Abstract
Rationale:
COVID-19 presentation is multifaceted and up to 44% of patients affected by COVID-19 experience musculoskeletal complaints, mostly in the form of diffuse aspecific arthromyalgias. Nevertheless, only a few cases of arthritis following SARS-CoV2 infection are reported.
Patient concerns:
A 27-year-old man affected by nail psoriasis presented with monoarthritis 2 weeks after being diagnosed with COVID-19.
Diagnoses:
Diagnostic work-up and differential diagnosis were made difficult by patient isolation, absence of lab tests, and his visit via telemedicine, even though signs of first metacarpophalangeal joint involvement were clear.
Interventions:
Due to the inefficacy of acetaminophen and nonsteroidal anti-inflammatory drugs, the patient was prescribed oral steroids with a rapid benefit.
Outcomes:
The patient's response to oral steroid was prompt and maintained even after therapy tapering. Even so, a formal diagnosis was not possible due to a difficult diagnostic work-up and lack of a long-term follow-up.
Lessons:
Like many other viral diseases, SARS-CoV2 can play as a causative agent or as a trigger for inflammatory arthritis development in predisposed individuals.
Keywords: case report, COVID-19, inflammatory arthritis, psoriatic arthritis, reactive arthritis
1. Introduction
Since its outbreak, COVID-19 has reshaped the way health care services are provided.[1] Its rapidity of spread among the population and potential evolution to a life-threatening disease are 2 key features of the burden SARS-CoV2 is exerting worldwide. Clinically, the main feature is pulmonary involvement leading to severe clinical features in almost 14% of the cases.[2] Few such cases further worsen, with the development of a multi-organ disease fueled by a systemic inflammatory response called cytokine storm.[3] However, the disease spectrum appears heterogeneous and like other cases of viral diseases, other organ systems can be clinically implicated.[4] As discussed elsewhere,[5] joint involvement ranging from arthralgia to chronic arthritis can accompany different viral infections. Although musculoskeletal complaints are relatively uncommon in endemic coronaviruses infections, up to 44% of patients affected by COVID-19 experience musculoskeletal complaints, mostly in the form of diffuse aspecific arthralgias and myalgias. In this paper, we discuss the case of a young man presenting with monoarthritis, compare it with similar cases published in the literature, and hypothesize on possible SARS-CoV2 roles in the development of inflammatory arthritis.
2. Case report
A 27-year-old man experienced acute swelling and pain of the first metacarpophalangeal (MCP) joint of the right hand 2 weeks after being diagnosed with COVID-19. He first managed his symptoms with Acetaminophen and oral nonsteroidal anti-inflammatory drugs (NSAIDs), ineffectively. His general practitioner (GP) prescribed him an oral steroid therapy, to be confirmed by a rheumatologist. The patient then contacted our rheumatology office and telephonically described his case. He stated that viral disease first presented with mild body temperature elevation (up to 37.3°C), flu-like symptoms, and mild, bilateral conjunctival injection, and was then submitted to a nasopharyngeal swab on the same day, which tested positive for SARS-CoV2. In absence of clinical features requiring hospitalization, the patient was thereafter isolated at home following local COVID-19 management guidelines.
The patient reported that his viral illness symptoms had subsided 5 days before arthritis onset. He reported no previous trauma or articular overuse. On history, his overall clinical status was good, except for a diagnosis of nail psoriasis at a young age without overt psoriatic cutaneous or joint involvement. He denied recent genitourinary or gastrointestinal infections or new skin lesions. Unfortunately, neither recent blood tests nor imaging studies were available. Necessitating visualizing the case, we were able to get in touch with the patient via video call and evaluate his hands and wrists. On observation, redness and swelling of the first right MCP were easily distinguishable while no other sign of inflammation was remarked (Fig. 1).
Figure 1.
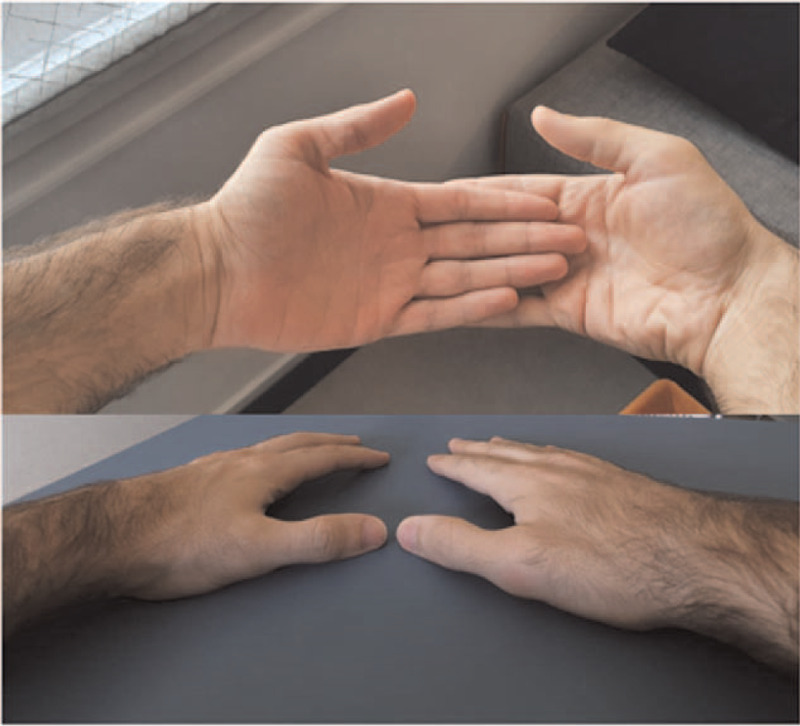
Swelling and redness of the first MCF of the right hand.
Due to the ineffectiveness of NSAIDs, we confirmed a short course of steroid therapy, starting with oral prednisone 10 mg/die with a rapid tapering schedule, confirming his GP decision. After steroid discontinuation, the patient reported the absence of pain or range of motion limitation and minimal residual swelling of the affected joint (Fig. 2). No other articular sites were subsequently affected by pain or swelling.
Figure 2.

Reduction of swelling and redness of the first MCF joint of the right hand after 10 d of oral steroid therapy.
3. Discussion
This is the first case of arthritis affecting a COVID-19 patient not suffering from known rheumatological conditions we happened to handle in our Rheumatology unit. In the attempt to retrieve similar cases and their management, we searched PubMed using the terms “Covid-19”, “coronavirus”, “arthritis”, “reactive arthritis”. The search included all articles published in the English language until November 11, 2020. Only cases describing arthritis clinically defined by signs of joint inflammation (dolor, rubor, calor, tumor, functio lesa, either alone or in combination) or defined by imaging modalities (ultrasounds and/or magnetic resonance) were taken into account. 4 cases of arthritis arising in COVID-19 patients unaffected by prior rheumatological disease were retrieved and compared to our case.[6–9]
Our patient suffered from monoarthritis that occurred 15 days after being diagnosed with COVID-19. This temporal window is matched by the other published cases, where the time ranged from 8 days after probable exposition to 25 days after viral symptoms onset. Overall, arthritis presented as a mono- or oligoarthritis of the lower limbs, irrespective of COVID-19 severity, and showed a good and rapid response to therapy with systemic NSAIDs and/or intra-articular glucocorticoids. In contrast, arthritis in our patient developed in the upper limbs, and was unresponsive to oral NSAIDs, even though he experienced a dramatic and rapid response to a short course of oral steroid therapy at a moderate-low dose. Clinical and epidemiological characteristics of COVID-19 and COVID-19-associated arthritis are summarized in Table 1.
Table 1.
Comparison between published cases of arthritis development following SARS-CoV2 infection and the case we present.
| Ono et al[6] | Parisi et al[7] | Saricaoglu et al[8] | Liew et al[9] | Present case | |
| Age | 50s | 58 | 73 | 47 | 27 |
| Sex | Male | Female | Male | Male | Male |
| Duration of COVID-19 | 21 d since swab positivity | 25 d since prodromic symptoms | 15 d since swab positivity | 1 wk since exposition | 15 d since swab positivity |
| Severity of COVID-19 | Need for intubation | Need for hospitalization | Need for hospitalization | Nonsevere | Nonsevere |
| Number of joints involved | 4 | 1 | 2 | 1 | 1 |
| Localization | I MTP and I IP, bilaterally | Ankle | Ankles | Knee | I MCP joint |
| Imaging | No erosions or enthesophytes on plain X-rays | Synovial hypertrophy and power Doppler signal on ultrasound | Normal findings on radiographic examination | Suprapatellar effusion with mild osteoarthritic changes on X-rays | n/a |
| Synovial fluid analysis | Mild inflammatory fluid, negative cultures, and monosodium urate research | n/a | n/a | Turbid yellow fluid, no crystals, negative bacterial cultures and negative fluid PCR and culture for SARS-CoV2 | n/a |
| Therapy | Oral NSAIDs and intra-articular corticosteroids | Oral NSAIDs | Oral NSAIDs | Oral NSAIDs and intra-articular corticosteroids | Oral steroids |
IP = interphalangeal, MCP = metacarpophalangeal, MTP = metatarsophalangeal, NSAIDs = nonsteroidal anti-inflammatory drugs.
3.1. A case of reactive arthritis?
Reactive arthritis (ReA) is a sterile, often transient arthritis occurring after a distant mucosal infection and is part of the spondyloarthritis (SpA) spectrum. ReA pathogenesis is not fully characterized, but it seems that direct microorganism dissemination, molecular mimicry, and/or host response against infection operating in the context of a strong genetic background is key to disease development.[10] The disease occurs 1 to 4 weeks after infection and affects more commonly young adults aged 20 to 40. Operating diagnostic criteria for ReA requires the presence of an asymmetric mono- or oligoarthritis, typically affecting lower limbs, associated with the evidence of a prior urogenital or gastrointestinal bacterial infection to label a diagnosis of “defined ReA”.[11] However, criteria strictness regarding specific causative agents is not free from limitations. ReA concept is a recent object of revisiting, especially through the observed epidemiological reduction of sex-transmitted disease in high-income countries as well as recognition of miscellaneous pathogens responsible for the disease.[12,13] Among miscellaneous responsible pathogens, some viruses are enlisted and the first COVID-19-related ReA cases start to emerge,[10] as we aforementioned.
Due to our patient at-home isolation, we were unable to perform further investigations and a proper examination to thoroughly consider the differential diagnosis in inflammatory arthritis.
Ultimately, given its epidemiological concordance, clinical presentation, and the occurrence of a temporal-related event such as SARS-CoV2 infection, the possibility that might represent a case of reactive arthritis secondary to COVID-19 infection must be taken into strong consideration.
Complicating facts, our patient suffers from nail psoriasis, a condition predisposing to psoriatic arthritis (PsA) among patients with psoriasis.[14] Back to the drawing board.
3.2. Viral triggers for PsA
Similar to most rheumatological conditions, inflammatory arthritis is the result of the combination of genetic susceptibility and environmental triggers.[15–17] The latter encompasses various stimuli, among which viral infections are proposed as aetiological triggers for autoimmunity.[18] This conclusion is drawn mainly from 2 observations. First, viral infections often precede the onset of autoimmune diseases.[19] Second, different viral genomes have been retrieved from autoimmune diseases tissue targets, that is, synovial membrane in inflammatory arthritis, more frequently in rheumatoid arthritis and PsA.[20,21]
Postulated mechanisms for the role of viruses in autoimmunity genesis are diverse, such as molecular mimicry, bystander activation, and viral infection persistence.[19,22]
The hypothesis of infections as PsA trigger has come a long way, and many data were gathered in a recent systematic review.[23] Most of our knowledge derives from data regarding Gram-positive and Gram-negative bacteria infections triggering PsA from psoriasis, most likely dysregulating the fragile immunological balance through Toll-like receptor (TLR)-2 and TLR-4 – mediated activation of the innate immune system.[23,24] Not as strong as bacteria's, evidence for viral stimuli in the development of PsA mainly comes from the high prevalence of PsA in sub-Saharan patients with HIV infections.[25] PsA susceptibility might be sustained by HIV pathogenesis, with the enrichment of IL-17+ CD8+ cells found in PsA synovium reflecting CD4+ T cells decrease and CD8+ T cells relative increase in HIV patients.[26,27] Other viral diseases have been linked to PsA, with the strongest association being those with Parvovirus B19, cytomegalovirus (CMV), and Epstein-Barr virus (EBV), as they or their genome were found in a higher proportion of PsA synovium than ReA synovium.[20,21] Once again, TLRs expressed by synoviocytes or other innate immune cells can be activated by antiviral compounds, notably TLR-3, -7, and -8, and may play a role in arthritis pathogenesis.[28] Being affected by nail psoriasis, our patient is a subject susceptible to joint involvement. In the context of genetic susceptibility, SARS-CoV2 infection may represent the precipitating event to PsA onset.
3.3. Addressing COVID-19
Since its outbreak, different immune dysregulations have been found to correlate with COVID-19.[29] Like HIV infection, lymphopenia is common among COVID-19 patients.[30] Notwithstanding, in SARS-CoV2 related disease CD8+ cells subset is more susceptible to infection induced-depletion, as opposed to HIV and its immunopathogenesis in PsA development.[31] TLR-3 and TLR-7 activation is presumed to be one of the first steps in SARS-Cov2 clearance.[32,33] Nevertheless, the SARS-CoV2 infection relationship with TLRs is nothing but straightforward, as both clinical and experimental evidence seems to indicate that some unexplained TLRs alteration, either as inhibition or dysfunction, could sustain the defective immune response to viral infection and subsequent severe clinical manifestations.[34] Despite the knowledge we possess about the viral role in inflammatory arthritis, COVID-19 appears to operate following different immunopathogenic pathways. Furthermore, other immunological alterations are observed in COVID-19 patients, such as regulatory cells dysfunction and increased circulating TH17 cytokines like IL-17 among all.[31,35,36] Lack of regulatory function likely associated with an increase in circulating IL-17, whose axis is implicated in PsA pathogenesis,[37,38] add complexity to the riddle of COVID-19 role in inflammatory arthritis development. Last but not least, human-viral molecular mimicry is supposed to be responsible for the multifaceted phenotype of COVID-19 disease.[39] Different autoimmune neurological and hematological conditions encountered as COVID-19 complications have been spotted as potential molecular mimicry results.[40] Among these, some cases of Guillain-Barré syndrome are supposed to bear a viral similarity to heat shock proteins (HSPs) as pathogenic mechanism.[41] HSPs are ubiquitous intracellular proteins that serve as chaperon proteins, MHC-peptide binding, and protein synthesis and folding.[42] They are upregulated in inflammatory states and are thought to balance immune tolerance.[43] However, these proteins are immunogenic and when inflammatory stimuli overcome regulatory factors, HSPs may aid break the tolerance.[43] In inflammatory arthritis, HSP-60 and HSP-70 are up-regulated and appear to stimulate synovial and peripheral T cell proliferation and activation.[44] Lucchese and Flöel[41] demonstrated that SARS-CoV2 shares an immunologically relevant sequence of peptides with HSP60. Though the same sequence of peptides is implicated in loss of immune tolerance in demyelinating diseases and not in inflammatory arthritis,[45] this association reduces the theoretical gap between COVID-19 and the development of inflammatory arthritis (Fig. 3).
Figure 3.
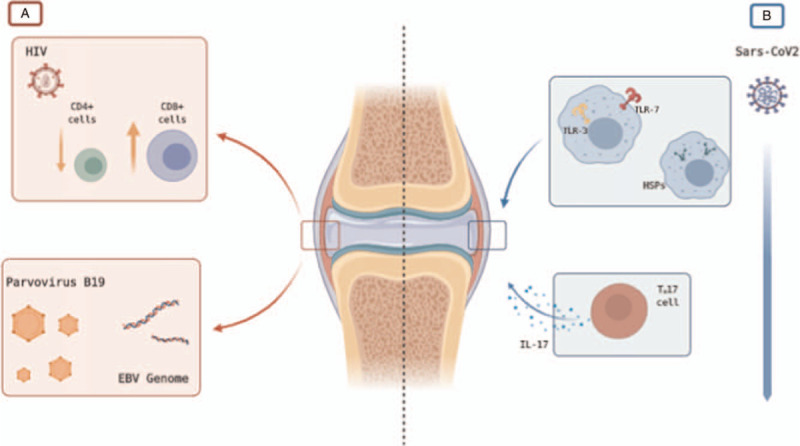
Comparison between (A) viral triggers in inflammatory arthritis and (B) possible mechanism behind COVID-19 induced inflammatory arthritis. (A) As discussed in the text, similarities between HIV-induced lymphocytes count alteration (i.e., decreased CD4+ cells count and a relative increase in CD8+ cells count) and inflammatory cells pattern in PsA synovium as well as the higher prevalence of HIV in sub-Saharan PsA patients fuel hypothesis on its role as a viral trigger for PsA.[26] The presence of replicating Parvovirus B19 and EBV and CMV genome in RA and PsA synovium is another element supporting viral triggers in arthritis development.[20,21] (B) Possible mechanisms through which SARS-CoV2 may serve as a trigger for inflammatory arthritis are dysregulation of TLRs activation,[34] molecular mimicry with HSPs,[41] and enhanced TH17 response[31] as well as dysfunction of regulatory cells.[35]
4. Conclusion
COVID-19 pandemic is posing difficulties in the management of patients both in hospital settings and territorial offices. Whether affected by chronic diseases or presenting with acute conditions not requiring urgent evaluation, many patients are assessed via telemedicine, that is, the remote delivery of healthcare services. This approach can help provide valuable solutions to the patients’ problems. One of the prices is the risk of leaving nonurgent complex cases and further investigations temporarily unresolved or postponed. Such is the case for our patient, whose resolutions of joint symptoms may serve as a temporal bridge for when an appropriate diagnostical work-up will be feasible to label this case either as reactive arthritis or psoriatic arthritis. Simple actions for complex times.
Author contributions
Conceptualization: Gilberto Cincinelli, Roberto Caporali.
Supervision: Roberto Caporali.
Writing – original draft: Gilberto Cincinelli, Raffaele Di Taranto, Francesco Orsini, Andrea Rindone.
Writing – review & editing: Gilberto Cincinelli, Antonella Murgo, Roberto Caporali.
Footnotes
Abbreviations: CD4+ = cluster of differentiation 4 positive, CD8+ = cluster of differentiation 8 positive, CMV = cytomegalovirus, EBV = Epstein-Barr virus, GP = general practitioner, HIV = human immunodeficiency virus, HSP = heat shock protein, IL-17+ = interleukin 17 positive, IP = interphalangeal, MCH = major histocompatibility complex, MCP = metacarpophalangeal, MRI = magnetic resonance imaging, MTP = metatarsophalangeal, NSAIDs = nonsteroidal anti-inflammatory drugs, PsA = psoriatic arthritis, RA = rheumatoid arthritis, ReA = reactive arthritis, SpA = spondyloarthritis, TH17 = T helper 17, TLR = toll-like receptor, US = ultrasonography.
How to cite this article: Cincinelli G, Di Taranto R, Orsini F, Rindone A, Murgo A, Caporali R. A case report of monoarthritis in a COVID-19 patient and literature review: simple actions for complex times. Medicine. 2021;100:23(e26089).
The current analysis is part of a project to collect observational data from rheumatological patients followed at the ASST Gaetano Pini-CTO. The project was approved by the Ethics Committee of the Geatano Pini Institute with approval number 141/2010. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
The authors declare that no funding was received for this study.
The authors have no conflicts of interest to disclosure.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Guerci C, Maffioli A, Bondurri AA, Ferrario L, Lazzarin F, Danelli P. COVID-19: How can a department of general surgery survive in a pandemic? Surgery 2020;167:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. [DOI] [PubMed] [Google Scholar]
- [5].Schett G, Manger B, Simon D, Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol 2020;16:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ono K, Kishimoto M, Shimasaki T, et al. Reactive arthritis after COVID-19 infection. RMD Open 2020;6:e001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parisi S, Borrelli R, Bianchi S, Fusaro E. Viral arthritis and COVID-19. Lancet Rheumatol 2020;2:e655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol 2021;93:192–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liew IY, Mak TM, Cui L, et al. A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 2020;26:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stavropoulos PG, Soura E, Kanelleas A, et al. Reactive arthritis. J Eur Acad Dermatol Venereol 2015;29:415–24. [DOI] [PubMed] [Google Scholar]
- [11].Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev 2014;13:546–9. [DOI] [PubMed] [Google Scholar]
- [12].Bentaleb I, Abdelghani KB, Rostom S, Amine B, Laatar A, Bahiri R. Reactive arthritis: update. Curr Clin Micro Rep 2020;7:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Misra R, Gupta L. Epidemiology: Time to revisit the concept of reactive arthritis. Nat Rev Rheumatol 2017;13:327–8. [DOI] [PubMed] [Google Scholar]
- [14].Langenbruch A, Radtke MA, Krensel M, Jacobi A, Reich K, Augustin M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol 2014;171:1123–8. [DOI] [PubMed] [Google Scholar]
- [15].Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 2018;6:01–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. [DOI] [PubMed] [Google Scholar]
- [17].Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res 2019;7:01–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Getts DR, Chastain EML, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev 2013;255:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 2006;19:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mehraein Y, Lennerz C, Ehlhardt S, et al. Detection of parvovirus B19 capsid proteins in lymphocytic cells in synovial tissue of autoimmune chronic arthritis. Mod Pathol 2003;16:811–7. [DOI] [PubMed] [Google Scholar]
- [21].Mehraein Y, Lennerz C, Ehlhardt S, Remberger K, Ojak A, Zang KD. Latent Epstein-Barr virus (EBV) infection and cytomegalovirus (CMV) infection in synovial tissue of autoimmune chronic arthritis determined by RNA- and DNA-in situ hybridization. Mod Pathol 2004;17:781–9. [DOI] [PubMed] [Google Scholar]
- [22].Perl A. Mechanisms of viral pathogenesis in rheumatic disease. Ann Rheum Dis 1999;58:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thrastardottir T, Love TJ. Infections and the risk of psoriatic arthritis among psoriasis patients: a systematic review. Rheumatol Int 2018;38:1385–97. [DOI] [PubMed] [Google Scholar]
- [24].Carrasco S, Neves FS, Fonseca MH, et al. Toll-like receptor (TLR) 2 is upregulated on peripheral blood monocytes of patients with psoriatic arthritis: a role for a gram-positive inflammatory trigger? Clin Exp Rheumatol 2011;29:958–62. [PubMed] [Google Scholar]
- [25].Njobvu P, McGill P. Psoriatic arthritis and human immunodeficiency virus infection in Zambia. J Rheumatol 2000;27:1699–702. [PubMed] [Google Scholar]
- [26].Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis 2010;10:470–8. [DOI] [PubMed] [Google Scholar]
- [27].Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol 2005;233:90–6. [DOI] [PubMed] [Google Scholar]
- [29].Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020;27:992.e3–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol 2020;20:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Poulas K, Farsalinos K, Zanidis C. Activation of TLR7 and innate immunity as an efficient method against COVID-19 pandemic: imiquimod as a potential therapy. Front Immunol 2020;11:1373.doi:10.3389/fimmu.2020.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Birra D, Benucci M, Landolfi L, et al. COVID 19: a clue from innate immunity. Immunol Res 2020;68:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Onofrio L, Caraglia M, Facchini G, Margherita V, De Placido S, Buonerba C. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 2020;6:FSO605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Muyayalo KP, Huang DH, Zhao SJ, Xie T, Mor G, Liao AH. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am J Reprod Immunol 2020;84:e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 2020;53:368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Taams LS, Steel KJA, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol 2018;14:453–66. [DOI] [PubMed] [Google Scholar]
- [38].McGonagle DG, McInnes IB, Kirkham BW, Sherlock J, Moots R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 2019;78:1167–78. doi:10.1136/annrheumdis-2019-215356. Epub 2019 Jul 5. Erratum in: Ann Rheum Dis. 2020;79:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, Jl Macario A, Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev 2020;19:102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 2020;16:413–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lucchese G, Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 2020;25:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev 1993;57:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van Eden W, Jansen MAA, Ludwig I, van Kooten P, van der Zee R, Broere F. The enigma of heat shock proteins in immune tolerance. Front Immunol 2017;8:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Spierings J, van Eden W. Heat shock proteins and their immunomodulatory role in inflammatory arthritis. Rheumatology (Oxford) 2017;56:198–208. [DOI] [PubMed] [Google Scholar]
- [45].Ruiz-Vázquez E, de Castro P. “2-6-11” motif in heat shock protein 60 and central nervous system antigens: a preliminary study in multiple sclerosis patients. J Physiol Biochem 2003;59:01–9. [DOI] [PubMed] [Google Scholar]


