Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, critically ill, hospital-acquired infections, superinfection
Abstract
OBJECTIVES:
To describe the epidemiology of superinfections (occurring > 48 hr after hospital admission) and their impact on the ICU and 28-day mortality in patients with coronavirus disease 2019 with acute respiratory distress syndrome, requiring mechanical ventilation.
DESIGN:
Retrospective analysis of prospectively collected observational data.
SETTING:
University-affiliated adult ICU.
PATIENTS:
Ninety-two coronavirus disease 2019 patients admitted to the ICU from February 21, 2020, to May 6, 2020.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The prevalence of superinfection at ICU admission was 21.7%, and 53 patients (57.6%) had at least one superinfection during ICU stay, with a total of 75 (82%) ventilator-associated pneumonia and 57 (62%) systemic infections. The most common pathogens responsible for ventilator-associated pneumonia were Pseudomonas aeruginosa (n = 26, 34.7%) and Stenotrophomonas maltophilia (n = 14, 18.7%). Bloodstream infection occurred in 16 cases, including methicillin-resistant Staphylococcus epidermidis (n = 8, 14.0%), Enterococcus species (n = 6, 10.5%), and Streptococcus species (n = 2, 3.5%). Fungal infections occurred in 41 cases, including 36 probable (30 by Candida albicans, six by C. nonalbicans) and five proven invasive candidiasis (three C. albicans, two C. nonalbicans). Presence of bacterial infections (odds ratio, 10.53; 95% CI, 2.31–63.42; p = 0.005), age (odds ratio, 1.17; 95% CI, 1.07–1.31; p = 0.001), and the highest Sequential Organ Failure Assessment score (odds ratio, 1.27; 95% CI, 1.06–1.63; p = 0.032) were independently associated with ICU or 28-day mortality.
CONCLUSIONS:
Prevalence of superinfections in coronavirus disease 2019 patients requiring mechanical ventilation was high in this series, and bacterial superinfections were independently associated with ICU or 28-day mortality (whichever comes first).
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported from Wuhan, China, as the cause of a respiratory illness defined coronavirus disease 2019 (COVID-19) (1). The clinical presentation of the disease is characterized by several potential complications, including acute respiratory distress syndrome (ARDS) requiring ICU admission and mechanical ventilation (MV) (2). Critically ill COVID-19 patients may suffer from bacterial, fungal, and viral superinfections that complicate their clinical course; however, little is known about the epidemiology of this complication in severe COVID-19 patients (3–5).
The primary objective of our study was to describe the epidemiology of superinfections in patients with severe COVID-19 admitted to the ICUs of the Brescia University Hospital in Lombardy, Italy, during the first wave of the SARS-CoV-2 pandemic. The secondary objectives were to analyze the impact of superinfections on ICU or 28-day mortality (whichever comes first) and on ICU length of stay (ICU-LOS) and hospital length of stay (HLOS).
MATERIALS AND METHODS
Study Site, Design, and Participants
In this single-center observational study, we retrospectively analyzed prospectively collected data concerning adult patients (> 18 yr old) with SARS-CoV-2 pneumonia and ARDS admitted to the ICU of the Brescia University Hospital from February 21, 2020, to May 6, 2020. SARS-CoV-2 infection diagnosis was established by clinical symptoms and reverse transcriptase-quantitative polymerase chain reaction (PCR) assay of a specimen collected on a nasopharyngeal swab, as indicated by the World Health Organization (6). Patients were managed according to the World Health Organization’s recommendations as well as locally developed guidelines (6, 7). Daily, an infectious disease specialist performed an ICU round to optimize antimicrobial use.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. As stated by Italian legislation, ethical review was not required due to retrospective nature of the present study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting results of this prospective cohort study (8).
Patients were treated according to the best available evidence with a combination of antiviral treatments and antibiotics when admitted to the hospital with respiratory failure due to COVID-19 infection (6). Antiviral treatments included a combination of lopinavir/ritonavir (or darunavir) and chloroquine or hydroxychloroquine, whereas antibiotic treatment started at hospital admission with ceftriaxone plus azithromycin. Considering the uncertainty of dexamethasone role in COVID-19 infection during the study period, the corticosteroid was given to patients that required at least high flow oxygen at a dosage of 20 mg/d for 10 days followed by 10 mg/d for another 10 days, based on the judgment of the clinician responsible for the patients. Patients with nonresponding ARDS in ICU received a high dosage of methylprednisolone (1 mg/kg/d) for 14 days. Patients who underwent noninvasive ventilation were suitable for tocilizumab administration, as reported earlier (9). Once in ICU with ARDS, patients received all the available medical treatment as reported earlier (10).
Definitions
Pulmonary infections included ventilator-associated pneumonia (VAP) and COVID-19-associated pulmonary aspergillosis (CAPA). Systemic infections included bloodstream infection (BSI) and invasive candidiasis (IC).
VAP was diagnosed in patients having received MV for at least 48 hours when the following two criteria were met: 1) clinically suspected VAP, defined as a new and persistent pulmonary infiltrate on chest radiograph associated with at least two of the following: temperature greater than or equal to 38°C, WBC count greater than or equal to 10 × 103/L, purulent tracheal secretions, increased minute ventilation, arterial oxygenation decline requiring modifications of the ventilator settings, and/or need for increased vasopressor infusion—for patients with ARDS, for whom demonstration of radiologic deterioration is difficult, at least two of the preceding criteria sufficed and 2) significant quantitative growth (≥ 104 colony-forming units/mL) of distal bronchoalveolar lavage samples (11).
We defined “proven” invasive fungal disease as 1) positive sterile materials, either positive culture from sterile materials (excluding BAL fluid, a paranasal or mastoid sinus cavity specimen, and urine) or histopathologic, cytopathologic, or direct microscopic examination or 2) positive DNA amplification by PCR (12). “Probable” invasive fungal disease was defined as the presence of a host factor, a clinical feature, and mycologic evidence (including Aspergillus galactomannan from bronchoalveolar lavage), as defined by Donnelly et al (12).
BSI was defined in the presence of at least one positive blood culture for bacteria. For coagulase-negative Staphylococci and other common skin contaminants, at least two consecutive blood cultures positive for the same pathogen were necessary to define BSI. The detection of Staphylococci other than Staphylococcus aureus in respiratory samples was not considered to represent infection.
Infections were categorized as “hospital”-acquired superinfections if diagnosed at least 48 hours after hospital admission and “ICU”-acquired superinfections if diagnosed after 72 hr after ICU admission. Data were collected prospectively, and the definition of superinfection episodes, as well as the diagnostic and therapeutic decisions, were taken in agreement with an infectious disease consultant. Furthermore, a “new” infection was defined if any of the following conditions occurred: 1) isolation of a new microorganism, 2) isolation of a previously detected microorganism with a different antimicrobial resistance (AMR) pattern, and 3) isolation of a previously detected microorganism after at least one negative test.
AMR was defined as resistance to methicillin for Staphylococcus species and to vancomycin-resistant Enterococcus (VRE) and by the production of extended-spectrum beta-lactamases for Enterobacterales (Escherichia coli, Morganella morganii, Enterobacter cloacae, Klebsiella species, Serratia marcescens). Nonfermenting gram-negative bacilli (NFGNB) included Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. Microorganisms were defined as multidrug-resistant (MDR) if resistant to greater than or equal to 1 drug in at least three classes of antibiotics (13).
Data Collection
All collected data were recorded on RedCap software (14) and subsequently de-identified. Demographic data included age, sex, weight, height, and comorbidity. Clinical data included daily Pao2/Fio2 ratio (P/F), daily Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score (SAPS) II, presence of MV, and drug treatments during ICU stay. Blood examinations included complete WBC count, coagulation profile, renal and liver function, C-reactive protein (CRP), procalcitonin, fibrinogen, d-dimer, serum ferritin, and lactate dehydrogenase. Microbiological investigations were planned weekly or performed on clinical judgment.
Statistical Methods
Quantitative variables were described using mean and sd, or median and interquartile range (IQR), as appropriate. Categorical variables were summarized as counts and percentages. Comparisons between superinfection versus no superinfection for all variables of interest were performed using the Wilcoxon-Mann-Whitney U test for quantitative variables and chi-square test, with p values computed with Monte Carlo simulation (B = 2,000), for qualitative ones. The association between ICU mortality or 28-day mortality and infection status was evaluated using logistic regression models. Results are reported as odds ratio (OR) and 95% CI. All tests were two-sided and assumed a 5% significance. All the analyses were performed using R Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Ninety-two patients (87.0% males) were enrolled in the study. The demographic and clinical characteristics of the study population are presented in Table 1. The median (IQR) age was 62.0 years (56.3–67.8); 98% of patients had at least one chronic comorbidity. Fifty-three patients (57.6%) had at least one superinfection (21 patients had one, 13 patients had two, five patients had three, and 14 patients had four or more episodes) for a total of 132 superinfections episodes (Supplementary Table 1, http://links.lww.com/CCX/A626). The prevalence of hospital-acquired superinfection at ICU admission was 21.7%, as shown in Supplementary Figure 1 (http://links.lww.com/CCX/A626).
TABLE 1.
Comparison of Demographic and Clinical Characteristics Between Patients With and Without Superinfection at ICU Admission
| Variables | No Superinfection (n = 39) | Superinfection (n = 53) | Total (n = 92) | p |
|---|---|---|---|---|
| Age, yr, median (IQR) | 60.00 (54.5–65.5) | 64.00 (58–70) | 62.00 (56.3–67.8) | 0.263 |
| Male sex, n (%) | 33 (84.6) | 47 (88.7) | 80 (87.0) | 0.742 |
| Number of comorbidities, n (%) | ||||
| None | 1 (2.6) | 1 (1.9) | 2 (2.2) | 0.688 |
| 1 | 31 (79.5) | 36 (67.9) | 67 (72.8) | |
| ≥ 2 | 7 (17.9) | 16 (30.2) | 23 (25.0) | |
| Preexisting pulmonary disease, n (%) | 2 (5.1) | 5 (9.4) | 7 (7.6) | 0.702 |
| Weight, kg, median (IQR) | 80.00 (72.9–87.1) | 85.00 (77.5–92.5) | 85.00 (76.5–93.5) | 0.254 |
| Severe obesitya, n (%) | 9 (23.7) | 16 (30.2) | 25 (27.5) | 0.632 |
| Simplified Acute Physiology Score II, median (IQR) | 33.50 (27.8–39.3) | 39.00 (28.6–49.4) | 34.50 (25.5–42.5) | 0.087 |
| Po2/Fio2, median (IQR) | 112.5 (83.8–141.3) | 82.2 (65.5–98.9) | 88.01 (65.8–110.24) | < 0.0001 |
| Hospital-ICU time, d, median (IQR)b | 1.00 (0–3.3) | 5.00 (1.5–8.5) | 3.00 (0–6.5) | 0.012 |
| Steroids, n (%) | 38 (97) | 52 (98) | 90 (98) | 0.999 |
| Steroids duration, d, median (IQR) | 4.50 (0–9.5) | 7.00 (3.8–10.3) | 7.00 (2.8–11.3) | 0.696 |
| Pre ICU noninvasive ventilation (included continuous positive airway pressure and bilevel positive airway pressure), n (%) | 17 (48.6) | 23 (65.7) | 40 (57.1) | 0.228 |
| Antibiotic at ICU admission, n (%) | 34 (87.2) | 47 (88.7) | 81 (88.0) | 1.000 |
IQR = interquartile range.
aSevere obesity is defined as a body mass index > 30 kg/m2.
bHospital-ICU time denotes the number of days between hospital admission and ICU admission.
At ICU admission, patients with superinfection had a higher SAPS II compared with patients without superinfection, a significantly lower median P/F (p < 0.001), and lower WBC (mean 11,420 vs 8,870 cells/μL; p = 0.035). There were no other differences in the two populations for the other variables, including blood work, steroid, and antibiotic use, and non invasive ventilation (NIV) application (Table 1 and Supplementary Table 2, http://links.lww.com/CCX/A626). In particular, 90 patients (98%) received steroids either before or during ICU stay, for a median (IQR) of 7.0 days (2.8–11.3); 81 patients (88.0%) were receiving antibiotics at ICU admission, and NIV was applied to 40 subjects (57.1%).
During ICU stay, patients with superinfection were more frequently tracheostomized (50.9% vs 15.4%; p = 0.001), were mechanically ventilated for a longer period of time (median [IQR], 12 d [6.0–18.0] vs 3 d [0–6.5]; p < 0.001), and received more frequently inhaled nitric oxide and neuromuscular blocking agents (Table 1). Superinfection patients did not differ from no superinfection patients in terms of steroid therapies, antiviral therapies, and tocilizumab administration (Supplementary Table 3, http://links.lww.com/CCX/A626).
Seventy-five (82%) VAP and 57 (62%) BSI were detected, as shown in Supplementary Table 4 (http://links.lww.com/CCX/A626). The most common pathogens responsible for VAP were P. aeruginosa (n = 26, 34.7%) and S. maltophilia (n = 14, 18.7%), with two cases of CAPA (Fig. 1A). Among systemic infections (n = 57), BSI occurred in 16 cases, including methicillin-resistant S. epidermidis (MRSE) (n = 8, 14.0%), Enterococcus species (n = 6, 10.5%), and Streptococcus species (n = 2, 3.5%). Fungal infections occurred in 41 cases, including 36 probable (30 by Candida albicans, six by C. nonalbicans) and five proven IC (three C. albicans, two C. nonalbicans) (Fig. 1B).
Figure 1.
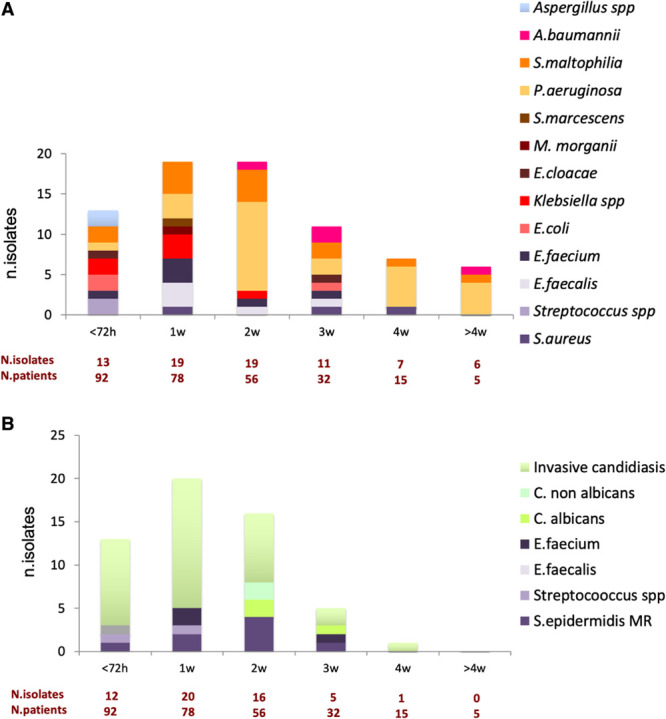
Representation of bacterial and fungal species responsible for hospital-acquired pneumonia and/or ventilator-associated pneumonia (A) and systemic infection, including bloodstream infections and invasive candidiasis (B) during ICU length of stay. A. baumannii = Acinetobacter baumannii, C. albicans = Candida albicans, C. nonalbicans = Candida nonalbicans, E. cloacae = Enterobacter cloacae, E. coli = Escherichia coli, E. faecalis = Enterococcus faecalis, E. faecium = Enterococcus faecium, M. morganii = Morganella morganii, P. aeruginosa = Pseudomonas aeruginosa, S. aureus = Staphylococcus aureus, S. epidermidis MR = Staphylococcus epidermidis methicillin-resistant, S. maltophilia = Stenotrophomonas maltophilia, S. marcescens = Serratia marcescens, spp = species.
Concerning the time of the onset of infections (Fig. 1; and Supplementary Table 4, http://links.lww.com/CCX/A626), the most common pathogens during the first 72 hours in ICU were multi-sensitive Enterobacterales (n = 5, 38.5%) for VAP (n = 13) and Candida species (n = 10, 83.3%) for systemic infections (n = 12). From day 4 to 7, seven of the 19 VAP (36.8%) were caused by NFGNB, seven (36.8%) by Gram-positive bacteria, and five (26.3%) by Enterobacterales. Among the 22 systemic infections, seven (31.8%) were BSI, four (18.1%) caused by Enterococcus species, two (9.1%) by MRSE, one (4.5%) by Streptococcus species, and 15 (68.1%) were probable IC. On week 2, 16 of the 19 VAP (84.2%) were caused by NFGNB. Of the 17 systemic infections, five (29.4%) were BSI (all caused by MRSE), and 12 (70.5%) were caused by Candida species (four proven IC and eight probable IC).
During the third week, there were 11 VAP and six (n = 6, 54.5%) were caused by NFGNB. Of the five systemic infections in 32 patients, two were BSI (40.0%, one Enterococcus, and one MRSE) and three were Candida species infections (60.0%, one proven and two probable IC). From week 4 onward, there were 13 VAP, mostly caused by NFGNB (n = 12, 92.3%) with a predominance of MDR P. aeruginosa (n = 9) and only one probable IC.
The onset of AMR bacterial strains is shown in Figure 2. A total of 15 (11.6%) episodes of superinfection in 12 patients were sustained by AMR microorganisms. The most frequent resistant microorganisms were MRSE (n = 8, 53.3%), Enterococcus species (n = 2, 13.3%), and MDR P. aeruginosa (n = 5, 33.3%).
Figure 2.
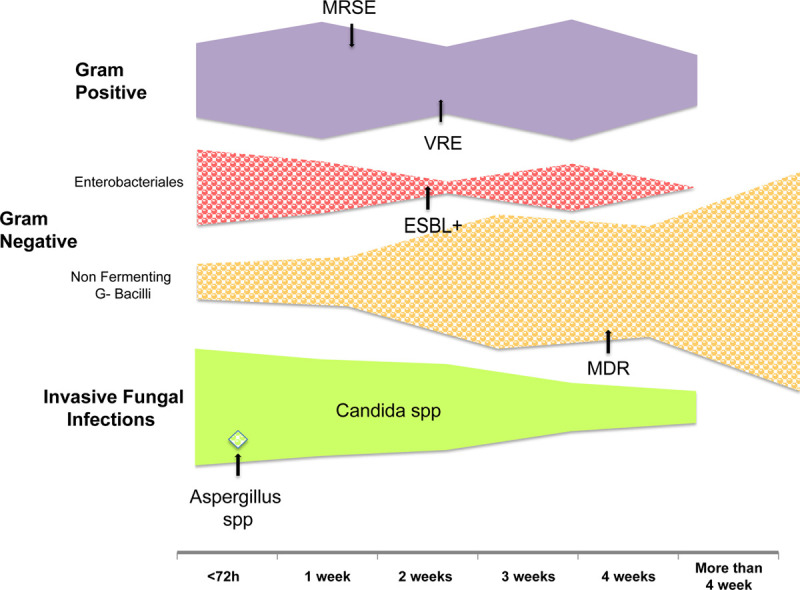
Graphical representation of the timeline of the different superinfection onset. The filling in square portion represents the hospital-acquired pneumonia and the ventilator-associated pneumonia, whereas the fulfill portion represents the systemic infection, including bloodstream infections and invasive candidiasis. The total width is proportional to the total amount of the superinfection. The arrows indicate the first appearance of antimicrobial resistance. ESBL = extended-spectrum beta-lactamase, MDR = multidrug-resistant, MRSE= methicillin-resistant Staphylococcus epidermidis, spp = species, VRE = vancomycin-resistant Enterococcus.
Concerning the secondary objectives, ICU-LOS was significantly longer in patients with superinfection (median [IQR], 15.0 d [9.5–20.5]) than in patients without (median [IQR], 5.0 d [2.0–8.0]), whereas HLOS was not. ICU and the 28-day mortality rates were also higher in patients with superinfection (45.3% vs 10.3%; p < 0.001) (Table 2). On univariate analysis, age, the highest daily SOFA score, the lowest daily Po2/Fio2, steroids duration, and the presence of any fungal or bacterial superinfection were associated with ICU mortality (Supplementary Table 5, http://links.lww.com/CCX/A626). On adjusted analysis (Table 3), bacterial infections (OR, 10.53; 95% CI, 2.31–63.42; p = 0.005), age (OR, 1.17; 95% CI, 1.07–1.31; p = 0.001), and the highest daily SOFA (OR, 1.27; 95% CI, 1.06–1.63; p = 0.032) score were independently associated with ICU mortality or 28-day mortality.
TABLE 2.
Unadjusted Analysis for Patients Outcomes
| Variables | No Superinfection (n = 39) | Superinfection (n = 53) | Total (n = 92) | p |
|---|---|---|---|---|
| ICU length of stay (d), median (IQR) | 5.00 (2.0–8.0) | 15.00 (9.5–20.5) | 10.00 (4–16) | < 0.001 |
| Hospital length of stay (d), median (IQR) | 21.00 (14.0–28.0) | 27.00 (16.0–38.0) | 23.50 (15.5–31.6) | 0.199 |
| ICU mortality, n (%) | 4 (10.3) | 24 (45.3) | 28 (30.4) | < 0.001 |
| 28-d mortality, n (%) | 6 (15.4) | 26 (49.1) | 32 (50.9) | 0.001 |
IQR = interquartile range.
TABLE 3.
Adjusted Analysis for Mortality (ICU Mortality or 28-d Mortality, Whichever Comes First)
| Variables | OR (95% CI) | p |
|---|---|---|
| Presence of fungal superinfection | 1.59 (0.39–6.77) | 0.515 |
| Presence of bacterial superinfection | 10.53 (2.31–63.42) | 0.005 |
| Male gender | 0.66 (0.10–5.82) | 0.678 |
| Age (yr) | 1.17 (1.07–1.31) | 0.001 |
| Worst daily Pao2/Fio2 | 0.98 (0.96–1.00) | 0.089 |
| Worst daily Sequential Organ Failure Assessment score | 1.27 (1.06–1.63) | 0.032 |
| ICU length of stay (d) | 0.94 (0.85–1.02) | 0.181 |
OR = odds ratio.
Boldface values indicate statistically significant covariates.
DISCUSSION
We describe the epidemiology of superinfections in a series of 92 consecutive patients with COVID-19–related ARDS admitted to ICU during the first wave of the COVID-19 outbreak in the North of Italy. We found a prevalence of superinfection of 21.7% at ICU admission, increasing to 57.6% during ICU stay, and a strong correlation between bacterial superinfection and mortality. This was observed despite the fact that no visitors were allowed in the ICU as per anti-COVID-19 policies.
Superinfections prevalence at ICU admission and during ICU stay is higher than what has been already reported in the literature, both in non-COVID19 ICU patients (8% to 22%) (15, 16) and in non-ICU patients with SARS-CoV2 infection (3% to 7.2%) (4, 17). Superinfection might be higher in COVID-19 patients due to different reasons. During the first wave of the SARS-CoV-2 pandemic, hospitals in Northern Italy faced an emergency situation that had never been seen in modern Western country history. We tripled the number of ICU beds in our hospital using inadequate space and employing non-well-trained nurses in ICU, with the consequent increased risks of inter-patient superinfection transmission. Furthermore, critically ill patients were frequently treated on the medical wards due to the lack of ICU beds, leading to a delayed ICU admission of very sick patients. Altogether, these conditions could explain an increased prevalence of superinfection both at ICU admission and during ICU stay.
Concerning superinfection etiology, we found a surprisingly high prevalence of Candida species as responsible for 75.9% of the superinfections during the first and the second week of ICU stay. Although the time of onset of IC is in line with the literature on critically ill non-COVID19 patients, where the peak of Candida species is reported between 5 and 12 days (18), in our population, IC prevalence is higher (19).
Known predisposing risk factors for IC are parenteral nutrition, presence of central venous line, corticosteroid therapy, long-term stay in the hospital, multiple Candida species colonization, antibiotic treatments, and MV (either invasive or noninvasive) (20, 21). Patients were likely exposed to these risk factors even before ICU admission during the COVID-19 pandemic as explained above, leading to patients with an already high risk of candidiasis at ICU admission. Interestingly, we observed CAPA in only two subjects at ICU admission and none during ICU stay. This is in contrast with the current literature that reports a prevalence of CAPA up to 35% (22–24). Our findings may be explained by the extensive use of echinocandin to treat proven and probable IC before and during ICU stay (25).
Concerning bacterial superinfections, we reported an prevalence of VAP of 34.8%, starting from the second week of ICU stay. Dudoignon et al (26) reported an prevalence of VAP in 37%, in line with our results, whereas Luyt et al (11) reported a much higher prevalence (86%) of VAP in patients with COVID-19–related ARDS requiring extracorporeal circulation. In our series, VAPs were caused mainly by Gram-negative bacteria, mostly caused by non-MDR P. aeruginosa starting from the second week, followed by MDR strains that account for 100% of the isolates after week 4, in line with previous studies. Pseudomonas species is known to be a pathogen responsible for secondary infection and late VAP in viral pneumonia (27), including COVID-19 (3–5, 17, 28–30).
We speculate a possible involvement of surfactant activity in the increased prevalence of P. aeruginosa-related VAP. The low concentrations of surfactant observed in patients with COVID-19–related ARDS might cause inflammation of distal airways and impaired surfactant function, predisposing for P. aeruginosa infection (31); this mechanism paired what has been observed in cystic fibrosis (32). On the other side, the increased rate of MDR strain of P. aeruginosa may be an effect of antimicrobial selection, probably magnified by suboptimal application of infection control measures due to ICU overpopulation and prolonged use of personal protective equipment (33).
In accordance with other reports, we found a high prevalence (16.3%) of BSI due to Gram-positive bacteria during the COVID-19 pandemic (28, 29). Most BSIs were due to MRSE (50.0%) and Enterococcus species (37.5%), with a high rate of vancomycin-resistant strains (VRE) (33.3%). The latter is higher when compared with our local experience in the pre-COVID-19 era. The high prevalence of Gram-positive related BSI could be explained by the lack of infection control measures in the context of the cohorting strategy and understaffing. Pre-COVID-19 studies demonstrated that the contact between patients and healthcare workers contaminated by VRE is one of the major risks for subsequent transmission (34). Besides, the administration of a third or fourth generation cephalosporin and the use of corticosteroids before ICU admission, widely used in our COVID-19 populations, are well-known risk factors for VRE colonization (35). Although there was no significant association between prior antibiotic use and development of infection in the ICU, it is possible that the extensive use of antimicrobials impacted the bacterial flora, leading to the selection of resistant strains and eventually determined infection only in the subset of superinfected patients. In our study, 88% of patients were receiving antibiotics before ICU admission, even if only 21.7% of the subjects had a demonstrated superinfection, in need of antibiotics. Recently, the World Health Organization recommended empiric treatment with antibiotics for patients with severe COVID-19, using host factors and local epidemiology to drive antibiotic choice (6). Previous reports have shown that antibiotic treatment is widely used for noncritical COVID-19 patients, even in the absence of any evidence of superinfection (3, 5). Our data suggest that empiric antibiotic treatment may be unnecessary for most patients at admission in ICU, and it should target subjects with clear signs and symptoms of infection (leukocytosis, an increase of CRP and/or procalcitonin, high SOFA score). Close monitoring of bacterial colonization is essential, facilitating targeted therapy with narrow-spectrum antibiotics. Empiric therapy of NFGNBs may be considered in the late stages of ICU stay.
ICU mortality in our patients (30.4%) is lower than what has been reported in a recent worldwide meta-analysis (41.6%) (36). In agreement with previous observations, we reported a relevant and significant impact of bacterial infections on the mortality of COVID-19 patients (4). The risk of death associated with bacterial infection was higher (OR, 10.53; 95% CI, 2.31–63.42) than what was reported in a meta-analysis (OR, 5.82; 95% CI, 3.4–9.9), which included ICU and non-ICU patients (5).
Our study has several limitations. This was a single-center ICU study and may not be generalizable to other ICU patients in Italy or elsewhere. The sample size was limited, and systematic testing for superinfections was not performed. The number of IC may be overestimated because we also accounted for probable infections. Furthermore, the low sample size limits inferences on the role of agents such as tocilizumab on the risk of secondary infections and larger prospective studies are needed to better study that relationship. However, data were collected prospectively, and the definition of superinfection episodes, as well as the diagnostic and therapeutic decisions, were taken in agreement with an infectious disease consultant.
CONCLUSIONS
In conclusion, we described the superinfection epidemiology in COVID-19 patients requiring MV, and we have reported a significant correlation between superinfections and ICU 28-day mortality. Our results could be useful to guide the choice of empirical therapy and suggest that adequate antimicrobial stewardship and optimization of the infection control measures could reduce the prevalence of superinfection and, therefore, mortality.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Matteelli and Piva contributed equally.
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Spedali Civili di Brescia University Hospital, Piazzale Spedali Civili di Brescia, 25123 Brescia, Italy.
REFERENCES
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020; 323:709–710 [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect. 2021; 27:9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. ; COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin Microbiol Infect. 2021; 27:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: A systematic review and meta-analysis. J Infect. 2020; 81:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization: COVID-19 Clinical management: living guidance. 2020 Available at: https://who.int. Accessed March 2021.
- 7.Lombardy Section Italian Society Infectious And Tropical Diseases. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020; 28:143–152 [PubMed] [Google Scholar]
- 8.Elm E von, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007; 335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020; 19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piva S, Filippini M, Turla F, et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J Crit Care. 2020; 58:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyt CE, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann Intensive Care. 2020; 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020; 71:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281 [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units. In: Annual epidemiological report for 2017. ECDC (Ed.). Stockholm, ECDC, 2019 [Google Scholar]
- 16.Vincent JL, Sakr Y, Singer M, et al. ; EPIC III Investigators. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020; 323:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020; 26:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankovszky P, Társy D, Öveges N, et al. Invasive Candida infections in the ICU: Diagnosis and therapy. J Crit Care Med (Targu Mures). 2015; 1:129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit Care. 2019; 23:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Zhu R, Luan Z, et al. Risk of invasive candidiasis with prolonged duration of ICU stay: A systematic review and meta-analysis. BMJ Open. 2020; 10:e036452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nat Rev Dis Primers. 2018; 4:18026. [DOI] [PubMed] [Google Scholar]
- 22.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020; 63:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: A prospective study. Clin Infect Dis. 2020. July 28. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020; 10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aruanno M, Glampedakis E, Lamoth F. Echinocandins for the treatment of invasive Aspergillosis: From laboratory to bedside. Antimicrob Agents Chemother. 2019; 63:e00399–e00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudoignon E, Caméléna F, Deniau B, et al. Bacterial pneumonia in COVID-19 critically ill patients: A case series. Clin Infect Dis. 2021; 72:905–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalanuria AA, Ziai W, Zai W, et al. Ventilator-associated pneumonia in the ICU. Crit Care. 2014; 18:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cataldo MA, Tetaj N, Selleri M, et al. ; INMICOVID-19 Co-infection Group. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming “collateral effect.” J Glob Antimicrob Resist. 2020; 23:290–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020; 50:e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020; 10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006; 36:423–435 [DOI] [PubMed] [Google Scholar]
- 32.Malhotra S, Hayes D, Jr, Wozniak DJ. Cystic fibrosis and Pseudomonas aeruginosa: The host-microbe interface. Clin Microbiol Rev. 2019; 32:e00138–e00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturdy A, Basarab M, Cotter M, et al. Severe COVID-19 and healthcare-associated infections on the ICU: Time to remember the basics? J Hosp Infect. 2020; 105:593–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson SS, Thom KA, Magder LS, et al. ; CDC Prevention Epicenters Program. Patient contact is the main risk factor for vancomycin-resistant Enterococcus contamination of healthcare workers’ gloves and gowns in the intensive care unit. Infect Control Hosp Epidemiol. 2018; 39:1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadimitriou-Olivgeris M, Drougka E, Fligou F, et al. Risk factors for enterococcal infection and colonization by vancomycin-resistant enterococci in critically ill patients. Infection. 2014; 42:1013–1022 [DOI] [PubMed] [Google Scholar]
- 36.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020; 75:1340–1349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


