Abstract
Background:
Vitamin K has long been regarded as a procoagulant drug by physicians, and concerns have been raised with regard to its effects on hemostasis. Although many studies have shown that vitamin K supplementation is safe for thrombotic events, the effect of vitamin K supplementation on the activities of vitamin K dependent procoagulation factors in healthy individuals is not available.
Objectives:
This study aimed to investigate whether vitamin K2 supplementation at recommended doses affects the activity of vitamin K dependent procoagulation factors in healthy individuals without any anticoagulation treatment.
Design:
Forty healthy volunteers between 25 and 40 years of age were recruited. Menaquinone-7 (MK-7) was administrated at 90 μg for 30 days. Prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and blood coagulation factors II, VII, IX, and X activities and Protein induced by vitamin K absence or antagonist-II (PIVKA-II) were measured on days 0 and 30 after MK-7 administration.
Results:
PT, APTT, and TT showed no significant differences on day 30 when compared with baseline. The activities of coagulation factors II, VII, IX, and X on day 30 showed no significant differences with those at baseline. PIVKA-II levels were unchanged after 30 days of MK-7 supplementation.
Conclusions:
MK-7 supplementation at recommended dosage does not affect vitamin K-dependent coagulation factors’ coagulation activity, and does not enhance the carboxylation of prothrombin in healthy individuals. This indicated that MK-7 administration does not alter hemostatic balance in healthy populations without anticoagulation treatment.
Keywords: coagulation activity, healthy individuals, menaquinone-7
1. Introduction
Vitamin K was first identified as a key factor in coagulation 80 years ago. The discovery of different isoforms of vitamin K elucidated the multi-functional role of vitamin K beyond coagulation. Natural vitamin K refers to a number of structurally related compounds including phylloquinone (vitamin K1) and menaquinones (K2 vitamins). Menaquinones are classified based on the length of their aliphatic side chain and are designated as MK-n, where n stands for the number of isoprenoid residues in the chain. Of all the menaquinones, menaquinone-7 (MK-7) is the most efficiently absorbed 1 and exhibited the greatest bioavailability,[1] becoming popular as a supplement for bone as well as vascular health. MK-7 serves as a cofactor in carboxylation process of certain protein-bound glutamate residues, wherein these are converted into gamma-carboxy glutamate (Gla) residues. These Gla residues are regarding essential for forming form calcium-binding sites for activating osteocalcin and matrix Gla proteins, which are considered beneficial in bone mineralization and cardiovascular health. Mounting evidence has shown beneficial effects of MK-7 in osteoporosis and cardiovascular disease.[2–4] The vitamin MK-7 is becoming popular as a supplement for bone and vascular health, and a dose of 100 to 120 μg is usually available as over-the-counter medication. However, it has been shown that it can interfere with anticoagulation therapy when used above 50 μg/day.[1] As MK-7 is widely used by healthy and normal population for preventing osteoporosis and cardiovascular disease, physicians’ have raised their concerns on whether MK-7 administration can alter the hemostatic balance by inducing a thrombotic tendency in healthy populations without anticoagulation treatment. Although thrombin generation in healthy subjects was unaffected with low dose MK-7 supplementation,[5] and whether MK-7 supplementation affects the activities of vitamin K dependent coagulation factors, and the carboxylation of coagulation factor in physiological state have not been elucidated.
The present study aimed to investigate the effects of MK-7 administration on carboxylation and coagulation activity of vitamin K dependent coagulation factors, and to clarify the plausible concern for the procoagulant effect of MK-7 in healthy population.
2. Methods
2.1. Subjects
Forty volunteers [18 men and 22 women; age range 25–40 years; height 166 ± 8.00 cm; body weight 57.33 ± 7.92 kg; and body mass index (BMI) 20.82 ± 1.54 kg/m2] were enrolled in this intervention study. Exclusion criteria were as follows: individuals with thrombotic and/or hemorrhagic events, who take vitamin K supplements and those that interfere with vitamin K and/or coagulation factor functions 2 weeks prior to the blood withdrawal, and who participates in another clinical study at the same time. There were no participant dropouts during the study. All participants included in the study have provided a written informed consent. The study protocol was approved by ethics committee and was in accordance with the Helsinki declaration. Trial registration code: ChiCTR1900028459. At http://www.chictr.org.cn/showproj.aspx?proj=27212.
The healthy individuals were given MK-7 90 μg/day (vitamin K soft gel, Sungen, China), which is a subclass of vitamin K2, for 30 days. Blood samples were withdrawn on days 0, 7 and 30 after the intake of MK-7 supplement.
2.2. Assay methods
The activated partial thromboplastin time (APTT) was measured through blood coagulation time method, and prothrombin time (PT) was determined by Quick's method.[6] The activities of vitamin K dependent coagulation factors II (FII:C), VII (FVII:C), IX (FIX:C), and X (FX:C) were measured with single-stage methods using factor deficient plasma. Serum protein induced by vitamin K absence or antagonist-II (PIVKA-II) levels were measured by electrochemiluminescence immunoassay and were expressed in milli-arbitrary units (mAUs). All assays except PIVKA-II were determined by automatic blood coagulation analyzer ACL TOP 700 (Instrumentation Laboratory) once in order to reduce potential variation among the others performed and done according to the manufacturer's instructions. Serum PIVKA-II levels were measured by an automatic chemiluminescence immunoassay analyzer (ARCHITECT I1000SR, Abbott).
2.3. Blood samples
Fasting venous blood was taken by venipuncture from the antecubital vein. For assays of APTT, PT, TT, FII:C, FVII:C, FIX:C, and FX:C, the blood samples were anticoagulated with 3.8% sodium citrate (9/1, blood/anticoagulant volume) and centrifuged at 3000 g for 10 minutes at room temperature. The plasma was separated and measured within 4 hours. For conducting PIVKA-II assay, blood samples were collected in heparinized vacutainer tubes and centrifuged at 3000 g for 10 minutes at room temperature.
2.4. Statistical analysis
Data are presented as means ± standard deviation. Paired sample t test was used to study the effects of vitamin K2 supplementation on coagulation parameters. Data was analyzed by IBM-SPSS version 21 and a P value of <.05 was considered to be statistically significant.
3. Results
3.1. Baseline characteristics
A total of 18 men (45%) and 22 females (55%) with an average age of 28 were enrolled. The baseline demographic characteristics, coagulation parameters (APTT, PT, and TT), and the activities of FII:C, FVII:C, FIX:C, and FX:C of all subjects were summarized in Table 1. All baseline coagulation factors were found to be in the normal range.
Table 1.
Baseline characteristics.
| Variables | Values |
| Male, n (%) | 18 (40) |
| Age(yr) | 28 (25–40) |
| Height (m) | 1.66 ± 0.08 |
| Weight (kg) | 57.33 ± 7.92 |
| BMI (kg/m2) | 20.82 ± 1.54 |
| APTT(25.1–36.5 s) | 33.51 ± 2.35 |
| PT (9.9–12.8 s) | 11.83 ± 0.61 |
| TT (10.3–16.6 s) | 15.08 ± 1.17 |
| FII:C (79–131%) | 99.96 ± 10.24 |
| FVII:C (50–129%) | 76.40 ± 12.33 |
| FIX:C (65–150%) | 99.65 ± 13.30 |
| FX:C (77–131%) | 92.01 ± 10.46 |
3.2. Routine coagulation assays
The APTT was 33.51 ± 2.35 s at baseline, 33.63 ± 2.14 s on day 7 and 33.53 ± 2.10 s on day 30, respectively. PT was 11.83 ± 0.61 s at baseline, 11.86 ± 0.57 s on day 7 and 11.97 ± 0.56 s on day 30, respectively. TT was 15.08 ± 1.17 s at baseline, 14.81 ± 0.94 s on day 7 and 14.83 ± 1.08 s on day 30, respectively.
All these coagulation parameters of healthy subjects showed no significant differences between the baseline and day 7 or day 30 after MK-7 intake (P > .05, Fig. 1).
Figure 1.
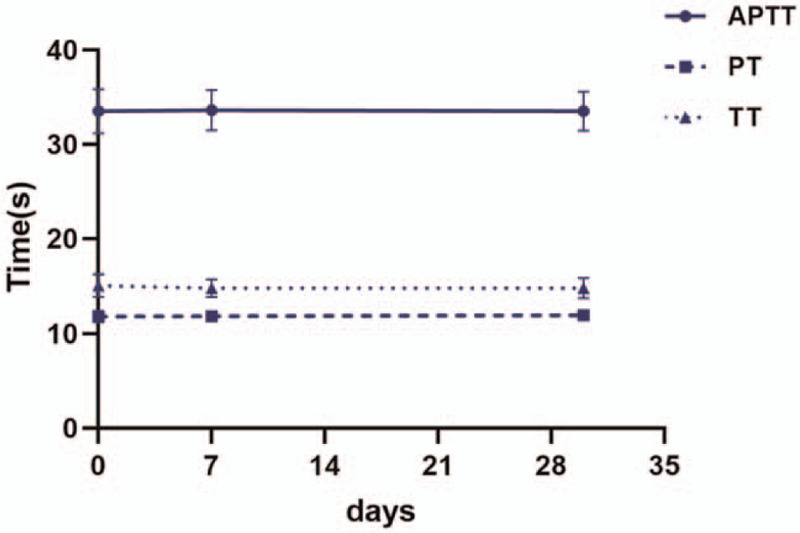
APTT, PT, and TT were measured at baseline, day 7 and day 30 after MK-7 supplement, and did not show significant differences at each time points.
3.3. Coagulation factors
The baseline activity of factor II was 99.96 ± 10.24%, which showed no significant change after MK-7 supplementation, and its activity was 99.97 ± 8.96% on day 7 (P = .99 vs baseline) and 97.28 ± 12.42% on day 30 (P = .24 vs baseline). The activities of coagulation factor VII, IX, and X on day 30 showed no significant differences at baseline (76.12 ± 15.82% vs 76.40 ± 12.33%, P = .92 for FVII: C; 97.65 ± 13.98% vs 99.65 ± 13.30%, P = .47 for FIX:C; 89.18 ± 10.76% vs 92.01 ± 10.46%, P = .1 for FX:C; Table 2).
Table 2.
Effect of MK-7 on coagulation factors activities in healthy subjects.
| Baseline | Day 7 | Day 30 | P∗ value | P# value | |
| FII:C(79–131) | 99.96 ± 10.24 | 99.97 ± 8.96 | 97.28 ± 12.42 | .99 | .24 |
| FVII:C(50–129) | 76.40 ± 12.33 | 77.72 ± 13.04 | 76.12 ± 15.82 | .54 | .92 |
| FIX:C(65–150) | 99.65 ± 13.30 | 98.19 ± 10.36 | 97.65 ± 13.98 | .46 | .47 |
| FX:C(77–131) | 92.01 ± 10.46 | 89.81 ± 10.55 | 89.18 ± 10.76 | .10 | .10 |
Baseline vs Day7.
Baseline vs Day30.
3.4. Coagulation factors of diluted plasma
On day 0 and day 30 of MK-7 supplementation, plasma was diluted at 1:10 ratio to assay the activity of coagulation factors. In the plasma diluted at 1:10 ratio, the baseline activity was 11.14 ± 1.53% for factor II, 8.71 ± 1.22% for factor VII, 11.57 ± 1.61% for factor IX, and 9.08 ± 1.63% for factor X. With MK-7 supplementation for 30 days, the activities of factors II, VII, IX, and X in plasma diluted at 1:10 ratio were 10.97 ± 1.55%, 8.73 ± 1.38%, 11.65 ± 1.54%, and 8.93 ± 1.13%, respectively. No significant variation was observed between day 30 and baseline with 10-fold plasma dilution (P = 0.61 for FVII:C, P = .95 for FIX:C, P = .65 for FIX:C, and P = .53 for FX:C, Fig. 2).
Figure 2.
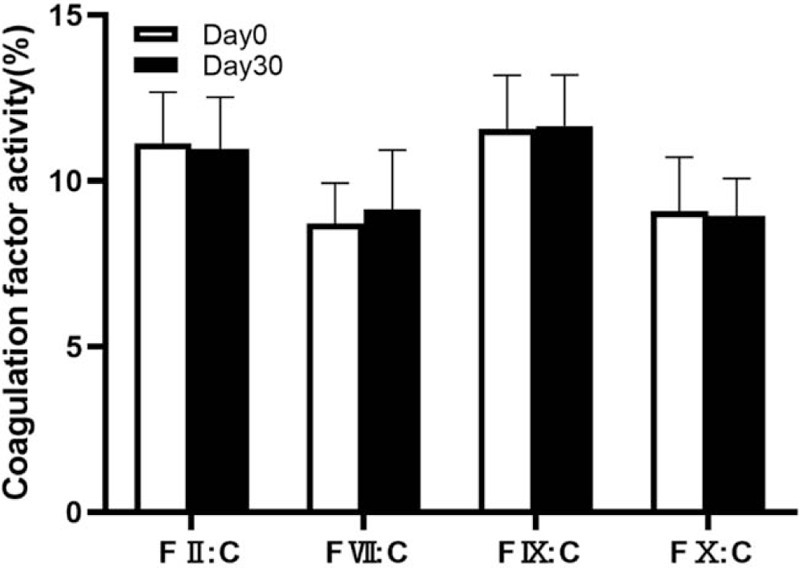
On day 0 and day30 of MK-7 administration, plasma was diluted at 1:10 ratio. Factor II (F II:C), factor VII (F VII:C), factor IX (F IX:C) and factor X (F X:C) activities showed similar decrement on day 0 and day 30.
3.5. PIVKA-II
In 40 healthy subjects, PIVKA-II at baseline was 22.87 ± 5.85 mAU/mL, and 22.26 ± 4.42 mAU/mL at day 30. The uncarboxylated prothrombin level at baseline and after 30 days of MK-7 supplementation showed no significant differences (P = .19, Fig. 3).
Figure 3.
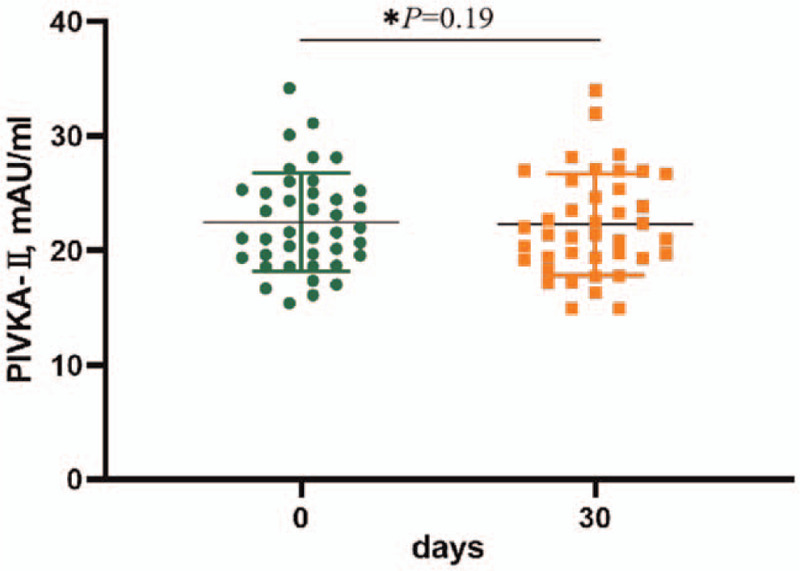
In the forty healthy subjects, the uncarboxylated prothrombin (PIVKA-II) levels did not change with 30 days MK-7 supplementation (P = .19).
3.6. Safety assessment
No volunteer complained of gastrointestinal discomfort, such as vomiting, diarrhea and abdominal pain. Swelling or pain in muscles did not occur in any individual, and no other adverse effects were observed.
4. Discussion
Vitamin K is well known for its function of activating the coagulation factors. Other essential functions of vitamin K is its contribution to bone metabolism and vascular calcification inhibition.[7,8] All these functions of vitamin K were carried out through carboxylation of a number of Gla-proteins by vitamin K. Gla-proteins in the extra-hepatic tissue, such as osteocalcin and matrix Gla-protein, are calcium binding and calcification inhibitor proteins, respectively, and showed only partial carboxylation in healthy adult population.[9,10] Vitamin K supplementation increased the carboxylation of these Gla-proteins, and believed to be beneficial to both bone and vascular health in general population.[11]
Although the notion of vitamin K supplementation in specific population group has been well acknowledged by nutritionists, concerns have been raised that vitamin K administration might influence the hemostatic profile by activating the pro-coagulation factors. As vitamin K is a pro-coagulation vitamin for most of the physicians in their first impression, such concerns might seem plausible. Although no increment of thrombotic events has been reported among vitamin K users, and long-term safety has been proved by many studies,[12,13] few data are available with regard to the influence of vitamin K on activation of vitamin K dependent coagulation factors in healthy population. Early study has shown that administration of vitamin K2 in the form of MK-4 to elderly patients with osteoporosis for 12 weeks showed no change in the generation of thrombin.[14] Elke et al[5] have found that low dose MK-7 supplementation had no effect on thrombin generation in healthy subjects. This study revealed that MK-7 supplementation did not affect the coagulation parameters, implying the unchanged hemostatic profile in healthy individuals taking MK-7. As regard to individuals receiving vitamin K antagonist, MK-7 supplements containing more than 50 μg/day may affect INR value,[1] MK-7 should be administrated with caution.
Triage theory proposed by McCan and Ames has suggested that, as a result of natural selection, vitamin K mainly functions for critical survival function, such as coagulation, which is protected at the expense of functions whose lack is only related with long term consequences, such as calcification or osteoporosis. Compared with other vitamin K dependent proteins, coagulation factors are preferentially carboxylated under normal physical conditions.[15] The dietary intake of vitamin K is believed to be sufficient to ensure the activation of coagulation factors,[7] and the present study is the first to reveal that additional MK-7 administration showed no overactivation of each factor. The activities of coagulation factors in plasma at 1:10 dilution were also measured to identify whether the activation was pronounced with MK-7. According to our study, the activities were proportionally decreased, showing similar trend with baseline.
Our study revealed a steady coagulation profile in healthy individuals taking extra vitamin K2. The reasonable explanation for the steady coagulation profile after taking vitamin K2 is that all Gla-containing coagulation factors are fully carboxylated at recommended dietary allowance levels, and excess vitamin K intake might not induce over carboxylation.[12] Our study showed that carboxylation of prothrombin could not increase with MK-7 administration in healthy individuals, strengthening the evidence for this assumption.
5. Conclusion
In conclusion, MK-7 supplementation at recommended dosage does not affect the hemostatic profile in healthy individuals. Steady coagulation profile is due to additional MK-7 supplementation, which does not induce overactivation of vitamin K dependent coagulation factors, and have been fully carboxylated at the time of dietary vitamin K intake. Our study might provide evidence in eliminating the concerns of hemostatic balance for MK-7 supplementation in healthy individuals to prevent bone and vascular diseases.
Author contributions
Conceptualization: JING TAN.
Formal analysis: Ruijun Ren.
Funding acquisition: JING TAN.
Investigation: Ruijun Ren, Jia Liu, Guo Cheng.
Methodology: Jia Liu.
Project administration: Ruijun Ren.
Software: Guo Cheng.
Writing – original draft: Ruijun Ren.
Writing – review & editing: JING TAN.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, Gla = gamma-carboxy glutamate, MK-7 = menaquinone-7, PIVKA-II = protein induced by vitamin K absence or antagonist-II, PT = prothrombin time, TT = thrombin time.
How to cite this article: Ren R, Liu J, Cheng G, Tan J. Vitamin K2 (Menaquinone-7) supplementation does not affect vitamin K-dependent coagulation factors activity in healthy individuals. Medicine. 2021;100:23(e26221).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Schurgers LJ, Teunissen KJ, Hamulyak K, et al. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. BLOOD 2007;109:3279–83. [DOI] [PubMed] [Google Scholar]
- [2].Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256–61. [DOI] [PubMed] [Google Scholar]
- [3].Knapen MH, Drummen NE, Smit E, Vermeer C. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int 2013;24:2499–507. [DOI] [PubMed] [Google Scholar]
- [4].Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J NUTR 2004;134:3100–5. [DOI] [PubMed] [Google Scholar]
- [5].Theuwissen E, Cranenburg EC, Knapen MH, et al. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr 2012;108:1652–7. [DOI] [PubMed] [Google Scholar]
- [6].Quick AJ. The prothrombin consumption time test. Am J Clin Pathol 1966;45:105–9. [DOI] [PubMed] [Google Scholar]
- [7].Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89–110. [DOI] [PubMed] [Google Scholar]
- [8].Wasilewski GB, Vervloet MG, Schurgers LJ. The bone-vasculature axis: calcium supplementation and the role of vitamin K. Front Cardiovasc Med 2019;6:06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schurgers LJ, Teunissen KJ, Knapen MH, et al. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol 2005;25:1629–33. [DOI] [PubMed] [Google Scholar]
- [10].Szulc P, Chapuy MC, Meunier PJ, et al. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 1993;91:1769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009;89:1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marles RJ, Roe AL, Oketch-Rabah HA. US Pharmacopeial convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr Rev 2017;75:553–78. [DOI] [PubMed] [Google Scholar]
- [13].Vissers LE, Dalmeijer GW, Boer JM, et al. Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc 2013;2:e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asakura H, Myou S, Ontachi Y, et al. Vitamin K administration to elderly patients with osteoporosis induces no hemostatic activation, even in those with suspected vitamin K deficiency. Osteoporos Int 2001;12:996–1000. [DOI] [PubMed] [Google Scholar]
- [15].McCann JC, Ames BN, Vitamin K. an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr 2009;90:889–907. [DOI] [PubMed] [Google Scholar]


