Abstract
OBJECTIVES:
Saddle pulmonary embolism is an uncommon type of venous thromboembolism that can lead to sudden hemodynamic collapse and death. Saddle pulmonary embolism can be difficult to recognize, and data on its presentation, clinical features, and associated complications are sparse. We sought to characterize patients with saddle pulmonary embolism.
DESIGN:
The Montage software (Nuance, Burlington, MA) was used to identify patients to create a retrospective cohort study.
SETTING:
Montefiore Medical Center from January 1, 2012, to December 31, 2018.
PATIENTS:
All subjects diagnosed with saddle pulmonary embolism in above time period.
INTERVENTIONS:
Charts were reviewed for demographics, diagnostics, laboratory data, presenting vital signs, inhospital mortality, 6-month survival, and prevalence of recurrent venous thromboembolism.
MEASUREMENTS AND MAIN RESULTS:
About 120 patients with saddle pulmonary embolism were identified. Median age was 61 years and 57.5% were women. Events were provoked by a transient risk factor in 43.3%. On presentation, median mean arterial pressures were normal (93 mm Hg). Only five of 120 of patients (4.2%) presented with vitals concerning for massive pulmonary embolism. We found a 9.2% inhospital mortality; an additional 8.6% died within 6 months of discharge. Inhospital mortality was higher in women (11.6%), compared with men (3.9%), but this was not significant (p = 0.28). In 10 patients, both ventilation/perfusion scans and computed tomography pulmonary angiogram were performed. None of the ventilation/perfusion scans diagnosed saddle pulmonary embolism. Thrombus was visualized in the right heart in eight of 105 (7.6%), and this group had a higher inhospital mortality (37.5%). Recurrent venous thromboembolism occurred in 13 of 85 of survivors (15.3%).
CONCLUSIONS:
Despite presenting without the accepted clinical criteria for massive pulmonary embolism, saddle pulmonary embolism has a very high inhospital mortality. Ventilation/perfusion scan is unable to diagnose saddle pulmonary embolism. Visualized right heart thrombi portend an even higher inhospital mortality.
Keywords: cardiovascular diseases, embolism, pulmonary embolism, thrombosis, vascular diseases, venous thromboembolism
Saddle pulmonary embolism (SPE) is a rare type of acute pulmonary embolism (PE) that can lead to sudden hemodynamic collapse and death. The definition of SPE is a visible thrombus located at the bifurcation of the main pulmonary artery, and a diagnosis of SPE suggests the possibility of hemodynamic instability. SPE has been associated with higher rates of cardiac arrest, cardiogenic shock, respiratory failure, and mean length of stay (1). For non-SPE, death attributed to PE has been reported in the range of 1–7.0% (2–4). SPE occurs in approximately 2.6–5.4% of all acute PE cases and has been thought to portend poorer outcomes without aggressive treatment (5, 6). Treatment options range from standard anticoagulation (AC), systemic thrombolysis, catheter-directed thrombolysis, and surgical embolectomy (6). Recent retrospective studies have questioned this assumption. Although one retrospective study showed worse 1-year overall mortality of SPE versus central PE in cancer patients (83.3% vs 41.2%) (7), other studies have shown an inhospital mortality that was not statistically different from matched non-SPE (5–9). These prior studies were limited by low sample sizes with only one study having more than 50 patients with SPE (5). This present study aims to characterize the presentation, diagnosis, management, and outcomes of 120 patients with SPE.
MATERIALS AND METHODS
Using the search terms “saddle,” “pulmonary,” and “embolus,” we searched through every CT scan and nuclear medicine scan report performed during January 1, 2012, to December 31, 2018, and recorded in our hospital radiology database (Montage Software, Nuance, Burlington, MA). The Institutional Review Board (IRB) of Albert Einstein College of Medicine approved the study (IRB number: 2019-10507). Medical records from these patients were individually and manually reviewed to confirm the presence of SPE. Clinical information extracted from patient charts included demographic data, imaging modality for SPE diagnosis, laboratory data, relevant past medical history such as venous thromboembolism (VTE) history, malignancy history, presenting vital signs, treatment received for SPE, hospital stay duration, inhospital mortality, and 6-month mortality. Simplified PESI (sPESI) scores were calculated for all patients to categorize severity of illness (10). Echocardiographic data performed during the index hospitalization were also extracted. Right heart strain was recorded if the echocardiogram reported right heart strain, right ventricular dilatation, or hypokinesis. Patients were also followed until last medical note, laboratory, or imaging. If found, transient risk factors for VTE were identified for each case. Risk factors were considered provoked (or transient) if they were present for no longer than 1 month prior to diagnosis of SPE. Recurrent VTE was recorded if they were found to have a second acute VTE episode after discharge or chronic PE.
Statistical analysis was performed using means with standard deviations and medians with interquartile range (IQR) for normal and nonnormal distribution of data, respectively. Two-tailed t tests or Mann-Whitney U tests were used for continuous variables as appropriate and Fisher exact or chi-square tests were used for categorical variables. Significance was assigned at p = 0.05.
RESULTS
Initial Montage database query yielded 395 patients. After manual review, only 120 patients were identified with acute SPE from January 1, 2012, to December 31, 2018. Demographic data are presented in Table 1. Median age at diagnosis was 61 years, with females comprising 57.5% of the patients; median body mass index was 33. There was a positive history of VTE in 18.3% of the study population. Clinical data on presentation included vital signs that showed normal median systolic blood pressure (SBP) and diastolic blood pressure, a normal respiratory rate, and a normal median oxygen saturation of 96% (IQR, 92–100). Median heart rate was above normal at 103 beats/min. Presenting mean heart rate (117 ± 19.23) was higher in patients who died in hospital than the mean heart rate (102 ± 19.4) in patients who survived, p = 0.03. Only 4.2% of patients presented with SBPs less than 90 mm Hg consistent with a massive PE. On presentation, 33 of 120 (27.5%) had an sPESI of 0; 51 of 120 (42.5%) had a score of 1; 24 of 120 (20.0%) had a score of 2; 10 of 120 (8.3%) had a score of 3; and two of 120 (1.7%) had a score of 4. The distribution of sPESI scores did not differ significantly between the patients who died in hospital when compared with those that survived, p = 0.16. Laboratory data at presentation showed a median hemoglobin of 12.8 g/dL (IQR, 11.1–14.2 mg/dL) and a median serum creatinine (Cr) of 1.0 mg/dL (IQR, 0.8–1.3 mg/dL). d-dimer laboratory data were only available for 27 patients and were elevated in all; median d-dimer was 11.67 µg/mL (IQR, 5.14–20.02 mg/dL). Patients who died had an average pro B-type Natriuretic Peptide of 6,478 ± 7,982 pg/mL compared with patients who survived hospitalization 2,394 ± 532 pg/mL, but this was not statistically significant (p = 0.17). The average Troponin-T in patients who died in hospital was 1.13 ± 3.01 ng/mL compared with patients who survived, 0.13 ± 0.26 ng/mL, but this was also not statistically significant.
TABLE 1.
Presenting Patient Characteristics
| Characteristic | n = 120 |
|---|---|
| Age, yr, median (IQR) | 61 (49–72) |
| Race | |
| White, n (%) | 28 (23.3) |
| Black, n (%) | 54 (45.0) |
| Other, n (%) | 38 (31.7) |
| Hispanic, n (%) | 28 (23.3) |
| Female, n (%) | 69 (57.5) |
| Prior history of venous thromboembolism, n (%) | 22 (18.3) |
| Vital signs | |
| Systolic blood pressure, median (IQR) | 126 (115–144) |
| Diastolic blood pressure, median (IQR) | 77 (64–87) |
| Heart rate, beats/min, median (IQR) | 103 (89–118) |
| Respiratory, rate/min, median (IQR) | 20 (18–24) |
| % Oxygen saturation, median (IQR) | 96 (92–100) |
| Body mass index, kg/m2, median (IQR), n = 104 | 33 (27.9–39.6) |
| Laboratory data | |
| Hemoglobin, g/dL, median (IQR) | 12.8 (11.1–14.2) |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.8–1.3) |
| d-dimer, µg/mL, mean (IQR), n = 27 | 11.67 (5.14–20.02) |
| Right heart strain (n = 105), n (%) | 70 (66.7) |
| pro B-type Natriuretic Peptide, pg/mL, median (IQR) | 1,175 (401–4,058) |
| Troponin, T ng/mL, median (IQR) | 0.04 (0.00–0.14) |
| DVT characteristics | |
| DVT laterality, n (%) | |
| Left | 29 (24.2) |
| Right | 32 (26.7) |
| Bilateral | 17 (14.2) |
| No DVT seen | 31 (25.8) |
| Not performed | 11 (9.2) |
| DVT location, n (%) | |
| Proximal | 38 (32) |
| Distal | 4 (3) |
| Both | 36 (29) |
| N/A | 42 (36) |
DVT = deep vein thrombosis, IQR = interquartile range.
Acute deep vein thrombosis (DVT) was found in 65.8% of patients, with 9.2% of patients not evaluated for DVT. Of the patients with an acute DVT, 74 of 78 of these patients (94.9%) had a proximal DVT defined as a DVT located in the popliteal vein or more proximal vessel, and 36 of 78 of patients (46.2%) with an acute DVT had both a proximal and distal DVT. The most proximal vein where a DVT was found on venous duplex was the femoral vein in 55.1% of patients where a DVT was diagnosed. These data are also noted in Table 1.
For 45 patients (37.5%), no cause was found, and the VTE was considered idiopathic. Cancer was present in 23 of the 120 patients (19.2%). The most common malignancy was lung cancer in four of 23 cancer patients. Events were provoked by a transient risk factor in 52 patients (43.3%). Surgery and hospitalization or immobility accounted for the majority of the provoked events, causing 42.3% and 34.6% events, respectively. Cerebral vascular accidents (CVAs) were the next most common at 11.5% of cases (6/52). Three of these cases were acute CVAs with concurrent new VTE, both diagnosed at admission to the hospital. Two had CVAs 1 week prior to diagnosis of SPE and had associated immobility. The last case also had a “recent” CVA at an outside institution with associated left-sided weakness and immobility. Pregnancy, hormone supplementation, heparin-induced thrombocytopenia, and May-Thurner syndrome accounted for one to two cases each (Fig. 1).
Figure 1.
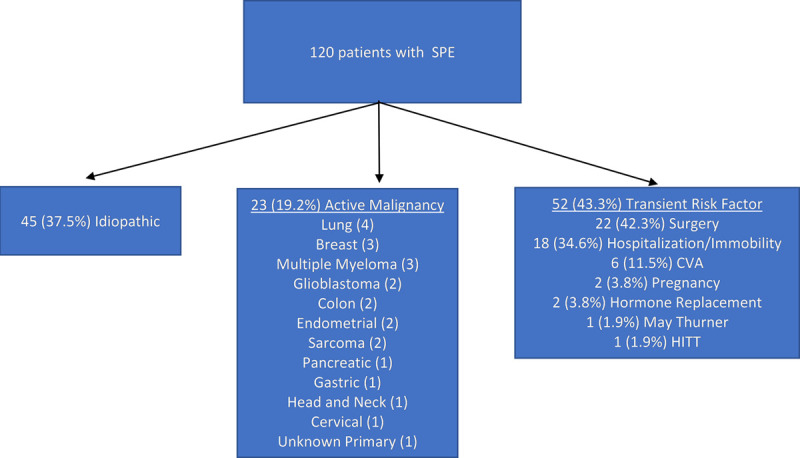
Etiology of each episode of saddle pulmonary embolism (SPE) was determined and listed under one of three categories: idiopathic, active malignancy, or transient risk factor. CVA = cerebral vascular accident, HITT = heparin-induced thrombocytopenia and thrombosis.
Diagnosis of SPE was done solely by computed tomography pulmonary angiogram (CTPA). Ten ventilation/perfusion scans were also performed prior to CTPA for 10 patients in the cohort. Ventilation/perfusion scans were ordered for different reasons. Two scans were ordered because of acute renal failure with an initial Cr of 1.85 and 1.6. Two scans were ordered, because a DVT was found. Four were ordered, because PE was suspected. One was ordered, because a ventilation/perfusion had diagnosed a PE at an outside institution. The 10th scan was done, because the patient refused to consent to contrast for a CTPA. The median-modified Geneva score of these patients was 6 with just two of the cases having a high-risk score. None of the ventilation/perfusion scans were able to detect the SPEs. Four were indeterminate and required further evaluation with CTPA, which led to the diagnosis of SPE. In six of 10 ventilation/perfusion scans, peripheral pulmonary emboli were diagnosed. A CTPA was performed in these six cases because of clinical factors, suggesting a larger clot burden or at the suggestion of the radiologist reading the ventilation/perfusion scan. About four of 10 ventilation/perfusion scans were indeterminate and suggested a CTPE be performed to evaluate further for PE. None of the ventilation/perfusion scans were able to diagnose SPE definitively.
Echocardiographic data were available in 105 patients. Right heart strain was noted in 66.7% of patients with an echocardiogram performed. For these patients, the average pulmonary artery pressure was 46.0 ±13.1 mm Hg. Right heart strain was associated with inpatient mortality, p = 0.04. Right atrial pressures were reported in 62 echocardiograms. About 67.7% of these patients had a right atrial pressure greater than 5 mm Hg. Right ventricular systolic pressure was also available in 58 patients. The median RVSP was 43.9 mm Hg (IQR, 34.5–51.7 mm Hg). All patients assessed with echocardiography with available RVSP readings had at least mild pulmonary hypertension. Eight patients (7.7%) had a thrombus visualized in the right side of the heart. Three of these eight patients (37.5%) died inhospital. Visualized thrombus was associated with inpatient mortality, p = 0.003. Thirty-four patients with echocardiographic data were not found to have right heart strain. None died in hospital; however, 6 months after discharge, five of these patients had died. Four died of an underlying malignancy, and the last died of complications from chronic mechanical ventilation.
Initial management of SPE included standard AC. Enoxaparin was the initial AC in 25.0%, and dalteparin was given to 3.3%. Most patients (69.2%) were initially anticoagulated with unfractionated heparin. About 50.0% of patients received one or more therapies in combination with AC: 19.2% of patients received systemic thrombolysis with tissue plasminogen activator (tPA), local catheter directed therapy in 13.3%, mechanical thrombectomy in 13.3%, and an inferior vena cava (IVC) filter in 34.2%. Use of systemic thrombolysis (p = 0.00008) and intubation (p = 1.2 × 10−9) was associated with inpatient mortality. IVC filter placement (p = 0.87) and ICU admission (p = 0.34) were not. tPA as sole initial treatment was used in 0.8% of patients. Two patients (1.7%) were not anticoagulated due to high risk of CNS hemorrhage.
About 71 patients (59.2%) received care in the ICU. Fifteen patients (13.0%) required transfusion during admission with the range of transfusions ranging from 1 to 33 units of packed RBCs (pRBCs). Half of the patients requiring transfusion required less than or equal to 2 units of pRBCs. Three patients required more than 10 units of pRBCs: two of these had received systemic tPA and one had a mechanical thrombectomy. The majority of patients (44.2%) discharged were prescribed warfarin. About 17.5% of patients were discharged on rivaroxaban, 8.3% on apixaban, 14.1% on enoxaparin, 5.0% on dalteparin, 1.7% on fondaparinux, and 0.83% on unfractionated heparin. The patient discharged on unfractionated heparin was discharged to inpatient hospice. Median length of stay was 9 days (range, 0–106 d).
Thirteen patients (10.8%) suffered a cardiac arrest, and 20 patients (16.7%) required intubation. The inhospital mortality rate for the entire cohort was 9.2% (11/120). Inhospital mortality of females diagnosed with SPE was 11.6% compared with 3.9% of males; however, this was not statistically different, p = 0.28. Of the patients who died in hospital, all of their deaths were attributed to PE or complications from PE.
Follow-up information was available on 105 of the 109 patients. Nine of the 105 patients (8.6%) with follow-up who were discharged did not survive 6 months; eight of the nine died from progressive malignancy, whereas the remaining patient died of sepsis, but none had a recurrent VTE during that time. Of the remaining 96 alive at 6 months, further follow-up information was available on 85. Recurrent VTE occurred in 13 of 85 patients (15.3%) followed after discharge. The median time to recurrence after discontinuation of AC was 136 days, and all patients had greater than 1 year of AC. Of these 85 patients followed, AC was stopped in 19 patients with recurrence in five of the 19 (26.3%) over 7 years. Ten of these 19 patients not on AC had a transient provoking risk factor, and therapy was discontinued by the physician. Of those 10, three developed a recurrent VTE (30%) within 4.5 years. Nine patients were not thought to have a transient factor but were still not on AC; two of these patients (22.2%) recurred. Of the 66 patients who remained on AC, eight (12.1%) still developed recurrent VTE. Four of these eight patients were on warfarin. Only one of these had a subtherapeutic international normalized ratio when they developed a recurrent VTE. One patient was only on prophylactic dose enoxaparin, two were on rivaroxaban, and one was on apixaban (Table 2). The median time to recurrent VTE for those on AC was 890.5 days. The cumulative prevalence of recurrent acute VTE was 3.5% at 1 year, 7.1% at 2 years, 10.6% at 3 years, 12.9% at 4 years, and 15.3% at 7 years.
TABLE 2.
Patient Outcomes
| Outcome | n = 120 |
|---|---|
| Cardiac arrest, n (%) | 13 (10.8) |
| ICU admission, n (%) | 71 (59.2) |
| Length of stay, d, median (range) | 9 (0–106) |
| Inhospital mortality, n (%) | 11/120 (9.2) |
| Therapy | |
| Mechanical thrombectomy | 16 (13.3) |
| Locally directed tPA | 16 (13.3) |
| Inferior vena cava filter placement, n (%) | 41 (34.2) |
| Systemic tPA, n (%) | 23 (19.2) |
| Died with 6 mo of discharge, n (%) | 9/105 (8.6) |
| Recurrent VTE, n = 85 (%) | 13 (15.3)* |
| Recurrent VTE on anticoagulation (n = 66) | 8 (12.1) |
| Warfarin | 4 |
| Rivaroxaban | 2 |
| Apixaban | 1 |
| Prophylactic dose enoxaparin | 1 |
| Recurrent VTE off anticoagulation (n = 19) | 5 (26.3) |
tPA = tissue plasminogen activator, VTE = venous thromboembolism.
DISCUSSION
We report on a large study population of SPE. The short-term inhospital mortality was high, 9.2% of diagnosed cases. Although difficult to compare across retrospective studies, inhospital mortality rates of previous studies (0–4.3%) were half of what was found in our study, but most of these studies had smaller sample size (5–9). Only one previous study had a sample size greater than 40 (5), but this study of 180 patients also had a lower inhospital mortality rate (4.3%) in patients diagnosed with SPE. The difference in mortality rates may be due to the different racial characteristics and associated comorbidities of the patient populations. In the study population by Alkinj et al (5), 96% identified as White, compared with only 23.3% of our study population. Previous studies have shown that Black Americans are 30–50% more likely to die of an acute PE; 45% of our study population identified as Black (11, 12). Furthermore, the rate of systemic thrombolysis in our study population was higher than Alkinj et al’s (5) (19.2% vs 10.0%), possibly suggesting a higher acuity leading to a higher mortality.
Previous retrospective studies reported the proportion of females having an SPE from 29% to 59.5% (5–9, 13). Our study found that females had a higher rate of inhospital mortality than males (11.9% vs 3.9%, p = 0.28), but this was not statistically significant. Females also comprised 57.5% of our study population, but this is not dissimilar to our typical inpatient gender ratios and Bronx population in the age range of 60–64 (14).
Presentation of the SPE was also highly variable. Presenting vital signs were not helpful in assessing this high mortality event. Only 4.2% of patients had initial vital signs consistent with massive PE with an SBP of less than 90 mm Hg. Right heart strain, tachycardia, and intubation requirement were associated with inpatient mortality, but the sPESI scores did not differ significantly between those that died in hospital and those that survived. Most importantly, evidence of a thrombus in the right heart, sometimes also referred to as clot-in-transit, was found in 7.6% of our cases and was associated with a mortality of 37.5%. The few prior reports on this, previously thought to be a very rare entity, noted a mortality of 12.5–45% (15, 16). The 34 patients who did not have right heart strain all survived their admission. SPE without right heart strain appears to have a similar mortality rate to peripheral PE, with a mortality rate of 1–4.9% (2–4). This may suggest that patients without right heart strain can be managed similarly to patients with peripheral PE.
Ventilation/perfusion scans did not diagnose SPE. In some of these patients, a diffuse decrease in perfusion was noted, but this could be mistaken for a negative ventilation/perfusion or read as indeterminate scan. In our series, the use of ventilation/perfusion scans led to a delay in diagnosis of SPE, and it is possible that a sooner diagnosis may have led to a more aggressive management strategy. Current American Society of Hematology guidelines recommend ventilation/perfusion scans for patients with low and intermediate pretest probability of PE and a positive d-dimer (17). Given our demonstration in this study of the prevalence of asymptomatic SPE patients and of the inability to diagnose SPE on ventilation/perfusion scans, this recommendation may have the potential of missing SPE with possible adverse clinical outcomes.
Some patients were managed with standard AC only. One patient was diagnosed and treated as an outpatient with a direct oral anticoagulant (DOAC), whereas other patients required systemic thrombolysis and monitoring. About 19.2% required systemic thrombolysis. Transfusions were required in 15 patients (13%). Although the majority of patients did not have bleeding, the rate of bleeding was greater than what is typically seen for treatment of VTE (0.6–1.8%) (18). This was almost certainly due to interventions such as tPA, catheter-directed thrombolysis, and mechanical thrombectomy, since all patients requiring transfusion of greater than 10 pRBCs had at least one of these procedures.
Recurrent VTE occurred in 15.3% of the 85 patients followed after discharge. Five of these patients were off AC when their current VTE recurred. Furthermore, these patients all had more than 1 year of AC. For both patients followed on AC and off AC, the prevalence of recurrent VTE appears to be high, 12.1% and 26.3%, respectively.
We recognize several limitations to our study. The retrospective nature of the study could have led to unknown confounding biases. In addition, the trial period of the study spanned from 2012 to 2018 during the period where DOACs and alternatives to warfarin were becoming more commonly used to treat VTE. It is unclear how this affected patient outcomes. Although none were able to definitively diagnose an SPE, only 10 patients had both a ventilation/perfusion scan and a CTPE, which could limit the generalizability of our conclusions on ventilation/perfusion scans. Finally, information was not entirely available for every patient in every category.
CONCLUSIONS
SPE is a rare diagnosis but has a range of clinical presentations. The majority of patients are not hypotensive or hypoxic on presentation, and saddle PE was not suspected initially. When diagnosed, there is a high associated inpatient mortality as we report here in a diverse, urban, minority patient population. Ventilation/perfusion scans may not be the most ideal next step in the evaluation of PE in low and intermediate pretest probability patients due to their inability to diagnose SPE. Right heart thromboembolism carries a significant inpatient mortality and may require more aggressive treatment.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Pathak R, Giri S, Karmacharya P, et al. Comparison between saddle versus non-saddle pulmonary embolism: Insights from nationwide inpatient sample. Int J Cardiol. 2015; 180:58–59 [DOI] [PubMed] [Google Scholar]
- 2.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992; 326:1240–1245 [DOI] [PubMed] [Google Scholar]
- 3.Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011; 57:700–706 [DOI] [PubMed] [Google Scholar]
- 4.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999; 353:1386–1389 [DOI] [PubMed] [Google Scholar]
- 5.Alkinj B, Pannu BS, Apala DR, et al. Saddle vs nonsaddle pulmonary embolism: Clinical presentation, hemodynamics, management, and outcomes. Mayo Clin Proc. 2017; 92:1511–1518 [DOI] [PubMed] [Google Scholar]
- 6.Ryu JH, Pellikka PA, Froehling DA, et al. Saddle pulmonary embolism diagnosed by CT angiography: Frequency, clinical features and outcome. Respir Med. 2007; 101:1537–1542 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf SW, Gladish G, Lenihan DJ, et al. Computerized tomographic finding of saddle pulmonary embolism is associated with high mortality in cancer patients. Intern Med J. 2010; 40:293–299 [DOI] [PubMed] [Google Scholar]
- 8.Sardi A, Gluskin J, Guttentag A, et al. Saddle pulmonary embolism: Is it as bad as it looks? A community hospital experience. Crit Care Med. 2011; 39:2413–2418 [DOI] [PubMed] [Google Scholar]
- 9.Choi KJ, Cha SI, Shin KM, et al. Central emboli rather than saddle emboli predict adverse outcomes in patients with acute pulmonary embolism. Thromb Res. 2014; 134:991–996 [DOI] [PubMed] [Google Scholar]
- 10.Jiménez D, Aujesky D, Moores L, et al. ; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010; 170:1383–1389 [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim SA, Stone RA, Obrosky DS, et al. Racial differences in 30-day mortality for pulmonary embolism. Am J Public Health. 2006; 96:2161–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998. Arch Intern Med. 2003; 163:1711. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998; 158:585–593 [DOI] [PubMed] [Google Scholar]
- 14.NYCdata. Population & Geography New York City (NYC) Population Estimates by Age, Mutually Exclusive Race and Hispanic Origin, and Sex. 2019. Available at: https://baruch.cuny.edu/nycdata/population-geography/pop-demography.htm. Accessed March 1, 2021
- 15.Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest. 2002; 121:806–814 [DOI] [PubMed] [Google Scholar]
- 16.Garvey S, Dudzinski DM, Giordano N, et al. Pulmonary embolism with clot in transit: An analysis of risk factors and outcomes. Thromb Res. 2020; 187:139–147 [DOI] [PubMed] [Google Scholar]
- 17.Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018; 2:3226–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013; 369:799–808 [DOI] [PubMed] [Google Scholar]


