Abstract
As they form, synapses go through various stages of maturation and refinement. These steps are linked to significant changes in synaptic function, potentially resulting in emergence and maturation of behavioral outputs. Synaptotagmins are calcium-sensing proteins of the synaptic vesicle exocytosis machinery, and changes in Synaptotagmin proteins at synapses have significant effects on vesicle release and synaptic function. Here, we examined the distribution of the synaptic vesicle protein Synaptotagmin 2a (Syt2a) during development of the zebrafish nervous system. Syt2a is widely distributed throughout the midbrain and hindbrain early during larval development but very weakly expressed in the forebrain. Later in development, Syt2a expression levels in the forebrain increase, particularly in regions associated with social behavior, and most intriguingly, around the time social behavior becomes apparent. We provide evidence that Syt2a localizes to synapses onto neurons implicated in social behavior in the ventral forebrain and show that Syt2a is colocalized with tyrosine hydroxylase, a biosynthetic enzyme in the dopamine pathway. Our results suggest a developmentally important role for Syt2a in maturing synapses in the forebrain, coinciding with the emergence of social behavior.
Keywords: social behavior, synapse, Synaptotagmin, tyrosine hydroxylase, ventral forebrain, zebrafish
Graphical Abstract
During brain development synapses form and are refined through the accumulation of different proteins. Here we examined the dynamic distribution of Synaptotagmin 2a (Syt2a) in the developing forebrain of zebrafish larvae with a focus on regions associated with social behavior. We show that Syt2a colocalized with Tyrosine Hydroxylase, a protein important for behavior. Further, we found that Syt2a localizes to synapses onto neurons implicated in social behavior, with an increase of Syt2a expression coinciding with the emergence of social behavior.
1 |. INTRODUCTION
During brain development, the assembly of synaptic protein complexes follows regional and temporal signals to form the synaptic connections necessary for a functional network. After initial assembly, synapses go through multiple stages of structural and functional development, leading to mature synapses that can maintain their overall protein compositions over long periods of time (Garner et al., 2006). A multitude of presynaptic proteins are involved in refining highly regulated synaptic properties by controlling the trafficking and release of synaptic vesicles (Rizo & Xu, 2015). Recent studies have added complexity to the maturation process at presynaptic synapses, by demonstrating switching of closely related proteins at already assembled synapses. For example, some synapses undergo a functional Synaptotagmin1 (Syt1) to Syt2 switch during development. This transition has been extensively studied at the Calyx of Held synapse in the mammalian auditory brainstem (Kochubey et al., 2016). Development of this large nerve terminal involves several steps during which initial synapses are formed, competing synapses are eliminated and the structure and function of remaining synapses refined. Fast transmitter release at these synapses initially depends on Syt1. Syt2 is expressed later, replacing Syt1 within a few days, and resulting in faster release kinetics (Kochubey et al., 2016). This example shows the importance of synaptic refinement: the identity of a specific Synaptotagmin protein present at a synapse determines its release properties.
Synaptotagmins are members of a large family of evolutionarily conserved, membrane-trafficking proteins, many of which are considered calcium ion sensors that control neurotransmitter release through the SNARE (SNAP Receptor) complex (Südhof, 2002). The large number of Synaptotagmins, with 17 Synaptotagmin genes in humans and 28 in zebrafish, provides many opportunities with which to fine tune presynaptic sensitivity and release properties. Often several different Synaptotagmins are expressed in the same tissue and appear to act redundantly after genetic elimination of one protein (Bouhours et al., 2017). Hence, the specific function of many Synaptotagmin genes remains elusive and the need for maintaining this large number of genes is speculative. Knowing the distribution of Syt2 might help to determine the function it plays. In mouse, Syt2 is highly expressed in the cerebellum, hindbrain, and spinal cord. Distinct subsets of neurons express Syt2 in the striatum, zona incerta, reticular nucleus of the thalamus, and ventromedial nucleus of the hypothalamus from postnatal days 14 (P14)-P16. A developmental change in Syt2 distribution was reported during the first two postnatal weeks in the mouse retina (Fox & Sanes, 2007), where Syt2 distribution either increased or decreased over time, depending on the specific cell type. Although Syt2 is mostly expressed in inhibitory neurons in the mouse forebrain, Syt2 can be present at both inhibitory and excitatory synapses, even within the same tissue (Chen et al., 2017; Fox & Sanes, 2007; Pang, Melicoff, et al., 2006). However, these studies have not linked expression pattern to specific behaviors.
Here, we investigated the expression profile of zebrafish Syt2a during various stages of zebrafish larval development. We focused on synaptic protein changes that occur over critical developmental periods, for instance, during establishment of complex behaviors. We recently demonstrated a requirement for the ventral telencephalon for social behavior (Stednitz et al., 2018), and a number of studies indicate that this behavior initiates around the second week of life (Dreosti et al., 2015; Larsch & Baier, 2018; Stednitz & Washbourne, 2020). Neurons in this region are largely cholinergic (Stednitz et al., 2018), and may be closely related to basal forebrain cholinergic neurons (BFCNs) associated with social behaviors in mammals (Berger-Sweeney et al., 2000). Our observations raise the question whether synaptic connections of the putative social circuit already exist prior to 14 days postfertilization (dpf) and if they do, which synaptic changes allow the behavior to emerge.
In zebrafish, genome duplication resulted in two paralogs of syt2, named syt2a and syt2b. We found a significant increase of the presynaptic protein Syt2a in the zebrafish forebrain during the second week of larval development, compared to relatively steady levels of other presynaptic and postsynaptic proteins. Syt2a forebrain expression is initially restricted to the olfactory bulb, posterior dorsal and ventral telencephalon, which then expands to broad and strong expression throughout the forebrain, showing a similar distribution to other synaptic markers. We found that the increase of forebrain Syt2a expression coincides with the temporal onset of social behavior. Further, we show that Syt2a and the catecholamine synthetic enzyme tyrosine hydroxylase (TH) colocalize on projections within the forebrain and on projections originating from dopaminergic neurons located in the diencephalon, both of which are important for social behaviors (Alger et al., 2011).
2 |. MATERIALS AND METHODS
2.1 |. Zebrafish husbandry and lines
Zebrafish embryos were obtained from natural spawning of wild-type (AB or AB/TU) or the transgenic lines Et(REX2-SCP1:GAL4FF)y321 [Et (y321)] (Marquart et al., 2015), Tg(UAS:GFP) (Kawakami et al., 2016), Tg(UAS:Syn1-GFP) (Easley-Neal et al., 2013), Tg(UAS:GFP-Geph) in the University of Oregon Zebrafish Facility. Embryos were staged at 28.5°C accordingly (Kimmel et al., 1994). All procedures were carried out under an approved protocol with the University of Oregon Animal Care and Use Committee.
To generate the transgenic line Tg(UAS:GFP-Geph), purified plasmid DNA encoding UAS:GFP-Geph was injected into fertilized wild-type (AB) zebrafish eggs at the one-cell stage together with RNA encoding Tol2 transposase, following standard protocols.
2.2 |. Syt2 homologs in the zebrafish
The nomenclature of the two zebrafish synaptotagmin 2 (syt2) homologs in different publications and databases has been controversial. Here, we justify our specific use of the syt2a and syt2b gene names. The syt2a (ZDB-GENE-060503-315) and syt2b (ZDB-GENE-090717-1) duplicates are located on Chromosomes 23 and 6 (Sanger zebrafish reference genome assembly GRCz11), respectively (Table 1). A knockdown of Syt2a (chr.6) leads to loss of znp-1 antibody labeling, showing that znp-1 selectively recognizes Syt2a (chr.6; Wen et al., 2010), previously designated Syt2b. However, because of the location of the gene syt2a on chr.23 in an environment that exclusively harbors b duplicates, syt2a was renamed to syt2b (Liu et al., 2015). We use the nomenclature proposed by Liu et al., 2015.
TABLE 1.
Gene reference sources
| Gene name | e!Ensembl location | NCBI reference sequence ID | Sanger gene ID | ZFIN gene ID | Antibody |
|---|---|---|---|---|---|
| syt2a | Chromosome 6: 55,174,657-55,197,620 |
XM_6830000.7 | not annotated | ZDB-GENE-090717-1 | znp-1 |
| syt2b | Chromosome 23: 6,232,895-6,313,808 |
XM_005174112.4 | ENSDARG00000025206 | ZDB-GENE-060503-315 | None |
Abbreviation:Syt2a, Synaptotagmin 2a
2.3 |. Cloning
We used the primers syt2a-F2: 5 ′-ACAACTCCACCGAGTCTGAG-3 ′ and syt2a-R1: 5′-ATACACAGACATGACCAGCG −3′ to clone a 592 bp fragment, amplified from zebrafish cDNA, into pCR-Blunt II-TOPO vector (ThermoFisher).
The construct UAS:GFP-Geph was generated using the Gateway system. The cDNA encoding zebrafish gephyrinb (a gift from Robert Harvey) was cloned into a p3E entry vector and combined with p5E-4xnr:UAS and pME-GFP into a Tol2 destination vector.
2.4 |. Immunohistochemistry
RNA in situ hybridization and immunohistochemistry were carried out on brain cryostat sections (16 μm) according to standard protocols (Westerfield, 2000). To optimize labeling of the anti-GluR 2/3 antibody, we used a “fresh-frozen” protocol entailing cryosectioning on nonfixed, frozen embryos. Cryosectioned tissues underwent a postsectioning fixation in 4% PFA-PBS solution for 8 min.
For immunohistochemistry, the following primary antibodies were used: Syn1/2, SV2, znp-1, Gephyrin, GluR2/3, TH, and GFP (see Table 2). Primary antibodies were revealed using the following secondary antibodies at a concentration of 1:750 (Alexa Fluor goat anti-mouse IgG1, IgG2a, and [H + L], Molecular Probes, coupled to 488, 546 or 633), Alexa Fluor 633 goat anti-rabbit, Molecular Probes, Alexa Fluor 488 goat anti-chicken, Molecular Probes and goat anti-rabbit Cy5 (IgG [H + L], Jackson ImmunoResearch Laboratories).
TABLE 2.
Antibody resources. Rabbit, Rb; mouse, Ms; chicken, Ch
| Target | Manufacturer | Catalog #, clone | Dilution | Species | Immunogen | RRID |
|---|---|---|---|---|---|---|
| Syn1/2 | Synaptic Systems | 106002 | 1:250 | Rb | Rat peptide, aa 2–28 | AB_2619773 |
| SV2 | DSHB | SP2/0 | 1:1000 | Ms | Synaptic vesicles Ommata electric organ | AB_2315387 |
| Syt2a | Abcam | Ab15403, znp-1 | 1:500 | Ms | Zebrafish lysates | AB_10013783 |
| Gephyrin | Abcam | Ab32206 | 1:1000 | Rb | Ms Peptide, aa 700-C-term | AB_2112628 |
| GluR2/3 | Millipore Sigma | AB1506 | 1:250 | Rb | Rat peptide, C-term EGYNVYGIESVKI | AB_90710 |
| TH | Millipore Sigma | AB152 | 1:500 | Rb | Denatured purified protein from rat | AB_390204 |
| GFP | Aves Labs | GFP-1020 | 1:1000 | Ch | Recombinant GFP | AB_10000240 |
Abbreviations: GluR2/3, glutamate receptor 2/3; Syt2a, Synaptotagmin 2a; SV2, Synaptic vesicle protein 2; Syn1/2, Synapsin1/2; TH, tyrosine hydroxylase
2.5 |. Microscopy
Images were taken on an inverted Nikon TU-2000 microscope with an EZ-C1 confocal system (Nikon) with either a ×20, ×60 water immersion, or ×100 oil immersion objective, a Zeiss LSM5 Pascal confocal microscope with ZEN software with ×20 or ×63 oil immersion objectives or a Leica SP8 confocal microscope with LasX software with ×40 water or ×63 oil immersion objectives. Images of in situ hybridizations were taken on a Zeiss Axioplan compound microscope with a Zeiss AxioCam MRc 5 digital camera.
2.6 |. Quantification of synaptic puncta
Cropped sections of the ×100 or ×63 images were saved as separate channels in grayscale bitmap format. Using the Image Pro Plus program (Media Cybernetics), puncta labeled by Synapsin 1/2, Gephyrin, and GluR 2/3 antibodies were identified manually and colocalization was quantified as performed previously (Hoy et al., 2013). Significance was determined using JMP software. A Tukey–Kramer honestly significant difference (HSD) for pairwise comparisons of least square means was applied to three time points across three regions of the brain with a p threshold of .05 (n ≥ 5 larvae, see Supplementary Tables 1 and 2 for exact n and p-values). A Student’s t test was applied to the analysis of puncta numbers on cell bodies anterior to the anterior commissure (AC) in excel. See figure legend for exact n. Neuropil intensity in cropped sections of ×20 images was measured in ∼250 μm2 areas of the AC and OT. The ratio of AC to OT was calculated for both Syt2a and Syn1/2 labeling at 6 dpf and 14 dpf. n = 5 larvae per age group. Significance was ascertained by Student’s t test. Data in the text and graphs are reported with standard error.
3 |. RESULTS
3.1 |. Syt2a distribution during early larval development is restricted to the midbrain and hindbrain
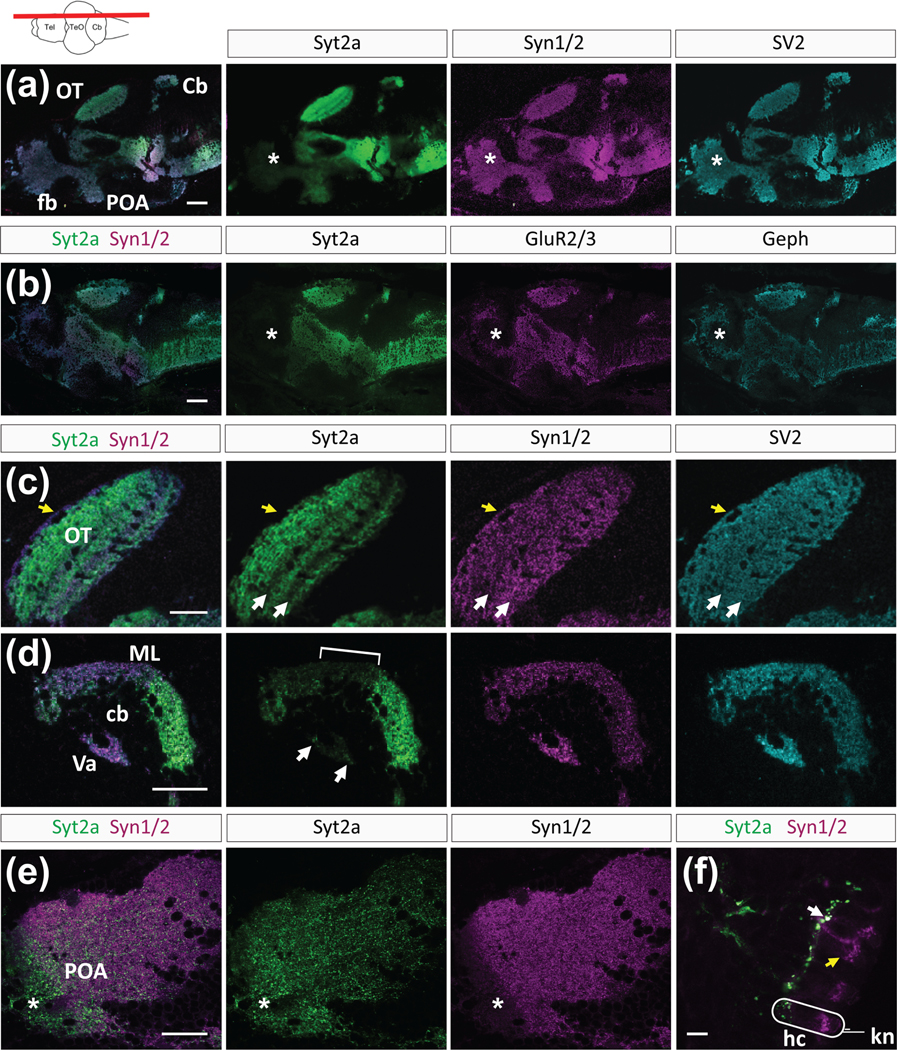
We examined the protein expression pattern of the presynaptic proteins Syt2a, Synaptic vesicle protein 2 (SV2), Synapsin1/2 (Syn1/2), and the postsynaptic proteins glutamate receptor 2/3 (GluR2/3) and Gephyrin (Geph) in the brain of zebrafish larvae at 6 dpf (Figure 1). SV2 and Syn1/2 are proteins associated with synaptic vesicles and are located in the presynaptic terminal. The postsynaptic proteins GluR2/3 and Geph are reliable markers of excitatory and inhibitory synapses, respectively. During larval development from preflexion to late flexion stages (6–14 dpf; Engeszer et al., 2007), immunolabeling for Syt2a was strong in the midbrain and hindbrain, showing that Syt2a expression is established early in these brain regions (Figure 1(a,b)). In contrast to the other synaptic markers that showed uniform distribution throughout the brain, Syt2a labeling was weak in the forebrain region (Figure 1(a,b), asterisks) at 6 dpf. The forebrain consists of the telencephalon, which processes sensory information, controls motor function, and is important for high order function, and the diencephalon, which relays sensory information, influences sensory perception and connects the endocrine and nervous systems (Vaz et al., 2019). The teleost ventral telencephalon, considered a homolog of the septal area, is a part of the limbic system in mammals (O’Connell & Hofmann, 2012). The weak Syt2a signal in the forebrain cannot be explained by weak expression of syt2a mRNA transcript, as our analysis showed that syt2a transcript was expressed in the forebrain at 6 dpf in comparable amounts to other brain regions (Figure S1). We conclude that syt2a transcript is not translated or translated only weakly, resulting in low protein levels in the forebrain at 6 dpf.
FIGURE 1.
Synaptic distribution of presynaptic proteins in the 6 dpf zebrafish brain. (a,b) Overlay and images of individual channels revealing the expression pattern of Syt2, Syn1/2, SV2, Geph, and GluR2/3. The forebrain (fb) region is marked by asterisks. (c) Higher magnification showing individual expression patterns of Syt2, Syn1/2, and SV2 in the optic tectum (OT). Two arrows mark the low Syt2 labeling in two synaptic layers. (d) High magnification of the cerebellum (cb) shows Syt2 labeling in the molecular layer (ML), but weaker signal in the medial part (bracket). Arrows point to punctate labeling in the Valvula cerebelli (Va). (e) The anterior part of the preoptic area (asterisk, POA) shows Syt2 labeling (green) but reduced Syn1/2 labeling (magenta). Yellow arrow marks absent Syt2a labeling in the outer layer. (f) In the lateral line, Syt2 is located in projections while Syn1/2 is found in hair cells (hc). White arrow points to colabeled synapses at the basal face of a hair cell. Yellow arrow points to Syn1/2 labeling in a hair cell. For orientation, a single hair cell (hc) and kinocilium (kn) are outlined in white. All sections except (f) are oriented anterior to the left, dorsal up. The level of the sections is depicted in a cartoon showing a dorsal view of a zebrafish brain (a–e) Scale bars are 100 μm in (a,b), 30 μm in (c–e), and 15 μm in (f)
In addition to the distinct weak forebrain labeling, we found other regional differences in Syt2a localization compared to other synaptic proteins. We detected layered Syt2a labeling in the optic tectum (OT) neuropil, with accumulation of Syt2a protein in the fiber-rich layers (Figure 1(c), arrows point to layers with weaker Syt2a labeling). Syn1/2 and SV2 did not show this layered pattern, but instead presented an even distribution throughout the OT neuropil. Further, Syt2a was absent from the outermost layer of the OT (yellow arrow), labeled by Syn1/2 and SV2. In the cerebellum (Cb), we found regionally distinct Syt2a distribution in the molecular layer (ML, Figure 1(d)), with sparse labeling in the medial portion of the Cb and strong labeling in the anterior and posterior Cb (Figure 1(d), bracket marks sparse labeling in medial portion). Further, Syt2a labeling was weak in the Valvula cerebelli (Va, Figure 1(d), arrows point to Syt2a puncta).
Although we found that Syt2a was often more restricted in its distribution as compared to Syn1/2, we found one exception in the anterior part of the preoptic area (POA), in which Syt2a showed broad and dense labeling throughout the entire POA, whereas Syn1/2 was very sparse (Figure 1(e), asterisk). Further, in lateral line neuromasts Syt2a was restricted to projections, whereas Syn1/2 puncta were distributed within the hair cells and on the projections (Figure 1(f)). Syt2a and Syn1/2 colocalized at the basal end of the sensory receptors, where the projections (green) synapse onto hair cells (magenta, Figure 1(f), white arrow). In summary, Syt2a is more selectively localized in the zebrafish nervous system at 6 dpf, as compared to other synaptic proteins, with the most apparent difference being very sparse expression in the forebrain.
3.2 |. Increased Syt2a expression in the forebrain during late larval development
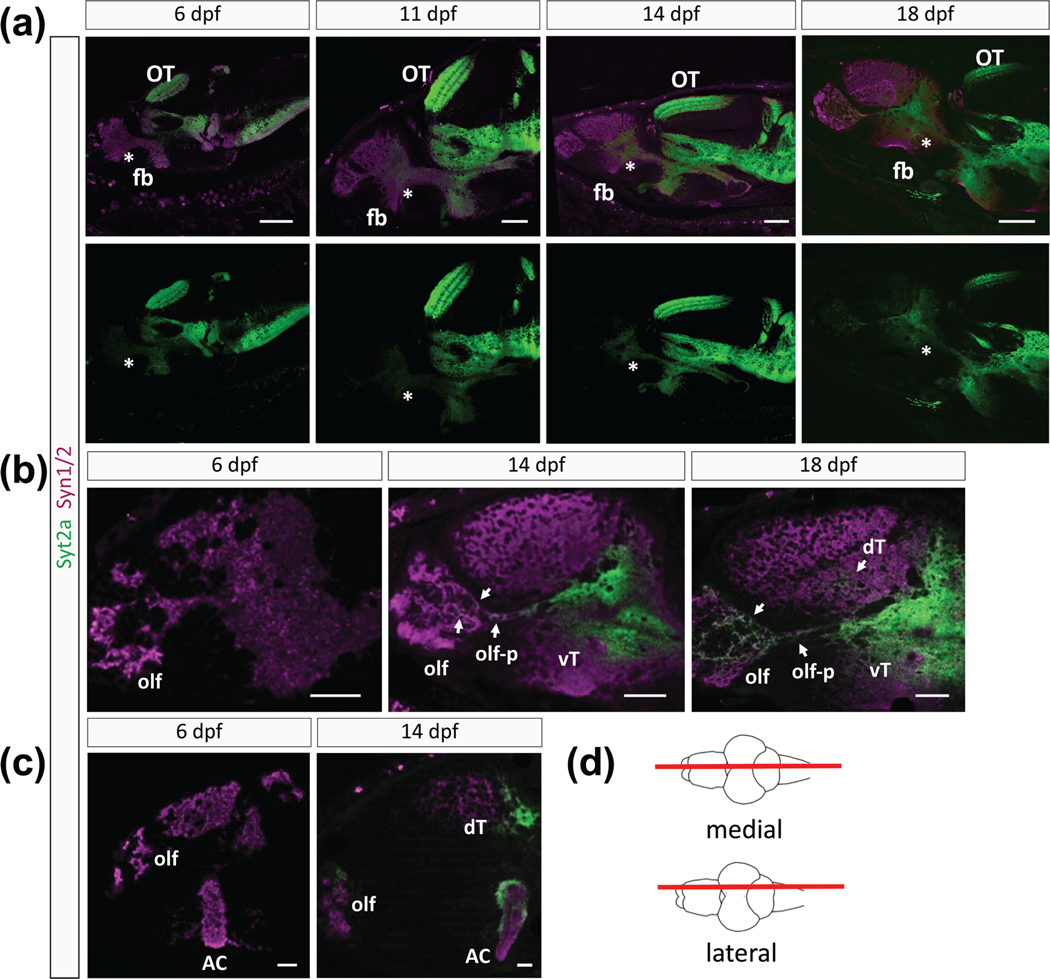
We found that Syt2a labeling in the forebrain increased during development (Figure 2). Whereas Syt2a was barely detectable in the forebrain at 6 dpf, Syt2a expression increased noticeably at 14 dpf, whereby the 14-fold lower intensity in the AC than midbrain (0.764 ± 0.205 for AC, 10.531 ± 1.701 for OT) increased until it reached an intensity at 18–20 dpf that appeared comparable to Syt2a expression level in the midbrain and hindbrain (Figure 2(a–c)). Despite the low level of Syt2a expression at 6 dpf, higher magnification images of the forebrain revealed sparse, punctate Syt2a protein in the olfactory bulb (olf), the AC and a small region of the dorsal telencephalon (not shown). The AC is a dense neuropil, nearly devoid of cell bodies, through which many projections of the telencephalon travel. Between 11 and 14 dpf, Syt2a expression in the ventral telencephalon broadened and expanded to regions adjacent to the initially weak Syt2a labeling (Figure 2(b,c)). Furthermore, Syt2a signal expanded more in the olfactory bulb (olf) and within olf projections (olf-p) at 14 dpf (Figure 2(b), arrows). At 18 dpf, Syt2a protein was present in most of the ventral telencephalon and expanded to the dorsal telencephalon.
FIGURE 2.
Differences in Syt2a and Syn1/2 expression during development. (a) Sections showing Syt2a and Syn1/2 expression in the brains of 6, 11, 14, and 18 dpf zebrafish larvae show that the Syn1/2 expression (magenta) throughout the forebrain (fb) appears not to change. In contrast, Syt2 expression (green) becomes more prominent over time selectively in the forebrain. Level of Syt2a expression in the optic tectum (OT) remains similar among different time points. Medial section (see cartoon in (d)). Asterisks mark the anterior forebrain. (b) Higher magnification of the forebrain region reveals robust and strong Syt2a expression in the olfactory bulb (olf), its projections (olf-p) and in the ventral telencephalon (vT) at 14 dpf, which broadens and becomes more visible in the dorsal telencephalon (dT) at 18 dpf. Arrows point to Syt2a puncta within these regions. (c) Lateral sections (see (d)) reveal an increase in Syt2a signal in the olf, dT, and the dorsal and lateral area of the anterior commissure (AC) between 6 and 14 dpf. (d) Cartoon showing a dorsal view of a zebrafish brain marking the level of the lateral and medial sections shown in the images. Scale bars are 100 μm in (a), 50 μm in (b), and 30 μm in (c)
We now compared the spatiotemporal distribution of Syt2a and Syn1/2 during forebrain development. As expected, we observed strong and uniform Syn1/2 labeling throughout the forebrain at 6 dpf (Figure 2(a–c)) with no noticeable change in distribution or expression levels at 11, 14, or 18 dpf (Figure 2(a)). Syn1/2 proteins are a reliable marker for presynaptic terminals, being present at almost all synapses in the vertebrate brain (Micheva et al., 2010). To quantify the degree of labeling in the forebrain as compared to other brain regions, we measured the fluorescence intensity of the AC and divided it by the intensity of labeling in the OT, as an “in image” control. The intensity ratio of Syt2aAC/OT labeling was about 10-fold lower than for Syn1/2AC/OT (0.067 ± 0.013 for Syt2aAC/OT, 0.66 ± 0.181 for Syn1/2AC/OT), confirming that Syt2a expression in the telencephalon is much weaker than Syn1/2 at 6 dpf. Syt2aAC/OT significantly increased 2.9-fold by 14 dpf (0.195 ± 0.038, p = .007), whereas Syn1/2AC/OT increased only 1.7-fold over the same period (1.16 ± 0.228, p = .09). Therefore our results suggest that although many synapses are already present within the forebrain at 6 dpf, as evidenced by Syn1/2 expression, most of these synapses do not contain Syt2a until an increase in Syt2a by 14 dpf, a time point at which social behavior is established (Dreosti et al., 2015; Stednitz & Washbourne, 2020).
The increased distribution of Syt2a during development is particularly evident in medial telencephalon sections (Figure 2(c,d)). Syt2a is expressed in the AC, the olfactory region (olf) and the dorsal telencephalon (dT) at 14 dpf. However, at 6 dpf, Syt2a is almost undetectable in these regions (Figure 2(c)). We note that Syt2a expression in the dorsal aspect and on the exterior portions of the AC was denser and appeared stronger compared to other regions within the AC (Figure 2(c)). We conclude that Syt2a is dynamically expressed in the forebrain during larval development, presumably through regulation of translation, because we did not see dramatic differences in mRNA levels across the brain (Figure S1).
3.3 |. Synapse composition in the AC shows dynamic developmental changes
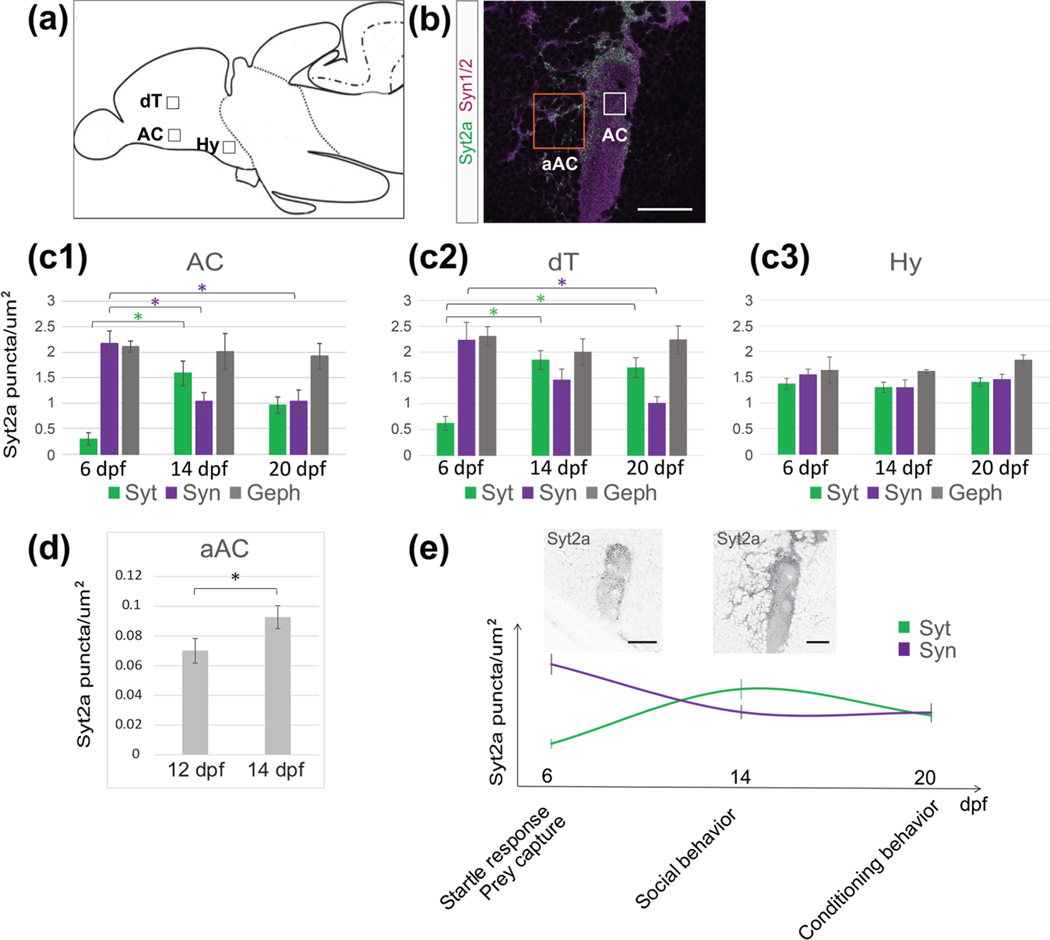
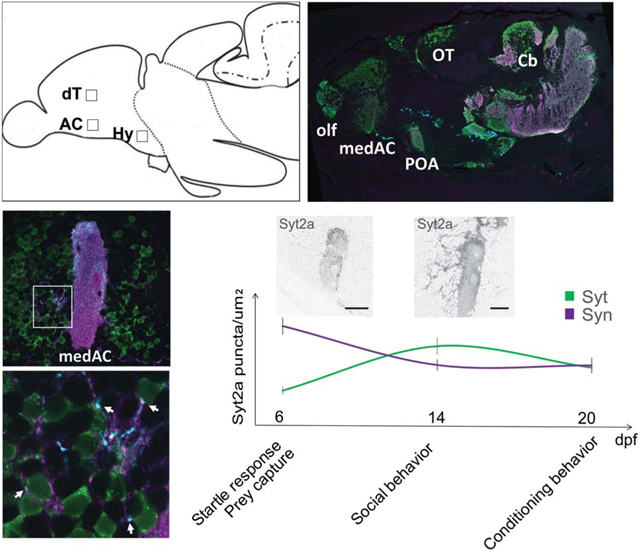
We quantified synaptic protein distribution in three selected regions of the forebrain (Figure 3(a,c–c2,e)) that are assumed to contribute to the social decision making network (Bshary et al., 2014), and found that the relative synaptic protein expression changed over development. We analyzed the density of Syt2a, Syn1/2 and Geph puncta in the dorsomedial part of the AC neuropil in the ventral telencephalon (AC, Figure 3(b), white square), a region in the posterior part of the dorsal telencephalon (dT, Figure 3(b)) and the anterior part of the hypothalamus (Hy, Figure 3(a)). We chose three time points, 6, 14, and 20 dpf that reflect different maturation states of zebrafish development. We found the most dramatic increase in Syt2a puncta density in the AC and dT (Figure 3(c1,c2), green) between 6 and 14 dpf, where Syt2a density peaks at 14 dpf. In contrast, the density of Syt2a puncta in the Hy remained at a similar level throughout this developmental period (Figure 3(c3)).
FIGURE 3.
Syt2a expression levels in the telencephalon change over time. (a) Cartoon showing a sagittal view of a zebrafish brain. Boxes mark the selected region of interest (ROI) used for synapse counts. (b) Image showing the anterior commissure (AC) labeled for Syt2 (green) and Syn1/2 (magenta) at 14 dpf. The white box shows the actual size of ROI used for counting. The orange box shows an ROI for a synapse count on neuronal cell bodies located adjacent to the AC (aAC). (c1–c3) Changes in synapse density of Syt2, Syn1/2, and Geph puncta at 6, 14, and 20 dpf in the anterior commissure (AC, 3c1), dorsal telencephalon (dT, 3c2), and hypothalamus (Hy, 3c3). Significance was determined using a Tukey–Kramer HSD, with *p < .05. (d) The number of Syt2 puncta on neuronal cell bodies in the aAC (see (b), orange box) increases between 12 and 14 dpf. Significance was determined using a Student’s t test, with *p < .05. n = 6 brains for 12 dpf and 5 brains for 14 dpf. (e) Graph shows Syt2a and Syn1/2 puncta density in the AC (see (c1–c3)) over time while marking behavioral milestones during zebrafish larval development. The two images show representative Syt2a expression at 6 and 14 dpf, respectively, demonstrating the increase in Syt2 puncta aAC. Scale bars are 30 μm in (b) and 10 μm in (e)
In contrast to Syt2a expression that is initially low and increases during development of the telencephalon, we found that Syn1/2 puncta density is already high at the earliest time point tested (Figure 3(c1–c3), purple). After peaking at a distinct development stage, the density of the presynaptic proteins Syt2a and Syn1/2 decreased later. In contrast, we found that the number of postsynaptic Geph puncta is stable across the three time points analyzed (Figure 3(c1–c3), gray). We conclude that the dynamic Syt2a expression levels in the telencephalon during development are consistent with an increasing number of synapses acquiring Syt2, whereas the localization of other synaptic proteins remains relatively stable over the same developmental period.
Consistent with the above observations, we noticed an increase in Syt2a puncta in a region adjacent to the AC (aAC, Figure 3(b), orange box), a region populated by neurons that are necessary for social behavior in the adult (Stednitz et al., 2018) and at 14 dpf (AT and PW, unpublished observation). We compared Syt2a puncta at 12 and 14 dpf, during which social behavior becomes more robust (Dreosti et al., 2015; Stednitz & Washbourne, 2020), and found a 20% increase in Syt2a puncta in the aAC region (Figure 3(d)). It is striking that Syt2a distribution becomes prominent and peaks at 14 dpf, a time at which social behavior is significantly strengthened (Figure 3(e)). These observations suggest that, during this time interval, synaptic properties are modulated, coinciding with the establishment of complex behaviors, including social orienting between fish, as well as detection of biological motion (Dreosti et al., 2015; Larsch & Baier, 2018; Roberts et al., 2013; Stednitz & Washbourne, 2020). However, this period is prior to the establishment of operant conditioning behavior in the third week of development (Valente et al., 2012).
3.4 |. Syt2a-positive synapses are localized on both somata and neurites of social behavior-relevant forebrain neurons
We previously identified a genetically defined population of lhx8a-positive neurons in the ventral forebrain of zebrafish larvae that is important for social behavior at 14 dpf (A. T., P. W., unpublished), as defined by ablation experiments similar to those in adult zebrafish (Stednitz et al., 2018). These cells are cholinergic (Stednitz et al., 2018), similar to an Lhx8-positive population of cells in the basal forebrain in mammals (Zhao et al., 2003). Despite the identification of these homologous neuronal populations in both fish and mammals, we know little about the neurons that synapse onto these BFCNs or what brain regions these neurons innervate.
As a marker for these BFCNs in the zebrafish, we took advantage of the transgenic line Et(y321), an enhancer trap located in the lhx8a gene, driving UAS:GFP protein expression in the ventral forebrain. We analyzed Syt2a distribution on these socially relevant BFCNsy321 and their projections at 14 dpf. The transgenic line expresses in two populations of neurons in the forebrain, one in the ventral telencephalon and another in the anterior part of the diencephalon, the POA of the hypothalamus (Hy, Figure 4(a)). The neuronal population in the telencephalon consists of two clusters, divided by the AC (Figure 4(a,b), arrow). As described earlier, Syt2a puncta were distributed throughout neurons adjacent to the AC (Figure 4(c–c2)). Although Syn1/2 showed an even distribution throughout the medial AC (medAC, Figure 4(b,c)), we found that Syt2a accumulates at the dorsal aspect of the medAC and on the external face of the neuropil (Figure 4(d, d1)), whereas the center and the ventral neuropil show less dense Syt2a expression. In the region anterior to the AC, we found Syt2a puncta localized at the cell bodies of BFCNsy321 (Figure 4(c–c2), arrows). All these puncta were also positive for Syn1/2, suggesting that these are assembled presynaptic terminals (Figure 4(c1,c2)). A few days later, at 20 dpf, we found BFCNsy321 in close proximity (arrows) or even engulfed (asterisk) by Syt2a positive AC neuropil (Figure 4(d,d1′)), suggesting that Syt2a positive AC neuropil dimensions expand over time. Another possibility is that more BFCNsy321 form in close apposition to the already established neuropil. We conclude that BFCNsy321 are in close proximity to the synaptic neuropil of the AC, and that Syt2a positive presynaptic terminals localize to the somata of BFCNsy321 in the ventral telencephalon.
FIGURE 4.
Syt2a puncta are localized on BFCNy321 somata and projections. (a) Sagittal view of a brain with Syt2 (cyan) and GFP (green) in a transgenic fish expressing GFP under control of the y321 enhancer trap, expressed in BFCNsy321. In the forebrain, the largest cluster of GFP positive neurons are located in the ventral telencephalon, in a region adjacent to the anterior commissure (AC) (arrow marks the AC), and the anterior hypothalamus (Hy). (b) Dorsal view of the forebrain showing a cluster of GFP positive neurons adjacent to the AC, and divided by the medial AC (medAC). (c) Lateral view of the medAC showing Syt2 (cyan) and Syn1/2 (magenta) within the GFP positive BFCNy321 domain. (c1) Magnification of the area marked by the box in (c) Arrows mark presynaptic terminals onto BFCNsy321 expressing GFP. (c2) Single neuron, see box in (c1), with Syt2 and Syn1/2 puncta on the neuronal soma (arrow). (d) Magnification of the dorsal part of the medAC, showing the close proximity of GFP positive neurons to the medAC at 20 dpf. (d1) High magnification with an asterisk marking a GFP positive neuron enclosed within the medAC neuropil. Arrows mark Syt2 and Syn1/2 positive puncta on GFP positive neurons. (e–e3) Mosaic expression of Syn1-GFP in BFCNy321 neurons and their neurites in the AC at 14 dpf. (e1–e3) High magnification shows colocalization of Syn1-GFP and Syt2a, marked by white arrows, a GFP negative Syt2a punctum is marked by a yellow arrow. Box in (e) shows the magnified area. (f–f3) Labeling of Syt2 with GFP-Geph fusion protein in BFCNsy321. High magnification of the boxed in area shows colocalization of Syt2a and GFP-Geph, marked by white arrows. A yellow arrow marks a GFP negative punctum. Scale bars are 100 μm in (a), 60 μm in (b), 30 μm in (c), 2 μm in (c1,c2), 4 μm in (d,d1), 30 μm in (e,f) and 5 μm in (e1,e2,f1,f2)
Because the AC neuropil is very dense and neurites from all BFCNsy321 are hard to follow, we expressed a Synapsin1-GFP fusion protein in a mosaic fashion using the transgenic line Et(y321);Tg(UAS: Syn1-GFP). This approach allowed us to follow individual neurites from BFCNsy321 and examine colocalization of Syt2a with zebrafish Synapsin1-GFP from a subset of BFCNsy321 in the medAC (Figure 4 (e–e3)). We found colocalization of Syt2a with presynaptic Syn1-GFP puncta, suggesting that Syt2a is located in the presynaptic terminals of BFCNsy321. In addition, we expressed a GFP-Gephyrin fusion protein in BFCNsy321 using the transgenic line Et(y321);Tg(UAS: GFP-Geph). In this case, we found that Syt2a puncta colocalized with postsynaptic GFP-Geph puncta on BFCNy321 neurites (Figure 4(f–f3)), suggesting that Syt2a is localized in presynaptic terminals formed onto projections from BFCNsy321 at (but not exclusively) inhibitory synapses. From these experiments, we conclude that Syt2a is localized at presynaptic terminals from BFCNsy321 and at presynaptic terminals that synapse onto BFCNy321 somata and neurites.
3.5 |. Syt2a and tyrosine hydroxylase colocalize at synapses
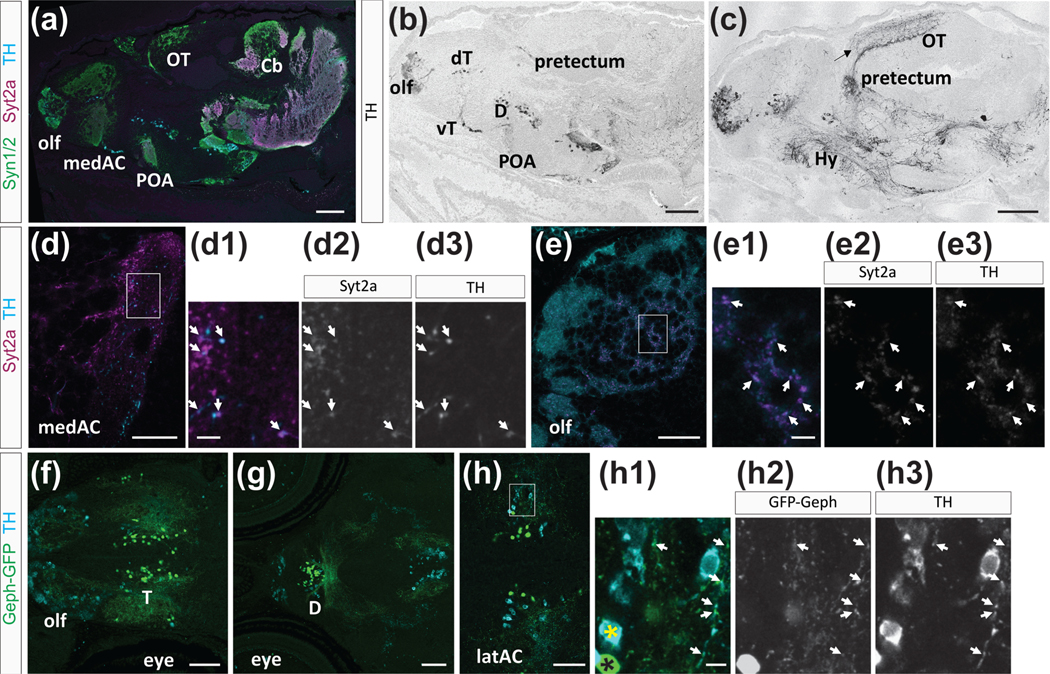
Dopaminergic neurons in the tegmentum that project anteriorly to ventral forebrain regions have been identified as important regulators of social behaviors in vertebrates (Arakawa & Ikeda, 1991). TH is the rate-limiting enzyme for catecholamine synthesis and is necessary for dopamine production. Therefore, we decided to examine whether there might be a “circuit” interaction between TH positive neurons and BFCNsy321. There are two th genes in zebrafish, th1 and th2. While th1 is expressed in the olfactory bulb, telencephalon, POA, posterior tuberculum, hypothalamus, and hindbrain, th2 is only expressed in the diencephalon and midbrain (Filippi et al., 2010; Yamamoto et al., 2010). To label all TH-positive neurons, we used an antibody that recognizes zebrafish TH1 and TH2 proteins (Yamamoto et al., 2010).
We found TH expression in cell bodies and projections in many brain regions (Figure 5(a–c)), similar to TH distribution in the adult zebrafish brain (Rink & Wullimann, 2002; Yamamoto et al., 2010). TH antibody labels neurons in the olfactory bulb, dorsal and ventral telencephalon, POA, hypothalamus, thalamus, and hindbrain (Figure 5(b,c)). We did not detect TH positive cell bodies in the OT, but found TH positive neurites in two bands located within the retino-recipient strata and deeper strata of the OT neuropil (Figure 5(c), arrow, (Arenzana et al., 2006)). The ventral telencephalon has only few TH expressing neurons, but we expected to see TH positive synaptic puncta in the medAC and in the region adjacent to the medAC (Figure 5 (d–d3)), as suggested by previous studies that traced the projections of dopaminergic neurons (Du et al., 2016). Indeed, we found TH puncta in and adjacent to the medAC, and these puncta colocalized with Syt2a, suggesting that TH is located in presynaptic terminals (Figure 5(d–d3)). Further, we found TH positive cell bodies distributed throughout the olfactory bulb and punctate TH expression on olfactory bulb projections (Figure 5(e–e3)). These results confirm that TH puncta are localized on projections and even at presynaptic terminals, and further suggest a localization of TH to Syt2a positive terminals in the ventral telencephalon.
FIGURE 5.
Tyrosine hydroxylase (TH) expression and colocalization with Syt2a. (a–c) Sagittal view of a brain at 14 dpf, showing TH expression in distinct neuronal clusters throughout the brain. (a) TH (cyan), Syt2a (magenta), and Syn1/2 (green) expression in the brain. (b,c) TH is expressed in neurons in the olfactory bulb (olf), dorsal (dT), and ventral telencephalon (vT), diencephalon (D), preoptic area (POA), and pretectum. TH projections are located throughout the brain. An arrow marks labeling in the optic tectum (OT). (d–d3) Syt2a and TH colocalize at synapses in the AC. Arrows mark Syt2a (d2) and TH (d3) colabeled puncta. (e–e3) In the olfactory bulb we find many Syt2 (e2) and TH (e3) co-expressing puncta (arrows). (f–h3) Dorsal view of the forebrain showing TH expression (cyan) and GFP-Geph positive (green) specifically in BFCNsy321. (f) Expression in the olf and telencephalon (T). (g) More posterior view showing TH expression and GFP-Geph positive BFCNsy321 in the diencephalon (D). (h–h3) Higher magnification of the telencephalic region. A yellow and black asterisk marks TH only and GFP-Geph only neurons, respectively. Arrows point to TH and GFP-Geph colocalization on projections from BFCNsy321. Area shown in (h–h3) is marked with a box in (h) Scale bars are 200 μm in (a–c), 30 μm in (d,e), 70 μm in (f,g), 50 μm in (h), and 5 μm in (d1,e1,h1)
Because dopamine is important for social behavior (Arakawa & Ikeda, 1991; Stednitz et al., 2018), we explored whether TH synaptic terminals are located on forebrain BFCNy321 cell bodies or their neurites. We did not detect TH in BFCNy321 cell bodies (Figure 5(h–h3); TH, black asterisk; BFCNy321, yellow asterisk), but found that TH positive neuronal cell bodies are often located in the vicinity of GFP-Geph expressing BFCNsy321 (5f-h3). Further, TH puncta frequently colocalized with GFP-Geph puncta (arrows in Figure 5(h–h3)), suggesting that inhibitory presynaptic terminals, located on the neurites of BFCNsy321, are also TH-positive. These studies suggest that Syt2a protein localizes to synapses in a region of the brain and at a time in development that is associated with the initiation of robust social behavior. Furthermore, Syt2a positive synapses in close apposition to neurites of BFCNsy321 in the ventral telencephalon show TH labeling, suggesting that they might release dopamine, a neurotransmitter associated with social behavior.
4 |. DISCUSSION
Among the many Synaptotagmin proteins that have been studied, Syt1 and Syt2 are the major proteins mediating fast and synchronous vesicular release at central synapses (Fernández-Chacón et al., 2001; Pang, Melicoff, et al., 2006; Pang, Sun, et al., 2006). Despite the similarity between Syt1 and Syt2, and their sometimes redundant functions, Syt2 release kinetics are substantially faster than release triggered by Syt1 (Xu et al., 2007), and thus differential expression of Syt1 and Syt2 has the potential to significantly alter the function of synapses during development. Here we have examined the expression pattern of one of the Syt2 homologs in the zebrafish brain during development, with a focus on brain regions implicated in social behavior.
We found that zebrafish Syt2a protein is more highly expressed in the midbrain at 6 dpf compared to lower Syt2 expression levels in the forebrain. Interestingly, we found that Syt2a levels in the forebrain change over an identified developmental period. Starting at 6 dpf, Syt2 forebrain protein expression increases and becomes robust at 14 dpf. Our data suggest that Syt2a protein is generated at low levels in the forebrain until about 14 dpf, when Syt2a protein synthesis is substantially upregulated. This is supported by the presence of syt2a transcript in the forebrain at 6 dpf (Figure S1). Our data suggest that existing presynaptic terminals in the forebrain change their protein composition and potentially also their electrophysiological properties as part of a synaptic refinement step around 14 dpf, thereby coinciding with the time at which social behavior becomes robust (Dreosti et al., 2015; Larsch & Baier, 2018; Stednitz & Washbourne, 2020). However, it is possible that the late onset of Syt2a expression in the forebrain is due to ascending projections from more posterior regions extending into the forebrain. Only a very limited number of ascending axonal projections into the subpallium have been reported during early larval development (Du et al., 2016; Tay et al., 2011). Further, a massive increase in projections into this region during later larval development that could account for the major increase in Syt2a protein in the telencephalon has not been reported. Although we cannot exclude this possibility, based on our observations, we favor a change in synaptic physiology as an explanation for the increase in Syt2a puncta in the telencephalon at 14 dpf. However, additional experiments that highlight the temporal and spatial distribution of projections growing into the telencephalon between 6 and 14 dpf would resolve this question.
In mice, Syt2 is widely expressed in the spinal cord, brainstem, and cerebellum at P15. In addition, similar to zebrafish, Syt2 is present in selected forebrain neurons, including most striatal neurons and some hypothalamic, cortical, and hippocampal neurons (Fox & Sanes, 2007; Pang, Melicoff, et al., 2006). However, the expression pattern of mouse Syt2 has not been extensively described at different developmental timepoints, and it is therefore unclear whether regions of the mouse brain might dramatically increase Syt2 expression over the course of development.
It is intriguing that the onset of Syt2a expression in the forebrain and the onset of robust social behavior coincide at around 14 dpf, raising the possibility that Syt2a is involved in the functional refinement of circuits by modulating calcium sensitivity and vesicle release properties of previously established synapses. We observed a significant increase in Syt2a protein expression in the ventral telencephalon, specifically in the AC and in regions adjacent to the AC associated with BFCNs. In previous work, we showed that this specific population of cholinergic neurons in the ventral forebrain is required for social behavior in adult zebrafish (Stednitz et al., 2018). Here, we show, that Syt2a is located at synapses onto somata and neurites of BFCNsy321, suggesting that Syt2a is important in this circuit and might define the developmental time line of social behavior acquisition. These findings are consistent with a suggested social behavior network (SBN; Geng & Peterson, 2019), consisting of several identified regions within the mammalian forebrain. Except for a few regions unique to mammals, the zebrafish forebrain shares most regions implicated in the mammalian SBN, suggesting that this network is highly conserved (Geng & Peterson, 2019). Different nomenclature in mammals and zebrafish complicates the direct anatomical comparison. However, the mammalian nucleus accumbens, lateral septum, striatum, and medial preoptic regions are homologous regions to the zebrafish ventral telencephalon and the preoptic region, both regions in which we find Syt2a protein at the time when social behavior becomes robust. The neurons upstream and downstream of BFCNsy321 have not been identified, a crucial future step to fully elucidating the SBN.
Studies in mouse, rat, and zebrafish have shown that dopamine is essential for social behavior (Arakawa & Ikeda, 1991; Homberg et al., 2016; Oliveri & Levin, 2019; Parker et al., 2013; Stednitz et al., 2018). We asked whether dopaminergic input might associate with BFCNsy321 in the ventral telencephalon, by examining TH expression at 14 dpf. TH is a major biosynthetic enzyme for dopamine and other catecholamines. Our experiments revealed several important points: (a) BFCNsy321 in the ventral telencephalon do not express TH, (b) TH puncta colocalize with Syt2a and GFP-Geph on neurites from BFCNsy321, and (c) there are several clusters of TH positive neurons that might be sources of TH positive presynaptic terminals onto BFCNsy321 in the ventral telencephalon.
The finding of an association between TH and Syt2a is consistent with observations in mammals, which showed that Syt2 colocalizes with Th mRNA in axons of dissociated superior cervical ganglion neurons. This study demonstrated that TH is locally synthesized and that TH protein accumulates with Synaptotagmin in presynaptic terminals (Gervasi et al., 2016), analogous to our results. Furthermore, we identified numerous clusters of neurons expressing TH, some of which had obvious projections to the ventral telencephalon. These include the olfactory bulb, the ventral telencephalon, and the diencephalon. Similar populations have been observed in mammals, suggesting that homologous populations of dopaminergic neurons might be participating in the regulation of social behavior in mammals.
Many dopaminergic clusters identified in mammals have corresponding clusters in the zebrafish (Filippi et al., 2012). However, the ventral tegmental area in the midbrain is one of the major sources of mesostriatal dopaminergic projections in mammals, but not in zebrafish (Rink & Wullimann, 2002). Neurons in the ventral tegmentum project into multiple limbic forebrain areas, including the nucleus accumbens, located in the ventral forebrain rostral to the preoptic region of the hypothalamus, a component of the SBN (Alger et al., 2011). This distribution is largely conserved among vertebrates (Smeets & González, 2000). In contrast, zebrafish lack dopaminergic neurons in the mesencephalon (Filippi et al., 2010; Rink & Wullimann, 2001). However, the zebrafish ascending diencephalic or the endosubpallium TH cluster have been assumed to be functionally homologous to some of the mesostriatal circuit system (Du et al., 2016; Filippi et al., 2012; Haehnel-Taguchi et al., 2018; Rink & Wullimann, 2002; Tay et al., 2011; Yamamoto & Vernier, 2011). Although it is possible that the projections onto the BFCNsy321come from any of the TH positive clusters, for instance the subpallium TH cluster (Tay et al., 2011), we hypothesize, based on our observations, that the ventral diencephalic cluster is a likely source of this input. Further experiments, such as retrograde tracing studies would be necessary to identify the dopaminergic cell cluster(s) projecting to the BFCNy321 neurons.
The aim of our study was to examine the distribution of Syt2a and other synaptic proteins during zebrafish development, in particular at and preceding the emergence of complex behaviors. We show that Syt2a localizes to synapses onto neurons implicated in social behavior and is colocalized with TH, important for social behavior. Our data demonstrate that Syt2a expression coincides with the emergence of social behavior and that Syt2a is at the right place and time to be an important participant in the refinement of synapses on neurons relevant for social behavior. In the future, it will be interesting to test whether brain region-specific deletion of Syt2 will significantly alter behaviors in the zebrafish, whether they manifest at a time point at which we first detect Syt2a protein at specific synapses on BFCNsy321 in the ventral telencephalon, and whether dopaminergic input into the ventral telencephalon is altered by this genetic manipulation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Robert Harvey (University of Southern California) for providing the plasmid encoding zebrafish gephyrin b, Adam Christensen for help with fish husbandry, the University of Oregon Zebrafish Facility staff for animal care, and the University of Oregon Histology Facility for preparing sections. This work was supported by National Institutes of Health grant R33MH104188 to P. W. and J. E.
Funding information
National Institutes of Health, Grant/Award Number: R33MH104188
Footnotes
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available from the following public data repository: https://doi.org/10.6084/m9.figshare.13151288.v2.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alger SJ, Juang C, & Riters LV (2011). Social affiliation relates to tyrosine hydroxylase immunolabeling in male and female zebra finches (Taeniopygia guttata). Journal of Chemical Neuroanatomy, 42(1), 45–55. 10.1016/j.jchemneu.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa O, & Ikeda T. (1991). Apomorphine effect on single and paired rat open-field behavior. Physiology & Behavior, 50(1), 189–194. 10.1016/0031-9384(91)90520-X. [DOI] [PubMed] [Google Scholar]
- Arenzana FJ, Arévalo R, Sánchez-González R, Clemente D, Aijón J, & Porteros A. (2006). Tyrosine hydroxylase immunoreactivity in the developing visual pathway of the zebrafish. Anatomy and Embryology, 211(4), 323–334. 10.1007/s00429-006-0084-2. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Stearns NA, Frick KM, Beard B, & Baxter MG (2000). Cholinergic basal forebrain is critical for social transmission of food preferences. Hippocampus, 10(6), 729–738. . [DOI] [PubMed] [Google Scholar]
- Bouhours B, Gjoni E, Kochubey O, & Schneggenburger R. (2017). Synaptotagmin2 (Syt2) drives fast release redundantly with Syt1 at the output synapses of parvalbumin-expressing inhibitory neurons. The Journal of Neuroscience, 37(17), 4604–4617. 10.1523/JNEUROSCI.3736-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R, Gingins S, & Vail AL (2014). Social cognition in fishes. Trends in Cognitive Sciences, 18(9), 465–471. 10.1016/j.tics.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Chen C, Arai I, Satterfield R, Young SM Jr., & Jonas P. (2017). Synaptotagmin 2 is the fast Ca(2+) sensor at a central inhibitory synapse. Cell Reports, 18(3), 723–736. 10.1016/j.celrep.2016.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Lopes G, Kampff AR, & Wilson SW (2015). Development of social behavior in young zebrafish. Frontiers in Neural Circuits, 9, 39–39. 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Guo Q, Shan M, Wu Y, Huang S, Zhao H, … Su B. (2016). Spatial and temporal distribution of dopaminergic neurons during development in zebrafish. Frontiers in Neuroanatomy, 10, 115–115. 10.3389/fnana.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley-Neal C, Fierro J Jr., Buchanan J, & Washbourne P. (2013). Late recruitment of synapsin to nascent synapses is regulated by Cdk5. Cell Reports, 3(4), 1199–1212. 10.1016/j.celrep.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Barbiano LADA, Ryan MJ, & Parichy DM (2007). Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Animal Behaviour, 74(5), 1269–1275. 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, & Südhof TC (2001). Synaptotagmin I functions as a calcium regulator of release probability. Nature, 410(6824), 41–49. 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Filippi A, Jainok C, & Driever W. (2012). Analysis of transcriptional codes for zebrafish dopaminergic neurons reveals essential functions of Arx and Isl1 in prethalamic dopaminergic neuron development. Developmental Biology, 369(1), 133–149. 10.1016/j.ydbio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Filippi A, Mahler J, Schweitzer J, & Driever W. (2010). Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. The Journal of Comparative Neurology, 518(4), 423–438. 10.1002/cne.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, & Sanes JR (2007). Synaptotagmin I and II are present in distinct subsets of central synapses. Journal of Comparative Neurology, 503(2), 280–296. 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Garner CC, Waites CL, & Ziv NE (2006). Synapse development: Still looking for the forest, still lost in the trees. Cell and Tissue Research, 326(2), 249–262. 10.1007/s00441-006-0278-1. [DOI] [PubMed] [Google Scholar]
- Geng Y, & Peterson RT (2019). The zebrafish subcortical social brain as a model for studying social behavior disorders. Disease Models & Mechanisms, 12(8), dmm039446. 10.1242/dmm.039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi NM, Scott SS, Aschrafi A, Gale J, Vohra SN, MacGibeny MA, Kar AN, Gioio AE, & Kaplan BB (2016). The local expression and trafficking of tyrosine hydroxylase mRNA in the axons of sympathetic neurons. RNA, 22(6), 883–895 Retrieved from http://rnajournal.cshlp.org/content/22/6/883.abstractN2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel-Taguchi M, Fernandes AM, Bohler M, Schmitt I, Tittel L, & Driever W. (2018). Projections of the diencephalospinal dopaminergic system to peripheral sense organs in larval zebrafish (Danio rerio). Frontiers in Neuroanatomy, 12, 20. 10.3389/fnana.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, VandenBroeke M, Youn J, Ellenbroek AK, Karel P, … Ellenbroek BA (2016). The role of the dopamine D1 receptor in social cognition: Studies using a novel genetic rat model. Disease Models & Mechanisms, 9(10), 1147–1158. 10.1242/dmm.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy JL, Haeger PA, Constable JR, Arias RJ, McCallum R, Kyweriga M, … Washbourne P. (2013). Neuroligin1 drives synaptic and behavioral maturation through intracellular interactions. The Journal of Neuroscience, 33(22), 9364–9384. 10.1523/JNEUROSCI.4660-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Asakawa K, Hibi M, Itoh M, Muto A, & Wada H. (2016). Gal4 driver transgenic zebrafish: Powerful tools to study developmental biology, organogenesis, and neuroscience. Advanced Genetics, 95, 65–87. 10.1016/bs.adgen.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, & Kane DA (1994). Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development, 120(2), 265. Retrieved from http://dev.biologists.org/content/120/2/265.abstract–276. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Babai N, & Schneggenburger R. (2016). A Synaptotagmin isoform switch during the development of an identified CNS synapse. Neuron, 91(5), 1183. 10.1016/j.neuron.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Larsch J, & Baier H. (2018). Biological motion as an innate perceptual mechanism driving social affiliation. Current Biology, 28(22), 3523–3532.e3524. 10.1016/j.cub.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Liu C, Hu J, Qu C, Wang L, Huang G, Niu P, … Wang H. (2015). Molecular evolution and functional divergence of zebrafish (Danio rerio) cryptochrome genes. Scientific Reports, 5, 8113. 10.1038/srep08113, https://www.nature.com/articles/srep08113#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart GD, Tabor KM, Brown M, Strykowski JL, Varshney GK, LaFave MC, Mueller T, Burgess SM, Higashijima SI, & Burgess HA (2015). A 3D searchable database of transgenic zebrafish Gal4 and Cre lines for functional neuroanatomy studies. Frontiers in Neural Circuits, 9, 78–78. 10.3389/fncir.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Busse B, Weiler NC, O’Rourke N, & Smith SJ (2010). Single-synapse analysis of a diverse synapse population: Proteomic imaging methods and markers. Neuron, 68(4), 639–653. 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science, 336(6085), 1154–1157. 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Oliveri AN, & Levin ED (2019). Dopamine D1 and D2 receptor antagonism during development alters later behavior in zebrafish. Behavioural Brain Research, 356, 250–256. 10.1016/j.bbr.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, … Südhof TC (2006). Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. The Journal of Neuroscience, 26(52), 13493–13504. 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Sun J, Rizo J, Maximov A, & Südhof TC (2006). Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. The EMBO Journal, 25(10), 2039–2050. 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Alberico SL, Miller AD, & Narayanan NS (2013). Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience, 255, 246–254. 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, & Wullimann MF (2001). The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Research, 889(1), 316–330. 10.1016/S0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rink E, & Wullimann MF (2002). Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Research Bulletin, 57(3), 385–387. 10.1016/S0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Rizo J, & Xu J. (2015). The synaptic vesicle release machinery. Annual Review of Biophysics, 44(1), 339–367. 10.1146/annurev-biophys-060414-034057. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Bill BR, & Glanzman DL (2013). Learning and memory in zebrafish larvae. Frontiers in Neural Circuits, 7, 126–126. 10.3389/fncir.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJAJ, & González A. (2000). Catecholamine systems in the brain of vertebrates: New perspectives through a comparative approach. Brain Research Reviews, 33(2), 308–379. 10.1016/S0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Stednitz SJ, McDermott EM, Ncube D, Tallafuss A, Eisen JS, & Washbourne P. (2018). Forebrain control of behaviorally driven social orienting in zebrafish. Current Biology, 28, 2445–2451.e3. 10.1016/j.cub.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stednitz SJ, & Washbourne P. (2020). Rapid progressive social development of zebrafish. Zebrafish, 17(1), 11–17. 10.1089/zeb.2019.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2002). Synaptotagmins: Why so many? Journal of Biological Chemistry, 277(10), 7629–7632. 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, Ryu S, Nitschke R, & Driever W. (2011). Comprehensive catecholaminergic projectome analysis reveals singleneuron integration of zebrafish ascending and descending dopaminergic systems. Nature Communications, 2, 171–171. 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A, Huang KH, Portugues R, & Engert F. (2012). Ontogeny of classical and operant learning behaviors in zebrafish. Learning & Memory, 19(4), 170–177. 10.1101/lm.025668.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz R, Hofmeister W, & Lindstrand A. (2019). Zebrafish models of neurodevelopmental disorders: Limitations and benefits of current tools and techniques. International Journal of Molecular Sciences, 20(6), 1296. 10.3390/ijms20061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Linhoff MW, McGinley MJ, Li G-L, Corson GM, Mandel G, & Brehm P. (2010). Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13906–13911. 10.1073/pnas.1008598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (2000). The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). (4th ed.). Eugene: Univ. of Oregon Press. https://zfin.org/zf_info/zfbook/zfbk.html. [Google Scholar]
- Xu J, Mashimo T, & Südhof TC (2007). Synaptotagmin-1, −2, and −9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron, 54(4), 567–581. 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ruuskanen JO, Wullimann MF, & Vernier P. (2010). Two tyrosine hydroxylase genes in vertebrates: New dopaminergic territories revealed in the zebrafish brain. Molecular and Cellular Neuroscience, 43(4), 394–402. 10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, & Vernier P. (2011). The evolution of dopamine systems in chordates. Frontiers in Neuroanatomy, 5, 21. 10.3389/fnana.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JLR, & Westphal H. (2003). The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proceedings of the National Academy of Sciences of the United States of America, 100(15), 9005–9010. 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.