Abstract
Hepatitis B virus (HBV) infection is a worldwide health problem with high morbidity and mortality rates. The therapeutic vaccine is a promising method of treatment, and HBV polymerase plays a vital role in viral replication. Therefore, a therapeutic vaccine that binds to HBV DNA polymerase may control HBV infection. We predicted and selected epitopes of polymerase using online databases and analysis software. We then performed molecular docking and peptide binding assays to evaluate the binding energies and affinities between polymerase epitopes and the HLA-A0201 molecule. Finally, we induced T cells from the peripheral blood mononuclear cells (PBMCs) of healthy donors using each epitope and quantified the functions of epitope-specific T cells by IFN-γELISPOT assay, T2 cell cytotoxicity assay, HepG2.2.15 cell cytotoxicity assay and HBV gene expression assays. Four epitopes (RVTGGVFLV, GLLGFAAPF, LLDDEAGPL and YMDDVVLGA) had low binding energy and two epitopes (RVTGGVFLV and GLLGFAAPF) had a high binding affinity. The T cells stimulated by two epitopes (GLLGFAAPF and HLYSHPIIL) had a greater ability to induce immune response and suppress HBV. The HBV DNA polymerase epitopes identified in this study are promising targets for designing an epitope-based therapeutic vaccine against HBV.
Keywords: bioinformatics, epitope, hepatitis B virus, polymerase, vaccine
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a worldwide health problem affecting more than 240 million individuals. Most individuals who are infected with HBV are at high risk for developing liver cirrhosis and hepatocellular carcinoma [1]. Approved therapies for chronic hepatitis B (CHB) include two classes of antiviral drugs: nucleos(t)ide analogs and interferon alpha (IFN-α) 2a [2]. Although these two classes of drugs inhibit viral replication, they do not eliminate the virus or its covalently closed circular DNA (cccDNA), the latter of which resides in the nucleus of infected cells [3]. In recent years, scientists have attempted to eliminate HBV and cccDNA using direct-acting antivirals (DAAs) and host-targeting agents (HTAs) [4, 5]. In contrast, therapeutic vaccination work by activating adaptive immunity and is a promising method for both disease prevention and therapy. Such vaccines have effectively induced tumor-specific T cells in patients with pre-cancerous conditions [6, 7].
HBV DNA polymerase (hereafter, “HBV polymerase” or “polymerase”) plays a vital role in viral replication [8]. Maintaining a high level of HBV cccDNA relies on viral replication, so the level of cccDNA in cells will decrease if the polymerase has been inhibited [9]. HBV polymerase interferes with HBV-mediated antagonization of IFN-α, which may explain why IFN-α therapy is not an effective treatment for CHB [10]. Currently the most effective CHB treatment, nucleos(t)ide analogs, suppresses viral replication by inhibiting HBV polymerase, demonstrating that targeting the enzyme is a potentially effective method of CHB treatment.
Chronic HBV infection is characterized by immune tolerance, especially the exhaustion of HBV-specific T cells [11]. Many strategies have been designed to overcome the immune tolerance of HBV [12–15]. A therapeutic vaccine should be able to compensate for immune tolerance. Usually, we can’t detect the HBV polymerase antigen and antibody in the blood, which implies that HBV polymerase rarely comes into contact with the immune system and induces immune tolerance. Therefore, therapeutic vaccines designed based on polymerase peptide epitopes may have the ability to efficiently overcome immune tolerance. Because CD8+ T cells can control HBV replication through cytotoxic and non-cytotoxic methods [16], polymerase-specific CD8+ T cells may be able to control viral replication without hepatocyte lysis.
In this study, we designed a therapeutic vaccine that targets HBV polymerase with the hope that it would induce an adaptive immune response that could control HBV infection. The human leukocyte antigen (HLA), also named "major histocompatibility complex (MHC), which can present antigenic peptide on the surface of cells. Then, the MHC-peptide compound stimulates T cells through T-cell receptors. Finally, the cellular immune response is activated. Because HLA-A0201 is the most common prevalent MHC-I allele in human [17], we choose HLA-A0201 to predict and identify HBV polymerase epitopes. We used molecular docking databases and analysis software to select polymerase epitopes and calculate the binding energies between the epitopes and the HLA-A0201 molecule. We then performed a peptide binding assay to validate the binding affinities between the polymerase epitopes and HLA-A0201. Next, T cells from human peripheral blood mononuclear cells (PBMCs) were induced by the selected epitopes. The immunogenicity of these epitope-specific T cells was measured by an IFN-γ ELISPOT assay and an in vitro T2 cells cytotoxicity assay, and the antiviral abilities of these epitope-specific T cells were determined by an HepG2.2.15 cells cytotoxicity assay and HBV gene expression assay.
RESULTS
Epitope prediction and selection
Both the IEDB database and the NetCTLpan 1.1server recommended the same 24 HBV polymerase epitopes (data not shown). Among these 24, the immunogenicity scores of 12 epitopes were positive, and all of the epitopes were nontoxic. Eight epitopes were 100% conserved in the genotype C protein sequence. The results are depicted in Table 1.
Table 1. Immunogenicity scores, toxicity and conservation of HBV DNA polymerase epitopes.
| Number | Sequence | Immunogenicity score | aConservation | Toxicity |
| 1 | CLFHIVNLI | 0.22707 | 77.78% | Non-Toxin |
| 2 | YAAVTHFLL | 0.21481 | 44.44% | Non-Toxin |
| 3 | RVTGGVFLV | 0.21088 | 100% | Non-Toxin |
| 4 | GLLGFAAPF | 0.20787 | 100% | Non-Toxin |
| 5 | HLPDRVHFA | 0.20368 | 100% | Non-Toxin |
| 6 | LLDDEAGPL | 0.18597 | 100% | Non-Toxin |
| 7 | YVIGSWGTL | 0.17599 | 100% | Non-Toxin |
| 8 | YMDDVVLGA | 0.11902 | 100% | Non-Toxin |
| 9 | GLSRYVARL | 0.0969 | 88.89% | Non-Toxin |
| 10 | GLYRPLLRL | 0.05052 | 88.89% | Non-Toxin |
| 11 | WILRGTSFV | 0.0468 | 100% | Non-Toxin |
| 12 | HLYSHPIIL | 0.04347 | 100% | Non-Toxin |
aAmino acid conservation was calculated based on the percentage of epitope sequence that matched the MHC-I genotype C protein sequence.
Molecular docking and peptide binding assay
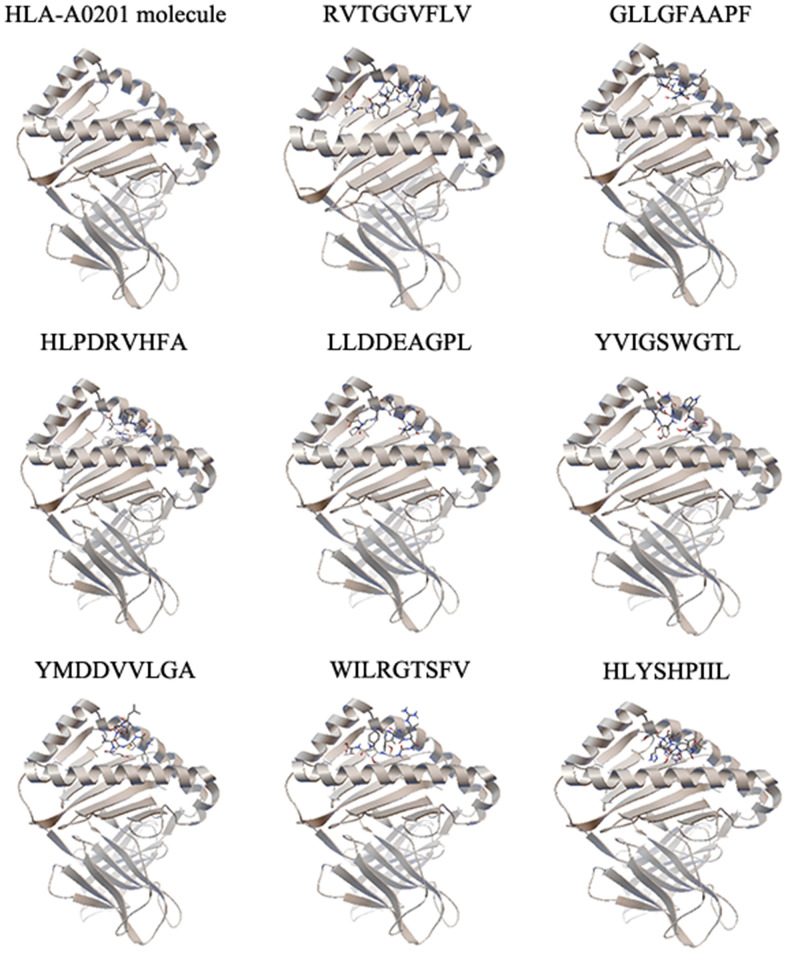
To identify the selected epitopes, we performed molecular docking and a peptide binding assay. The binding energies between epitopes and the HLA-A0201 molecule were calculated by molecular docking (Table 2 and Figure 1). We identified the four epitopes (RVTGGVFLV, GLLGFAAPF, LLDDEAGPL and YMDDVVLGA) with the lowest binding energies and selected them from further study. The binding affinities between the selected epitopes and the HLA-A0201 molecule were validated by a peptide binding assay. As shown in Table 3, two epitopes (RVTGGVFLV and GLLGFAAPF) had the highest binding affinities to the HLA-A0201 molecule.
Table 2. HBV DNA polymerase epitope binding energies.
| Number | Sequence | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| 1 | RVTGGVFLV | 8.33a | 13.94 | 10.67 | 16.01 | 8.91 |
| 2 | GLLGFAAPF | 1.58 | 1.86 | 0.64 | 8.7 | 7.45 |
| 3 | HLPDRVHFA | 22.79 | 48.45 | 30.97 | 131.21 | 54.74 |
| 4 | LLDDEAGPL | 13.16 | 6.65 | 4.88 | 5.01 | 20.39 |
| 5 | YVIGSWGTL | 25.66 | 20.01 | 17.64 | 21.9 | 31.56 |
| 6 | YMDDVVLGA | 9.8 | 25.87 | 25.09 | 14.86 | 11.3 |
| 7 | WILRGTSFV | 53.88 | 96.64 | 50.06 | 26.57 | 24.09 |
| 8 | HLYSHPIIL | 59.14 | 162.05 | 56.68 | 69.09 | 36.03 |
aBinding energies between HBV DNA polymerase epitopes and the HLA-A0201 molecule.
Figure 1.
The molecular docking results of each epitope. Each epitope binds to the groove of the HLA-A0201 molecule between the α1 and α2 domains, which is shown by secondary structure. One representative of five independent experiments is shown.
Table 3. HBV DNA polymerase epitope fluorescence indices.
| Number | sequence | FIsa |
| 1 | RVTGGVFLV | 1.0647 |
| 2 | GLLGFAAPF | 1.3822 |
| 3 | HLPDRVHFA | 0.2920 |
| 4 | LLDDEAGPL | 0.5106 |
| 5 | YVIGSWGTL | 0.8953 |
| 6 | YMDDVVLGA | 0.4242 |
| 7 | WILRGTSFV | 0.4790 |
| 8 | HLYSHPIIL | 0.6279 |
aFluorescence index (FI) was calculated as described in the Methods section. One representative of 3 independent experiments is shown.
In vitro human IFN-γ ELISPOT assay
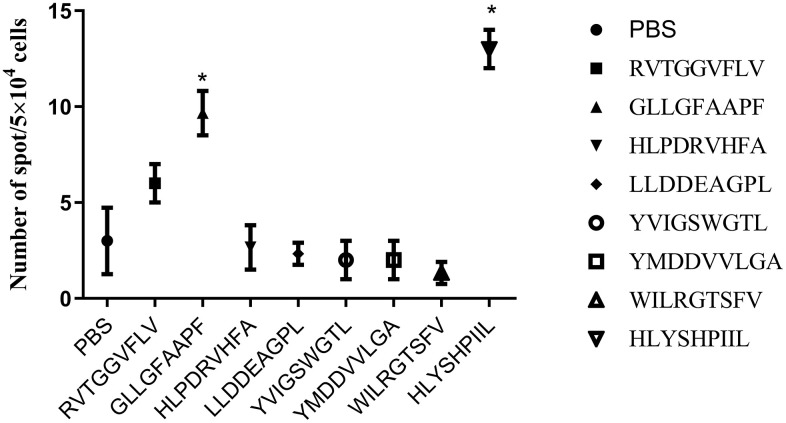
Because cytotoxic T lymphocytes (CTLs) are known to produce the Th1 cytokine IFN-γ, we quantified epitope-specific T cell responses by measuring IFN-γ secretion with an ELISPOT assay (Figure 2). PBMCs induced by phosphate buffer saline (PBS) were used as a negative control. We concluded that two epitopes (GLLGFAAPF and HLYSHPIIL) generated strong epitope-specific T-cell responses by inducing more IFN-γ secretion than was measured in control (P<0.05).
Figure 2.
ELISPOT assay results of each epitope-specific T cell. We measured the IFN-γ released by T cells obtained from the peripheral blood mononuclear cells (PBMCs) of healthy donors. The number of spots is presented as mean±SD. The mean ± SD from three independent experiments is shown. *Statistically significant compared to the control group.
In vitro cytotoxicity assay against T2 cells
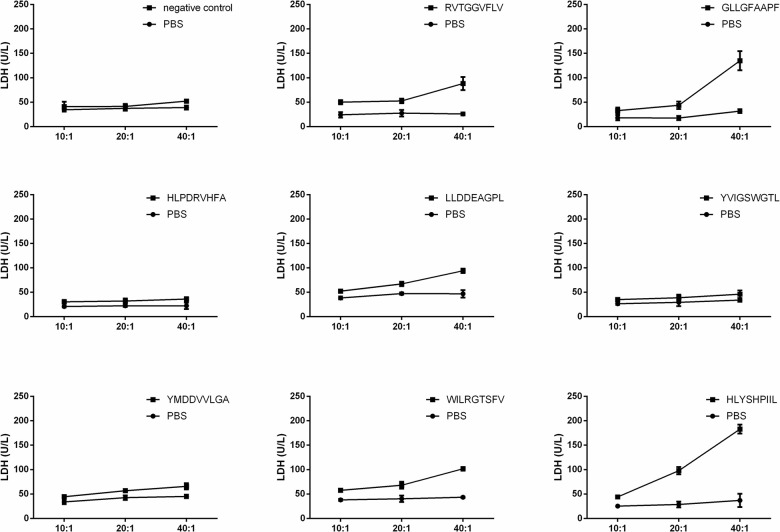
Epitope-specific T cells could lyse antigen-infected T2 cells. We validated the function of epitope-specific T cells by quantifying their cytotoxic activity using a lactate dehydrogenase (LDH) release assay. As shown in Figure 3, the T cells stimulated by the epitopes GLLGFAAPF and HLYSHPIIL exhibited higher cytotoxic activity toward T2 cells compared to T cells stimulated by other epitopes.
Figure 3.
T2 cell cytotoxicity assay of each epitope-specific T cell type. T2 cells were pulsed with peptide and lysed by epitope-specific T cells obtained from peripheral blood mononuclear cells (PBMCs). The mean ± SD from 3 independent experiments is shown.
In vitro cytotoxicity assay against HepG2.2.15 cells
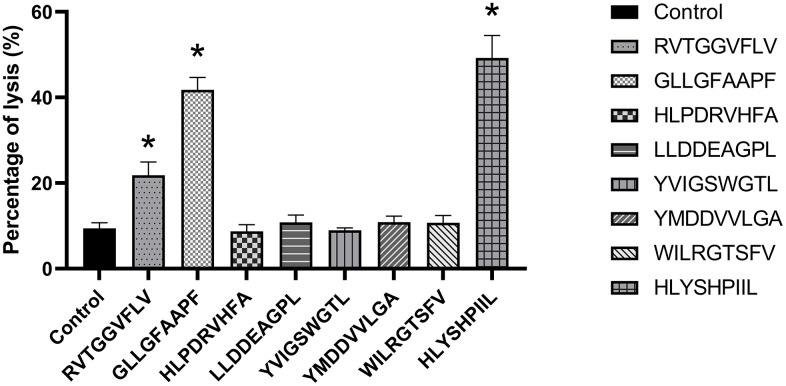
In order to determine the antiviral abilities of epitopes, the function of epitope-specific T cells was detected by HepG2.2.15 cell cytotoxicity assay. PBMCs induced by PBS were used as a negative control. As shown in Figure 4, when the ratio of epitope-specific T-cells versus HepG2.2.15 cells was 20:1, the T cells stimulated by the epitopes RVTGGVFLV, GLLGFAAPF and HLYSHPIIL exhibited higher cytotoxic activities against HepG2.2.15 cells compared to control group (P<0.05).
Figure 4.
HepG2.2.15 cell cytotoxicity assay of each epitope-specific T cell type. HepG2.2.15 cells were lysed by epitope-specific T cells obtained from peripheral blood mononuclear cells (PBMCs). The mean ± SD from 3 independent experiments is shown. *Statistically significant compared to the control group.
HBV gene expression assays
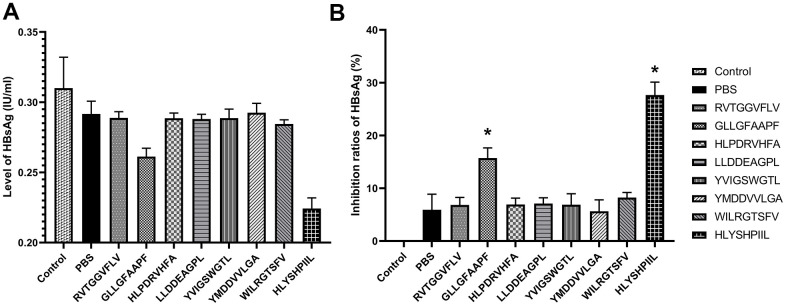
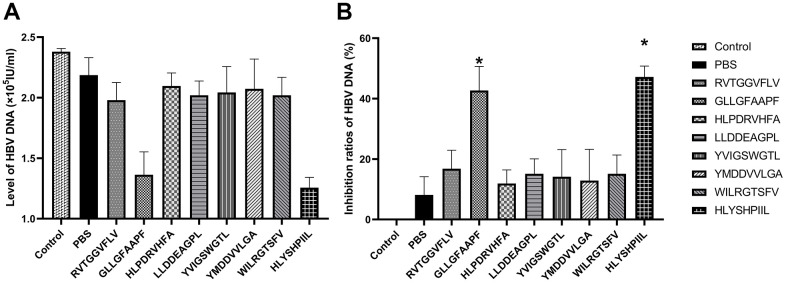
We also determined the antiviral abilities of epitopes using HBV gene expression assays. The antiviral abilities of epitope-specific T cells were validated by calculating the inhibition ratios of HBsAg and HBV-DNA. The control group was HepG2.2.15 cells cultured without T cells, and the PBS group was HepG2.2.15 cells co-cultured with T cells stimulated by PBS. The HBsAg level of each group was shown in Figure 5A, and as shown in Figure 5B, the T cells stimulated by the epitopes GLLGFAAPF and HLYSHPIIL exhibited higher activities to inhibit the expression of HBsAg compared to the PBS group (P<0.05). The HBV-DNA level of each group was shown in Figure 6A, and as shown in Figure 6B, the T cells stimulated by the epitopes GLLGFAAPF and HLYSHPIIL had higher abilities to eliminate HBV-DNA compared to the PBS group (P<0.05), which was consistent to HBsAg inhibition assay.
Figure 5.
HBV gene expression assay. HepG2.2.15 cells were co-cultured with epitope-specific T cells. The HBsAg level of each group was shown in (A), and the inhibition ratio of HBsAg was shown in (B). The control group was HepG2.2.15 cells cultured without T cells, and the PBS group was HepG2.2.15 cells co-cultured with T cells stimulated by PBS. The mean ± SD from 3 independent experiments is shown. *Statistically significant compared to the PBS group.
Figure 6.
HBV gene expression assay. HepG2.2.15 cells were co-cultured with epitope-specific T cells. The HBV-DNA level of each group was shown in (A), and the inhibition ratio of HBV-DNA was shown in (B). The control group was HepG2.2.15 cells cultured without T cells, and the PBS group was HepG2.2.15 cells co-cultured with T cells stimulated by PBS. The mean ± SD from 3 independent experiments is shown. *Statistically significant compared to the PBS group.
DISCUSSION
In this study, we predicted and identified epitopes of the HBV polymerase protein with the assistance of online databases and analysis software. Eight epitopes conformed to all criteria and were selected for molecular docking and in vitro validation. Molecular docking analysis revealed four low binding-energy epitopes (RVTGGVFLV, GLLGFAAPF, LLDDEAGPL and YMDDVVLGA), and the peptide binding assay showed that two epitopes (RVTGGVFLV and GLLGFAAPF) have a high binding affinity for HLA-A0201. An in vitro human IFN-γ ELISPOT assay and a T2 cells cytotoxicity assay revealed that two epitopes (GLLGFAAPF and HLYSHPIIL) have a greater ability to induce and stimulate epitope-specific T cells than do the remaining epitopes. A HepG2.2.15 cells cytotoxicity assay showed that three epitopes (RVTGGVFLV, GLLGFAAPF and HLYSHPIIL) had a higher ability to stimulate epitope-specific T cells. Furthermore, in HBV gene expression assays, the results revealed that T cells induced by two epitopes (GLLGFAAPF and HLYSHPIIL) had greater ability to inhibit the expression of HBsAg and HBV-DNA. These epitopes will be further validated by our subsequent studies and might become the basis of polymerase-specific therapeutic vaccines. Although the RVTGGVFLV epitope has a high binding affinity for HLA-A0201 and a high immunogenicity in HepG2.2.15 cells cytotoxicity assay, it had a lesser ability to induce epitope-specific T cells in the IFN-γ ELISPOT, T2 cells cytotoxicity assays and HBV gene expression assays. That the RVTGGVFLV epitope has a high binding affinity to HLA-A0201 implies that it will be presented to the cell surface by the MHC molecule. But a necessary step to induce epitope-specific T cells is that the MHC-epitope compound binds to the T cell receptor and activates it. Although the RVTGGVFLV epitope has a high ability to induce epitope-specific T cells in HepG2.2.15 cells cytotoxicity assay, the ability of RVTGGVFLV epitope is obvious weaker than the other two epitopes (GLLGFAAPF and HLYSHPIIL).
Previously, we selected HBV polymerase epitopes with the help of online databases and analysis tools [18]. We validated these epitopes using molecular docking and a peptide binding assay. The purpose of that study was to select the HBV polymerase epitopes that affect the most HBV-infected individuals in regions of high HBV prevalence. In this study, we selected HBV polymerase epitopes that commonly affect Chinese people and validated these epitopes through in vitro experiments.
Chronic HBV infection is a worldwide disease that has high morbidity and mortality rates [3]. Many novel therapeutic methods have been explored in recent years [5], including DAAs and HTAs. Most DAAs are drugs that target and inhibit the steps of viral replication. A drawback to this strategy is that selection and mutation enable viruses to develop drug resistance. Furthermore, drug side effects are inevitable. HTAs, such as innate immune ligands, cellular inhibitors of apoptosis proteins, check-point inhibitors and therapeutic vaccines control viruses indirectly through the immune system [19]. HBV therapeutic vaccines are a promising strategy to inhibit viral replication and eliminate cccDNAs, but the impairment of the immune response and the exhaustion of T cells have limited their utilities [20]. Epitope-based vaccines are designed to induce a precise immune response against antigens and are more efficient than traditional vaccines. Epitopes predicted by bioinformatics tools should be confirmed through in vitro and in vivo experiments [21]. To surmount these barriers, our present study selected and validated HBV polymerase epitopes that may have the potential to be used in epitope-based vaccines.
Many HBV protective vaccines have been constructed based on the preS, surface and core proteins, but these vaccines have no therapeutic effect on individuals with chronic HBV infection. An HBV polymerase-based vaccine may have the ability to overcome these barriers. In CHB patients, preS, surface and core proteins abound in blood and hepatocytes. They are in constant contact with the immune system, which leads to immune tolerance of these proteins. Thus, vaccines constructed using these proteins are ineffective at eliciting an immune response. In contrast, HBV polymerase mainly hides inside hepatocytes and within HBV, and is rarely in contact with the immune system. Therefore, T cells do not build up a tolerance to the polymerase protein, so vaccines containing polymerase epitopes might not elicit immune tolerance. In addition, preS, surface and core proteins are not required for viral replication, and their elimination by the immune system does not control HBV infection. However, polymerase plays a vital role in HBV replication.
The primary CHB treatment, nucleos(t)ide analogs, targets HBV polymerase and inhibits viral replication, which suggests that HBV infection can be controlled if there is a method that can inhibit polymerase. An HBV polymerase-based vaccine may have the ability to control HBV infection by inducing the immune system to target the HBV polymerase protein. A novel vaccine comprising particulate surface and core antigens, with the saponin-based ISCOMATRIX adjuvant, induces both antigen-specific CD8+T cells and antibodies [22]. An antigen-antibody (HBsAg-HBIG) complex therapeutic vaccine candidate with alum as adjuvant (YIC) has been investigated in phase III clinical trial, but the results indicated that overstimulation with YIC decreased the efficacy of the vaccine [23]. In HBV-tolerant mice, a novel particulate vaccine could effectively suppress HBsAg by inducing a humoral immune response. [24]. Moreover, a recent study reported that a yeast-based vaccine candidate expressing X, surface and core antigens could elicit a functional adaptive immune response [25]. Besides, a heat-inactivated, yeast-based T-cell vaccine, GS-4774, could significantly stimulate effective immune response. [26]. Another study tested an HBV DNA vaccine, HB-110, and found that the vaccine exhibited a weaker HBV-specific T-cell induction and HBeAg seroconversion in Korean patients than did the HB-100 vaccine in Caucasian patients [27].
A therapeutic vaccine specifically targeting the HBV DNA polymerase protein has never been reported except in our previous study [18], in which we selected polymerase epitopes in silico. TG1050, a non-replicating adenovirus encoding a large fusion protein composed of partial HBV core, polymerase and envelope proteins, has been validated in vivo. The results indicated that TG1050 induced both splenic and intrahepatic functional T cells in mice [28]. Because HBV surface and core proteins are not critical in HBV replication, the effect of TG1050 may be attributed to polymerase-specific T cells induced by the polymerase component of this vaccine.
The in vivo and in vitro experiments were necessary to characterize the epitopes predicted in silico [29, 30]. The epitopes in this study were predicted and identified by molecular docking validated with a peptide binding assay and characterized using IFN-γ ELISPOT, T2 cells cytotoxicity, HepG2.2.15 cells cytotoxicity and HBV gene expression assays. Our data indicated that two of these epitopes have the ability to induce and stimulate T cells. In our forthcoming study, epitopes identified in this study will become the basis of novel DNA vaccines [31]. The effectiveness of these DNA vaccines in eliminating the virus will be determined in HBV-transgenic mice.
Our present study has predicted and selected epitopes of the HBV polymerase protein and these epitopes have been validated by in vitro experiments. This is a novel and promising strategy for designing therapeutic vaccines. It is possible that a vaccine designed based on the epitopes identified in this study will specially target HBV polymerase and control HBV infection, achieving the same effect of nucleos(t)ide analogues but without drug resistance and the life-long administration of medicine.
MATERIALS AND METHODS
Epitope prediction
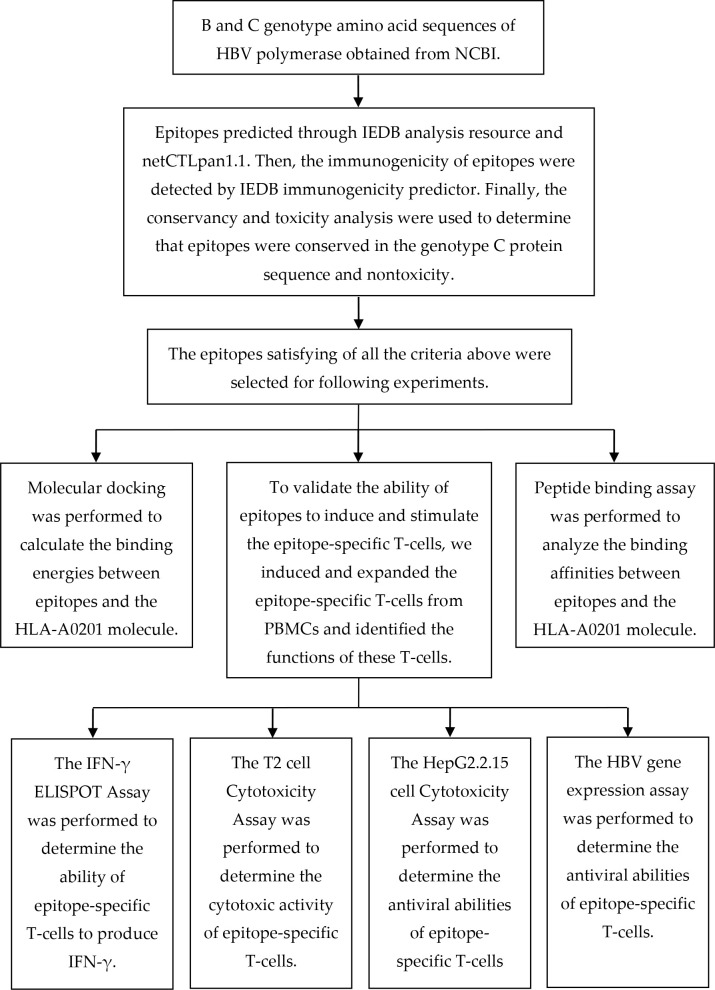
The epitope prediction was performed based on our previous study [18]. The B genotype protein sequence of HBV polymerase was obtained from the National Center for Biotechnology Information (NCBI) (GenBank accession no. BAS53332.1). To predict epitopes that could be presented on the surface of cells, we utilized the MHC-I epitope prediction tools. We selected a nonapeptide within the HLA-A0201 MHC-I allele because most MHC-I epitopes are nonapeptides, and HLA-A0201 is the most prevalent MHC-I allele in human. Other parameters were set to default. The MHC-I epitopes were predicted on 8/7/2016 using the Immune Epitope Database (IEDB) analysis resource consensus tool [32], which combines predictions from NetMHC(3.4) [33, 34], SMM [35] and Comblib [36]. We also used the NetCTLpan 1.1 server to predict peptide MHC-I binding, proteasomal C-terminal cleavage and TAP transport efficiency [37]. We picked the first 30 epitopes (about 3.6%) recommended by each algorithm, and the epitopes repeatedly appeared in first 30 epitopes (predicted by each algorithm) were selected for further analysis. We then used the IEDB immunogenicity predictor [38] to select the epitopes that had a positive immunogenicity value. Finally, we utilized the conservation and toxicity [39] analysis to determine which epitopes were nontoxic and 100% conserved in the genotype C protein sequence (GenBank accession no. BAQ95565.1). A flow chart depicting the process of predicting and validating T-cell epitopes is shown in Figure 7.
Figure 7.
A flow chart illustrating the process of predicting and validating T-cell epitopes.
Molecular docking
To calculate the binding energies between HBV polymerase epitopes and the HLA-A0201 molecule, we performed molecular docking on the basis of our previous study [18]. Briefly, we downloaded the molecular structure of HLA-A0201 from the RCSB Protein Data Bank and predicted the structures of epitopes using PEP-FOLD2.0 [40, 41]. This yielded five models of structures. The docking analysis was performed by AutoDock4.2 [42]. The number of points in the x-dimension was 60, in the y-dimension was 40 and in the z-dimension was 54. The center grid box was set at x = 0.196, y = 2.472 and z = 20.594. The other parameters were set to default. We calculated the binding energy score and visualized the docking consequence for interaction force analysis. The lower binding energy indicated that epitope was easier bound to HLA-A0201 molecule and harder to be separated.
Peptide synthesis and cell lines
Epitopes selected by in situ methods should be validated by in vitro experiments. The epitopes met all the criteria above were synthesized and purified to greater than 99% purity. High-performance liquid chromatography (HPLC) and mass spectrometry were used to analyze the purity and molecular weight of peptides (Shanghai Biotech BioScience and Technology Co, Ltd; Shanghai, China). The TAP-deficient T2 cell line was purchased from the American Type Culture Collection (Manassas, VA, USA).
Binding affinity of candidate peptides to HLA-A0201
To analyze the binding affinity between epitopes and the HLA-A0201 molecule, we performed a peptide binding assay according to our previous study [18]. Briefly, T2 cells (1×106/well) were incubated with peptides (100 μM) in 24-well plates for 18 hours at 37° C. T2 cells without peptides served as a background control. After being washed with phosphate buffer saline (PBS) three times, cells were stained with FITC-conjugated anti-HLA-A2 mAb BB7.2 and analyzed using a BD AccuriC6 flow cytometer (Becton Dickinson, Mountain View, CA, USA). The mean fluorescence index (MFI) was recorded [43]. We calculated the fluorescence index (FI) as follows: FI = (MFI with individual peptides - background MFI)/(background MFI). Peptides with an FI of greater than 1 were deemed high-affinity epitopes.
In vitro expansion of epitope-specific T cells from PBMCs of healthy donors
To validate the ability of epitopes to induce and stimulate the epitope-specific T cells, we used PBMCs from the blood of healthy donors. The blood was collected after we obtained written informed consent in accordance with procedures approved by the Human Research Ethics Board of the First Affiliated Hospital of Wenzhou Medical University(2017 No. 46) and in accordance with the ethical standards of the Helsinki Declaration. PBMCs were obtained through centrifugation at a Ficoll-Paque density gradient and suspended in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μM streptomycin. PBMCs were stained by FITC-conjugated anti-HLA-A2 mAb and analyzed by flow cytometry. HLA-A0201-positive PBMCs were used for the expansion of epitope-specific T cells. PBMCs were cultured with each peptide (50 μM) and recombinant interleukin 2 (100 U/ml; Sigma, USA). Half of the culture medium was replaced with fresh RPMI-1640 medium every 3 or 4 days. The function of epitope-specific T cells was tested by IFN-γ ELISPOT, T2 cells cytotoxicity, HepG2.2.15 cells cytotoxicity and HBV gene expression assays on the 20th day.
In vitro human IFN-γ ELISPOT assay
In order to measure the ability of epitope-specific T cells to produce IFN-γ, the ELISPOT assay was performed according to the instructions of the kit (Invitrogen, Carlsbad, CA, USA). Epitope-specific T cells(5x105/ml) were added to plates. An automated ELISPOT plate reader was used to quantify IFN-γ release by counting the number of spots present.
Cytotoxicity assay against T2 cells
T2 cell is (transporter associated with antigen processing, TAP) deficient cell line. When T2 cell is cultured with different epitopes, the HLA-A0201 molecule on surface of T2 cell will bind to different epitopes, the epitope-HLA-A0201 complex can be recognized by epitope-specific T cells and corresponding T2 cell will be lysed. In order to determine the cytotoxic activity of epitope-specific T cells, we measured LDH release based on the instructions of a non-radioactive cytotoxicity assay kit (Promega, USA). Epitope-specific T cells served as effector cells, and T2 cells loaded with or without each peptide served as target cells. The ratios of effector cells versus target cells were set to 10:1, 20:1 and 40:1. The effector cells were co-cultured with target cells (1×105/well) at 37° C for 5 hours, and LDH release was measured.
Cytotoxicity assay against HepG2.2.15 cell
In order to determine the antiviral abilities of epitope-specific T-cells, we detect the cytotoxicity of epitope-specific T cells against HepG2.2.15 cell. The epitope-specific T-cells served as effector cells, and the HepG2.2.15 cells served as target cells. The ratios of epitope-specific T-cells versus HepG2.2.15 cells were set to 5:1, 10:1, 20:1. The effector cells and target cells were incubated together in 96-well plates at 37° C for 12 hours, and each well was added to total volume of 200 ul. The cytotoxicity activity of epitope-specific T-cells was evaluated based on LDH release using a non-radio-active cytotoxicity assay kit (Promega, US). The percentage of lysis was calculated as formula: (experimental release- target cell spontaneous release- effector cell spontaneous release)/(target cell maximum release- target cell spontaneous release).
HBV gene expression assay
The antiviral abilities of epitopes were also determined by HBV gene expression assay. The epitope-specific T-cells and HepG2.2.15 cells were co-cultured in 24-well plates at 37° C for 72 hours. The epitope-specific T-cells served as effector cells, and the HepG2.2.15 cells served as target cells. The ratio of effector cells versus target cells was set to 10:1, the density of target cell was 1×105/well. After 72 hours cocultivation, the supernatants were collected to determine the concentrations of HBV surface antigen (HBsAg) and HBV-DNA. The HBsAg concentration was detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Shanghai Siic Kehua Biotech Co., Ltd.). And the HBV-DNA concentration was detected by real-time fluorescence quantitative PCR according to the manufacturer’s protocol (Yaneng Bioscience (Shenzhen) Co., Ltd.). The control group was HepG2.2.15 cells cultured without T cells, and the PBS group was HepG2.2.15 cells co-cultured with T cells stimulated by PBS. The inhibition ratios of HBsAg and HBV-DNA were calculated as the formula: (1- experimental group/ control group)×100%.
Statistics
All experiments were performed independently 3 times, and the results were reported as mean±SD. We conducted the statistical analysis using a Student's t-test. A P value <0.05 was considered significant. All statistical analyses were conducted using SPSS 19.0 software.
Footnotes
AUTHOR CONTRIBUTIONS: Jinming Wu and Juzeng Zheng conceived and designed the experiments; Juzeng Zheng, Ziqiang Xia and Yilun Xu performed the experiments; Juzeng Zheng, Yilun Xu, Zhanfan Ou, and Xianfan Lin analyzed the data; Sisi Jin and Yang Liu contributed analysis tools; Jinming Wu, Juzeng Zheng, Ziqiang Xia, Yilun Xu, Zhanfan Ou, Xianfan Lin, Sisi Jin and Yang Liu contributed to writing the paper.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was supported by the Wenzhou Science and Technology Bureau under grant number Y20190115, Y20140682.
REFERENCES
- 1.Wong MC, Huang JL, George J, Huang J, Leung C, Eslam M, Chan HL, Ng SC. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019; 16:57–73. 10.1038/s41575-018-0055-0 [DOI] [PubMed] [Google Scholar]
- 2.Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019; 18:827–44. 10.1038/s41573-019-0037-0 [DOI] [PubMed] [Google Scholar]
- 3.Hong X, Kim ES, Guo H. Epigenetic regulation of hepatitis B virus covalently closed circular DNA: Implications for epigenetic therapy against chronic hepatitis B. Hepatology. 2017; 66:2066–77. 10.1002/hep.29479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselah T, Loureiro D, Boyer N, Mansouri A. Targets and future direct-acting antiviral approaches to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol. 2019; 4:883–92. 10.1016/S2468-1253(19)30190-6 [DOI] [PubMed] [Google Scholar]
- 5.Gehring AJ, Protzer U. Targeting Innate and Adaptive Immune Responses to Cure Chronic HBV Infection. Gastroenterology. 2019; 156:325–37. 10.1053/j.gastro.2018.10.032 [DOI] [PubMed] [Google Scholar]
- 6.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016; 16:219–33. 10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
- 7.Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018; 359:1355–60. 10.1126/science.aar7112 [DOI] [PubMed] [Google Scholar]
- 8.Clark DN, Hu J. Unveiling the roles of HBV polymerase for new antiviral strategies. Future Virol. 2015; 10:283–95. 10.2217/fvl.14.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE 4th, Xiong S, Brosgart CL, Chen SS, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004; 126:1750–58. 10.1053/j.gastro.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wu M, Zhang X, Zhang W, Zhang Z, Chen L, He J, Zheng Y, Chen C, Wang F, Hu Y, Zhou X, Wang C, et al. Hepatitis B virus polymerase impairs interferon-α-induced STA T activation through inhibition of importin-α5 and protein kinase C-δ. Hepatology. 2013; 57:470–82. 10.1002/hep.26064 [DOI] [PubMed] [Google Scholar]
- 11.Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol. 2015; 12:258–63. 10.1038/cmi.2014.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Dong A, Xiao J, Zhou X, Mi H, Xu H, Zhang J, Wang B. Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice. Cell Mol Immunol. 2016; 13:850–61. 10.1038/cmi.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao HJ, Han QJ, Wang G, Lin A, Xu DQ, Wang YQ, Zhao LH, Tian ZG, Zhang J. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8+ effector memory T cells. Gut. 2019; 68:2032–43. 10.1136/gutjnl-2017-315588 [DOI] [PubMed] [Google Scholar]
- 14.Bian Y, Zhang Z, Sun Z, Zhao J, Zhu D, Wang Y, Fu S, Guo J, Liu L, Su L, Wang FS, Fu YX, Peng H. Vaccines targeting preS1 domain overcome immune tolerance in hepatitis B virus carrier mice. Hepatology. 2017; 66:1067–82. 10.1002/hep.29239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Z, Kong X, Li F, Wei H, Sun R, Tian Z. IL-12-based vaccination therapy reverses liver-induced systemic tolerance in a mouse model of hepatitis B virus carrier. J Immunol. 2013; 191:4184–93. 10.4049/jimmunol.1203449 [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, Chou WM, Wettengel JM, Malo A, et al. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016; 150:194–205. 10.1053/j.gastro.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 17.Middleton D, Williams F, Meenagh A, Daar AS, Gorodezky C, Hammond M, Nascimento E, Briceno I, Perez MP. Analysis of the distribution of HLA-A alleles in populations from five continents. Hum Immunol. 2000; 61:1048–52. 10.1016/s0198-8859(00)00178-6 [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, Lin X, Wang X, Zheng L, Lan S, Jin S, Ou Z, Wu J. In Silico Analysis of Epitope-Based Vaccine Candidates against Hepatitis B Virus Polymerase Protein. Viruses. 2017; 9:112. 10.3390/v9050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol. 2016. (Suppl 1); 64:S117–31. 10.1016/j.jhep.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 20.Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol. 2011; 54:1286–96. 10.1016/j.jhep.2010.12.031 [DOI] [PubMed] [Google Scholar]
- 21.Weber CA, Mehta PJ, Ardito M, Moise L, Martin B, De Groot AS. T cell epitope: friend or foe? Immunogenicity of biologics in context. Adv Drug Deliv Rev. 2009; 61:965–76. 10.1016/j.addr.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchmann P, Dembek C, Kuklick L, Jäger C, Tedjokusumo R, von Freyend MJ, Drebber U, Janowicz Z, Melber K, Protzer U. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice. Vaccine. 2013; 31:1197–203. 10.1016/j.vaccine.2012.12.074 [DOI] [PubMed] [Google Scholar]
- 23.Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH, Zhen Z, Wang HF, Gong HY, et al. , and YIC Efficacy Trial Study Team. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013; 59:450–56. 10.1016/j.jhep.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 24.Zhang TY, Guo XR, Wu YT, Kang XZ, Zheng QB, Qi RY, Chen BB, Lan Y, Wei M, Wang SJ, Xiong HL, Cao JL, Zhang BH, et al. A unique B cell epitope-based particulate vaccine shows effective suppression of hepatitis B surface antigen in mice. Gut. 2020; 69:343–54. 10.1136/gutjnl-2018-317725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King TH, Kemmler CB, Guo Z, Mann D, Lu Y, Coeshott C, Gehring AJ, Bertoletti A, Ho ZZ, Delaney W, Gaggar A, Subramanian GM, McHutchison JG, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One. 2014; 9:e101904. 10.1371/journal.pone.0101904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boni C, Janssen HL, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, Alfieri A, Brillo F, Fisicaro P, et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology. 2019; 157:227–241.e7. 10.1053/j.gastro.2019.03.044 [DOI] [PubMed] [Google Scholar]
- 27.Yoon SK, Seo YB, Im SJ, Bae SH, Song MJ, You CR, Jang JW, Yang SH, Suh YS, Song JS, Kim BM, Kim CY, Jeong SH, Sung YC. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015; 35:805–15. 10.1111/liv.12530 [DOI] [PubMed] [Google Scholar]
- 28.Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, Kratzer R, Lelu-Santolaria K, Evlachev A, Meritet JF, Schlesinger Y, Villeval D, Strub JM, et al. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015; 64:1961–71. 10.1136/gutjnl-2014-308041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Gu Y, Bian L, Shi Y, Cai Y, Chen Y, Chen H, Qian L, Wu X, Xu K, Mallone R, Davidson HW, Yu L, et al. Characterization of immune response to novel HLA-A2-restricted epitopes from zinc transporter 8 in type 1 diabetes. Vaccine. 2016; 34:854–62. 10.1016/j.vaccine.2015.10.108 [DOI] [PubMed] [Google Scholar]
- 30.Wen J, Duan Z, Jiang L. Identification of a dengue virus-specific HLA-A*0201-restricted CD8+ T cell epitope. J Med Virol. 2010; 82:642–48. 10.1002/jmv.21736 [DOI] [PubMed] [Google Scholar]
- 31.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005; 175:633–39. 10.4049/jimmunol.175.2.633 [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B. Immune epitope database analysis resource. Nucleic Acids Res. 2012; 40:W525–30. 10.1093/nar/gks438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen M, Lundegaard C, Worning P, Lauemøller SL, Lamberth K, Buus S, Brunak S, Lund O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003; 12:1007–17. 10.1110/ps.0239403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8-11. Nucleic Acids Res. 2008. (Suppl 2); 36:W509–12. 10.1093/nar/gkn202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters B, Sette A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics. 2005; 6:132. 10.1186/1471-2105-6-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, Peters B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008; 4:2. 10.1186/1745-7580-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stranzl T, Larsen MV, Lundegaard C, Nielsen M. NetCTLpan: pan-specific MHC class I pathway epitope predictions. Immunogenetics. 2010; 62:357–68. 10.1007/s00251-010-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calis JJ, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, Keşmir C, Peters B. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput Biol. 2013; 9:e1003266. 10.1371/journal.pcbi.1003266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GP, and Open Source Drug Discovery Consortium. In silico approach for predicting toxicity of peptides and proteins. PLoS One. 2013; 8:e73957. 10.1371/journal.pone.0073957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Maupetit J, Derreumaux P, Tufféry P. Improved PEP-FOLD Approach for Peptide and Miniprotein Structure Prediction. J Chem Theory Comput. 2014; 10:4745–58. 10.1021/ct500592m [DOI] [PubMed] [Google Scholar]
- 41.Thévenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tufféry P. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012; 40:W288–93. 10.1093/nar/gks419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009; 30:2785–91. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan ZL, Wang ZB, Guo JL, Liu WQ, Hu J, Li J, Wang SN, Li Q, Wen JS. Two novel squamous cell carcinoma antigen-derived HLA-A*0201-binding peptides induce in vitro and in vivo CD8+ cytotoxic T lymphocyte responses. Int J Oncol. 2013; 42:1482–92. 10.3892/ijo.2013.1834 [DOI] [PubMed] [Google Scholar]