Abstract
Background:
Obesity and insulin resistance (IR) are common in polycystic ovary syndrome (PCOS), which contribute to reproductive and metabolic abnormalities. Metformin increases insulin sensitivity, but it is associated with unsatisfied benefits of weight loss. Recent studies have reported that glucagon-like peptide 1 (GLP-1) receptor agonists improve IR and reduce weight in women with PCOS. We conducted a systematic review and meta-analysis to compare the effects between GLP-1 receptor agonists and metformin, and between GLP-1 receptor agonist-metformin combination and GLP-1 receptor agonists in overweight/obese women with PCOS on anthropometric, metabolic, reproductive outcomes.
Methods:
Databases including PubMed, EMBASE, Web of Science, and Cochrane Library were selected to search for randomized controlled trials (RCTs) published in English up to March 2020. Eligible studies were identified according to the inclusion criteria. The primary outcomes included menstrual frequency, body mass index (BMI), total testosterone, and the homeostatic model assessment of insulin resistance. GRADE criteria were implemented to assess the quality of evidence for primary outcomes.
Results:
Seven RCTs were selected for analysis, comprising 464 overweight/obese women with PCOS. In the low-quality evidence, a meta-analysis demonstrated that GLP-1 receptor agonists showed better effects relative to metformin on the reduction of body mass index (mean difference − 1.72; 95% confidence interval −2.46 to −0.99, P < .001) and homeostatic model assessment of insulin resistance (standard mean difference −0.37; 95% confidence interval − 0.60,− 0.15, P = .001). Moreover, the combination therapy exhibited similar effects on primary outcomes relative to GLP-1 receptor agonist alone. GLP-1 receptor agonists were also found to be associated with lower abdominal girth compared to metformin. A meta-analysis of gastrointestinal discomfort showed no significant difference between GLP-1 receptor agonist and metformin therapies, and between the combination therapy and GLP-1 receptor agonist alone.
Conclusions:
GLP-1 receptor agonists appear to be more beneficial for weight loss and IR improvement compared to metformin for overweight/obese women with PCOS. However, the combination treatment displays comparable effects with GLP-1 receptor agonist alone. The incidence of gastrointestinal discomforts was similar in different groups. However, the quality of the body of evidence is “low.” Further prospective RCTs and cost-effectiveness analyses are also warranted to guide GLP-1 receptor agonists to treat women with PCOS.
Keywords: glucagon-like peptide 1 receptor agonists, insulin resistance, metformin, polycystic ovary syndrome, weight loss
1. Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder characterized by chronic anovulation, hyperandrogenism, and polycystic ovary morphology. Although obesity is not a diagnostic criterion for PCOS, it contributes substantially to reproductive and metabolic abnormalities in women with PCOS.[1,2] Multiple studies have shown that weight loss can help PCOS women resume spontaneous menstruation, reduce circulating androgen levels, and improve glucose and lipid metabolism.[3,4] Moreover, weight loss conduces to an increased pregnancy rate in women with PCOS.[5] Patients with obesity and PCOS can obtain these benefits by losing as little as 5% of the initial weight.[6] Lifestyle modification is an integral part of PCOS treatment. Increased exercise and dietary habit changes help patients lose weight and reduce the risk of cardiovascular disease and diabetes, but it is often challenging for patients to persist in life. Metformin, an insulin sensitizer, is commonly used in combination with lifestyle modification to treat women with PCOS. The improvement of insulin sensitivity by metformin is associated with its ability to decrease androgen levels, increase ovulation rate, and improve glucose tolerance. However, the effects of metformin on weight loss are not satisfactory to women with PCOS.[7,8]
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of novel anti-diabetes agents, which are incretin mimetics share similar effects of GLP-1, including glucose-dependent enhancement of insulin secretion and islet B cells proliferation.[9] GLP-1 receptor agonists have shown effective improvement in insulin resistance (IR) and impaired glucose tolerance,[10] which is also associated with weight loss due to delayed gastric emptying and increased satiety via a central action.[11] Exenatide and liraglutide have recently been used for the treatment of PCOS, which led to significant weight loss and improved glucose metabolism in patients with PCOS compared to placebo.[12–14] Moreover, liraglutide was associated with reduced plasma cardiovascular risk biomarkers (eg, MR-proANP and MR-proADM) in patients with PCOS.[15]
Randomized controlled trials (RCTs) have been reported to compare the efficacy and safety of GLP-1 receptor agonist and metformin in the treatment of women with PCOS.[16–21] The results showed that GLP-1 receptor agonist showed better weight loss effect on PCOS than metformin. However, it was reported by Salamun et al that the weight loss effect in a combination of GLP-1 receptor agonist and metformin was similar to the PCOS patients using metformin as single therapy.[22] GLP-1 receptor agonist, metformin, or a combined treatment can improve metabolic abnormalities in patients with PCOS, but the differences among groups are varied in studies. In addition, the small sample size might limit the accuracy of all the existing randomized controlled trials. Therefore, in this work, we aimed to compare the efficacy and safety between GLP-1 receptor agonist and metformin, and between GLP-1 receptor agonist combined with metformin and GLP-1 receptor agonist alone in the treatment of PCOS, and to comprehensively evaluate the effectiveness and safety of GLP-1 receptor agonist in the treatment of PCOS.
2. Methods
2.1. Search strategy
Electronic databases (PubMed, EMBASE, Web of Science, and Cochrane Library) were searched until March 2020. The following MeSH terms and Emtree terms were employed: “polycystic ovary syndrome,” “glucagon-like peptide-1 receptor,” “glucagon-like peptide-1 receptor agonists,” “liraglutide” and “exenatide.” The following is an example of the search strategy used on PubMed: (((“Polycystic Ovary Syndrome”[Mesh]) OR ((((((Ovary Syndrome, Polycystic) OR (Syndrome, Polycystic Ovary)) OR (Stein-Leventhal Syndrome)) OR (Polycystic Ovarian Syndrome)) OR (Ovarian Syndrome, Polycystic)) OR (Stein Leventhal Syndrome))) AND ((((“Glucagon-Like Peptide-1 Receptor”[Mesh]) OR ((((((Glucagon Like Peptide 1 Receptor) OR (Peptide-1 Receptor, Glucagon-Like)) OR (GLP-1R Receptor)) OR (GLP-1 Receptor)) OR (Receptor, GLP-1)) OR (Receptor, Glucagon-Like Peptide-1))) OR (“Liraglutide”[Mesh])) OR (“Exenatide”[Mesh]))) AND ((clinical[tiab] AND trial[tiab]) OR “clinical trials as topic”[mesh] OR “clinical trial”[pt] OR random∗[tiab] OR “random allocation”[mesh] OR “therapeutic use”[sh]).
We also searched reference lists of all eligible articles and previous reviews on relevant topics for additional studies. The findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
2.2. Inclusion and exclusion criteria
Eligible studies were accepted if they complied with the following inclusion criteria: Participants: patients with a diagnosis of PCOS; Intervention(s): GLP-1 receptor agonist (GLP-1 RA) alone or the combination of GLP-1 RA and metformin were applied to PCOS patients; comparison(s): comparison between GLP-1 RA and metformin, between GLP-1 RA combined with metformin and GLP-1 RA alone; Outcomes: changes in obesity, menstrual frequency, metabolic or endocrine parameters, and adverse events; study: RCTs with results published in English. Single-arm study without a control group or studies with a comparison between GLP-1 RA and placebo were excluded.
2.3. Data extraction and risk of bias of included studies
Two reviewers (MRL and DXS) independently and simultaneously screened studies’ titles and abstracts for eligibility. Each of them independently assessed the full text of the potentially relevant studies for inclusion. Discrepancies were resolved by consensus after discussion. The same reviewer also extracted the data independently. They recorded the characteristics of the included studies and outcome measures, including the authors, publication year, study region and design, the definition of PCOS, the number of participants, mean age, body mass index (BMI) of participants, interventions, duration, and measurement of variables of interest. Standard errors were converted to standard deviations.
Primary outcomes, secondary outcomes, and adverse events were analyzed. The primary outcomes included menstrual frequency (MFR), BMI, total testosterone (TT), and the homeostatic model assessment of insulin resistance (HOMA-IR). The secondary outcomes included abdominal girth (AG), sex hormone-binding globulin, free androgen index (FAI), androstenedione, dehydroepiandrosterone sulphate (DHEAS), fasting blood glucose, fasting insulin, triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and high-sensitivity C-reactive protein (hsCRP).
Two reviewers (MRL and WYF) independently assessed the risk of bias for RCTs using the Cochrane Collaboration's tool,[23] which addresses 7 domains of bias: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other bias.
2.4. Quality assessment
The quality of evidence for the meta-analysis's primary outcomes was independently assessed by 2 authors (MRL and WYF) using GRADE criteria. According to the study design, risk of bias, indirectness, inconsistency, imprecision, and publication bias, the quality of evidence was rated to be high, moderate, low, and very low qualities.
2.5. Statistical analysis
Statistical analysis was performed using Review Manager version 5.3 (Cochrane Collaboration, Software Update). Continuous data are expressed as mean differences (MD) or standard mean differences (SMD). Moreover, dichotomous data are expressed as risk ratio (RR) with 95% confidence intervals (CIs). Heterogeneity was evaluated by the χ2 test and expressed by I2 values. An I2 statistic >50% was considered to show substantial heterogeneity. All the analyses in our study were performed using random-effects models because of more conservative estimates. Corresponding 95% CIs and P values were calculated. Two-tailed P values <0.05 were considered statistically significant. Sensitivity analysis was carried out to examine changes in pooled effect size by serially excluding each study.
3. Results
3.1. Selected studies
The literature search and study selection procedure are shown in Figure 1. A total of 289 published studies were identified, and after the removal of duplicates, title and abstract screening, and further evaluation, 10 studies were retrieved for full-text assessment. Among these studies, 2 articles were based on the same study population, and 2 articles did not meet the inclusion criteria. Finally, a total of 7 studies were eligible for the meta-analysis. Six RCTs reported data on GLP-1 RA versus metformin, and 3 RCTs reported data on the combination of GLP-1 RA and metformin to compare with GLP-1 RA alone.
Figure 1.
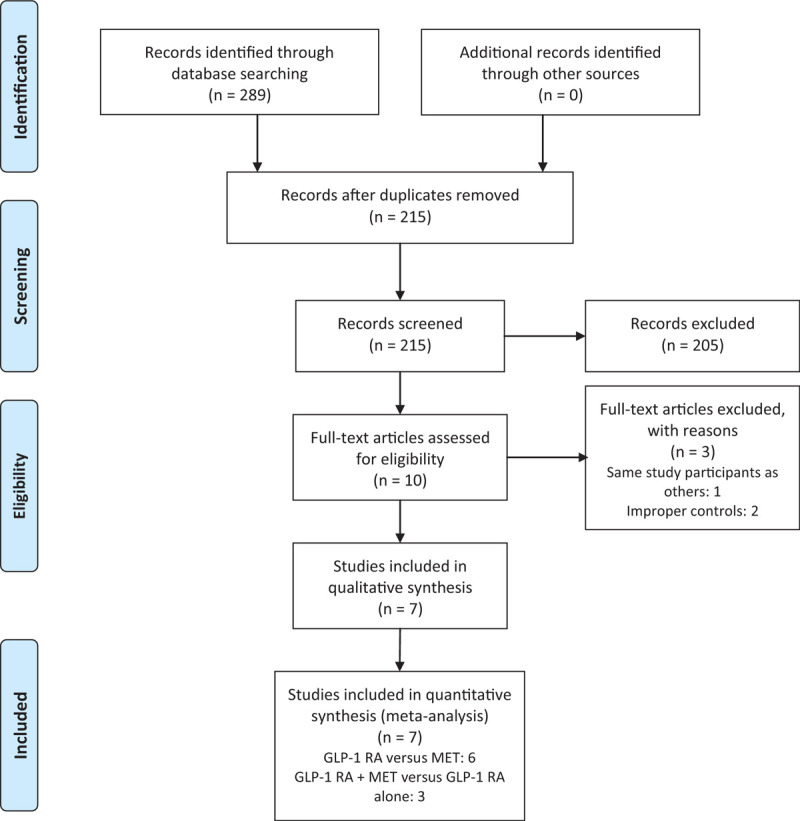
Flow diagram of study selection.
3.2. Characteristics of included studies and risk of bias
The characteristics of the included RCTs are summarized in Table 1. Four studies defined PCOS based on the criteria of Rotterdam,[16,19–21] and the other 3 studies diagnosed PCOS using the National Institutes of Health (NIH) criterion.[17,18,24] Four RCTs compared GLP-1 RA with metformin.[18–21] One RCT compared the combination of GLP-1 RA and metformin with the same dosage GLP-1 RA alone.[24] The other 2 studies were 3-arm trials, including GLP-1 RA group, metformin group, and the combined GLP-1 RA and metformin group.[16,17] Only 2 of these 3 arms meeting the inclusion criteria were included in the appropriate meta-analysis. Patients in 3 RCTs received exenatide (10 μg bid),[16,20,21] and patients in the other 4 RCTs received liraglutide (1.2 mg qd).[17–19,24] The 2 drugs were both administrated by subcutaneous injection.
Table 1.
The characteristics of the included studies.
| Author (year) | Country | Diagnosis | Type | Interventions (sample size) | Mean age, y | Mean BMI, kg/m2 | Duration (w) | Outcomes |
| Comparison between GLP-1 RA versus metformin | ||||||||
| Elkind-Hirsch et al (2008)[16] | USA | ASRM-ESHRE | RCT | EX 10 μg bid (20) | 28.2 (4.9) | 39.9 (5.8) | 24w | MFR, BMI, AG, TT, DHEA-S, SHBG, FAI, HOMA-IR, TC, TG, HDL-C, LDL-C, hsCRP, TNF-α, IL-6 |
| MET 1.0 g bid (20) | 27.7 (6.7 | 41.3 (8.1) | ||||||
| Jensterle Sever et al (2014)[17] | Slovenija | NIH | RCT | LIRA 1.2 mg qd (13) | 12w | MFR, BMI, AG, TT, DHEA-S, SHBG, AD, FBG, FINS, HOMA-IR, TC, TG, HDL-C, LDL-C | ||
| MET 1.0 g bid (14) | 31.3 (9.4) | 36.6 (3.5) | ||||||
| Jensterle et al (2015)[18] | Slovenija | NIH | RCT | LIRA 1.2 mg qd (17) | 29.5 (7.7) | 40.8 (6.1) | 12w | MFR, BMI, AG, TT, DHEA-S, AD, SHBG, FAI, HOMA-IR, TC, TG, HDL-C, LDL-C |
| MET 1.0 g bid (15) | 25.3 (5.2) | 38.2 (7) | ||||||
| Jensterle et al (2015∗)[19] | Slovenija | ASRM-ESHRE | RCT | LIRA 1.2 mg qd (15) | 36.7 (5.6) | 12w | MFR, BMI, AG, TT, DHEA-S, AD, SHBG, FAI, FBG, FINS, HOMA-IR | |
| MET 1.0 g bid (15) | 39.4 (6.9) | |||||||
| Liu et al (2017)[20] | China | ASRM-ESHRE | RCT | EX 10 μg bid (88) | 27.9 (2.7) | 29.2 (3.1) | 12w | MFR, BMI, AG, TT, DHEA-S, SHBG, AD, FAI, FBG, FINS, HOMA-IR, TC, TG, HDL-C, LDL-C, hsCRP |
| MET 1.0 g bid (88) | 27.7 (3.8) | 28.3 (1.9) | ||||||
| Zheng et al (2017)[21] | China | ASRM-ESHRE | RCT | EX 10 μg bid (41) | 27.7 (3.4) | 29.2 (4.2) | 12w | MFR, BMI, AG, TT, DHEA-S, SHBG, FAI, FBG, FINS, HOMA-IR, TC, TG, HDL-C, LDL-C, hsCRP |
| MET 1.0 g bid (41) | 28.2 (3.9) | 29 (4.1) | ||||||
| Comparison between GLP-1 RA + metformin and GLP-1 RA alone | ||||||||
| Elkind-Hirsch et al (2008)[16] | USA | ASRM-ESHRE | RCT | EX 10 μg bid + MET 1.0 g bid (20) | 32.1 (3.1) | 41.2 (7.6) | 24w | MFR, BMI, AG, TT, DHEA-S, SHBG, FAI, HOMA-IR, TC, TG, HDL-C, LDL-C, hsCRP, TNF-α, IL-6 |
| EX 10 μg bid (20) | 28.2 (4.9) | 39.9 (5.8) | ||||||
| Jensterle Sever et al (2014)[17] | Slovenija | NIH | RCT | LIRA 1.2 mg qd + MET 1.0 g bid (13) | 31.1 (5.1) | 37.6 (5.1) | 12w | MFR, BMI, AG, TT, DHEA-S, SHBG, AD, FBG, FINS, HOMA-IR, TC, TG, HDL-C, LDL-C |
| LIRA 1.2 mg qd (13) | 31.5 (6.4) | 39.3 (4.2) | ||||||
| Jensterle et al (2016)[24] | Slovenija | NIH | RCT | LIRA 1.2 mg qd + MET 1.0 g bid (22) | 30.4 (4.2) | 37.7 (4) | 12w | Weight, BMI, AG, TT, DHEA-S, SHBG, AD, FBG, FINS, HOMA-IR |
| LIRA 1.2 mg qd (22) | 30.3 (4.6) | 36.7 (5.1) | ||||||
AD = androstenedione, AG = abdominal girth, BMI = body mass index, DHEA-S = dehydroepiandrosterone sulphate, EX = exenatide, FAI = free androgen index, FBG = fasting blood glucose, FINS = fasting insulin, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, LDL-C = low-density lipoprotein cholesterol, LIRA = liraglutide, MET = metformin, MFR = Menstrual frequency, SHBG = sex hormone-binding globulin, TC = total cholesterol, TG = triglyceride, TT = serum total testosterone.
All studies were assessed as having some risk of bias (Fig. 2). One RCT described an unspecific randomization procedure and had an unclear risk of bias.[20] The other 6 RCTs described adequate methods of random sequence generation: RAND program in Excel or computer-generated randomization list. None of these studies described an acceptable method of allocation concealment, so it was considered that there was an uncertain risk of selection bias. All of the included studies were open-label, but they were considered to be at low risk because outcome measures were objective results. The blinding of outcome assessors was not clearly described in all trials. The risk of bias was thus unclear in this regard. One RCT was judged to be at high risk of attrition bias because of incomplete outcome data.[21] The rate of lost follow-up was >20%, and the reasons for the loss are unbalanced in 2 groups. The other studies were at low risk of attrition bias, and 2 RCTs adopted intent-to-treat population analysis.[16,18] All the studies were considered at low risk of reporting bias and other biases.
Figure 2.
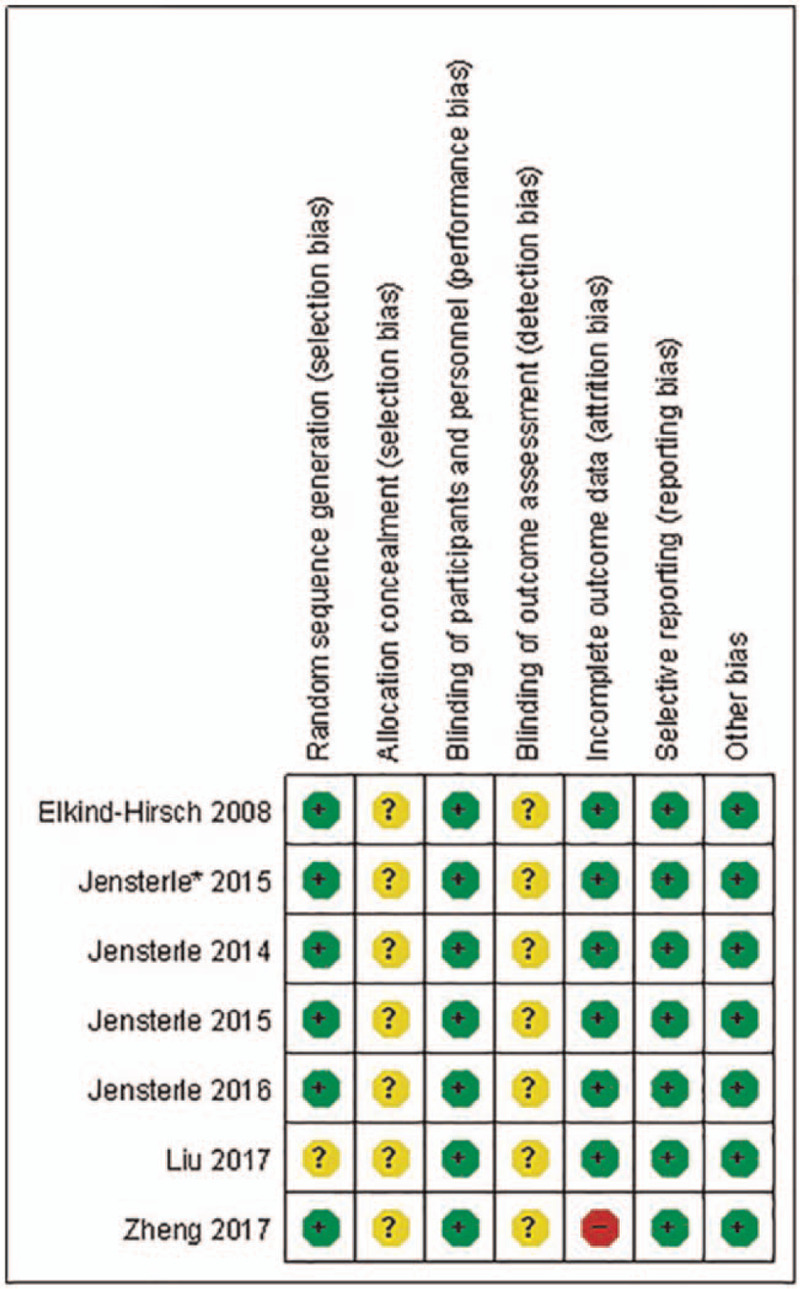
Risk of bias summary.
3.3. Primary outcomes
3.3.1. Comparison between GLP-1 RA versus metformin
Six RCTs were included in the present meta-analysis.[16–21] GLP-1 RA were significantly associated with lower BMI (MD −1.72; 95% CI −2.46 to −0.99; I2 = 0%, P < .001) and HOMA-IR (SMD −0.37; 95% CI −0.60 to −0.15; I2 = 0%, P = .001) compared with metformin, however, there were no significant differences on MFR and TT (SMD 0.04; 95% CI −0.24 to 0.32; I2 = 28%, P = .77) between GLP-1 RA and metformin (Fig. 3). All 6 studies reported the data of MFR, and a random-effects model indicated no differences between 2 groups with significant heterogeneity (P < .001, I2 = 85%). Sensitivity analyses were conducted in which each study was deleted to test the stability of the data. When excluding the study conducted by Liu et al,[20] substantial change in heterogeneity test (P = .15, I2 = 41.0%) was found in MFR, but not in the estimate of effect size (MD 0.04; 95% CI −0.07 to 0.15; P = .46). Five RCTs were included in the meta-analysis for HOMA-IR except one, as it reported data as median and interquartile range.[17]
Figure 3.

A meta-analysis of GLP-1 receptor agonists versus metformin for primary outcomes. (A) BMI = body mass index, (B) MFR = menstrual frequency, (C) TT = serum total testosterone, (D) HOMA-IR = homeostasis model assessment of insulin resistance.
Patients in studies reported by Liu et al and Zheng et al were provided diet and exercise instruction,[20,21] whereas patients in the other 4 studies were given an unrestricted diet and unprescribed exercise.[16–19] A subgroup analysis was conducted to explore the effect of lifestyle intervention on the primary outcomes. The results showed that GLP-1 RA + lifestyle modification versus metformin + lifestyle modification exhibited better effects on reducing BMI (MD −1.88; 95% CI −2.68 to −1.08; I2 = 0%, P < .001) and HOMA-IR (SMD −0.46; 95% CI −0.73 to −0.19; I2 = 0%, P = .007). However, no significant difference was observed on MFR and TT between GLP-1 RA and metformin with or without lifestyle intervention (see Figure, Supplemental Content, which shows the subgroup analysis of primary outcomes).
3.3.1.1. Subgroup analysis
Patients in studies reported by Liu et al and Zheng et al were provided diet and exercise instruction [20,21], whereas patients in the other 4 studies were given an unrestricted diet and unprescribed exercise[16–19]. A subgroup analysis was conducted to explore the effect of lifestyle intervention on the primary outcomes. The results showed that GLP-1 RA + lifestyle modification versus metformin + lifestyle modification exhibited better effects on reducing BMI (MD −1.88; 95% CI −2.68 to −1.08; I2 = 0%, P < 0.00001) and HOMA-IR (SMD −0.46; 95% CI −0.73 to −0.19; I2 = 0%, P = 0.0007). However, no significant difference was observed on MFR and TT between GLP-1 RA and metformin with or without lifestyle intervention (see Additional file 1).
3.3.2. Comparison between GLP-1 RA plus metformin and GLP-1 RA alone
Only 3 RCTs were included in the present meta-analysis.[16,17,24] Three RCTs reported data of BMI, TT, and HOMA-IR. Two RCTs reported data of MFR. As shown in Figure 4, no significant differences were found on MFR, BMI, TT, and HOMA-IR between GLP-1 RA + metformin and GLP-1 RA alone, and no significant heterogeneity was found in studies.
Figure 4.
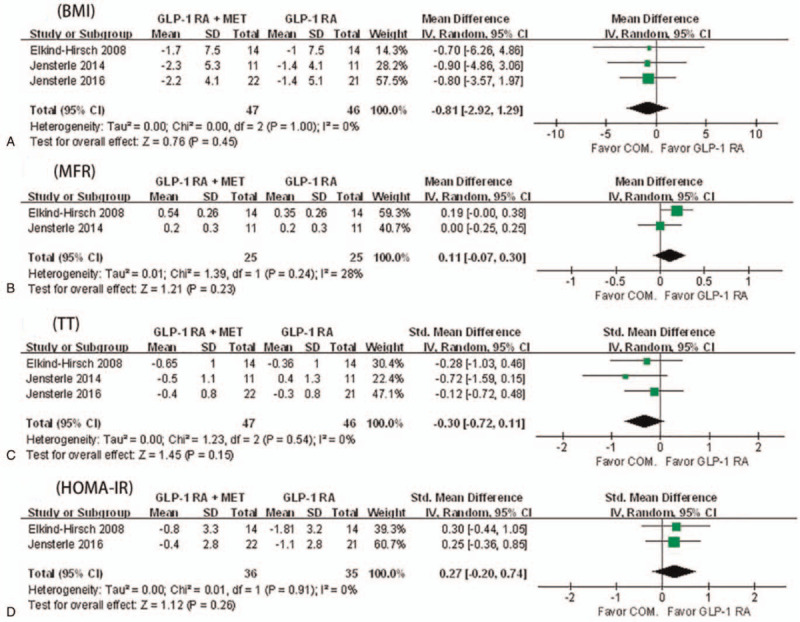
A meta-analysis of GLP-1 receptor agonists + metformin versus GLP-1 receptor agonists alone for primary outcomes. (A) BMI = body mass index, (B) MFR = menstrual frequency, (C) TT = serum total testosterone, (D) HOMA-IR = homeostasis model assessment of insulin resistance.
Subgroup analysis cannot be conducted due to the limited number of articles.
3.4. Secondary outcomes
3.4.1. Comparison between GLP-1 RA versus metformin
As shown in Table 2, GLP-1 RA exhibited more benefits for reducing AG (MD −3.54; 95% CI −5.65 to −1.43; I2 = 0%, P < .001) than metformin. Only 3 RCTs reported the data of hsCRP, and high heterogeneity (P = .002, I2 = 89%) was found in studies. Elkind-Hirsch et al reported that hsCRP increased after treatment in both groups,[16] whereas Liu et al and Zheng et al reported that hsCRP significantly decreased after treatment in both groups.[20,21] We could not draw a valid conclusion regarding hsCRP. No significant differences were found in other endocrine or metabolic parameters between 2 groups, and no significant heterogeneity was found in studies.
Table 2.
Meta-analysis results of secondary outcomes.
| Studies | Sample | MD/SMD (95% CI) | Heterogeneity test | |||
| Outcomes | (N) | size (N) | P | I2 (%) | P | |
| Comparison between GLP-1 RA versus metformin | ||||||
| AG | 6 | 329 | MD –3.54 (–5.65––1.43) | .98 | 0 | .001 |
| SHBG | 5 | 304 | SMD 0.03 (–0.20–0.25) | .99 | 0 | .80 |
| FAI | 5 | 304 | SMD 0.07 (–0.16–0.30) | .53 | 0 | .54 |
| AD | 3 | 80 | SMD 0.06 (–0.38–0.50) | .53 | 0 | .79 |
| DHEAS | 5 | 171 | SMD –0.07 (–0.37–0.23) | .79 | 0 | .64 |
| FBG | 3 | 248 | SMD –0.02 (–0.27–0.23) | 1 | 0 | .85 |
| FINS | 3 | 248 | SMD –0.26 (–0.72–0.19) | .08 | 60 | .26 |
| TC | 5 | 302 | SMD 0.06 (–0.17–0.28) | .85 | 0 | .63 |
| TG | 4 | 277 | SMD 0.08 (–0.21–0.36) | .30 | 19 | .60 |
| HDL-c | 5 | 302 | SMD –0.12 (–0.36–0.12) | .38 | 4 | .33 |
| LDL-c | 5 | 302 | SMD –0.07 (–0.29–0.16) | .99 | 0 | .56 |
| Comparison between GLP-1 RA + metformin and GLP-1 RA alone | ||||||
| AG | 3 | 93 | MD –4.74 (–11.65–0.13) | .89 | 0 | .18 |
| SHBG | 2 | 71 | SMD 0.36 (–0.11–0.83) | .82 | 0 | .13 |
| FAI | 1 | 28 | SMD –0.03 (–0.77–0.71) | .94 | ||
| AD | 2 | 65 | SMD –0.75 (–1.26––0.25) | .71 | 0 | .05 |
| DHEAS | 2 | 50 | SMD –0.18 (–0.74–0.37) | .81 | 0 | .51 |
| FBG | 1 | 43 | SMD –0.58 (–1.19–0.03) | .06 | ||
| FINS | 1 | 43 | SMD 0.35 (–0.25–0.96) | .25 | ||
| TC | 2 | 50 | SMD –0.50 (–1.06–0.07) | .84 | 0 | .08 |
| TG | 1 | 28 | SMD –0.37 (–1.12–0.38) | .33 | ||
| HDL-c | 2 | 50 | SMD –0.09 (–0.98–0.81) | .11 | 60 | .85 |
| LDL-c | 2 | 50 | SMD –0.47 (–1.03–0.09) | .90 | 0 | .10 |
AD = androstenedione, AG = abdominal girth, DHEA-S = dehydroepiandrosterone sulphate, FAI = free androgen index, FBG = fasting blood glucose, FINS = fasting insulin, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, SHBG = sex hormone-binding globulin, TC = total cholesterol, TG = triglyceride.
3.4.2. Comparison between GLP-1 RA plus metformin and GLP-1 RA alone
As shown in Table 2, there were no significant differences found in anthropometric, endocrine, or metabolic parameters between the 2 groups, and no significant heterogeneity was found in studies.
3.5. Adverse events
The adverse events were reported by all the articles assessed in this study. The most frequent adverse events were mild or moderate gastrointestinal (GI) discomforts, including nausea, diarrhea, bloating, vomiting, stomachache, and constipation. Furthermore, headaches and fatigue were also common adverse effects. As shown in Table 3, no significant difference was observed in the meta-analysis of GI side effect between GLP-1 RA and metformin therapies, and between the combination therapy and GLP-1 RA alone (Table 3). Those adverse events occurred at a higher incidence during the initial weeks of treatment, and gradual dose titration reduced the GI side effects related to drug treatment.
Table 3.
Meta-analysis results of adverse events.
| Studies | Sample | RR (95% CI) | Heterogeneity test | |||
| Adverse events | (N) | Size (N) | P | I2 (%) | P | |
| Comparison between GLP-1 RA versus metformin | ||||||
| Nausea | 6 | 387 | 1.35 (0.75–2.44) | .11 | 45 | .32 |
| Diarrhea | 6 | 387 | 0.43 (0.12–1.60) | .06 | 52 | .21 |
| Bloating | 3 | 298 | 0.82 (0.29–2.32) | .60 | 0 | .71 |
| Vomiting | 4 | 330 | 1.37 (0.54–3.44) | .95 | 0 | .51 |
| Stomachache | 4 | 330 | 0.86 (0.20–3.67) | .27 | 23 | .84 |
| Constipation | 5 | 360 | 0.31 (0.09–1.07) | .66 | 0 | .06 |
| Headache | 4 | 129 | 4.28 (0.95–19.36) | .97 | 0 | .06 |
| Fatigue | 3 | 298 | 1.10 (0.19–6.22) | .25 | 29 | .91 |
| Comparison between GLP-1 RA + metformin and GLP-1 RA alone | ||||||
| Nausea | 3 | 110 | 1.35 (0.82–2.23) | .11 | 54 | .24 |
| Diarrhea | 3 | 110 | 1.08 (0.57–2.03) | .52 | 0 | .81 |
| Vomiting | 2 | 84 | 0.33 (0.08–1.35) | .07 | 70 | .12 |
| Headache | 3 | 110 | 0.82 (0.26–2.57) | .81 | 0 | .73 |
CI = confidence interval, RR = risk ratio.
3.6. Quality of evidence
Quality assessment of the evidence for selected primary outcomes is shown in Table 4. The quality of evidence for BMI, MFR, TT, and HOMA-IR values was relatively low. All studies were open-label and were judged under an unclear risk of allocation concealment (selection bias). One study was at high risk of attrition bias. In addition, small total sample sizes and wide 95% CIs reduced the overall quality of the evidence.
Table 4.
Quality assessment of the evidence for selected primary outcomes.
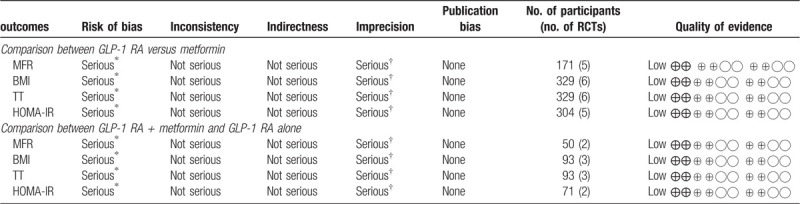
Downgraded 1 level due to unclear risk of bias in the domains of allocation concealment, and blinding.
Downgraded 1 level due to imprecision.
BMI = body mass index, HOMA-IR = homeostatic model assessment of insulin resistance, MFR = menstrual frequency, TT = total testosterone.
4. Discussion
The present systematic review and meta-analysis identified 7 RCTs, including 464 overweight/obese women with PCOS that evaluated the impact of GLP-1 RA or GLP-1 RA-metformin combination on anthropometric, endocrine, or metabolic outcomes. In comparison with metformin, the treatment of GLP-1 RA is associated with lower BMI, HOMA-IR, and AG. Moreover, subgroup analysis for primary outcomes suggested that the combination of drug treatment and lifestyle intervention is beneficial for the improvement of BMI and HOMA-IR. Other outcomes, including MFR, TT, DHEAS, androstenedione, FAI, fasting blood glucose, fasting insulin, hsCRP, total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol, did not differ significantly between GLP-1 RA and metformin treatment. Studies on GLP-1 RA combined with metformin compared with GLP-1 RA alone exhibited similar effects on all outcomes. Mild or moderate GI discomfort was found to be the most frequent adverse event. Moreover, the meta-analysis of gastrointestinal discomforts showed no significant difference between GLP-1 RA and metformin therapies, and between the combination therapy and GLP-1 RA alone.
It is well known that the pathogenesis of PCOS has not yet been elucidated, and the current treatment methods are mainly based on symptoms and experience. Although obesity and IR are not the diagnostic criteria of PCOS, they are common symptoms in PCOS patients and associated with reproductive (infertility, miscarriage, and pregnancy complications) and metabolic (prediabetes, type 2 diabetes mellitus, metabolic syndrome, cardiometabolic) abnormalities.[25–27] Therefore, lifestyle modification and insulin sensitizer are recommended for PCOS patients in all guidelines.[28–30] Metformin is the most commonly used insulin sensitizer in the treatment of PCOS. It increases insulin sensitivity by decreasing gluconeogenesis, lipogenesis, and enhancing glucose uptake in the liver, skeletal muscle, adipose tissue, and ovaries.[31,32] Moreover, metformin inhibits ovarian androgen production and improves the reproductive outcomes of PCOS, which may also be associated with weight loss.[33] However, its therapeutic effects are not satisfactory. A new approach can reduce body weight and improve IR at the same time seems to be more appealing.
Comparisons of single and combined treatment with GLP-1 RA and metformin in PCOS have attracted the attention of researchers since Elkind-Hirsch et al first conducted a 3-arm RCT to compare the combination of exenatide and metformin with the treatment using exenatide or metformin alone in overweight women with PCOS.[16] Two-arm RCTs comparing between GLP-1 RA and metformin were also conducted later by other researchers. The present meta-analysis suggests that GLP-1 RA exhibits more benefits for reducing weight and improving IR compared with metformin, as well as reducing AG, which is often used as an indicator of the abdominal distribution of body fat. Abdominal adiposity contributes more to endocrine, metabolic, and reproductive dysfunctions than subcutaneous fat, and are more common in patients with PCOS. Accordingly, 2 studies have reported the effects of GLP-1 RA on reproductive function in patients with PCOS. Liu et al[20] found that exenatide may contribute to increasing natural pregnancy rates in overweight/obese women with PCOS after 12 weeks of intervention. Salamun et al[22] reported that low-dose liraglutide added to metformin was superior to metformin alone in increasing the in vitro fertilization pregnancy rate in infertile obese women with PCOS after 12 weeks of intervention. More researches are needed to elucidate the precise effect of GLP-1RA on female reproduction.
Hyperandrogenism is a critical characteristic of PCOS and involved in the pathogenesis of PCOS. A vicious circle is formed by hyperandrogenism, obesity, and IR. Excessive androgen in patients with PCOS promotes fat deposition in the abdomen. At the same time, obesity further facilitates androgen secretion by the ovaries and adrenals directly by local cytokines and oxidative stress or indirectly caused by IR and compensatory hyperinsulinemia.[34] It has been reported that GLP-1 RA can improve hyperandrogenism in PCOS women,[13] which may be related to weight loss and improvement of IR. However, the present work has indicated that no significant differences were found in TT, DHEAS, AD, sex hormone-binding globulin, and FAI between GLP-1 RA and metformin therapy. Weight loss can help PCOS women resume spontaneous menstruation, but GLP-1 RA was not superior to metformin for improving MFR. The mechanism of androgen reduction by GLP-1 receptor agonist still needs to be further explored.
Both GLP-1 RA and metformin therapy can reduce weight and improve IR in patients with PCOS. Still, the present meta-analysis has demonstrated that the combination of metformin and GLP-1 RA did not make women with obesity and PCOS obtain more therapeutic benefits compared with GLP-1 RA alone. Although there is no high heterogeneity in studies, only 3 RCTs were included in this meta-analysis. We found that patients who participated in the study of Elkind-Hirsch et al used short-acting exenatide for 24 weeks,[16] whereas patients participated in 2 studies of Jensterle et al used long-acting liraglutide for 12 weeks.[17,24] Elkind-Hirsch et al reported no significant differences were found between exenatide plus metformin and single exenatide in terms of BMI, TT, HOMA-IR, and so on.[16] However, Jensterle et al reported liraglutide plus metformin was superior to single liraglutide in reducing weight and androstenedione.[24] Due to the limited number of studies, subgroup analysis was not performed in this work.
In addition to the comparison of the efficacy of different drugs, the other key consideration in whether to recommend GLP-1 RA or metformin or combination in the treatment of overweight/obese PCOS patients is the safety of drugs. Similar to metformin, the most common side effect of GLP-1 RA is mild to moderate GI discomfort, which can be reduced by gradual dose titration. Our present study suggests that there is no significant difference between GLP-1 RA and metformin therapies, and between the combination therapy and GLP-1 RA alone. The adverse events mainly occur in the first few weeks of drug treatment. Therefore, single GLP-1 RA treatment or a combination of GLP-1 RA and metformin can be considered as a safe approach as the treatment of PCOS.
A similar meta-analysis has been conducted before our study.[35] The meta-analysis by Han included 375 patients and 8 RCTs, 5 were English literatures, 3 were Chinese literatures, all comparing GLP-1 RA with metformin, and reported that the treatment of GLP-1 RA was more effective in improving
IR and reducing BMI, and AG, however, GLP-1 RA was associated with a higher incidence of nausea and headache than metformin. Our present study included 6 RCTs published in English comparing GLP-1 RA with metformin, and we found that GI discomfort showed no significant difference between GLP-1 RA and metformin therapies.
The strengths of this systematic review and meta-analysis are the novelty of the clinically relevant comparison of GLP-1 RA with metformin, and comparison of GLP-1 RA-metformin combination with single GLP-1 RA, which provide a comprehensive evaluation of GLP-1 RA in PCOS treatment. We searched for publications as completely as possible. However, only articles published in English were eligible, which might have led to selective bias. Due to a limited number of identified RCTs, publication bias assessment and subgroup analysis were not conducted, most of which involved small sample sizes. The study duration was short-term (mostly 12 weeks) and lack of the evaluation of cost-effectiveness. Different regions, diagnostic criteria of PCOS, and GLP-1 receptor agonist types were also observed in these studies. Additionally, HOMA-IR, not the hyperinsulinemic-euglycemic clamp technique, was used as a monitoring indicator of IR in all included studies.
5. Conclusions
In this systematic review and meta-analysis, we report that GLP-1 receptor agonists or GLP-1 receptor agonist-metformin combination appear to offer more benefits in weight loss and IR improvement compared with metformin for women with obesity and PCOS. Considering the current evidence of efficacy and safety, GLP-1 receptor agonist may be a novel and effective medicine for the treatment of overweight/obese patients with PCOS. However, given the limitations of published studies, further investigation based on a larger scale, consolidated baseline BMI range, and PCOS phenotypes, long-term RCTs, comparison of single and combined treatment with GLP-1 receptor agonists and metformin, as well as the cost-effectiveness analysis is required to guide GLP-1 receptor agonists better as the treatment of PCOS.
Acknowledgments
The authors thank their collaborators for their contribution to this study.
Author contributions
Conceptualization: Ruilin Ma, Aijun Sun.
Data curation: Ruilin Ma, Xuesong Ding, Yanfang Wang.
Formal analysis: Aijun Sun.
Methodology: Xuesong Ding, Aijun Sun.
Software: Ruilin Ma, Xuesong Ding, Yanfang Wang, Yan Deng.
Writing – original draft: Ruilin Ma, Xuesong Ding, Yanfang Wang.
Writing – review & editing: Ruilin Ma, Xuesong Ding, Yan Deng, Aijun Sun.
Supplementary Material
Footnotes
Abbreviations: AG = abdominal girth, BMI = body mass index, DHEAS = dehydroepiandrosterone sulphate, FAI = free androgen index, GLP-1 RA = glucagon-like peptide-1 receptor agonist, GLP-1 receptor agonist = glucagon-like peptide-1 receptor agonist, HOMA-IR = homeostatic model assessment of insulin resistance, hsCRP = high-sensitivity C-reactive protein, IR = insulin resistance, LDL-c = low-density lipoprotein cholesterol, MD = mean differences, MFR = menstrual frequency, PCOS = polycystic ovary syndrome, RCT = randomized controlled trial, SMD = standard mean differences, TT = total testosterone.
How to cite this article: Ma R, Ding X, Wang Y, Deng Y, Sun A. The therapeutic effects of glucagon-like peptide-1 receptor agonists and metformin on polycystic ovary syndrome: a protocol for systematic review and meta-analysis. Medicine. 2021;100:23(e26295).
Ethics approval and consent to participate: Not applicable.
The authors report no conflicts of interest.
Funding: Funding information is not available.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Meier RK. Polycystic ovary syndrome. Nurs Clin North Am 2018;53:407–20. [DOI] [PubMed] [Google Scholar]
- [2].Barber TM, Hanson P, Weickert MO, et al. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health 2019;13:1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update 2015;21:560–74. [DOI] [PubMed] [Google Scholar]
- [4].Lim SS, Hutchison SK, Van Ryswyk E, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3:Cd007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998;13:1502–5. [DOI] [PubMed] [Google Scholar]
- [6].Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism 2019;92:108–20. [DOI] [PubMed] [Google Scholar]
- [7].Dumitrescu R, Mehedintu C, Briceag I, et al. Metformin-clinical pharmacology in PCOs. J Med Life 2015;8:187–92. [PMC free article] [PubMed] [Google Scholar]
- [8].Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ (Clinical research ed ) 2003;327:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sfairopoulos D, Liatis S, Tigas S, et al. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones (Athens) 2018;17:333–50. [DOI] [PubMed] [Google Scholar]
- [10].Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19:524–36. [DOI] [PubMed] [Google Scholar]
- [11].Vilsbøll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed ) 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nylander M, Frossing S, Kistorp C, et al. Liraglutide in polycystic ovary syndrome: a randomized trial, investigating effects on thrombogenic potential. Endocr Connect 2017;6:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frossing S, Nylander M, Chabanova E, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metab 2018;20:215–8. [DOI] [PubMed] [Google Scholar]
- [14].Moreno JL, Willett KC, Desilets AR. Exenatide as a novel weight loss modality in patients without diabetes. Ann Pharmacother 2012;46:1700–6. [DOI] [PubMed] [Google Scholar]
- [15].Frøssing S, Nylander M, Kistorp C, et al. Effect of liraglutide on atrial natriuretic peptide, adrenomedullin, and copeptin in PCOS. Endocr Connect 2018;7:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elkind-Hirsch K, Marrioneaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:2670–8. [DOI] [PubMed] [Google Scholar]
- [17].Jensterle Sever M, Kocjan T, Pfeifer M, et al. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 2014;170:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jensterle M, Kravos NA, Pfeifer M, et al. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones (Athens) 2015;14:81–90. [DOI] [PubMed] [Google Scholar]
- [19].Jensterle M, Salamun V, Kocjan T, et al. Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: a pilot randomized study. J Ovarian Res 2015;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu X, Zhang Y, Zheng SY, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017;87:767–74. [DOI] [PubMed] [Google Scholar]
- [21].Zheng S, Zhang Y, Long T, et al. Short term monotherapy with exenatide is superior to metformin in weight loss, improving insulin resistance and inflammation in Chinese overweight/obese PCOS women. Obes Med 2017;7:15–20. [Google Scholar]
- [22].Salamun V, Jensterle M, Janez A, et al. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol 2018;179:01–11. [DOI] [PubMed] [Google Scholar]
- [23].Higgins J. Cochrane handbook for systematic reviews of interventions version 5.1. 0 (Updated on 2011). Cochrane Collab. 2015. Available at: http://www.cochrane-handbook.org. Accessed January, 2015. [Google Scholar]
- [24].Jensterle M, Goricar K, Janez A. Metformin as an initial adjunct to low-dose liraglutide enhances the weight-decreasing potential of liraglutide in obese polycystic ovary syndrome: Randomized control study. Exp Ther Med 2016;11:1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction (Cambridge, England) 2010;140:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lainez NM, Coss D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019;160:2719–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ormazabal V, Nair S, Elfeky O, et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol 2018;131:e157–71. [DOI] [PubMed] [Google Scholar]
- [29].Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chinese guideline for diagnosis and management of polycystic ovary syndrome. Chin J Obstet Gynecol 2018;53:02–6. [DOI] [PubMed] [Google Scholar]
- [31].Jenkins AJ, Welsh P, Petrie JR. Metformin, lipids and atherosclerosis prevention. Curr Opin Lipidol 2018;29:346–53. [DOI] [PubMed] [Google Scholar]
- [32].Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs 2015;75:1071–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007;356:551–66. [DOI] [PubMed] [Google Scholar]
- [34].Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 2007;18:266–72. [DOI] [PubMed] [Google Scholar]
- [35].Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online 2019;39:332–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


