Abstract
Cancer immunotherapies have revolutionized how we can treat adult malignancies and are being translated to pediatric oncology. Chimeric antigen receptor T-cell therapy and bispecific antibodies targeting CD19 have shown success for the treatment of pediatric patients with B-cell acute lymphoblastic leukemia. Anti-GD2 monoclonal antibody has demonstrated efficacy in neuroblastoma. In this review, we summarize the immunotherapeutic agents that have been approved for treating childhood cancers and provide an updated review of molecules expressed by pediatric cancers that are under study or are emerging candidates for future immunotherapies. Advances in our knowledge of tumor immunology and in genome profiling of cancers has led to the identification of new tumor-specific/associated antigens. While cell surface antigens are normally targeted in a major histocompatibility complex (MHC)-independent manner using antibody-based therapies, intracellular antigens are normally targeted with MHC-dependent T cell therapies. Glypican 2 (GPC2) and B7-H3 (CD276) are two cell surface antigens that are expressed by a variety of pediatric tumors such as neuroblastoma and potentially can have a positive impact on the treatment of pediatric cancers in the clinic.
Keywords: pediatric cancer, glypican 2 (GPC2), B7-H3, monoclonal antibody, chimeric antigen receptor (CAR)
1. Introduction
Cancer is the second leading cause of death among children aged 1 to 14 in the United States. It is estimated that 11,050 new cases of childhood cancer may be diagnosed and over 1,000 children may die from cancer in 2020 (Siegel, Miller, & Jemal, 2020). There are major categories of childhood cancers, into which diseases are grouped based on their anatomic locations. Hematological malignancies include blood, bone marrow, and lymphoid system cancers. Brain tumors are intracranial nervous system cancers, while solid tumors generally include extracranial and non-hematologic cancers (X. Chen, Pappo, & Dyer, 2015). Chemotherapy and radiation treatment approaches have improved cure rates in childhood cancers, but children with relapsed/refractory cancer have treatment options often limited to further intensified chemotherapy or radiation. Novel therapeutic approaches are desperately needed to improve survival rates and to reduce toxicities.
Immunotherapy represents a new frontier in treating cancers. Although the field has come of age, we have only recently begun to witness it thriving. The emergence of therapeutic monoclonal antibodies (mAbs) has propelled the clinical applications of immune checkpoint blockade and chimeric antigen receptor (CAR) T-cell therapy, both of which have achieved notable success in treating pediatric cancer, particularly hematological malignancies. Here, we highlight therapeutic targets in childhood cancer, including those that have resulted in immunotherapies that are approved by the US Food and Drug Administration (FDA). However, immunotherapy has yet to be widely used for pediatric brain tumors and solid tumors, partially due to the lack of proper targets that are constantly expressed in tumors yet largely absent in healthy tissues. The discovery of new therapeutic targets in childhood cancers is imperative for the continued development of targeted immunotherapy for childhood cancer. We review emerging immunotherapeutic targets including cell surface antigens such as glypican 2 (GPC2) and B7-H3 (CD276) that are major histocompatibility complex (MHC)-independent, and intracellular antigens that are MHC-restricted, focusing on their development as they make their way into clinical applications, as well as challenges associated with each target.
2. Approved immunotherapies for childhood cancers
Over the past several years, immunotherapy has been introduced as a treatment for pediatric cancers. In fact, several therapies have been approved for different malignancies. Multiple approaches targeting B-lymphocyte antigen CD19, including bispecific antibody and CAR T-cell therapy, are now used in children with B-cell acute lymphoblastic leukemia (ALL). Disialoganglioside GD2 has been well-researched as a target in neuroblastoma, including in an FDA-approved monoclonal antibody. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are checkpoint inhibitors that are being used for treatment in multiple childhood cancers. A list of currently approved immunotherapies for childhood cancer are shown in Table 1. Moreover, enormous efforts are being made to explore more immunotherapeutic approaches targeting these established antigens, clinical trials that are recruiting within the past 5 years in the US are listed in Table 2.
Table 1. FDA Approved immunotherapies for childhood cancers.
B-ALL: B-cell Acute lymphoblastic leukemia; HL: Hodgkin lymphoma; CRC: Colorectal Cancer
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) | Reference |
|---|---|---|---|---|---|---|
| NCT01471782 | Clinical Study With Blinatumomab in Pediatric and Adolescent Patients With Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia | CD19 | 1/2 | B-ALL | Blinatumomab, anti-CD19 BiTE | (von Stackelberg, et al., 2016) |
| NCT02435849 | Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B-cell ALL and High Risk B-cell ALL at First Relapse. | CD19 | 2 | B-ALL | Tisagenlecleucel (CTL019); CAR T cell | (Maude, et al., 2018) |
| NCT00026312 | Isotretinoin With or Without Dinutuximab, Aldesleukin, and Sargramostim Following Stem Cell Transplant in Treating Patients With Neuroblastoma | GD2 | 3 | Localized/Regional Neuroblastoma Recurrent Neuroblastoma Stage 4/4S Neuroblastoma |
Aldesleukin Dinutuximab Isotretinoin Sargramostim |
(A. L. Yu, et al., 2010) |
| NCT02332668 | A Study of Pembrolizumab (MK-3475) in Pediatric Participants With an Advanced Solid Tumor or Lymphoma (MK-3475–051/KEYNOTE-051) | PD1 | 1/2 | Melanoma Lymphoma Classical HL Microsatellite-instabilityhigh Solid Tumor |
Pembrolizumab (MK-3475) Humanized IgG4 monoclonal antibody |
(Geoerger, et al., 2020) |
| NCT02060188 | An Investigational Immuno-therapy Study of Nivolumab, and Nivolumab in Combination With Other Anti-cancer Drugs, in Colon Cancer That Has Come Back or Has Spread | PD1 | 2 | Microsatellite Unstable/Stable CRC Mismatch Repair Proficient/Deficient CRC |
Ipilimumab Nivolumab Cobimetinib Daratumumab anti-LAG-3 antibody |
(Overman, et al., 2017) |
| NCT00094653 | MDX-010 Antibody, MDX-1379 Melanoma Vaccine, or MDX-010/MDX-1379 Combination Treatment for Patients With Unresectable or Metastatic Melanoma | CTLA-4 | 3 | Melanoma Metastases | MDX-010 (anti-CTLA4) monoclonal antibody MDX-1379 (gp100) Melanoma Peptide Vaccine |
(Hodi, et al., 2010) |
Table 2. Pediatric immunotherapy clinical trials involving the established immunotherapeutic targets that are currently active/recruiting in the US since 2016.
B-ALL: B-cell Acute lymphoblastic leukemia; HL: Hodgkin lymphoma; NHL: Non- Hodgkin lymphoma; CLL: Chronic lymphocytic leukemia; DIPG: Diffuse Intrinsic Pontine Glioma
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
|---|---|---|---|---|---|
| NCT04544592 | UCD19 CarT in Treatment of Pediatric B-ALL and B-NHL | CD19 | ½ | B-cell Acute Lymphoblastic Leukemia B-cell Non Hodgkin Lymphoma |
CD19CAR-CD3Zeta-4-1BB-Expressing Allogenic TLymphocyte Cells |
| NCT03186118 | Pilot Study of T-APCs Following CAR T Cell Immunotherapy for CD19+ Leukemia | CD19 | 1 | CD19+ Acute Leukemia | T-cell Antigen Presenting Cells expressing truncated CD19 (T-APC) |
| NCT04276870 | Phase 2 Trial of CD19 Redirected Autologous CAR T Cells (CART19) for Orphan Indications of Pediatric B Cell Acute Lymphoblastic Leukemia (B ALL) | CD19 | 2 | Hypodiploid or t(17;19) B-ALL Infants With Very High Risk KMT2A B-ALL Patients With Central Nervous System Relapse |
Murine CART19 |
| NCT03792633 | Study of huCART19 for Very High-Risk (VHR) Subsets of Pediatric B-ALL | CD19 | 2 | B-ALL | huCART19 |
| NCT03684889 | CD19-specific CAR T Cells With a Fully Human Binding Domain for CD19+ Leukemia or Lymphoma | CD19 | 1/2 | B-cell Leukemia B-cell Lymphoma |
SCRI-huCAR19v1 SCRI-huCAR19v2 |
| NCT03573700 | Evaluation of CD19-Specific CAR Engineered Autologous T-Cells for Treatment of Relapsed/Refractory CD19+ Acute Lymphoblastic Leukemia | CD19 | 1/2 | B-relapsed/refractory ALL | Cyclophosphamide Fludarabine; Mesna CD19- specific CAR T-cells (SJCAR19 product) |
| NCT03467256 | CD19 T-CAR for Treatment of Children and Young Adults With r/r B-ALL | CD19 | 1/2 | B-ALL | CD19 CAR T-Cell Fludarabine Cyclophosphamide Tocilizumab |
| NCT03774654 | CD19.CAR Allogeneic NKT for Patients With Relapsed or Refractory B-Cell Malignancies (ANCHOR) | CD19 | 1 | B-cell NHL CLL |
CD19.CAR-aNKT cells Cyclophosphamide Fludarabine |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT03016377 | Administration of Autologous CAR-T CD19 Antigen With Inducible Safety Switch in Patients With Relapsed/Refractory ALL | CD19 | 1/2 | B-ALL | iC9-CAR19 cells Rimiducid Cyclophosphamide Fludarabine |
| NCT03768310 | CD19.CAR-multiVSTs for Patients With CD19+ B-ALL or NHL Undergoing Related Allogeneic HSCT | CD19 | 1 | B-ALL B-cell NHL |
CD19.CAR-multiVST |
| NCT03056339 | Umbilical & Cord Blood (CB) Derived CAR-Engineered NK Cells for B Lymphoid Malignancies | CD19 | 1/2 | B-ALL B-cell NHL |
Fludarabine; Cyclophosphamide Mesna iC9/CAR.19/IL15-Transduced CB-NK Cells AP1903 |
| NCT03743246 | A Study to Evaluate the Safety and Efficacy of JCAR017 in Pediatric Subjects With Relapsed/Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (B-ALL) and B-cell Non-Hodgkin Lymphoma (B-NHL) | CD19 | 1/2 | B-ALL B-cell NHL |
JCAR017 Fludarabine Cyclophosphamide |
| NCT04196413 | GD2 CAR T Cells in Diffuse Intrinsic Pontine Gliomas(DIPG) & Spinal Diffuse Midline Glioma(DMG) | GD2 | 1 | Glioma of Spinal Cord Glioma of Brainstem | GD2 CAR T cells Fludarabine Cyclophosphamide |
| NCT03721068 | Study of CAR T-Cells Targeting the GD2 With IL-15+iCaspase9 for Relapsed/Refractory Neuroblastoma | GD2 | 1 | Neuroblastoma | iC9.GD2.CAR.IL-15 T-cells Cyclophosphamide Fludarabine |
| NCT04099797 | C7R-GD2.CAR T Cells for Patients With GD2-expressing Brain Tumors (GAIL-B) | GD2 | 1 | DIPG High Grade Glioma |
(C7R)-GD2.CART cells Cyclophosphamide Fludarabine |
| NCT03635632 | C7R-GD2.CART Cells for Patients With Relapsed or Refractory Neuroblastoma and Other GD2 Positive Cancers (GAIL-N) | GD2 | 1 | Neuroblastoma Osteosarcoma Ewing Sarcoma Rhabdomyosarcoma |
(C7R)-GD2.CART cells Cyclophosphamide Fludarabine |
| NCT03294954 | GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children With Neuroblastoma (GINAKIT2) | GD2 | 1 | Neuroblastoma | GD2-CAR NKT cells Cyclophosphamide Fludarabine |
| NCT03860207 | Study of the Safety and Efficacy of Humanized 3F8 Bispecific Antibody (Hu3F8-BsAb) in Patients With Relapsed/Refractory Neuroblastoma, Osteosarcoma and Other Solid Tumors | GD2 | 1/2 | Neuroblastoma Osteosarcoma Other Solid Tumor Cancers |
Humanized 3F8 Bispecific Antibody |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT03363373 | Naxitamab for High-Risk Neuroblastoma Patients With Primary Refractory Disease or Incomplete Response to Salvage Treatment in Bone and/or Bone Marrow | GD2 | 2 | Neuroblastoma | GM-CSF + Naxitamab |
| NCT03690869 | REGN2810 in Pediatric Patients With Relapsed, Refractory Solid, or Central Nervous System (CNS) Tumors and Safety and Efficacy of REGN2810 in Combination With Radiotherapy in Pediatric Patients With Newly Diagnosed or Recurrent Glioma | PD-1 | 1/2 | Relapsed/refractory Solid Tumor Central Nervous System Tumor DIPG High Grade Glioma |
REGN2810 Radiation: Conventional or hypofractionated Re-irradiation |
| NCT03843294 | Tumor Associated Antigen Specific T Cells (TAA-T) With PD1 Inhibitor for Lymphoma | PD-1 | 1 | Hodgkin Lymphoma Diffuse Large B Cell Lymphoma |
TAA-T cells Nivolumab |
| NCT03407144 | Safety and Efficacy of Pembrolizumab (MK-3475) in Children and Young Adults With Classical Hodgkin Lymphoma | PD-1 | 2 | HL | Pembrolizumab Chemotherapy drugs Radiotherapy |
| NCT02992964 | Pilot Study of Nivolumab in Pediatric Patients With Hypermutant Cancers | PD-1 | 1/2 | Refractory or Recurrent Hypermutated Malignancies Biallelic Mismatch Repair Deficiency (bMMRD) Patients |
Nivolumab |
| NCT02914405 | Phase I Study of 131-I mIBG Followed by Nivolumab & Dinutuximab Beta Antibodies in Children With Relapsed/Refractory Neuroblastoma (MiniVan) | PD-1/GD2 | 1 | Neuroblastoma | Nivolumab Ch14.18/CHO |
| NCT03605589 | Pembro + Blina Combination in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Leukemia or Lymphoma | CD19/PD-1 | 1 | B-cell Leukemia B-cell Lymphoma |
Blinatumomab Pembrolizumab |
| NCT03769467 | Tabelecleucel in Combination With Pembrolizumab in Subjects With Epstein-Barr Virus-associated Nasopharyngeal Carcinoma (EBV+ NPC) | CD19/PD-1 | 1/2 | Epstein-Barr Virus Infections Epstein-Barr Viraemia Epstein-Barr Virus-associated Nasopharyngeal Carcinoma |
tabelecleucel pembrolizumab |
| NCT02879695 | Blinatumomab and Nivolumab With or Without Ipilimumab in Treating Patients With Relapsed or Refractory CD19+ Precursor B-Lymphoblastic Leukemia | CD19/PD-1/CTLA-4 | 1 | B-ALL | Blinatumomab Ipilimumab Nivolumab |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT04465643 | Neoadjuvant Nivolumab Plus Ipilimumab for Newly Diagnosed Malignant Peripheral Nerve Sheath Tumor | PD-1/CTLA-4 | 1 | Nerve Sheath Tumors | Nivolumab Ipilimumab |
| NCT03837899 | Durvalumab and Tremelimumab for Pediatric Malignancies | PD-1/CTLA-4 | 1 | Pediatric Cancer Solid Tumor Pediatric Hematological Malignancies |
Durvalumab / Tremelimumab Combination Therapy |
| NCT04323046 | Immunotherapy (Nivolumab and Ipilimumab) Before and After Surgery for the Treatment of Recurrent or Progressive High Grade Glioma in Children | PD-1/CTLA-4 | 1 | Glioblastoma Malignant Glioma Grade III Glioma |
Ipilimumab Nivolumab |
2.1. CD19
CD19 is possibly the most successful immunotherapeutic target. CD19 is a type I transmembrane protein of the immunoglobulin superfamily (K. Wang, Wei, & Liu, 2012), and functions in B cell receptor-dependent and independent signaling (Fujimoto, Poe, Inaoki, & Tedder, 1998). CD19 is universally expressed in the B-cell lineage but is not expressed on pluripotent stem cells. Additionally, its expression is sustained during neoplastic transformation. For these reasons, CD19 has long been studied as a marker for leukemia and lymphoma, as it is expressed on a wide variety of hematological malignancies (Scheuermann & Racila, 1995). Several therapies targeting CD19 are FDA approved in children with hematological malignancies. Blinatumomab is a bi-specific T-cell engager (BiTE) antibody directed against CD19 and CD3 on T cells. It is constructed from single-chain variable fragments (scFvs) of both CD19 and CD3 antibodies, which are connected by a serine-glycine linker. The binding of the antibody redirects T cells to CD19-positive neoplastic cells and results in directed cytotoxicity (Löffler, et al., 2000). An open-label, single-arm phase I/II trial of blinatumomab in children with relapsed/refractory B-cell precursor ALL demonstrated antileukemic activity with 27/70 patients achieving complete remission (von Stackelberg, et al., 2016).
Successful CAR T cells in clinic have thus far also been targeted against CD19, including the first FDA approved CAR T cell system, tisagenlecleucel. In the pivotal trial, 75 pediatric patients with relapsed/refractory B-cell ALL received a weight-adjusted dose of CAR T cells including a CD3ζ signaling domain and 4–1BB co-stimulatory domain (Maude, et al., 2018). At 6 months, the rate of event-free survival was 73%. CAR T cells were detectable in peripheral blood for up to 20 months after treatment. Sequential therapy of CD19 CAR T cells and allogenic hematopoietic stem-cell transplant achieved long-term disease control (median follow-up 4.8 years) in children and young adults with B-ALL (Nirali N. Shah, et al., 2021). Many strategies to further engineer CD19 CAR T cells for improved efficacy are currently being studied, including the use of a humanized binding domain (Brudno, et al., 2020), different hinge and transmembrane domains (Alabanza, et al., 2017), co-expression of cytokines (Štach, et al., 2020), and “off-the-shelf” manufacturing (Curran, et al., 2020; Shen, Pham, Wu, Munson, & Aftab, 2019). Additionally, trials are underway to expand the use of CD19 CAR T cell therapy to other B-cell leukemias and lymphomas.
2.2. GD2
GD2 is a diasialoganglioside that is expressed on tumors derived from the neuroectoderm, like melanoma and neuroblastoma (Schulz, et al., 1984). GD2 was identified as a neuroblastoma tumor antigen with therapeutic potential in the mid-1980s after uniform GD2 expression was detected in tumors of all clinical stages of neuroblastoma (Z. L. Wu, Schwartz, Seeger, & Ladisch, 1986). Additionally, GD2 expression is largely restricted in healthy tissues, found only in neurons, skin melanocytes, and peripheral pain fibers (Svennerholm, et al., 1994). The high tumor-association of GD2 has made it a promising target in neuroblastoma for monoclonal antibodies.
Dinutuximab (ch14.18) is a chimeric monoclonal antibody that was FDA approved in 2015 for the treatment of high-risk neuroblastoma, in combination with 13-cis-retinoic acid (RA), interleukin-2 (IL-2) and granulocyte macrophage colony-stimulating factor (GM-CSF). The mouse monoclonal antibody 14.18 was isolated by hybridoma (Mujoo, Cheresh, Yang, & Reisfeld, 1987) and a subsequent mouse-human chimeric antibody, dinutuximab. was produced to reduce immunogenicity associated with murine antibodies (Gillies, Lo, & Wesolowski, 1989). Dinutuximab was first investigated as a single agent in a clinical trial of 334 patients with Stage 4 neuroblastoma (Simon, et al., 2004). While the study determined the side effects of monoclonal antibody to be manageable, the study found there was no significant improvement in event-free survival after dinutuximab treatment. A separate phase III clinical trial of 226 patients with high-risk neuroblastoma that responded to induction therapy and stem-cell transplantation, administered dinutuximab in combination with GM-CSF, IL-2, and RA and reported a significant improvement in event-free and overall survival as compared to standard therapy (A. L. Yu, et al., 2010). This was the pivotal trial in FDA approval of dinutuximab. However, though considered manageable, the side effects associated with dinutuximab are significant and include neuropathic pain, hypotension, hypersensitivity, and capillary leak syndrome, among others (Simon, et al., 2004; A. L. Yu, et al., 2010). Long-term follow-up of the study confirmed that dinutuximab improved survival, though the differences in survival between treatment groups lessened over time as a result of late relapses (A. L. Yu, et al., 2021). Additionally, dinutuximab remains a second-line treatment for neuroblastoma, indicated only in cases where at least partial response to first-line multimodal therapy has occurred.
Dinutuximab has been cloned into Chinese Hamster Ovary (CHO) cells for production, which results in a more favorable glycosylation pattern with fewer N-glycolylneuraminic acids, avoiding clearance by xeno-autoantibodies (Ladenstein, et al., 2013). The ch14.18/CHO (named as dinutuximab beta), has the same binding as dinutuximab and complement-dependent cytotoxicity with improved antibody-dependent cell-mediated cytotoxicity (Zeng, et al., 2005). Dinutuximab beta was approved by the European Medicines Agency for the treatment of neuroblastoma in 2017 and is currently under investigation in the United States. The results from an ongoing phase III clinical trial (NCT01704716) demonstrate that dinutuximab beta is associated with an improved survival in children with high-risk neuroblastoma (Ladenstein, et al., 2020).
Several other antibodies to GD2 have been isolated in the past several decades and remain investigational in clinical trials. The murine monoclonal antibody 3F8 was isolated in the mid-1980s (N.-K. V. Cheung, et al., 1985) and was first investigated in a phase I clinical trial for metastatic neuroblastoma or melanoma a few years later, with anti-tumor responses in 7 of 17 patients (N. K. Cheung, et al., 1987). Humanized 3F8 (naxitimab) maintains the binding ability and causes more potent antibody-dependent cell-mediated cytotoxicity (N.-K. V. Cheung, Guo, Hu, Tassev, & Cheung, 2012). Naxitimab has been granted Orphan Drug Status by the FDA and several phase II trials for high-risk neuroblastoma are currently recruiting.
CARs constructed using dinutuximab are currently under investigation. There have been several different approaches towards CAR T cell therapy targeting GD2. A first-generation CAR, containing scFv of the 14.2Ga murine antibody and a CD3ζ signaling domain, was expressed in Epstein-Barr virus-specific cytotoxic T lymphocytes (EBV-CTLs) and activated T cells (ATCs) (Louis, et al., 2011; Pule, et al., 2008). A phase I clinical trial (NCT00085930) of GD2 EBV-CTLs and GD2 ATCs showed a complete remission in 3 out of 19 high-risk neuroblastoma patients and demonstrated persistence of GD2 ATCs of up to 192 weeks (Louis, et al., 2011). More recently, several phase I clinical trials have been completed (NCT02107963) or are currently underway (NCT04539366) to investigate a third generation GD2 CAR T cells, comprising the 14.2Ga scFv, OX40 and CD28 co-stimulatory domains, the CD3ζ domain, and an inducible caspase 9 kill switch (Di Stasi, et al., 2011). Another approach co-expressing the GD2 CAR and interleukin-15 in natural killer (NK) T cells (X. Xu, et al., 2019) is also currently under investigation (NCT03294954). The initial report demonstrated that GD2 CAR-NKT cells expanded to clinical scale and induced regression of bone metastases in one patient among three enrolled children (Heczey, et al., 2020). Recently, SynNotch CAR T cells using GD2 as the “gate” and B7-H3 as the secondary target demonstrated anti-tumor activity in a metastatic neuroblastoma mouse model (Moghimi, et al., 2021). Overall, GD2 CAR T cells for neuroblastoma and other childhood cancers remain an active area of research, with the need for larger-scale clinical trials to confirm clinical relevance and efficacy.
While GD2 is a well-established immunotherapeutic target for neuroblastoma and other childhood cancers, there remain several challenges to effective GD2-targeted treatments. The expression of GD2 is not ideal for a tumor antigen, as it is also expressed on neurons, melanocytes, and peripheral pain fibers. On-target, off-tumor effects due to the presence of GD2 on pain fibers may be the cause of the high levels of pain reported by patients receiving anti-GD2 monoclonal antibodies. Additionally, approved anti-GD2 monoclonal antibody therapy, dinutuximab, is indicated only in combination with GM-CSF, IL-2, and RA, all of which can be associated with toxic side effects and cytokine release syndrome. GD2 CAR is a promising approach, but face challenges in long-term persistence, and require further investigation to be a widely used treatment in childhood cancer.
2.3. PD1/CTLA-4
Many tumors are able to prevent recognition by the immune system by expressing immune checkpoints, which inhibit T cell activation when they bind their T cell receptors. Activated T cells express programmed cell death protein 1 (PD-1) which, when it binds to its ligand PD-L1, prevents a T cell response, thus acting as an immune checkpoint. PD-L1 is frequently expressed on tumor cells, including neuroblastoma, osteosarcoma, and Ewing’s sarcoma (Pinto, et al., 2017), allowing the tumor to suppress T cell activity and escape immune detection. Pembrolizumab is a humanized antibody that binds PD-1, blocking its interaction with PD-L1. As of 2020, pembrolizumab is approved for pediatric use in relapsed/refractory classic Hodgkin’s lymphoma (HL), primary mediastinal large B-cell lymphoma, Merkel cell carcinoma, and microsatellite instability-high and tumor mutational burden-high cancers. An open-label, single-arm phase I/II trial of pembrolizumab in pediatric patients with various patients demonstrated that it is well-tolerated and determined a recommended pediatric dose; however, only HL saw clear anti-tumor activity (Geoerger, et al., 2020). Nivolumab is another human monoclonal antibody that binds PD-1 (C. Wang, et al., 2014). It is currently approved for pediatric patients 12 years or older for microsatellite instability-high or mismatch repair deficient colorectal cancer, though the relevant phase II trial was not for pediatric patients (Overman, et al., 2017). A recently published phase I/II trial for children with relapsed/refractory solid tumors or lymphoma demonstrated tolerability of nivolumab in pediatric patients, but again showed clinical efficacy only in lymphoma (Davis, et al., 2020). A third fully human monoclonal antibody, cemiplimab, is currently being evaluated for pediatric relapsed/refractory solid or central nervous system tumors in a Phase I/II trial (NCT0369089).
CTLA-4 is another immune checkpoint that has been the target of immune checkpoint blockade therapy. Normally, CTLA-4 is expressed on T cells and functions in preventing autoimmunity (Jain, Nguyen, Chambers, & Kang, 2010). Ipilimumab is a fully human anti-CTLA-4 monoclonal antibody that is FDA approved for the treatment of unresectable or metastatic melanoma and microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer in children 12 years or older. At the time of approval, ipilimumab had been investigated in two pediatric clinical trials. A phase I dose-finding trial included 33 pediatric patients with advanced solid tumors determined that ipilimumab was safely administered in children with similar toxicities to those reported in adults, though it did not show anti-tumor efficacy as a single agent (Merchant, et al., 2016). A phase II study administered ipilimumab to pediatric patients with unresectable stage III or IV melanoma demonstrated anti-tumor activity and determined ipilimumab has a similar safety profile in adults and children (Geoerger, et al., 2017).
While immune checkpoint therapy has been shown to be effective in adults, the same has not been demonstrated in pediatric populations. The mutational landscape of tumors generally influences the strength of the T cell response against tumors; however, one of the hallmarks of most pediatric cancers is their relative lack of mutations (Campbell, et al., 2017). Tumor escape can occur when tumors develop compensatory mechanisms in response to single approach immunotherapy. Thus, combination therapy targeting both PD-1 and CTLA-4 has attracted significant attention (Puri & Shafique, 2020), and several trials are currently underway investigating PD-1/CTLA-4 inhibitor combinations in pediatric populations.
3. Emerging cell surface targets for pediatric hematologic malignancies
The treatment landscape for pediatric hematologic malignancies is evolving faster than ever before. Particularly, immunotherapy is the front and center in the battle to control these diseases. Studies focusing on CD19 currently represent the bulk of clinical investigations for hematologic malignancies. However, CD19 is only a viable target in about half of hematologic malignancies, and some patients may develop resistance to CD19-targeted immunotherapies. Here, we briefly summarize other emerging targets for treating hematological malignancies and the latest promising clinical developments. The list of clinical trials focusing on these emerging targets is provided in Table 3.
Table 3. Clinical trials involving emerging immunotherapeutic targets for pediatric hematological malignancies that are currently active/recruiting in the US since 2016.
B-ALL: B-cell Acute lymphoblastic leukemia; HL: Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma; AML: Acute Myeloid Leukemia
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
|---|---|---|---|---|---|
| NCT01516580 | Intergroup Randomized Trial for Children or Adolescents With B-Cell Non Hodgkin Lymphoma or B-Acute Leukemia: Rituximab Evaluation in High Risk Patients | CD20 | 3 | Mature B-cell Leukemia Burkitt-type |
Rituximab Chemotherapy Drugs |
| NCT00659425 | CAT-8015 in Children, Adolescents and Young Adults With Acute Lymphoblastic Leukemia or Non-Hodgkin’s Lymphoma | CD20 | 1 | B-ALL B-cell NHL |
CAT-8015 (Moxetumomab Pasudotox) |
| NCT04075266 | A Study of Ocrelizumab in Children and Adolescents With Relapsing-Remitting Multiple Sclerosis | CD20 | 2 | Multiple Sclerosis | Ocrelizumab |
| NCT03019640 | Umbilical Cord Blood NK Cells, Rituximab, High-Dose Chemotherapy, and Stem Cell Transplant in Treating Patients With Recurrent or Refractory B-Cell NHL | CD20 | 2 | Lymphoma | Autologous HSCT Cord Blood-derived Expanded Allogeneic Natural Killer Cells Rituximab Chemotherapy Drugs |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT02877303 | Blinatumomab and Combination Chemotherapy as Frontline Therapy in Treating Patients With B Acute Lymphoblastic Leukemia | CD19/CD20 | 2 | B-ALL B Lymphoblastic Lymphoma |
Blinatumomab Ofatumumab Rituximab Chemotherapy Drugs |
| NCT04049383 | CAR-20/19-T Cells in Pediatric Patients With Relapsed/Refractory B Cell ALL (CAR-20/19-T) | CD19/CD20 | 1 | B-ALL | CAR-20/19-T cells |
| NCT03913559 | Inotuzumab Ozogamicin for Children With MRD Positive CD22+ Lymphoblastic Leukemia | CD22 | 2 | B-ALL | Inotuzumab Ozogamicin Chemotherapy Drugs |
| NCT03104491 | Inotuzumab Ozogamicin Post-Transplant For Acute Lymphocytic Leukemia | CD22 | 1/2 | Cluster of Differentiation Antigen (CD22)-Positive ALL |
Inotuzumab Ozogamicin |
| NCT02315612 | Anti-CD22 Chimeric Receptor T Cells in Pediatric and Young Adults With Recurrent or Refractory CD22-expressing B Cell Malignancies | CD22 | 1 | Follicular Lymphoma ALL NHL Large Cell Lymphoma |
CD22 (M971BBz)-CAR |
| NCT04088864 | CD22-CAR T Cells in Children and Young Adults With B Cell Malignancies | CD22 | 1 | B Cell Lymphoma B-ALL | Fludarabine Cyclophosphamide Autologous CD22 CAR T |
| NCT02650414 | CD22 Redirected Autologous T Cells for ALL | CD22 | 1 | B Cell Leukemias B Cell Lymphomas |
CART22 cells transduced with a lentiviral vector to express anti-CD22 scFv TCRz:41BB |
| NCT04571138 | A Pediatric and Young Adult Trial of Genetically Modified T Cells Directed Against CD22 for Relapsed/Refractory Leukemia or Lymphoma | CD22 | 1/2 | Leukemia Lymphoma | SCRI-CAR22v2 |
| NCT03241940 | Phase I Dose Escalation Study of CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory B Cell Malignancies | CD19/CD22 | 1 | B-ALL Recurrent Adult ALL Recurrent/Refractory Childhood ALL |
CAR T-Cell Therapy Cyclophosphamide Fludarabine Phosphate |
| NCT03448393 | CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies | CD19/CD22 | 1 | ALL B-Cell Leukemia B-Cell Lymphoma Lymphoma, Non-Hodgkin |
CD19/CD22 CAR T-Cells Fludarabine Cyclophosphamide |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT04303520 | Anti-CD19/CD22 Bispecific CAR-T Cell Therapy for CD19-positive ALL | CD19/CD22 | 1 | CD19-positive ALL | CD19/CD22 CAR-T cells Fludarabine Cyclophosphamide |
| NCT04204161 | A Clinical Study of CAR-T Cells Treatment for Children With CD19+/CD22+ R/R ALL and Lymphoma | CD19/CD22 | 1 | ALL B-cell lymphoma |
CAR-T19/CAR-T22 |
| NCT03614858 | CD19/CD22-targeted Chimeric Antigen Receptor Engineered T Cell (CART) in B-Cell Acute Lymphoblastic Leukemia | CD19/CD22 | 1/2 | B-Cell Leukemia | CART-19/22 |
| NCT03330691 | A Feasibility and Safety Study of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Leukemia | CD19/CD22 | 1 | Leukemia Lymphoma |
Patient-derived CD19- and CD22 specific CAR |
| NCT04029038 | Modified Immune Cells (CD19-CD22 CAR T Cells) in Treating Patients With Recurrent or Refractory CD19 Positive, CD22 Positive Leukemia or Lymphoma | CD19/CD22 | 1/2 | B-ALL Lymphoma |
CD19/CD22 Chimeric Antigen Receptor T-cells Cyclophosphamide Fludarabine |
| NCT03241940 | Phase I Dose Escalation Study of CD19/CD22 CAR T Cells in Children and Young Adults With Recurrent or Refractory B Cell Malignancies | CD19/CD22 | 1 | B-ALL | CAR T-Cell Therapy Drug: Cyclophosphamide Drug: Fludarabine Phosphate |
| NCT03244306 | A Phase 1 Study of CD22-CAR TCell Immunotherapy for CD22+ Leukemia and Lymphoma (PLAT-04) | CD22 | 1 | Leukemia | Patient-derived CD22-specific chimeric antigen receptor T-cells expressing an EGFRt |
| NCT01780662 | Brentuximab Vedotin and Gemcitabine Hydrochloride in Treating Younger Patients With Relapsed/Refractory Hodgkin Lymphoma |
CD30 | 1/2 | HL | Brentuximab Vedotin Gemcitabine Hydrochloride |
| NCT01979536 | Brentuximab Vedotin or Crizotinib and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II-IV Anaplastic Large Cell Lymphoma | CD30 | 2 | Anaplastic Large Cell Lymphoma Ann Arbor Stage II-IV Noncutaneous Childhood Anaplastic Large Cell Lymphoma |
Brentuximab Vedotin Chemotherapy drugs |
| NCT02690545 | Study of CD30 CAR for Relapsed/Refractory CD30+ HL and CD30+ NHL | CD30 | 1/2 | Lymphoma Lymphoma, Non-Hodgkin |
ATLCAR.CD30 cells |
| NCT02917083 | CD30 CAR T Cells, Relapsed CD30 Expressing Lymphoma (RELY-30) (RELY-30) |
CD30 | 1 | HL NHL |
|
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT04288726 | Allogeneic CD30.CAR-EBVSTs in Patients With Relapsed or Refractory CD30-Positive Lymphomas | CD30 | 1 | Extranodal NK/T-Cell Lymphoma, Nasal Type Classical HL |
CD30.CAR-EBVST cells |
| NCT02927769 | A Study of Nivolumab Plus Brentuximab Vedotin in Patients Between 5 and 30 Years Old, With Relapsed or Refractory HL | CD30/PD-1 | 2 | Hodgkin Disease | Biological: Nivolumab Biological: brentuximab vedotin |
| NCT03907488 | Immunotherapy (Nivolumab or Brentuximab Vedotin) Plus Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage III-IV Classic Hodgkin Lymphoma | CD30/PD-1 | 3 | HL | Brentuximab Vedotin (CD30) Filgrastim/Pegfilgrastim (G-CSF) Nivolumab (PD-1) Chemotherapy drugs |
| NCT04074746 | Bispecific Antibody AFM13 Combined With NK Cells for Patients With Recurrent or Refractory CD30 Positive Hodgkin or Non-Hodgkin Lymphomas | CD30/CD16A | 1 | HL B-cell NHL |
Anti-CD30/CD16A Monoclonal Antibody AFM13 Genetically Engineered Lymphocyte Therapy Chemotherapy drugs |
| NCT03737955 | Fractionated Gemtuzumab Ozogamicin in Treating Measurable Residual Disease in Patients With AML | CD33 | 2 | AML | Gemtuzumab Ozogamicin |
| NCT03971799 | Study of Anti-CD33 Chimeric Antigen Receptor-Expressing T Cells (CD33CART) in Children and Young Adults With Relapsed/Refractory AML |
CD33 | 1/2 | AML | CD33CART |
| NCT03537599 | Daratumumab and Donor Lymphocyte Infusion in Treating Participants With Relapsed Acute Myeloid Leukemia After Stem Cell Transplant | CD38 | 1/2 | AML | Daratumumab |
| NCT03860844 | Isatuximab in Combination With Chemotherapy in Pediatric Patients With Relapsed/Refractory ALL or AML | CD38 | 2 | ALL AML | Isatuximab Chemotherapy drugs |
| NCT04678336 | CD123 Redirected T Cells for AML in Pediatric Subjects | CD123 | 1 | AML | CART123 CAR T cells |
| NCT04158739 | Flotetuzumab for the Treatment of Pediatric Recurrent or Refractory Acute Myeloid Leukemia |
CD123 | 1 | AML | Cytarabine Flotetuzumab |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT03739606 | Flotetuzumab in Treating Patients With Recurrent or Refractory CD123 Positive Blood Cancer | CD123 | 2 | CD123 positive blood cancers | Anti-CD123/CD3 Monoclonal Antibody MGD006 |
| NCT02159495 | Genetically Modified T-cell Immunotherapy in Treating Patients With Relapsed/Refractory Acute Myeloid Leukemia and Persistent/Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm | CD123 | 1 | AML | CD123CAR-CD28-CD3zeta-EGFRt-expressing T cell cyclophosphamide Fludarabine Phosphate |
| NCT04318678 | CD123-Directed Autologous T-Cell Therapy for Acute Myelogenous Leukemia (CATCHAML) | CD123/CD20 | 1 | AML | CD123-CAR T Cyclophosphamide Fludarabine;Mesna Rituximab |
3.1. CD20
CD20 is a B cell differentiation antigen widely expressed on the surface of developing B-cells, as well as many adult B-cell malignancies. Rituximab, a chimeric anti-CD20 mAb, was the first approved antibody for use in human cancer (e.g. B-cell lymphomas). Although anti-CD20 therapies have had a limited success in pediatric cancer in the past as CD20 is not commonly expressed in either ALL or pediatric B-cell lymphomas (Jeha, et al., 2006), recent data shows that induction chemotherapy for pediatric ALL may result in upregulation of CD20 (Dworzak, et al., 2008), providing rationale for targeting CD20 in pediatric ALL. A phase III trial (NCT01516580) administered rituximab with chemotherapy and demonstrated that the combination was effective in treating high-risk, high-grade, mature B-cell non-Hodgkin’s lymphoma (NHL) in children and adolescents, with long-term remission occurring in 95% of patients (Minard-Colin, et al., 2020).
3.2. CD22
CD22 is universally expressed on the surface of most pediatric B-cell ALL (Campana, et al., 1985), and therefore has been explored as a potential immunotherapeutic target. Epratuzumab is the first humanized anti-CD22 IgG1 antibody. In pediatric ALL, epratuzumab is not administered as a single agent but it is rather combined to an established chemotherapy in clinical trials (Raetz, et al., 2008). Another anti-CD22 antibody, HB22.7, which binds to the ligand-binding domains of CD22, is under investigation (Sullivan-Chang, O’Donnell, & Tuscano, 2013). Upon antibody binding, CD22 is internalized, and therefore, immunotoxins and antibody-drug conjugates (ADCs) targeting CD22 have been developed and have generated promising results. Moxetumomab pasudotox is a recombinant immunotoxin constructed from a high-affinity anti-CD22 antibody fragment fused to a bacteria toxin (pseudomonas exotoxin). In a clinical trial, moxetumomab pasudotox has induced complete responses in 11/47 (23%) pediatric patients with relapsed/refractory B-cell ALL (Wayne, et al., 2017) (NCT00659425). The use of inotuzumab ozogamicin, which combines an anti-CD22 mAb with a cytotoxic agent that induces double-strand DNA breaks, is now approved for use in relapsed/refractory B-cell precursor ALL (Kantarjian, et al., 2016). Combotox, a 1:1 mixture of two immunotoxins synthesized by coupling deglycosylated ricin A chain to monoclonal antibodies that bind CD22 and CD19, has shown complete remission in 3/17 children with ALL (Herrera, et al., 2009).
CD22-targeted CAR T cell therapy has been investigated in patients, and is of interest as antigen loss of CD19 is one of the causes of CD19-targeted therapy resistance. In a phase I trial of a CD22-targeted CAR, a dose-dependent antileukemic response was seen in patients with CD19-negative/dim or CD19-positive relapsed B-cell ALL (Fry, et al., 2018). With ongoing enrollment, a 70% of complete remission rate was achieved among 58 patients infused with the CD22-targeted CAR T cells (N. N. Shah, et al., 2020). The use of bispecific CARs targeting CD19 and CD22 is another approach to prevent antigen escape (Qin, et al., 2018; Schneider, et al., 2017). Multiple clinical studies are currently performed in pediatric B-cell ALL patients using CD19/CD22-targeted CAR T cells (NCT03448393, NCT03330691). In addition, the sequential combination of CD19 and CD22 CAR T cells has been explored to treat patients with relapsed/refractory B-cell ALL, and the relapse-free survival is prolonged (Liu, et al., 2021).
3.3. CD30
CD30 is a member of the tumor necrosis factor (TNF) receptor superfamily. CD30 expression is restricted to a small percentage of activated B and T cells in healthy individuals. In tumor cells, CD30 expression is most commonly associated with lymphoid malignancies, for example, anaplastic large cell lymphoma (ALCL) (Leoncini, et al., 1990) and Reed-Sternberg cells in HL (Stein, et al., 1985). Treating ALCL and HL with anti-CD30 antibodies has generally been unsuccessful (Ansell, et al., 2007; Forero-Torres, et al., 2009), but the development of the ADC brentuximab vedotin (SGN-35) is a promising CD30-targeted therapy. Brentuximab vedotin consists of chimeric CD30 mAb brentuximab (cAC10) and an antimitotic agent (monomethyl auristatin E or MMAE). SGN-35 demonstrated long-term disease control in a subset of patients with relapsed or refractory HL (R. Chen, et al., 2016), and this success leads to the approval of SGN-35 for treating patients with HL after failed autologous stem cell transplantation. Recently, the efficacy of SGN-35 has been evaluated in pediatric patients with HL and ALCL. In a phase II trial by the Children’s Oncology Group, pediatric and young adult patients with HL were treated with SGN-35 and gemcitabine, and the complete remission rate was 57% (24/42) (Cole, et al., 2018). This trial has encouraged a randomized phase II study for pediatric ALCL that compares the use of SGN-35 to the anaplastic lymphoma kinase (ALK) inhibitor crizotinib and combination chemotherapy (NCT01979536). The results have not yet been reported. Two parallel phase I/II studies (NCT02690545 and NCT02917083) of CD30-targeted CAR T cells in children and adults with relapsed/refractory HL are ongoing. Their recent report of adult patients who received fludarabine-based lymphodepletion followed by CD30 CAR T cells had a high rate of durable responses with satisfying safety profile (Ramos, et al., 2020), providing insights for children enrolled in the studies.
3.4. CD33
CD33 is a myeloid differentiation antigen found on blasts of 85–90% of adult and childhood acute myeloid leukemia (AML) as well as on normal myeloid progenitors and myelocytes (Schwonzen, Diehl, Dellanna, & Staib, 2007). CD33 is expressed on a majority of AML cells but is absent on normal hematopoietic stem cells (Arndt, et al., 2013; Hoyer, Grogg, Hanson, Gamez, & Dogan, 2008), making it an ideal target for AML therapy. Efforts to target CD33 therapeutically have focused on gemtuzumab ozogamicin (GO), a selective anti-CD33 antibody-calicheamicin conjugate (Godwin, Gale, & Walter, 2017), which was approved by FDA for treatment of elderly patients with AML in 2000. In 2010, GO was withdrawn from the US and European markets after it failed to show improved efficacy versus standard of care in an adult AML phase III trial (SWAG S0106), as well as concerns about hepatic veno-occulsive disease. However, recently there has been renewed interest in GO. A report showed that combination of GO with busulfan/cyclophosphamide for poor-risk CD33+ pediatric AML did not increase the risk of veno-occlusive disease (Satwani, et al., 2012). Data from a large randomized trial in pediatric patients with AML demonstrated that administering GO in addition to conventional chemotherapy was associated with a significantly improved event-free survival and relapse-free survival (Gamis, et al., 2014). A CD3×CD33 BiTE named AMG330, similar to blinatumomab, was engineered to target AML and was shown to be very effective in recruiting and activating autologous T cells in preclinical study (Laszlo, et al., 2014). A phase I clinical trial of AMG330 (NCT02520427) in adults with relapsed/refractory AML is under way, no clinical early phase studies have yet been reported in pediatric patients with AML. Moreover, preclinical studies have demonstrated the feasibility of generating CAR T cells targeting CD33 (Kenderian, et al., 2015; O’Hear, Heiber, Schubert, Fey, & Geiger, 2015). A first-in-child CD33-targeted CAR T cell phase I trial (NCT03971799) for children and young adults with relapsed or refractory AML is currently ongoing.
3.5. CD38
CD38, a type II transmembrane glycoprotein, is expressed on thymocytes, activated T cells and terminally differentiated B cells, but expressed at very low levels on normal lymphoid and myeloid cells and on non-hematopoietic tissues. Since its expression is uniformly high on myeloma cells (Lin, Owens, Tricot, & Wilson, 2004), CD38 is a good target for novel therapeutic strategies. Daratumumab is an US FDA-approved human immunoglobulin mAb that targets CD38 and is well tolerated and effective in relapsed multiple myeloma (Lokhorst, et al., 2015; van de Donk, et al., 2016). Recent studies demonstrated that CD38 is robustly expressed in pediatric T-cell ALL, and daratumumab is effective in vivo using PDX models of human T cell-ALL (Bride, et al., 2018; Tembhare, et al., 2020), suggesting CD38 can be further developed as an immunotherapeutic target for pediatric T cell-ALL. As such, daratumumab is being evaluated in combination with standard chemotherapies in the treatment of pediatric patients with T cell-ALL (NCT03384654).
3.6. CD123
CD123, also referred to as IL-3 receptor α-chain, is highly expressed in many hematological malignancies including AML (Munoz, et al., 2001) and blastic plasmacytoid dendritic cell neoplasm (BPDCN) (Julia, et al., 2013). A report from the Children’s Oncology Group showed that children and young adults with high CD123 expression had inferior clinical outcomes compare to patients with lower CD123 expression (Lamble, et al., 2019). The negative influence of CD123 on clinical outcome of pediatric AML was further demonstrated (Kandeel, Madney, Eldin, & Shafik, 2021). Importantly, its expression in erythroid progenitors and hematopoietic cells is either low or undetectable (S. Huang, et al., 1999), making CD123 an attractive therapeutic target. SL-401 (also called tagraxofusp), a recombinant fusion protein that links IL-3 to a truncated diphtheria toxin payload, was effective and well tolerated in adult patients with BPDCN (Frankel, et al., 2014). Following the FDA’s 2018 approval of SL-401 for treating adult BPDCN patients, a recent study reported SL-401 in pediatric patients with BPDCN was well tolerated and produced a promising response (Sun, et al., 2018). Bispecific antibodies targeting CD3 and CD123 have been designed and evaluated in preclinical and early clinical research. Flotetuzumab (MGD-006), a CD3 and CD123 dual affinity retargeting antibody (DART), has shown clinical activity in adult patients with AML (Uy, et al., 2017), and is now the first bispecific antibody for AML being studied in pediatric patients. Lastly, CD123-targeted CAR T trials for patients with relapsed/refractory AML are currently ongoing. The Mustang Bio MB-102 (NCT02159495), currently under investigation at City of Hope Medical Center, has shown promising anti-leukemic activity (Budde, et al., 2017). The trial is now enrolling children and young adults with relapsed/refractory CD123+ leukemia. A first-in-child CD123 CAR T phase 1 study (NCT04318678) is also open at the St. Jude Children’s Research Hospital.
4. Emerging cell surface immunotherapeutic targets for pediatric solid tumors
Metastatic or recurrent solid tumors in pediatric patients still have a less than 20% survival (Ward, DeSantis, Robbins, Kohler, & Jemal, 2014). In order to improve survival and decrease treatment side effects, novel treatments, such as immunotherapy, are desperately needed. Here, we review the emerging cell surface molecules that may lead to the development of new targeted therapies for pediatric solid tumors. The current clinical studies on the discussed cell surface targets are listed in Table 4.
Table 4. Clinical trials involving emerging immunotherapeutic targets for pediatric CNS and solid tumors that are currently active/recruiting in the US since 2016.
CNS: Central Nervous System; DIPG: Diffuse Intrinsic Pontine Glioma
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
|---|---|---|---|---|---|
| NCT04715191 | Interleukin-15 and -21 Armored Glypican-3-speccific Chimeric Antigen Receptor Expressed in T Cells for Pediatric Solid Tumors | GPC3 | 1 | Liver Cancer Rhabdomyosarcoma Malignant Rhabdoid Tumor Liposarcoma Wilms Tumor Yolk Sac Tumor |
15.21.GPC3-CAR T cells Cytoxan Fludara |
| NCT02932956 | Glypican 3-specific chimeric antigen receptor expressed in T cells for patients with pediatric solid tumors (GAP) | GPC3 | 1 | pediatric liver cancer | GPC3 CAR T cells Cytoxan Fludara |
| NCT04377932 | Interleukin-15 Armored Glypican 3-specific Chimeric Antigen Receptor Expressed in T Cells for Pediatric Solid Tumors | GPC3 | 1 | Liver Cancer Rhabdomyosarcoma Wilms Tumor Yolk Sac Tumor |
AGAR T cells Cytoxan Fludara |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT04167618 | 177Lu-DTPA-Omburtamab Radioimmunotherapy for Recurrent or Refractory Medulloblastoma | B7-H3 | 1/2 | Medulloblastoma | 177Lu-DTPA-omburtamab |
| NCT03275402 | 131I-omburtamab Radioimmunotherapy for Neuroblastoma Central Nervous System/Leptomeningeal Metastases | B7-H3 | 2/3 | Neuroblastoma CNS Metastases Leptomeningeal Metastases |
131I-omburtamab |
| NCT04022213 | A Study of the Drug I131-Omburtamab in People With Desmoplastic Small Round Cell Tumors and Other Peritoneum Tumors | B7-H3 | 2 | Desmoplastic Small Round Cell Tumor Peritoneal Cancer |
131 I-omburtamab Radiation |
| NCT04432649 | Targeting CD276 (B7-H3) Positive Solid Tumors by 4SCAR-276 | B7-H3 | 1/2 | Solid Tumor | 4SCAR-276 |
| NCT04483778 | B7H3 CAR T Cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | B7-H3 | 1 | Solid Tumor | second generation 4-1BBζ B7H3-EGFRt-DHFR a second generation 4-1BBζ CD19-Her2tG |
| NCT04185038 | Study of B7-H3-Specific CAR T Cell Locoregional Immunotherapy for Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma and Recurrent or Refractory Pediatric Central Nervous System Tumors |
B7-H3 | 1 | Pediatric Central Nervous System Tumors DIPG Diffuse Midline Glioma |
SCRI-CARB7H3(s); B7H3-specific chimeric antigen receptor (CAR) T cells |
| NCT04510051 | CAR T Cells After Lymphodepletion for the Treatment of IL13Rα2 Positive Recurrent or Refractory Brain Tumors in Children | IL-13Rα2 | 1 | Malignant Brain Neoplasm Recurrent/Refractory Malignant Brain Neoplasm |
IL13Ralpha2-specific Hinge-optimized 4-1BB CAR Truncated CD19-expressing T |
| NCT02208362 | Genetically Modified T-cells in Treating Patients With Recurrent or Refractory Malignant Glioma |
IL-13Rα2 | 1 | Glioblastoma Malignant Glioma Grade II/III Glioma |
IL-13Rα-specific Hingeoptimized 4-1BB CAR/Truncated CD19-expressing T cells |
| NCT03638167 | EGFR806-specific CAR T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric Central Nervous System Tumors | EGFR | 1 | Pediatric CNS tumors | EGFR806-specific chimeric antigen receptor (CAR) T cell |
| NCT03618381 | EGFR806 CAR T Cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | EGFR | 1 | Pediatric Solid Tumors | 2nd generation 4-1BBζ EGFR806-EGFRt |
| NCT04616560 | Trastuzumab Deruxtecan for the Treatment of HER2+ Newly Diagnosed or Recurrent Osteosarcoma | Her2 | 2 | Osteosarcoma | Trastuzumab Deruxtecan |
| Identifier | Study name | Target | Phase | Disease (s) | Intervention (s) |
| NCT00902044 | Her2 Chimeric Antigen Receptor Expressing T Cells in Advanced Sarcoma | Her2 | 1 | Sarcoma | HER2 CAR T cells Fludarabine,Cyclophosphamide |
| NCT03500991 | HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors |
Her2 | 1 | Central Nervous System Tumor, Pediatric |
HER2-specific chimeric antigen receptor (CAR) T cell |
| NCT02442297 | T Cells Expressing HER2-specific Chimeric Antigen Receptors(CAR) for Patients With HER2-Positive CNS Tumors (iCAR) | Her2 | 1 | Brain Tumor | HER2-specific T cells |
| NCT02470091 | Denosumab in Treating Patients With Recurrent or Refractory Osteosarcoma | RANKL | 2 | Osteosarcoma | Denosumab |
| NCT01343043 | A Pilot Study of Genetically Engineered NY-ESO-1 Specific NY-ESO-1c259T in HLA-A2+ Patients With Synovial Sarcoma | NY-ESO-1 | 1 | Neoplasms | NY-ESO-1(c259)T Cells |
| NCT03240861 | Genetically Engineered PBMC and PBSC Expressing NY-ESO-1 TCR After a Myeloablative Conditioning Regimen to Treat Patients With Advanced Cancer |
NY-ESO-1 | 1 | HLA-A*0201 Positive Cells Present NY-ESO-1 Positive Unresectable Tumors |
Cell Therapy Chemotherapy |
| NCT02650986 | Gene-Modified T Cells With or Without Decitabine in Treating Patients With Malignancies Expressing NY-ESO-1 |
NY-ESO-1 | 1/2 | Advanced/Metastatic NY-ESO-1-positive Tumors | TGFbDNRII-transduced Autologous Tumor Infiltrating Lymphocytes |
| NCT02789228 | Research Study Utilizing Expanded Multi-antigen Specific Lymphocytes for the Treatment of Solid Tumors (REST) | WT1/PRAME/Survivin | 1 | Solid tumors | Tumor associated antigen lymphocytes (TAA-CTL) |
| NCT03652545 | Multi-antigen T Cell Infusion Against Neurooncologic Disease (REMIND) | WT1/PRAME/Survivin | 1 | Brain tumors | TAA-T |
| NCT04044768 | Spearhead 1 Study in Subjects With Advanced Synovial Sarcoma or Myxoid/Round Cell Liposarcoma |
MAGE-A4 | 2 | Synovial Sarcoma Myxoid Liposarcoma |
ADP-A2M4 |
4.1. Glypicans
Glypicans have a 60–70 kDa core protein with varying numbers of heparan sulfate (HS) glycosaminoglycan chains (Jorge Filmus & Selleck, 2001; Ho & Kim, 2011). Glypicans are anchored to the cell surface by a glycosylphosphatidylinositol (GPI) anchor (Bernfield, et al., 1999). All glypicans have 14 absolutely conserved cysteines which form 7 disulfide bonds that stabilize the globular three-dimensional structure of the core protein (J. Filmus, Capurro, & Rast, 2008; Ho & Kim, 2011). In total, there are six glypican family members found in humans, named glypican 1 (GPC1) through glypican 6 (GPC6).
4.1.1. GPC3
GPC3 is an oncofetal protein that is overexpressed in hepatoblastomas and Wilms tumors, among other pediatric solid embryonal tumors (Toretsky, et al., 2001), germ cell tumors like yolk sac tumors (YST) (Esheba, Pate, & Longacre, 2008), and a minority of rhabdomyosarcoma (RMS) (Chan, et al., 2013). Various immunotherapeutic approaches targeting GPC3 in adult cancers have been investigated, offering a potential means to treat these pediatric solid embryonal tumors. A phase I clinal trial (UMIN000006357) in Japan demonstrated that the GPC3 peptide vaccination induced a GPC3-specific CTL responses in 7/18 patients, and the use of GPC3-peptide vaccine could prevent the recurrence of pediatric solid tumors, especially hepatoblastomas (Tsuchiya, et al., 2017). A clinical trial of GPC3-targeted CAR T cells for treating patients with pediatric solid tumors (NCT02932956) is ongoing.
4.1.2. GPC2
4.1.2.1. Biology of GPC2
GPC2 protein, referred to as HSPG M13, was first identified as a unique core protein by SDS-PAGE from the membrane fraction of proteoglycans derived from postnatal day 0 rat brain (Herndon & Lander, 1990). The GPC2 gene is at locus 7q22.1 (J. Filmus, et al., 2008) and encodes for a 579 amino acid protein, though amino acids 555–579 comprise a propeptide that is removed in the mature form. The C-terminus has the GPI attachment site, at G554, and the N-terminus encodes a signal peptide, from M1 to S24. The structural features of GPC2 are shown in Fig. 1A.
Fig. 1.
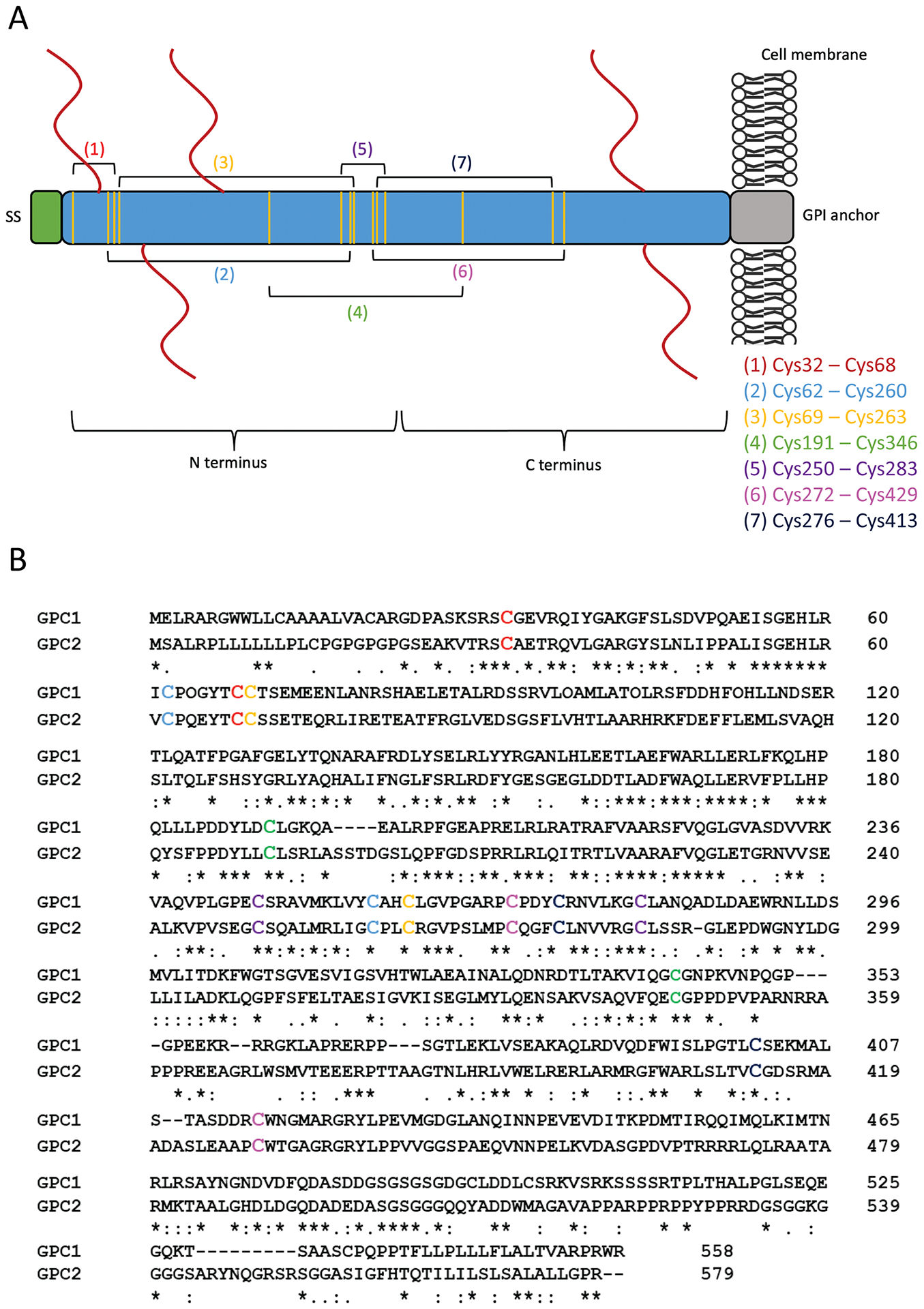
Structure and sequence of GPC2. (A) Schematic of the GPC2 protein. The C terminus is located at the cell surface; the N terminus is distal to the cell surface. The mature protein is 579 amino acids. The N-terminal signal peptide (SS) comprises the first 24 amino acids. The glycosylphosphatidylinositol (GPI) anchor attachment is located at G554. The predicted heparan sulfate (HS) chains are shown by red lines at S55, S92, S155, S500, and S502. The conserved cysteine residues are represented by the vertical yellow lines. Brackets drawn between yellow lines denote the 7 predicted disulfide bonds. The specific arrangement of the bonds is listed. (B) The sequence alignment of GPC1 and GPC2. The cysteine residues involved in the formation of disulfide bonds are shown in color corresponding to (A). The serine-glycine residues that serve as HS attachment sites on the GPC2 core protein are shown in black boxes.
The crystal structure of GPC2 has yet to be solved, but the structure of the GPC1 core protein has been previously reported (Svensson, Awad, Hakansson, Mani, & Logan, 2012). Here, we performed a multiple sequence alignment with GPC1 and GPC2 (UniProt accession numbers: P35052 and Q8N158, respectively) to predict the location of the GPC2 disulfide bonds, based on the structure model reported for GPC1. The alignment is shown in Fig. 1B and the predicted disulfide bond arrangement is described in Fig. 1A. GPC2 is predicted to have five HS insertion sites (predicted by UniProt; accession number Q8N158). It has long been thought that all glypicans carry two highly conserved HS chains near the cell surface (Bernfield, et al., 1999; J. Filmus, et al., 2008; Ho & Kim, 2011; Song & Filmus, 2002). Indeed, GPC2 is predicted to have two HS insertion sites at the C-terminus (S500 and S502). However, it is also predicted that GPC2 has three additional insertion sites at the N-terminus (S55, S92, S155), a feature unique among the glypicans. HS chains are attached to the core protein by tetrasaccharide linker to a serine that is N-terminal to a glycine (Fig. 1B). The linker is composed of a xylose (O-glycosylated to the serine), two galactose residues, and a glucuronic acid (GlcA) (Hacker, Nybakken, & Perrimon, 2005; Turnball, Powell, & Guimond, 2001). From there, a chain of repeating N-acetylglucosamine and glucuronic acid disaccharides (-GlcNA-GlcA-) forms. After polymerization, HS chains undergo a number of enzyme-catalyzed modifications, including epimerization and sulfation (Esko & Selleck, 2002). Previous work by our lab has shown that particular HS chain modifications are required for Wnt binding (Gao, Xu, Liu, & Ho, 2016). The Wnt binding motif requires a length of at least 6 saccharide residues and the presence of 6-O-sulfations; 3-O-sulfations are not required but enhance Wnt binding (Gao, et al., 2016). The presence of the unique N-terminal HS chains on GPC2, as well as the modified composition of those chains, likely contributes to the function of GPC2.
Outside of pediatric cancers, GPC2 is thought to function in axonal growth and guidance in the developing nervous system. GPC2 is expressed by neurons immediately after the terminal mitosis and disappears by the time the final synaptic contact is made (Stipp, Litwack, & Lander, 1994), suggesting that GPC2 functions in the motile behaviors of neurons. Immunostaining of cryostat sections of the P0 rat brain confirmed that GPC2 expression occurs in a specific spatio-temporal manner, where GPC2 expression correlates with the timing of axonal outgrowth (Ivins, Litwack, Kumbasar, Stipp, & Lander, 1997). Indeed, GPC2 was shown to be expressed on neuronal growth cones both by immunostaining of cultured retinal neurons and by SDS-PAGE performed on the proteoglycan fraction isolated from growth cone particles (Ivins, et al., 1997). Other HSPGs have been implicated in axon guidance (Johnson, et al., 2004) and disruption of HS chain synthesis in embryonic mice revealed the necessity of HS in axon guidance (Inatani, Irie, Plump, Tessier-Lavigne, & Yamaguchi, 2003). The importance of HS in axon guidance may give context to the GPC2’s uniquely high number of HS chains. However, the precise mechanisms by which it functions has yet to be studied, and there are multiple speculative ways in which GPC2 may function in neuronal development.
4.1.2.2. GPC2 expression and signaling in neuroblastoma
Recently, GPC2 has been studied as a neuroblastoma tumor antigen (Bosse, et al., 2017; N. Li, Fu, Hewitt, Dimitrov, & Ho, 2017). In addition to high GPC2 mRNA expression (Orentas, et al., 2012), GPC2 protein expression has been confirmed in multiple neuroblastoma cell lines by Western blotting (N. Li, et al., 2017). Our lab isolated a human single domain antibody (LH7) by phage display, which binds GPC2 with high affinity. Immunohistochemistry (IHC) using LH7 confirmed GPC2 expression in a majority (52%) of neuroblastoma tumor tissues (N. Li, et al., 2017). Western blotting with another antibody showed GPC2 expression in neuroblastoma primary tumors, patient-derived xenografts (PDX), and a majority of neuroblastoma cell lines tested (Bosse, et al., 2017). We have recently isolated a high-affinity mouse monoclonal anti-GPC2 antibody (CT3) (Ho, Li, & Fleming, 2019). IHC using the CT3 mAb also showed that GPC2 is overexpressed in neuroblastoma, when compared to normal nerve tissue (N. Li, Spetz, & Ho, 2020). Importantly, there is a correlation between high GPC2 and poor neuroblastoma prognosis, including poorer overall survival, found by integrating clinical and genomic data from neuroblastoma patients (N. Li, et al., 2017).
MYCN amplification has long been associated with poor prognosis in neuroblastoma, including overall survival (Brodeur, Seeger, Schwab, Varmus, & Bishop, 1984; Seeger, et al., 1985). Amplification of MYCN in neuroblastoma is associated with the overexpression of the protein it encodes, N-myc (Brodeur, 2003). GPC2 expression might be related to MYCN amplification in neuroblastoma (Bosse, et al., 2017; N. Li, et al., 2017). Stratification of high-risk neuroblastoma tumor datasets revealed that MYCN amplification is associated with higher GPC2 expression (Bosse, et al., 2017). The GPC2 promoter contains a canonical E-box motif that N-myc binds in multiple MYCN-amplified neuroblastoma cell lines, suggesting that N-myc may directly upregulate GPC2 protein expression. Conversely, depletion of N-myc in MYCN-amplified neuroblastoma cell lines downregulated GPC2 protein expression (Bosse, et al., 2017). Genetically silencing GPC2 in neuroblastoma cell lines resulted in suppression of N-myc expression (N. Li, et al., 2017).
It is possible that N-myc overexpression in neuroblastoma also occurs as a downstream effect of Wnt/ß-catenin signaling, with GPC2 playing a regulatory role. The Wnt/ß-catenin pathway is known to function in pathogenesis in numerous cancers (Clevers & Nusse, 2012), thus possibly playing a role in neuroblastoma proliferation. The Wnt signaling pathway was shown to be GPC2 associated in an independent pathway analysis performed on high-risk neuroblastoma RNAseq data (Bosse, et al., 2017). Additionally, GPC3 has been established as a co-receptor in the canonical Wnt signaling pathway in HCC (Cappuro, Xiang, Lobe, & Filmus, 2005; Gao & Ho, 2011; Gao, et al., 2015; N. Li, et al., 2019b). The possible roles of GPC2 in modulating Wnt/ß-catenin signaling to increase neuroblastoma proliferation are described in Fig. 2.
Fig. 2.
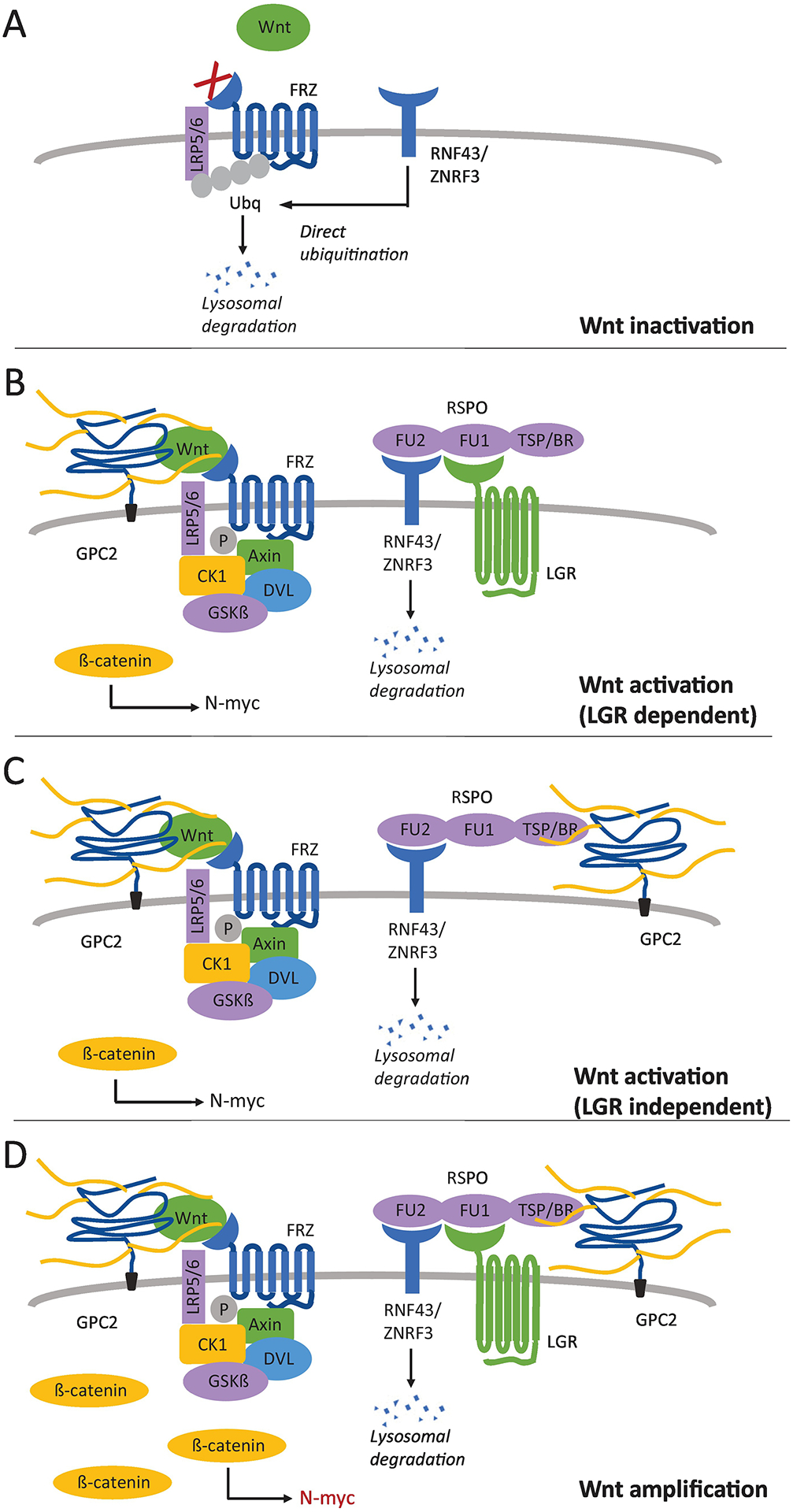
Model of GPC2’s role in Wnt signaling. (A) Without binding of RSPO, RNF43/ZNRF3 inactivate Wnt receptors at the cell surface by direct ubiquitination. This prevents Wnt from binding to LRP5/6 and FRZ, leading to a low signal. (B) RSPO can bind RNF43/ZNRF3 through its furin-like domain 1 (FU1), preventing the inactivation of Wnt receptors, if another RSPO receptor is present. The FU2 domain of RSPO can bind LGRs, leading to the internalization and degradation of RNF43/ZNRF3, and maintaining active levels of Wnt receptors at the cell surface. When Wnt binds to its receptors at the cell surface, the interaction is stabilized by GPC2, and accumulation of ß-catenin in the cytoplasm ultimately leads to the expression of downstream target genes (N-myc). (C) In the absence of LGRs, GPC2 can act as a RSPO co-receptor by binding the thrombospondin type 1 domain (TSP) and basic region (BR) of RSPO. Similarly, this leads to degradation of RNF43/ZNRF3, and activation of the Wnt signal. (D) RSPO can simultaneously bind both LGR and GPC2, ultimately leading to an amplification of the Wnt signal and increased expression of N-myc.
The canonical Wnt signaling pathway, and the role of GPC3 in modulating the pathway, has been described (Kolluri & Ho, 2019; N. Li, et al., 2019b). Briefly, Wnt binds to two co-receptors: Frizzled (FZD), a seven-pass transmembrane G protein receptor, and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), a single-pass transmembrane receptor. This causes Axin, normally part of a destruction complex for ß-catenin, to associate with LRP5/6, causing an accumulation of ß-catenin in the cytoplasm. ß-catenin is a co-activator of T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, causing transcription of downstream target genes. In HCC, GPC3 stabilizes the interaction of Wnt with its receptor FRZ, forming a signaling complex of GPC3, Wnt, FRZ, and LRP5/6 on the cell surface (Capurro, Martin, Shi, & Filmus, 2014; Gao & Ho, 2011; N. Li, et al., 2019a), increasing ß-catenin in the cytoplasm and ultimately upregulating the expression of downstream cell proliferation target genes. Given the closely related structure and functions of the glypican family members, it is possible that GPC2 acts to modulate Wnt/ß-catenin signaling in the same manner as GPC3, with MYCN as a downstream target gene in neuroblastoma. Coimmunoprecipitation has shown that GPC2 can interact with Wnt3a (N. Li, et al., 2017). Additionally, neuroblastoma cell lines that express GPC2 also express ß-catenin and levels of active ß-catenin decrease when neuroblastoma cell lines are treated with an anti-GPC2 antibody. This suggests that GPC2 has a stimulatory effect on Wnt signaling to upregulate expression of N-myc protein.
More recent studies show that glypicans may play several different regulatory roles in the Wnt signaling pathway (Fig. 2). Proteins called R-spondins (RSPOs) can amplify Wnt signals (Lebensohn & Rohatgi, 2018). Levels of available Wnt receptors (FRZ and LRP5/6) on the cell surface are kept low in the presence of ZNRF3 and RNF43, transmembrane E3 ligases, which inactivate FRZ and LRP5/6 through direct ubiquitination. RSPOs can interact with ZNRF3/RNF43, causing their endocytosis and subsequent lysosomal degradation, thus activating Wnt receptors at the cell surface and amplifying the response. However, RSPOs also have to simultaneously bind at least one other receptor in order to cause the degradation of ZNRF3/RNF43, usually a leucine-rich repeat-containing G-protein coupled receptor (LGR). Alternatively, it has recently been shown that RSPOs can interact with HSPGs, in particular glypicans, this interaction can trigger RSPO potentiation of Wnt signaling in the absence of LGRs (Lebensohn & Rohatgi, 2018). Additionally, the interaction of RSPOs with HSPGs can amplify the signal response in the presence of LGRs. Glypicans may be able to amplify Wnt signaling by interacting with RSPOs to increase the availability of Wnt receptors.
4.1.2.3. Expression of GPC2 in other childhood cancers
In addition to neuroblastoma, high GPC2 mRNA expression was found in additional childhood cancers including desmoplastic round small cell tumors, Ewing’s sarcoma, osteosarcoma, rhabdomyosarcoma, and ALL (Orentas, et al., 2012). Medulloblastoma and retinoblastoma also displayed increased GPC2 levels similar to that of neuroblastoma (Bosse, et al., 2017). Group 4 medulloblastoma, which is also known to have poorer outcomes in the presence of MYCN amplification, has higher GPC2 mRNA expression than other medulloblastoma groups. (Bosse, et al., 2017). This group can also have MYCN amplification, where MYCN-amplified cases have a significantly worse outcome (Kool, et al., 2012). Additionally, MYCN amplification is one of the most common mutations in retinoblastoma (N. Wu, et al., 2017).
4.1.2.4. GPC2 as a therapeutic target for treating childhood cancers
GPC2 shows promise as a therapeutic target in neuroblastoma for several reasons. First, its expression is extremely tumor specific. IHC using the LH7 single-domain antibodies showed no specific staining in any normal tissues, including in essential organs (N. Li, et al., 2017). Additional IHC analysis from another anti-GPC2 antibody further confirmed highly restricted GPC2 expression in normal tissues, even in other tissues derived from the neural crest (Bosse, et al., 2017). Unlike GD2, which is expressed at low levels on normal nerves and caused neuropathic pain in dinutuximab immunotherapy (A. L. Yu, et al., 2010), GPC2-targeted treatments may avoid this side effect. Second, neuroblastoma appears to be dependent on GPC2 for cell proliferation. Our work indicates that GPC2 regulates cancer cell proliferation through its action in the Wnt signaling pathway. Components of the Wnt signaling pathway are attractive therapeutic targets because Wnt frequently directly influences tumor cell proliferation and differentiation (Madan & Virshup, 2015). GPC3-targeted immunotoxins and CAR T cells have been proven to be potentially effective treatments for HCC, due in part to their inhibition of Wnt signaling (Gao, et al., 2015; D. Li, et al., 2020). Inhibition of Wnt by targeting GPC2 may prove to be just as effective in treating neuroblastoma and other pediatric malignancies.
Several immunotherapies have been explored that target GPC2 for the treatment of neuroblastoma. Our lab has constructed CAR T cells targeting GPC2 using the LH7 single domain, CD8α hinge and transmembrane domains, a 4-1BB co-stimulatory domain, and a CD3ζ signaling domain in the cytoplasm (N. Li, et al., 2017). The LH7 CAR T cells potently killed GPC2-positive neuroblastoma cell lines in a luminescence-based in vitro cytolytic assay and regressed disseminated neuroblastoma tumors in mice. We constructed immunotoxins using the LH7 single domain antibody and a fragment of pseudomonas exotoxin, which were able to regress neuroblastoma tumors in mice (N. Li, et al., 2017). An ADC constructed from the DNA damaging agent pyrolobenzodiazepine and D3-GPC2-IgG1, an anti-GPC2 IgG converted from an anti-GPC2 Fab from a human library, also demonstrated neuroblastoma cytotoxicity in a murine PDX model (Bosse, et al., 2017). All three GPC2-targeted therapies support the use of GPC2 as a therapeutic target in neuroblastoma and other GPC2-positive pediatric cancers.
4.2. B7-H3 (CD276)
4.2.1. Biology of B7-H3
B7 homolog 3 (B7-H3, CD276) is an immune checkpoint member that belongs to B7-CD28 family (Picarda, Ohaegbulam, & Zang, 2016). B7-CD28 superfamily consist of seven members that belong to 3 groups by phylogenetic classification: group I contains B7-1, B7-2, and B7h; group II consists of PD-L1 and PD-L2; and group III includes B7-H3 and B7x (B7-H4) (Hofmeyer, Ray, & Zang, 2008; Zang, et al., 2003). Human B7-H3 (2Ig-B7-H3) was cloned in 2001, which is 316 amino acid in length and shares 20–27% amino acid identity with other B7 family members (Chapoval, et al., 2001). The 2Ig-B7-H3, a type I transmembrane protein, consists of a signal peptide in its NH2-terminus, single extracellular V-like and C-like (VC) Ig domains, a transmembrane region, and a cytoplasmic tail (Fig. 3). Subsequently, another long isoform of B7-H3, termed as 4Ig-B7-H3 (B7-H3b), was identified by sequencing and confirmed by mAbs recognition on molecules of human dendritic cell (Steinberger, et al., 2004b; M. Sun, et al., 2002). 4Ig-B7-H3 consists of two sets of VC-like Ig domains, forming a structure of single peptide-IgV-IgC-IgV-IgC-TM-cytoplasmic tail, and the sequences of the two IgV-IgC pairs are highly homologous (95% identity) because of exon duplication and differential splicing (Fig. 3). Both human B7-H3 isoforms are located in chromosome 15, and 4Ig-B7-H3 was finally found as the dominantly expressed form in human tissues with no functional difference compared with 2Ig-B7-H3 (Y. H. Zhou, et al., 2007). In contrast, mouse B7-H3 homolog contained only one short isoform, 2Ig-B7-H3, which contains a single copy of IgV-IgC domains, with 88% identity and 93% similarity compared to the human counterpart. Mouse B7-H3 was identified with 315 amino acids in length, and located in in chromosome 9 (M. Y. Sun, et al., 2002). Structural analysis of B7-H3 genes indicated that gene duplication events occurred after the separation of mouse and human ancestors, though the functional implication of this gene duplication event is unclear. Recently, crystal structure of murine B7-H3 at a 3 Å resolution has been solved, which provides a model for the formulation of the IgV-IgC domains in external domains. They revealed that the FG loop of the IgV domain was crucial for B7-H3 to inhibit T cell proliferation (Vigdorovich, et al., 2013).
Fig. 3.
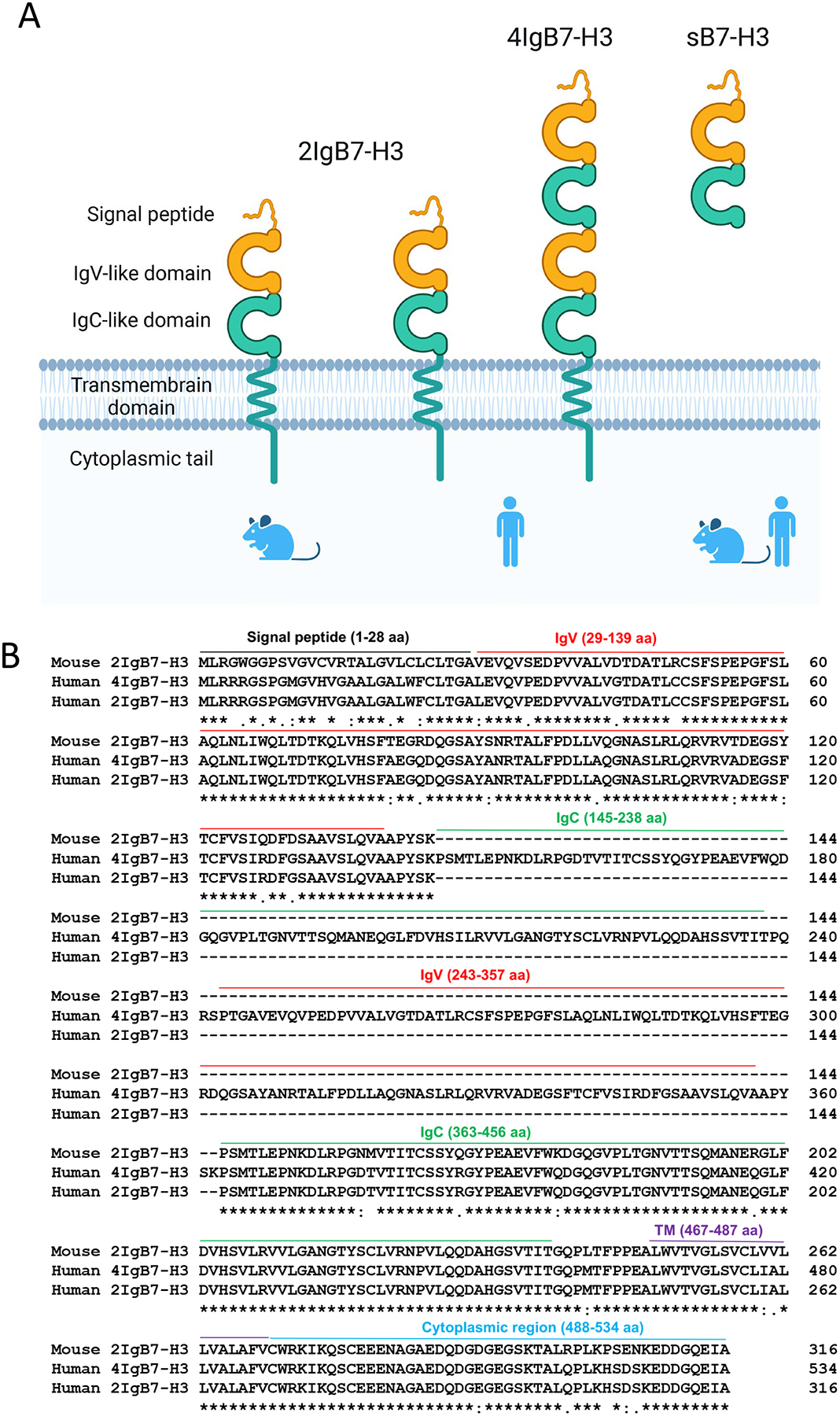
Identification of B7-H3 isoforms. (A) B7-H3 is a type I transmembrane protein that belongs to the Ig superfamily. 2IgB7-H3 exists in both human and mouse, whereas 4IgB7-H3 only exists in human. 4Ig-B7-H3 isofom consists of two pairs of VC-like Ig domains, forming a structure of single peptide-IgV-IgC-IgV-IgC-TM-cytoplasmic tail. In addition, soluble isoform B7-H3 (sB7-H3) exists in both mouse and human serum/plasma. (B) Sequence alignment of human 4IgB7-H3 with 2IgB7-H3 isoform of both human and mouse. The leader peptide is indicated by black solid line; IgV- and IgC-like domains are indicated by red and green lines; transmembrane region is indicated by purple line; cytoplasmic tail is indicated by blue line.
Like other members of B7-CD28 superfamily such as B7-H4, soluble isoform B7-H3 (sB7-H3) was also found in normal human serum/plasma samples and supernatants of monocytes, DCs, and activated T cells culture (G. B. Zhang, et al., 2008). sB7-H3 is shed from B7-H3 expressed on the surface of these activated cells by a matrix metalloproteinase (MMP). Another sB7-H3 isoform was produced by alternative splicing from the 4th intron of B7-H3. It contains an additional 4 amino acids, encoded by the intron sequence, at the C-terminus compared to the ectodomain of 2Ig-B7-H3 (W. Chen, et al., 2013). Furthermore, the concentrated sB7-H3 was able to compete with recombinant B7-H3Ig protein for binding to a putative B7-H3 receptor on the activated T cells, indicating sB7-H3 was an active form which might respond to the surface-bound B7-H3 (G. B. Zhang, et al., 2008). Increasing evidence has shown an association between increased sB7-H3 levels and unfavorable prognosis in cancer patients, implying that sB7-H3 expression can serve as a tumor biomarker for clinical diagnosis (F. Xu, et al., 2017; G. Zhang, et al., 2009; L. Zhao, et al., 2017). A previous report showed significantly higher level of sB7-H3 in osteosarcoma (OS) patients in comparison with healthy people, which suggest serum sB7-H3 could be used for OS diagnosis (L. Wang, et al., 2018). These clinical evidence have demonstrated that high levels of sB7-H3 from tumors may correlate with tumor progression and poor prognosis.
4.2.2. B7-H3 expression in childhood cancers
4Ig-B7-H3 encodes a 110-kDa protein. B7-H3 mRNA is found ubiquitously expressed in most tissues both in human and mouse and in various carcinoma cells (M. Sun, et al., 2002). However, B7-H3 protein is not constitutively expressed. B7-H3 is not detectable in peripheral blood mononuclear cells (M. Sun, et al., 2002), whereas induced expression of B7-H3 is found on dendritic cells (DCs) and monocytes upon in vitro stimulation (Chapoval, et al., 2001; Steinberger, et al., 2004a). Indeed, B7-H3 can be induced on DCs by the key TH1 cytokine interferon γ (IFN-γ) and suppressed by TH2 cytokine Interleukin-4 (IL-4) (Suh, et al., 2003). This result indicates that B7-H3 may provide a negative feedback loop during the amplification phase of TH1 reaction, but not affect TH2 responses. B7-H3 protein expression in elevated in multiple adult malignancies and correlates with poor prognosis, including prostate cancer (Zang, et al., 2007), ovarian carcinomas (J. Zhang, et al., 2017), oral squamous carcinoma (Z. Li, Liu, Que, & Tang, 2019), lung adenocarcinoma (T. T. Yu, Zhang, Lu, & Wang, 2018), pancreatic cancer (Inamura, et al., 2018), craniopharyngioma (C. Chen, et al., 2019), and AML (Guery, et al., 2015). Using immunohistochemical tissue microarray, Zhang et al. reported that 93 out of 103 tested ovarian tumor tissues express B7-H3, which is mainly distributed on cell membranes and in the cytoplasm (Zang, et al., 2010). Moreover, various levels of B7-H3 were also observed in some tumor-adjacent normal tissues, though with less staining intensity compared to tumor tissues (Z. Zhang, et al., 2020). In contrast, limited B7-H3 expression was found in human normal tissues including prostate, breast, placenta, lung, liver, colon, and lymphoid organs (Maachani, et al., 2020; Seaman, et al., 2017; Z. Zhang, et al., 2020). In addition to cancer cells, B7-H3 was detected in the endothelium of tumor-associated vasculature in 44% of ovarian carcinomas patients, and a high expression level was associated with a significantly shorter survival time and a higher incidence of recurrence (Zang, et al., 2010). Moreover, a study demonstrated that B7-H3 protein expression is increased in tumor vessels of human lung, breast, colon, endometrial, renal and ovarian cancer, but not in the angiogenic vessels of normal ovary (Seaman, et al., 2017). These findings make B7-H3 a potentially ideal therapeutic target for the tumor microenvironment.
Importantly, B7-H3 is highly expressed in pediatric solid tumors, including neuroblastoma, central nervous system (CNS) malignancies and bone cancers (Du, et al., 2019b). B7-H3 was detected in a panel of neuroblastoma cell lines, including SK-N-BE, SH-SY-5Y, GI-LI-N, HTLA230, and ACN (Castriconi, et al., 2004). Staining with human B7-H3 specific mAb 376.96, Du et al. showed high protein level of B7-H3 in cryosectioned human NB sample as well as human NB cell lines (CHLA-255, IMR- 32, LAN-1, and SKNLP) (Du, et al., 2019b). Moreover, B7-H3 was found to be present in NB cell lines-derived exosomes which can promote cancer growth and dissemination (Marimpietri, et al., 2013).
B7-H3 has been found in multiple pediatric glial and nonglial CNS tumors, and its abnormal expression was correlated with tumor grade according to immunohistochemical staining intensity (Maachani, et al., 2020). Moreover, high B7-H3 mRNA expression in pediatric gliomas and ependymomas is significantly associated with shorter survival rate (Maachani, et al., 2020). It has been observed that the B7-H3 staining was more intense in high-grade than in low-grade tumor tissues from glioma patients (Maachani, et al., 2020). IHC data shows majority of pediatric brain tumors tissues, including medulloblastoma, high-grade gliomas and diffuse intrinsic pontine glioma (DIPG) are B7-H3 positive (R. G. Majzner, et al., 2019). Increased B7-H3 expression was reported in 91.8% (56/61) of the osteosarcoma lesions, and its level was correlated with poor prognosis in patients with osteosarcoma (L. Wang, et al., 2013). Beyond osteosarcoma, high expression B7-H3 was also found in Ewing’s sarcoma (Orentas, et al., 2012), myeloma (D. D. Zhao, et al., 2013), and osteoblastomas (Al-Sukaini, et al., 2017). These findings indicated that B7-H3 may serve as a novel target for immunotherapy against bone cancers.
4.2.2. B7-H3-mediated signaling pathways
B7-H3 has been proven to be involved in tumorigenesis. A clarification of its role and underlying molecular mechanisms is beneficial for the development of effective clinical utilizations (Fig. 4).
Fig. 4.
Potential role of B7-H3 in immunity and tumorigenesis. In innate immunity, B7-H3 inhibited cytolytic activity of NK cells, whereas promoted proinflammatory cytokine production in activated monocytes/macrophages. In adoptive immune response, B7-H3 protein could induce T-cell proliferation and enhance IFN-γ and IL-10 production. In a B7-H3 deficient mouse model, B7-H3 is a negative regulator that preferentially affects TH1 responses. Both membrane B7-H3 isoforms and soluble B7-H3 were widely expressed in tumor cells and tumor microenvironment, playing an impressive proliferative role in the tumorigenesis. Mechanically, B7-H3 was involved in regulating tumor anti-apoptosis, pro-proliferation, angiogenesis, and tumor microenvironment through JAK2/STAT3, NF-κB, or PI3K/Akt signaling pathways. Therefore, these findings indicated that B7-H3 may serve as a novel target for cancer immunotherapy.
4.2.2.1. The roles of B7-H3 in regulating immune responses
The B7-H3 pathway plays a dual role in regulating the innate immune responses. B7-H3 has been shown to inhibit NK cell activation. In ovarian cancer, MiR-29c downregulated tumor B7-H3 expression to inhibit NK cell exhaustion and associated with glycolysis of cancer cell (Deng, Wu, Zhang, Jin, & Miao, 2021). Neuroblastoma is one of the most common childhood solid tumors, and the infusion of activated NK cells involved in its immunotherapy. It has been reported that neuroblastoma-associated cell surface 4Ig-B7-H3 molecules expression coved NK cell-mediated lysis by interacting with an undefined inhibitory receptor expressed on NK cells (Castriconi, et al., 2004). In contrast, B7-H3 was demonstrated as a costimulatory of innate immunity by accumulating proinflammatory cytokine production in monocytes/macrophages via both TLR4- and TLR2-dependent mechanisms (G. Zhang, et al., 2010). Therefore, further studies are required to elucidate the role of B7-H3 in innate immunity.
In adoptive immune response, T cell co-stimulation and co-inhibition, generated mainly by the interaction between B7 family ligands and their receptors in CD28 family, is crucial for T cell-mediated antitumor immunity. The immunological function of B7-H3 has been controversial. Initial studies reported that synthetic soluble B7-H3 protein could bind to activated T cells and co-stimulated their proliferation and interferon γ (IFN-γ) production, indicating B7-H3 is a co-stimulatory molecule participating in T cell immune responses (Chapoval, et al., 2001; M. Y. Sun, et al., 2002). B7-H3 protein was able to induce T-cell proliferation and enhance IFN-γ and IL-10 production (G. B. Zhang, et al., 2004). However, several studies have contradicted those findings. B7-H3 down-regulated T cell responses by binding to an inhibitory receptor (Ling, et al., 2003; Steinberger, et al., 2004a). The study of B7-H3 deficient mice also supports that B7-H3 negatively regulate TH1 responses (Suh, et al., 2003). Therefore, further studies are needed to clarify the in vivo functions of B7-H3. Both co-stimulation and co-inhibition roles of B7-H3 suggested that two B7-H3 receptors with different functions might exist to regulate its immune reaction. However, the actual B7-H3 receptor remains unknown despite human fusion B7-H3-Ig molecules recognizing a putative receptor expressed on activated CD4+ and CD8+ T cells. Recently, the triggering receptor expressed on myeloid cell-like transcript 2 (TREML2/TLT-2) has been described as a costimulatory receptor of murine B7-H3 and enhance T cell response (Hashiguchi, et al., 2008), but it does not recognize human B7-H3 (Leitner, et al., 2009). In contrast, Leitner et al. claimed their data do not support TLT-2 acts as a receptor for murine B7-H3 (Leitner, et al., 2009).
4.2.2.2. The signaling of B7-H3 in cancer
Beyond dual immunomodulatory effects of B7-H3, accumulated data have begun to focus on its novel non-immunological roles in cancer development. It has been recently reported that B7-H3 plays an impressive proliferative role in the tumorigenesis, and its underlying mechanisms is possibly related to anti-apoptosis, pro-proliferation, angiogenesis, and tumor microenvironment (X. Zhou, et al., 2019). Although the mechanism of how B7-H3 promotes tumors independent of immunomodulation is not thoroughly understood, JAK2/STAT3, NF-κB, PI3K/Akt signaling pathway are reported to be involved in this non-immunological regulation.
The JAK2/STAT3 pathway is associated with anti-apoptosis process of cancer cells. It was found that silencing B7-H3 reduces the ability of ovarian tumor cell invasion, migration and proliferation, but increases cell apoptosis (upregulated Bax, caspase-8, cleaved caspase-8 and decreased Bcl-2, Bcl-xl, MMP-2). In contrast, overexpression of B7-H3 enhances ovarian tumor cell capacities in invasion, migration and proliferation via JAK2/STAT3 pathway (J. Zhang, et al., 2017). Moreover, ectopic expression of B7-H3 can boost anti-apoptosis of colorectal tumor cell by JAK2/STAT3, which increases the expression of Bcl-2 and Bcl-xL (T. Zhang, Jiang, Zou, Liu, & Hua, 2015). Furthermore, overexpression of B7-H3 can enhance glioma cell proliferation via the activation of the JAK2/STAT3/Slug signaling pathway. Meanwhile, B7-H3 induces epithelial–mesenchymal transition (EMT) processes as evidenced by decrease of E-cadherin and increase of MMP-2/-9 expression, which results in invasion of glioma cells (Zhong, et al., 2020). Taken together, these finding suggested that B7-H3 has the potential to be a useful prognostic marker of tumor, and JAK2/STAT3 pathway is mainly involved in the anti-apoptotic mechanism mediating by tumor B7-H3.
NF-κB, a crucial transcription factor in innate and adaptive immunity, is associated with tumorigenesis, cancer cell invasion and metastasis (Maier, et al., 2010). Previous studies have shown that B7-H3 expression positively regulated expression of metastasis-associated proteins, MMP-2, Stat3, and the level of secreted interleukin-8 (IL-8) (Tekle, et al., 2012) in the tumoral microenvironment. Importantly, exogenous soluble B7-H3 promotes the invasion and metastasis of pancreatic cancer cells through the TLR4/NF-κB pathway, resulting in increased expression of IL-8 and vascular endothelial cell growth factor (VEGF) (Xie, et al., 2016). Consistent with this result, another group reported that B7-H3 promoted colorectal cancer angiogenesis by upregulating VEGFA expression through activating the NF-κB pathway (R. Wang, et al., 2020).
PI3K/Akt signaling pathway is involved in invasion of cancer cells, such as breast cancer, gastric adenocarcinoma, and lung cancer cells (H. Li, Zhang, Liu, & Yin, 2014; X. Wu, et al., 2014). B7-H3 enhanced the migration and invasion of human bladder cancer cells through the PI3K/Akt/STAT3 signaling pathway, and STAT3 served as a downstream modulator of the Akt (Y. Li, et al., 2017). In addition, B7-H3 has been found to drive oral squamous carcinoma (OSCC) progression via upregulation of PI3K/Akt/mTOR signaling, resulting in increased expression of HIF-1α, which regulates the reprogramming of cancer metabolism in favor of aerobic glycolysis (Z. Li, et al., 2019).
In a study of B7-H3 deficient mice, the transgenic adenocarcinoma of the mouse prostate (TRAMP)+ mice lacking B7-H3 showed significant tumor enlargement (Kreymborg, et al., 2015). In contrast to TRAMP+B7-H3wt mice, a higher abundance of neoplastic epithelium and increased atypia was found in TRAMP+B7H3−/− mice. Moreover, they also observed that B7-H3−/− had no obvious direct effect on effector CD4+ or CD8+ T cell proliferation or CD8+ T cell or NK cell-mediated cytotoxicity, but significantly accumulate proliferative activity of FoxP3+ T cells (Treg), which may potentially suppress immune environment. In the study of autoimmune disease, B7-H3 deficient mice had dramatically less inflammation, decreased pathogenesis, and restricted disease development in both experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) models, and decreased IFN-γ and IL-17 production (Luo, et al., 2015). Thus, B7-H3 play an important role in regulating immune microenvironment (TME) and promoting T cells differentiation (Th1, Th2, and Th17).
Beyond tumor cells, stromal cell types within the TME are genetically stable, which play an active role in facilitating tumor progression, metastasis, as well as restricting T cell entry into the TME (“EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma,” 2012). Thus, novel treatment strategies to improve anti-tumor activity should combine anticancer and antistroma agents. B7-H3 may become a dual-compartment therapeutic target since it is broadly overexpressed by both tumor cells and tumor-associated vasculature (Mesri, et al., 2013; Seaman, et al., 2017). Besides being found in the tumor vasculature, sB7-H3 has an influence on TME. The supernatant concentrated sB7-H3 was able to bind to a putative B7-H3 receptor on the activated T cells (G. Zhang, et al., 2008). Moreover, pancreatic tumor cells could release sB7-H3 to the extracellular medium, enhancing the invasion and metastasis of pancreatic tumor cells in vitro and in vivo (Xie, et al., 2016). In addition, B7-H3 can act as a co-inhibitor on T-cells in TME, thereby increasing immune evasion. 4Ig-B7-H3 molecule expressed on neuroblastoma cells was found to protect tumor cells from lysing mediated by NK cells (Castriconi, et al., 2004), which helps tumor escape from the innate immune system.
4.2.3. B7-H3 as a therapeutic target in childhood cancers
Due to its high expression in many different cancers, B7-H3 has been studied as a target for several different therapeutic approaches in preclinical and clinical trials. A murine monoclonal antibody, 8H9, was used to identify B7-H3 expression in neuroectodermal, mesenchymal, and epithelial cancers (Modak, Kramer, Gultekin, Guo, & Cheung, 2001). Subsequently, an affinity-matured humanized construct of 8H9 (hu8H9-6m) was shown to have increased affinity and mediated antibody-dependent cell-mediated cytotoxicity in LAN-1 neuroblastoma cells (Ahmed, et al., 2015). Epitope mapping revealed that hu8H9-6m binds to the FG loop of B7-H3 (Ahmed, et al., 2015), which has been previously shown to be critical in B7-H3’s role in inhibiting T cell proliferation (Vigdorovich, et al., 2013), suggesting that 8H9-based therapy may act in modulating B7-H3 activity as an immune checkpoint. 8H9 (omburtamab) has been labeled with radioactive iodine and omburtamab-based radioimmunotherapy is currently being investigated in children with brain and central nervous system cancers (NCT04167618, NCT03275402, NCT01502917, NCT00089245) and peritoneal cancers (NCT0402213). A published case study reporting three children with embryonal tumor with multilayered rosettes (ETMR) from a trial that recruited patients with brain and central nervous system cancers (NCT00089245) demonstrated that 131I-omburtamab is well-tolerated in children and may have a therapeutic effect (Bailey, et al., 2019).
MGA271 is another anti-B7-H3 antibody that was humanized and Fc-optimized (Loo, et al., 2012). MGA271 demonstrated potent antibody-dependent cell-mediated cytotoxicity in vitro against multiple tumor lines and showed anti-tumor activity in vivo in a renal cell carcinoma xenograft mouse model. MGA271 (enoblituzumab) has since been studied in several clinical trials, including in one trial for children with B7-H3-positive malignant solid tumors, including neuroblastoma, osteosarcoma, rhabdomyosarcoma, Ewing’s sarcoma, Wilms tumor, and desmoplastic small round cell tumor. Recently, a B7-H3-targeted antibody-drug conjugate (ADC) has shown remarkably antitumor activity against a panel of pediatric solid tumor PDX models (Kendsersky, et al., 2021).
Several different CAR T cell constructs targeting B7-H3 have been developed. A second-generation CAR T construct with MGA271 scFv and a 4-1BB co-stimulatory domain caused tumor regression in preclinical mouse xenograft models of medulloblastoma, osteosarcoma, Ewing’s sarcoma (Robbie G. Majzner, et al., 2019) and in atypical teratoid/rhabdoid tumors (Theruvath, et al., 2020). Second-generation CAR T cells constructed with the scFv from the anti-B7-H3 monoclonal antibody 376.96 and either a CD28 or 4-1BB co-stimulatory domain showed anti-tumor efficacy in xenograft models of pancreatic cancer, ovarian cancer, and neuroblastoma (Du, et al., 2019a). Of particular interest, while both constructs showed potent activity, 4-1BB co-stimulation promoted better activity against tumor cells expressing PD-L1, providing resistance to the PD-1/PD-1 checkpoint (Du, et al., 2019a). The same B7-H3 CAR T cells also controlled glioblastoma tumor growth in a xenograft mouse model (Nehama, et al., 2019). Third-generation B7-H3 CAR T cells constructed with the 8H9 scFv and both CD28 and 4-1BB co-stimulatory domains also demonstrated efficacy in a glioblastoma mouse model (Tang, et al., 2019), as well as in a model of extranodal NK/T cell lymphoma (Zheng, et al., 2020). Another third-generation CAR T cell constructed with the scFv from J42, a monoclonal antibody generated via hybridoma, exhibited antitumor efficacy in acute myeloid leukemia and melanoma (Z. Zhang, et al., 2020). Another group co-expressed a PD-1 receptor on B7-H3 CAR T cells, and showed improved antitumor activity in a lung cancer mouse model (B. Huang, et al., 2020). Tandem second-generation 4-1BB CAR T cells targeting B7-H3 and CD70 had improved efficacy compared to nonspecific CARS in lung cancer and melanoma (Yang, et al., 2020). There are currently three clinical trials investigating B7H3 CAR T cells in children, one using a fourth generation construct (NCT04432649) and two using second generation constructs (NCT04483778, NCT04185038). These clinical trials commenced in late 2019 or 2020 and have not reported results, however the preclinical success of CAR T cells targeting B7-H3 suggest that it may be a useful therapeutic for a variety of cancers.
The high expression of B7-H3 in various types of cancers makes it an appealing target for therapeutic approaches, as such therapies may be useful in treating a wide variety of disease. However, B7-H3 is also known to have ubiquitous low-level expression in normal tissues, raising concerns about potential on-target, off-tissue effects. However, the antibody MGA271 showed minimal binding to normal tissues, as compared to tumor tissues (Loo, et al., 2012). Of note, activation of the MGA271 CAR T cells discussed above was proportional to B7-H3 antigen density, suggesting that there may be a “therapeutic window” that avoids negative effects of inadvertently targeting low-level B7-H3 effects on normal tissues (Robbie G. Majzner, et al., 2019). Taken together, many preclinical and early clinical results of B7-H3-targeted therapies suggest that B7-H3 is a promising therapeutic target for many childhood cancers and warrants further investigation.
4.3. L1CAM (CD171)
L1 cell adhesion molecule (L1CAM), also called CD171, is a neuronal adhesion protein that is overexpressed in neuroblastoma. Monoclonal antibody CE7 preferentially recognizes to a tumor-specific epitope of L1CAM (Novak-Hofer, et al., 1992), and that binding is likely dependent on glycosylation (Hong, et al., 2014; Meli, et al., 1999). In a phase 1 clinical trial of first-generation CE7 CD8+ CAR T cells to treat patients with recurrent neuroblastoma, on-target off-tumor toxicity was not observed, though only one of six patients showed clinical response possibly due to the lack of CD4+ helper T cells and co-stimulation (Park, et al., 2007). To improve therapeutic efficacy, a second-generation (2G) CE7 CAR with 4-1BB and a third-generation (3G) CE7 CAR includes CD28 and 4-1BB costimulatory domains were developed (Kunkele, et al., 2017). Both 2G and 3G CE7 CAR T cells showed antitumor effects in cell and mouse models. In this study, there was no significant clinical toxicity after infusion of up to 1 × 108/kg CE7 2G CAR T cells in non-human primates. A phase 1 trial with the CE7 2G CAR is currently undergoing at Seattle Children’s Hospital for recurrent/refractory high-risk neuroblastoma patients (NCT02311621). Patients received anti-L1CAM CAR T cells in a 1:1 ratio of CD4:CD8 T cells. Researchers recently found that exposure of CD4+ CE7 CAR T cells to tumor-derived extracellular vesicles significantly impaired tumor cytotoxicity of CAR T cells in preclinical neuroblastoma models (Ali, et al., 2020), suggesting that cell- independent tumor-suppressive effects should be considered for CAR T cell-based therapy for solid tumors.
4.4. IL-13Rα2
IL-13 is a Th2 cytokine that binds to the low-affinity IL-13Rα1 receptor in most cells, and then joins IL4Rα to form a heterodimer complex, which mediates signal transduction through the JAK/STAT6 pathway (Arima, et al., 2005). IL-13 can also bind to a high-affinity IL-13Rα2 receptor, which is subsequently internalized (Wynn, 2003). This interaction induces production of TGFβ1 through the AP-1 transcription factor (Fichtner-Feigl, Strober, Kawakami, Puri, & Kitani, 2006). IL-13Rα2 is expressed in approximately 83% of pediatric brain tumors (Kawakami, Kawakami, Takahashi, Abe, & Puri, 2004). Specifically, IL-13Rα2-positive staining was found in all (11/11) high-grade astrocytoma, 79% (26/33) low-grade astrocytoma, 4 of 6 medulloblastoma, and 2 of 3 ependymoma samples. Moreover, it is also strongly expressed in diffuse intrinsic pontine glioma (DIPG), a fatal childhood brain cancer (Berlow, et al., 2018; Bhardwaj, Suzuki, Leland, Joshi, & Puri, 2018). In a phase 1 trial of newly diagnosed DIPG, a peptide vaccine against the glioma-associated antigen, EphA2, IL-13Rα2 and survivin were targeted (Pollack, et al., 2014). The vaccination was well tolerated with preliminary indications of clinical response. Patients had an overall survival of 55.2 weeks, which shows a significant increase comparing with control levels of 39–43 weeks. These results suggest that the targeting of IL-13Rα2 may represent a potential target for treating pediatric brain tumors.
4.5. EGFR
Epidermal growth factor variant III (EGFRvIII), the most common variant of EGFR, is found in many adult tumor types but absent in the majority of normal tissues. The expression of EGFRvIII was found in approximately 20% of pediatric gliomas (Bax, et al., 2009). Also, EGFRvIII expression was detected in 6 of 11 DIPG cases, which is similar to the staining pattern seen in glioblastoma multiforme from adult trials (G. Li, et al., 2012). A phase 1 trial utilizing the EGFRvIII peptide vaccine rindopepimut in children with DIPG is underway at Stanford University (NCT01058850). mAb806 was initially raised against the EGFRvIII variant, but also binds to full-length EGFR that is expressed as a result of gene amplification (Luwor, et al., 2001). However, it does not recognize EGFR expressed on normal cells (Jungbluth, et al., 2003), likely due to changes in accessibility of the mAb806 epitope between tumor-expressed and wild-type EGFR (Orellana, et al., 2019). Second-generation EGFR806-CAR T cells regressed tumors in a glioma xenograft mouse model, and that CAR T cell activation was upon tumor-expressed EGFR stimulation (Ravanpay, et al., 2019). A clinical trial of the EGFR806-CAR in treating pediatric brain tumors is underway (NCT03638167).
4.6. HER2
Epidermal growth factor receptor 2 (Her2) is amplified in many adult solid tumors like breast cancer. The anti-Her2 mAb trastuzumab (Herceptin) has been a successful example of tumor immunotherapy. However, its use in pediatric solid tumors has been restricted. The expression of Her2 has been found in Wilm’s tumor, medulloblastoma, and osteosarcoma (Ebb, et al., 2012; Nellan, et al., 2018; Ragab, Samaka, & Shams, 2010). Trastuzumab was safe when in combination with chemotherapy in a phase 2 trial of osteosarcoma patients, yet clinical response was not significant (Ebb, et al., 2012). As this study did not discriminate based on Her2 positivity, a randomized study of patients with Her2-positive disease would be required in the future. Although the first clinical trial of Her2-targeted CAR T cells in adult cancer resulted in a fatal outcome likely due to high T cell dose and low-level of Her2 expression in the lung (Morgan, et al., 2010), numerous efforts have been made to improve safety of Her2 CAR T cells in patients. In a phase 1 dose-escalation trial (NCT00902044), intravenous administration of up to 1 × 108/kg Her2 CAR T cells in patients with Her2+ sarcoma was safe and showed clinical benefits in some patients (Navai, et al., 2019). Particularly, they reported a promising tumor response in a child with refractory bone marrow-metastatic rhabdosarcoma in this trial (Hegde, et al., 2020). This patient had a complete response for 12 months then relapsed after 6 months off therapy. A second remission was achieved after one cycle of lymphodepletion and Her2 CAR T cells. Response consolidation with additional CAR T cell infusion includes anti-PD1 mAb pembrolizumab to improve efficacy. Their studies suggest that the Her2 CAR T cells given in combination with fludarabine/cyclophosphamide lymphodepletion induce endogenous immune reactivity. Another trial of Her2-specific CAR T cell using locoregional administration for Her2-positive recurrent/refractory pediatric brain tumors is ongoing at Seattle Children’s Hospital (NCT03500991).
4.7. ALK
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase. ALK mutations have been identified in both familial and sporadic neuroblastoma (Mosse, et al., 2008). Moreover, ALK amplification is detected in approximately 15–20% of neuroblastoma patients (De Brouwer, et al., 2010). ALK shows restricted expression in central and peripheral nervous system during development (Iwahara, et al., 1997). ALK-targeted CAR T cells demonstrated in vitro activity but had limited antitumor efficacy in xenograft models of neuroblastoma (Walker, et al., 2017). This is likely due to cell surface ALK expression is below the threshold of antigen density required for CAR activity.
4.8. RANKL
The cytokine receptor activator of nuclear factor-κB ligand (RANKL) is a member of the tumor necrosis factor (TNF) family. RANKL is expressed on the surface of osteoblasts and is essential for osteoclast formation, function, and survival. Denosumab is a RANKL antibody and is used to treat adults with multiple myeloma and cancer patients with bone metastases (Fizazi, et al., 2009). RANKL was found in 75% of high-grade osteosarcomas in a clinical study including children, and its expression correlated with poor response to neoadjuvant chemotherapy (Lee, et al., 2011). Denosumab may have an ability in the treatment of osteosarcoma and is currently being evaluated in a phase 2 clinical trial (NCT02470091) in children with recurrent or refractory osteosarcoma.
5. Emerging MHC restricted immunotherapeutic target in pediatric cancer
While optimal antigens have not been well defined for many pediatric cancers, several known major histocompatibility complex (MHC)-restricted cancer antigens have been identified on pediatric tumors. Cancer testis antigens (CTAs) are recently discovered proteins expressed on several malignancies. There are also non-CTA MHC-restricted antigens in pediatric cancers. Here, we review several attractive MHC-restricted targets in pediatric malignancies.
5.1. WT1
Wilm’s tumor gene 1 (WT1), a zinc finger transcription factor, was originally identified as a tumor suppressor gene in children. The expression of WT1 is broadly detected in childhood ALL (Boublikova, et al., 2006), and in various pediatric tumors (Oue, et al., 2011), indicating that WT1 could be a potential therapeutic target in pediatric malignancies. A recent report suggests that WT1 could predict relapse in pediatric AML following hematopoietic stem cell transplantation (D. X. Deng, et al., 2021). WT1 peptide vaccination trials in adult patients with leukemia demonstrated WT1 vaccination could induce WT1-specific immunity and result in cancer regression (Keilholz, et al., 2009; Oka, et al., 2004). WT1 peptide vaccination has been investigated in childhood cancer. Initial results suggest that WT1 vaccination has therapeutic potential in children with solid tumors or leukemia (Hashii, et al., 2012; Oka, et al., 2004). Large studies are needed on the application of WT1 vaccination to prevent tumor recurrence in children.
5.2. Survivin
Survivin is an inhibitor of apoptosis protein expressed in a large number of adult malignancies, but also has low-level expression in normal tissues. Survivin expression has also been evaluated in pediatric tumors. It was found to be expressed in 10–50% of cells from primary medulloblastoma tumors (Bodey, Bodey, Siegel, & Kaiser, 2004), and in over 80% of primary rhabdomyosarcoma tumors (Caldas, Holloway, Hall, Qualman, & Altura, 2006). Survivin is also associated with therapy outcome in childhood de novo AML (Tamm, et al., 2004). In addition, the expression of survivin splice variant 2B correlates with refractory disease and poor outcome in pediatric AML (Moore, et al., 2014). Survivin-specific T cells isolated from cancer patients can mediate tumor lysis (Cheever, et al., 2009). Recently, a pilot trail of vaccination with survivin-derived peptide in HLA-A2+ children with recurrent high-grade gliomas is well tolerated, and showed preliminary evidence of modest clinical response (Pollack, et al., 2016).
5.3. CTAs
Cancer testis antigens (CTAs) are restricted to testicular germ cells and placenta trophoblasts with minimum expression in normal adult somatic cells (Simpson, Caballero, Jungbluth, Chen, & Old, 2005). Several CTAs are re-expressed in a variety of adult epithelial cancers as well as pediatric cancers (e.g. neuroblastoma, sarcoma). CTAs are considered as immunogenic proteins since many members can elicit spontaneous cellular and humoral immune responses in cancer patients (van der Bruggen, et al., 1991). Because of their restricted expression in normal tissues and immunogenic potentials, CTAs have been considered candidate targets for cancer immunotherapy. Here, we focus on the development of some promising CTAs for treating childhood cancers.
5.3.1. NY-ESO-1
New York esophageal squamous cell carcinoma 1 (NY-ESO-1) is a well-known CTA with re-expression in various adult cancers. Previous studies have reported the expression of NY-ESO-1 (30–82%) in patients with neuroblastoma (Wolfl, et al., 2005), and in rhabdomyosarcoma and osteogenic sarcoma cell lines (Jacobs, et al., 2007). A phase 1 trial combining decitabine with a dendritic cell vaccine targeting NY-ESO-1 demonstrated that the vaccine was well-tolerated and resulted in antitumor activity in some pediatric patients with relapsed/refractory neuroblastoma and sarcoma (Krishnadas, et al., 2015). Particularly, NY-ESO-1 is highly expressed in a majority of synovial sarcomas (Lai, et al., 2012). In a pilot phase 1 trial (NCT01343043) of genetically engineered NY-ESO-1 specific NY-ESO-1c259T in HLA-A2+ patients with synovial sarcoma, 4/6 patients receiving treatment had objective responses, and studies in children and adults are ongoing.
5.3.2. PRAME
PRAME, also known as preferentially expressed antigen in melanoma, encodes the human leucocyte antigen (HLA)-A*24 restricted cytotoxic T lymphocytes in metastatic cutaneous melanoma (Ikeda, et al., 1997). PRAME is expressed at high levels in many adult malignancies. Since it is only expressed at low levels in a few normal tissues including testes, adrenals, ovaries and endometrium, PRAME is considered to be an attractive candidate for tumor immunotherapy. PRMAE overexpression has also been identified in pediatric ALL (Y. H. Zhang, et al., 2017), childhood AML (Steinbach, Hermann, Viehmann, Zintl, & Gruhn, 2002), neuroblastoma (Oberthuer, Hero, Spitz, Berthold, & Fischer, 2004), and medulloblastoma (Orlando, et al., 2018). However, the downregulation of HLA-class I molecules by neuroblastoma cells limits the application of PRAME-specific HLA-class I-restricted cytotoxic T lymphocytes (CTLs) (Raffaghello, et al., 2005). Recently, a group has demonstrated that neuroblastoma cells upregulated HLA-class I expression on their surface upon exposure to activated NK cells, and NK-modulated neuroblastoma cells were recognized by PRAMESLLQHLIGL/A2-specific CTL clones (Spel, et al., 2015). Their findings suggest that NK cells may serve as adjuvant for CTL-based immunotherapy of neuroblastoma. The SLL peptide is presented in context of HLA-A*02, which is found in up to 48.4% of Caucasians and 22.6% of black ethnic groups (Gonzalez-Galarza, et al., 2015). CAR T cells specific for the PRAME-derived SLL peptide controlled tumor growth in a orthotopic medulloblastoma mouse model (Orlando, et al., 2018).
5.3.3. MAGEs
The melanoma antigen gene (MAGE) protein family is a large, highly conserved group of proteins that share a common MAGE homology domain. MAGEs have been found to be expressed in some pediatric cancers including neuroblastoma, osteosarcoma, rhabdomyosarcoma, medulloblastoma (Dalerba, et al., 2001; Jacobs, et al., 2007; Scarcella, et al., 1999). In a phase 1 trial of decitabine/dendritic cell vaccine targeting MAGEs, majority of patients with neuroblastoma and sarcoma developed a response to the overlapping peptides derived from MAGE-A1, MAGE-A3, and NY-ESO-1 post-vaccination (Krishnadas, et al., 2015).
6. Future Remarks: Challenges and Opportunities
Immunotherapy is changing the treatment landscape in pediatric cancers. Current advances in the field are building upon the success of blinatumomab and CD19-targeted CAR T cells in treating pediatric leukemia. The success of immunotherapy is largely based on the choice of target. Unlike ALL or CLL in which the tumor cells universally express CD19 or CD22, solid tumors rarely express tumor-specific antigens. It is more common to find a tumor-associated antigen (e.g. GD2, B7-H3, Her2, EGFR) where the antigen is enriched on tumors but is also expressed at low levels on normal tissues. Normal tissue expression carries the risk of on-target, off-tumor toxicities, which underscores the importance of finding a safe tumor-specific/associated antigen that is not expressed in vital tissues/organs. Recently, GPC2 is found to be highly expressed in neuroblastoma but has restricted expression in normal tissues except testis, appearing to be an optimal immunotherapeutic target. Moreover, activation of proto-oncogenes is a common theme in childhood cancers. Transcription factors (e.g. MYCN) compose the largest class of oncogenes identified in pediatric tumors. GPC2 is frequently overexpressed in MYCN-amplified neuroblastoma cells. The GPC2-targeted immunotherapeutic strategies have yielded impressive results in preclinical studies through stimulation of immune cells and inhibition of oncogenic pathway. Thus, it is desirable for immune targets to be involved in tumor growth/survival.
Another major obstacle of immunotherapy in solid tumors is the immunosuppressive TME. Solid tumors are composed of infiltrating stromal cells and immune cells in addition to malignant cells. In pediatric cancer, tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs) are two of the most prominent infiltrating cells. MDSCs produce immunosuppressive factors such as interleukin-10 (IL-10), prostaglandin E2, and TGF-β. To overcome these limitations, combination of adoptive T cell therapy with checkpoint blockade or depletion of suppressive factors in the TME have been explored in preclinical and early clinical studies. Recently, B7-H3 has been emerging as a potential therapeutic target that engages both tumor and stroma cells in the TME. The strategy that targets B7-H3 to destroy the dual-compartment in the TME is attractive for treating multiple childhood cancers as well as other adult solid tumors.
In the field of immunotherapy, the pediatric cancer cells can gain resistance to the monoclonal antibody by losing expression of the target antigen. This is also the case for resistance of CD19-targeted CAR T cells. In other cases, neutralizing antibodies have been identified against the therapeutic antibody, which reduce its pharmacokinetics and response. While it is challenging to develop a “magic bullet” solution to these obstacles, combination therapy targeting different antigens and/or distinct immunological mechanisms may provide more effective strategies, and ultimately will increase patient survival and improve life quality for children with cancer.
Acknowledgments
This work was supported by the Intramural Research Program of NIH, Center for Cancer Research (CCR), National Cancer Institute (NCI) (Z01 BC010891 and ZIA BC010891 to M.H.). The authors thank the NIH Fellows Editorial Board for reviewing and editing the manuscript. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The antibodies targeting glypican-2 (GPC2) or B7-H3 are available for licensing, in a wide range of fields of use, from the National Cancer Institute, NIH. If you are interested in obtaining a license, please contact the principal investigator Dr. Mitchell Ho at homi@mail.nih.gov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
M.H., N.L. and D.L. are inventors on patents describing antibodies to glypican 2 (GPC2) and/or B7-H3 that are assigned to the National Institutes of Health (NIH): international patent application PCT/US2017/043112 , “Monoclonal antibodies targeting glypican-2 (GPC2) and use thereof”; international patent applications no. PCT/US2019/045338, “High affinity monoclonal antibodies targeting glypican-2 and uses thereof”; and international patent application no. PCT/US2020/056601, “High affinity nanobodies targeting B7-H3 (CD276) for treating multiple solid tumors”. The remaining author has no competing interests.
References
- Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo H-F, Larson SM, & Cheung N-KV (2015). Humanized Affinity-matured Monoclonal Antibody 8H9 Has Potent Antitumor Activity and Binds to FG Loop of Tumor Antigen B7-H3. Journal of Biological Chemistry, 290, 30018–30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sukaini A, Hornicek FJ, Peacock ZS, Kaban LB, Ferrone S, & Schwab JH (2017). Immune Surveillance Plays a Role in Locally Aggressive Giant Cell Lesions of Bone. Clin Orthop Relat Res, 475, 3071–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, Yang S, & Kochenderfer JN (2017). Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol Ther, 25, 2452–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Toews K, Schwiebert S, Klaus A, Winkler A, Grunewald L, Oevermann L, Deubzer HE, Tuns A, Jensen MC, Henssen AG, Eggert A, Schulte JH, Schwich E, Rebmann V, Schramm A, & Kunkele A (2020). Tumor-Derived Extracellular Vesicles Impair CD171-Specific CD4(+) CAR T Cell Efficacy. Front Immunol, 11, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, Keler T, Graziano R, Blanset D, Yellin M, Fischkoff S, Assad A, & Borchmann P (2007). Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol, 25, 2764–2769. [DOI] [PubMed] [Google Scholar]
- Arima K, Sato K, Tanaka G, Kanaji S, Terada T, Honjo E, Kuroki R, Matsuo Y, & Izuhara K (2005). Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem, 280, 24915–24922. [DOI] [PubMed] [Google Scholar]
- Arndt C, von Bonin M, Cartellieri M, Feldmann A, Koristka S, Michalk I, Stamova S, Bornhauser M, Schmitz M, Ehninger G, & Bachmann M (2013). Redirection of T cells with a first fully humanized bispecific CD33-CD3 antibody efficiently eliminates AML blasts without harming hematopoietic stem cells. Leukemia, 27, 964–967. [DOI] [PubMed] [Google Scholar]
- Bailey K, Pandit-Taskar N, Humm JL, Zanzonico P, Gilheeney S, Cheung NV, & Kramer K (2019). Targeted radioimmunotherapy for embryonal tumor with multilayered rosettes. J Neurooncol, 143, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax DA, Gaspar N, Little SE, Marshall L, Perryman L, Regairaz M, Viana-Pereira M, Vuononvirta R, Sharp SY, Reis-Filho JS, Stavale JN, Al-Sarraj S, Reis RM, Vassal G, Pearson AD, Hargrave D, Ellison DW, Workman P, & Jones C (2009). EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res, 15, 5753–5761. [DOI] [PubMed] [Google Scholar]
- Berlow NE, Svalina MN, Quist MJ, Settelmeyer TP, Zherebitskiy V, Kogiso M, Qi L, Du Y, Hawkins CE, Hulleman E, Li XN, Gultekin SH, & Keller C (2018). IL-13 receptors as possible therapeutic targets in diffuse intrinsic pontine glioma. PLoS One, 13, e0193565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, & Zako M (1999). Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem, 68, 729–777. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Suzuki A, Leland P, Joshi BH, & Puri RK (2018). Identification of a novel role of IL-13Rα2 in human Glioblastoma multiforme: interleukin-13 mediates signal transduction through AP-1 pathway. J Transl Med, 16, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey B, Bodey V, Siegel SE, & Kaiser HE (2004). Survivin expression in childhood medulloblastomas: a possible diagnostic and prognostic marker. In Vivo, 18, 713–718. [PubMed] [Google Scholar]
- Bosse KR, Raman P, Zhu Z, Lane M, Martinez D, Heitzeneder S, Rathi KS, Kendsersky NM, Randall M, Donovan L, Morrissy S, Sussman RT, Zhelev DV, Feng Y, Wang Y, Hwang J, Lopez G, Harenza JL, Wei JS, Pawel B, Bhatti T, Santi M, Ganguly A, Khan J, Marra MA, Taylor MD, Dimitrov DS, Mackall CL, & Maris JM (2017). Identification of GPC2 as an Oncoprotein and Candidate Immunotherapeutic Target in High-Risk Neuroblastoma. Cancer Cell, 32, 295–309 e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublikova L, Kalinova M, Ryan J, Quinn F, O’Marcaigh A, Smith O, Browne P, Stary J, McCann SR, Trka J, & Lawler M (2006). Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia, 20, 254–263. [DOI] [PubMed] [Google Scholar]
- Bride KL, Vincent TL, Im SY, Aplenc R, Barrett DM, Carroll WL, Carson R, Dai Y, Devidas M, Dunsmore KP, Fuller T, Glisovic-Aplenc T, Horton TM, Hunger SP, Loh ML, Maude SL, Raetz EA, Winter SS, Grupp SA, Hermiston ML, Wood BL, & Teachey DT (2018). Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood, 131, 995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM (2003). Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer, 3, 203–216. [DOI] [PubMed] [Google Scholar]
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, & Bishop JM (1984). Amplification of N-myc in Untreated Human Neuroblastomas Correlates With Advanced Disease Stage. Science, 224, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, Roschewski M, Dean R, Cachau R, Youkharibache P, Patel R, Hansen B, Stroncek DF, Rosenberg SA, Gress RE, & Kochenderfer JN (2020). Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med, 26, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, Weng L, Del Real M, Hernandez R, Marcucci E, Shepphird JK, Wang X, Wood B, Marcucci G, Brown CE, & Forman SJ (2017). Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood, 130, 811–811. [Google Scholar]
- Caldas H, Holloway MP, Hall BM, Qualman SJ, & Altura RA (2006). Survivin-directed RNA interference cocktail is a potent suppressor of tumour growth in vivo. J Med Genet, 43, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana D, Janossy G, Bofill M, Trejdosiewicz LK, Ma D, Hoffbrand AV, Mason DY, Lebacq AM, & Forster HK (1985). Human B cell development. I. Phenotypic differences of B lymphocytes in the bone marrow and peripheral lymphoid tissue. J Immunol, 134, 1524–1530. [PubMed] [Google Scholar]
- Campbell BB, Light N, Fabrizio D, Zatzman M, Fuligni F, de Borja R, Davidson S, Edwards M, Elvin JA, Hodel KP, Zahurancik WJ, Suo Z, Lipman T, Wimmer K, Kratz CP, Bowers DC, Laetsch TW, Dunn GP, Johanns TM, Grimmer MR, Smirnov IV, Larouche V, Samuel D, Bronsema A, Osborn M, Stearns D, Raman P, Cole KA, Storm PB, Yalon M, Opocher E, Mason G, Thomas GA, Sabel M, George B, Ziegler DS, Lindhorst S, Issai VM, Constantini S, Toledano H, Elhasid R, Farah R, Dvir R, Dirks P, Huang A, Galati MA, Chung J, Ramaswamy V, Irwin MS, Aronson M, Durno C, Taylor MD, Rechavi G, Maris JM, Bouffet E, Hawkins C, Costello JF, Meyn MS, Pursell ZF, Malkin D, Tabori U, & Shlien A (2017). Comprehensive Analysis of Hypermutation in Human Cancer. Cell, 171, 1042–1056 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuro MI, Xiang Y, Lobe C, & Filmus J (2005). Glypican-3 Promotes the Growth of Hepatocellular Carcinoma Stimulating Canonical Wnt Signaling. Cancer Research, 65, 6245–6254. [DOI] [PubMed] [Google Scholar]
- Capurro M, Martin T, Shi W, & Filmus J (2014). Glypican-3 Binds to Frizzled and Plays a Direct Role in the Stimulation of Canonical Wnt Signaling. J Cell Sci, 127, 1565–1575. [DOI] [PubMed] [Google Scholar]
- Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, Moretta A, & Bottino C (2004). Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A, 101, 12640–12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ES, Pawel BR, Corao DA, Venneti S, Russo P, Santi M, & Sullivan LM (2013). Immunohistochemical expression of glypican-3 in pediatric tumors: an analysis of 414 cases. Pediatr Dev Pathol, 16, 272–277. [DOI] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, & Chen L (2001). B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol, 2, 269–274. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, & Matrisian LM (2009). The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res, 15, 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wang Y, Zhong K, Jiang C, Wang L, Yuan Z, Nie C, Xu J, Guo G, Zhou L, Yang M, & Tong A (2019). Frequent B7-H3 overexpression in craniopharyngioma. Biochem Biophys Res Commun, 514, 379–385. [DOI] [PubMed] [Google Scholar]
- Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Huebner D, Fong A, & Younes A (2016). Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood, 128, 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, Li F, Zhou Z, Zhao M, & Liu H (2013). Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One, 8, e76965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Pappo A, & Dyer MA (2015). Pediatric solid tumor genomics and developmental pliancy. Oncogene, 34, 5207–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N-KV, Guo H, Hu J, Tassev DV, & Cheung IY (2012). Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. OncoImmunology, 1, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N-KV, Saarinen UM, Neely JE, Landmeier B, Donovan D, & Coccia PF (1985). Monoclonal Antibodies to a Glycolipid Antigen on Human Neuroblastoma Cells. Cancer Research, 45, 2642–2649. [PubMed] [Google Scholar]
- Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, Spitzer T, Strandjord SE, Coccia PF, & Berger NA (1987). Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. Journal of Clinical Oncology, 5, 1430–1440. [DOI] [PubMed] [Google Scholar]
- Clevers H, & Nusse R (2012). Wnt/beta-catenin signaling and disease. Cell, 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cole PD, McCarten KM, Pei Q, Spira M, Metzger ML, Drachtman RA, Horton TM, Bush R, Blaney SM, Weigel BJ, & Kelly KM (2018). Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin’s lymphoma (AHOD1221): a Children’s Oncology Group, multicentre single-arm, phase 1–2 trial. Lancet Oncol, 19, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran KJ, Sauter CS, Kernan NA, Prockop SE, Boulad F, Perales M, Giralt SA, Riviere I, Wang X, Boelens J-J, Sadelain M, Brentjens R, & O’Reilly RJ (2020). Durable Remission Following “Off-the-Shelf” Chimeric Antigen Receptor (CAR) T-Cells in Patients with Relapse/Refractory (R/R) B-Cell Malignancies. Biology of Blood and Marrow Transplantation, 26, S89. [Google Scholar]
- Dalerba P, Frascella E, Macino B, Mandruzzato S, Zambon A, Rosolen A, Carli M, Ninfo V, & Zanovello P (2001). MAGE, BAGE and GAGE gene expression in human rhabdomyosarcomas. Int J Cancer, 93, 85–90. [DOI] [PubMed] [Google Scholar]
- Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ, & Mackall CL (2020). Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. The Lancet Oncology, 21, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM, Lakeman A, Vandesompele J, Hoebeeck J, Van Maerken T, De Paepe A, Laureys G, Schulte JH, Schramm A, Van Den Broecke C, Vermeulen J, Van Roy N, Beiske K, Renard M, Noguera R, Delattre O, Janoueix-Lerosey I, Kogner P, Martinsson T, Nakagawara A, Ohira M, Caron H, Eggert A, Cools J, Versteeg R, & Speleman F (2010). Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res, 16, 4353–4362. [DOI] [PubMed] [Google Scholar]
- Deng DX, Wen JJ, Cheng YF, Zhang XH, Xu LP, Wang Y, Yan CH, Chen YH, Chen H, Han W, Wang FR, Wang JZ, Qin YZ, Liu KY, Huang XJ, Zhao XS, & Mo XD (2021). Wilms’ tumor gene 1 is an independent prognostic factor for pediatric acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation. BMC Cancer, 21, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Wu D, Zhang Y, Jin Z, & Miao J (2021). MiR-29c downregulates tumor-expressed B7-H3 to mediate the antitumor NK-cell functions in ovarian cancer. Gynecol Oncol. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, & Brenner MK (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med, 365, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene K, Herrera SG, Xu Y, Sun C, Chen Y, Ma X, Ferrone CR, Pylayeva-Gupta Y, Yeh JJ, Liu R, Savoldo B, Ferrone S, & Dotti G (2019a). Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell, 35, 221–237.e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene K, Herrera SG, Xu Y, Sun C, Chen Y, Ma X, Ferrone CR, Pylayeva-Gupta Y, Yeh JJ, Liu R, Savoldo B, Ferrone S, & Dotti G (2019b). Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell, 35, 221–237 e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzak MN, Schumich A, Printz D, Potschger U, Husak Z, Attarbaschi A, Basso G, Gaipa G, Ratei R, Mann G, & Gadner H (2008). CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood, 112, 3982–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. (2012). J Hepatol, 56, 908–943. [DOI] [PubMed] [Google Scholar]
- Ebb D, Meyers P, Grier H, Bernstein M, Gorlick R, Lipshultz SE, Krailo M, Devidas M, Barkauskas DA, Siegal GP, Ferguson WS, Letson GD, Marcus K, Goorin A, Beardsley P, & Marina N (2012). Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children’s oncology group. J Clin Oncol, 30, 2545–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esheba GE, Pate LL, & Longacre TA (2008). Oncofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. Am J Surg Pathol, 32, 600–607. [DOI] [PubMed] [Google Scholar]
- Esko JD, & Selleck SB (2002). Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem, 71, 435–471. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, & Kitani A (2006). IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med, 12, 99–106. [DOI] [PubMed] [Google Scholar]
- Filmus J, Capurro M, & Rast J (2008). Glypicans. Genome Biol, 9, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J, & Selleck SB (2001). Glypicans: proteoglycans with a surprise. Journal of Clinical Investigation, 108, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, & Jun S (2009). Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol, 27, 1564–1571. [DOI] [PubMed] [Google Scholar]
- Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, Bosly A, Pinter-Brown L, Kennedy D, Sievers EL, & Gopal AK (2009). A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol, 146, 171–179. [DOI] [PubMed] [Google Scholar]
- Frankel AE, Woo JH, Ahn C, Pemmaraju N, Medeiros BC, Carraway HE, Frankfurt O, Forman SJ, Yang XA, Konopleva M, Garnache-Ottou F, Angelot-Delettre F, Brooks C, Szarek M, & Rowinsky E (2014). Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood, 124, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, & Mackall CL (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med, 24, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Poe JC, Inaoki M, & Tedder TF (1998). CD19 regulates B lymphocyte responses to transmembrane signals. Semin Immunol, 10, 267–277. [DOI] [PubMed] [Google Scholar]
- Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, Hirsch BA, Kahwash SB, Heerema-McKenney A, Winter L, Glick K, Davies SM, Byron P, Smith FO, & Aplenc R (2014). Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol, 32, 3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, & Ho M (2011). The role of glypican-3 in regulating Wnt in hepatocellular carcinomas. Cancer Rep, 1, 14–19. [PMC free article] [PubMed] [Google Scholar]
- Gao W, Tang Z, Zhang YF, Feng M, Qian M, Dimitrov DS, & Ho M (2015). Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun, 6, 6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Xu Y, Liu J, & Ho M (2016). Epitope mapping by a Wnt-blocking antibody: evidence of the Wnt binding domain in heparan sulfate. Sci Rep, 6, 26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoerger B, Bergeron C, Gore L, Sender L, Dunkel IJ, Herzog C, Brochez L, Cruz O, Nysom K, Berghorn E, Simsek B, Shen J, & Pappo A (2017). Phase II study of ipilimumab in adolescents with unresectable stage III or IV malignant melanoma. European Journal of Cancer, 86, 358–363. [DOI] [PubMed] [Google Scholar]
- Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, Laetsch TW, Petrilli AS, Ebinger M, Toporski J, Glade-Bender J, Nicholls W, Fox E, DuBois SG, Macy ME, Cohn SL, Pathiraja K, Diede SJ, Ebbinghaus S, & Pinto N (2020). Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. The Lancet Oncology, 21, 121–133. [DOI] [PubMed] [Google Scholar]
- Gillies SD, Lo KM, & Wesolowski J (1989). High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods, 125, 191–202. [DOI] [PubMed] [Google Scholar]
- Godwin CD, Gale RP, & Walter RB (2017). Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia, 31, 1855–1868. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, Teles e Silva AL, Ghattaoraya GS, Alfirevic A, Jones AR, & Middleton D (2015). Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res, 43, D784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery T, Roumier C, Berthon C, Renneville A, Preudhomme C, & Quesnel B (2015). B7-H3 protein expression in acute myeloid leukemia. Cancer Med, 4, 1879–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, & Perrimon N (2005). Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol, 6, 530–541. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, & Azuma M (2008). Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses (vol 105, pg 10495, 2008). Proceedings of the National Academy of Sciences of the United States of America, 105, 14744–14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashii Y, Sato-Miyashita E, Matsumura R, Kusuki S, Yoshida H, Ohta H, Hosen N, Tsuboi A, Oji Y, Oka Y, Sugiyama H, & Ozono K (2012). WT1 peptide vaccination following allogeneic stem cell transplantation in pediatric leukemic patients with high risk for relapse: successful maintenance of durable remission. Leukemia, 26, 530–532. [DOI] [PubMed] [Google Scholar]
- Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, Ghatwai N, Dakhova O, Liu B, Raveh-Sadka T, Chauvin-Fleurence CN, Xu X, Ngai H, Di Pierro EJ, Savoldo B, Dotti G, & Metelitsa LS (2020). Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med, 26, 1686–1690. [DOI] [PubMed] [Google Scholar]
- Hegde M, Joseph SK, Pashankar F, DeRenzo C, Sanber K, Navai S, Byrd TT, Hicks J, Xu ML, Gerken C, Kalra M, Robertson C, Zhang H, Shree A, Mehta B, Dakhova O, Salsman VS, Grilley B, Gee A, Dotti G, Heslop HE, Brenner MK, Wels WS, Gottschalk S, & Ahmed N (2020). Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun, 11, 3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon ME, & Lander AD (1990). A Diverse Set of Developmentally Regulated Proteoglycans is Expressed in the Rat Central Nervous System. Neuron, 4, 949–961. [DOI] [PubMed] [Google Scholar]
- Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG, Aquino V, Ghetie V, Vitetta ES, & Schindler J (2009). A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol, 31, 936–941. [DOI] [PubMed] [Google Scholar]
- Ho M, & Kim H (2011). Glypican-3: a new target for cancer immunotherapy. Eur J Cancer, 47, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Li N, & Fleming B (2019). HIGH AFFINITY MONOCLONAL ANTIBODIES TARGETING GLYPICAN-2 AND USES THEREOF. In.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, & Urba WJ (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyer KA, Ray A, & Zang X (2008). The contrasting role of B7-H3. Proc Natl Acad Sci U S A, 105, 10277–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Stastny M, Brown C, Chang WC, Ostberg JR, Forman SJ, & Jensen MC (2014). Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother, 37, 93–104. [DOI] [PubMed] [Google Scholar]
- Hoyer JD, Grogg KL, Hanson CA, Gamez JD, & Dogan A (2008). CD33 detection by immunohistochemistry in paraffin-embedded tissues: a new antibody shows excellent specificity and sensitivity for cells of myelomonocytic lineage. Am J Clin Pathol, 129, 316–323. [DOI] [PubMed] [Google Scholar]
- Huang B, Luo L, Wang J, He B, Feng R, Xian N, Zhang Q, Chen L, & Huang G (2020). B7-H3 specific T cells with chimeric antigen receptor and decoy PD-1 receptors eradicate established solid human tumors in mouse models. OncoImmunology, 9, 1684127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Chen Z, Yu JF, Young D, Bashey A, Ho AD, & Law P (1999). Correlation between IL-3 receptor expression and growth potential of human CD34+ hematopoietic cells from different tissues. Stem Cells, 17, 265–272. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T, & Coulie PG (1997). Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity, 6, 199–208. [DOI] [PubMed] [Google Scholar]
- Inamura K, Takazawa Y, Inoue Y, Yokouchi Y, Kobayashi M, Saiura A, Shibutani T, & Ishikawa Y (2018). Tumor B7-H3 (CD276) Expression and Survival in Pancreatic Cancer. J Clin Med, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Irie F, Plump AS, Tessier-Lavigne M, & Yamaguchi Y (2003). Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science, 302, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Litwack ED, Kumbasar A, Stipp CS, & Lander AD (1997). Cerebroglycan, a Developmentally Regulated Cell-Surface Heparan Sulfate Proteoglycan, Is Expressed on Developing Axons and Growth Cones. Developmental Biology, 184, 320–332. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, & Yamamoto T (1997). Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene, 14, 439–449. [DOI] [PubMed] [Google Scholar]
- Jacobs JF, Brasseur F, Hulsbergen-van de Kaa CA, van de Rakt MW, Figdor CG, Adema GJ, Hoogerbrugge PM, Coulie PG, & de Vries IJ (2007). Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer, 120, 67–74. [DOI] [PubMed] [Google Scholar]
- Jain N, Nguyen H, Chambers C, & Kang J (2010). Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A, 107, 1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeha S, Behm F, Pei D, Sandlund JT, Ribeiro RC, Razzouk BI, Rubnitz JE, Hijiya N, Howard SC, Cheng C, & Pui CH (2006). Prognostic significance of CD20 expression in childhood B-cell precursor acute lymphoblastic leukemia. Blood, 108, 3302–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, & Van Vactor D (2004). Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol, 14, 499–504. [DOI] [PubMed] [Google Scholar]
- Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, Dereure O, Wetterwald M, d’Incan M, Grange F, Cornillon J, Tertian G, Maubec E, Saiag P, Barete S, Templier I, Aubin F, & Dalle S (2013). Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol, 169, 579–586. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, Ritter G, Cohen L, Scanlan MJ, Cavenee WK, & Old LJ (2003). A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci U S A, 100, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel EZ, Madney Y, Eldin DN, & Shafik NF (2021). Overexpression of CD200 and CD123 is a major influential factor in the clinical course of pediatric acute myeloid leukemia. Exp Mol Pathol, 118, 104597. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gokbuget N, O’Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E, & Advani AS (2016). Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med, 375, 740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Kawakami K, Takahashi S, Abe M, & Puri RK (2004). Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer, 101, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, & Scheibenbogen C (2009). A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood, 113, 6541–6548. [DOI] [PubMed] [Google Scholar]
- Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, June CH, & Gill S (2015). CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia, 29, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendsersky NM, Lindsay J, Kolb EA, Smith MA, Teicher BA, Erickson SW, Earley EJ, Mosse YP, Martinez D, Pogoriler J, Krytska K, Patel K, Groff D, Tsang M, Ghilu S, Wang Y, Seaman S, Feng Y, Croix BS, Gorlick R, Kurmasheva R, Houghton PJ, & Maris JM (2021). The B7-H3-Targeting Antibody-Drug Conjugate m276-SL-PBD Is Potently Effective Against Pediatric Cancer Preclinical Solid Tumor Models. Clin Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri A, & Ho M (2019). The Role of Glypican-3 in Regulating Wnt, YAP, and Hedgehog in Liver Cancer. Front Oncol, 9, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, & Pfister SM (2012). Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol, 123, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymborg K, Haak S, Murali R, Wei J, Waitz R, Gasteiger G, Savage PA, van den Brink MR, & Allison JP (2015). Ablation of B7-H3 but Not B7-H4 Results in Highly Increased Tumor Burden in a Murine Model of Spontaneous Prostate Cancer. Cancer Immunol Res, 3, 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, & Lucas KG (2015). A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother, 64, 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkele A, Taraseviciute A, Finn LS, Johnson AJ, Berger C, Finney O, Chang CA, Rolczynski LS, Brown C, Mgebroff S, Berger M, Park JR, & Jensen MC (2017). Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin Cancer Res, 23, 466–477. [DOI] [PubMed] [Google Scholar]
- Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Ash S, Laureys G, Brock P, Michon JM, Owens C, Trahair T, Chi Fung Chan G, Ruud E, Schroeder H, Beck-Popovic M, Schreier G, Loibner H, Ambros P, Holmes K, Castellani MR, Gaze MN, Garaventa A, Pearson ADJ, & Lode HN (2020). Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenstein R, Weixler S, Baykan B, Bleeke M, Kunert R, Katinger D, Pribill I, Glander P, Bauer S, Pistoia V, Michon J, Garaventa A, & Lode HN (2013). Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: a SIOPEN Phase 1 study. MAbs, 5, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, Rosenberg SA, Miettinen MM, & Lee CC (2012). NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol, 25, 854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamble AJ, Eidenschink Brodersen L, Alonzo TA, Wang J, Gerbing RB, Pardo L, Sung L, Tasian SK, Cooper TM, Kolb EA, Aplenc R, Loken MR, & Meshinchi S (2019). Correlation of CD123 Expression Level with Disease Characteristics and Outcomes in Pediatric Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Blood, 134, 459–459. [Google Scholar]
- Laszlo GS, Gudgeon CJ, Harrington KH, Dell’Aringa J, Newhall KJ, Means GD, Sinclair AM, Kischel R, Frankel SR, & Walter RB (2014). Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood, 123, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebensohn AM, & Rohatgi R (2018). R-spondins can potentiate WNT signaling without LGRs. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Jung JS, Kim DH, Lim JS, Kim MS, Kong CB, Song WS, Cho WH, Jeon DG, Lee SY, & Koh JS (2011). RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr Blood Cancer, 56, 738–743. [DOI] [PubMed] [Google Scholar]
- Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, Pfistershammer K, & Steinberger P (2009). B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. European Journal of Immunology, 39, 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini L, Del Vecchio MT, Kraft R, Megha T, Barbini P, Cevenini G, Poggi S, Pileri S, Tosi P, & Cottier H (1990). Hodgkin’s disease and CD30-positive anaplastic large cell lymphomas--a continuous spectrum of malignant disorders. A quantitative morphometric and immunohistologic study. Am J Pathol, 137, 1047–1057. [PMC free article] [PubMed] [Google Scholar]
- Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, Su L, Wu X, Zhou J, Mackay S, Kramer J, Duan Z, Yang H, Kolluri A, Hummer AM, Torres MB, Zhu H, Hall MD, Luo X, Chen J, Wang Q, Abate-Daga D, Dropublic B, Hewitt SM, Orentas RJ, Greten TF, & Ho M (2020). Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Mitra SS, Monje M, Henrich KN, Bangs CD, Nitta RT, & Wong AJ (2012). Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J Neurooncol, 108, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang B, Liu Y, & Yin C (2014). EBP50 inhibits the migration and invasion of human breast cancer cells via LIMK/cofilin and the PI3K/Akt/mTOR/MMP signaling pathway. Med Oncol, 31, 162. [DOI] [PubMed] [Google Scholar]
- Li N, Fu H, Hewitt SM, Dimitrov DS, & Ho M (2017). Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc Natl Acad Sci U S A, 114, E6623–E6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Spetz M, & Ho M (2020). The Role of Glypicans in Cancer Progression and Therapy. J Histochem Cytochem, 22155420933709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y, Feng M, Lee B, Gao W, & Ho M (2019a). A frizzled-like cysteine rich domain in glypican-3 mediates Wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y, Feng M, Lee B, Gao W, & Ho M (2019b). A Frizzled-Like Cysteine-Rich Domain in Glypican-3 Mediates Wnt Binding and Regulates Hepatocellular Carcinoma Tumor Growth in Mice. Hepatology, 70, 1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo G, Song J, Cai Z, Yang J, Chen Z, Wang Y, Huang Y, & Gao Q (2017). B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J Cancer, 8, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu J, Que L, & Tang X (2019). The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer, 10, 5770–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Owens R, Tricot G, & Wilson CS (2004). Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol, 121, 482–488. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, & Collins M (2003). Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics, 82, 365–377. [DOI] [PubMed] [Google Scholar]
- Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, Pan J, Yu X, Chen B, Wu T, Chang AH, & Tong C (2021). Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol. [DOI] [PubMed] [Google Scholar]
- Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, & Bargou RC (2000). A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood, 95, 2098–2103. [PubMed] [Google Scholar]
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NW, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, & Richardson PG (2015). Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med, 373, 1207–1219. [DOI] [PubMed] [Google Scholar]
- Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, Son T, Chen Y, Easton AN, Li JC, Rillema JR, Licea M, Fieger C, Liang TW, Mather JP, Koenig S, Stewart SJ, Johnson S, Bonvini E, & Moore PA (2012). Development of an Fc-Enhanced Anti-B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clinical Cancer Research, 18, 3834–3845. [DOI] [PubMed] [Google Scholar]
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, & Brenner MK (2011). Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood, 118, 6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Zhu G, Xu H, Yao S, Zhou G, Zhu Y, Tamada K, Huang L, Flies AD, Broadwater M, Ruff W, van Deursen JM, Melero I, Zhu Z, & Chen L (2015). B7-H3 Promotes Pathogenesis of Autoimmune Disease and Inflammation by Regulating the Activity of Different T Cell Subsets. PLoS One, 10, e0130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, & Scott AM (2001). Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2–7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res, 61, 5355–5361. [PubMed] [Google Scholar]
- Maachani UB, Tosi U, Pisapia DJ, Mukherjee S, Marnell CS, Voronina J, Martinez D, Santi M, Dahmane N, Zhou Z,Hawkins C, & Souweidane MM (2020). B7-H3 as a Prognostic Biomarker and Therapeutic Target in Pediatric central nervous system Tumors. Transl Oncol, 13, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B, & Virshup DM (2015). Targeting Wnts at the source--new mechanisms, new biomarkers, new drugs. Mol Cancer Ther, 14, 1087–1094. [DOI] [PubMed] [Google Scholar]
- Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, & Wirth T (2010). NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett, 295, 214–228. [DOI] [PubMed] [Google Scholar]
- Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, Sotillo E, Labanieh L, Lee DW, Orentas RJ, Dimitrov DS, Zhu Z, Croix BS, Delaidelli A, Sekunova A, Bonvini E, Mitra SS, Quezado MM, Majeti R, Monje M, Sorensen PHB, Maris JM, & Mackall CL (2019). CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clinical Cancer Research, 25, 2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, Sotillo E, Labanieh L, Lee DW, Orentas RJ, Dimitrov DS, Zhu Z, Croix BS, Delaidelli A, Sekunova A, Bonvini E, Mitra SS, Quezado MM, Majeti R, Monje M, Sorensen PHB, Maris JM, & Mackall CL (2019). CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res, 25, 2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, Mauri P, Melioli G, & Pistoia V (2013). Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One, 8, e75054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, & Grupp SA (2018). Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med, 378, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli ML, Carrel F, Waibel R, Amstutz H, Crompton N, Jaussi R, Moch H, Schubiger PA, & Novak-Hofer I (1999). Anti-neuroblastoma antibody chCE7 binds to an isoform of L1-CAM present in renal carcinoma cells. Int J Cancer, 83, 401–408. [DOI] [PubMed] [Google Scholar]
- Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, Delbrook C, Lodish M, Bishop R, Wolchok JD, Streicher H, & Mackall CL (2016). Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin Cancer Res, 22, 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri M, Birse C, Heidbrink J, McKinnon K, Brand E, Bermingham CL, Feild B, Fitzhugh W, He T, Ruben S, & Moore PA (2013). Identification and characterization of angiogenesis targets through proteomic profiling of endothelial cells in human cancer tissues. PLoS One, 8, e78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard-Colin V, Auperin A, Pillon M, Burke GAA, Barkauskas DA, Wheatley K, Delgado RF, Alexander S, Uyttebroeck A, Bollard CM, Zsiros J, Csoka M, Kazanowska B, Chiang AK, Miles RR, Wotherspoon A, Adamson PC, Vassal G, Patte C, Gross TG, European Intergroup for Childhood Non-Hodgkin, L., & Children’s Oncology, G. (2020). Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N Engl J Med, 382, 2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak S, Kramer K, Gultekin SH, Guo HF, & Cheung NK (2001). Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res, 61, 4048–4054. [PubMed] [Google Scholar]
- Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, Hogarty MD, Barrett DM, Shimada H, & Asgharzadeh S (2021). Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun, 12, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AS, Alonzo TA, Gerbing RB, Lange BJ, Heerema NA, Franklin J, Raimondi SC, Hirsch BA, Gamis AS, & Meshinchi S (2014). BIRC5 (survivin) splice variant expression correlates with refractory disease and poor outcome in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer, 61, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, & Rosenberg SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther, 18, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, & Maris JM (2008). Identification of ALK as a major familial neuroblastoma predisposition gene. Nature, 455, 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Cheresh DA, Yang HM, & Reisfeld RA (1987). Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res, 47, 1098–1104. [PubMed] [Google Scholar]
- Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, Brunet S, & Sierra J (2001). Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica, 86, 1261–1269. [PubMed] [Google Scholar]
- Navai SA, Derenzo C, Joseph S, Sanber K, Byrd T, Zhang H, Mata M, Gerken C, Shree A, Mathew PR, Dakhova O, Salsman V, Hicks J, Yi Z, Wu M-F, Wang T, Grilley B, Rooney C, Brenner M, Heslop H, Gee A, Gottschalk S, Ahmed N, & Hegde M (2019). Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Research, 79, LB-147–LB-147. [Google Scholar]
- Nehama D, Di Ianni N, Musio S, Du H, Patané M, Pollo B, Finocchiaro G, Park JJH, Dunn DE, Edwards DS, Damrauer JS, Hudson H, Floyd SR, Ferrone S, Savoldo B, Pellegatta S, & Dotti G (2019). B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine, 47, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, Foreman NK, Warren KE, & Lee DW (2018). Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. Journal for ImmunoTherapy of Cancer, 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak-Hofer I, Amstutz HP, Haldemann A, Blaser K, Morgenthaler JJ, Blauenstein P, & Schubiger PA (1992). Radioimmunolocalization of neuroblastoma xenografts with chimeric antibody chCE7. J Nucl Med, 33, 231–236. [PubMed] [Google Scholar]
- O’Hear C, Heiber JF, Schubert I, Fey G, & Geiger TL (2015). Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica, 100, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberthuer A, Hero B, Spitz R, Berthold F, & Fischer M (2004). The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res, 10, 4307–4313. [DOI] [PubMed] [Google Scholar]
- Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, Hosen N, Yoshihara S, Wu F, Fujiki F, Murakami M, Masuda T, Nishida S, Shirakata T, Nakatsuka S, Sasaki A, Udaka K, Dohy H, Aozasa K, Noguchi S, Kawase I, & Sugiyama H (2004). Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A, 101, 13885–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana L, Thorne AH, Lema R, Gustavsson J, Parisian AD, Hospital A, Cordeiro TN, Bernado P, Scott AM, Brun-Heath I, Lindahl E, Cavenee WK, Furnari FB, & Orozco M (2019). Oncogenic mutations at the EGFR ectodomain structurally converge to remove a steric hindrance on a kinase-coupled cryptic epitope. Proc Natl Acad Sci U S A, 116, 10009–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentas RJ, Yang JJ, Wen X, Wei JS, Mackall CL, & Khan J (2012). Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front Oncol, 2, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando D, Miele E, De Angelis B, Guercio M, Boffa I, Sinibaldi M, Po A, Caruana I, Abballe L, Carai A, Caruso S, Camera A, Moseley A, Hagedoorn RS, Heemskerk MHM, Giangaspero F, Mastronuzzi A, Ferretti E, Locatelli F, & Quintarelli C (2018). Adoptive Immunotherapy Using PRAME-Specific T Cells in Medulloblastoma. Cancer Res, 78, 3337–3349. [DOI] [PubMed] [Google Scholar]
- Oue T, Uehara S, Yamanaka H, Takama Y, Oji Y, & Fukuzawa M (2011). Expression of Wilms tumor 1 gene in a variety of pediatric tumors. J Pediatr Surg, 46, 2233–2238. [DOI] [PubMed] [Google Scholar]
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine J-M, Maglinte GA, Kopetz S, & André T (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. The Lancet Oncology, 18, 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, & Jensen MC (2007). Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther, 15, 825–833. [DOI] [PubMed] [Google Scholar]
- Picarda E, Ohaegbulam KC, & Zang X (2016). Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res, 22, 3425–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto N, Park JR, Murphy E, Yearley J, Mcclanahan T, Annamalai L, Hawkins DS, & Rudzinski ER (2017). Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatric Blood & Cancer, 64, e26613. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Normolle DP, Connelly AK, Dibridge S, Mason G, Whiteside TL, & Okada H (2016). Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J Neurooncol, 130, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Potter DM, Connelly AK, Dibridge SA, Whiteside TL, & Okada H (2014). Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol, 32, 2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, & Brenner MK (2008). Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine, 14, 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, & Shafique M (2020). Combination checkpoint inhibitors for treatment of non-small-cell lung cancer: an update on dual anti-CTLA-4 and anti-PD-1/PD-L1 therapies. Drugs Context, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Ramakrishna S, Nguyen S, Fountaine TJ, Ponduri A, Stetler-Stevenson M, Yuan CM, Haso W, Shern JF, Shah NN, & Fry TJ (2018). Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22. Mol Ther Oncolytics, 11, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL, Adamson PC, & Children’s Oncology Group Pilot, S. (2008). Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol, 26, 3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, Wang X, Ferrone S, & Pistoia V (2005). Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene, 24, 4634–4644. [DOI] [PubMed] [Google Scholar]
- Ragab SM, Samaka RM, & Shams TM (2010). HER2/neu expression: a predictor for differentiation and survival in children with Wilms tumor. Pathol Oncol Res, 16, 61–67. [DOI] [PubMed] [Google Scholar]
- Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, Wang T, Shea TC, Rooney CM, Dittus C, Park SI, Gee AP, Eldridge PW, McKay KL, Mehta B, Cheng CJ, Buchanan FB, Grilley BJ, Morrison K, Brenner MK, Serody JS, Dotti G, Heslop HE, & Savoldo B (2020). Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol, 38, 3794–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanpay AC, Gust J, Johnson AJ, Rolczynski LS, Cecchini M, Chang CA, Hoglund VJ, Mukherjee R, Vitanza NA, Orentas RJ, & Jensen MC (2019). EGFR806-CAR T cells selectively target a tumor-restricted EGFR epitope in glioblastoma. Oncotarget, 10, 7080–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satwani P, Bhatia M, Garvin JH Jr., George D, Dela Cruz F, Le Gall J, Jin Z, Schwartz J, Duffy D, van de Ven C, Foley S, Hawks R, Morris E, Baxter-Lowe LA, & Cairo MS (2012). A Phase I study of gemtuzumab ozogamicin (GO) in combination with busulfan and cyclophosphamide (Bu/Cy) and allogeneic stem cell transplantation in children with poor-risk CD33+ AML: a new targeted immunochemotherapy myeloablative conditioning (MAC) regimen. Biol Blood Marrow Transplant, 18, 324–329. [DOI] [PubMed] [Google Scholar]
- Scarcella DL, Chow CW, Gonzales MF, Economou C, Brasseur F, & Ashley DM (1999). Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clin Cancer Res, 5, 335–341. [PubMed] [Google Scholar]
- Scheuermann RH, & Racila E (1995). CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma, 18, 385–397. [DOI] [PubMed] [Google Scholar]
- Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, Kaiser A, Dropulic B, & Orentas RJ (2017). A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer, 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, & Reisfeld RA (1984). Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res, 44, 5914–5920. [PubMed] [Google Scholar]
- Schwonzen M, Diehl V, Dellanna M, & Staib P (2007). Immunophenotyping of surface antigens in acute myeloid leukemia by flow cytometry after red blood cell lysis. Leuk Res, 31, 113–116. [DOI] [PubMed] [Google Scholar]
- Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, Tessarollo L, Smith SW, Degrado S, Borkin D, Jain N, Scheiermann J, Feng Y, Wang Y, Li J, Welsch D, DeCrescenzo G, Chaudhary A, Zudaire E, Klarmann KD, Keller JR, Dimitrov DS, & St Croix B (2017). Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell, 31, 501–515 e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, & Hammond D (1985). Association of Multiple Copies of the N-myc Oncogene With Rapid Progression of Neuroblastomas. N Engl J Med, 313, 1111–1116. [DOI] [PubMed] [Google Scholar]
- Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, Ombrello A, Steinberg SM, Martin S, Delbrook C, Hoffman L, Little L, Ponduri A, Qin H, Qureshi H, Dulau-Florea A, Salem D, Wang HW, Yuan C, Stetler-Stevenson M, Panch S, Tran M, Mackall CL, Stroncek DF, & Fry TJ (2020). CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol, 38, 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, Wolters PL, Steinberg SM, Baker EH, Delbrook CP, Stetler-Stevenson M, Fry TJ, Stroncek DF, & Mackall CL (2021). Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. Journal of Clinical Oncology, JCO.20.02262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RR, Pham CD, Wu M, Munson DJ, & Aftab BT (2019). CD19 chimeric antigen receptor (CAR) engineered epstein-barr virus (EBV) specific T cells – an off-the-shelf, allogeneic CAR T-cell immunotherapy platform. Cytotherapy, 21, S11. [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2020). Cancer statistics, 2020. CA Cancer J Clin, 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, & Berthold F (2004). Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol, 22, 3549–3557. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, & Old LJ (2005). Cancer/testis antigens, gametogenesis and cancer. Nature Reviews Cancer, 5, 615–625. [DOI] [PubMed] [Google Scholar]
- Song HH, & Filmus J (2002). The Role of Glypicans in Mammalian Development. Biochim Biophys Acta, 1573, 241–246. [DOI] [PubMed] [Google Scholar]
- Spel L, Boelens JJ, van der Steen DM, Blokland NJ, van Noesel MM, Molenaar JJ, Heemskerk MH, Boes M, & Nierkens S (2015). Natural killer cells facilitate PRAME-specific T-cell reactivity against neuroblastoma. Oncotarget, 6, 35770–35781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štach M, Ptáčková P, Mucha M, Musil J, Klener P, & Otáhal P (2020). Inducible secretion of IL-21 augments anti-tumor activity of piggyBac-manufactured chimeric antigen receptor T cells. Cytotherapy. [DOI] [PubMed] [Google Scholar]
- Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, & et al. (1985). The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood, 66, 848–858. [PubMed] [Google Scholar]
- Steinbach D, Hermann J, Viehmann S, Zintl F, & Gruhn B (2002). Clinical implications of PRAME gene expression in childhood acute myeloid leukemia. Cancer Genet Cytogenet, 133, 118–123. [DOI] [PubMed] [Google Scholar]
- Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, & Knapp W (2004a). Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol, 172, 2352–2359. [DOI] [PubMed] [Google Scholar]
- Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, & Knapp W (2004b). Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. Journal of Immunology, 172, 2352–2359. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Litwack ED, & Lander AD (1994). Cerebroglycan: An Integral Membrane Heparan Sulfate Proteoglycan That Is Unique to the Developing Nervous System and Expressed Specifically during Neuronal Differentiation. J Cell Biol, 124, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, & Mak TW (2003). The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol, 4, 899–906. [DOI] [PubMed] [Google Scholar]
- Sullivan-Chang L, O’Donnell RT, & Tuscano JM (2013). Targeting CD22 in B-cell malignancies: current status and clinical outlook. BioDrugs, 27, 293–304. [DOI] [PubMed] [Google Scholar]
- Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, & Dong C (2002). Characterization of mouse and human B7-H3 genes. J Immunol, 168, 6294–6297. [DOI] [PubMed] [Google Scholar]
- Sun MY, Richards S, Prasad DVR, Mai XM, Rudensky A, & Dong C (2002). Characterization of mouse and human B7-H3 genes. Journal of Immunology, 168, 6294–6297. [DOI] [PubMed] [Google Scholar]
- Sun W, Liu H, Kim Y, Karras N, Pawlowska A, Toomey D, Kyono W, Gaynon P, Rosenthal J, & Stein A (2018). First pediatric experience of SL-401, a CD123-targeted therapy, in patients with blastic plasmacytoid dendritic cell neoplasm: report of three cases. J Hematol Oncol, 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L, Boström K, Fredman P, Jungbjer B, Lekman A, Månsson J-E, & Rynmark B-M (1994). Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism, 1214, 115–123. [DOI] [PubMed] [Google Scholar]
- Svensson G, Awad W, Hakansson M, Mani K, & Logan DT (2012). Crystal structure of N-glycosylated human glypican-1 core protein: structure of two loops evolutionarily conserved in vertebrate glypican-1. J Biol Chem, 287, 14040–14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, Karawajew L, Ludwig WD, & Wuchter C (2004). High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res, 10, 3737–3744. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, Zhu Y, Zhang G, Guo G, Tong A, & Zhou L (2019). B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol Ther Oncolytics, 14, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle C, Nygren MK, Chen YW, Dybsjord I, Nesland JM, Maelandsmo GM, & Fodstad O (2012). B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer, 130, 2282–2290. [DOI] [PubMed] [Google Scholar]
- Tembhare PR, Sriram H, Khanka T, Chatterjee G, Panda D, Ghogale S, Badrinath Y, Deshpande N, Patkar NV, Narula G, Bagal B, Jain H, Sengar M, Khattry N, Banavali S, Gujral S, & Subramanian PG (2020). Flow cytometric evaluation of CD38 expression levels in the newly diagnosed T-cell acute lymphoblastic leukemia and the effect of chemotherapy on its expression in measurable residual disease, refractory disease and relapsed disease: an implication for anti-CD38 immunotherapy. J Immunother Cancer, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A, Majzner RG, Xu P, Mueller S, Yecies DW, Finetti MA, Williamson D, Johann PD, Kool M, Pfister S, Hasselblatt M, Frühwald MC, Delattre O, Surdez D, Bourdeaut F, Puget S, Zaidi S, Mitra SS, Cheshier S, Sorensen PH, Monje M, & Mackall CL (2020). Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nature Medicine, 26, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toretsky JA, Zitomersky NL, Eskenazi AE, Voigt RW, Strauch ED, Sun CC, Huber R, Meltzer SJ, & Schlessinger D (2001). Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol, 23, 496–499. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Hosono A, Yoshikawa T, Shoda K, Nosaka K, Shimomura M, Hara J, Nitani C, Manabe A, Yoshihara H, Hosoya Y, Kaneda H, Kinoshita Y, Kohashi K, Yoshimura K, Fujinami N, Saito K, Mizuno S, & Nakatsura T (2017). Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology, 7, e1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnball J, Powell A, & Guimond S (2001). Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol, 11, 75–82. [DOI] [PubMed] [Google Scholar]
- Uy GL, Godwin J, Rettig MP, Vey N, Foster M, Arellano ML, Rizzieri DA, Topp MS, Huls G, Lowenberg B, Martinelli G, Paolini S, Ciceri F, Carrabba MG, Ballesteros-Merino C, Bifulco CB, Lelièvre H, La Motte-Mohs R, Li D, Sun J, Jacobs K, Spohn K, Lonsdale N, Tran K, Baughman J, Shannon M, Fox B, Bonvini E, Wigginton J, Davidson-Moncada J, & DiPersio JF (2017). Preliminary Results of a Phase 1 Study of Flotetuzumab, a CD123 x CD3 Bispecific Dart® Protein, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood, 130, 637–637. [Google Scholar]
- van de Donk NW, Moreau P, Plesner T, Palumbo A, Gay F, Laubach JP, Malavasi F, Avet-Loiseau H, Mateos MV, Sonneveld P, Lokhorst HM, & Richardson PG (2016). Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood, 127, 681–695. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, & Boon T (1991). A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science, 254, 1643–1647. [DOI] [PubMed] [Google Scholar]
- Vigdorovich V, Ramagopal UA, Lázár-Molnár E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG, & Almo SC (2013). Structure and T cell inhibition properties of B7 family member, B7-H3. Structure, 21, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O’Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Rheingold SR, Cooper TM, Zwaan CM, Barnette P, Messina C, Michel G, DuBois SG, Hu K, Zhu M, Whitlock JA, & Gore L (2016). Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol, 34, 4381–4389. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, Lopomo P, Vigny M, Fry TJ, Orentas RJ, & Mackall CL (2017). Tumor Antigen and Receptor Densities Regulate Efficacy of a Chimeric Antigen Receptor Targeting Anaplastic Lymphoma Kinase. Mol Ther, 25, 2189–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Thudium KB, Han M, Wang X-T, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, Singh S, Wong S, Garner N, Leblanc H, Bunch RT, Blanset D, Selby MJ, & Korman AJ (2014). In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and In Vivo Toxicology in Non-Human Primates. Cancer Immunology Research, 2, 846–856. [DOI] [PubMed] [Google Scholar]
- Wang K, Wei G, & Liu D (2012). CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol, 1, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kang FB, Zhang GC, Wang J, Xie MF, & Zhang YZ (2018). Clinical significance of serum soluble B7-H3 in patients with osteosarcoma. Cancer Cell International, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, Cao N, Liu L, & Zhang Y (2013). B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One, 8, e70689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi T, & Chen W (2020). B7-H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis, 11, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, DeSantis C, Robbins A, Kohler B, & Jemal A (2014). Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin, 64, 83–103. [DOI] [PubMed] [Google Scholar]
- Wayne AS, Shah NN, Bhojwani D, Silverman LB, Whitlock JA, Stetler-Stevenson M, Sun W, Liang M, Yang J, Kreitman RJ, Lanasa MC, & Pastan I (2017). Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia. Blood, 130, 1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, Ernestus K, & Berthold F (2005). Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother, 54, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Jia D, Bates B, Basom R, Eberhart CG, & MacPherson D (2017). A mouse model of MYCN-driven retinoblastoma reveals MYCN-independent tumor reemergence. J Clin Invest, 127, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen Y, Li G, Xia L, Gu R, Wen X, Ming X, & Chen H (2014). Her3 is associated with poor survival of gastric adenocarcinoma: Her3 promotes proliferation, survival and migration of human gastric cancer mediated by PI3K/AKT signaling pathway. Med Oncol, 31, 903. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Schwartz E, Seeger R, & Ladisch S (1986). Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res, 46, 440–443. [PubMed] [Google Scholar]
- Wynn TA (2003). IL-13 effector functions. Annu Rev Immunol, 21, 425–456. [DOI] [PubMed] [Google Scholar]
- Xie C, Liu D, Chen Q, Yang C, Wang B, & Wu H (2016). Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway. Sci Rep, 6, 27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Yi J, Wang F, Wang W, Wang Z, Xue J, & Luan X (2017). Involvement of soluble B7-H3 in combination with the serum inflammatory cytokines interleukin-17, −8 and −6 in the diagnosis of hepatocellular carcinoma. Oncol Lett, 14, 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Huang W, Heczey A, Liu D, Guo L, Wood M, Jin J, Courtney AN, Liu B, Di Pierro EJ, Hicks J, Barragan GA, Ngai H, Chen Y, Savoldo B, Dotti G, & Metelitsa LS (2019). NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin Cancer Res, 25, 7126–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Tang X, Zhang Z, Gu L, Wei H, Zhao S, Zhong K, Mu M, Huang C, Jiang C, Xu J, Guo G, Zhou L, & Tong A (2020). Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics, 10, 7622–7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, & Group C. s. O. (2010). Anti-GD2 Antibody With GM-CSF, interleukin-2, and Isotretinoin for Neuroblastoma. N Engl J Med, 363, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, Hank JA, Batova A, London WB, Tenney SC, Smith M, Shulkin BL, Parisi M, Matthay KK, Cohn SL, Maris JM, Bagatell R, Park JR, & Sondel PM (2021). Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin Cancer Res, 27, 2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TT, Zhang T, Lu X, & Wang RZ (2018). B7-H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther, 11, 4693–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Loke P, Kim J, Murphy K, Waitz R, & Allison JP (2003). B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A, 100, 10388–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, & Allison JP (2010). Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol, 23, 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, & Allison JP (2007). B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A, 104, 19458–19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, Lewis G, Ladenstein R, & Lode HN (2005). Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol, 42, 1311–1319. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hou J, Shi J, Yu G, Lu B, & Zhang X (2008). Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology, 123, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, Redmond HP, Wang JH, & Zhang X (2010). B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol, 185, 3677–3684. [DOI] [PubMed] [Google Scholar]
- Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, & Zhang X (2009). Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer, 66, 245–249. [DOI] [PubMed] [Google Scholar]
- Zhang GB, Chen YJ, Shi Q, Ma HB, Ge Y, Wang Q, Jiang Z, Xu Y, & Zhang XG (2004). Human recombinant B7-H3 expressed in E. coli enhances T lymphocyte proliferation and IL-10 secretion in vitro. Acta Biochim Biophys Sin (Shanghai), 36, 430–436. [DOI] [PubMed] [Google Scholar]
- Zhang GB, Hou JQ, Shi JF, Yu GH, Lu BF, & Zhang XG (2008). Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology, 123, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu L, Han S, Li Y, Qian Q, Zhang Q, Zhang H, Yang Z, & Zhang Y (2017). B7-H3 is related to tumor progression in ovarian cancer. Oncol Rep, 38, 2426–2434. [DOI] [PubMed] [Google Scholar]
- Zhang T, Jiang B, Zou ST, Liu F, & Hua D (2015). Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol, 21, 1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Lu AD, Yang L, Li LD, Chen WM, Long LY, Zhang LP, & Qin YZ (2017). PRAME overexpression predicted good outcome in pediatric B-cell acute lymphoblastic leukemia patients receiving chemotherapy. Leuk Res, 52, 43–49. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, Zheng M, Huang J, Zhong K, Zhao S, Tang M, Zhou T, Yang H, Guo G, Zhou L, Xu J, & Tong A (2020). B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol Ther Oncolytics, 17, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DD, Lin L, Ge Q, Li ZY, He XP, Xu KL, Lu J, & Huang XJ (2013). [Relation of B7-H3 molecule expression in multiple myeloma with poor prognosis and bone destruction]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 21, 637–642. [DOI] [PubMed] [Google Scholar]
- Zhao L, Xie C, Liu D, Li T, Zhang Y, & Wan C (2017). Early Detection of Hepatocellular Carcinoma in Patients with Hepatocirrhosis by Soluble B7-H3. J Gastrointest Surg, 21, 807–812. [DOI] [PubMed] [Google Scholar]
- Zheng M, Yu L, Hu J, Zhang Z, Wang H, Lu D, Tang X, Huang J, Zhong K, Wang Z, Li Y, Guo G, Liu S, Tong A, & Yang H (2020). Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Translational Oncology, 13, 100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Tao B, Chen Y, Guo Z, Yang X, Peng L, Xia X, & Chen L (2020). B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Onco Targets Ther, 13, 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ouyang S, Li J, Huang X, Ai X, Zeng Y, Lv Y, & Cai M (2019). The novel non-immunological role and underlying mechanisms of B7-H3 in tumorigenesis. J Cell Physiol, 234, 21785–21795. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Chen YJ, Ma ZY, Xu L, Wang Q, Zhang GB, Xie F, Ge Y, Wang XF, & Zhang XG (2007). 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens, 70, 96–104. [DOI] [PubMed] [Google Scholar]


