individual BW and pen feed intake were recorded weekly to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F). Blood was collected on days 0, 14, and 43 and analyzed for indicators of liver and kidney health. Nitrogen (N)-balance was conducted immediately following the growth performance period to determine the effect of DON on nutrient utilization. Blood and urine samples collected during N balance were analyzed for DON content. Feeding DON reduced (P < 0.05) ADFI and ADG from days 0 to 28 compared with CONT, after which there was no effect of diet on ADFI and ADG. The G:F was lower (P < 0.05) in DON5 fed pigs compared with all treatments during days 0 to 7; however, no treatment effects on G:F was observed from days 8 to 42. Nitrogen retention was lower (P < 0.05) in DON3 and DON5 compared with DON1-fed pigs. Nitrogen retention efficiency was higher (P < 0.05) in DON1 compared with DON3 and DON5 and protein deposition for DON1 pigs was higher (P < 0.05) than all treatments. There were no treatment effects on indicators of liver and kidney health. As dietary DON intake increased, concentration of DON in blood and urine increased. Overall, although there was an initial decrease in ADG and ADFI in pigs receiving diets containing >1 ppm DON, pig performance recovered after a period of time, whereas nutrient utilization continued to be affected after recovery of performance. Moreover, the lack of DON on G:F indicates that the negative effects of DON on growth performance are largely due to reduced feed intake. Overall, although pigs maybe capable of adapting to intake of DON-contaminated diets, their final body weight will be reduced when fed diets containing >1 ppm DON.
Keywords: average daily gain, average daily feed intake, deoxynivalenol, gain:feed, nitrogen balance, swine
Introduction
Mycotoxins are secondary metabolites of fungi that cause adverse physiological effects in humans and animals when ingested. Although many mycotoxins have been identified, the mycotoxin deoxynivalenol (DON) is one of the most significant mycotoxins in agriculture, as it contaminates common feed grains, such as corn, wheat, oats, and barley (Rotter et al., 1996; Chaytor et al., 2011). In North America, it was reported that 85% of all grain samples analyzed and about 90% of complete feed samples contained DON (Gruber-Dorninger et al., 2019). There is evidence that DON consumption in animals results in reduced feed intake, digestive dysfunction (e.g., gastroenteritis, gastrointestinal tract lesions, reduced nutrient absorption), immune suppression, and reduced growth performance (Pinton et al., 2008; Serviento et al., 2018). Although all species respond to DON exposure, pigs are particularly susceptible (Wu et al., 2010). Previous studies have reported that DON ingestion in pigs causes a reduction in average daily feed intake (ADFI) and body weight gain (Goyarts et al., 2005; Ghareeb et al., 2015). This has also been shown to cause intestinal damage, which may lead to reduced intestinal barrier function and increased susceptibility to enteric pathogens, further resulting in reduced nutrient absorption and utilization (Pinton et al., 2009; Ghareeb et al., 2015). To date, most studies on the effects of DON in swine have been completed in weaned pigs with the assumption that the physiological effects of consuming DON are highest in the young animal (Dersjant-Li et al., 2003). As reported by Andretta et al. (2012), the average initial age of pigs used in DON challenge studies was 44 d, with approximately 64% of these studies completed in the nursery phase, 18% in the grower phase, and only 3% in the finisher phase. Moreover, the majority of studies have examined the impact of DON over a relatively short period of time (Dersjant-Li et al., 2003). Therefore, there is a need to evaluate the impact of DON contamination in older pigs (finishing pigs) and over an extended period. It may be possible that due to the overall higher feed intake in the finishing period, the effects of DON may be greater in this production stage. However, it has also been suggested that pigs may adapt to DON-contaminated feed (Rotter et al., 1994).
A necessary step in mitigating the effects of DON in swine production starts with accurately determining DON content in feed ingredients and complete feeds. Unfortunately, there are high discrepancies and variability when it comes to testing for DON as previously reported (Whitaker et al., 2000; Beaulieu et al., 2009; Kong et al., 2015). Sampling methods such sampling time and location, batches sampled, and the type of samples (grain dust vs. actual grains) will all affect the accuracy of test results (Whitaker et al., 2000). Harvesting and storage conditions have with also been reported to play a role in the inconsistencies analysis DON concentration in diet or ingredient samples (Champeil et al., 2004). With these challenges in mind, it is worth considering the potential for determining DON exposure through analysis of biological samples. For example, previous studies have shown that there is a strong correlation between the intake of DON and its presence in excretions (e.g., urine; Dänicke and Brezina, 2013). Therefore, the objectives of the present study were to determine the effect of long-term exposure of finishing pigs to DON-contaminated diets on growth performance, nutrient utilization, organ health, and DON content in biological samples.
Materials and Methods
The experimental protocol used in the present study was reviewed and approved by the Animal Research Ethics Board of the University of Saskatchewan (no. 20130054) and followed the guidelines of Canadian Council on Animal Care (CCAC, 2009).
Animals, housing, diets, and experimental design
A total of 200 mixed-sex finishing pigs (Camborough Plus × C337; PIC, Canada) with initial body weight (BW) of 76.6 ± 3.9 kg were used in a 42-d study at the Prairie Swine Centre, Inc. (Saskatoon, SK, Canada). The pigs were group housed in pens (five pigs per pen) in environmentally controlled rooms. The pens were randomly assigned to one of four dietary treatments (n = 10 pens/treatment; Table 1), which consisted of a control diet (CONT) containing no (or 0 ppm) DON or a diet containing 1, 3, or 5 ppm DON (DON1, DON3, or DON5, respectively). All diets were wheat-barley-soybean meal-based and formulated to be isonitrogenous and isoenergetic and met or exceeded nutrient requirements according to National Research Council (NRC, 2012). The DON-contaminated diets were formulated by replacing DON-free wheat with appropriate amounts of DON-contaminated wheat and wheat screenings. Prior to diet manufacture, the concentration of DON and other mycotoxins was determined in ingredients by Central Testing Laboratory, Ltd. (Winnipeg, MB, Canada) in composite samples prepared from single batches of the ingredients. All diets were made in separate batches, and diets with the least amount of DON were made first to prevent contamination. Pigs were fed ad libitum and had free access to water for the duration of the study.
Table 1.
Ingredient composition and calculated and analyzed nutrient content of experimental diets1 (as-fed basis)
| Ingredient, % | CONT | DON1 | DON3 | DON5 |
|---|---|---|---|---|
| Barley | 44.0 | 44.0 | 44.0 | 44.0 |
| Wheat | 40.0 | 33.3 | 20.0 | 6.7 |
| DON wheat2 | 0.0 | 4.9 | 14.8 | 24.7 |
| Wheat screenings3 | 0.0 | 1.7 | 5.2 | 8.6 |
| Soybean meal | 10.0 | 10.0 | 10.0 | 10.0 |
| Canola oil | 3.5 | 3.5 | 3.5 | 3.5 |
| l-Lysine–HCl | 0.30 | 0.30 | 0.30 | 0.30 |
| dl-Methionine | 0.07 | 0.07 | 0.07 | 0.07 |
| l-Threonine | 0.10 | 0.10 | 0.10 | 0.10 |
| Limestone | 0.8 | 0.8 | 0.8 | 0.8 |
| Dicalcium phosphate | 0.5 | 0.5 | 0.5 | 0.5 |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin/mineral premix4 | 0.2 | 0.2 | 0.2 | 0.2 |
| Calculated nutrient content5 | ||||
| ME, kcal/kg | 3282 | 3282 | 3282 | 3282 |
| Dry matter, % | 86.49 | 86.51 | 86.54 | 86.58 |
| Crude protein, % | 15.9 | 15.9 | 15.9 | 16.0 |
| Lysine, % SID6 | 0.76 | 0.76 | 0.76 | 0.76 |
| Calcium, % | 0.50 | 0.50 | 0.50 | 0.50 |
| Phosphorus, % | 0.48 | 0.48 | 0.48 | 0.48 |
| Analyzed nutrient content | ||||
| Growth performance diet | ||||
| Dry matter, % | 88.9 | 88.2 | 88.3 | 88.8 |
| Crude protein, % | 14.6 | 14.2 | 13.5 | 14.8 |
| N-balance diet7 | ||||
| Dry matter, % | 88.5 | 88.1 | 88.9 | 88.7 |
| Crude protein, % | 14.5 | 14.6 | 14.1 | 14.7 |
1CONT, 0 DON; DON1, 1 ppm DON; DON3, 3 ppm DON; DON5, 5 ppm DON.
2DON wheat contains 6.9 ppm DON (Central Testing Laboratory, Winnipeg, MB, Canada).
3Wheat screenings contain 32.8 ppm DON (Central Testing Laboratory, Winnipeg MB, Canada).
4Supplied per kg of complete diet; vitamin A, 8,000 IU; vitamin D, 1,500 IU; vitamin E, 30 IU; menadione, 2.5 mg; vitamin B12, 0.025 mg; thiamine, 1.00 mg; biotin, 0.10 mg; niacin, 20 mg; riboflavin, 4 mg; pantothenate; 12 mg; folic acid, 0.50 mg; pyridoxine, 2.0 mg; Fe, 100 mg; Zn, 100 mg; Mg, 40 mg; Cu, 15 mg; Se, 0.30 mg; and I, 1 mg.
5Nutrient content of diets based on the nutrient content of feed ingredients according to NRC (2012) and analysis of nutrient content of wheat screenings (Central Laboratory Testing, Ltd, Winnipeg, MB, Canada).
6SID, standardized ileal digestible.
7Nitrogen balance diet contained 0.4% celite as a marker added at the expense of wheat.
Growth performance and nitrogen balance
Individual pig BW were measured at the start of the experiment (initial BW) and weekly for the duration of the study for determination of average daily gain (ADG). Each week, feed intake was recorded and the ADFI determined. The gain to feed ratio (gain:feed [G:F]) was calculated from the weekly ADG and ADFI.
On day 35 of the growth performance period, one barrow per pen (identified and marked at the start of the experiment for serial blood sampling and nitrogen [N]-balance) was individually housed in metabolism crates (56” × 58.5”) in a temperature-controlled room (21 ± 2 °C) for N-balance collection (n = 10/treatment). The selected pigs remained on their original dietary treatment during the N-balance period and the same experimental diets were fed except for the inclusion of celite as an indigestible marker at the expense of uncontaminated wheat. Pigs were fed at 2.8 × maintenance energy requirement for metabolizable energy (197 kcal/kg BW0.60/d; NRC, 2012) and fed in two equal meals each day at 0700 and 1500 h. After a 7-d dietary and environmental adaptation, total urine and fresh-fecal samples were collected over a 2-d N-balance period. During the sample collection period, urine was collected quantitatively over two 24-h periods via urine jars placed beneath the urine collection trays containing a sufficient amount of HCl to maintain pH < 3 (Columbus et al., 2014). At the end of each 24-h period, urine was weighed and a 5% aliquot was sampled and stored at −20 °C. Fresh-fecal samples were taken daily by rectal palpation and immediately stored at −20 °C. At the end of the 2-d sample collection period, urine samples were thawed and pooled for each pig, filtered with glass wool to remove any debris, and a 5% subsample was obtained and stored at −20 °C until further analysis. Similarly, fecal samples were thawed, pooled for each pig and homogenized, and a subsample taken and stored at −20 °C until further analysis.
Blood sampling
Blood samples were obtained from the representative pigs selected at the start of the experiment on days 0, 14 (growth performance period), and 43 (on day 1 of N-balance period, 3 to 4 h after the morning meal) via jugular puncture into heparin-coated and additive-free tubes (5 mL; BD vacutainer tubes, Mississauga, ON, Canada). Blood collected into additive-free tubes was allowed to clot, and then blood samples were then centrifuged at 2,500 × g for 15 min to harvest plasma and serum samples, after which they were stored at −20 °C until further analyses.
Analytical procedures
Analysis of feed, fecal, and urine samples
Dry matter content of the diet and fecal samples were analyzed according to method 930.15 of AOAC (2007). Nitrogen content in diet, feces, and urine samples were analyzed using an automatic analyzer (LECO FP 528; MI, USA; AOAC, 2007; Method 990.03). The acid-insoluble ash content of both diet and fecal samples were analyzed according to the method described previously (Van Keulen and Young, 1977). The complete experimental diets were analyzed for DON and other mycotoxins at the University of Natural Resources and Life Sciences, Vienna (Tulln, Austria; Table 2) according to Sulyok et al. (2020). All analyses were performed in duplicate.
Table 2.
Analyzed mycotoxin contents of experimental diets1 (as-fed basis)
| Finisher diet | ||||||||
|---|---|---|---|---|---|---|---|---|
| Growth performance diets | N-balance diets | |||||||
| Mycotoxin, ppm | CONT | DON1 | DON3 | DON5 | CONT | DON1 | DON3 | DON5 |
| Deoxynivalenol | 0.11 | 1.34 | 3.59 | 5.72 | 1.56 | 1.32 | 3.09 | 4.94 |
| 3-acetyldeoxynivalenol | ND2 | ND | ND | ND | ND | ND | ND | ND |
| 15-acetyldeoxynivalenol | ND | ND | ND | ND | ND | ND | ND | ND |
| HT-2 toxin | ND | ND | ND | 0.050 | ND | ND | ND | 0.03 |
| Nivalenol | 0.15 | 0.18 | 0.53 | 0.64 | 0.12 | 0.11 | 0.12 | 0.08 |
| Ochratoxin A | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.03 | 0.07 | 0.09 |
| Zearalenone | ND | 0.002 | 0.009 | 0.014 | 0.003 | 0.002 | 0.009 | 0.013 |
| Total Ergot alkaloids | 0.99 | 0.57 | 1.03 | 1.26 | 0.24 | 0.16 | 0.39 | 0.67 |
1CONT, 0 DON; DON1, 1 ppm DON; DON3, 3 ppm DON; DON5, 5 ppm DON.
2ND, not detected or below limit of detection.
Serum analysis for kidney and liver metabolites
Serum harvested from blood samples taken on days 0 and 14 of the growth performance period and day 43 (N-balance period) were analyzed for key indicators of liver and kidney function and health using an automatic blood chemistry analyzer (Prairie Diagnostic Services).
Deoxynivalenol analysis in serum and urine samples
All mycotoxin analysis in biological matrices was performed at the laboratory of the BIOMIN Research Center (Tulln, Austria). Analytical standards for DON were acquired commercially (Romer Labs GmbH, Tulln, Austria). Phosphate-buffered saline (PBS) was obtained from Sigma–Aldrich (Vienna, Austria), methanol (MeOH) and acetic acid from VWR International (Vienna, Austria), and acetonitrile from Chem-Lab NV (Zedelgem, Belgium). In urine, direct quantification of DON was performed. The determination of the analytes was performed on a QTRAP 6500 using an HPLC–MS/MS-based method described previously (Schwartz-Zimmermann et al., 2017). The samples were measured in duplicate. Serum samples obtained from pigs during the N-balance collection were subjected to indirect quantification of DON via enzymatic pretreatment. To this end, 35 mg of β-glucuronidase (Escherichia coli, Type IX-A; Sigma–Aldrich) was dissolved in 2.5-mL PBS and added at 50 µL per 100-µL serum. After incubation for 18 h (37 °C, 80 rpm), 300 µL of MeOH/acetic acid (99.8/0.2, v/v) was added. HPLC–MS/MS analysis was performed as described for urine samples.
Statistical Analyses
All data were verified for normality using the PROC UNIVARIATE (SAS Institute, Cary, NC, Version 9.4), and outliers were tested using the studentized residual analysis. The growth performance data were initially analyzed as a repeated measure with treatment, time, and treatment × time interactions included in the model; however, since there were no significant treatment × time interactions, the data were reanalyzed as a randomized complete block design with the fixed effect of dietary treatment (CONT, DON1, DON3, and DON5) and block (room) as a random variable (PROC MIXED, SAS Institute, Version 9.4). The N-balance data were also analyzed as a randomized complete block design with the fixed effect of dietary treatments (CONT, DON1, DON3, and DON5) and the random variable was the block (room) (PROC MIXED, SAS Institute, Version 9.4). The blood chemistry data were analyzed as a repeated measure with day as the repeated variable. Regression analysis was used to describe relationships between DON intake and ADG, total BW gain relative to CONT, urinary DON output, and DON content in serum (PROC REG, SAS Institute, Version 9.4). The Tukey–Kramer mean separation test was used to separate means, and differences between means were considered significant at P ≤ 0.05 and a trend toward significance was considered at 0.05 > P < 0.10.
Results
Calculated vs. analyzed DON levels in diets
Although the majority of diets contained similar DON content compared with formulated values, the high DON content in the CONT diet used in the N balance (Table 2) was unexpected and further indicates the variability in DON content and the difficulty in accurately determining DON content in dietary ingredients, as the same ingredients, including the same clean wheat, DON-contaminated wheat, and DON-contaminated wheat screenings, were used in all diets.
Growth performance
Growth performance data are presented in Table 3. Initial BW (day 0) was not different among the dietary treatments (P > 0.05). BW was reduced in DON3 and DON5-fed pigs by day 7 of the study compared with CONT, with the greatest reduction observed with DON5 (P > 0.05). This reduction in BW was maintained throughout the study. For the duration of the study, ADG in DON1-fed pigs was not different than pigs receiving the CONT diet (P > 0.05). From days 0 to 7, DON3-fed pigs had reduced growth compared with both CONT- and DON1-fed pigs (P < 0.05) but were not different from CONT-fed pigs from days 8 to 42 (P > 0.05). Pigs fed DON5 had reduced ADG from days 0 to 21 compared with all other dietary treatments (P < 0.05). From days 22 to 28, ADG of DON5-fed pigs was not different than DON1- and DON3-fed pigs (P > 0.05). From days 29 to 42, there were no differences in ADG among dietary treatments (P > 0.05). Overall (days 0 to 42), ADG was reduced in DON3- and DON5-fed pigs compared with both CONT- and DON1-fed pigs, with the greatest reduction observed with DON5 (P < 0.05). There was no impact of DON1 on ADFI compared with CONT (P > 0.05). From days 0 to 7, DON3-fed pigs had reduced ADFI compared with both CONT- and DON1-fed pigs (P < 0.05), after which no difference was observed (P > 0.05). In DON5-fed pigs, ADFI was reduced from days 0 to 28 compared with all other dietary treatments (P < 0.05), after which no difference was observed (P > 0.05). Overall (days 0 to 42), ADFI was only reduced in DON5 fed pigs (P < 0.05). G:F was reduced in DON5-fed pigs from days 0 to 7 compared with all other dietary treatments (P < 0.05), which were not different from each other (P > 0.05). There was no effect (P > 0.05) of dietary treatment on G:F from days 8 to 42 or overall (days 0 to 42).
Table 3.
Growth performance of finisher pigs fed graded levels of deoxynivalenol1
| Dietary treatments2 | ||||||
|---|---|---|---|---|---|---|
| Item | CONT | DON1 | DON3 | DON5 | SEM | P-value3 |
| BW, kg | ||||||
| Initial | 76.9 | 77.0 | 76.3 | 76.0 | 1.18 | NS |
| Day 7 | 85.4a | 84.8a | 83.0b | 80.8c | 0.34 | <0.001 |
| Day 14 | 95.3a | 95.3a | 92.4b | 88.7c | 0.42 | <0.001 |
| Day 21 | 103.4a | 103.8a | 99.8b | 95.7c | 0.50 | <0.001 |
| Day 28 | 112.1a | 111.9a | 107.8b | 103.0c | 0.53 | <0.001 |
| Day 35 | 119.7a | 119.8a | 114.9b | 110.4c | 0.63 | <0.001 |
| Day 42 | 126.7a | 126.9a | 123.6b | 118.5c | 0.80 | <0.001 |
| ADG, kg/d | ||||||
| Days 0–7 | 1.27a | 1.18a | 0.93b | 0.60c | 0.05 | <0.001 |
| Days 8–14 | 1.40ab | 1.49a | 1.33b | 1.13c | 0.04 | <0.001 |
| Days 15–21 | 1.17ab | 1.21a | 1.06b | 1.01c | 0.04 | 0.004 |
| Days 22–28 | 1.24a | 1.17ab | 1.15ab | 1.04b | 0.04 | 0.033 |
| Days 29–35 | 1.08 | 1.12 | 1.01 | 1.06 | 0.04 | NS |
| Days 36–42 | 1.06 | 1.00 | 1.20 | 1.14 | 0.06 | NS |
| Overall | 1.19a | 1.20a | 1.12b | 1.00c | 0.02 | <0.001 |
| ADFI, kg/d | ||||||
| Days 0–7 | 2.59a | 2.59a | 2.22b | 1.70c | 0.06 | <0.001 |
| Days 8–14 | 2.98a | 3.07a | 2.89a | 2.55b | 0.07 | <0.001 |
| Days 15–21 | 3.03a | 3.03a | 2.88a | 2.56b | 0.05 | <0.001 |
| Days 22–28 | 3.25a | 3.19a | 3.13a | 2.85b | 0.05 | <0.001 |
| Days 29–35 | 3.22 | 3.20 | 3.19 | 3.04 | 0.06 | NS |
| Days 36–42 | 3.19 | 3.11 | 3.36 | 3.05 | 0.08 | NS |
| Overall | 2.99a | 3.06a | 2.94a | 2.60b | 0.05 | <0.001 |
| G:F, kg/kg | ||||||
| Days 0–7 | 0.49a | 0.46a | 0.41a | 0.34b | 0.02 | <0.001 |
| Days 8–14 | 0.47 | 0.49 | 0.47 | 0.44 | 0.01 | NS |
| Days 15–21 | 0.38 | 0.40 | 0.37 | 0.40 | 0.01 | NS |
| Days 22–28 | 0.38 | 0.36 | 0.37 | 0.36 | 0.02 | NS |
| Days 29–35 | 0.33 | 0.35 | 0.32 | 0.35 | 0.01 | NS |
| Days 36–42 | 0.33 | 0.32 | 0.36 | 0.37 | 0.01 | NS |
| Overall | 0.40 | 0.39 | 0.38 | 0.38 | 0.01 | NS |
1Values are least squares means (n, 10/treatment).
2CONT, 0 DON; DON1, 1 ppm DON; DON3, 3 ppm DON; DON5, 5 ppm DON.
3NS, not significant.
a,b,cMeans within a row without a common superscript are significantly different (P < 0.05).
Relationship between dietary DON intake and BW gain
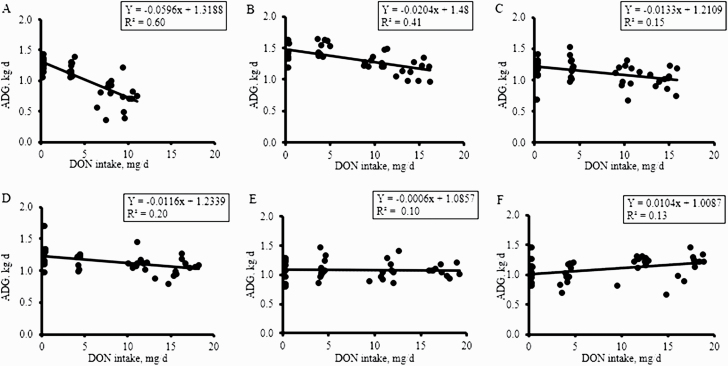
A linear regression model was applied to study the relationship between DON intake and ADG (Figure 1). There was a negative relationship between DON intake and ADG, such that as DON intake increased, there was a linear reduction in ADG. This relationship was consistent from days 0 to 35; however, the strength of the impact of DON intake on ADG reduced consistently and was lowest from days 28 to 35 with a slope not different than zero (Figure 1E).
Figure 1.
Regression analysis of the relationship between DON intake and weekly ADG. The figures represent days 0 to 7 (A), days 8 to 14 (B), days 15 to 21 (C), days 22 to 28 (D), days 29 to 35 (E), and days 36 to 42 (F). The coefficient of determination (R2) values range from 0.1 to 0.6, suggesting a high level of variance in the dependent variable (ADG). Points on graph represent an experimental pen (n = 10 pens per treatment).
Nitrogen balance
The results for the N-balance study are presented in Table 4. It is important to note that the CONT diet used in the N balance had unexpectedly high DON content and so results should be interpreted appropriately. Average daily N intake for pigs fed DON3 and DON5 was not different but were both lower (P < 0.05) than DON1 and CONT diet. Urinary N output from CONT, DON3, and DON5 diets were not different (P > 0.05), but the pigs fed DON1 had (P < 0.05) lower urinary N output compared with the other dietary treatments. Fecal N output was not different between DON1, DON3, and DON5, but was significantly (P < 0.05) higher in the CONT diet. The apparent total tract digestibility of N was significantly (P < 0.05) lower in the CONT diet compared with all the other dietary treatments. Nitrogen retention was lower (P < 0.05) in pig fed CONT, DON3, and DON5 diets compared with DON1. As such, N retention efficiency was higher (P < 0.05) in DON1 compared with CONT, DON3, and DON5, and therefore, protein deposition (PD) for DON1 pigs was higher (P < 0.05) than pigs fed CONT, DON3, and DON5 diets.
Table 4.
Nitrogen balance in pigs fed diets containing graded levels of deoxynivalenol1
| Dietary treatments2 | ||||||
|---|---|---|---|---|---|---|
| Item, g/d | CONT | DON1 | DON3 | DON5 | SEM | P-value |
| N intake | 67.84a | 68.45a | 63.48b | 63.32b | 1.27 | 0.024 |
| Fecal N output | 14.10a | 8.82b | 9.28b | 9.26b | 1.05 | 0.005 |
| Urinary N output | 25.53a | 15.19b | 28.38a | 22.69ab | 3.59 | 0.015 |
| ATTD3 of N, % | 79.27a | 87.12b | 85.41b | 85.44b | 1.56 | 0.009 |
| N retained | 28.30b | 44.40a | 25.85b | 31.34b | 3.17 | <0.001 |
| PD4 | 176.89b | 277.78a | 161.56b | 195.99b | 19.81 | <0.001 |
| N retention efficiency5,% | 41.68b | 65.12a | 40.62b | 49.64b | 4.75 | <0.001 |
1Values are least squares means (n, 10/treatment).
2CONT, 0 DON; DON1, 1 ppm DON; DON3, 3 ppm DON; DON5, 5 ppm DON.
3ATTD, apparent total tract digestibility.
4PD, N retained × 6.25.
5Nitrogen retention efficiency calculated as the N retained divided by N intake and expressed as a percentage.
a,bMeans within a row without a common superscript are significantly different (P < 0.05).
Blood metabolites
Key indicators of kidney and liver health and function are presented in Supplementary Table 1. There was no effect (P > 0.05) of dietary DON content on the selected liver and kidney blood parameters. For most of the analyzed metabolites, there was a significant effect of day, except for potassium and creatine kinase that tended to be different (P = 0.075 and 0.073, respectively) and gamma-glutamyl transferase that was not affected (P > 0.05) by day. These differences are considered unrelated to dietary treatment as there was no significant diet × day interaction.
Concentration of DON in serum and urine
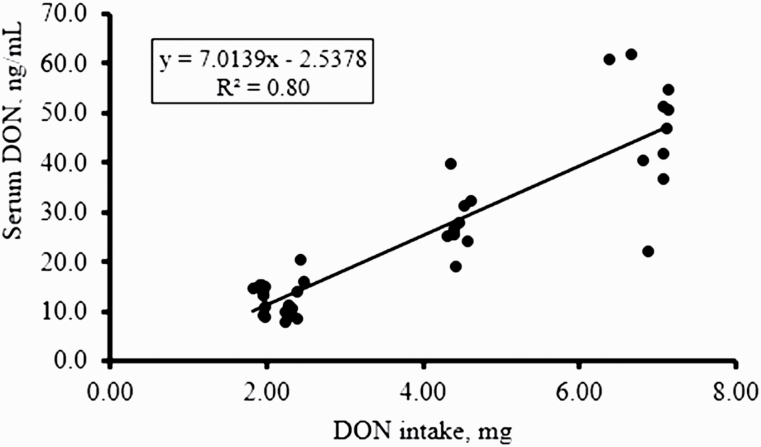
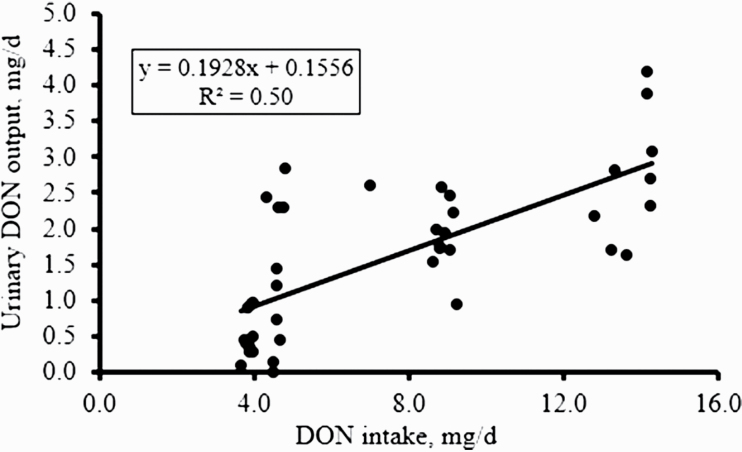
A linear regression model was used to analyze the relationship between DON intake and DON concentration in serum and urine as shown in Figures 2 and 3, respectively. As dietary DON intake increased, the amount of DON in the blood increased (P < 0.05; R2 = 0.80). The same relationship was observed for urine, where as dietary DON intake increased, there was an increase in DON excretion in urine (P < 0.05; R2 = 0.50).
Figure 2.
Regression analysis of the relationship between DON intake and serum DON concentration (ng/mL). The blood samples were taken during the nitrogen balance period (3 to 4 h after a single meal) of the experiment and analyzed for DON concentration (n = 10 pigs per treatment). Data are expressed as the DON intake after a single meal and the serum DON concentration (ng/mL) after that meal. The coefficient of determination (R2) of the regression curve is 0.80 at P < 0.05.
Figure 3.
Regression analysis of the relationship between deoxynivalenol (DON) intake and DON output in urine (n = 10 pigs per treatment). The urine samples were collected during the nitrogen balance period of the experiment over 24 h and analyzed for DON content. Data are expressed as the DON intake per day (mg/d) and the urine DON output (mg/d) and the coefficient of determination (R2) of the regression curve is 0.50 at P < 0.05.
Discussion
In general, DON intake causes reduced performance and can have potentially negative impacts on animal health. The majority of studies on the effects of mycotoxins in swine are performed in young animals with the assumption that the physiological effects of consuming mycotoxin contaminated feed are highest in the young animal (Dersjant-Li et al., 2003; Chen et al., 2008; Savard et al., 2015). Moreover, previous studies have examined the impact of mycotoxins over a relatively short period of time (Accensi et al., 2006; Alizadeh et al., 2015; Dänicke et al., 2017). The objective of the present study was to examine the effects of feeding graded levels of DON to finisher (75 to 120 kg) pigs on growth performance, nutrient utilization, and overall health status over a 43-d period.
In the present study, the diets were formulated to target dietary DON levels of 0, 1, 3, and 5 ppm. For the most part, the formulated and analyzed levels of the DON in the complete diets were similar. One exception to this is the analyzed DON content (1.56 ppm) versus targeted content (0 ppm) in the CONT diet used in the N-balance study. These inconsistencies in accurately determining DON levels in complete diets have been previously reported (Patience et al., 2014; Kong et al., 2016) and could be due to the mechanisms by which Fusarium infects grain and proliferates in spots or localized portions of the grain batch during storage, leading to uneven distribution of Fusarium growth in samples (Swamy et al., 2002; Kong et al., 2012, 2015).
In the current study, upon initial exposure to DON-contaminated diets, there was a 27% and 53% reduction in ADG in pigs fed the DON3 and DON5 diets, respectively, which agrees with Serviento et al. (2018) who fed pigs between 70 and 95 kg BW with DON-contaminated diets (4.8 ppm) and observed a 40% to 60% reduction in ADG. We also observed a consistent reduction in ADG during the first 28 d of DON exposure, which was expected and in agreement with previous studies examining the effect of DON intake in pigs (Kong et al., 2015; Serviento et al., 2018; Nguyen-Ba et al., 2020). There was limited or no effect of DON intake on G:F, which suggests that the reduced growth performance is largely due to the reduction in feed intake and not due to reduced nutrient utilization. This observation is in agreement with Goyarts et al. (2005) who demonstrated that pigs fed a DON-contaminated diet for 11 wk had a 15% reduction in feed intake and 13% reduction in weight gain, but no difference in G:F. Likewise, Pastorelli et al. (2012) determined that approximately 85% of the reduction in ADG during mycotoxicosis is due to the observed reduction ADFI, with the remainder due to G:F. The present study evaluated a single mycotoxin (DON) and in much older pigs (finisher pigs), whereas Pastorelli et al. (2012) included studies examining different mycotoxins, alone or in combination, as well as studies examining mycotoxicosis in postweaned pigs, indicating there may be a difference in response due to age and physiological stage.
Previous studies have suggested that pigs may be able to adapt to DON intake, with the depression in feed intake and growth being most severe in the first week after exposure (Pollmann et al., 1985; Foster et al., 1986; Rotter et al., 1994; Serviento et al., 2018). Indeed, Serviento et al. (2018) observed an immediate reduction in ADG and ADFI upon initial exposure to a DON-contaminated diet, which gradually recovered over a 7-d period. Likewise, in their meta-analysis, Dersjant-Li et al. (2003) demonstrated a strong linear relationship between DON intake and the reduction in ADG in pigs; however, the strength of this relationship (i.e., R2 value) was lower when the duration of the study was longer, suggesting adaptation and recovery of performance. We observed a similar response in the current study when examining the linear response of ADG to DON intake, with a strong relationship immediately after exposure which weakened over time. Rotter et al. (1994) fed up to 3 ppm DON to young pigs and observed a decreased weight gain over the initial 7 d; however, growth performance did not differ among groups by the end of the 4-wk study. This is similar to the observed recovery period in the current study, in which ADG had started to recover by 28 d post-DON exposure. Serviento et al. (2018) also observed a rapid and significant decrease in feed intake in both grower and finisher pigs when exposed to DON-contaminated diets, which recovered after approximately 7 d. Overall, it appears that pigs do have the ability to adapt to DON intake. The overall reduction in BW gain observed in a meta-analysis by Dersjant-Li et al. (2003) was approximately 20% with 5 ppm DON. The length of time available to recover postexposure probably plays a role, as in the current study, we only observed a 6% reduction in BW in DON-fed pigs.
N balance can be used as an indicator of the efficiency of nutrient utilization, specifically of dietary protein for PD (i.e., lean gain). It has been suggested that DON intake can interfere with PD (Swamy et al., 2003). Unfortunately, in the current study, we were not aware of DON contamination in the CONT diet used for N balance. We, therefore, lack a true control diet to compare the results of the other diets. If we assume that the DON1 response is similar to a DON-free diet (based on similar growth performance results as CONT-fed pigs), then it would appear that DON intake reduced N retention. The response of pigs fed the CONT diet is similar to DON3 and DON5, despite having similar DON content to the DON1 diet. It is possible that this observation is indicative of the initial response in nutrient utilization to a low-level DON contamination. Overall, N utilization may be impaired with DON exposure; however, this was not evident in measures of G:F in the current study.
There is some evidence suggesting that, once absorbed, DON can cause kidney and liver damage and can suppress immune function, resulting in decreased ability to resist disease challenge (Chaytor et al., 2011). As a reduction in immune function and organ damage may not be evident in growth performance, we evaluated pig health via measurement of key indicators of liver and kidney health. In the current study, we saw no evidence of kidney and liver damage, as evaluated using a standard veterinary liver/kidney health panel. Similarly, previous studies observed little or no effect on hematological, biochemical, or immune response in pigs fed diets containing DON (Dänicke et al., 2004b; Goyarts et al., 2005; Accensi et al., 2006) or DON combined with aflatoxin (Chaytor et al., 2011). Moreover, many of the negative effects observed in the study by Goyarts et al. (2005) on blood biochemistry were due more to the restricted feeding regime (i.e., pair-fed pigs simulating the effect of DON on feed intake) than to feeding DON-contaminated diets.
Given the difficulties with obtaining consistent and/or reliable measures of DON in feedstuffs and feed, we evaluated whether DON content in biological samples could be used as an indicator of actual DON ingestion in pigs. It is known that levels of DON in plasma increase after intake of DON-contaminated diets, reaching a peak between 1.5 (Goyarts and Dänicke, 2006) and 4 h (Dänicke et al., 2004b) postintake. In the current study, we confirmed that DON ingestion can be determined through analysis of DON in blood serum samples taken between 3 and 4 h postingestion. The level of DON in serum was correlated with DON intake and could, therefore, be used to determine the degree of DON exposure under controlled conditions. The higher than expected DON content in the diets used for the N-balance measures was confirmed by serum DON concentration, with pigs fed CONT diets having detectable levels of DON in serum (Figure 2). The main route of excretion of ingested and absorbed DON is through urine as previously reported by Frobose et al. (2017) who determined urinary DON output in pigs fed DON-contaminated diets with dietary DON-detoxifying agents. In the current study, DON concentration in urine was highly correlated with actual DON ingestion. Dänicke et al. (2004a) reported >50% DON recovery in urine, confirming that urine is a major route of excretion. They also found that DON concentration in urine increased in DON-fed vs. control pigs. With the results of the current study, we provide evidence that urinary DON (24-h period) can also be used to estimate actual DON exposure in pigs under controlled conditions. Still, when interpreting DON levels in biological matrices, emphasis needs to be put on influencing factors such as suitable analytical methods, sampling time point, and individual variations in metabolism (Dänicke and Brezina, 2013).
Conclusion
In summary, we observed an initial reduction in ADFI and ADG upon introduction of diets containing >1 ppm DON to finisher pigs; however, performance (i.e., ADG, ADFI) recovered after 28 d, suggesting that pigs may be able to adapt to consumption of DON-contaminated diets. The lack of negative effect of DON intake on G:F suggests that the effect of DON intake on ADG is largely due to the reductions in feed intake. The consumption of diets containing DON up to 5 ppm appears to have little impact on organ function. Overall, although pigs maybe capable of adapting to intake of DON-contaminated diets, BW remains low compared with pigs fed up to 1 ppm DON.
Supplementary Material
Acknowledgments
Funding for this study was provided by the Saskatchewan Ministry of Agriculture and the Canada-Saskatchewan Growing Forward 2 bi-lateral agreement (20170002), the Barley Development Commission of Saskatchewan, BIOMIN Holding GmbH, and Mitacs (IT12203). General program funding for the Prairie Swine Centre, Inc. is provided by the Government of Saskatchewan, Saskatchewan Pork Development Board, Alberta Pork, Manitoba Pork, and Ontario Pork. The authors acknowledge the assistance of the staff and students of the Prairie Swine Centre, Canadian Feed Research Centre, and the University of Saskatchewan.
Glossary
Abbreviations
- ATTD
apparent total tract digestibility
- AST
aspartate aminotransferase
- BW
body weight
- CCK
cholecystokinin
- CTK
creatine kinase
- DON
deoxynivalenol
- G:F
gain:feed
- GGT
gamma-glutamyl transferase
- GLDH
glutamate dehydrogenase
- HPLC
high-pressure liquid chromatography
- PBS
phosphate-buffered sodium
- PD
protein deposition
Conflict of interest statement
V.N. is an employee of BIOMIN Holding GmbH. All other authors declare no conflicts of interest.
Literature Cited
- Accensi, F., Pinton P., Callu P., Abella-Bourges N., Guelfi J. F., Grosjean F., and Oswald I. P.. . 2006. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets. J. Anim. Sci. 84:1935–1942. doi: 10.2527/jas.2005-355 [DOI] [PubMed] [Google Scholar]
- Alizadeh, A., Braber S., Akbari P., Garssen J., and Fink-Gremmels J.. . 2015. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins (Basel) 7:2071–2095. doi: 10.3390/toxins7062071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretta, I., Kipper M., Lehnen C. R., Hauschild L., Vale M. M., and Lovatto P. A.. . 2012. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 6:1476–1482. doi: 10.1017/S1751731111002278 [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) . 2007. Official methods of analysis of AOAC International. 18th ed. Gaithersburg (MD): AOAC International. [Google Scholar]
- Beaulieu, A. D., Patience J. F., and Gillis D.. . 2009. The efficacy of eight different feed additives on mitigating the effects of deoxynivalenol (DON). In: 28th Annual Centralia Swine Research Update, 28 January 2009, Kirkton-Woodham Community Centre, Ontario, Canada. [Google Scholar]
- Canadian Council on Animal Care (CCAC) . 2009. Guidelines on the care and use of farm animals in research, teaching and testing. Ottawa (Canada): CCAC. [Google Scholar]
- Champeil, A., Fourbet J. F., and Doré T.. . 2004. Effects of grain sampling procedures on Fusarium mycotoxin assays in wheat grains. J. Agric. Food Chem. 52:6049–6054. doi: 10.1021/jf049374s [DOI] [PubMed] [Google Scholar]
- Chaytor, A. C., See M. T., Hansen J. A., de Souza A. L., Middleton T. F., and Kim S. W.. . 2011. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 89:124–135. doi: 10.2527/jas.2010-3005 [DOI] [PubMed] [Google Scholar]
- Chen, F., Ma Y., Xue C., Ma J., Xie Q., Wang G., Bi Y., and Cao Y.. . 2008. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. J. Vet. Sci. 9:39–44. doi: 10.4142/jvs.2008.9.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus, D. A., Lapierre H., Htoo J. K., and de Lange C. F.. . 2014. Nonprotein nitrogen is absorbed from the large intestine and increases nitrogen balance in growing pigs fed a valine-limiting diet. J. Nutr. 144:614–620. doi: 10.3945/jn.113.187070 [DOI] [PubMed] [Google Scholar]
- Dänicke, S., Beineke A., Berk A., and Kersten S.. . 2017. Deoxynivalenol (DON) contamination of feed and grinding fineness: Are there interactive implications on stomach integrity and health of piglets? Toxins (Basel) 9:16. doi: 10.3390/toxins9010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dänicke, S., and Brezina U.. . 2013. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 60:58–74. doi: 10.1016/j.fct.2013.07.017 [DOI] [PubMed] [Google Scholar]
- Dänicke, S., Goyarts T., Valenta H., Razzari E., and Bohm J.. . 2004a. On the effects of deoxynivalenol (DON) in pig feed on growth performance, nutrients utilization and DON metabolism. J. Anim. Feed Sci. 13:539–556. doi: 10.22358/jafs/67624/2004 [DOI] [Google Scholar]
- Dänicke, S., Valenta H., and Doll S.. . 2004b. On the toxicokinetics and metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 58:169–180. doi: 10.1080/00039420410001667548 [DOI] [PubMed] [Google Scholar]
- Dersjant-Li, Y., Verstegen M. W. A., and Gerrits W. J. J.. . 2003. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 16:223–229. doi: 10.1079/NRR200368 [DOI] [PubMed] [Google Scholar]
- Foster, B. C., Trenholm H. L., Friend D. W., Thompson B. K., and Hartin K. E.. . 1986. Evaluation of different sources of deoxynivalenol (vomitoxin) fed to swine. Can. J. Anim. Sci. 66:1149–1154. doi. 10.4141/cjas86-128 [DOI] [Google Scholar]
- Frobose, H. L., Stephenson E. W., Tokach M. D., DeRouchey J. M., Woodworth J. C., Dritz S. S., and Goodband R. D.. . 2017. Effects of potential detoxifying agents on growth performance and deoxynivalenol (DON) urinary balance characteristics of nursery pigs fed DON-contaminated wheat. J. Anim. Sci. 95:327–337. doi: 10.2527/jas.2016.0664 [DOI] [PubMed] [Google Scholar]
- Ghareeb, K., Awad W. A., Böhm J., and Zebeli Q.. . 2015. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 35:327–337. doi: 10.1002/jat.3083 [DOI] [PubMed] [Google Scholar]
- Goyarts, T., and Dänicke S.. . 2006. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pigs. Toxic. Lett. 163:171–182. doi: 10.1016/j.toxlet.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Goyarts, T., Dänicke S., Rothkötter H. J., Spilke J., Tiemann U., and Schollenberger M.. . 2005. On the effects of a chronic deoxynivalenol intoxication on performance, haematological and serum parameters of pigs when diets are offered either for ad libitum consumption or fed restrictively. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52:305–314. doi: 10.1111/j.1439-0442.2005.00734.x [DOI] [PubMed] [Google Scholar]
- Gruber-Dorninger, C., Jenkins T., and Schatzmayr G.. . 2019. Global mycotoxin occurrence in feed: A ten-year study. Toxins 11:375. doi: 10.3390/toins11070375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C., Park S., and Kim B. G.. . 2012. Evaluation of a mycotoxin adsorbent in swine diets containing barley naturally contaminated with Fusarium mycotoxins. Rev. Colom. Pecu. 29:169–177. doi: 10.17533/udea.rccp.v29n3a02 [DOI] [Google Scholar]
- Kong, C., Shin S. Y., Park C. S., and Kim B. G.. . 2015. Effects of feeding barley naturally contaminated with Fusarium mycotoxins on growth performance, nutrient digestibility, and blood chemistry of gilts and growth recoveries by feeding a non-contaminated diet. Asian-Australas. J. Anim. Sci. 28:662–670. doi: 10.5713/ajas.14.0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C., Park C. S., and Kim B. G.. . 2016. Evaluation of a mycotoxin adsorbent in swine diets containing barley naturally contaminated with Fusarium mycotoxins. Rev. Colomb. Cienc. Pecu. 29:169–177. doi: 10.17533/udea.rccp.v29n3a02 [DOI] [Google Scholar]
- National Research Council (NRC) . 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Nguyen-Ba, H., Taghipoor M., and van Milgen J.. . 2020. Modelling the feed intake response of growing pigs to diets contaminated with mycotoxins. Animal 14(Suppl 2):s303–s312. doi: 10.1017/S175173112000083X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli, H., Van Milgen J., Lovatto P., and Montagne L.. . 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X [DOI] [PubMed] [Google Scholar]
- Patience, J. F., Myers A. J., Ensley S., Jacobs B. M., and Madson D.. . 2014. Evaluation of two mycotoxin mitigation strategies in grow-finish swine diets containing corn dried distillers grains with solubles naturally contaminated with deoxynivalenol. J. Anim. Sci. 92:620–626. doi: 10.2527/jas.2013-6238 [DOI] [PubMed] [Google Scholar]
- Pinton, P., Accensi F., Beauchamp E., Cossalter A., Callu P., Grosjean F., and Oswald I. P.. . 2008. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune response. Toxicol. Let. 177:215–222. doi: 10.1016/j.toxlet.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Pinton, P., Nougayrède J. P., Del Rio J. C., Moreno C., Marin D. E., Ferrier L., Bracarense A. P., Kolf-Clauw M., and Oswald I. P.. . 2009. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 237:41–48. doi: 10.1016/j.taap.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Pollmann, D. S., Koch B. A., Seitz L. M., Mohr H. E., and Kennedy G. A.. . 1985. Deoxynivalenol-contaminated wheat in swine diets. J. Anim. Sci. 60:239–247. doi: 10.2527/jas1985.601239x [DOI] [PubMed] [Google Scholar]
- Rotter, B. A., Prelusky D. B., and Pestka J. J.. . 1996. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 48:1–34. doi: 10.1080/009841096161447 [DOI] [PubMed] [Google Scholar]
- Rotter, B. A., Thompson B. K., Lessard M., Trenholm H. L., and Tryphonas H.. . 1994. Influence of low-level exposure to Fusarium mycotoxins on selected immunological and hematological parameters in young swine. Fundam. Appl. Toxicol. 23:117–124. doi: 10.1006/faat.1994.1087 [DOI] [PubMed] [Google Scholar]
- Savard, C., Provost C., Alvarez F., Pinilla V., Music N., Jacques M., Gagnon C. A., and Chorfi Y.. . 2015. Effect of deoxynivalenol (DON) mycotoxin on in vivo and in vitro porcine circovirus type 2 infections. Vet. Microbiol. 176:257–267. doi: 10.1016/j.vetmic.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Schwartz-Zimmermann, H. E., Hametner C., Nagl V., Fiby I., Macheiner L., Winkler J., Dänicke S., Clark E., Pestka J. J., and Berthiller F.. . 2017. Glucuronidation of deoxynivalenol (DON) by different animal species: Identification of iso-DON glucuronides and iso-deepoxy-DON glucuronides as novel DON metabolites in pigs, rats, mice, and cows. Arch. Toxicol. 91:3857–3872. doi: 10.1007/s00204-017-2012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviento, A. M., Brossard L., and Renaudeau D.. . 2018. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behavior in finishing pigs. J. Anim. Sci. 96:5209–5221. doi: 10.1093/jas/sky378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulyok, M., Stadler D., Steiner D., and Krska R.. . 2020. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 412:2607–2620. doi: 10.1007/s00216-020-02489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy, H. V., Smith T. K., MacDonald E. J., Boermans H. J., and Squires E. J.. . 2002. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 80:3257–3267. doi: 10.2527/2002.80123257x [DOI] [PubMed] [Google Scholar]
- Swamy, H. V., Smith T. K., MacDonald E. J., Karrow N. A., Woodward B., and Boermans H. J.. . 2003. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 81:2792–2803. doi: 10.2527/2003.81112792x [DOI] [PubMed] [Google Scholar]
- Van Keulen, J. V., and Young B. A.. . 1977. Evaluation of acid insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44:282–287. doi: 10.2527/jas1977.442282x [DOI] [Google Scholar]
- Whitaker, T. B., Hagler W. M., Giesbrecht F. G., and Johansson A. S.. . 2000. Sampling, sample preparation, and analytical variability associated with testing wheat for deoxynivalenol. J. AOAC Int. 83:1285–1292. doi: 10.1093/jaoac/83.5.1285 [DOI] [PubMed] [Google Scholar]
- Wu, Q., Dohnal V., Huang L., Kuca K., and Yuan Z.. . 2010. Metabolic pathways of trichothecenes. Drug Metab. Rev. 42:250–267. doi: 10.1080/03602530903125807 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.