Abstract
Background (Trial Design):
The incidence rate of gestational diabetes is high. In the long run, it harms the health of both the mother and child. In order to understand the distribution of hematological cells with gestational diabetes mellitus (GDM), a longitudinal cohort study was conducted from 2012 to 2018.
Methods:
A longitudinal case control study of 1860 pregnant women was conducted between 2012 and 2018. Data of hematological parameters at 11 time points of gestational stage were obtained from a laboratory database. Repeated measures analysis and independent t-test were used to analyze the effect of the hematological parameters on GDM.
Results:
The trend of blood cells fluctuated with gestational age in normal controls but was more remarkable in GDM. Compared with the controls, blood neutrophils, lymphocytes, and monocytes augmented in the second trimester but decreased in the third trimester; platelet (PLT) and thrombocytocrit increased throughout the three trimesters, and red blood cell (RBC) was abundant in the last 2 trimesters in GDM.
Conclusions:
Peripheral blood leukocytes, platelets, and erythrocytes were significantly different during gestation between GDM and normal controls. Inflammation may also be involved in GMD.
Keywords: gestational diabetes mellitus, hematological parameters, inflammatory
1. Introduction
The prevalence of gestational diabetes mellitus, defined as carbohydrate intolerance first diagnosed during pregnancy, ranges from 1% to 14% of all pregnancies. It is not merely a temporary condition, but a high-risk factor for type 2 diabetes mellitus, hypertension, and atherosclerotic disease for gravidas and their babies.[1–4] It is unclear why some gravidas are unable to balance insulin needs and thus develop GDM. In women, GDM tends to present with an inflammatory response, which is likely to play a role in insulin resistance.[5,6] Studies have revealed increased circulating levels of the inflammatory markers, fibrinogen, C-reactive protein and leukocyte, in GDM women, thereby showing that the maternal inflammatory response is established during the earliest phases of implantation.[4,7,8]
Platelets and erythrocytes, like leukocytes, also play an important role in the inflammatory response. Activated platelets can release considerable quantities of chemokines and express a multitude of immune receptors on their membranes that are involved with inflammation by binding to neutrophils.[9–11] Erythrocytes have surface complement receptors, thereby attaching to small-circulation immune complexes. Thus, they have an important role in removing immune complexes from the circulation in persistent infections and some autoimmune diseases. Previous studies have reported independent associations of hematological parameters with the risk for incident type 2 diabetes, although limited data are available on the associations of these parameters with GDM. Clinical studies have shown that the blood cell count is related to insulin resistance and metabolic syndrome, which may be a high-risk factor for GDM.[12–15] These findings led us to conclude that the elevated levels of platelets and erythrocytes in peripheral blood do not necessarily serve as a unique marker of chronic hypoxia or blood coagulation, but may be related to inflammation — a marked characteristic of insulin resistance and GDM. Previous studies have shown that hypertensive disorders of pregnancy are associated with increased systemic inflammation, as depicted by the platelets in gravidas.[16] However, it is necessary to further explore the association between GDM and hematological parameters. We conducted a longitudinal case control study to test the following hypotheses:
-
1.
sub-clinical inflammation may favour the development of gestational diabetes;
-
2.
an increase in inflammatory cells, such as platelets and leukocytes, during early pregnancy is associated with GDM; and
-
3.
an increase of blood glucose in turn affects the change of blood cell composition.
In this report, we aimed to assess the nature of leukocytes, platelets, and erythrocytes from GDM and normal controls at different phases of pregnancy. We explored the significant changes in the characteristics of hematological cells of both GDM and normal controls during pregnancy and evaluated whether inflammation in GDM was observed.
2. Methods
2.1. Sample collection
The study was approved by the Ethics Committee of Guangzhou Women and Children's Medical Center. Informed written consent was obtained from all participants. We conducted a longitudinal study and enrolled all pregnant women who visited the antenatal clinic and were hospitalized at Guangzhou Women and Children's Medical Center from July 2012 to September 2018. Women with no fetal malformations or other pregnancy complications with single pregnancies comprised the normal control group; those with GDM comprised the case group. Initial screening for GDM was performed with a 1-hour 50-g oral glucose challenge test (OGTT) at 24 to 28 weeks of gestation. If the glucose challenge test value was equal to, or greater than, 7.8 mmol/L, a 3-hour 75-g OGTT was administered. If 2 of the 4 values obtained from the OGTT were at, or above, the cut-off levels (fasting glucose ≥5.6 mmol/L, 1-hour glucose ≥10.3 mmol/L, 2-hour glucose ≥8.6 mmol/L, or 3-hour glucose ≥6.7 mmol/L), gestational diabetes mellitus was diagnosed.[2]
2.2. Population characteristics
The inclusion criteria for the case group was based according to the diagnostic criteria for gestational diabetes mellitus (GDM) formulated by American Diabetes Association (ADA) in 2011. On the other hand, inclusion criteria for the control group was as follows: no active liver, kidney, heart, lung blood, or metabolic diseases during pregnancy. In total, 1878 gestational women met the criteria for inclusion in the study, which comprised 872 controls (normal pregnancies) and 1006 GDM. Eighteen patients were excluded from the study owing to a lack of outcome data. Of the 1860 women, 43 (2.3%) had one data of complete blood counts available for analysis. Furthermore, 357 (19.2%) had 2, 876 (47.1%) had 3, 436 (23.4%) had 4, 110 (5.9%) had 5, 26 (1.4%) had 6, 7 (0.4%) had 7, and 4 (0.2%) had 8 data for analysis.
2.3. Data analysis
Data regarding pregnancy characteristics and complete blood counts at various gestational stages were obtained from maternal health booklets (official medical records for outpatient pregnant women), inpatient medical records, and the clinical laboratory database. The automated blood cell counter, Cell-Dyn 3700 (Abbott Diagnostics, Santa Clara, CA, USA), was used for complete blood counts. A biochemical analyzer (7170A, Hitachi High-Technologies Corporation Japan) was used to detect the plasma glucose levels.
2.4. Statistical analyses
Statistical analysis was performed using SPSS for Windows (Version 21.0, Chicago, IL; SPSS Inc) and SAS (Version 9.0, SAS Institute Inc., Cary, NC). A P value <.05 (two-tailed) was considered to be statistically significant. For analyses of the blood cell counts, data were recorded at the following time points: 4–8, 9–12, 13–16, 17–20, 21–24, 25–28, 29–32, 33–36, and 37 weeks of gestation to delivery, and at 1 and 7 weeks postpartum, for the line chart to analyze the trend of blood cells throughout pregnancy. Furthermore, we also analyzed the effect of the parameters on GDM using repeated measures analysis of variance. One-way analysis of variance (ANOVA) was used to compare the continuous variables for the following time points: 4–12, 13–28, and 29 to 41 weeks of gestation, and 1 week postpartum. For all statistical analyses, the time points were chosen to reflect the ranges of gestational ages of the study sample without overlap for individual patients; the data were expressed as mean ± SD.
3. Results
3.1. Clinical and age characteristics of the 2 groups
The mean age of the study population was 28.7 ± 4.0. Manifestations of GDM were more likely to present with the older age group than with the normal control (30.60 ± 4.0 vs 27. 0 ± 3.3 years old, P < .001, Table 1). General characteristics of the study population are shown in Table 1. Compared with the normal group, older patients with a family history of type 2 diabetes mellitus were more likely to present with GDM (P < .05). Premature rupture of membranes, hydramnios, premature or cesarean delivery, macrosomia, and icterus neonatorum were adverse outcomes of GDM (P < .05). Moreover, gravidity, parity, body mass index, family history of hypertension, and icterus neonatorum were not significantly different between the 2 groups.
Table 1.
General characteristics of the study population. (m ± sd).
| Characteristic | GDM | Control | P value |
| n | 1006 | 854 | |
| Age (yr) | 30.60 ± 4.0 | 27. 0 ± 3.3 | .00 |
| Gravidity | 1.75 ± 1.11 | 1.57 ± 0.09 | .42 |
| Parity | 0.25 ± 0.60 | 0.13 ± 0.35 | .32 |
| BMI (kg/m2) | 21.19 ± 1.86 | 20.45 ± 2.18 | .11 |
| Family history of type 2 DM (%) | 25 | 7 | .02 |
| Family history of hypertension (%) | 22 | 18 | .65 |
| PROM (%) | 44 | 23 | .04 |
| Hydramnios (%) | 14 | 0 | .04 |
| Premature delivery (%) | 14 | 0 | .04 |
| Macrosomia (%) | 8 | 0 | .17 |
| Cesarean (%) | 28 | 9 | .03 |
| Icterus neonatorum (%) | 11 | 2 | .25 |
BMI = body mass index, DM = diabetes mellitus, GDM = gestational diabetes mellitus, PROM = premature rupture of membranes.
3.2. Trend analysis of hematological parameters in gestation
All complete blood cell results, from 4 to 41 weeks of gestation until 7 weeks postpartum, were obtained. Figures 1–3 illustrate the relationship in the blood cell count and 2 groups, along with the development of gestational stage. As a result, leukocyte count was significantly different at various gestational stages. The number of leukocytes increased from weeks 4 to 24 of gestation and reached a plateau, lasting for 22 weeks. Furthermore, from weeks 21 to 42, there was a peak at week 1 postpartum. Subsequently, leukocyte count decreased remarkably at week 7 postpartum in normal pregnancies. In comparison, the trend of GDM was different from the normal control; the plateau lasted only 4 weeks, from weeks 16 to 20, then decreased until delivery with a lower peak than that of the control at week 1 postpartum (Fig. 1).
Figure 1.
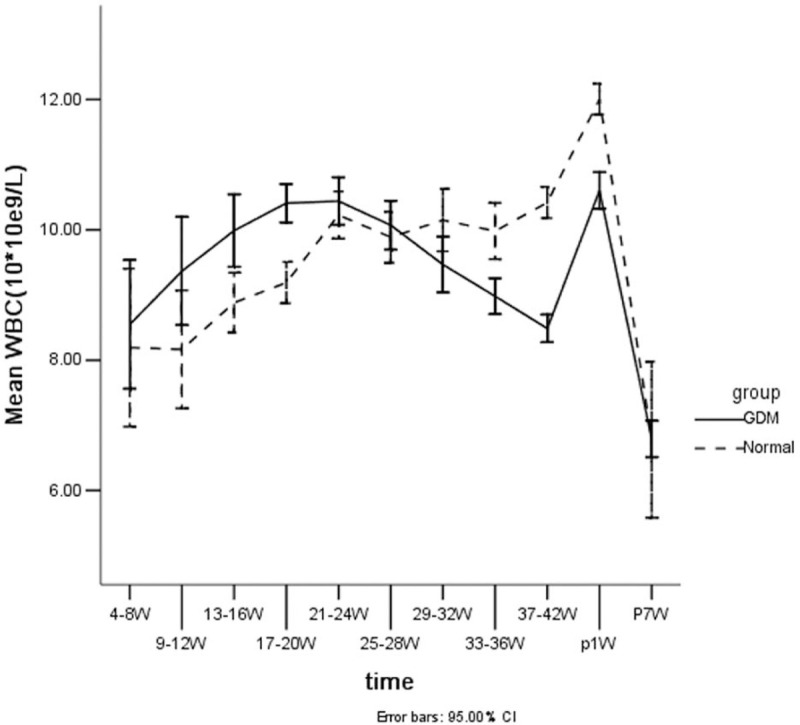
Line trends of leukocyte counts vs gestational age (from 4–41 weeks of gestation, and 1 to 7 weeks postpartum) with GDM and normal pregnancy. The changes in the WBC of the 2 groups and at all time points for each group were significantly different (P < .01).
Figure 3.
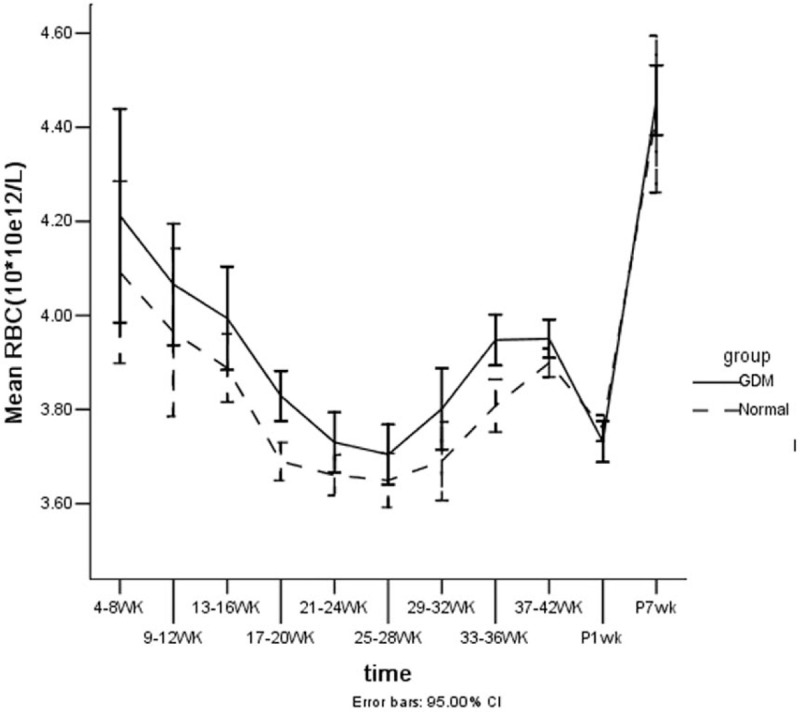
Line trends of erythrocyte counts vs gestational age (from 4 to 41 weeks of gestation, and 1 to 7 weeks postpartum) with GDM and normal pregnancy. The changes in the RBC of the 2 groups and at all times points for each group were significantly different (P < .01).
The trend of PLT slightly fluctuated with gestational age in the normal control group, but was more remarkable at various gestational stages of GDM. It reached the plateau at weeks 16 and 20, and then descended until delivery. There was also a peak at week 1 postpartum in the two groups (Fig. 2).
Figure 2.
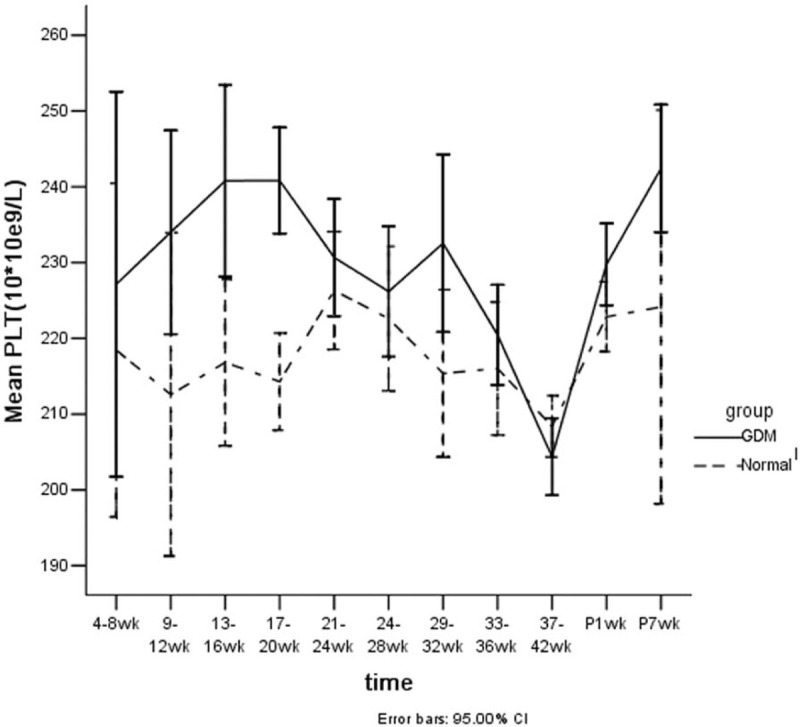
Line trends of platelet counts vs gestational age (from 4 to 41 weeks of gestation, and 1 to 7 weeks postpartum) with GDM and normal pregnancy. The changes in the PLT of the 2 groups and at all times points for each group were significantly different (P < .01).
The red blood cell (RBC) decreased continuously from weeks 4 to 28 of gestation, then subsequently increased until week 42. After delivery, it began to decrease at week 1 postpartum, then increased to a top at week 7 in 2 groups. The trend was similar among the 2 groups, but the RBC in the GDM group was higher than that of the normal control (Fig. 3).
3.3. Repeated-measures analysis of variance of the 2 groups
Repeated-measures ANOVA showed that the changes in the parameters (e.g., WBC, RBC and PLT) of both groups were significantly different (P < .01); that the changes in the parameters of WBC, neutrophils (Neu), monocytes (Mono), RBC, mean cell volume (MCV), and mean corpuscular hemoglobin (MCH) for all time points in each group were significantly different (P < .01); and that there were no significant interactions among groups and gestational time (P > .05, Table 2).
Table 2.
ANOVA for repeated-measures analysis.
| Parameter | Group/time | Num DF∗ | Den DF† | F | P |
| WBC | group | 1 | 906 | 31.09 | .00 |
| Time | 1 | 906 | 37.70 | .00 | |
| Neu | group | 1 | 906 | 24.43 | .00 |
| Time | 1 | 906 | 50.52 | .00 | |
| Lym | group | 1 | 906 | 14.45 | .00 |
| Time | 1 | 906 | 1.64 | .200 | |
| Mono | group | 1 | 905 | 25.56 | .00 |
| Time | 1 | 905 | 12.27 | .00 | |
| Eos | group | 1 | 905 | 33.71 | .00 |
| Time | 1 | 905 | 1.55 | .213 | |
| Baso | group | 1 | 905 | 11.45 | .00 |
| Time | 1 | 905 | 0.49 | .48 | |
| RBC | group | 1 | 906 | 18.92 | .00 |
| Time | 1 | 906 | 99.51 | .00 | |
| MCV | group | 1 | 906 | 41.15 | .00 |
| Time | 1 | 906 | 30.18 | .00 | |
| MCH | group | 1 | 906 | 30.26 | .00 |
| Time | 1 | 906 | 25.90 | .00 | |
| PLT | group | 1 | 906 | 27.17 | .00 |
| Time | 1 | 906 | 0.39 | .53 | |
| MPV | group | 1 | 901 | 10.35 | .00 |
| Time | 1 | 901 | 2.76 | .10 | |
| PCT | group | 1 | 901 | 11.01 | .00 |
| Time | 1 | 901 | 0.19 | .66 |
Num DF = numerator degrees of freedom.
Den DF = denominator degrees of freedom.
ANOVA = analysis of variance, Baso = basophil(s), Eos = eosinophil, Lym = lymphocyte, MCH = mean corpuscular hemoglobin, MCV = mean cell volume, Mono = monocytes, MPV = mean platelet volume, Neu = neutrophils, PCT = platelet crit, WBC = white blood cell.
3.4. One-way ANOVA of the continuous variables in the GDM group
According to the results shown in Figures 1–3 and the clinical scale about the pregnancy, we grouped the cases and normal control into 4 subgroups by gestational age (first trimester: g4∼12w, second trimester: g13∼28w, third trimester: g29∼41w, postpartum: p1–7d). Compared with the normal control group, the leukocyte parameters (except Basophil, which was lower) of the GDM group were higher in the first trimester [Eosinophils and Basophil, P < .05; WBC, Neu, lymphocyte (Lym) and Mono, P > .05] and in the second trimester (WBC, Neu, Lym, Mono, Eos and Baso, P < .01), but significantly lower in the last trimester (P ≤ .01, except for Lym and Eos, which were higher) and postpartum (P < .001, except for Eos, which was higher). (Partial results in Table 3).
Table 3.
The blood cell parameters (mean ± SD) in the GDM group and the control group from week 4 of gestation to 1 week postpartum.
| GDM | Control group | |||||
| Gestational age | Parameters | n | mean ± SD | n | mean ± SD | P |
| g4∼12w | WBC (×109/L) | 45 | 8.98 ± 2.08 | 38 | 8.18 ± 2.11 | .08 |
| NEU (×109/L) | 6.05 ± 1.71 | 5.56 ± 2.23 | .27 | |||
| PLT (×109/L) | 230.80 ± 44.25 | 215.05 ± 44.89 | .11 | |||
| RBC (×1012L) | 4.13 ± 0.41 | 4.02 ± 0.39 | .19 | |||
| g13∼28w | WBC (×109/L) | 448 | 10.34 ± 2.28 | 433 | 9.51 ± 2.30 | .00 |
| NEU (×109/L) | 7.55 ± 1.94 | 6.91 ± 2.02 | .00 | |||
| PLT (×109/L) | 237.25 ± 51.55 | 219.00 ± 47.69 | .00 | |||
| RBC (×1012L) | 3.82 ± 0.43 | 3.71 ± 0.30 | .00 | |||
| g29∼41w | WBC (×109/L) | 1006 | 8.99 ± 2.40 | 854 | 10.27 ± 2.67 | .00 |
| NEU (×109/L) | 6.35 ± 2.18 | 7.63 ± 2.47 | .00 | |||
| PLT (×109/L) | 215.47 ± 57.39 | 211.91 ± 48.91 | .15 | |||
| RBC (×1012L) | 3.89 ± 0.45 | 3.84 ± 0.36 | .00 | |||
| p1–7d | WBC (×109/L) | 527 | 10.61 ± 3.23 | 530 | 12.01 ± 2.78 | .00 |
| NEU (×109/L) | 8.06 ± 2.95 | 9.07 ± 2.47 | .00 | |||
| PLT (×109/L) | 229.77 ± 63.10 | 222.85 ± 53.90 | .06 | |||
| RBC (×1012L) | 3.73 ± 0.51 | 3.76 ± 0.32 | .28 | |||
GDM = gestational diabetes mellitus.
Comparing the platelet parameters of the GDM group with those of the control group in the 4 GDM subgroups, the following were obtained: (a) in the first trimester, there were no differences in the parameters between the GDM and control groups; (b) in the second trimester, the GDM group presented significantly higher RBC, PLT and platelet crit (PCT) (P < .002) and MCV, mean platelet volume (MPV) and MCH (P < .001) than the control group; (c) In the last trimester, the GDM group presented significantly higher RBC, PCT, RDW (red cell distribution width) and PDW (platelet distribution width) (P ≤ .003) and lower Hb (hemoglobin), HCT (hematocrit), MCV and MCH (P ≤ .006) than control group; and (d) postpartum, the GDM group presented significantly higher mean corpuscular hemoglobin concentration (MCHC) and RDW (P ≤ .03) and lower Hb, HCT, MCV, MCH and MPV than the control group (P ≤ .03). (Partial results in Table 3).
Comparing the erythrocyte parameters of the GDM group with those of the control group in the 4 GDM subgroups, the following were obtained: (a) there were no differences in the erythrocyte parameters between the GDM and control groups in the first trimester; (b) in the second trimester, the GDM group presented significantly higher RBC (P < .001) and lower MCV and MCH (P < .001) than the control groups; (c) in the last trimester, the GDM group presented significantly higher RBC and RDW (P ≤ .003) and lower Hb, HCT, MCV and MCH (P ≤ .006) than the control groups; and (d) postpartum, the GDM group presented significantly higher MCHC and RDW (P ≤ .03) and lower Hb, HCT, MCV and MCH than the control groups (P ≤ .001) (partial results in Table 3).
Some similarities existed between the GDM group and the control group in terms of the platelet and erythrocyte parameters in the second trimester (PLT, MPV, PCT, RBC, MCV, MCH; P < .05), last trimester (PCT, PDW; RBC, Hb, HCT, MCV, MCH, RDW; P < .05), and postpartum (MPV, Hb, HCT, MCV, MCH, MCHC, RDW; P < .05). It is noteworthy that the levels of Eos, RBC, PLT and Baso, MCV of the parameters of the GDM were higher and lower respectively in all times during the pregnancy than control group; while, the levels of Neu and Mono with GDM were higher in the first and the second trimesters and lower in the last trimesters and the postpartum than that of the control group.
4. Discussion
Increasing data shows that platelet is significantly important in various inflammatory reactions.[9–11] Neutrophils are primary effectors of the immune response against invading pathogens but are also central mediators of inflammatory and sterile injury. Recent studies have demonstrated that inflammatory functions of neutrophils mostly rely on active thrombocytosis. When combined with platelets, neutrophils initiate normal migration, subsequently contributing to the inflammatory process. In fact, aspirin can prevent a heart attack because it interferes with the activity of platelets. In this study, neutrophils, platelets, and RBCs are all predominantly increased in women with GDM; the changes of the 3 cells between the 2 groups and of the pregnant times in each group were significantly different. Increasing blood neutrophils and platelets imply that inflammation was contributory in the development of the GDM, especially in the first 2 trimesters. An increased leukocyte count (10.34 ± 2.28 vs 9.51 ± 2.30 × 109/L; P < .001), which was consistent with Wolf's data (WBC 10.5 ± 2.2 vs 9.2 ± 2.2 × 109/L; P < .01),[11] was associated with a risk for GDM in early pregnancy.
Limited data are available on the associations of platelet and erythrocyte parameters with GDM. The levels of PLT, PCT and PDW were higher and the level of MPV was lower in the GDM group, as compared to that of the control group, before week 24 of gestation. Furthermore, levels of Neu, Lym, Mono, Eos, RBC, PLT and Baso, and the MCV and MPV of the patients were higher in the GDM group and lower in the control group, especially before week 24. This finding implied that peripheral blood cells may be high-risk factors for the development of GDM, although the increase in the number of these 3 cell types was not significant for the 2 groups during the first trimester; the reason may be that the cases were too small in this phase.
We also demonstrated that older women were more likely to develop GDM, and there was a statistically significant linear trend between increasing age and GDM incidence in those 2 groups (Table 1). Therefore, we recommend that older gravidas should screen blood cells before week 24, as it may be a useful and timely strategy for obstetricians to manage high-risk pregnant women.
The dysregulation of inflammatory response, especially before the early second trimester, provided further support to the hypothesis that inflammation contributes to the development of GDM.[2,4,5] The inflammation factors of C-reactive protein and fibrinogen have been shown to be significantly higher in women with GDM. However, the results of other studies differ from those of our study. Chen's study showed that GDM was associated with Hb, HCT and erythrocyte levels only at the first prenatal visit.[17] GDM had lower platelet counts and higher MPV values.[18] No statistically significant difference was observed in the platelet count between the 2 groups.[19] These conflicting results are possibly due to the differences between methods and/or equipment used for automated blood counts and as well as the inadequacy of sample sizes. In our research, the complete blood count changed at different gestational times; variable observation times for gestational trimesters may have contributed to varying results. Conversely, the elevated glucose levels increased the RBC count, and the primary changes possibly led to the changes in other blood constituents. This may be due to the complexity of the human microenvironment.
5. Conclusion
In conclusion, we showed that the leukocyte, platelet, and erythrocyte parameters in GDM changed significantly, along with the development of gestation. This finding suggests that a mild inflammatory and unbalanced immune response occurs in GDM. The abnormal activation of the immune system may play a role in the etiology of GDM. The hematological parameters may be the potential predictors of GDM before the emergence of symptoms. It may provide a new strategy for obstetrician to management high risk pregnant woman timely. However, it need a prospective longitudinal study to elucidate relationship of inflammation progresses with the develop GDM.
Acknowledgments
We are grateful to the staff from our hospital laboratory for help with the data analyses and for providing the data on blood cell parameters.
Author contributions
Conceptualization: Yonggang Zhang, Yipeng Zhang, Limin Zhao.
Data curation: Yanyan Shang.
Formal analysis: Yanyan Shang.
Methodology: Yonggang Zhang, Yipeng Zhang, Limin Zhao.
Project administration: Dabao He, Jiying Chen.
Supervision: Dabao He, Jiying Chen.
Writing – original draft: Yonggang Zhang.
Writing – review & editing: Yipeng Zhang, Limin Zhao.
Footnotes
Abbreviations: ANOVA = analysis of variance, Baso = basophil(s), Eos = eosinophil, GDM = gestational diabetes mellitus, Hb = hemoglobin, HCT = hematocrit, Lym = lymphocyte, MCH = mean corpuscular hemoglobin, MCHC = mean corpuscular hemoglobin concentration, MCV = mean cell volume, Mono = monocytes, MPV = mean platelet volume, Neu = neutrophils, OGTT = oral glucose challenge test, PCT = platelet crit, PDW = platelet distribution width, PLT = platelet, RBC = red blood cell, RDW = red cell distribution width, WBC = white blood Cell.
How to cite this article: Zhang Y, Zhang Y, Zhao L, Shang Y, He D, Chen J. Distribution of complete blood count constituents in gestational diabetes mellitus. Medicine. 2021;100:23(e26301).
This work was supported by the National Natural Science Foundation of China (81801474), the National Natural Science Fund of China (81871716), the Science and Technology Fund of Shenzhen (JCYJ20180306172502097), Science and technology special fund of Longhua District (2017096) and The Science and Technology Fund of Guangzhou (201707010019). The funding source has no role in study just supplied the fund.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Artzi NS, Shilo S, Hadar E, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med 2020;26:71–6. [DOI] [PubMed] [Google Scholar]
- [2].McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47.doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- [3].Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am 2019;48:479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018;19:3342.doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Homayouni A, Bagheri N, Mohammad-Alizadeh-Charandabi S, et al. Prevention of gestational diabetes mellitus (GDM) and probiotics: mechanism of action: a review. Curr Diabetes Rev 2020;16:538–45. [DOI] [PubMed] [Google Scholar]
- [6].Poola-Kella S, Steinman RA, Mesmar B, et al. Gestational diabetes mellitus: post-partum risk and follow up. Rev Recent Clin Trials 2018;13:05–14. [DOI] [PubMed] [Google Scholar]
- [7].Alamolhoda SH, Yazdkhasti M, Namdari M, et al. Association between C-reactive protein and gestational diabetes: a prospective study. J Obstet Gynaecol 2020;40:349–53. [DOI] [PubMed] [Google Scholar]
- [8].Liu Y, Sun X, Tao J, et al. Gestational diabetes mellitus is associated with antenatal hypercoagulability and hyperfibrinolysis: a case control study of Chinese women. J Matern Fetal Neonatal Med 2020;01–4. doi: 10.1080/14767058.2020.1818202. [DOI] [PubMed] [Google Scholar]
- [9].Khodadi E. Platelet function in cardiovascular disease: activation of molecules and activation by molecules. Cardiovasc Toxicol 2020;20:01–10. [DOI] [PubMed] [Google Scholar]
- [10].Fashami MA, Hajian S, Afrakhteh M, et al. Is there an association between platelet and blood inflammatory indices and the risk of gestational diabetes mellitus? Obstet Gynecol Sci 2020;63:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pretorius E, Olumuyiwa-Akeredolu OO, Mbotwe S, et al. Erythrocytes and their role as health indicator: using structure in a patient-orientated precision medicine approach. Blood Rev 2016;30:263–74. [DOI] [PubMed] [Google Scholar]
- [12].Mahdiani A, Kheirandish M, Bonakdaran S. Correlation between white blood cell count and insulin resistance in type 2 diabetes. Curr Diabetes Rev 2019;15:62–6. [DOI] [PubMed] [Google Scholar]
- [13].Kuo TY, Wu CZ, Lu CH, et al. Relationships between white blood cell count and insulin resistance, glucose effectiveness, and first- and second-phase insulin secretion in young adults. Medicine (Baltimore) 2020;99:e22215.doi: 10.1097/MD.0000000000022215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oliveira CC, Roriz AKC, Ramos LB, et al. Blood count parameters as a marker for metabolic syndrome in older adults. Exp Gerontol 2017;96:123–6. [DOI] [PubMed] [Google Scholar]
- [15].Lee YJ, Shin YH, Kim JK, et al. Metabolic syndrome and its association with white blood cell count in children and adolescents in Korea: the 2005 Korean National Health and Nutrition Examination Survey. Nutr Metab Cardiovasc Dis 2010;20:165–72. [DOI] [PubMed] [Google Scholar]
- [16].Han C, Huang P, Lyu M, et al. Oxidative stress and preeclampsia-associated prothrombotic state. Antioxidants (Basel) 2020;9:1139.doi: 10.3390/antiox9111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang H, Zhu C, Ma Q, et al. Variations of blood cells in prediction of gestational diabetes mellitus. J Perinat Med 2015;43:89–93. [DOI] [PubMed] [Google Scholar]
- [18].Erdoğan S, Ozdemir O, Doğan HO, et al. Liver enzymes, mean platelet volume, and red cell distribution width in gestational diabetes. Turk J Med Sci 2014;44:121–5. [DOI] [PubMed] [Google Scholar]
- [19].Bozkurt N, Yilmaz E, Biri A, et al. The mean platelet volume in gestational diabetes. J Thromb Thrombolysis 2006;22:51–4. [DOI] [PubMed] [Google Scholar]


