Abstract
SETTING:
Rural Eastern Cape, South Africa.
OBJECTIVE:
To identify steps in the TB preventive care cascade from routinely collected data among TB patients at a district hospital prior to the implementation of a novel TB program.
DESIGN:
This was a retrospective study. We adapted the TB prevention cascade to measure indicators routinely collected at district hospitals for TB using a cascade framework to evaluate outcomes in the cohort of close contacts.
RESULTS:
A total of 1,722 charts of TB patients were reviewed. The majority of patients (87%) were newly diagnosed with no previous episodes of TB. A total of 1,548 (90%) patients identified at least one close contact. A total of 7,548 contacts were identified with a median of 4.9 (range 1–16) contacts per patient. Among all contacts identified, 2,913 (39%) were screened for TB. Only 15 (0.5%) started TB preventive therapy and 122 (4.4%) started TB treatment. Nearly 25% of all medical history and clinical information was left unanswered among the 1,722 TB charts reviewed.
CONCLUSION:
Few close contacts were screened or started on TB preventive therapy in this cohort. Primary care providers for TB care in district health facilities should be informed of best practices for screening and treating TB infection and disease.
Keywords: post-exposure, latent tuberculosis, infection treatment, close contacts
Abstract
CONTEXTE :
Le Cap Est rural, le Cap, Afrique du Sud.
OBJECTIF :
Identifier les étapes de la cascade de soins préventifs de la TB à partir des données de routine recueillies parmi des patients dans un hôpital de district avant la mise en œuvre d’un nouveau programme TB.
SCHÉMA :
Ceci était une étude rétrospective. Nous avons adapté la cascade de prévention de la TB pour mesurer les indicateurs recueillis en routine dans les hôpitaux de district pour la TB en utilisant un cadre en cascade afin d’évaluer les résultats dans la cohorte des contacts étroits.
RÉSULTATS :
Un total de 1 722 dossiers de patients TB a été revu. La majorité des patients (87%) avait un diagnostic nouveau sans épisode de TB préalable. Un total de 1 548 (90%) patients ont identifié au moins un contact étroit ; 7 548 contacts ont été identifiés avec une médiane de 4,9 (fourchette 1–16) contacts par patient. Parmi tous les contacts identifiés, 2 913 (39%) ont eu une recherche de TB. Seulement 15 (0,5%) ont initié le traitement préventif et 122 (4,4%) ont mis en route le traitement de TB. Près de 25% de tous les antécédents et autres informations cliniques n’était pas remplis dans les 1 722 dossiers TB revus.
CONCLUSION :
Peu de contacts étroits ont été dépistés ou mis sous traitement préventif de TB dans cette cohorte. Les prestataires de soins de santé primaires pour la TB dans les structures de santé des districts doivent être informés des meilleures pratiques pour le dépistage et le traitement de la TB infection et maladie.
Although curable and preventable, TB remains the leading infectious killer of adults in the world.1 Globally, one billion people are estimated to be infected with Mycobacterium tuberculosis, and 5–15% are at risk of becoming ill with TB disease.1 TB is characterized by an often sub-clinical, asymptomatic, non-infectious phase (TB infection), followed by progression to a symptomatic and infectious clinical disease phase (TB disease).2 TB preventive therapy (TPT) is a highly effective method to prevent progression from TB infection to TB disease: for example, a 6-month course of daily isoniazid (INH; a component drug of TPT) reduces TB disease risk by 60–90%.3–9 Targeting TPT to high-risk groups is a crucial strategy to control the TB epidemic, as it is the only way to halt the progression of TB infection to TB disease and reduce the reservoir of potential cases of TB disease.2,10 People living with HIV (PLWH) and household contacts of patients with TB are high-risk groups who should receive TPT after symptom screening and ruling out TB disease.11 While TPT use in PLWH has expanded globally, TPT in household contacts is inadequately deployed in most high TB burden countries, despite being indicated in most national guidelines.
Care cascades are useful frameworks for diseases when people go through multiple interventions or steps of care from initial diagnosis to achieving a desired outcome. HIV care cascades have frequently been used in research, and are now routinely used to describe target outcomes, such as the 90-90-90 target set by UNAIDS.12 Care cascades have enhanced the monitoring and evaluation of programs to help identify where best to target interventions to improve screening, diagnosis, treatment initiation, and treatment management (or in the case of HIV, viral suppression). Due to improved HIV care in the past decade, UNAIDS has set higher targets of 95-95-95, i.e., 95% of PLWH should know their status; 95% of people should know their status on treatment; and 95% of people with suppressed viral loads should be treatment.12 Care cascades have recently been incorporated into TB care to identify gaps and areas for improvement.13–18
The Zero TB Initiative (ZTBI) is a global coalition of policymakers, clinicians, municipalities and local governments, and civil society groups committed to TB elimination using a comprehensive set of interventions.19 The ZTBI has proposed three TB care cascades under the moniker: “Search-Treat-Prevent” for programs to monitor and evaluate important process indicators among certain risk groups.19 The ZTBI prevention cascade has been implemented in Peru, where accompaniment of household contacts significantly improved the evaluation of contacts and increased TPT use.15 In Pakistan, 800 contacts of drug-resistant patients were enrolled in a TPT program, where 92% were screened, 80% of those eligible for TPT initiated TPT, and 70% of those completed treatment.20 Thus, when providing TPT as part of a program, high rates of screening, initiation, and completion of treatment can be obtained.
The prevention cascade involves six steps (Figure 1). First, the target population must be established in order to calculate the number of evaluations completed among the target population (Step 1). Then, to determine the TB disease diagnosis, one can calculate the number of individuals with TB disease divided by the number with complete TB evaluation (Step 2). Step 3 evaluates the prescription – the number prescribed TPT over the number eligible for preventive therapy. Step 4 measures the uptake of preventive therapy, and Step 5 evaluates the completion of preventive therapy. Finally, Step 6 evaluates TB-free survival 1 year later of those deemed not to have TB disease at initial evaluation.
FIGURE 1.
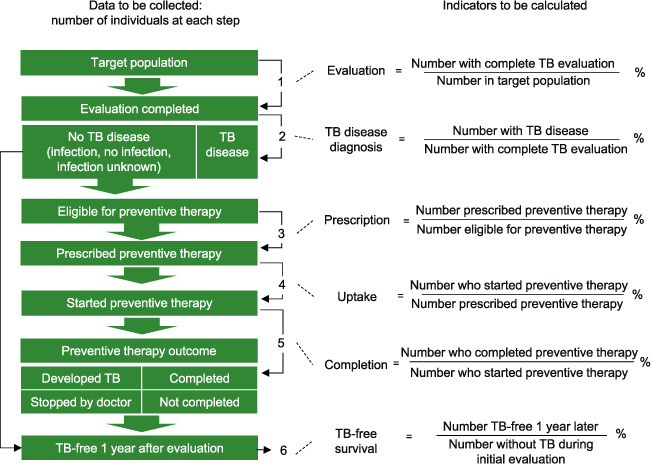
Zero TB Initiative: A best-practice framework of program indicators for monitoring a comprehensive approach to the TB epidemic, 2017. TPT = TB preventive therapy.
Our objective was to identify steps of the TB preventive care cascade, which could be calculated, from routinely collected data among TB patients at a rural hospital in Eastern Cape, South Africa, prior to the implementation of a novel TB program. A secondary aim was to identify the amount of missing data. Enumerating the losses at each step of the care cascade will allow us to direct appropriate interventions as part of a novel TB program, specifically looking to close the gap in losses among household contacts in the TB prevention cascade.
METHODS
Design
This is a retrospective study of a cohort of close contacts using index patient medical history. All available charts for index patients initiating drug-susceptible TB (DS-TB) treatment at the hospital between January 1, 2017 and March 2, 2019 were included. We have adapted the prevention cascade to measure indicators routinely collected at district hospitals for DS-TB. We applied the ZTBI process indicator framework to examine outcomes in the cohort of close contacts, calculating the percentage of contacts who completed each step out of those eligible to complete it.
Population
The southern part of the King Sabata Dalindyebo (KSD) sub-district of the OR Tambo District of the Eastern Cape Province of South Africa is served by a single district hospital. This is a rural government facility that serves as a referral center for 14 primary care clinics and two community health centers. The hospital provides basic hospital and primary health care (PHC) services to a catchment population of 125,000 people and 20,000 households spread over an area of 1200 km2 in the southern part of the KSD sub-district.21
The sub-district is underdeveloped and poor, with an average annual household income of 14 600 South African rands (US$960), a 78% unemployment rate, and access to running water for less than 50% of the population.21 Accessing health clinics is difficult for many, who are limited by topography, poor road infrastructure and natural barriers such as rivers, especially during the rainy season.
The KSD sub-district has a high burden of TB and HIV disease. Based on the nearly 1,050 patients initiated on TB treatment in the Zithulele catchment population in 2017, a conservative estimate of the TB disease incidence rate is approximately 840/100,000. Just under half (49%) of patients diagnosed with TB were HIV-positive, and 30% had extra-pulmonary TB.
Sources of data
Between March 2018 and December 2019, trained study personnel, supervised by the Principal Investigator (BvdW), abstracted information from the medical charts of the index patients at the hospital. Data were entered into an electronic REDCap database (Vanderbilt University, Nashville, TN, USA) to ensure accuracy of data using automatic data checks and value limits within certain variables, and radio buttons to prevent errors.22 Information regarding close contacts included whether any contacts were identified, if any contacts were symptomatically screened, if any started INH preventive therapy (IPT), and if any started TB treatment. A National Department of Health four-symptom screen was employed to screen household contacts for symptoms of TB and includes questions regarding cough for more than 2 weeks, fever, drenching night sweats, and unexplained weight loss.23 At the time of analysis, after screening negative, all children under the age of 5 years and all PLWH were eligible for TPT.23
Data analysis
SAS v9.4 (SAS Institute, Cary, NC, USA) and Excel (Microsoft, Redmond, WA, USA) were used for data analysis. Descriptive statistics were used to summarize index patient demographic and clinical characteristics. The primary analysis assessed the TB prevention cascade. We calculated proportions to summarize each step of the cascade when data were available.
Ethics
Approval was obtained from Harvard Medical School, Boston, MA, USA, as well as Walter Sisulu University, Mthatha, South Africa. Eastern Cape Department of Health (Bhisho, South Africa) also gave permission for the chart review to take place.
RESULTS
A total of 1,722 charts of DS-TB patients were reviewed. The mean age of index patients was 34.5 years, with half being female. Most patients (1,498/1,722, 87%) were newly diagnosed with no previous episodes of TB. The majority (65%) of patients had pulmonary TB, 31% had extrapulmonary TB and 4% had both pulmonary and extrapulmonary TB, including lymphatic, abdominal, pleural, and TB of the spine.
Of 1,722 index patients, 1,548 (90%) had identified at least one close contact in their medical chart (Figure 2). A total of 7,548 contacts were identified, with a median of 4.9 (interquartile range 3–6) contacts per index patient. Among all contacts identified, 2,913/7,548 (39%) were screened for TB. Of 1,722 index patients, 603 (35%) reported at least one contact screened. Fifteen out of 2,913 (0.5%) contacts started TB preventive therapy and 122/2,791 (4.4%) started TB treatment. No data were available regarding timing of screening nor dates for when TPT or TB treatment was initiated in the contacts.
FIGURE 2.
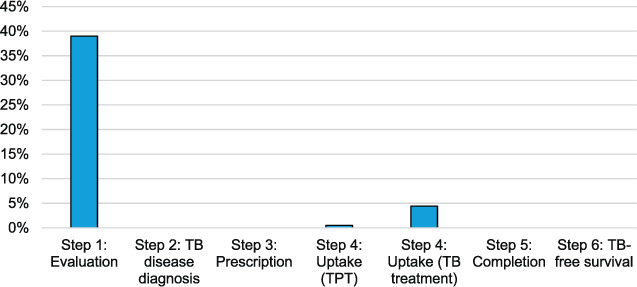
District hospital TB preventive care cascade.
DISCUSSION
Using the framework of the ZTBI preventive care cascade, only 39% of household contacts were screened, and less than 1% started TPT. An additional 4.4% started TB disease treatment. A major challenge was that many variables collected had missing data in the medical charts, pointing to the need for better documentation for TB close contacts. Not only was documentation sparse, but many steps of the ZTBI preventive care cascade could not be calculated, as the charts did not have space or relevant questions to record this vital information.
Similar to the losses in the treatment cascade in South Africa reported by Naidoo et al.,13 we found big losses at each step in the prevention cascade. There was a large drop in the number of contacts identified to the number of contacts screened — an area for significant improvement. Many contacts who are identified are not screened, likely due to difficulties in patients bringing family members to the clinic or hospital for screening, or for hospital and clinic staff accessing transportation to get into the community.24,25 Compared with results from two other preventive care cascades (in drug-resistant cohorts), we found similarly large losses.26,27 The biggest drop was in screening for TPT uptake; however, it is worth noting that at the time these data were collected, only children under 5 years and PLWH were eligible for IPT. Thus, not everyone screened was eligible for IPT as per South African government guidelines.28 We were unable to determine an accurate denominator for those eligible for IPT, as the ages of contacts were often not listed, and the HIV status of contacts was not documented.
Multiple studies, including meta-analyses, have found that TPT is an underutilized strategy for controlling TB in high-burden countries.29 Scaling up TPT is part of the United Nations High-Level Meeting on TB priorities, and high-burden countries must interrupt transmission through a multipronged approach, including TPT use among household contacts.30 TPT has been shown to be very effective among PLWH, two meta-analyses have shown greater than a 60% reduction in TB among HIV-infected adults after IPT.31 Additionally, TB control should also focus on reducing ongoing transmission, and expansion of IPT should include efficient TB screening and monitoring (such as monitoring for INH resistance). Therefore, collecting the necessary data to monitor steps in the TB prevention care cascade is essential to improving quality of care for patients and contacts, as without this, it is difficult to measure improvements in care or gather baseline information.17 Initiating registers or individual charts for close contacts could alleviate some of the missing data and methodological issues we encountered.13
Limitations
This study is not without limitations. First, little in the index patient’s chart provides information about household contacts. Additionally, of the information collected about household contacts, little pertains to the TB preventive care cascade. Our objective, however, was to conduct a baseline assessment of the ZTBI preventive TB care cascade to inform us on what key variables should be collected as part of a strengthened, comprehensive TB program. Per discussions with hospital physicians, 39% of contacts screened was surprisingly high, as they rarely see close contacts for screening except in cases of drug-resistant TB. These physicians expect many of the close contacts deemed ‘screened’ are likely to be based on reports by index patients of close contacts who are either asymptomatic or who have cough, fever, night sweats, or unexplained weight loss. Thus, a true in-person screening is likely even less than the 39% reported in the reviewed charts.
CONCLUSION
In our study, few household contacts were screened or started on TPT. However, conducting a baseline assessment prior to an intervention can provide clarity both to planning what variables researchers should collect in order to reduce missing data, and where to emphasize program investment and planning to maximize impact and reach.
ACKNOWLEDGEMENTS
The authors thank B Speelman and K Radjabova for their assistance in data entry.
References
- 1.World Health Organization Geneva, Switzerland: WHO, 2018. Global tuberculosis report, 2018; p. 243. [Google Scholar]
- 2.Rangaka MX, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet. 2015;386(10010):2344–2353. doi: 10.1016/S0140-6736(15)00323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox GJ, et al. Preventive therapy for latent tuberculosis infection-the promise and the challenges. Int J Infect Dis. 2017;56:68–76. doi: 10.1016/j.ijid.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Stagg HR, et al. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161(6):419–428. doi: 10.7326/M14-1019. [DOI] [PubMed] [Google Scholar]
- 5.Akolo C, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smieja MJ, et al. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;(2):CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danel C, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 8.Ayieko J, et al. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta–analysis. BMC Infect Dis. 2014;14(1):91. doi: 10.1186/1471-2334-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golub JE, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21(11):1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 11.Fox GJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS 90-90-90 treatment for all. Geneva, Switzerland: UNAIDS; 2020. https://www.unaids.org/en/resources/909090 Accessed May 2020. [Google Scholar]
- 13.Naidoo P, et al. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(Suppl_7):S702–S713. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbaraman R, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019;16(2):e1002754. doi: 10.1371/journal.pmed.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen CM, et al. Tuberculosis household accompaniment to improve the contact management cascade: a prospective cohort study. PLoS One. 2019;14(5):e0217104. doi: 10.1371/journal.pone.0217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsdurf H, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 17.Cazabon D, et al. Quality of tuberculosis care in high burden countries: the urgent need to address gaps in the care cascade. Int J Infect Dis. 2017;56:111–116. doi: 10.1016/j.ijid.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaraman R, et al. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLoS Med. 2016;13(10):e1002149. doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zero TB Initiative A best-practice framework of program indicators for monitoring a comprehensive approach to the tuberculosis epidemic. Boston, MA, USA: ZTBI; 2017. https://www.zerotbinitiative.org/resources-for-coalitions Accessed November 2019. [Google Scholar]
- 20.Malik AA, et al. Tuberculosis preventive therapy for individuals exposed to drug-resistant tuberculosis: feasibility and safety of a community-based delivery of fluoroquinolone-containing preventive regimen. Clin Infect Dis. 2020;70(9):1958–1965. doi: 10.1093/cid/ciz502. [DOI] [PubMed] [Google Scholar]
- 21.Wazimap District-level household information. Johannesburg, South Africa: Wazimap; 2018. https://wazimap.co.za/profiles/ward-21507027-king-sabata-dalindyebo-ward-27-21507027/ Accessed April 2020. [Google Scholar]
- 22.Harris PA, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South Africa Department of Health In: Tuberculosis guidelines. Pretoria, South Africa: SA DoH; 2019. Treatment for latent TB infection in South Africa; p. 55. [Google Scholar]
- 24.Sullivan BJ, Esmaili BE, Cunningham CK. Barriers to initiating tuberculosis treatment in sub-Saharan Africa: a systematic review focused on children and youth. Glob Health Action. 2017;10(1):1290317. doi: 10.1080/16549716.2017.1290317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou G, et al. Barriers to hospital and tuberculosis programme collaboration in China: context matters. Glob Health Action. 2015;8:1–9. doi: 10.3402/gha.v8.27067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black F, Amien F, Shea J. An assessment of the isoniazid preventive therapy programme for children in a busy primary healthcare clinic in Nelson Mandela Bay Health District, Eastern Cape Province, South Africa. S Afr Med J. 2018;108(3):217–223. doi: 10.7196/SAMJ.2018.v108i3.12639. [DOI] [PubMed] [Google Scholar]
- 27.van de Water B et al. Prevention care cascade in persons exposed to drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2020;24(12):1305–1306. doi: 10.5588/ijtld.20.0296. [DOI] [PubMed] [Google Scholar]
- 28.South African Department of Health Pretoria, South Africa: SA DoH, 2014. National Tuberculosis Management Guidelines 2014; p. 120. [Google Scholar]
- 29.Churchyard GJ, et al. Tuberculosis preventive therapy: an underutilised strategy to reduce individual risk of TB and contribute to TB control. S Afr Med J. 2014;104(5):339–343. doi: 10.7196/samj.8290. [DOI] [PubMed] [Google Scholar]
- 30.Churchyard GJ, Swindells S. Controlling latent TB tuberculosis infection in high-burden countries: a neglected strategy to end TB. PLOS Med. 2019;16(4):e1002787. doi: 10.1371/journal.pmed.1002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood R, Bekker LG. Isoniazid preventive therapy for tuberculosis in South Africa: an assessment of the local evidence base. S Afr Med J. 2014;104(3):174–177. doi: 10.7196/samj.7968. [DOI] [PubMed] [Google Scholar]


