Abstract
OBJECTIVE:
To evaluate care cascades for programmatic active case finding and latent TB infection (LTBI) management in young child TB contacts (aged <5 years) in Victoria, Australia.
DESIGN:
This was a retrospective review of public health surveillance data to identify contacts of all pulmonary TB cases notified from 2016 to 2019.
RESULTS:
Contact tracing identified 574 young child contacts of 251 pulmonary TB cases. Active TB was found in 28 (4.9%) contacts, none of whom had previously received bacille Calmette-Guérin vaccination, and 529 were tested for TB infection using the tuberculin skin test (TST). The overall TST positivity was 15.3% (95% CI 0.1–0.2). Among the 574 children, 150 (26.1%) were close contacts of sputum smear-positive cases and 25 (16.7%) of these were not referred to TB clinics. Of the 125 referred, 81 were considered to have LTBI, 79 agreed to commence TB preventive treatment (TPT) and 71 (89.9%) completed TPT. Following completion of TPT, no child was subsequently diagnosed with active TB.
CONCLUSION:
There was a high yield from active case finding and uptake of TPT. Notable losses in the cascade of care occurred around referral to tertiary clinics, but high treatment completion rates and good outcomes were found in those prescribed treatment.
Keywords: contact tracing, child, tuberculosis, preventive therapy, active case finding
Abstract
OBJECTIF :
Evaluer les cascades de soins pour la recherche active programmatique des cas et la prise en charge de l’infection tuberculeuse latente (ITL) chez les contacts de jeunes patients atteints de TB âgés de <5 ans dans l’état de Victoria, Australie.
SCHÉMA :
Revue rétrospective de données de surveillance de santé publique afin d’identifier les contacts de tous les cas de TB pulmonaire notifiés de 2016 à 2019.
RÉSULTATS :
Le traçage des contacts a identifié 574 jeunes enfants tous contacts de 251 cas de TB pulmonaire. Une TB active a été trouvée chez 28 enfants contacts (4,9%), aucun n’ayant reçu le vaccin bacille Calmette-Guérin, et 529 ont été testés à la recherche d’infection TB avec un test cutané à la tuberculine (TCT). La positivité d’ensemble du TCT a été de 15,3% (IC 95% 0,1–0,2). Parmi les 574 enfants, 150 (26,1%) étaient des contacts étroits de cas à frottis de crachats positifs et 25 (16,7%) d’entre eux n’ont pas été référés dans des structures TB. Sur les 125 enfants référés, 81 ont été considérés comme ayant une ITL, 79 ont accepté de débuter un traitement préventif (TPT) et 71 (89,9%) l’ont terminé. Aucun enfant n’a ensuite eu de diagnostic de TB active.
CONCLUSION :
La recherche active de cas et la couverture TPT ont eu un rendement élevé. Des pertes notables dans la cascade de soins sont survenues lors de référence aux structures tertiaires, mais ceux qui ont reçu un traitement ont eu un taux élevé d’achèvement et de bons résultats.
Young children aged <5 years are recognised to be at high risk for acquisition of TB and progression to active disease following close contact in both high and low TB endemic settings.1–4 The consequences of active TB in children are substantial, with higher risk of severe and disseminated forms of disease than in adults, and more rapid progression to disease after infection.4 For young children with evidence of infection only at the time of screening, TB preventive treatment (TPT) is highly protective against the development of active TB.4 Contact tracing of young children after exposure to TB is therefore a global priority, both for early identification of active disease and for intervention with TPT to avoid progression.5
Despite these acknowledged needs and widely adopted policies, contact tracing remains under-prioritised in many settings, even among the highest-priority groups such as young children.6 Where programmatic contact tracing is implemented, there is also a need for stronger monitoring and evaluation in order to identify gaps in service delivery and opportunities for improving outcomes.5,7
The state of Victoria, Australia, is a low-incidence, low-transmission setting, with high case notification and centralised programmatic management of TB, including contact tracing.3,8 Young children in Victoria represented 35.3% of all TB cases among children ( <15 years) in Australia in 2013.9 The purpose of this analysis was to review TB contact tracing activity and outcomes among young child contacts, and evaluate the cascades of care for active case finding and provision of TPT.
METHODS
The Victorian Tuberculosis Program (VTP) manages TB in the entire state of Victoria in Australia. Data for all notified active TB cases, TB contacts and people identified with latent TB infection (LTBI) are collected by the VTP and stored electronically in the Public Health Events Surveillance System. The notification of active TB cases by both clinicians and laboratories is mandatory in Victoria.
We extracted data for children aged <5 years who were the contacts of active TB cases notified from 1 January 2016 to 31 December 2019 from the Public Health Electronic Surveillance System. Data included date of birth, sex, country of birth, type of contact (close contact or other), bacille Calmette-Guerin (BCG) immunisation status, tuberculin skin test (TST) or interferon-gamma release assay (IGRA) results, treatment of LTBI and treatment outcome. LTBI was defined as evidence of TB infection on either the TST or an IGRA, and no evidence of active disease.
During the study period, the VTP nurses identified and traced contacts of active TB cases, assessed them for symptoms of active TB and tested them for TB infection, usually by performing TST in young child contacts. Where possible, close contacts were tested for TB infection within days of being identified (baseline) and again at 8–12 weeks after last infectious contact (break of contact) if they were negative. Casual contacts were tested at the break of contact. Close contact was defined as children who have had frequent, prolonged and close contact in an enclosed environment with an infectious case, such as all people living in the same dwelling; relatives and friends who have frequent, prolonged and close contact; and childcare colleagues who share the same indoor area on a daily basis. Casual contacts were defined as children who had a minimum exposure to an infectious index case (cumulatively 4–8 h) such as being exposed at a party or by a visiting relative or friend.
We defined a positive TST as an induration of ⩾5 mm at 48–72 h; IGRA results were reported according to manufacturer’s guidelines. We defined a TST conversion as any increase of >5 mm diameter induration on a child with an initial TST result of <5 mm. As per VTP guidelines, children with a negative TST or IGRA at break of contact and with no history of BCG were referred to immunisation clinics for consideration of BCG vaccination.
While local and international guidelines emphasise household contacts of people with pulmonary TB, VTP policy has historically expanded to include testing of children at lower epidemiologic risk, such as casual contacts, and contacts of non-transmissible forms of TB.
The testing of this population has been termed ‘opportunistic testing’ and is based on the rationale of possible overlap of risk factors in a vulnerable population. We collated results from opportunistic testing separately to allow evaluation of the yield and outcome from this approach.
According to local policy, all child contacts with a positive TST or IGRA should be referred to specialist paediatric TB clinics for further assessment and consideration of TPT. In addition, local guidelines recommend that all young children ( <5 years of age) who are close contacts of sputum smear-positive pulmonary TB cases are referred, regardless of initial TST result, for clinical review and consideration of primary prophylaxis until break-of-contact TST is completed, following exclusion of active disease. Close contacts with negative TST or IGRA results at baseline testing are commenced on prophylaxis and treatment is stopped when they test negative again at the break of contact. This is known as window TB preventive therapy (window TPT). Nurses assess contacts for a referral to specialist clinics using the TB guidelines, with individualised case discussion with specialist paediatricians.
Children with negative TST or IGRA test who have no evidence of TB disease may be referred for BCG vaccination by the clinicians. The notification of BCG vaccination and LTBI by clinicians and laboratories outside the TB programme is not mandatory in Victoria.
Children commenced on LTBI treatment are supervised by nurses through home visits and phone calls. The children are also reviewed periodically by the physicians at specialist clinics. All contacts of TB are also recorded on the statewide TB Surveillance System used for mandatory notification to ensure that any suspected TB reactivation is captured. All children with suspected TB were assessed by expert paediatric services. Notification of all forms of TB, whether microbiologically confirmed or not, is mandatory for both clinicians and laboratories in Victoria. All notified cases during the study period were included in this analysis. In Victoria, TB diagnostic assessment includes the routine use of culture and genomic testing of appropriate specimens, with routine whole-genome sequencing of culture-positive isolates, accompanied by radiological and clinical examination.
We performed a cascade of care analysis, following contacts from the time of identification through to completion of management for active or latent TB.
Ethics statement
The approval from a Human Research Ethics Committee was not required under the rules of our institutions, as data were collected and used under the legislative authority of the Public Health and Wellbeing Act 2008.
Statistical analysis
Data preparation and statistical analysis were performed using Stata/SE v15.0 (Stata Corp, College Station, TX, USA). Descriptive statistics were used to describe the demographic data (age, sex, country of birth) and clinical characteristics (TST/IGRA results, BCG status, active TB) of the participants. We conducted pre-planned subgroup analysis to compare the yield of TB infection testing in different cohorts of epidemiological significance — contacts of sputum smear-positive cases, contacts of sputum smear-negative cases and children tested opportunistically. TST positivity was calculated using proportions and 95% confidence intervals (CIs). Pearson’s χ2 test was performed to test the association between categorical variables. P values were considered significant at P < 0.05. Univariate and multivariate logistic regression analyses were performed to consider associations with test outcomes. Variables significant at P < 0.1 were included in the multivariable model.
RESULTS
Between 2016 and 2019, we identified 574 children aged <5 years who were contacts of 251 index cases with pulmonary TB. Following clinical assessment, 28 (4.9%) children were diagnosed with active TB, of whom 26 were born in Australia. All 28 children had pulmonary TB (none had cavities) and seven also had extrapulmonary involvement. None of 107 contact children who had previously received BCG had active TB detected. Children aged <2 years had the highest risk of developing active TB following exposure (7.0%), while low risk was observed in those aged older (3.7%). Among 28 children with active disease, 10 (35.7%) were culture-positive – all isolates were fully susceptible – and 18 (64.3%) were culture-negative. Three children were tested for HIV infection and all were negative.
Of the 574 young child contacts, 543 (94.6%) were tested for TB infection: 510 (93.9%) using TST only, 14 (2.6%) using IGRA only and 19 (3.5%) using both TST and IGRA. TST was positive in 81/529 (15.3%) contacts tested. The characteristics of the contacts of pulmonary TB index cases who had TST performed are given in Table 1. We observed a trend towards higher proportion of TST positivity with older age. The proportion of TST positivity was higher in children born in a high TB incidence country than in those born in a low TB incidence country (40.7% vs. 14.1%). TST conversion at “break of contact” occurred in 18/262 (6.9%) contacts who were retested after an initial negative test.
TABLE 1.
TST prevalence among young child TB contacts by characteristics of contact and index case
| Variable | Contacts of pulmonary TB | Contacts of smear-positive TB | Contacts of bacteriologically negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Positive n | Prevalence % (95% CI) | Total | Positive n | Prevalence % (95% CI) | Total | Positive n | Prevalence % (95% CI) | |
| TST results | 529 | 81 | 15.3 (12.5–18.7) | 139 | 43 | 30.9 (23.7–9.2) | 191 | 17 | 8.9 (5.6–13.9) |
| Age, years | |||||||||
| <1 | 82 | 7 | 8.5 (4.1–16.9) | 26 | 7 | 26.9 (13.1–47.3) | 37 | 0 | 0 |
| 1 | 101 | 12 | 11.9 (6.8–19.8) | 25 | 5 | 20.0 (8.4–40.7) | 38 | 3 | 7.9 (2.5–22.2) |
| 2 | 157 | 26 | 16.6 (11.5–23.3) | 34 | 11 | 32.4 (18.6–50.0) | 43 | 6 | 14.0 (6.3–28.1) |
| 3 | 121 | 24 | 19.8 (13.6–28.0) | 35 | 13 | 37.1 (22.7–54.4) | 41 | 5 | 12.2 (5.1–26.5) |
| 4 | 68 | 12 | 17.7(10.3–28.7) | 19 | 7 | 36.8 (18.2–60.5) | 32 | 3 | 9.4 (3.0–25.9) |
| Sex | |||||||||
| Male | 234 | 37 | 15.8 (11.7–21.1) | 69 | 20 | 29.0 (19.4–40.9) | 82 | 7 | 8.5 (4.1–17.0) |
| Female | 249 | 43 | 17.3 (13.1–22.5) | 70 | 23 | 32.9 (22.8–44.8) | 108 | 10 | 9.3 (5.0 -16.5) |
| Unknown | 46 | 1 | 2.2 (0.3–14.2) | — | — | — | 1 | 0 | 0 |
| Country of birth | |||||||||
| High TB incidence* | 27 | 11 | 40.7 (23.9–60.1) | 12 | 9 | 75.0 (43.1–92.2) | 12 | 2 | 16.7 (3.9–49.7) |
| Low TB incidence | 477 | 67 | 14.1(11.2–17.5) | 124 | 33 | 26.6 (19.5–35.2) | 164 | 15 | 9.2 (5.6–14.7) |
| Unknown | 25 | 3 | 12.0 (3.8–31.9) | 3 | 1 | 33.3 (2.5–90.7) | 15 | 0 | 0 |
| Type of contact | |||||||||
| Casual | 65 | 7 | 10.1 (4.9–19.9) | — | — | — | 13 | 1 | 7.7 (1.0–41.4) |
| Close | 456 | 70 | 15.5 (12.4–19.1) | 139 | 43 | 30.9 (23.7–39.2) | 177 | 15 | 8.5 (5.2–13.6) |
| Church | 8 | 4 | 50.0 (18.5–81.5) | — | — | — | 1 | 1 | 100.0 |
| BCG status | |||||||||
| No | 353 | 34 | 9.6 (7.0–13.2) | 99 | 24 | 24.2 (16.7–33.8) | 150 | 5 | 3.3 (1.4–7.8) |
| Yes | 80 | 40 | 50.0 (39.1–60.9) | 22 | 14 | 63.6 (41.7–81.1) | 27 | 11 | 40.7 (23.8–60.2) |
| Unknown | 96 | 7 | 7.3 (3.5–14.6) | 18 | 5 | 27.8 (11.7–52.9) | 14 | 1 | 7.1 (0.9–39.2) |
* WHO-estimated TB incidence rate of ⩾100 cases per 100 000 population per annum.6
TST = tuberculin skin test; CI = confidence interval; BCG = bacille Calmette-Guérin.
During the study period, 150 close contacts of sputum smear-positive index cases were identified. Of these, 139 contacts (92.7%) had TST performed and 43 (30.9%) were positive. Table 1 shows also the close contacts of sputum smear-positive index cases. In this cohort, children born in high TB incidence countries were more likely to test positive than those born in low TB incidence countries (75.0% vs. 26.6%; P < 0.05). TST conversion occurred in 8/82 (9.8%) contacts who were retested (not shown in Table 1).
There were 210 contacts of bacteriological negative/clinically diagnosed index cases (Table 1). A total of 191 children underwent TST and 17 (8.9%) were positive. In this group TST positivity was high among the BCG-vaccinated (40.7%) and among those aged 2 years (14.0%).
A total of 32 children were contacts of other bacteriological positive cases (such as culture, bronchial washings and polymerase chain reaction testing; not shown in Table 1). Of these 32 contacts, 30 underwent TST and 1 (3.3%) had a positive result.
Table 2 shows the results of multiple logistic regression performed to examine factors associated with TST positivity. Country of birth was not included in the multivariate analysis, as it is closely correlated to BCG status. After adjusting for the effect of age, BCG status, and sputum smear status of the index case in the model, an increased odd of a positive TST remained significant among the contacts aged <2 years and contacts of sputum smear-positive cases, while BCG vaccination was protective.
TABLE 2.
Univariate and multivariate analysis of predictors of TST positivity in contacts of pulmonary TB cases
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Male sex (reference group: female) | 1.1 (0.7–1.8) | 0.667 | ||
| Age <2 years (reference group: ⩾2 years) | 1.9 (1.1–3.3) | 0.024 | 1.9 (1.0–3.6) | 0.053 |
| High TB incidence country of birth (reference group: low TB incidence country) | 4.2 (1.9–9.5) | <0.0001 | ||
| BCG-vaccinated (reference group: non-BCG-vaccinated) | 9.4 (5.3–16.5) | <0.0001 | 9.1 (5.0–16.4) | <0.0001 |
| Close contact (reference group: all other contacts) | 1.1 (0.6–2.2) | 0.787 | ||
| Contacts of sputum smear-positive cases (reference group: all other contacts) | 2.9 (1.7–5.1) | <0.0001 | 3.6 (1.9–6.8) | <0.0001 |
TST = tuberculin skin test; OR = odds ratio; CI = confidence interval; BCG = bacille Calmette-Guérin.
All the close contacts of sputum smear-positive cases were eligible for referral, regardless of test result. Casual contacts of sputum smear-positive and contacts of sputum smear-negative cases with evidence of infection were eligible for referral. Among the 150 close contacts of sputum smear-positive cases, 25 (16.7%) were not referred to TB clinics, while there were higher rates of clinic referral than recommended in local guidelines among the other three groups. No child who did not attend clinic was later diagnosed with active TB.
A total of 150 children were identified as close contacts of sputum smear-positive index cases (Figure 1), of which 144 (96%) were tested for LTBI. Among 125 close contacts of sputum smear-positive cases referred, 81 were considered to have LTBI, and of these, 79 were commenced on TPT and 2 declined. All children with a positive test were referred. Of the 79 children commenced on TPT, 26 (32.9%) were given a window TPT. Eighteen (14.4%) were commenced on active TB treatment, 24 (19.2%) were considered not to have been recently infected and were discharged without further treatment, and two (1.6%) failed to attend clinical appointments. Of the 24 children who were discharged, three had been previously treated for LTBI. Of the 79 children commenced on LTBI treatment, 8 (10.1%) discontinued therapy prior to completion. Following completion of LTBI treatment, no child was subsequently diagnosed with active TB. The mean follow-up time was 30.7 months, with a total of 1448.7 person-years for all the contacts of PTB cases. All active cases completed treatment.
FIGURE 1.
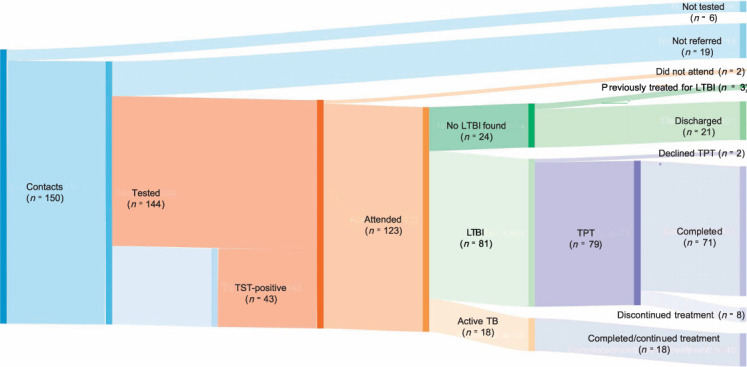
Cascade of care for close contacts of sputum smear-positive index cases. *The TST-positive cohort (n = 43) is a subset of those 125 who were referred. Some children with negative TB test at baseline were referred (see Methods).
Figure 2 shows the comparison of four groups: close contacts of sputum smear-positive cases, casual contacts of sputum smear-positive cases (included contacts found in congregate settings such as childcare, churches, and schools), contacts of sputum smear-negative cases and children who underwent opportunistic testing. Age, history of BCG vaccination, birth in a high incidence TB setting and close household contact were predictive of TST reactivity, with country of birth and vaccination status being highly correlated. In multivariate analysis, vaccination status was more strongly associated with TST reactivity, and country of birth was therefore removed.
FIGURE 2.
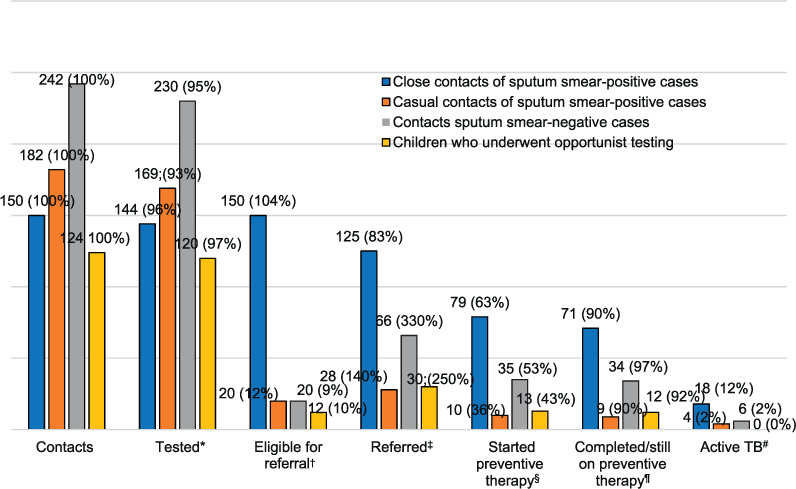
Cascade of care for contacts of TB patients. To calculate percentages, the following were numerators and denominators were used: *number tested/number of contacts; †number eligible/number tested; ‡number referred/number eligible; §number who started TPT/number referred; ¶number who completed or were still on TPT/number who started TPT; #number of persons with active TB/number of contacts.
Altogether 698 children were identified for LTBI investigation, of whom 663 (95%) children were tested. Of the 663 children tested, 99 (14.9%) had a positive TB test (93 were TST-positive and 6 were IGRA-positive). A total of 37 contacts received IGRA, of whom 21 had both IGRA and TST. Of the 21 contacts who received both IGRA and TST, 12 were TST-positive, and none were positive on IGRA.
A total of 249 children were referred to the specialist clinics, of whom 137 (55.0%) were commenced on TPT, 28 (11.2%) on active TB treatment, 19 (7.6%) were referred to BCG immunisation clinics and 65 (26.0%) were discharged. Four children with positive TB test were not referred. At the time of reporting, 126 children have ether completed or are still on LTBI treatment. All 28 children diagnosed with active TB completed treatment (this is not shown in the graph). Among the children not referred to the clinic, no child was later diagnosed with active TB.
The proportion of children tested for LTBI among the contacts of smear-positive and smear-negative cases was similar, 96.0% and 95.0% respectively. Of the 124 children who underwent opportunistic screening, 12 (9.7%) were infected and there was no active TB found in this cohort. Eligible means positive LTBI test for other groups except the contacts of smear-positive cases.
DISCUSSION
In low TB incidence countries such as Australia, active detection and treatment of TB disease and infection among contacts is a critical role of the TB programme. The high yield of around 1 in 20 young child contacts having active TB at the time of initial screening in itself highlights the importance of prompt contact tracing for active case finding.4 This yield is similar to that reported previously from Victoria and from many other settings, emphasising that the household is a high-risk setting irrespective of whether in a low or high TB endemic country.1–3 The prevalence of LTBI among young child contacts of TB cases in Victoria that we found – 15.3% overall and 30.9% if the index case was sputum smear-positive – is lower than that reported from other low TB incidence countries. The reported prevalence of LTBI among young child contacts from California and more recently, from New York City, NY, USA, was respectively 45% and 49%.1,10 In our setting, the inclusion of lower-risk contacts from casual and school contexts and the early detection of index cases may have contributed to the lower than expected prevalence of infection.
The WHO and the Australian national TB guidelines recommend that all young children who are close contacts of a sputum smear-positive pulmonary TB case should be referred to a TB specialist medical practitioner and if they do not have active TB, be considered for commencement of TPT regardless of TST result until infection is excluded at break of contact.11,12 Our review found that, while almost all of the contacts were reviewed by nurses in the community, 17% of eligible child contacts were not referred to the specialist TB clinic follow-up for consideration for TPT, including 14 contacts aged <2 years. Of the 25 children not referred, 22 tested negative at the initial testing and three children were not tested at all. Children returning negative tests after exposure also had community assessment from specialist TB nurses, and may have not been assessed in clinics due to assessments of low risk. No child in this category was subsequently diagnosed with active disease. On the other hand, contacts of sputum smear-negative cases, including children with negative test for infection were often referred, although this is not recommended by the current guidelines. Nurses appeared to refer some children based on perceived higher-risk scenarios, including prolonged or heavy exposure or where there was evidence of transmission to others.
The significant increased odds of a positive TST among the contacts aged <2 years and contacts of sputum smear positive cases following adjusting for the effect of age, BCG status supports our local practice. When screening for TB infection, we prioritise close contact of sputum smear-positive cases and children aged <2 years.
Some children who were TST-positive and had a history of BCG were given an IGRA. A negative IGRA was considered as negative LTBI test even when the TST was positive. A TST cut-off of 5 mm is associated with poor specificity in BCG-vaccinated children aged <5 years.13 As most children from high TB incidence countries are BCG-vaccinated, country of birth was not included in the multivariate analysis.
A significant proportion of children tested for infection by the programme staff were identified as opportunistic contacts. They had a lower prevalence of LTBI and no active TB cases were identified. However, even in this cohort, 10% were found to have LTBI, which is considerably higher than the general Australian population. This population may remain an important group to detect and treat with TPT in a low TB endemic setting aiming for TB elimination.14
Important programmatic indicators for contact tracing include coverage of contact tracing, along with uptake and completion of TPT for all eligible contacts.11 The rates of uptake and completion of LTBI treatment are high in young child contacts in our setting, consistent with experience in similar resource-rich, low-endemic countries.15 As the populations with LTBI recommended for TPT expand to include larger numbers of adults and others at lower individual risk, it may be more challenging to reach and maintain such high rates under programmatic conditions.16
CONCLUSION
Routine screening of child TB contacts in Victoria continues to be important for early detection and treatment of active TB cases alone. The implementation of TPT in high-risk populations require ongoing efforts to maintain high rates of evaluation, uptake and completion. Efforts to sustain and further strengthen successful TPT implementation are relevant for all eligible populations and regimen options.
ACKNOWLEDGEMENTS
The authors would like to thank the clinical nurse consultants for performing contact investigation. Figure 1 was produced by S Bogart using sankeyMATIC (www.sankeymatic.com).
Footnotes
Conflict of interests: none declared.
References
- 1.Lobato MN, Royce SE, Mohle-Boetani JC. Yield of source-case and contact investigations in identifying previously undiagnosed childhood tuberculosis. Int J Tuberc Lung Dis. 2003;7(Suppl 3):S391–S396. [PubMed] [Google Scholar]
- 2.Fox GJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyo N, Tay EL, Denholm JT. Evaluation of tuberculin skin testing in tuberculosis contacts in Victoria, Australia, 2005–2013. Public Health Action. 2015;5:188–193. doi: 10.5588/pha.15.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez L, et al. The risk of tuberculosis in children after close exposure: A systematic review and individual-participant meta-analysis. Lancet. 2020;395:973–984. doi: 10.1016/S0140-6736(20)30166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham SM. The management of infection with Mycobacterium tuberculosis in young children post-2015: an opportunity to close the policy-practice gap. Exp Rev Resp Med. 2017;11:41–49. doi: 10.1080/17476348.2016.1267572. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Global tuberculosis report, 2020. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 7.Al Abri S et al. Tools to implement the World Health Organization End TB Strategy: Addressing common challenges in high and low endemic countries. Int J Infect Dis. 2020;92(Suppl):S60–S68. doi: 10.1016/j.ijid.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Globan M, et al. Molecular epidemiology of tuberculosis in Victoria, Australia, reveals low level of transmission. Int J Tuber Lung Dis. 2016;20:652–658. doi: 10.5588/ijtld.15.0437. [DOI] [PubMed] [Google Scholar]
- 9.Teo SS, et al. The epidemiology of tuberculosis in children in Australia, 2003–2012. Med J Aust. 2015;203:440. doi: 10.5694/mja15.00717. [DOI] [PubMed] [Google Scholar]
- 10.Slutsker JS, et al. Using reports of latent tuberculosis infection among young children to identify tuberculosis transmission in New York City, 2006–2012. Am J Epidem. 2018;187:1303–1310. doi: 10.1093/aje/kwx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization WHO consolidated guidelines on tuberculosis – tuberculosis preventive treatment. Geneva, Switzerland: WHO; 2020. [PubMed] [Google Scholar]
- 12.Communicable Diseases Network Australia National guidelines for the public health management of TB. Canberra ACT, Australia: Australian Department of Health; 2015. [Google Scholar]
- 13.Seddon JA, et al. The impact of BCG vaccination on tuberculin skin test responses in children is age dependent: evidence to be considered when screening children for tuberculosis infection. Thorax. 2016;71(10):932–939. doi: 10.1136/thoraxjnl-2015-207687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale K, et al. Estimating the prevalence of latent tuberculosis in a low-incidence setting: Australia. Eur Respir J. 2018;52:1801218. doi: 10.1183/13993003.01218-2018. [DOI] [PubMed] [Google Scholar]
- 15.Erkens CG, et al. Monitoring latent tuberculosis infection diagnosis and management in the Netherlands. Eur Respir J. 2016;47:1492–1501. doi: 10.1183/13993003.01397-2015. [DOI] [PubMed] [Google Scholar]
- 16.Stock D. National position statement for the management of latent tuberculosis infection. Commun Dis Intell Q Rep. 2017;41(3):E204–208. [PubMed] [Google Scholar]


