Abstract
Fibroblasts are a highly heterogeneous population in tumor microenvironment. PDGFR-β+ fibroblasts, a subpopulation of activated fibroblasts, have proven to correlate with cancer progression through multiple of mechanisms including inducing angiogenesis and immune evasion. However, the prognostic role of these cells in solid tumors is still not conclusive. Herein, we carried out a meta-analysis including 24 published studies with 6752 patients searched from PubMed, Embase and EBSCO to better comprehend the value of such subpopulation in prognosis prediction for solid tumors. We noted that elevated density of intratumoral PDGFR-β+ fibroblasts was remarkably associated with worse overall survival (OS) and disease-free survival (DFS) of patients. In subgroup analyses, the data showed that PDGFR-β+ fibroblast infiltration considerably decreased OS in non-small cell lung cancer (NSCLC), breast and pancreatic cancer, and reduced DFS in breast cancer. In addition, increased number of PDGFR-β+ fibroblasts appreciably correlated with advanced TNM stage of patients. In conclusion, PDGFR-β+ fibroblast infiltration deteriorates survival in human solid tumors especially in NSCLC, breast and pancreatic cancer. Hence, they may offer a practicable prognostic biomarker and a potential therapeutic strategy for these patients.
Keywords: tumor-infiltrating PDGFR-β+ fibroblasts, worse prognosis, solid tumor, meta-analysis
INTRODUCTION
Tumor microenvironment (TME) is linked closely with the initiation, promotion and progression of cancer. Fibroblasts, the major composition of cancer stroma, are often activated by various stimuli such as some cytokines secreted by tumor cells in the TME [1]. Increasing research has documented that tumor-infiltrating fibroblasts could facilitate cancer progression through a multitude of mechanisms including inducing angiogenesis and immune suppression [2].
PDGFR-β, a major regulatory protein for mesenchymal cells such as fibroblasts and mesangial cells, is becoming a pivotal controller of these cells in TME of numerous malignancies including breast and prostate cancer [3]. Recently, many studies have demonstrated that PDGFR-β was frequently upregulated in fibroblasts in tumor stroma [4], and could well represent the activation status of fibroblasts [5]. In the last decades, although a great number of researchers have investigated the association between intratumoral PDGFR-β+ fibroblasts and prognosis in human solid tumors, their results were controversial [6]. It needs further investigation, in addition, the potential of intratumoral PDGFR-β+ fibroblasts as a practicable prognostic biomarker and targeted strategy is required to be explored.
In this study, we carried out a meta-analysis to quantitatively assess the correlation between tumor-infiltrating PDGFR-β+ fibroblasts and clinical outcomes in solid tumors, and found that high density of intratumoral PDGFR-β+ fibroblasts was remarkably associated with worse overall survival (OS) and disease-free survival (DFS) of patients. In subgroup analyses, PDGFR-β+ fibroblast infiltration considerably decreased OS in non-small cell lung cancer (NSCLC), breast and pancreatic cancer, and reduced DFS in breast cancer of patients. Moreover, increased number of PDGFR-β+ fibroblasts appreciably correlated with advanced TNM stage of patients. Hence, we may offer a practicable prognostic biomarker and a potential therapeutic strategy for these patients.
MATERIALS AND METHODS
Literature search
PubMed, Embase and EBSCO were retrieved to evaluate the PDGFR-β+ fibroblast infiltration and clinical outcomes in solid tumors from January 1980 to November 2020. The keywords for searching strategy were: (fibroblasts [Title/Abstract] OR PDGFR-β [Title/Abstract]) AND (tumor [Title/Abstract] OR cancer [Title/Abstract] OR carcinoma [Title/Abstract] OR neoplasms [Title/Abstract]) AND (survival [Title/Abstract] OR prognosis [Title/Abstract]).
Inclusion and exclusion criteria
Studies included in this meta-analysis should meet the following inclusion criteria: (1) been published as original articles in English; (2) investigated human subjects; (3) tested PDGFR-β+ fibroblasts in primary tumor lesions; (4) supplied hazard ratios (HRs), or Kaplan – Meier curves exhibiting the association between PDGFR-β+ fibroblasts and OS, and/or DFS.
The exclusion criteria were that studies haven’t been published as research article or full text such as case report, commentary, letter and conference abstract. Studies without sufficient data for hazard ratios (HRs) calculation or detecting fibroblasts in metastatic tissues, or not with marker ‘PDGFR-β’ were also excluded.
Endpoints
OS and DFS were considered as the primary and second endpoint respectively in this meta-analysis.
Data extraction
Two authors (GM.H. and KF.Z.) independently extracted data such as number of patients, follow-up time, method applied for quantifying PDGFR-β+ fibroblasts as well as the cut-off value for identifying increased density of such subpopulation. OS, DFS and clinicopathological features including primary tumor, lymph node, distant metastasis (TNM) stage as well as tumor differentiation were obtained from the text, tables and Kaplan – Meier curves.
Quality evaluation
Two authors adopted Newcastle–Ottawa Scale (NOS) [7] to assess the quality of individual research independently, and achieved consensus with the assistant of the third or more authors. Six or above that the study scored was regarded as high quality.
Subgroup analyses
In this study, the subgroup analyses between PDGFR-β+ fibroblasts infiltration and OS or DFS were conducted according to tumor types.
Statistical analysis
Relevant data were combined into hazard ratios (HRs) for OS, DFS, and odds ratios (ORs) for clinicopathological features such as TNM stage, lymph node metastasis, tumor differentiation with STATA 12.0 respectively based on the random-effect model if statistical heterogeneity was considerable [8], otherwise, the fixed-effect model was adopted [9]. We also used sensitivity test, Begg’s funnel plot and Egger’s analysis [10] to investigate the impact of each research on overall result and publication bias respectively. We considered that there was statistical significance when P value was less than 0.05.
RESULTS
Search results and characteristics of studies
Flow chart diagram of study selection was stated in Supplementary Figure 1. We finally included 24 researches with 6752 patients in this meta-analysis [11–34], and then assessed these included cohort researches with Newcastle–Ottawa Scale (NOS). Characteristics of researches being appropriate for data integration were exhibited in Table 1 and Supplementary Table 1.
Table 1. Features of individual included research.
| Research | Year | Type of tumor | Patients’ No. | M / F | Median age (range) (year) | Cut-off value | PDGFR-β+ fibroblast: (H/L) | TNM stage | Median follow-up (months) | Clinical outcome | Quality score (NOS) |
| Park, C.K. et al [11] | 2016 | Breast cancer | 524 | 0/524 | <50: 55.8%; ≥50: 44.2% | ≥ 10% of the stroma /HPF | 153/489 | I - III | NR | OS, DFS | 8 |
| Park, S.Y. et al [12] | 2015 | Breast cancer | 642 | 0/642 | ≤50: 60.3%; >50: 39.7% |
≥ 10% of the stroma /HPF | 153/489 | I - III | 68.3 ± 30.1 | OS, DFS | 8 |
| Kim, H.M. et al [13] | 2016 | Malignant phyllodes tumor of breast | 16 | 0/16 | 47.6 ± 12.9 | ≥ 30% of the stroma /HPF | 5/11 | NR | NR | OS, DFS | 8 |
| Jung, Y.Y. et al [14] | 2015 | Breast cancer | 642 | 0/642 | ≤50: 60.3%; >50: 39.7% |
≥ 10% of the stroma /HPF | 23/619 | I - III | 68.3 ± 30.1 | OS, DFS | 7 |
| Paulsson, J. et al [15] | 2009 | Breast cancer | 289 | 0/289 | 64.2 (27, 96) | ≥ 10% of stromal fibroblasts /HPF | 100/189 | I - III | 106 (0, 207) | OS, DFS | 8 |
| Kilvaer, T.K. et al [16] | 2019 | NSCLC | 513 | 343/170 | <65: 42.5%; ≥65: 57.5% |
Score ≥2 | 202/311 | IA - IIIB | NR | OS | 7 |
| Kanzaki, R. et al [17] | 2018 | NSCLC | 92 | 78/14 | 60.2 | ≥ 5% of the stroma /HPF | 65/27 | IA - IV | 187 (48, 260) | OS, DFS | 8 |
| Donnem, T. et al [18] | 2008 | NSCLC | 335 | 255/80 | 67 (28, 85) | Score ≥ 2.5 | 69/262 | I - IIIA | 96 (10, 179) | OS | 7 |
| Kilvaer, T.K. et al [19] | 2018 | NSCLC | 499 | 161/338 | <65: 42.9%; ≥65: 57.1% |
≥ 10% of the stroma /HPF | 199/300 | IA - IIIA | 48.0 (1, 137) | OS | 7 |
| Chu, J.S. et al [20] | 2013 | Hepatic carcinoma | 93 | 77/16 | ≤50: 33.3%; >50: 66.7% |
≥ 50% of stroma /10HPF | 18/75 | III | (1, 58) | OS | 6 |
| Zhang, X.F. et al [21] | 2017 | Intrahepatic cholangiocarcinoma | 41 | NR | NR | ≥ 20% of the stroma /HPF | 33/8 | I - IV | 15.7 (1.3, 63.2) | OS | 6 |
| Chen, L. et al [22] | 2011 | Hepatic carcinoma | 63 | 59/4 | 48.9 (30, 73) | ≥ 26% of the stroma /HPF | 43/20 | I - IV | 46.7 (40.3, 62.1) | OS, DFS | 7 |
| Sayaka, Y. et al [23] | 2012 | Pancreatic adenocarcinoma | 26 | 18/8 | 61.5 (45, 81) | NR | 13/13 | I - IVB | NR | OS | 6 |
| Kurahara, H. et al [24] | 2016 | Pancreatic cancer | 120 | 71/49 | ≤70: 60.8%; >70: 39.2% |
score > 2 | 59/61 | NR | 29.2 | OS | 6 |
| Hagglof, C. et al [25] | 2010 | Prostate cancer | 244 | 244/0 | 74 (51, 95) | mean density ≥1.0 | 66/178 | NR | (1, 300) | OS | 7 |
| Nordby, Y. et al [26] | 2017 | Prostate cancer | 529 | 529/0 | 62 (47, 75) | mean density ≥1.50 | 262/267 | I - IV | 148.8 (18, 240) | DFS | 6 |
| Frodin, M. et al [27] | 2017 | Renal cell carcinoma | 287 | 162/125 | (37, 89) | NR | 144/143 | I - IV | NR | OS | 8 |
| Shim, M. et al [28] | 2015 | Renal cell carcinoma | 758 | 536/222 | 55 (47, 64) | ≥ 33% of the stroma /HPF | 302/456 | I - II | 29.5 (21.5, 39.6) | DFS | 7 |
| Corvigno, S. et al [29] | 2016 | Ovarian cancer | 154 | 0/154 | 60 (22, 84) | ≥ 10% of the stroma /HPF | 79/75 | I - IV | 28 (0.03, 162.5) | OS | 7 |
| Mezheyeuski, A. et al [30] | 2016 | Colorectal cancer | 372 | 182/190 | (18, 75) | ≥ 50% of the stroma /HPF | NR | IV | 9 (7.8, 10.2) | OS | 7 |
| Yonemori, K. et al [31] | 2011 | Angiosarcoma | 34 | 9/25 | 68 (16, 96) | Score ≥1 | 30/4 | I - III | 26.7 (0.3, 152.6) | OS | 7 |
| Ha, S.Y. et al [32] | 2014 | Esophageal squamous cell carcinoma | 116 | 112/4 | <65: 26.7%; ≥65: 73.3% |
≥ 50% of the stroma /HPF | 63/53 | I - IV | 30 (0, 108) | OS, DFS | 6 |
| Moreno, L. et al [33] | 2013 | Ependymoma | 24 | 15/9 | (1.5, 64.9) | ≥ 50% of the stroma /HPF | 7/17 | IV | 32.3 (2.1, 59.1) | OS | 6 |
| Sun, W.Y. et al [34] | 2015 | Thyroid papillary carcinoma | 339 | NR | NR | ≥ 50% of the stromal cells /HPF | 72/267 | NR | NR | OS, DFS | 6 |
OS: overall survival; DFS: disease-free survival; TNM, Tumor, Lymph Node, Metastasis; NR: not reported; HPF: high power field. M: male; F: female.
Meta-analyses
OS
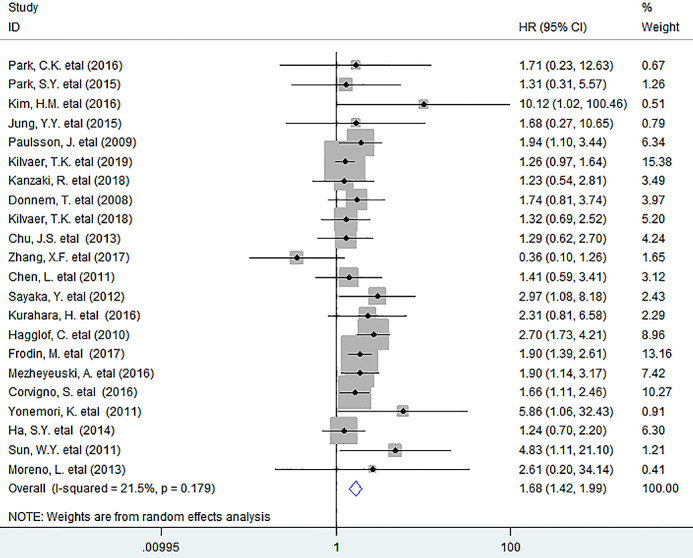
In this study, we noted that increased number of tumor-infiltrating PDGFR-β+ fibroblasts remarkably reduced OS (HR = 1.68, 95% CI 1.42 to 1.99, P < 0.001) in human solid tumors, with little heterogeneity being detected among included researches (I2 = 21.5%, P = 0.179) (Figure 1).
Figure 1.
Forest plots describing HR of the association between PDGFR-β+ fibroblast infiltration and OS in solid tumors. HRs: hazard ratios; OS: overall survival.
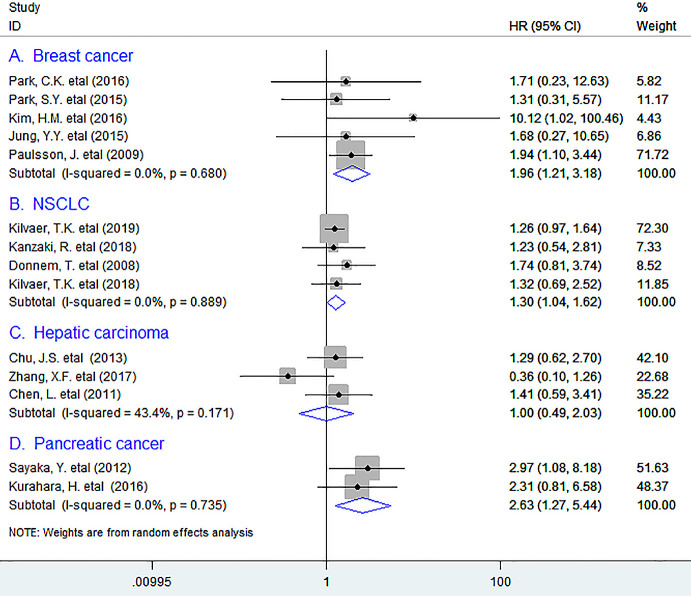
In subgroup analyses based on tumor types, the pooled data indicated that high density of PDGFR-β+ fibroblasts within tumor was markedly associated with reduced OS in breast cancer (BC) (HR = 1.96, 95% CI 1.21 to 3.18, P = 0.006), with no heterogeneity being observed; Similar results were observed between PDGFR-β+ fibroblasts and OS in non-small cell lung cancer (NSCLC) (HR = 1.30, 95% CI 1.04 to 1.62, P = 0.021), and pancreatic cancer (PC) (HR = 2.63, 95% CI 1.27 to 5.44, P = 0.009).(Figure 2) However, we were unable to obtain a combined result for several types of tumor including ovarian cancer, renal cell carcinoma, colorectal cancer (CRC), esophageal squamous cell carcinoma, angiosarcoma and thyroid papillary carcinoma as there was only one study that supplied sufficient data for such type of tumor.
Figure 2.
Subgroup analyses describing HRs of the association between PDGFR-β+ fibroblast infiltration and OS in breast cancer (A), NSCLC (B), Hepatic carcinoma (C), and pancreatic cancer (D). HRs: hazard ratios; OS: overall survival.
DFS
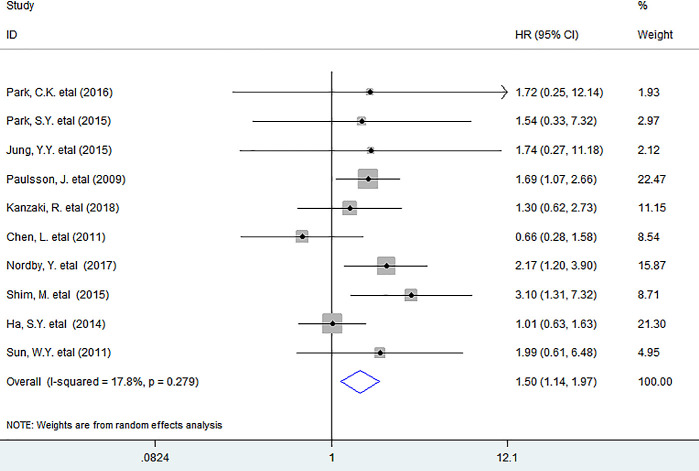
Pooled data showed that the infiltration of PDGFR-β+ fibroblasts appreciably decreased DFS (HR = 1.50, 95% CI 1.14 to 1.97, P = 0.004) of patients (Figure 3).
Figure 3.
Forest plots describing HR of the association between PDGFR-β+ fibroblast infiltration and DFS in solid tumors. HRs: hazard ratios; DFS: disease-free survival.
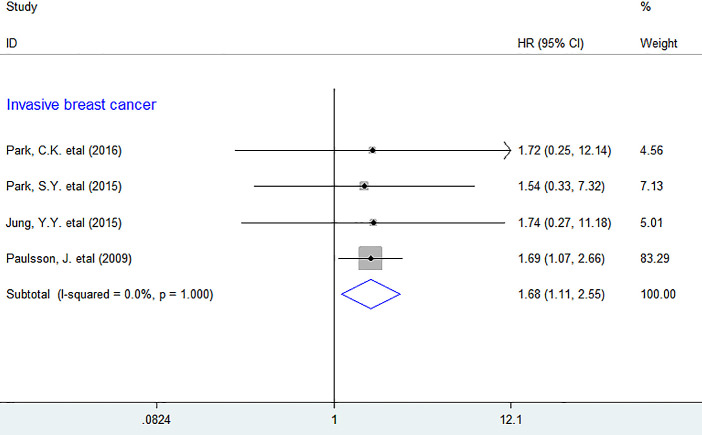
In subgroup analyses, we discovered that increased number of intratumoral PDGFR-β+ fibroblasts was considerably associated with lower DFS in BC (HR = 1.68, 95% CI 1.11 to 2.55, P = 0.014), with little heterogeneity being detected (I2 = 0%, P = 1.000). (Figure 4) However, there was no sufficient data for other types of tumor, so we were unable to obtain the combined result.
Figure 4.
Subgroup analyses describing HRs of the association between PDGFR-β+ fibroblast infiltration and DFS. HRs: hazard ratios; DFS: disease-free survival.
In addition, we found that elevated density of those cells was remarkably associated with advanced TNM stage (OR = 0.47, 95% CI 0.25 to 0.86, P = 0.015), but not with lymph node metastasis or tumor differentiation of patients (Supplementary Figure 2).
Sensitivity analyses
Sensitivity analyses revealed that each individual study didn’t have impact on overall result for OS or DFS (Supplementary Figure 3).
Publication bias
Funnel plot and Egger’s tests indicated that no potential publication bias existed between tumor-infiltrating PDGFR-β+ fibroblasts and OS (P = 0.305) or DFS (P = 0.727) (Supplementary Figure 4).
DISCUSSION
Although multitudinous researchers have correlated tumor-infiltrating PDGFR-β+ fibroblasts and survival in human solid tumors for the past decades, the results were inconsistent even controversial. In this study, we noted that PDGFR-β+ fibroblast infiltration significantly decreased survival in solid tumors especially in BC, NSCLC and PC. In addition, increased number of PDGFR-β+ fibroblasts remarkably correlated with advanced TNM stage. Hence, we harbor the idea that this is the first to exhibit the important prognostic value of tumor-infiltrating PDGFR-β+ fibroblasts in human solid tumors.
We considered that the following evidence can probably explain the negative correlation between intratumoral PDGFR-β+ fibroblasts and prognosis of patients. First, tumor-infiltrating fibroblasts can trigger proliferation, survival and invasion of tumor cells by releasing a variety of growth factors, cytokines, chemokines and degradation of extracellular matrix proteins including matrix metalloproteinases (MMPs) (e.g. MMP9) [35, 36]; Second, they can also produce hydrogen peroxide to induce carcinogenesis, promote epithelial-mesenchymal transition of tumor cells, [37] and induce CD73+γδTreg cell differentiation via IL-6 secretion thereby facilitating immune suppression [38]. More importantly, in vivo experiments have indicated that the activation of PDGFR-β in fibroblasts mediated by its ligand (PDGF-β) can promote the accumulation and expansion of these cells in primary tumor thereby prompting cancer progression [39, 40]. In addition, PDGFR-β+ fibroblasts can stimulate tumor growth through inducing angiogenesis by generating proangiogenic factors such as VEGF [41]. Furthermore, they can dampen antitumor immunity and promote cancer immune evasion via secreting immunosuppressive cytokines including TGF-β1 [41], and recruiting MDSCs through CCL2 released in the TME [42]. Hence, it is rational to conclude that the PDGFR-β+ fibroblasts are prone to foster tumor progression and decrease survival.
Several limitations existed in the meta-analysis. For example, morphometric analyses applied for assessment of PDGFR-β+ fibroblasts in individual included studies were inconsistent. In addition, there was no sufficient data for OS in certain types of tumor, we were therefore unable to obtain pooled results for them.
In conclusion, PDGFR-β+ fibroblast infiltration deteriorates survival in human solid tumors especially in NSCLC, breast and pancreatic cancer. They may therefore provide a practicable prognostic biomarker and a potential therapeutic strategy.
Ethics approval and consent to participate
Ethical approval is not required for this article.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the members of the departments who helped in this study.
Abbreviations
- OS
overall survival
- DFS
disease-free survival
- HRs
hazard ratios
- ORs
odds ratios
- Cl
confidence interval
- TNM
Tumor, Lymph Node, Metastasis
- BC
breast cancer
- NSCLC
non-small cell lung cancer
- PC
pancreatic cancer
- CRC
colorectal cancer
- ECM
extracellular matrix
- TME
tumor microenvironment
- NR
not reported
- HPF
high power field
Footnotes
AUTHOR CONTRIBUTIONS: GM.H. got involved in the design and coordination of the study project, extracted data and drafted the manuscript. LM.H. and KF.Z. engaged in data extraction. LW.M., F.X. and SM.W. took part in data analysis. T.Z. participated in the design of research. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: The authors have declared that no conflicts of interest exist.
FUNDING: This work was funded by the National Natural Science Foundation of China (Grant No. 81702803, GMH) and was also partly supported by Shaoxing Science and Technology Plan Project (2018C30055, LMH; 2018C30075, KFZ).
This corresponding author has a verified history of publications using a personal email address for correspondence
REFERENCES
- 1.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006; 6:392–401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016; 16:582–98. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 3.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008; 22:1276–312. 10.1101/gad.1653708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash J. Cancer-Associated Fibroblasts: perspectives in Cancer Therapy. Trends Cancer. 2016; 2:277–79. 10.1016/j.trecan.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014; 211:1503–23. 10.1084/jem.20140692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsson J, Ehnman M, Östman A. PDGF receptors in tumor biology: prognostic and predictive potential. Future Oncol. 2014; 10:1695–708. 10.2217/fon.14.83 [DOI] [PubMed] [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–05. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 8.Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health. 1988; 9:123–60. 10.1146/annurev.pu.09.050188.001011 [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007; 28:105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CK, Jung WH, Koo JS. Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer Res Treat. 2016; 159:55–69. 10.1007/s10549-016-3929-2 [DOI] [PubMed] [Google Scholar]
- 12.Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015; 149:727–41. 10.1007/s10549-015-3291-9 [DOI] [PubMed] [Google Scholar]
- 13.Kim HM, Lee YK, Koo JS. Expression of CAF-Related Proteins Is Associated with Histologic Grade of Breast Phyllodes Tumor. Dis Markers. 2016; 2016:4218989. 10.1155/2016/4218989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung YY, Lee YK, Koo JS. Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. Tumour Biol. 2015; 36:8685–95. 10.1007/s13277-015-3594-9 [DOI] [PubMed] [Google Scholar]
- 15.Paulsson J, Sjöblom T, Micke P, Pontén F, Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirström K, Ostman A. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am J Pathol. 2009; 175:334–41. 10.2353/ajpath.2009.081030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilvaer TK, Rakaee M, Hellevik T, Vik J, Petris L, Donnem T, Strell C, Ostman A, Busund LR, Martinez-Zubiaurre I. Differential prognostic impact of platelet-derived growth factor receptor expression in NSCLC. Sci Rep. 2019; 9:10163. 10.1038/s41598-019-46510-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanzaki R, Ose N, Kawamura T, Funaki S, Shintani Y, Minami M, Takakura N, Okumura M. Stromal PDGFR-β Expression is Associated with Postoperative Survival of Non-Small Cell Lung Cancer Patients Receiving Preoperative Chemo- or Chemoradiotherapy Followed by Surgery. World J Surg. 2018; 42:2879–86. 10.1007/s00268-018-4560-7 [DOI] [PubMed] [Google Scholar]
- 18.Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008; 3:963–70. 10.1097/JTO.0b013e3181834f52 [DOI] [PubMed] [Google Scholar]
- 19.Kilvaer TK, Rakaee M, Hellevik T, Østman A, Strell C, Bremnes RM, Busund LT, Dønnem T, Martinez-Zubiaurre I. Tissue analyses reveal a potential immune-adjuvant function of FAP-1 positive fibroblasts in non-small cell lung cancer. PLoS One. 2018; 13:e0192157. 10.1371/journal.pone.0192157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu JS, Ge FJ, Zhang B, Wang Y, Silvestris N, Liu LJ, Zhao CH, Lin L, Brunetti AE, Fu YL, Wang J, Paradiso A, Xu JM. Expression and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2013; 32:16. 10.1186/1756-9966-32-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XF, Dong M, Pan YH, Chen JN, Huang XQ, Jin Y, Shao CK. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum Pathol. 2017; 65:92–100. 10.1016/j.humpath.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Shi Y, Jiang CY, Wei LX, Lv YL, Wang YL, Dai GH. Coexpression of PDGFR-alpha, PDGFR-beta and VEGF as a prognostic factor in patients with hepatocellular carcinoma. Int J Biol Markers. 2011; 26:108–16. 10.5301/JBM.2011.8322 [DOI] [PubMed] [Google Scholar]
- 23.Yuzawa S, Kano MR, Einama T, Nishihara H. PDGFRβ expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol. 2012; 29:2824–30. 10.1007/s12032-012-0193-0 [DOI] [PubMed] [Google Scholar]
- 24.Kurahara H, Maemura K, Mataki Y, Sakoda M, Shinchi H, Natsugoe S. Impact of p53 and PDGFR-β Expression on Metastasis and Prognosis of Patients with Pancreatic Cancer. World J Surg. 2016; 40:1977–84. 10.1007/s00268-016-3477-2 [DOI] [PubMed] [Google Scholar]
- 25.Hägglöf C, Hammarsten P, Josefsson A, Stattin P, Paulsson J, Bergh A, Ostman A. Stromal PDGFRbeta expression in prostate tumors and non-malignant prostate tissue predicts prostate cancer survival. PLoS One. 2010; 5:e10747. 10.1371/journal.pone.0010747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordby Y, Richardsen E, Rakaee M, Ness N, Donnem T, Patel HR, Busund LT, Bremnes RM, Andersen S. High expression of PDGFR-β in prostate cancer stroma is independently associated with clinical and biochemical prostate cancer recurrence. Sci Rep. 2017; 7:43378. 10.1038/srep43378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frödin M, Mezheyeuski A, Corvigno S, Harmenberg U, Sandström P, Egevad L, Johansson M, Östman A. Perivascular PDGFR-β is an independent marker for prognosis in renal cell carcinoma. Br J Cancer. 2017; 116:195–201. 10.1038/bjc.2016.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shim M, Song C, Park S, Choi SK, Cho YM, Kim CS, Ahn H. Prognostic significance of platelet-derived growth factor receptor-β expression in localized clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2015; 141:2213–20. 10.1007/s00432-015-2019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corvigno S, Wisman GB, Mezheyeuski A, van der Zee AG, Nijman HW, Åvall-Lundqvist E, Östman A, Dahlstrand H. Markers of fibroblast-rich tumor stroma and perivascular cells in serous ovarian cancer: inter- and intra-patient heterogeneity and impact on survival. Oncotarget. 2016; 7:18573–84. 10.18632/oncotarget.7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezheyeuski A, Bradic Lindh M, Guren TK, Dragomir A, Pfeiffer P, Kure EH, Ikdahl T, Skovlund E, Corvigno S, Strell C, Pietras K, Ponten F, Mulder J, et al. Survival-associated heterogeneity of marker-defined perivascular cells in colorectal cancer. Oncotarget. 2016; 7:41948–58. 10.18632/oncotarget.9632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonemori K, Tsuta K, Ando M, Hirakawa A, Hatanaka Y, Matsuno Y, Chuman H, Yamazaki N, Fujiwara Y, Hasegawa T. Contrasting prognostic implications of platelet-derived growth factor receptor-β and vascular endothelial growth factor receptor-2 in patients with angiosarcoma. Ann Surg Oncol. 2011; 18:2841–50. 10.1245/s10434-011-1640-4 [DOI] [PubMed] [Google Scholar]
- 32.Ha SY, Yeo SY, Xuan YH, Kim SH. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma. PLoS One. 2014; 9:e99955. 10.1371/journal.pone.0099955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno L, Popov S, Jury A, Al Sarraj S, Jones C, Zacharoulis S. Role of platelet derived growth factor receptor (PDGFR) over-expression and angiogenesis in ependymoma. J Neurooncol. 2013; 111:169–76. 10.1007/s11060-012-0996-z [DOI] [PubMed] [Google Scholar]
- 34.Sun WY, Jung WH, Koo JS. Expression of cancer-associated fibroblast-related proteins in thyroid papillary carcinoma. Tumour Biol. 2016; 37:8197–207. 10.1007/s13277-015-4684-4 [DOI] [PubMed] [Google Scholar]
- 35.Bruzzese F, Hägglöf C, Leone A, Sjöberg E, Roca MS, Kiflemariam S, Sjöblom T, Hammarsten P, Egevad L, Bergh A, Ostman A, Budillon A, Augsten M. Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15. Cancer Res. 2014; 74:3408–17. 10.1158/0008-5472.CAN-13-2259 [DOI] [PubMed] [Google Scholar]
- 36.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005; 120:303–13. 10.1016/j.cell.2004.12.018 [DOI] [PubMed] [Google Scholar]
- 37.Chan JS, Tan MJ, Sng MK, Teo Z, Phua T, Choo CC, Li L, Zhu P, Tan NS. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017; 8:e2562. 10.1038/cddis.2016.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu G, Cheng P, Pan J, Wang S, Ding Q, Jiang Z, Cheng L, Shao X, Huang L, Huang J. An IL6-Adenosine Positive Feedback Loop between CD73+ γδTregs and CAFs Promotes Tumor Progression in Human Breast Cancer. Cancer Immunol Res. 2020; 8:1273–86. 10.1158/2326-6066.CIR-19-0923 [DOI] [PubMed] [Google Scholar]
- 39.Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009; 69:369–78. 10.1158/0008-5472.CAN-08-2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008; 5:e19. 10.1371/journal.pmed.0050019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014; 159:55–72. 10.1016/j.imlet.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 42.Powell DW. Myofibroblasts: paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000; 111:271–92. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.