PURPOSE
Liquid biopsy–based biomarkers, including circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), are increasingly important for the characterization of metastatic breast cancer (MBC). The aim of the study was to explore CTCs and ctDNA dynamics to better understand their potentially complementary role in describing MBC.
METHODS
The study retrospectively analyzed 107 patients with MBC characterized with paired CTCs and ctDNA assessments and a second prospective cohort, which enrolled 48 patients with MBC. CTCs were immunomagnetically isolated and ctDNA was quantified and then characterized through next-generation sequencing in the retrospective cohort and droplet digital polymerase chain reaction in the prospective cohort. Matched pairs variations at baseline, at evaluation one (EV1), and at progression were tested through the Wilcoxon test. The prognostic role of ctDNA parameters was also investigated.
RESULTS
Mutant allele frequency (MAF) had a significant decrease between baseline and EV1 and a significant increase between EV1 and progression. Number of detected alterations steadily increased across timepoints, CTCs enumeration (nCTCs) significantly increased only between EV1 and progression. MAF dynamics across the main altered genes was then investigated. Plasma DNA yield did not vary across timepoints both in the retrospective cohort and in the prospective cohort, while the short fragments fraction showed a potential role as a prognostic biomarker.
CONCLUSION
nCTCs and ctDNA provide complementary information about prognosis and treatment benefit. Although nCTCs appeared to assess tumor biology rather than tumor burden, MAF may be a promising biomarker for the dynamic assessment of treatment response and resistance.
BACKGROUND
Despite the advances in prevention and antineoplastic treatments, breast cancer (BC) is still the most frequently diagnosed cancer in women. Among all new cases, 6%-7% are diagnosed with de-novo metastatic disease and approximately 30% of patients initially diagnosed in earlier stages eventually relapse in distant sites.1-3
CONTEXT
Key Objective
With a steady decrease in costs and the noninvasive nature of testing, liquid biopsy has a growing potential role in the management of metastatic breast cancer. However, the optimal way of integrating different biomarkers remains unclear. The study explored the different dynamics of circulating tumor DNA (ctDNA) and circulating tumor cell enumeration (nCTCs) to better describe their specific features and potential ways of integration for future clinical algorithms based on the longitudinal evolution of liquid biopsy characteristics.
Knowledge Generated
ctDNA provides a more quantitative, real-time assessment of tumor burden and treatment benefit. Furthermore, ctDNA, when analyzed at a single gene level, can provide insights on treatment resistance. nCTCs likely describe the underlying metastatic biology.
Relevance
ctDNA can be used to monitor treatment response and anticipate clinical progression; nCTCs provide an overall biologic readout of the disease's clinical aggressiveness.
The growing scalability and the steady decrease in costs have favored the investigation of new clinical algorithms based not only on baseline (BL) liquid biopsy characteristics but also on their longitudinal evolution.4
Circulating tumor cells (CTCs) were the first modern liquid biopsy marker deployed in clinical practice because of their strong prognostic value. However, their longitudinal implementation is still debated.5-7 In this context, the characterization of circulating tumor DNA (ctDNA) has proven useful for treatment selection and encouraging data support its potential role in clinical trial enrollment. By contrast, it has been observed how different information could be obtained by ctDNA according to the analytic workflow.8-11
Notwithstanding the potentials of both liquid biopsy techniques, little is known about their dynamics across longitudinal evaluations. To better grasp potential specificities and confounding factors and therefore enhance their deployment and integration in clinical practice, we analyzed the variations in CTCs enumeration and ctDNA-detected features in patients receiving treatment for metastatic breast cancer (MBC) to comprehensively explore the composite nature of liquid biopsy biomarkers.
METHODS
Study Population and Ethical Approval
The study was based on two distinct cohorts. The retrospective NU16B06 cohort was analyzed for hypothesis generation on the overall dynamics comparison of liquid biopsy–derived parameters. Patients were characterized for CTCs (Data Supplement), total plasma DNA levels (DNA yield) and ctDNA sequencing through the Guardant360 (Guardant Health, Redwood City, CA) next-generation sequencing platform (Data Supplement).12-14 Patients with ≥ 5 CTC/7.5 mL of blood were defined as stage IVaggressive, whereas patients with < 5 CTC/7.5 mL of blood were defined as stage IVindolent.6
Subsequently, the prospective CRO-2018-56 cohort was used to validate the DNA yield findings and to further characterize the different components of plasma DNA and their clinical meaning through droplet digital polymerase chain reaction (ddPCR) (Data Supplement).15
The NU16B06 cohort
This retrospective cohort consisted of 107 patients with MBC longitudinally characterized for CTCs and ctDNA at the Thomas Jefferson University (Philadephia, PA) and the Robert H. Lurie Comprehensive Cancer Center at Northwestern University (Chicago, IL). Patients were enrolled between 2013 and 2019 under the Investigator Initiated Trial NU16B06 independently from treatment line. BL staging was performed according to the investigators' choice. CTCs and ctDNA collection were performed before treatment start (BL), at progression (progressive disease), and at the first clinical evaluation, with a median of 3 months after the BL timepoint (evaluation one [EV1]). ctDNA analysis comprised the number of detected alterations (NDA), mutant allele frequency (MAF), and DNA yield. The Investigator Initiated Study was approved by the institutional review board under the protocol number NU16B06.
The CRO-2018-56 study
To validate the findings for DNA yield, 48 women with hormone receptor–positive human epidermal growth factor receptor 2 (HER2)-negative (luminal-like) MBC were prospectively enrolled through a multicenter pragmatic study between 2018 and 2019. Patients were eligible for endocrine therapy (ET) as first-line treatment and could have received both ET and chemotherapy (CT) in the adjuvant and neoadjuvant settings. Samples were collected at BL and after 3 months concomitantly with imaging evaluation (EV1) and were analyzed through ddPCR for the detection of small, medium, and long fragments of the gene coding for Beta-Actin (ACTB). The study was approved by the ethics committee under the CEUR-2018-Sper-056-CRO protocol.
Statistical Analysis
Clinical and pathologic variables were reported using descriptive analyses. Categorical variables were reported as frequency distributions, whereas continuous variables were described through median and interquartile ranges (IQRs). Matched pairs variations of CTCs enumeration (nCTCs), NDA, MAF, and DNA yield were tested across three timepoints: BL, EV1, and progression. Wilcoxon signed rank test was used for continuous variables, whereas categorical variables were investigated through the McNemar test. Progression-free survival (PFS) was defined as the time from BL to progression (defined through imaging) or death for any cause, whichever came first. Patients without an end point event at the last follow-up visit were censored. Differences in survival were tested by logrank test and Cox regression with 95% CI and represented by Kaplan-Meier estimator plot. Statistical analysis was conducted using StataCorp 2016 Stata Statistical Software: Release 15.1 (College Station, TX), R (version 3.3.1; The R foundation for Statistical Computing, Vienna, Austria) and JMP (version 14; SAS Institute, Cary, NC).
RESULTS
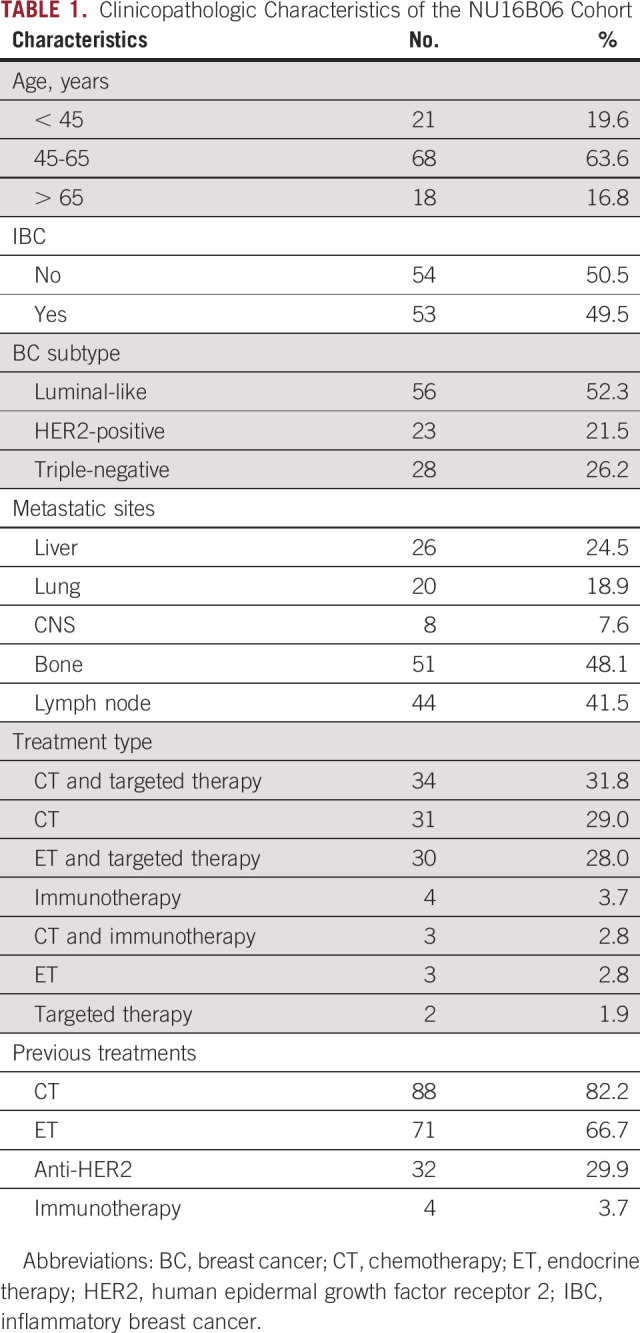
The 107-patient NU16B06 cohort was characterized for nCTCs and ctDNA at BL, EV1, and progression. Median age at BL was 55 years (IQR 46-63). Luminal-like was the most represented subtype (56 patients, 52%), followed by triple-negative BC (28 patients, 26%) and HER2-positive (23 patients, 22%) (Table 1). Fifty-three patients (50%) were diagnosed with inflammatory BC (Table 1). The most common metastatic site was bone (51 patients, 48%), followed by lymph nodes (44 patients, 42%) and liver (26 patients, 25%) (Table 1). The main treatment option was CT (N = 65, 60.8), as single agent (N = 31, 28.9%) or in association (anti-HER2 agents: N = 18, 16.8%; mammalian target of rapamycin [mTOR] inhibitors: N = 9, 8.4%) (Table 1). ET was prescribed in 33 patients (30.8%). Fulvestrant and aromatase inhibitors were the main ET backbones (N = 19, 17.8%, and N = 14, 13.1%, respectively). ET was combined with cyclin-dependent kinase (CDK) inhibitors in 21 patients (19.6%), with mTOR inhibitors in 7 (6.5%), and with anti-HER2 agents in five patients (4.7%) (multiple combinations were possible) (Table 1). CT was the most common previous treatment type (N = 88, 82.2%), 71 patients had received previous ET (66.4%), and 32 anti-HER2 agents (29.9%). Eleven patients (10.3%) were not exposed to previous treatments.
TABLE 1.
Clinicopathologic Characteristics of the NU16B06 Cohort
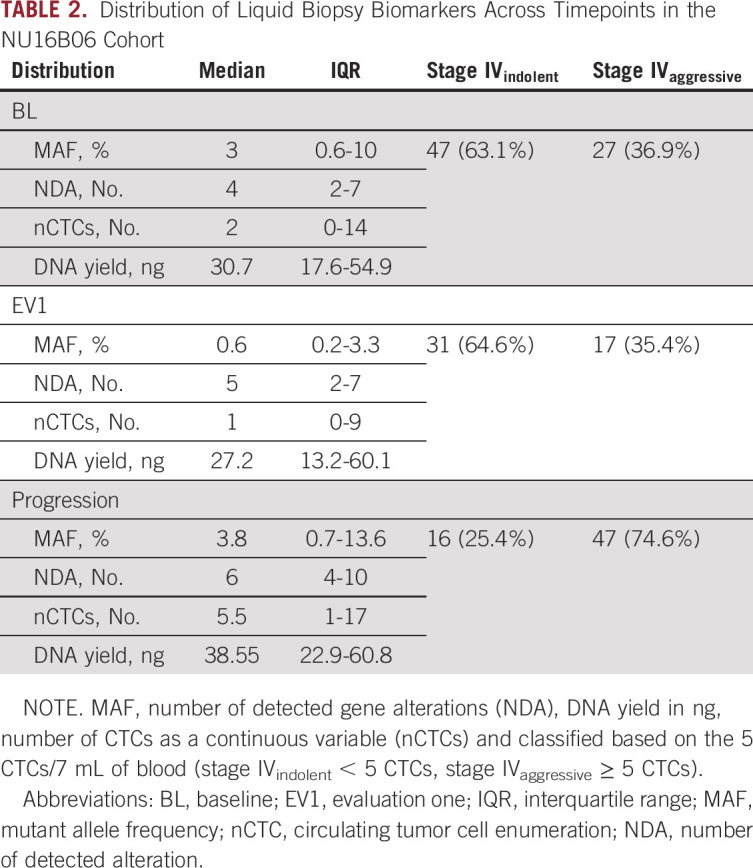
nCTCs at BL were performed in 74 patients; 37% was classified as stage IVaggressive (27 patients), whereas the proportion was 75% at progression (47 patients) (Table 2). Median time to the first evaluation was 3 months (IQR 2-4).
TABLE 2.
Distribution of Liquid Biopsy Biomarkers Across Timepoints in the NU16B06 Cohort
nCTCs, NDA, and MAF Showed Differential Dynamics Across Timepoints
Median MAF at BL was 3, NDA was 4, and nCTCs was 2 (Table 2). At EV1, the median MAF was 0.6, NDA was 5, and nCTCs was 1 (Table 2). At progression, the median MAF was 3.8, NDA was 6, and nCTCs was 5.5 (Table 2; Figs 1A, 1C, and 1E).
FIG 1.
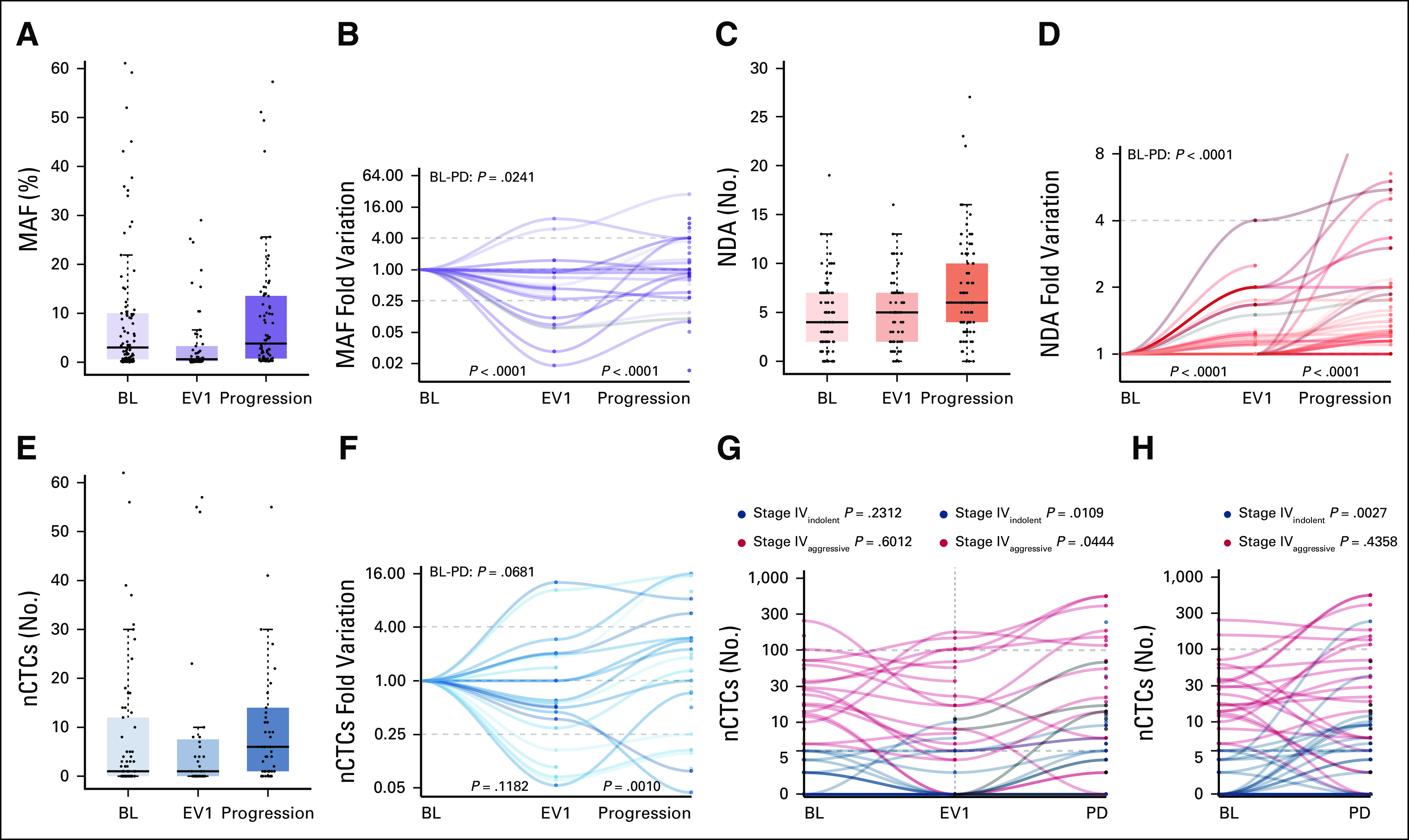
MAF, NDA, and nCTCs distribution and dynamics across the three investigated timepoints (BL, first clinical evaluation [EV1], and progression) and nCTCs dynamics across the three investigated timepoints according to BL CTCs status (stage IVindolent v stage IVaggressive) (G and H). (A, C, and E) Median, interquartile range, and outliers are described for the overall biomarker distribution at each timepoint through box and whiskers plots. (B, D, and F) Biomarker dynamics was then plotted for each patient. MAF initially decreased between BL and EV1, whereas it increased at progression (P < .0001). This trend was observed both in the (A) overall distribution and (B) at a single patient's level normalized on the BL levels. NDA had a steady increase across all timepoints (P < .0001) in the (C) general cohort and (D) at a single patient's level. (E) nCTCs did not vary significantly at EV1 (P = .1182) and it generally increased significantly at progression (P = .0010), (F) although some patients experienced a decrease. In the stage IVaggressive subgroup, (G) a significant increase was observed only between EV1 and progression (P = .0444), and a significant increase was observed in the stage IVindolent subgroup both (G) between EV1 and progression (P = .0109) and (H) BL and PD (P = .0027). (G) No significant differences were observed in either subgroup between BL or EV1. BL, baseline; CTC, circulating tumor cell; EV1, evaluation one; MAF, mutant allele frequency; nCTC, circulating tumor cell enumeration; NDA, number of detected alteration; PD, progressive disease.
With serial assessments, MAF significantly decreased and NDA significantly increased between BL and EV1 (decreased and increased, respectively, P < .0001), whereas both were significantly increased between EV1 and progression (P < .0001) and BL and progression (P = .0241 and P < .0001, respectively) (Figs 1B and 1D).
No significant variations were observed for nCTCs between BL and EV1 and BL and progression (Fig 1F). A significant increase was observed between EV1 and progression (P = .0010) (Fig 1F).
The cohort was then stratified into stage IVindolent and stage IVaggressive.6 Although the general trend was confirmed in the stage IVaggressive subgroup (Figs 1G and 1H), a significant increase was observed in the stage IVindolent subgroup both between EV1 and progression (P = .0109) (Fig 1H) and between BL and progression (P = .0027) (Fig 1G). No significant differences were observed in either subgroup between BL and EV1 (Fig 1H).
MAF Was a Composite Measure Comprising Genes With Different Dynamics
TP53, PIK3CA, MET, ERBB2, EGFR, MYC, NF1, ESR1, ARID1A, and NOTCH1 were the main altered genes at BL (Fig 2A), whereas across all timepoints, TP53, PIK3CA, ERBB2, MET, EGFR, and ESR1 were the most represented. A considerable proportion of patients at BL had more than one alteration for TP53 and PIK3CA (14 and 12, respectively) (Data Supplement).
FIG 2.
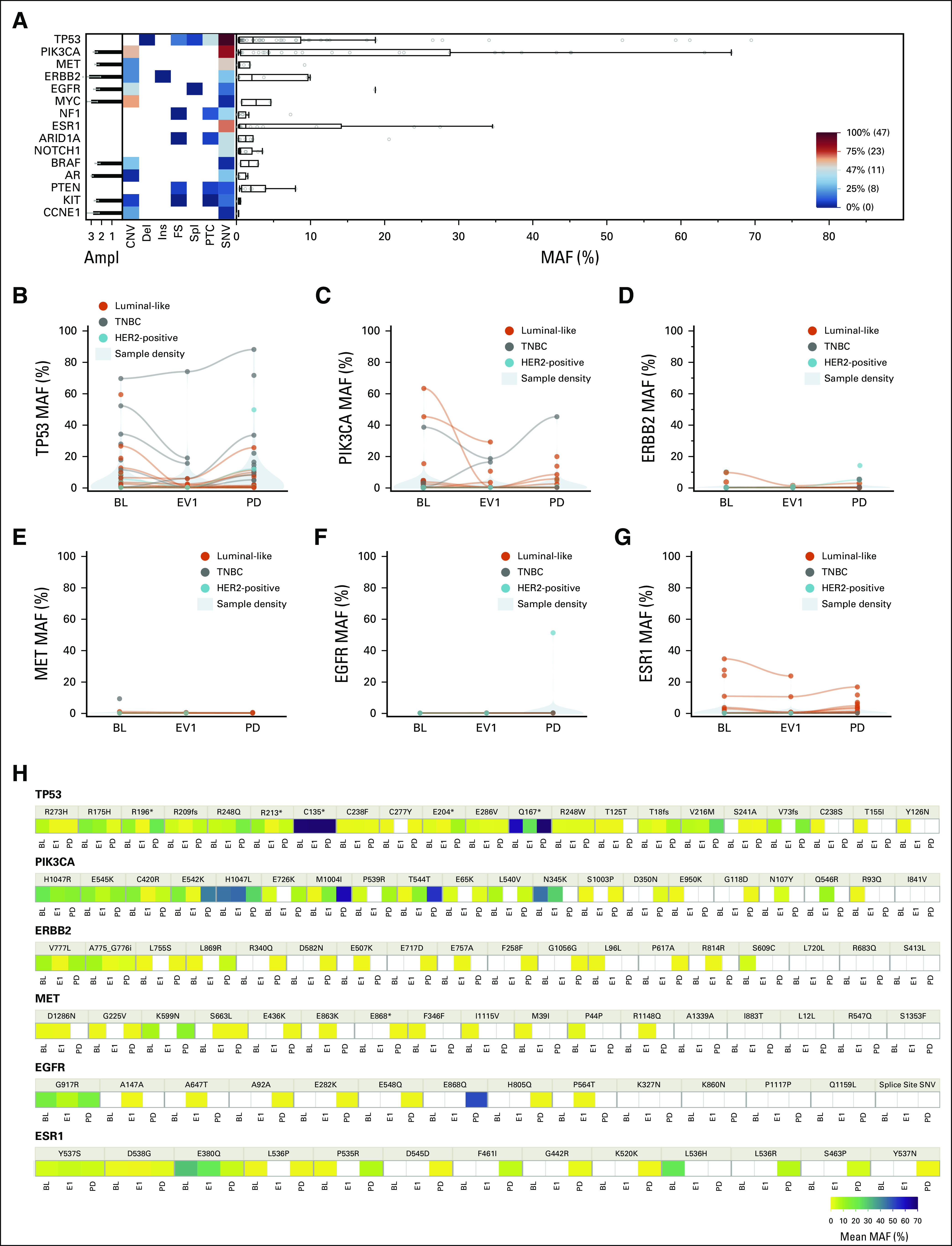
(A) Landscape plot at BL, and (B-G) MAF variations of the top six mutated genes across timepoints as a whole and (H) broken down at variant level. Detected genomic alterations at BL are detailed in the (A) landscape plot as a heat map by type of alteration, MAF, and Ampl (from 1+ to 3+). Variants are classified as CNV, del, ins, FS, spl, PTC, and SNV. Boxplots (right) show the median and interquartile range for MAF, whereas the histograms (left) show the mean Ampl with standard deviation. Shown in the bottom right is a scale for the heat map; variants under the median prevalence are marked as blue, and above the median are depicted in red. (A) TP53 and PIK3CA aberrations were the most represented and (B, C, and H) their MAF varied across all timepoints consistently with the overall MAF. (D, G, and H) An increase in ERBB2 and ESR1 MAF was significant at progression especially in the luminal-like subtype (orange). Ampl, amplification; BL, baseline; CNV, copy-number variants; del, deletion; EV1, evaluation one; FS, frameshift; HER2, human epidermal growth factor receptor 2; ins, insertion; MAF, mutant allele frequency; PD, progressive disease; PTC, premature termination codons; SNV, single nucleotide variants; spl, splicing variants; TNBC, triple-negative breast cancer.
A significant increase in MAF between EV1 and progression was observed for TP53 (P = .0053), PIK3CA (P = .0457), ERBB2 (P = .0456), and ESR1 (P = .0016) (Figs 2B-2D and 2G). Similarly, an increase between BL and progression was highlighted for TP53 (P = .0283), PIK3CA (P = .0456), and ESR1 (P = .0003) (Figs 2B, 2C, and 2G). No significant variations in MAF were observed for EGFR and MET across all timepoints (Fig 2F). Notably, no new ESR1 alteration was observed in EV1, whereas a significant occurrence of ERBB2 alterations was observed for HER2-negative patients (McNemar test P = .0253). For descriptive purposes, the median MAF across all detected gene variants is shown in Figure 2H.
Plasma DNA Yield Did Not Vary Across Timepoints and Was Not Correlated With MAF and NDA
To better understand the value of ctDNA characterization over total plasma DNA, the NU16B06 samples were also characterized for DNA yield. Median DNA yield at BL was 30.7 ng, at EV1 was 27.2 ng, and at progression was 38.55 ng (Table 2). No significant differences were observed between BL and EV1 or between EV1 and progression (Fig 3A). No correlation was observed with MAF or NDA at BL (Figs 3B and 3C).
FIG 3.
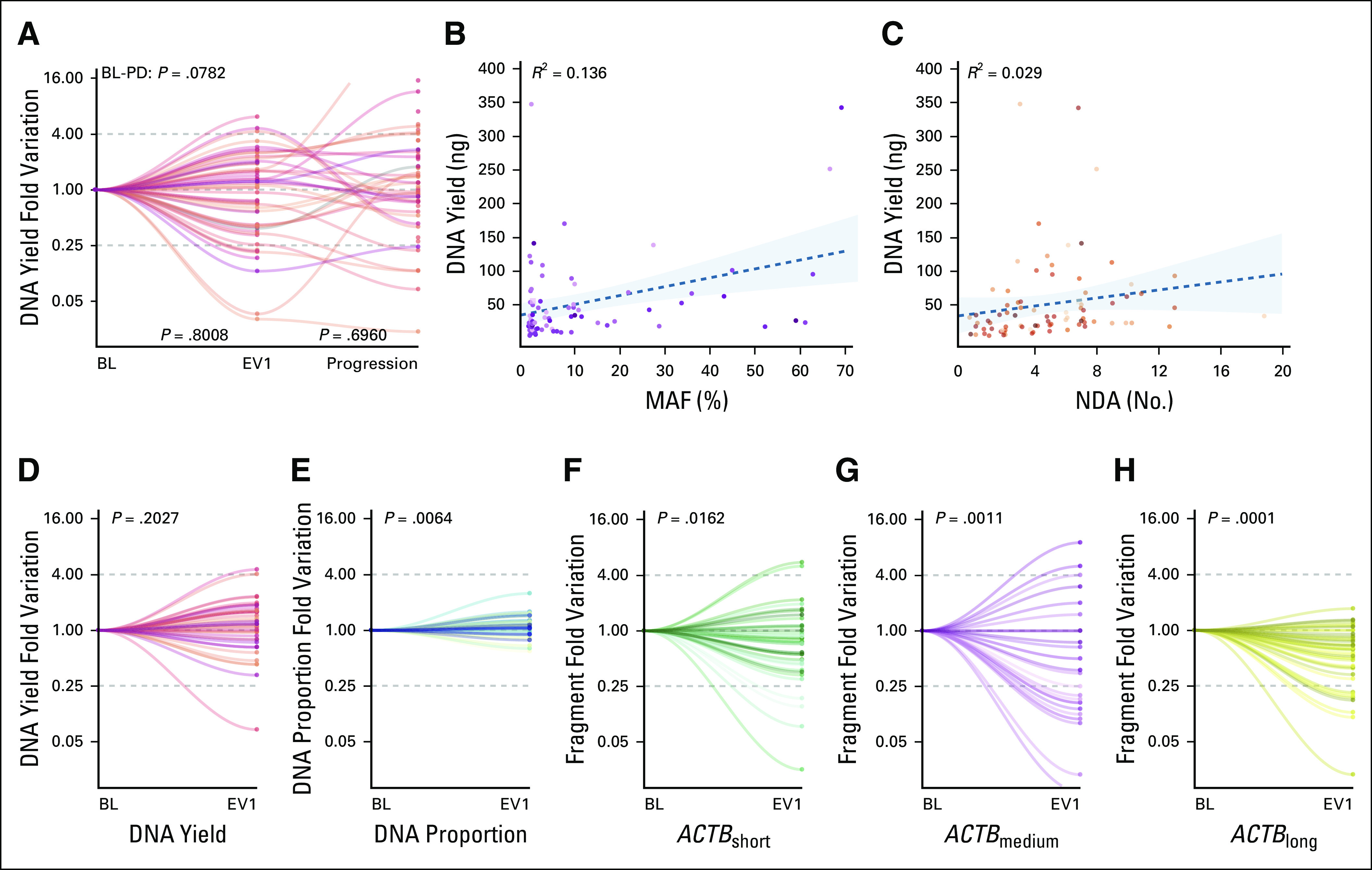
(A) DNA yield dynamics in the NU16B06 cohort. (B and C) Scatter plot of the correlation between MAF and NDA with DNA yield measured through the Qubit system. (D) DNA yield dynamics in the CRO-2018-56 cohort, (E) together with the early dynamics of the DNA proportion and (F, G, and H) different ACTB fragments. No significant differences were observed for (A) DNA yield in the NU16B06 cohort across study timepoints and (B) no correlation was observed between DNA yield and MAF or NDA at BL. (D) No significant variations were confirmed in the CRO-2018-56 prospective cohort for DNA yield, whereas (E) a significant increase in DNA proportion was observed between BL and EV1. A consistent decrease was observed for (F) ACTBshort, (G) ACTBmedium, and (H) ACTBlong. BL, baseline; EV1, evaluation one; MAF, mutant allele frequency, NDA, number of detected alteration; PD, progressive disease.
The DNA yield dynamics were, moreover, analyzed in the prospective CRO-2018-56 cohort. Forty-eight patients with luminal-like MBC who were candidates for first-line ET were enrolled and their blood samples were collected at BL and at EV1 (first CT scan after 3 months). The most common regimen was ET plus CDK4/6 inhibitors (92%), whereas only 6% of patients received ET as a single agent. Further patients' characteristics are shown in the Data Supplement. Consistent with what was observed in the unselected NU16B06 cohort, no differences were observed for DNA yield between BL and EV1 (P = .2027) (Fig 3D).
Short Plasma DNA Fragments Showed Different Dynamics and Clinical Outcome
Plasma DNA originates from different sources through shedding or cell lysis. The former is more likely to be related to ctDNA and is composed by short fragments. The latter is predominantly derived by leukocytes and usually consists of longer fragments. For exploratory purposes, a ddPCR analysis was performed on the CRO-2018-56 cohort to evaluate potential differences within the overall DNA yield by measuring the different fractions of the ACTB DNA fragments (136 bp, 420 bp, and 2,000 bp, respectively, of ACTBshort, ACTBmedium, and ACTBlong) and their proportion (ACTBshort/ACTBshort plus ACTBmedium plus ACTBlong).15
A significant increase in DNA proportion was observed between BL and EV1 (P = .0064) (Fig 3E) because of significant decreased levels not only in ACTBshort (71% of cases, P = .0162) (Fig 3F), but also for ACTBmedium (66% of cases, P = .0011) (Fig 3G) and ACTBlong (78% of cases, P = .0001) (Fig 3H).
Since ACTBmedium and ACTBlong were mainly derived by cytolysis of neutrophils, the white blood cell count dynamics was also investigated.15 As expected, because of the mechanism of CDK4/6 inhibitors, a significant drop in neutrophil count was observed between BL and EV1 (decrease in 94% of cases, P < .0001), whereas a decrease in lymphocyte count was trended toward significance (decrease in 65% of cases, P = .0615).
For exploratory purposes, the prognostic impact of BL DNA yield was tested in terms of PFS. Patients with a DNA yield higher than the 75th percentile experienced a similar prognosis with respect to the lower percentiles (P = .9325) (Fig 4A). Patients with a DNA proportion in the top quartile at BL had a significantly worse outcome (PFS at 6 months 56% v 90%, P = .0007) (Fig 4B). The prognostic impact of a ≥ 20% decrease between BL and EV1 was then investigated across the different ACTB fragments lengths. Although a significant impact was observed for ACTBshort (hazard ratio [HR]: 3.82; 95% CI, 1.29 to 11.29; P = .0153) (Fig 4C), no significant difference was observed for ACTBmedium and ACTBlong (HR, 1.95; 95% CI, 0.67 to 5.67; P = .2212 and HR, 1.04; 95% CI, 0.13 to 8.00; P = .9712, respectively). The prognostic impact of ACTBshort was also retained after correction for ACTBmedium and ACTBlong in multivariate analysis (HR, 5.24; 95% CI, 1.06 to 25.97; P = .0423) (data not shown).
FIG 4.
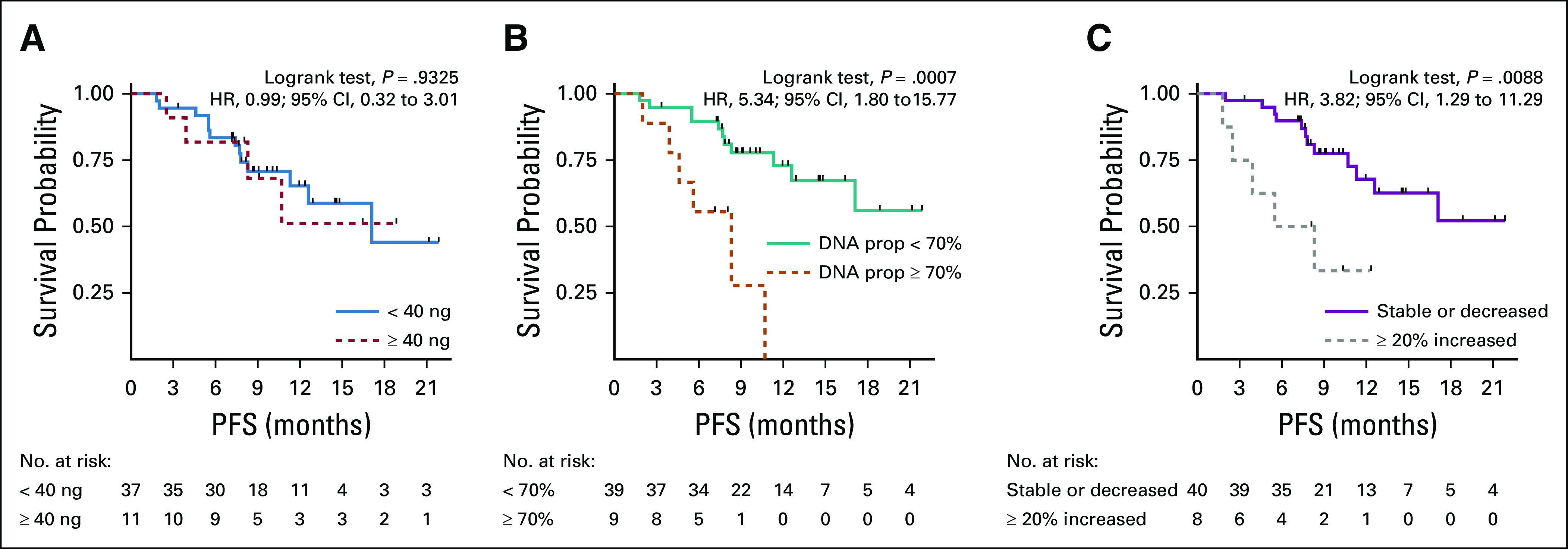
Kaplan-Meier plots for the impact on PFS of different plasma DNA-based biomarkers. Plasma DNA concentration measure with (A) Qubit, (B) DNA prop and (C) 20% increase in ACTBshort between baseline and evaluation one. No significant impact was observed on PFS for (A) the plasma DNA concentration, (B) whereas a ≥ 70% DNA prop and (C) a ≥ 20% increase in ACTBshort had an unfavorable prognostic impact. DNA prop, DNA proportion; HR, hazard ratio; PFS, progression-free survival.
DISCUSSION
This study analyzed the dynamic behavior of liquid biopsy biomarkers with respect to treatment response, with the goal of integrating different information that could potentially guide BL treatment choices and serial assessments after treatment initiation.
The study found significant differences between CTCs and ctDNA through MAF and NDA characterization. Importantly, MAF appeared to follow treatment response versus progression. By contrast, NDA increased steadily across timepoints, whereas nCTCs increased only at the time of clinical progression.
CTCs enumeration was the first clinically deployed liquid biopsy biomarker and, although the prognostic implications were consistently confirmed, monitoring results have been controversial.5-7 The SWOG 0500 phase III trial was the first attempt to use CTCs as a longitudinal clinical decision-making tool.7 The study was negative with respect to early change of CT regimen for patients with persistently high CTCs, jeopardizing the clinical utility of longitudinal CTCs characterization. However, the study lacked a precision medicine approach for treatment selection and biology-defined sampling timeframes.7
Similarly, the CirCe01 trial investigated whether it was possible to discontinue a potentially noneffective treatment based on CTCs dynamics in patients with MBC treated beyond the second line.16 The study confirmed the prognostic impact of BL stage IVaggressive on overall survival, but not for PFS.16 It, moreover, observed that patients with ≥ 5 CTCs/7.5 mL (Stage IV aggressive) at BL and with either < 5 CTCs/7.5 mL at the second cycle or a relative decrease of at least 70% of the BL CTCs enumeration experienced a longer PFS.16
Interestingly, consistent results were observed in this study's NU16B06 cohort (HR, 2.04; 95% CI, 0.96 to 4.35; P = .0653).
The study reported here further highlighted more nuanced trend of nCTCs since an increase was observed only at progression. These results may suggest that, although MAF could be more suitable for real-time disease monitoring, nCTCs could be more likely linked to metastatic biology, in particular in the stage IVindolent population. Previous studies suggested that nCTCs is a composite biomarker comprising different subpopulation at different stages of epithelial to mesenchymal transition and that patients who respond to therapy have a proportional decrease of the mesenchymal subpopulation. Patients who experience progressive disease show an increased number of mesenchymal CTCs.17,18 CTCs were, moreover, associated with distinctive biologic features such as mutations and metastatic organotropism.19,20
This study then analyzed how single genes can differently account for the overall MAF, demonstrating that a limited set of genes (ie, TP53 and PIK3CA) actually contributed to the overall measure. Consistent results on ctDNA dynamics were reported in the BEECH study, which highlighted a decrease in ctDNA after 8 days of treatment, while the longitudinal characterization of 21 patients treated with pyrotinib confirmed that the mean allele fraction at each timepoint was correlated with tumor size by computed tomography, with a lead time of 8 to 16 weeks in progression detection.21,22
By contrast, the increasingly high sensitivity of sequencing technologies can introduce potentially confounding factors such as the detection of somatic mutations deriving from normal tissues, in particular clonal hematopoiesis of indeterminate potential.23,24 This could also explain the higher incidence of co-occurring TP53 mutations, with respect to public databases. In our study, we did not concurrently sequence paired white blood cells to rule out clonal hematopoiesis of indeterminate potential.
Ma et al,22 moreover, suggested that a broad gene characterization is needed to correct potential biases deriving by their biologic role and treatment-derived selective pressure. Although genes encompassing truncal mutations (eg, TP53 and PIK3CA) were generally in line with the overall MAF trend, ESR1 and ERBB2 mutations were mainly a later event with a rising MAF and incidence because of the onset of treatment resistance. Although genetic alterations of ESR1 have an established role as resistance biomarkers, other genes such as ERBB2 in HER2-negative patients still need to be fully explored.8,25 It has been reported that patients with luminal-like MBC who acquired ctDNA-detectable ERBB2 alterations during the course of ET had promising responses with the use of tyrosine kinase inhibitors such as neratinib.25,26
Our study, moreover, suggested that the DNA yield fluorometric measurement was not correlated with MAF and NDA and did not vary across treatment timepoints, excluding its potential as a low-cost biomarker. Based on the proportion between plasma-detectable short fragments of ACTB, the study suggested a prognostic impact of the BL DNA proportion over the total plasma concentration. By contrast, this approach showed potential caveats for its longitudinal utilization as the genomic DNA fraction could be affected by drug-related events such as leukopenia and neutropenia, representing a potential confounding factor in the interpretation of the DNA proportion dynamics. Nonetheless, the study suggested how only the short fragment fraction was actually linked to prognosis, independent from the genomic DNA one, supporting the proof of concept that the ctDNA fraction should be accurately selected for a proper liquid biopsy–based disease characterization.
There are several limitations of this study. Since current clinical next-generation sequencing platforms are mainly based on targeted gene panels, MAF could have been underestimated if (1) the driver gene was not included in the panel, or (2) in the presence of two separate subclonal populations not sharing high-MAF mutations.
The retrospective cohort, moreover, was focused on standard, EpCAM-based nCTCs rather than an in-depth CTCs characterization, which could be a limiting factor as demonstrated by previous studies.17,18
The retrospective cohort was large but heterogenous both in terms of disease subtype and treatment line. Although this may increase the generalizability of the findings, it may also have introduced potential biases derived by specific biologic features. By contrast, the prospective cohort is highly homogeneous, but this may in turn jeopardize the results' applicability in other treatment settings.
In conclusion, the study suggests that both CTCs and ctDNA provide complementary information about prognosis and treatment benefit. nCTCs describe the underlying metastatic biology, whereas ctDNA provides a more quantitative, real-time assessment of tumor burden and treatment benefit. In addition, serial ctDNA measurements can be analyzed for early detection of clinically significant resistance alterations.
Lorenzo Gerratana
Consulting or Advisory Role: Lilly, Novartis
Travel, Accommodations, Expenses: Menarini Silicon Biosystems
Alessandra Franzoni
Consulting or Advisory Role: Lilly, Novartis
Lisa E. Flaum
Consulting or Advisory Role: Seattle Genetics, Novartis
Speakers' Bureau: Seattle Genetics, Novartis, AstraZeneca
William John Gradishar
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Pfizer, Puma Biotechnology
Amir Behdad
Honoraria: Thermo Fisher Scientific, Bayer, Roche China, Lilly
Speakers' Bureau: Bayer, Thermofisher Scientific Biomarkers, Lilly, Roche China
Travel, Accommodations, Expenses: Bayer, Foundation Medicine, Pfizer
Hushan Yang
Stock and Other Ownership Interests: Illumina, Pfizer, Oriomics
Travel, Accommodations, Expenses: Oriomics
Other Relationship: NIH/NCI
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Lilly, Pfizer, Pierre Fabre, Daiichi Sankyo
Consulting or Advisory Role: Roche, Amgen, Lilly, Novartis, Pfizer, Eisai
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene
Massimo Cristofanilli
Honoraria: Pfizer, Foundation Medicine
Consulting or Advisory Role: Novartis, CytoDyn, Lilly, Foundation Medicine, Menarini
Research Funding: Lilly, Angle, Merck
No other potential conflicts of interest were reported.
DISCLAIMER
The funding sources had no role in the study design, data collection, data analysis, interpretation, or writing of the manuscript.
SUPPORT
Supported by the Lynn Sage Cancer Research Foundation, OncoSET Precision Medicine Program, Ministry of Health Grant Ricerca Finalizzata (Grant No.: RF-2016-02362544) and Italian League for the Fight against Cancer (LILT) Healthcare research 2018—5 × mille program. REDCap support was funded in part by a Clinical and Translational Science Award (CTSA) grant from the National Institutes of Health Grant No. UL1TR001422.
AUTHOR CONTRIBUTIONS
Conception and design: Lorenzo Gerratana, Andrew A. Davis, Giuseppe Damante, Amir Behdad, Fabio Puglisi, Massimo Cristofanilli
Financial support: Fabio Puglisi, Massimo Cristofanilli
Administrative support: Qiang Zhang
Provision of study materials or patients: Debora Basile, Youbin Zhang, William John Gradishar, Hushan Yang, Fabio Puglisi, Massimo Cristofanilli
Collection and assembly of data: Lorenzo Gerratana, Andrew A. Davis, Qiang Zhang, Debora Basile, Giovanna Rossi, Kimberly Strickland, Alessandra Franzoni, Lorenzo Allegri, Zhaomei Mu, Youbin Zhang, Lisa E. Flaum, William John Gradishar, Amir Behdad, Hushan Yang
Data analysis and interpretation: Lorenzo Gerratana, Andrew A. Davis, Alessandra Franzoni, Lorenzo Allegri, Youbin Zhang, William John Gradishar, Leonidas C. Platanias, Massimo Cristofanilli
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lorenzo Gerratana
Consulting or Advisory Role: Lilly, Novartis
Travel, Accommodations, Expenses: Menarini Silicon Biosystems
Alessandra Franzoni
Consulting or Advisory Role: Lilly, Novartis
Lisa E. Flaum
Consulting or Advisory Role: Seattle Genetics, Novartis
Speakers' Bureau: Seattle Genetics, Novartis, AstraZeneca
William John Gradishar
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Pfizer, Puma Biotechnology
Amir Behdad
Honoraria: Thermo Fisher Scientific, Bayer, Roche China, Lilly
Speakers' Bureau: Bayer, Thermofisher Scientific Biomarkers, Lilly, Roche China
Travel, Accommodations, Expenses: Bayer, Foundation Medicine, Pfizer
Hushan Yang
Stock and Other Ownership Interests: Illumina, Pfizer, Oriomics
Travel, Accommodations, Expenses: Oriomics
Other Relationship: NIH/NCI
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Lilly, Pfizer, Pierre Fabre, Daiichi Sankyo
Consulting or Advisory Role: Roche, Amgen, Lilly, Novartis, Pfizer, Eisai
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene
Massimo Cristofanilli
Honoraria: Pfizer, Foundation Medicine
Consulting or Advisory Role: Novartis, CytoDyn, Lilly, Foundation Medicine, Menarini
Research Funding: Lilly, Angle, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Bonotto M, Gerratana L, Poletto E, et al. : Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist 19:608-615, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennecke H, Yerushalmi R, Woods R, et al. : Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271-3277, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Gerratana L, Zhang Q, Shah AN, et al. : Performance of a novel next generation sequencing circulating tumor DNA (ctDNA) platform for the evaluation of samples from patients with metastatic breast cancer (MBC). Crit Rev Oncol Hematol 145:102856, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, et al. : Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781-791, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Pierga J-Y, Reuben J, et al. : The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol 134:39-45, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Smerage JB, Barlow WE, Hortobagyi GN, et al. : Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32:3483-3489, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Leary B, Hrebien S, Morden JP, et al. : Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 9:896, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buono G, Gerratana L, Bulfoni M, et al. : Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat Rev 73:73-83, 2019 [DOI] [PubMed] [Google Scholar]
- 10.André F, Ciruelos E, Rubovszky G, et al. : Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med 380:1929-1940, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Gerratana L, Davis AA, Shah AN, et al. : Emerging role of genomics and cell-free DNA in breast cancer. Curr Treat Options Oncol 20:68, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Lanman RB, Mortimer SA, Zill OA, et al. : Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10:e0140712, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes SA, Beare D, Boutselakis H, et al. : COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res 45:D777-D783, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zill OA, Banks KC, Fairclough SR, et al. : The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res 24:3528-3538, 2018 [DOI] [PubMed] [Google Scholar]
- 15.van Dessel LF, Vitale SR, Helmijr JCA, et al. : High-throughput isolation of circulating tumor DNA: A comparison of automated platforms. Mol Oncol 13:392-402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helissey C, Berger F, Cottu P, et al. : Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: The observational step of the CirCe01 phase III trial. Cancer Lett 360:213-218, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Bulfoni M, Gerratana L, Del Ben F, et al. : In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res 18:30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, Bardia A, Wittner BS, et al. : Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339:580-584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis AA, Zhang Q, Gerratana L, et al. : Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res 21:137, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerratana L, Davis AA, Polano M, et al. : Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. Eur J Cancer 143:147-157, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Hrebien S, Citi V, Garcia-Murillas I, et al. : Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol 30:945-952, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma F, Guan Y, Yi Z, et al. : Assessing tumor heterogeneity using ctDNA to predict and monitor therapeutic response in metastatic breast cancer. Int J Cancer 146:1359-1368, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Riedlinger GM, Jalloul N, Poplin E, et al. : Detection of three distinct clonal populations using circulating cell-free DNA: A cautionary note on the use of liquid biopsy. JCO Precis Oncol 2019. https://doi.org/10.1200/PO.19.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razavi P, Li BT, Brown DN, et al. : High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med 25:1928-1937, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medford AJ, Dubash TD, Juric D, et al. : Blood-based monitoring identifies acquired and targetable driver HER2 mutations in endocrine-resistant metastatic breast cancer. NPJ Precis Oncol 3:18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma CX, Bose R, Gao F, et al. : Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer. Clin Cancer Res 23:5687-5695, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


