Abstract
Background
It remains unclear whether people with non‐muscle invasive bladder cancer (NMIBC) benefit from intravesical gemcitabine compared to other agents in the primary or recurrent setting following transurethral resection of a bladder tumor. This is an update of a Cochrane Review first published in 2012. Since that time, several randomized controlled trials (RCTs) have been reported, making this update relevant.
Objectives
To assess the comparative effectiveness and toxicity of intravesical gemcitabine instillation for NMIBC.
Search methods
We performed a comprehensive literature search of the Cochrane Library, MEDLINE, Embase, four other databases, trial registries, and conference proceedings to 11 September 2020, with no restrictions on the language or status of publication.
Selection criteria
We included RCTs in which participants received intravesical gemcitabine for primary or recurrent NMIBC.
Data collection and analysis
Two review authors independently assessed the included studies and extracted data for the primary outcomes: time to recurrence, time to progression, grade III to V adverse events determined by the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0), and the secondary outcomes: time to death from bladder cancer, time to death from any cause, grade I or II adverse events determined by the CTCAE v5.0 and disease‐specific quality of life. We performed statistical analyses using a random‐effects model and rated the certainty of the evidence using GRADE.
Main results
We included seven studies with 1222 participants with NMIBC across five comparisons. This abstract focuses on the primary outcomes of the three most clinically relevant comparisons.
1. Gemcitabine versus saline: based on two years' to four years' follow‐up, gemcitabine may reduce the risk of recurrence over time compared to saline (39% versus 47% recurrence rate, hazard ratio [HR] 0.77, 95% confidence interval [CI] 0.54 to 1.09; studies = 2, participants = 734; I2 = 49%; low‐certainty evidence), but the CI included the possibility of no effect. Gemcitabine may result in little to no difference in the risk of progression over time compared to saline (4.6% versus 4.8% progression rate, HR 0.96, 95% CI 0.19 to 4.71; studies = 2, participants = 654; I2 = 53%; low‐certainty evidence). Gemcitabine may result in little to no difference in the CTCAE grade III to V adverse events compared to saline (5.9% versus 4.7% adverse events rate, risk ratio [RR] 1.26, 95% CI 0.58 to 2.75; studies = 2, participants = 668; I2 = 24%; low‐certainty evidence).
2. Gemcitabine versus mitomycin: based on three years' follow‐up (studies = 1, participants = 109), gemcitabine may reduce the risk of recurrence over time compared to mitomycin (17% versus 40% recurrence rate, HR 0.36, 95% CI 0.19 to 0.69; low‐certainty evidence). Gemcitabine may reduce the risk of progression over time compared to mitomycin (11% versus 18% progression rate, HR 0.57, 95% CI 0.32 to 1.01; low‐certainty evidence), but the CI included the possibility of no effect. We are very uncertain about the effect of gemcitabine on the CTCAE grade III to V adverse events compared to mitomycin (RR 0.51, 95% CI 0.13 to 1.93; very low‐certainty evidence). The analysis was only based on recurrent NMIBC.
3. Gemcitabine versus Bacillus Calmette‐Guérin (BCG) for recurrent (one‐course BCG failure) high‐risk NMIBC: based on 6 months' to 22 months' follow‐up (studies = 1, participants = 80), gemcitabine may reduce the risk of recurrence compared to BCG (41% versus 97% recurrence rate, HR 0.15, 95% CI 0.09 to 0.26; low‐certainty evidence) and progression over time (16% versus 33% progression rate, HR 0.45, 95% CI 0.27 to 0.76; low‐certainty evidence). We are very uncertain about the effect of gemcitabine on the CTCAE grade III to V adverse events compared to BCG (RR 1.00, 95% CI 0.21 to 4.66; very low‐certainty evidence).
In addition, the review provides information on the comparison of gemcitabine versus BCG and gemcitabine versus one‐third dose BCG.
Authors' conclusions
Based on findings of this review, gemcitabine may have a more favorable impact on recurrence and progression‐free survival than mitomycin but we are very uncertain as to how major adverse events compare. The same is true when comparing gemcitabine to BCG in individuals with high risk disease who have previously failed BCG. The underlying low‐ to very low‐certainty evidence indicates that our confidence in these results is limited; the true effects may be substantially different from these findings; therefore, better quality studies are needed.
Plain language summary
Intravesical gemcitabine for non‐muscle invasive bladder cancer
Review question
In people with tumors of the superficial layer of the urinary bladder (namely non‐muscle invasive bladder cancer [NMIBC]), how does gemcitabine that is put into the bladder compare to other medicines after the tumor has been removed?
Background
NMIBC can be taken out of the bladder using small instruments and a light source (called transurethral surgery). However, these tumors often come back (recurrence) with an aggressive feature such as spread into the deep layers of the bladder. To prevent this, we can put various medicines into the bladder. In this review, we wanted to know whether gemcitabine (a chemotherapy medication) was better or worse than other medicines.
Study characteristics
The evidence is current to 11 September 2020. We included only studies in which chance determined whether people received gemcitabine or other medicines. We found seven studies with 1222 participants. Two studies compared gemcitabine versus saline. One study compared gemcitabine versus mitomycin (a chemotherapy medication). Three studies compared gemcitabine versus BCG (Bacillus Calmette‐Guérin; a medicine used to help keep cancer from growing). One study compared gemcitabine versus one‐third dose BCG.
Key results
Gemcitabine may reduce the risk of recurrence over time, but may have a similar effect on progression (cancer getting worse) and severe unwanted effects compared to saline. Gemcitabine may prevent recurrence and progression compared to mitomycin. We are very unsure about the effect of gemcitabine on the severe unwanted effects compared to mitomycin. In people who had a high‐risk NMIBC with the cancer coming back after one course of treatment with BCG, gemcitabine may cause less tumor recurrence and progression compared to giving BCG again. We are very unsure about the effect of gemcitabine on the severe unwanted effects compared to BCG retreatment. The review also includes information on how gemcitabine compares to BCG and how it compares to one‐third dose BCG.
Reliability of the evidence
The reliability of the evidence was low or very low for most of the treatments we compared, meaning that we were often uncertain about whether the findings were true. Further research will likely change these findings.
Summary of findings
Summary of findings 1. Gemcitabine compared to saline.
| Patient or population: participants with non‐muscle invasive bladder cancer (607 men, 127 women) Country: Germany, Turkey, and the US Setting: multicenter, likely inpatients Intervention: gemcitabine Comparison: saline | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with saline | Risk difference with gemcitabine | ||||
|
Time to recurrence (absolute effect size estimates based on recurrence rate at 4 years) Follow‐up: range 2–4 years MCID: 5% absolute difference |
734 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | HR 0.77 (0.54 to 1.09) | Moderate | |
| 470 per 1000e | 83 fewer per 1000 (180 fewer to 29 more) | ||||
|
Time to progression (absolute effect size estimates based on progression rate at 4 years) Follow‐up: range 2–4 years MCID: 5% absolute difference |
654 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b,c | HR 0.96 (0.19 to 4.71) | Low | |
| 48 per 1000e | 2 fewer per 1000 (39 fewer to 159 more) | ||||
|
Grade III–V adverse events
assessed with: 1 study: measured as serious adverse events; 1 study: CTCAE version 3.0 and version 4.0 Follow‐up: range 1–3 months MCID: 5% absolute difference |
668 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | RR 1.26 (0.58 to 2.75) | Study population | |
| 47 per 1000 | 12 more per 1000 (20 fewer to 83 more) | ||||
|
Time to death from bladder cancer
(absolute effect size estimates based on death rate at 2 years) Follow‐up: 2 years MCID: 3% absolute difference |
328 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | HR 0.98 (0.02 to 49.40) | Low | |
| 6 per 1000f | 0 fewer per 1000 (6 fewer to 251 more) | ||||
|
Time to death from any cause (absolute effect size estimates based on death rate at 4 years) Follow‐up: range 2–4 years MCID: 3% absolute difference |
734 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d | HR 0.62 (0.39 to 1.00) | Low | |
| 121 per 1000 e | 44 fewer per 1000 (72 fewer to 0 fewer) | ||||
|
Grade I or II adverse events
assessed with:
1 study: measured as serious adverse events; 1 study: CTCAE version 3.0 and version 4.0 Follow‐up: range 1–3 months MCID: 5% absolute difference |
668 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | RR 1.13 (0.87 to 1.45) | Study population | |
| 246 per 1000 | 32 more per 1000 (32 fewer to 111 more) | ||||
| Disease‐specific quality of life | Not reported | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; HR: hazard ratio; MCID: minimal clinically important difference; n: number of participants; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for study limitations: high risk of selective reporting and other bias.
bNot downgraded further for moderate inconsistency; this contributed to the decision to downgrade twice overall.
cDowngraded one level for imprecision: confidence intervals crossed a clinically important threshold and no effect.
dDowngraded two levels for imprecision: confidence intervals crossed a clinically important threshold and no effect; wide confidence intervals
eBaseline risk for recurrence, progression, and death from any cause came from Messing 2018.
fBaseline risk for death from bladder cancer come from Böhle 2009.
Summary of findings 2. Gemcitabine compared to mitomycin.
| Patient or population: participants with non‐muscle invasive bladder cancer1 (93 men, 16 women) Country: Italy Setting: single center, likely inpatients Intervention: gemcitabine Comparison: mitomycin | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with mitomycin | Risk difference with gemcitabine | ||||
|
Time to recurrence Follow‐up: 3 years MCID: 5% absolute difference |
109 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
HR 0.36 (0.19 to 0.69) | Study population | |
| 400 per 1000 | 232 fewer per 1000 (308 fewer to 103 fewer) | ||||
|
Time to progression Follow‐up: 3 years MCID: 5% absolute difference |
109 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | HR 0.57 (0.32 to 1.01) | Study population | |
| 182 per 1000 | 74 fewer per 1000 (120 fewer to 2 more) | ||||
|
Grade III–V adverse events (local adverse events which result in delay intravesical treatment were regarded as Grade III–V complications) Follow‐up: 3 years MCID: 5% absolute difference |
109 (1 RCT) | ⊕⊝⊝⊝ Verylowa,d | RR 0.51 (0.13 to 1.93) | Study population | |
| 109 per 1000 | 53 fewer per 1000 (95 fewer to 101 more) | ||||
| Time to death from bladder cancer | Not reported | — | — | — | |
| Time to death from any cause | Not reported | — | — | — | |
|
Grade I or II adverse events (local adverse events which did not result in delay intravesical treatment) Follow‐up: 3 years MCID: 5% absolute difference |
109 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
RR 0.53 (0.37 to 0.78) | Study population | |
| 727 per 1000 | 342 fewer per 1000 (458 fewer to 160 fewer) | ||||
| Disease‐specific quality of life | Not reported | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; HR: hazard ratio; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1The analysis was only based on participants with recurrent non‐muscle invasive bladder cancer; the only included trial did not include participants with primary (untreated) disease.
aDowngraded one level for study limitations: unclear or high risk of bias on one or more domains.
bDowngraded one level for imprecision: outcome based on only a single study of a small number of participants.
cDowngraded one level for imprecision: confidence intervals crossed a clinically important threshold and no effect.
dDowngraded two levels for imprecision: confidence intervals crossed a clinically important threshold and no effect; wide confidence intervals.
Summary of findings 3. Gemcitabine compared to BCG for recurrent (one‐course BCG failure) non‐muscle invasive bladder cancer.
| Patient or population: participants with recurrent (1‐course BCG failure) high‐risk non‐muscle invasive bladder cancer (49 men, 31 women) Country: Italy Setting: multicenter, likely inpatients Intervention: gemcitabine Comparison: BCG | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with BCG | Risk difference with gemcitabine | ||||
|
Time to recurrence Follow‐up: range 6–22 months MCID: 5% absolute difference |
80 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | HR 0.15 (0.09 to 0.26) | Study population | |
| 970 per 1000 | 561 fewer per 1000 (699 fewer to 372 fewer) | ||||
|
Time to progression Follow‐up: range 6–22 months MCID: 5% absolute difference |
80 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | HR 0.45 (0.27 to 0.76) | Study population | |
| 325 per 1000 | 163 fewer per 1000 (224 fewer to 67 fewer) | ||||
|
Grade III–V adverse events
assessed with: CTCAE version 3.0 Follow‐up: range 6–22 months MCID: 5% absolute difference |
80 (1 RCT) | ⊕⊝⊝⊝ Verylowa,c | RR 1.00 (0.21 to 4.66) | Study population | |
| 75 per 1000 | 0 fewer per 1000 (59 fewer to 275 more) | ||||
|
Time to death from bladder cancer Follow‐up: range 6–22 months MCID: 3% absolute difference |
80 (1 RCT) | ⊕⊝⊝⊝ Verylowa,c |
HR 0.04 (0.01 to 2.25) | Study population | |
| 17 per 1000 | 16 fewer per 1000 (17 fewer to 21 more) | ||||
| Time to death from any cause | Not reported | — | — | — | — |
|
Grade I or II adverse events
assessed with: CTCAE version 3.0 Follow‐up: range 6–22 months MCID: 5% absolute difference |
80 (1 RCT) | ⊕⊝⊝⊝ Verylowa,c | RR 0.92 (0.48 to 1.77) | Study population | |
| 325 per 1000 | 26 fewer per 1000 (169 fewer to 250 more) | ||||
| Disease‐specific quality of life | Not reported | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; MCID: minimal clinically important difference; HR: hazard ratio; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for study limitations: high risk of bias on one or more domains.
bDowngraded one level for imprecision: outcome based on only a single study of a small number of participants.
cDowngraded two levels for imprecision: confidence intervals crossed a clinically important threshold and no effect; wide confidence intervals.
Background
Description of the condition
Under 2018 GLOBOCAN data, urothelial carcinoma of the bladder is the 10th most common malignancy worldwide, with 549,393 new cases and 200,000 cancer‐related deaths (Bray 2018). In 2020, an estimated 81,400 new cases of bladder cancer will be diagnosed in the US, with 17,982 estimated deaths occurring during this same period (Siegel 2020). Risk factors for developing bladder cancer include male sex, white race, smoking, personal or family history of bladder cancer, pelvic radiation, environmental/occupational exposures, exposure to certain drugs, chronic infection, irritation of the urinary tract, and certain medical conditions including obesity and diabetes (DeGeorge 2017). Most people with bladder cancer are diagnosed during diagnostic testing resulted from hematuria. In people in whom bladder cancer is suspected, computer tomography urography is used to assess the whole urinary tract, and cystoscopy is used to assess the lower urinary tract (Helenius 2015). At presentation, approximately 75% of patients have a non‐muscle invasive disease, and 25% have muscle‐invasive or metastatic disease. Non‐muscle invasive tumors can be either papillary or non‐papillary. Those papillary tumors that are confined to the innermost layer of the bladder (urothelium) are designated Ta tumors, while those that have invaded the basement membrane beneath this layer, the lamina propria, are designated T1 tumors. Most tumors diagnosed are Ta tumors (Burger 2008). People who present with T1 are at higher risk due to the greater propensity of these tumors to recur and progress. Non‐papillary tumors include carcinoma in situ (CIS), a flat, high‐grade tumor that commonly presents concurrently with papillary tumors and has a high risk of progression (Hansel 2013; Sylvester 2006).
The initial management of non‐muscle invasive bladder cancer (NMIBC) is transurethral resection (TUR) to remove all visible tumors, and depth includes the muscularis propria. After the initial transurethral surgery, 50% to 70% of tumors have recurred (Perlis 2013), and 10% to 30% of tumors are progressing (grade and stage progression) within five years (Lamm 2014). Factors associated with recurrence and progression include high stage, high grade, large tumor size, multifocality, high number of the previous recurrence, presence of concomitant CIS, lymphovascular invasion, and histologic variants (Kamat 2016). Therefore, frequent cystoscopic surveillance is required for detecting early recurrence, but this procedure may impact the person's quality of life and has considerable implications for health care in terms of cost.
Description of the intervention
To overcome the problem of tumor recurrence, anti‐tumor agents may be instilled into the bladder for a short time to bathe the tumor cells. This is called intravesical therapy and is frequently used as an adjunctive following TUR. The objective is to eradicate residual tumor cells missed in the original resection and to prevent or delay tumors from recurring or progressing to more invasive disease (Babjuk 2019; Peyton 2019). Therefore, intravesical therapy has an essential role in the management of NMIBC. For intravesical drug instillation, usually a two‐way catheter is sterilely inserted into the bladder. When the bladder is completely drained, anti‐tumor agents such as Bacillus Calmette‐Guérin (BCG), or chemotherapeutic drugs (e.g. mitomycin, epirubicin, or gemcitabine) are passed into the bladder through the catheter and the drug solution retained for 1.5 hours to 2 hours. After that, the participant voids to remove the drug solution. Gemcitabine 2 g in 50 mL or 100 mL of saline can be used once a week for six weeks (namely induction therapy) (Addeo 2010; Bendary 2011; Di Lorenzo 2010; Gontero 2013; Porena 2010), or immediate single instillation after transurethral resection of bladder tumor (TURBT) (Böhle 2009; Messing 2018), in NMIBC.
Adverse events of the intervention
Adverse events from intravesical anti‐tumor agent instillation can be divided into local and systemic. The common local adverse events are urinary frequency, urinary urgency, dysuria, hematuria, bladder or pelvic pain, and prostatitis. However, most of these are usually self‐limiting (Griffin 2013). Systemic adverse events are rare and primarily result in myelosuppression (Griffin 2013). However, studies have reported that gemcitabine induced no higher than grade III Common Terminology Criteria for Adverse Events (CTCAE) for local and systemic adverse events. The most reported adverse events are voiding dysfunction, pain, hematuria, pyrexia, and alopecia (Böhle 2009; Maffezzini 2009; Messing 2018).
How the intervention might work
Gemcitabine is a pyrimidine antimetabolite that inhibits DNA synthesis by inhibition of DNA polymerase and ribonucleotide reductase, cell cycle‐specific for the S‐phase of the cycle (also blocks cellular progression at G1/S‐phase). Gemcitabine is phosphorylated intracellularly by deoxycytidine kinase to gemcitabine monophosphate, which is further phosphorylated to active metabolites gemcitabine diphosphate and gemcitabine triphosphate. Gemcitabine diphosphate inhibits DNA synthesis by inhibiting ribonucleotide reductase; gemcitabine triphosphate is incorporated into DNA and inhibits DNA polymerase. These metabolites are responsible for the cytotoxic action of gemcitabine by blocking DNA synthesis and leading to programmed cell death (apoptosis) (Laufer 2003; Mini 2006). Gemcitabine has several pharmacologic properties that are conducive for its use as an intravesical agent in the management of NMIBC. First, gemcitabine has demonstrated activity in killing cultured bladder cancer cells in vitro (Kilani 2002). Second, the low molecular weight and the high lipid solubility allow sufficient uptake into malignant urothelial cells for cytotoxicity in vivo (Sternberg 2000). Third, gemcitabine has a high plasma clearance so that any drug that does enter the systemic circulation after intravesical administration will be quickly eliminated, reducing the risk of systemic toxicity (Cozzi 1999; Laufer 2003).
Why it is important to do this review
There are two systematic reviews on this topic (Jones 2012; Ye 2018). The systematic review and meta‐analysis by Ye and colleagues, which included randomized controlled trials (RCTs) and retrospective observational studies, concluded that intravesical gemcitabine instillation may have similar efficacy and lower incidence of dysuria and hematuria compared with BCG (Ye 2018). However, this review had many inherent limitations. One previous Cochrane Review for gemcitabine for the treatment of NMIBC based on RCTs demonstrated that intravesical gemcitabine therapy had similar effects in intermediate‐risk patients, but less effective in high‐risk patients and superior in BCG‐refractory patients compared to intravesical BCG therapy. Also, the Cochrane Review reported that single‐dose intravesical instillation after transurethral surgery is ineffective compared to saline (Jones 2012). After publication of this review, Cochrane introduced more rigorous methodology, which included assessment of risk of bias and production of 'Summary of findings' tables (the GRADE approach; Schünemann 2017). Furthermore, the results of several randomized trials for gemcitabine have been reported since the Jones 2012 review. Therefore, the previous review must be considered outdated. This is an update of the Cochrane Review first published in 2012 (Jones 2012).
Objectives
To assess the comparative effectiveness and toxicity of intravesical gemcitabine instillation for NMIBC.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs. We excluded quasi‐randomized and non‐randomized studies, cohort studies, case series, cross‐over trials, and cluster‐randomized trials. We did not exclude studies on the basis of publication status or language.
Types of participants
We included studies that used participants with NMIBC (Stage 0a, Stage 0is and Stage I) (Babjuk 2019; NCCN guideline 2019), with any tumor grade (Epstein 1998; Humphrey 2016) as determined via cross‐sectional imaging, cystoscopic appearance, or biopsy. We included studies irrespective of intravesical therapy dose or schedule. Participants who received prior intravesical therapy and failed to respond, such as BCG‐refractory participants, were also eligible. We excluded participants with previous or concurrent upper urinary tract or prostatic urethral urothelial cancer, cancers other than bladder, and previous systemic treatment or radiation therapy for any cancer.
Types of interventions
We investigated the following comparisons of experimental versus comparator interventions.
Experimental interventions
Intravesically administered gemcitabine
All participants had undergone TUR prior to receiving the intervention.
Comparator interventions
Observation (no intervention).
Intravesically administered placebo or non‐chemotherapeutic drugs (e.g. saline).
Intravesically administered chemotherapy other than gemcitabine.
Intravesically administered full dose BCG (excluded in case of single intravesical therapy immediate after TUR).
Intravesically administered 1/3 dose BCG (excluded in case of single intravesical therapy immediate after TUR).
All participants had undergone TUR prior to receiving the intervention.
Comparisons
Intravesically administered gemcitabine versus observation (no intervention).
Intravesically administered gemcitabine versus intravesically administered placebo or non‐chemotherapeutic drugs (e.g. saline).
Intravesically administered gemcitabine versus intravesically administered chemotherapy other than gemcitabine.
Intravesically administered gemcitabine versus intravesically administered BCG (excluded in case of single intravesical therapy immediate after TUR).
Intravesically administered gemcitabine versus intravesically administered 1/3 of BCG
Concomitant interventions were the same in the experimental and comparator groups to establish fair comparisons. We also analyzed those studies separately in which patients with recurrent disease that had failed a given intravesical agent were re‐exposed to that same agent in the control group. If we included a study with more than two intervention arms, we only included experimental and comparator intervention groups that met the eligibility criteria of the review. We listed all treatment arms in the Characteristics of included studies table.
Types of outcome measures
We did not use the measurement of the outcomes included in this review as an eligibility criterion for considering studies.
Primary outcomes
Time to recurrence (time‐to‐event outcome).
Time to progression (time‐to‐event outcome).
Grade III to V adverse events (dichotomous outcome).
Secondary outcomes
Time to death from bladder cancer (time‐to‐event outcome).
Time to death from any cause (time‐to‐event outcome).
Grade I or II adverse events (dichotomous outcome).
Disease‐specific quality of life (continuous outcome).
Method and timing of outcome measurement
-
Time to recurrence: measured from the time of randomization to the time of the recurrence.
Definition of recurrence: any type of recurrence; judged based on imaging modalities (e.g. computed tomography), cystoscopy, or histopathologic proof of recurrence.
-
Time to progression: measured from the time of randomization to the time of the progression.
Definition of progression: increase in T stage from CIS (Stage 0is) or Ta (Stage 0a) to T1 (Stage I), development of T2 or greater (≥ stage II) or lymph node disease or distant metastasis, or an increase in tumor grade from low to high (Lamm 2014).
Grade III to V adverse events: determined by the CTCAE v5.0 (CTCAE), occurring at any time during treatment (e.g. hematuria that required hospitalization for catheter irrigation [grade III], life threatening/disabling [grade IV], and death [grade V]).
Time to death from bladder cancer: measured from the time of randomization to the time of death due to bladder cancer.
Time to death from any cause: measured from the time of randomization to the time of death due to any cause.
Grade I or II adverse events: measured by CTCAE v5.0 (CTCAE), occurring at any time during treatment (e.g. asymptomatic hematuria [grade I] and symptomatic hematuria requiring temporary bladder irrigation [grade II]).
Quality of life: measured by validated instruments such as the European Organisation for Research and Treatment of Cancer (EORTC) core quality‐of‐life questionnaire version 3.0 (QLQ C‐30), 12‐item Short Form (SF‐12), 36‐item Short Form (SF‐36), or Functional Assessment of Cancer Therapy (FACT) questionnaire.
If the authors did not use the CTCAE v5.0, we graded the adverse events as described in their respective studies. We defined a clinically meaningful minimal duration of follow‐up as three months (12 weeks). If we were unable to retrieve the necessary information to assess time‐to‐event outcomes, we tried to assess the number of events per total number of included participants in each relevant study for dichotomized outcomes at one year, two years, three years, and five years after administering intravesical therapy.
We considered a 5% absolute risk difference as clinically important for time to recurrence, time to progression, and grade I to V adverse events. We considered a 3% absolute risk difference as clinically important for time to death from bladder cancer and time to death from any cause. We used published threshold for disease‐specific quality of life instruments (e.g. EORTC QLQ‐C30: minimal clinically important difference: 10; Osoba 1998).
Search methods for identification of studies
We performed comprehensive searches, applying no restrictions on the language of publication or publication status.
Electronic searches
We assessed the search strategies used for the previous reviews and amended them to incorporate changes in medical subject heading terminology and added additional databases. All searches were from inception to 11 September 2020. See Appendix 1 for the full search strategies.
The Cochrane Library (Wiley): 2020, Issue 9.
MEDLINE (via OvidSP): 1946 to 11 September 2020.
MEDLINE In Process & Epub Ahead of Print (via Ovid): searched 11 September 2020.
Embase (via OvidSP): 1947 to 11 September 2020.
Web of Science Core Collection (via Thomson Reuters): 1990 to 11 September 2020.
LILACS (Latin American and the Caribbean Health Sciences Literature; via Virtual Health Library): 1982 to 11 September 2020.
Scopus (via Elsevier): 1960 to 11 September 2020.
OpenGrey (Native Interface): 1980 to 11 September 2020.
We searched the following trials registers.
ClinicalTrials.gov (clinicaltrials.gov/): 2008 to 11 September 2020.
World Health Organization International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/): 2009 to 11 September 2020.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, reviews, and meta‐analyses. We also contacted the authors of included trials to identify any further studies that we may have missed. We searched the abstract proceedings of any relevant meetings conducted during 2017 to 2020 by the American Urological Association, European Association of Urology (EAU), and American Society of Clinical Oncology to search for unpublished studies.
Data collection and analysis
In this review, we followed the methodologic recommendations given by Cochrane (Higgins 2017a).
Selection of studies
We used reference management software to identify and remove potential duplicate records (EndNote), and then imported these references into Covidence, a web‐based program for systematic review development. When more than one report of the same trial was available, we included the most up‐to‐date publication in the analysis. If a study had more than one publication, we grouped publications so that each study, rather than each publication, was the unit of interest. Two review authors (ECH, PM) independently scanned the abstract or title (or both) of the records retrieved, to determine which studies should be assessed further. Two review authors (ECH, PM) investigated all potentially relevant records as full text; mapped records to studies; and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a). We planned to resolve any discrepancies through consensus or recourse to a third review author (JHJ). If resolution of a disagreement was not possible, we planned to designate the study as 'awaiting classification' and to contact study authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a data extraction form that we piloted ahead of time.
For studies that fulfilled the inclusion criteria, two review authors (ECH, PM) independently extracted the following information, which we report in the Characteristics of included studies table.
Study design.
Study dates (if dates were not available then this was reported as such).
Study settings and country.
Participant inclusion and exclusion criteria (including participant comorbidities, disease stage, pretreatment).
Participant details, baseline demographics (including participant age, disease stage).
Number of participants by study and by study arm.
Details of relevant experimental and comparator interventions (including dose, frequency, and duration).
Definitions of relevant outcomes, method and timing of outcome measurement, and any relevant subgroups.
Study funding sources.
Declarations of interest by primary investigators.
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes such as adverse events, we attempted to obtain numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes such as quality‐of‐life scores, we attempted to obtain means and standard deviations or data necessary to calculate this information (Hozo 2005). For time‐to‐event outcomes, we extracted the hazard ratio (HR) from published data according to published guidance (Parmar 1998; Tierney 2007), with corresponding measures of variance or data necessary to calculate this information.
We planned to resolve any disagreements by discussion, or, if required, by consultation with a third review author (JHJ or VN).
We provided information, including trial identifier, about potentially relevant ongoing studies in the Characteristics of ongoing studies table.
We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data‐set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (ECH and PM) independently assessed the risks of bias for each included study. We resolved disagreements by consensus, or by consulting with a third review author (PD). We used the Cochrane 'Risk of bias' assessment tool for the following domains (Higgins 2017b).
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias (e.g. baseline imbalance).
We judged 'Risk of bias' domains as 'low risk,' 'high risk,' or 'unclear risk.' We presented the results of this assessment graphically. For selection bias (random sequence generation and allocation concealment) and reporting bias (selective reporting), we evaluated the risks of bias at a trial level.
For performance bias (blinding of participants and personnel), we defined all outcomes as similarly susceptible to performance bias and assessed them in one group.
For detection bias (blinding of outcome assessments), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes.
We defined the following outcome measures as subjective.
Time to recurrence.
Time to progression.
Time to death from bladder cancer.
Grade I or II adverse events.
Disease‐specific quality of life.
We defined the following outcomes as objective.
Grade III to V adverse events.
Time to death from any cause.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and presented the judgment for each outcome separately when reporting our findings in the 'Risk of bias' tables. If appropriate, we created groups of outcomes with similar reporting characteristics (e.g. grade III to V events and any adverse events) to facilitate both the 'Risk of bias' ratings and presentation. We further summarized the risk of attrition bias across domains for each outcome in each included study, as well as across the studies and domains for each outcome, in accordance with the approach for the summary assessment of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017b).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes measured on the same scale, we estimated the intervention effect using the mean difference (MD) with a 95% CI. For continuous outcomes measuring the same underlying concept (e.g. disease‐specific quality of life), but using different measurement scales, we planned to report the standardized mean difference (SMD) with 95% CIs. We expressed time‐to‐event data as HRs with 95% CIs. We analyzed the data using Review Manager 5 (Review Manager 2014).
Unit of analysis issues
The unit of analysis was each individual participant. We planned to consider the level at which randomization occurred, and the multiple observations of the same outcome. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined study groups to create a single pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute multiple times (if possible, splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlations arising from the same set of participants being in multiple comparisons (Higgins 2011).
Dealing with missing data
We obtained missing data from corresponding study authors, if feasible, and performed intention‐to‐treat analyses of data were available. Otherwise, we performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐ups, and withdrawals), and the critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs. We also used the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted the I2 statistic as follows (Deeks 2017):
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: may indicate considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting. As we included only one or two studies in each comparison in our review, we could not use funnel plots to assess any small‐study effects.
Data synthesis
We performed data synthesis using Review Manager 5 (Review Manager 2014) in accordance with the guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a). In the meta‐analyses, we used a random‐effects model. For dichotomous outcomes, we used the Mantel‐Haenszel method. For continuous outcomes, we used the inverse variance method. For time‐to‐event outcomes, we used the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity; therefore, where sufficient data were available, we planned to perform the following predefined subgroup analyses.
Risk (low risk versus intermediate risk versus high risk) according to EORTC and EAU risk classification system (Babjuk 2019; Sylvester 2006).
Dose of gemcitabine (e.g. 2000 mg versus 1000 mg).
If EAU risk categories were not available, and if there were sufficient data, we planned to perform subgroup analyses based on (Babjuk 2019; Sylvester 2006):
number of tumors (one versus more than one);
tumor size (less than 3 cm versus 3 cm or greater);
tumor stage (Ta versus T1);
presence of CIS (absent or present);
tumor grade (Grade 1 versus Grades 2 and 3 or low grade versus high grade);
primary versus recurrent disease.
We used the test for subgroup differences in Review Manager 5 to compare subgroup analyses if there was at least one study with the data available for our predefined subgroups (Review Manager 2014). Furthermore, unless the trial(s) were stratified for the subgroups, we downgraded the certainty of evidence.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size, if applicable.
Restricting the analysis by taking the risk of bias into account and excluding studies classified as having a high risk or unclear risk of bias.
Summary of findings and assessment of the certainty of the evidence
We presented 'Summary of findings' tables, reporting the following outcomes listed according to priority.
Time to recurrence.
Time to progression.
Grade III to V adverse events.
Time to death from bladder cancer.
Time to death from any cause.
Grade I or II adverse events.
Disease‐specific quality of life.
We presented the findings and the certainty of the available evidence according to GRADE methodology (Schünemann 2017). We assessed the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria related, not only to internal validity (risk of bias, inconsistency, imprecision, and publication bias), but also to external validity (directness of results) (Guyatt 2008). Two review authors (MAH, ECH, or JHJ) independently rated the certainty of the evidence for each outcome as 'high,' 'moderate,' 'low,' or 'very low.' We resolved discrepancies by consensus, or, if needed, by the arbitration of a third review author (PD). We presented a summary of the evidence for the main outcomes in summary of findings table, which we generated using the GRADEpro GDT (gradepro.org). This table provides key information about the best estimate of the magnitude of an effect in relative terms and presents absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of our overall confidence in the effect estimates for each outcome (Guyatt 2011; Schünemann 2017).
Results
Description of studies
We identified 1002 records through electronic database searching and four records in existing systematic review.
Results of the search
After removal of duplicates, we screened the titles and abstracts of 650 records, and excluded 615 obviously irrelevant records. We screened 35 full‐text records (22 studies), and excluded 10 records (10 studies) that did not meet the inclusion criteria or were not relevant to the review. We included seven studies (19 records) in the review. The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 1).
1.
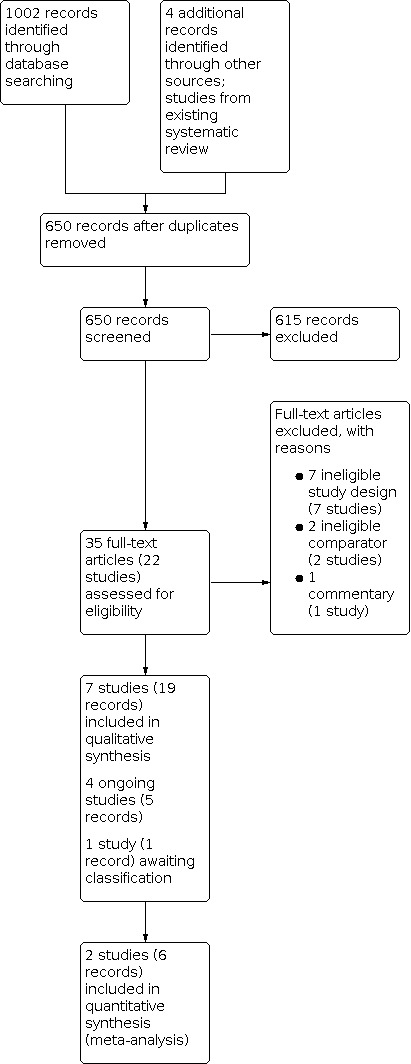
Study flow diagram.
Included studies
Details of included studies are presented in the Characteristics of included studies table; Table 4; and Table 5.
1. Baseline characteristics of the included study.
| Study name | Trial period | Setting/country | Description of participants | Intervention(s) and comparator(s) | Treatment schedule | Duration of follow‐up | Age (mean ± SD) (years) | Gender (men/women, %) | Disease type |
| Addeo 2010 | 2003–2005 | Single center/Italy | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/50 mL saline | 6‐week induction course + maintenance 10 monthly treatments during first year | Median 36 months | 64.9 ± 10.55 | 46 (85.2)/8 (14.8) | Recurrent disease |
| Comparator: mitomycin 40 mg/50 mL saline | 4‐week induction course + maintenance 10 monthly treatments during first year | 67.9 ± 10.2 | 47 (85.5)/8 (14.5) | ||||||
| Bendary 2011 | 2006–2008 | Single center/Egypt | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/50 mL saline | 6‐week induction course | Range 3–18 months (mean 10.8 ± 27 months) | Overall 56.2 ± 11.18 | NA | Primary without CIS disease |
| Comparator: BCG 6 × 108 CFU/50 mL saline | NA | ||||||||
| Böhle 2009 | 2004–2005 | Multicenter (24 centers)/Germany and Turkey | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/100 mL saline | Single instillation | median 23.6 months (range 0–46 months) | 63.2 ± 11.9 | 127 (76.5)/39 (23.5) | Primary and recurrent disease, both |
| Comparator: 100 mL saline | 66.3 ± 11 | 136 (84)/26 (16) | |||||||
| Di Lorenzo 2010 | 2006–2008 | Multicenter/Italy | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/50 mL saline | Twice weekly (days 1 and 4) for 6‐week induction course + maintenance 3 weekly instillations at 3, 6, and 12 months. | Median 15.2 months (range 6–22 months) | 69.3 ± 8.4 | 27 (67.5)/13 (32.5) | Recurrent disease (BCG failure; high‐risk disease only) |
| Comparator: BCG (Connaught strain, 81 mg/50 mL saline) | 6‐week induction course + maintenance 3 weekly instillations at 3, 6, and 12 months | median 15.8 months (range 7–21 months) | 71.4 ± 7.9 | 22 (55)/18 (45) | |||||
| Gontero 2013 | 2006–2010 | Multicenter (3 centers)/multicountry (Italy, Germany, and the US) | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/50 mL saline | 6‐week induction course + maintenance monthly treatments up to 1 year | 1 year | 67.4 ± 9.4 | 53 (86.9)/8 (13.1) | Primary and recurrent disease, both (intermediate‐risk disease only) |
| Comparator: 1/3 dose BCG (Connaught strain, 27 mg/50 mL saline) | 6‐week induction course + maintenance 3 weekly instillations at 3, 6, and 12 months | 67.5 ± 9.8 | 50 (84.7)/9(15.3) | ||||||
| Messing 2018 | 2008–2012 | Multicenter (23 centers)/the US | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/100 mL saline | Single instillation | 4 years | Median: 66 (IQR 59–74) | 163 (81)/38 (19) | Primary and recurrent disease, both |
| Comparator: 100 mL saline | Median: 66 (IQR 59–75) | 181 (88)/24 (12) | |||||||
| Porena 2010 | 2004–2006 | Single center/Italy | Participants with NMIBC who underwent TURBT | Intervention: gemcitabine 2000 mg/50 mL saline | 6‐week induction course + maintenance therapy 3, 6, 12, 18, 24, 30, and 36 months | Mean 44 months | 70.2 ± 5.5 | 26 (81.3)/6 (18.7) | Primary disease (high‐risk disease only) |
| Comparator: BCG (Tice strain) 5 × 108 CFU/50 mL saline | 68.7 ± 10.2 | 28 (87.5)/4 (12.5) |
BCG: Bacillus Calmette‐Guérin; CFU: colony‐forming units; CIS: carcinoma in situ; IQR: interquartile range; NA: not available; NMIBC: non‐muscle invasive bladder cancer; SD: standard deviation; TURBT: transurethral resection of the bladder tumor.
2. Participants in the included study.
| Study name | Interventions and comparators | Screened/eligible (n) | Randomized (n) | Analyzed (n): efficacy | Analyzed (n): safety | Finishing trial (n (%)) |
| Addeo 2010 | Intervention: gemcitabine 2000 mg/50 mL saline | 120/109 | 54 | 54 | 54 | 54 (100) |
| Comparator: mitomycin 40 mg/50 mL saline | 55 | 55 | 55 | 55 (100) | ||
| Bendary 2011 | Intervention: gemcitabine 2000 mg/50 mL saline | NA/80 | 40 | NA | NA | NA |
| Comparator: BCG 6 × 108 CFU/50 mL saline | 40 | NA | NA | NA | ||
| Böhle 2009 | Intervention: gemcitabine 2000 mg/100 mL saline | NA/355 | 179 | Primary outcome; 166/secondary outcome; 124 | 166 | 41 (22.9) |
| Comparator: 100 mL saline | 176 | Primary outcome; 162/secondary outcome; 124 | 162 | 47 (26.7) | ||
| Di Lorenzo 2010 | Intervention: gemcitabine 2000 mg/50 mL saline | 92/80 | 40 | 40 | 40 | 40 (100) |
| Comparator: BCG (Connaught strain, 81 mg/50 mL saline) | 40 | 40 | 40 | 40 (100) | ||
| Gontero 2013 | Intervention: gemcitabine 2000 mg/50 mL saline | 120/118 | 59 | 41 | 41 | 41 (100) |
| Comparator: 1/3 dose BCG (Connaught strain, 27 mg/50 mL saline) | 59 | 47 | 47 | 47 (100) | ||
| Messing 2018 | Intervention: gemcitabine 2000 mg/100 mL saline | NA/416 | 207 | 201 | 165 | 102 (49.3) |
| Comparator: 100 mL saline | 209 | 205 | 175 | 113 (54.1) | ||
| Porena 2010 | Intervention: gemcitabine 2000 mg/50 mL saline | 74/64 | 32 | 32 | 32 | 32 (100) |
| Comparator: BCG (Tice strain) 5 × 108 CFU/50 mL saline | 32 | 32 | 32 | 32 (100) | ||
| Intervention: gemcitabine | 611 | — | — | 310a | ||
| Comparator: mitomycin | 55 | — | — | 55 (100) | ||
| Comparator: BCG | 171 | — | — | 119a | ||
| Comparator: saline | 385 | — | — | 160 (62.3) | ||
| Grand total | 1222 | — | — | 644 (52.7)b | ||
BCG: Bacillus Calmette‐Guérin; CFU: colony‐forming units; n: number of participants; NA: not available. aBendary 2011 did not report the number of participants who finished trial. bCalculated without Bendary 2011.
Source of data
We included six published studies and one abstract proceeding (Bendary 2011). All studies were published in English. We attempted to contact all corresponding authors of included trials to obtain additional information on study methodology and results, and received replies from four (Böhle 2009; Cao 2011; Di Lorenzo 2010; Gontero 2013; see Appendix 2).
Study design and settings
All studies were parallel RCTs. Two studies were reported as 'double‐blinded' (Böhle 2009; Messing 2018). Three studies were open‐label trials (Di Lorenzo 2010; Gontero 2013; Porena 2010). The remaining two trials had no information regarding blinding. All studies were probably conducted in an inpatient setting. Four studies were multicenter (Böhle 2009; Di Lorenzo 2010; Gontero 2013; Messing 2018), while three studies were single center trials (Addeo 2010; Bendary 2011; Porena 2010). The studies were performed from 2003 to 2012.
Participants
We included 1222 randomized participants (gemcitabine 611, mitomycin 55, BCG 171, saline 385), of which 644 completed the trials (gemcitabine 310, mitomycin 55, BCG 119, saline 160) (Table 5). However, one study that compared gemcitabine to BCG did not report the number of participants who completed the trial in each group (Bendary 2011). All studies included men and women.
All studies included participants with NMIBC, but two studies included small numbers of participants with muscle‐invasive bladder cancer in the analysis (7.7% in Böhle 2009, 3.7% in Messing 2018). These two studies originally intended to include the primary and recurrent NMIBC (Böhle 2009; Messing 2018). The remaining studies included each different disease type (recurrent NMIBC: Addeo 2010; primary NMIBC without CIS: Bendary 2011; high‐risk BCG failure recurrent NMIBC: Di Lorenzo 2010; intermediate‐risk primary and recurrent NMIBC: Gontero 2013; and high‐risk primary NMIBC: Porena 2010). Most exclusion criteria included active urinary tract infection, previous pelvic radiation therapy for any malignancy, or prior treatment for any malignancy.
Interventions
Two studies used gemcitabine as an intravesical dose of 2000 mg mixed with 100 mL saline (Böhle 2009; Messing 2018), and the remaining studies administered gemcitabine as an intravesical dose of 2000 mg mixed with 50 mL saline. Each study used different treatment schedule (Table 4). Most studies used intervention as induction and maintenance therapy (Addeo 2010; Di Lorenzo 2010; Gontero 2013; Porena 2010), while two studies used single instillation (Böhle 2009; Messing 2018), and one study used induction therapy only (Bendary 2011).
Comparators
Studies used six different comparators, namely saline, mitomycin, Connaught strain BCG, Tice strain BCG, BCG without a mention for a type of strain and one‐third dose Connaught strain BCG. All comparators were also administrated intravesically. Two studies administered saline as an intravesical dose of 100 mL (Böhle 2009; Messing 2018). One study used mitomycin 40 mg mixed with 50 mL saline as a comparator (Addeo 2010). Two studies administered Connaught strain BCG at 81 mg (Di Lorenzo 2010) and 27 mg (Gontero 2013), mixed with 50 mL saline. One study administered Tice strain BCG at 5 × 108 colony‐forming units (CFU) mixed with 50 mL saline(Porena 2010). One study administered BCG without mentioning the type of strain at 6 × 108 CFU mixed with 50 mL saline (Bendary 2011). Four studies used the same treatment schedule to that of the intervention (Bendary 2011; Böhle 2009; Messing 2018; Porena 2010), while remaining studies used different treatment schedules to the intervention.
Comparisons
We included five comparisons in this review: two studies compared gemcitabine to saline for primary and recurrent NMIBC (Böhle 2009; Messing 2018), one study compared gemcitabine to mitomycin for recurrent NMIBC (Addeo 2010), two studies compared gemcitabine to BCG in two different disease type (i.e. for primary high‐risk NMIBC [Porena 2010] and for recurrent [one‐course BCG failure] high‐risk NMIBC [Di Lorenzo 2010]), and one study compared gemcitabine to one‐third dose BCG for primary and recurrent intermediate‐risk NMIBC (Gontero 2013).
Outcomes
We identified all primary outcomes in each of the included studies for four comparisons. We extracted approximate HRs for time to recurrence using the Tierney 2007 method from three studies (Addeo 2010; Gontero 2013; Porena 2010), and for time to progression using Parmar 1998 method from three studies (Böhle 2009; Di Lorenzo 2010; Gontero 2013). For Addeo 2010, we regarded local adverse events that resulted in delay intravesical treatment as grade III to V complications and the regarded others as grade I or II complications. For Böhle 2009, we regarded severe adverse events as grade III to V complications and the others as grade I or II complications. Porena 2010 reported adverse events of gemcitabine and BCG, but we could not grade the adverse events in accordance with CTCAE. We were unable to obtain additional data from the authors, therefore we did not include this study in the analysis of this outcome. The remaining studies rated the complications by CTCAE v3.0, which is quite similar that of CTCAE v5.0 (Di Lorenzo 2010; Gontero 2013; Messing 2018).
In terms of secondary outcomes, two studies reported time to death from bladder cancer (Böhle 2009; Di Lorenzo 2010), and we extracted approximate HR using the Tierney 2007 method. Two studies reported time to death from any cause (Böhle 2009; Messing 2018), but we extracted approximate HR from one study (Böhle 2009). Gontero 2013 also reported one event of death from any cause, but we could not extract the HR since the study did not report which intervention (i.e. gemcitabine or one‐third BCG) the participant received. Five studies reported Grade I or II adverse events (Addeo 2010; Böhle 2009; Di Lorenzo 2010; Gontero 2013; Messing 2018). One study reported disease‐specific quality of life (Gontero 2013).
Two trials provided relevant data for predefined subgroup analysis (Böhle 2009; Messing 2018).
Funding sources and conflicts of interest
One study reported no funding source (Bendary 2011), and three reported the funding source (one supported by the national cancer prevention program [Addeo 2010], one supported by a pharmaceutical company which involved in the whole study process [Böhle 2009], and one supported by the national program and partially pharmaceutical company, but not participated in study analysis [Messing 2018]). The remaining trials did not mention a funding source. Two studies reported no conflicts of interest (Addeo 2010; Di Lorenzo 2010), and two reported their conflicts of interest (Böhle 2009; Messing 2018). The remaining studies did not mention conflicts of interest.
Excluded studies
We excluded 10 studies. Two were single arm studies which evaluated the effect of gemcitabine on solitary low‐risk NMIBC (Brausi 2011) and BCG refractory NMIBC (Dalbagni 2006). One study was a conference proceeding with non‐randomized study design (Gantz 2018). Two studies had an ineligible comparator; Cao 2011 combined different drugs as a comparator and Gardmark 2005 (which was included in the previous version of this review as an included study [Jones 2012]), used different dose and schedules of gemcitabine as a comparator. Böhle 2010 was commentary for Böhle 2009. Four studies from China were non‐randomized (Dong 2017; Lin 2016; Sun 2016; Xia 2019).
We presented details of excluded studies in the Characteristics of excluded studies table.
Studies awaiting classification and ongoing trials
We found one study awaiting classification (Xiaohong 2015; Characteristics of studies awaiting classification table), and four ongoing studies, which did not provide usable outcome data at the time that this review was written (ChiCTR1900026643; NCT00192049; NCT02695771; NCT04172675; Characteristics of ongoing studies table).
Risk of bias in included studies
Detailed results of the 'Risk of Bias' assessment are shown in Figure 2, and the judgments for individual domains are provided in the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Since Bendary 2011 provided insufficient data (abstract only) to judge risk of bias, we rated this study at unclear risk of bias for all domains except blinding of outcome assessment for objective outcomes.
Allocation
Random sequence generation
We judged five studies at low risk of bias because these trials used appropriate methods of random sequence generation (Böhle 2009; Di Lorenzo 2010; Gontero 2013; Messing 2018; Porena 2010). We rated the remaining studies at unclear risk of bias (Addeo 2010; Bendary 2011).
Allocation concealment
We rated four studies at low risk of bias since allocation was performed centrally (Böhle 2009; Di Lorenzo 2010; Gontero 2013; Messing 2018). We judged the remaining studies at unclear risk of bias (Addeo 2010; Bendary 2011; Porena 2010).
Blinding
Blinding of participants and personnel (performance bias)
We rated Böhle 2009 and Messing 2018 at low risk of bias because participants and personnel were blinded. We rated Di Lorenzo 2010; Gontero 2013; and Porena 2010 at high risk of bias since these were open‐label trials. The remaining studies were at unclear risk of bias.
Blinding of outcome assessment (detection bias)
Susceptible (subjective) outcomes (time to recurrence, time to progression, time to death from bladder cancer, grade I or II adverse events, disease‐specific quality of life)
We rated Böhle 2009 and Messing 2018 at low risk of bias because outcome assessors were blinded. We rated Di Lorenzo 2010; Gontero 2013; and Porena 2010 at high risk of bias since these were open‐label trials. The remaining studies were at unclear risk of bias.
Not susceptible (objective) outcomes (time to death from any cause, grade III to V adverse events)
We rated all trials at low risk of bias because blinding was unlikely to influence the outcome in any of the studies.
Incomplete outcome data
Time to recurrence and time to progression
We judged six studies at low risk of bias, because almost all randomized participants included in the analysis (Addeo 2010; Böhle 2009; Di Lorenzo 2010; Gontero 2013; Messing 2018; Porena 2010). The remaining study was at unclear risk of bias.
Grade III to V adverse events
We rated five studies at low risk of bias (Addeo 2010; Böhle 2009; Di Lorenzo 2010; Messing 2018; Porena 2010). One study was at high risk of bias because substantial proportions of participants were excluded from the final analysis (Gontero 2013). Bendary 2011 was at unclear risk of bias.
Time to death from bladder cancer
Only two studies investigated this outcome and were at low risk of bias (Böhle 2009; Di Lorenzo 2010). We did not rate this domain for the remaining studies, because these studies did not address this outcome (Addeo 2010; Gontero 2013; Messing 2018; Porena 2010). We report the risk of bias as unclear in the table because this is the default value. Bendary 2011 was at unclear risk of bias (abstract).
Time to death from any cause
We rated two studies at low risk of bias (Böhle 2009; Messing 2018). The remaining studies did not investigate this outcome and rated as unclear risk of bias which is a default value (Addeo 2010; Di Lorenzo 2010; Gontero 2013; Porena 2010). Bendary 2011 was at unclear risk of bias (abstract).
Grade I or II adverse events
We rated five studies at low risk of bias (Addeo 2010; Böhle 2009; Di Lorenzo 2010; Messing 2018; Porena 2010). One study was at high risk of bias because substantial proportions of participants were excluded from the final analysis (Gontero 2013). Bendary 2011 was at unclear risk of bias (abstract).
Disease‐specific quality of life
Only one study addressed this outcome and we judged it at high risk of bias (Gontero 2013).
Selective reporting
Three studies were at low risk of bias as they reported all outcomes according to their protocol (Gontero 2013; Messing 2018; Porena 2010). Two studies were at unclear risk of bias because the protocol for each study was not available (Addeo 2010; Di Lorenzo 2010). We rated one study as high risk of bias because the results of primary and secondary outcomes were different between protocol (unpublished data) and report, and one outcome was analyzed by post hoc due to lower event rates (Böhle 2009). Bendary 2011 was at unclear risk of bias (abstract).
Other potential sources of bias
We rated three studies at high risk of bias because treatment schedules differed between intervention and comparator in the two studies (Addeo 2010; Di Lorenzo 2010), and there was possibility of a difference in concomitant treatment instillations (BCG) in one study (Böhle 2009). The remaining studies were at low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Two studies were included in the meta‐analysis (Böhle 2009; Messing 2018). While the remaining trials used different comparators and different clinical scenarios for NMIBC, we considered it inappropriate to pool and meta‐analyze the data because of considerable clinical heterogeneity (see Table 4).
1. Gemcitabine versus saline
Two studies compared gemcitabine versus saline for primary and recurrent NMIBC (Böhle 2009; Messing 2018; Table 1).
Primary outcomes
Time to recurrence
Gemcitabine may reduce the risk of recurrence over time compared to saline (HR 0.77, 95% CI 0.54 to 1.09; studies = 2, participants = 734; I2 = 49%; low‐certainty evidence; Analysis 1.1), but the CI included the possibility of no effect. Based on the control event risk of 470 per 1000 participants as drawn from Messing 2018 at four years of follow‐up, this would result in 83 fewer recurrences (95% CI 180 fewer to 29 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations because one study had a high risk of selective reporting and other bias, and imprecision, given that the CI was also consistent with a small or no increase in the risk of recurrence. The observed inconsistency contributed to the decision to downgrade by two levels overall.
1.1. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 1: Time to recurrence
Time to progression
Gemcitabine may result in little to no difference in the risk of progression over time compared to saline (HR 0.96, 95% CI 0.19 to 4.71; studies = 2, participants = 654; I2 = 53%; low‐certainty evidence; Analysis 1.2). Based on the control event risk of 48 per 1000 participants as drawn from Messing 2018 at four years of follow‐up, this corresponds to two fewer progressions (95% CI 39 fewer to 159 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations because one study had a high risk of selective reporting and other bias, and imprecision, given that the CI was also consistent with an appreciable increase in the risk of progression. We did not downgrade for inconsistency because heterogeneity may have come from different gemcitabine and saline irrigation times between two studies (Böhle 2009; Messing 2018).
1.2. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 2: Time to progression
Grade III to V adverse events
Gemcitabine may result in little to no difference in the CTCAE grade III to V adverse events compared to saline (RR 1.26, 95% CI 0.58 to 2.75; studies = 2, participants = 668; I2 = 24%; low‐certainty evidence; Analysis 1.3). Based on the control event risk of 47 per 1000 participants in the trials and one month' to three months' follow‐up, this corresponds to 12 more adverse events (95% CI 20 fewer to 83 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision, given that the CI was also consistent with an appreciable increase in CTCAE grade III to V adverse events.
1.3. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 3: Grade III–V adverse events
Secondary outcomes
Time to death from bladder cancer
We are very uncertain about the effects of gemcitabine on the risk of death from bladder cancer over time compared to saline (HR 0.98, 95% CI 0.02 to 49.40; studies = 1, participants = 328; very low‐certainty evidence; Analysis 1.4). Based on the control event risk of 6 per 1000 participants as drawn from Böhle 2009 at two years of follow‐up, this would result in 0 fewer death from bladder cancer (95% CI 6 fewer to 251 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations (downgraded one level), and very serious imprecision (downgraded two levels).
1.4. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 4: Time to death from bladder cancer
Time to death from any cause
We are very uncertain about the effects of gemcitabine on the risk of death from any cause over time compared to saline (HR 0.62, 95% CI 0.39 to 1.00; studies = 2, participants = 734; I2 = 0%; very low‐certainty evidence; Analysis 1.5). Based on the control event risk of 121 per 1000 participants as drawn from Messing 2018 at four years of follow‐up, this corresponds to 44 fewer deaths from any cause (95% CI 72 fewer to 0 fewer) per 1000 participants. We downgraded the certainty of the evidence due to study limitations (downgraded one level), and very serious imprecision (downgraded two levels).
1.5. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 5: Time to death from any cause
Grade I or II adverse events
Gemcitabine may result in little to no difference in CTCAE grade I or II adverse events compared to saline (RR 1.13, 95% CI 0.87 to 1.45; studies = 2, participants = 668; I2 = 0%; low‐certainty evidence; Analysis 1.6). Based on the control event risk of 246 per 1000 participants in the trials and one month' to three months' follow‐up, this corresponds to 32 more adverse events (95% CI 32 fewer to 111 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations, and imprecision, given that the CI was also consistent with an appreciable increase in CTCAE grade I or II adverse events.
1.6. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 6: Grade I or II adverse events
Disease‐specific quality of life
We found no studies that reported disease‐specific quality of life.
Subgroup analysis
We performed preplanned subgroup analyses (stratified tumor grade) with regard to primary outcomes.
Tumor grade: low versus high
See Analysis 1.7.
1.7. Analysis.

Comparison 1: Gemcitabine versus saline, Outcome 7: Time to recurrence (subgroup analysis)
Time to recurrence
Of the 543 participants, 430 had low‐grade tumor (gemcitabine n = 208; saline n = 222) and 113 had high‐grade tumor (gemcitabine n = 57; saline n = 56). The HR of time to recurrence with gemcitabine was 0.75 (95% CI 0.38 to 1.46), for participants who had low‐grade tumor, and 0.74 (95% CI 0.43 to 1.28) for those who had high‐grade tumor. The test for interaction was not significant (P = 0.98; I2 = 0%).
Sensitivity analysis
We performed a sensitivity analysis using Messing 2018, which was at low risk of bias, overall.
See Table 6
3. Gemcitabine compared to saline (sensitivity analysis based on risk of bias).
| Patient or population: participants with non‐muscle invasive bladder cancer (344 men, 62 women) Country: US Setting: multicenter (23 centers), likely inpatients Intervention: gemcitabine Comparison: saline | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with salinea | Risk difference with gemcitabine | ||||
|
Time to recurrence Follow‐up: 4 years MCID: 5% absolute difference |
406 (1 RCT) | ⊕⊕⊕⊝ Moderateb | HR 0.66 (0.48 to 0.90) | Study population | |
| 470 per 1000 | 128 fewer per 1000 (207 fewer to 35 fewer) | ||||
|
Time to progression Follow‐up: 4 years MCID: 5% absolute difference |
406 (1 RCT) | ⊕⊕⊕⊕ High | HR 0.51 (0.17 to 1.50) | Study population | |
| 48 per 1000 | 23 fewer per 1000 (40 fewer to 23 more) | ||||
|
Grade III–V adverse events
assessed with: CTCAE version 3.0 and version 4.0 Follow‐up: 1 month MCID: 5% absolute difference |
340 (1 RCT) | ⊕⊕⊕⊝ Moderateb | RR 0.71 (0.20 to 2.46) | Study population | |
| 34 per 1000 | 10 fewer per 1000 (27 fewer to 50 more) | ||||
| Time to death from bladder cancer | Not reported | — | — | — | |
|
Time to death from any cause Follow‐up: 4 years MCID: 3% absolute difference |
406 (1 RCT) | ⊕⊕⊝⊝ Lowc | HR 0.68 (0.36 to 1.27) | Study population | |
| 121 per 1000 | 37 fewer per 1000 (76 fewer to 30 more) | ||||
|
Grade I or II adverse events
assessed with: CTCAE version 3.0 and version 4.0 Follow‐up: 1 month MCID: 5% absolute difference |
340 (1 RCT) | ⊕⊕⊕⊝ Moderateb | RR 1.20 (0.86 to 1.66) | Study population | |
| 269 per 1000 | 54 more per 1000 (38 fewer to 177 more) | ||||
| Disease‐specific quality of life | Not reported | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; HR: hazard ratio; MCID: minimal clinically important difference; n: number of participants; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aBaseline risk came from Messing 2018. bDowngraded one level for imprecision: confidence intervals crossed the assumed threshold of a clinically important difference. cDowngraded two levels for imprecision: confidence intervals crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence intervals.
Time to recurrence
Gemcitabine probably reduces the risk of recurrence over time compared to saline (HR 0.66, 95% CI 0.48 to 0.90; participants = 406; moderate‐certainty evidence). Based on the control event risk of 470 per 1000 participants, this corresponds to 128 fewer recurrences (95% CI 207 fewer to 35 fewer) per 1000 participants. We downgraded the certainty of the evidence due to imprecision, given that the CI was also consistent with a small or no reduction in the risk of recurrence over time.
Time to progression
Gemcitabine results in little to no difference in the risk of progression over time compared to saline (HR 0.51, 95% CI 0.17 to 1.50; participants = 406; high‐certainty evidence). Based on the control event risk of 48 per 1000 participants, this would result in 23 fewer progressions (95% CI 40 fewer to 23 more) per 1000 participants.
Grade III to V adverse events
Gemcitabine probably results in little to no difference in CTCAE grade III to V adverse events compared to saline (RR 0.71, 95% CI 0.20 to 2.46; participants = 340; moderate‐certainty evidence). Based on the control event risk of 34 per 1000 participants, this corresponds to 10 fewer adverse events (95% CI 27 fewer to 50 more) per 1000 participants. We downgraded the certainty of the evidence due to imprecision, given that the CI was also consistent with an increase in CTCAE grade III to V adverse events.
Time to death from bladder cancer
The study did not address the time to death from bladder cancer.
Time to death from any cause
Gemcitabine may reduce the risk of death from any cause over time compared to saline (HR 0.68, 95% CI 0.36 to 1.27; participants = 406; low‐certainty evidence), but the CI included the possibility of no effect. Based on the control event risk of 121 per 1000 participants, this corresponds to 37 fewer deaths from any cause (95% CI 76 fewer to 30 more) per 1000 participants. We downgraded the certainty of the evidence due to imprecision, given that the CI was consistent both with a reduction in the risk of death from any cause over time as well as an increase in the risk of death from any cause (i.e. wide CIs).
Grade I or II adverse events
Gemcitabine probably increases CTCAE grade I or II adverse events slightly compared to saline (RR 1.20, 95% CI 0.86 to 1.66; participants = 340; moderate‐certainty evidence), but the CI included the possibility of no effect. Based on the control event risk of 269 per 1000 participants, this corresponds to 54 more adverse events (95% CI 38 fewer to 177 more) per 1000 participants. We downgraded the certainty of the evidence due to imprecision, given that the CI was also consistent with a small or no reduction in CTCAE grade I or II adverse events.
2. Gemcitabine versus mitomycin
One study compared gemcitabine versus mitomycin for recurrent NMIBC (Addeo 2010; Table 2). There was no data available for gemcitabine versus mitomycin for primary NMIBC.
Primary outcomes
Time to recurrence
Gemcitabine may reduce the risk of recurrence over time compared to mitomycin (HR 0.36, 95% CI 0.19 to 0.69; studies = 1, participants = 109; low‐certainty evidence; Analysis 2.1). Based on the control event risk of 400 per 1000 participants in the trial included in this analysis and at three years of follow‐up, this would result in 232 fewer recurrences (95% CI 308 fewer to 103 fewer) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision (outcome based on only a single study of a small number of participants).
2.1. Analysis.

Comparison 2: Gemcitabine versus mitomycin, Outcome 1: Time to recurrence
Time to progression
Gemcitabine may reduce the risk of progression over time compared to mitomycin (HR 0.57, 95% CI 0.32 to 1.01; studies = 1, participants = 109; low‐certainty evidence; Analysis 2.2), but the CI included the possibility of no effect. Based on the control event risk of 182 per 1000 participants in the trial included in this analysis and at three years of follow‐up, this corresponds to 74 fewer progressions (95% CI 120 fewer to 2 more) per 1000 participants. We downgraded the certainty of the evidence, due to study limitations and imprecision, the CI was also compatible with a small or no increase in the risk of progression.
2.2. Analysis.

Comparison 2: Gemcitabine versus mitomycin, Outcome 2: Time to progression
Grade III to V adverse events
We are very uncertain about the effect of gemcitabine on the CTCAE grade III to V adverse events compared to mitomycin (RR 0.51, 95% CI 0.13 to 1.93; studies = 1, participants = 109; very low‐certainty evidence; Analysis 2.3). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and very serious imprecision (downgraded two levels).
2.3. Analysis.

Comparison 2: Gemcitabine versus mitomycin, Outcome 3: Grade III–V adverse events
Secondary outcomes
Time to death from bladder cancer
We found no studies that reported the time to death from bladder cancer.
Time to death from any cause
We found no studies that reported time to death from any cause.
Grade I or II adverse events
Gemcitabine may reduce the CTCAE grade I or II adverse events compared to mitomycin (RR 0.53, 95% CI 0.37 to 0.78; studies = 1, participants = 109; low‐certainty evidence; Analysis 2.4). Based on the control event risk of 727 per 1000 participants in the trial included in this analysis and at three years of follow‐up, this corresponds to 342 fewer adverse events (95% CI 458 fewer to 160 fewer) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision (outcome based on only a single study of a small number of participants).
2.4. Analysis.

Comparison 2: Gemcitabine versus mitomycin, Outcome 4: Grade I or II adverse events
Disease‐specific quality of life
We found no studies that reported disease‐specific quality of life.
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
3. Gemcitabine versus BCG
One study compared gemcitabine versus BCG for primary high‐risk NMIBC (Porena 2010; summary of findings table provided as Table 7); we did not identify studies in participants with recurrent disease.
4. Gemcitabine compared to BCG.
| Patient or population: participants with non‐muscle invasive bladder cancer1 (54 men, 10 women) Country: Italy Setting: single center, likely inpatients Intervention: gemcitabine Comparison: BCG | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with BCG | Risk difference with Gemcitabine | ||||
|
Time to recurrence Follow‐up: mean 44 months MCID: 5% absolute difference |
64 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | HR 10.07 (4.48 to 22.63) | Study population | |
| 478 per 1000 | 521 more per 1000 (468 more to 522 more) | ||||
|
Time to progression Follow‐up: mean 44 months MCID: 5% absolute difference |
64 (1 RCT) | ⊕⊝⊝⊝ Verylowa,c | Not estimable | No events | No events |
| Grade III–V adverse events | Not reported | — | — | — | — |
| Time to death from bladder cancer | Not reported | — | — | — | — |
| Time to death from any cause | Not reported | — | — | — | — |
| Grade I or II adverse events | Not reported | — | — | — | — |
| Disease‐specific quality of life | Not reported | — | — | — | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; MCID: minimal clinically important difference; HR: hazard ratio; RCT: randomized controlled trial. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1The analysis was only based on participants with primary high‐risk non‐muscle invasive bladder cancer; the only included study did not include participants with recurrent disease.
aDowngraded one level for study limitations: unclear or high risk of bias on one or more domains.
bDowngraded one level for imprecision: outcome based on only a single study of a small number of participants.
cDowngraded two levels for imprecision: no events in either arm.
Primary outcomes
Time to recurrence
Gemcitabine may increase the risk of recurrence over time compared to BCG (HR 10.07, 95% CI 4.48 to 22.63; studies = 1, participants = 64; low‐certainty evidence; Analysis 3.1). Based on the control event risk of 478 per 1000 participants in the trial included in this analysis and at mean 44 months' follow‐up, this corresponds to 521 more recurrences (95% CI 468 more to 522 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision (outcome based on only a single study of a small number of participants).
3.1. Analysis.

Comparison 3: Gemcitabine versus Bacillus Calmette‐Guérin (BCG), Outcome 1: Time to recurrence
Time to progression
We are very uncertain about the effect of gemcitabine on the risk of progression over time compared to BCG (HR: not estimable, no events in either arm; studies = 1, participants = 64; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and very serious imprecision (downgraded two levels); there were no events in either arm.
3.2. Analysis.

Comparison 3: Gemcitabine versus Bacillus Calmette‐Guérin (BCG), Outcome 2: Time to progression
Grade III to V adverse events
Porena 2010 reported adverse events of gemcitabine and BCG, but we could not grade them in accordance with CTCAE. Therefore, we were unable to estimate this outcome.
Secondary outcomes
Time to death from bladder cancer
We found no studies that reported the time to death from bladder cancer.
Time to death from any cause
We found no studies that reported time to death from any cause.
Grade I or II adverse events
Porena 2010 reported adverse events of gemcitabine and BCG, but we could not grade them in accordance with CTCAE. Therefore, we were unable to estimate this outcome.
Disease‐specific quality of life
We found no studies that reported disease‐specific quality of life.
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
4. Gemcitabine versus BCG for recurrent (one‐course BCG failure) high‐risk NMIBC
One study compared gemcitabine versus BCG for recurrent high‐risk NMIBC (Di Lorenzo 2010; Table 3) in participants who had previously undergone one course of BCG treatment and recurred.
Primary outcomes
Time to recurrence
Gemcitabine may reduce the risk of recurrence over time compared to BCG (HR 0.15, 95% CI 0.09 to 0.26; studies = 1, participants = 80; low‐certainty evidence; Analysis 4.1). Based on the control event risk of 970 per 1000 participants in the trial included in this analysis and at six months' to 22 months' follow‐up, this corresponds to 561 fewer recurrences (95% CI 699 fewer to 372 fewer) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision (outcome based on only a single study of a small number of participants).
4.1. Analysis.

Comparison 4: Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer, Outcome 1: Time to recurrence
Time to progression
Gemcitabine may reduce the risk of progression over time compared to BCG (HR 0.45, 95% CI 0.27 to 0.76; studies = 1, participants = 80; low‐certainty evidence; Analysis 4.2). Based on the control event risk of 325 per 1000 participants in the trial included in this analysis and at six months' to 22 months' follow‐up, this corresponds to 163 fewer progressions (95% CI 224 fewer to 67 fewer) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision (outcome based on only a single study of a small number of participants).
4.2. Analysis.

Comparison 4: Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer, Outcome 2: Time to progression
Grade III to V adverse events
We are very uncertain about the effect of gemcitabine on the CTCAE grade III to V adverse events compared to BCG (RR 1.00, 95% CI 0.21 to 4.66; studies = 1, participants = 80; very low‐certainty evidence; Analysis 4.3). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and very serious imprecision (downgraded two levels), the CI was consistent both with an appreciable reduction as well as an appreciable increase in CTCAE grade III to V adverse events (i.e. wide CIs).
4.3. Analysis.

Comparison 4: Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer, Outcome 3: Grade III–V adverse events
Secondary outcomes
Time to death from bladder cancer
We are very uncertain about the effect of gemcitabine on the risk of death from bladder cancer over time compared to BCG (HR 0.04, 95% CI 0.01 to 2.25; studies = 1, participants = 80; very low‐certainty evidence; Analysis 4.4). Based on the control event risk of 17 per 1000 participants in the trial included in this analysis and at six months' to 22 months' follow‐up, this corresponds to 16 fewer deaths from bladder cancer (95% CI 17 fewer to 21 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations (downgraded one level) and very serious imprecision (downgraded two levels), the CI was consistent both with an appreciable reduction as well as an appreciable increase in the risk of time to death from bladder cancer (i.e. wide CIs).
4.4. Analysis.

Comparison 4: Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer, Outcome 4: Time to death from bladder cancer
Time to death from any cause
We found no studies that reported time to death from any cause.
Grade I or II adverse events
We are very uncertain about the effect of gemcitabine on the CTCAE grade I or II adverse events compared to BCG (RR 0.92, 95% CI 0.48 to 1.77; studies = 1 participants = 80; very low‐certainty evidence; Analysis 4.5). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and imprecision (downgraded two levels), the CI was consistent both with an appreciable reduction as well as an appreciable increase in CTCAE grade I or II adverse events (i.e. wide CIs).
4.5. Analysis.

Comparison 4: Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer, Outcome 5: Grade I or II adverse events
Disease‐specific quality of life
We found no studies that reported disease‐specific quality of life.
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
5. Gemcitabine versus one‐third dose BCG
One study compared gemcitabine versus one‐third dose BCG in participants with either primary and recurrent intermediate‐risk NMIBC (Gontero 2013; summary of findings table provided in Table 8).
5. Gemcitabine compared to one‐third dose BCG.
| Patient or population: participants with non‐muscle invasive bladder cancer (103 men, 17 women) Country: Italy, Germany, and the US Setting: multicenter (3 centers), likely inpatients Intervention: gemcitabine Comparison: 1/3 dose BCG | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with 1/3 dose BCG | Risk difference with gemcitabine | ||||
|
Time to recurrence Follow‐up: 1 year MCID: 5% absolute difference |
118 (1 RCT) | ⊕⊝⊝⊝ Verylowa,b | HR 1.17 (0.57 to 2.42) | Study population | |
| 237 per 1000 | 34 more per 1000 (94 fewer to 243 more) | ||||
|
Time to progression Follow‐up: 1 year MCID: 5% absolute difference |
118 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | HR 1.63 (0.39 to 6.83) | Study population | |
| 51 per 1000 | 31 more per 1000 (31 fewer to 250 more) | ||||
|
Grade III–V adverse events
assessed with: CTCAE version 3.0 Follow‐up: 1 year MCID: 5% absolute difference |
88 (1 RCT) | ⊕⊝⊝⊝ Verylowa,d | Not estimable | No events | No events |
| Time to death from bladder cancer | Not reported | — | — | — | — |
| Time to death from any cause | Not reported | — | — | — | — |
|
Grade I or II adverse events
assessed with: CTCAE version 3.0 Follow‐up: 1 year MCID: 5% absolute difference |
88 (1 RCT) | ⊕⊝⊝⊝ Verylowa,b | RR 0.84 (0.49 to 1.46) | Study population | |
| 404 per 1000 | 65 fewer per 1000 (206 fewer to 186 more) | ||||
|
Disease‐specific quality of life
assessed with: EORTC QLQ‐C30 (Global health status scale: higher score represents better functioning) Scale: 0–100 Follow‐up: 1 year MCID: 10e |
88 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — | The mean disease‐specific quality of life was 80.9 | MD 4.5 higher (1.6 lower to 10.6 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; CTCAE: Common Terminology Criteria for Adverse Events; EORTC QLQ‐C30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for study limitations: high risk of bias on one or more domains. bDowngraded two levels for imprecision: confidence intervals crossed a clinically important threshold and no effect; wide confidence intervals. cDowngraded one level for imprecision: confidence intervals crossed a clinically important threshold and no effect. dDowngraded two levels for imprecision: no events in either arm. eMCID: came from Osoba 1998.
Primary outcomes
Time to recurrence
We are very uncertain about the effect of gemcitabine on recurrence over time compared to one‐third dose BCG (HR 1.17, 95% CI 0.57 to 2.42; studies = 1, participants = 118; very low‐certainty evidence; Analysis 5.1). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and imprecision (downgraded two levels), the CI was consistent both with an appreciable reduction in the risk of recurrence over time as well as an appreciable increase in the risk of recurrence (i.e. wide CIs).
5.1. Analysis.

Comparison 5: Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG), Outcome 1: Time to recurrence
Time to progression
Gemcitabine may result in little to no difference in the risk of progression over time compared to one‐third dose BCG (HR 1.63, 95% CI 0.39 to 6.83; studies = 1, participants = 118; low‐certainty evidence; Analysis 5.2). Based on the control event risk of 51 per 1000 participants in the trial included in this analysis and at one year' follow‐up, this corresponds to 31 more progressions (95% CI 31 fewer to 250 more) per 1000 participants. We downgraded the certainty of the evidence due to study limitations and imprecision, the CI was consistent with an appreciable increase in the risk of progression.
5.2. Analysis.

Comparison 5: Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG), Outcome 2: Time to progression
Grade III to V adverse events
We are very uncertain about the effect of gemcitabine on the CTCAE grade III to V adverse events compared to one‐third dose BCG (RR: not estimable, no events in either arm; studies = 1, participants = 88; very low‐certainty evidence; Analysis 5.3). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and imprecision (downgraded two levels). There were no events in either arm.
5.3. Analysis.

Comparison 5: Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG), Outcome 3: Grade III–V adverse events
Secondary outcomes
Time to death from bladder cancer
We found no studies that reported time to death from bladder cancer.
Time to death from any cause
Gontero 2013 reported one death from any cause, but we could not extract HR since the study did not report which intervention the participant received (i.e. gemcitabine or one‐third BCG).
Grade I or II adverse events
We are very uncertain about the effect of gemcitabine on CTCAE grade I or II adverse events compared to one‐third dose BCG (RR 0.84, 95% CI 0.49 to 1.46; studies = 1, participants = 88; very low‐certainty evidence; Analysis 5.4). We downgraded the certainty of the evidence due to study limitations (downgraded one level) and imprecision (downgraded two levels); the CI was consistent both with an appreciable reduction as well as an appreciable increase in CTCAE grade I or II adverse events (i.e. wide CIs).
5.4. Analysis.

Comparison 5: Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG), Outcome 4: Grade I or II adverse events
Disease‐specific quality of life
Gemcitabine may result in similar disease‐specific quality of life compared to one‐third dose BCG (MD 4.50, 95% CI –1.60 to 10.60; studies = 1, participants = 88; low‐certainty evidence; Analysis 5.5). We downgraded the certainty of the evidence due to study limitations and imprecision, given the CI was also consistent with the improvement of quality of life.
5.5. Analysis.

Comparison 5: Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG), Outcome 5: Disease‐specific quality of life
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
Discussion
Summary of main results
This latest update of a prior Cochrane Review (Jones 2012) includes seven studies with 1222 randomized participants across five comparisons for evaluating the effect of gemcitabine compared to other agents in the NMIBC.
Gemcitabine versus saline
In people with primary and recurrent NMIBC, gemcitabine may reduce the risk of recurrence and may have a similar effect on the risk of progression over time compared to saline. We are very uncertain about the effects of gemcitabine on the risk of death from bladder cancer and death from any cause overtime. In terms of adverse events, gemcitabine may have similar CTCAE grade I to V adverse events compared to saline. Based on the preplanned subgroup analysis, we are uncertain about the effect of gemcitabine according to the tumor grade. This result should be interpreted with caution because the included study was not designed to assess subgroup effects.
Based on the predefined sensitivity analysis of the one study judged at low risk of bias (Messing 2018), findings were similar. We found no information about the disease‐specific quality of life.
Gemcitabine versus mitomycin
In people with recurrent NMIBC previously treated with BCG or epirubicin (one of the intravesical chemotherapeutic drug which is used in NMIBC), gemcitabine may reduce the risk of recurrence over time and may reduce the risk of progression over time. In terms of adverse events, gemcitabine may reduce CTCAE grade I or II adverse events compared to mitomycin, but we are very uncertain about the effect of gemcitabine on CTCAE grade III to V adverse events. We found no information about time to death from bladder cancer, time to death from any cause, and disease‐specific quality of life.
Gemcitabine versus BCG
In participants with primary high‐risk NMIBC, gemcitabine may increase the risk of recurrence over time, but we are very uncertain about the effect on the risk of progression compared to BCG. We found no information about other outcomes.
Gemcitabine versus BCG for recurrent high‐risk NMIBC after one‐course BCG failure
After one‐course BCG failure, gemcitabine may reduce the risk of recurrence and progression over time compared to BCG. However, we are very uncertain about the effect on grade I to V CTCAE adverse events and the risk of death from bladder cancer. We found no information about time to death from any cause and disease‐specific quality of life.
Gemcitabine versus one‐third dose BCG
In participants with primary and recurrent intermediate‐risk NMIBC, we are very uncertain about the effect of gemcitabine on the risk of recurrence and grade I to V CTCAE adverse events. Gemcitabine may have a similar effect on the risk of progression over time and disease‐specific quality of life compared to one‐third dose BCG. We found no information about time to death from bladder cancer and time to death from any cause.
Overall completeness and applicability of evidence
The studies included in this review may deserve the following consideration.
The findings of this review were based on fairly narrow evidence base on seven unique trials. Only one or two trials informed each of the five comparisons and all trials were conducted in Europe (four studies from Italy) or the US. Similar studies performed by other investigators in other countries would be valuable in validating these findings.
Although our review intended to evaluate the effect of intravesical gemcitabine in NMIBC, two studies included a small subset of patients who were ultimately found to have muscle‐invasive disease (Böhle 2009 7.7% and Messing 2018; 3.7%). Since we were unable to exclude this group of patients from the analysis. their contribution was small and this also resembles routine clinical practice we included these studies (available analysis data set) in our review and also did not downgrade for indirectness. .
There are multiple factors that affect the effect of intravesical instillation therapy such as the dose of anti‐tumor agents, dwell time of anti‐tumor agents, and instillation schedule. In addition, tumor factors such as the number of tumors, tumor size, stage, presence of CIS, and grade can influence the effectiveness of treatment. In this review, each study used a different dose and schedule, and some of the studies did not provide relevant information, thus we could not conduct preplanned subgroup analysis except for one comparison (Analysis 1.7).
There was moderate heterogeneity between the studies in terms of time to recurrence and time to progression (Analysis 1.1; Analysis 1.2). This probably results from different dwell times of gemcitabine and saline. However, despite the different dwell times of gemcitabine, the effect estimate favored gemcitabine, and we could apply this result to clinical practice.
Based on current evidence‐based guidelines (Babjuk 2019), after TUR of bladder tumor, people should undergo immediate postoperative instillation of mitomycin C followed by an induction course of anti‐tumor agents, namely BCG, with or without maintenance therapy according to their risk of recurrence. As none of the included studies used this comparison, which is considered the standard of care, these issues limit clinical applicability.
Our ability to assess the longer‐term outcomes of gemcitabine compared to other agents was limited given that the follow‐up duration in some of the studies was 12 weeks or less than two years (mean or median). The evidence to assess the efficacy and safety of gemcitabine over such a short term appears insufficient to provide assurance of long‐term outcomes.
The findings of this systematic review were limited to evidence from RCTs of low to very low certainty. The consideration of non‐randomized studies may have provided some evidence for additional outcomes such as adverse events (Schünemann 2013). Also, while we believe this to be unlikely, it is possible that they could have provided complementary results with higher certainty than those from RCTs.
Quality of the evidence
We judged the overall risk of bias of the included trials as unclear except for one trial (Messing 2018). Some studies raised concerns about performance and detection bias, and others have reporting and other biases (e.g. baseline imbalance and different treatment schedules). We judged the certainty of the evidence as low to very low for most outcomes due to these study limitations as well as wide CIs that crossed assumed clinically important thresholds resulting in the imprecision of the results. Moreover, several outcomes based on only a single study of a small number of participants.
Potential biases in the review process
Despite a comprehensive search strategy with no publication or language restrictions, we may have missed additional RCTs that may be unpublished or were published in languages other than English (or both). We translated all Chinese literature into English using Google translator; therefore, the lack of human double‐data abstraction may be considered a potential source of bias. The small number of studies included in this review was insufficient to generate funnel plots; therefore, the risk of publication bias may have been underestimated.
Böhle 2009 and Messing 2018 included some people with clinical stage T2 disease (i.e. muscle invasive bladder tumor). Since the T2 tumors are more aggressive than NMIBC, this could be a source of bias, possibly resulting in an underestimate of the effect size.
For the interpretation of clinically important effect sizes, we used absolute effect estimates that were informed by the input of expert clinicians on our team; unless there were publish thresholds (as was the case for quality‐of‐life instruments), we used 5% for the most patient‐important primary outcomes and 3% for the secondary outcomes of time to death from bladder cancer and time to death from any cause. We recognize that different thresholds might lead to different interpretations and have, therefore, made all our judgments as transparent as possible.
Agreements and disagreements with other studies or reviews
We found only two systematic reviews that investigated the effect of gemcitabine compared to BCG (Ye 2018) and mitomycin (Li 2020a). Ye 2018 included 365 participants from five trials, both randomized and non‐randomized, and concluded that intravesical gemcitabine may have a similar effect on the recurrence (RR 1.17, 95% CI 0.83 to 1.67), progression (RR 1.02, 95% CI 0.42 to 2.56), and any adverse events (RR 0.55, 95% CI 0.25 to 1.20) compared to BCG. However, this review did not consider clinical heterogeneity of included studies (i.e. meta‐analysis with regard to primary high‐risk and intermediate‐risk bladder cancer) and used RR for time to event outcomes, thereby questioning the appropriateness of pooling. Moreover, it provided no information of a priori registered protocol and risk of bias of included studies. Li 2020a reported that gemcitabine was more effective than mitomycin in terms of recurrence and adverse events. Although, the author explicitly mentioned that they included RCTs only, some studies were not RCTs. With regard to analysis, it had the same issues identified with Ye 2018. They did not consider clinical heterogeneity between included studies. Recently, two systematic reviews which included participants with NMIBC not responsive to intravesical BCG were published (Kamat 2020; Li 2020b). They included all studies regardless of the study design, however, they found no additional RCTs to the ones that we included. These two reviews can help the reader understand the current best body of evidence; however, our confidence must be very low about the results from study designs other than RCTs given the inherent study limitations of non‐randomized studies.
In addition, none of the existing systematic reviews (including the previous version of this Cochrane Review) provided a GRADE rating, which we consider critical to any systematic review (Jones 2012; Li 2020a; Ye 2018). This updated Cochrane Review used rigorous methodology, exhaustive literature search, and assessment of the certainty of the evidence using GRADE, thereby providing the most reliable evidence summary. Furthermore, our interpretation focused on clinically relevant (rather and statistically significant) findings and provided absolute effect size estimates for all dichotomous and time‐to‐event outcomes.
Authors' conclusions
Implications for practice.
Based on the findings of low‐ to very‐low certainty evidence, gemcitabine may reduce recurrence over time in primary and recurrent non‐muscle invasive bladder cancer (NMIBC) compared to saline and mitomycin. Gemcitabine may have similar Common Terminology Criteria for Adverse Events (CTCAE) grade III to V adverse events compared to saline. Gemcitabine may be inferior in terms of recurrence compared to BCG, but in people who have recurred after BCG treatment, it may be superior in terms of risk of recurrence and progression over time. Compared to one‐third dose BCG, we are very uncertain about the effect of gemcitabine on the recurrence and CTCAE grade III to V adverse events, but may have similar effects on the risk of progression.
Implications for research.
More adequately powered and high‐quality trials using the same treatment schedule between gemcitabine and other intravesical agent including one‐third dose of BCG (NCCN guideline 2019), reporting outcomes in subgroups according to the risk classification system, patient‐reported quality of life data and long‐term outcomes such as mortality would be useful. Moreover, in the era of BCG shortage, there is a need for trials of gemcitabine versus other novel intravesical agents (Sari 2020).
What's new
| Date | Event | Description |
|---|---|---|
| 18 April 2021 | New search has been performed | In this update, we added 2 new studies and excluded 1 study included in the previous review due to an unsuitable comparator. We applied current MECIR standards and GRADE to assess the certainty of the evidence. The conclusions of this review have changed. |
| 18 April 2021 | New citation required and conclusions have changed | In this update, we added 2 new studies and excluded 1 study included in the previous review due to an unsuitable comparator. We applied current MECIR standards and GRADE to assess the certainty of the evidence. The conclusions of this review have changed. |
History
Protocol first published: Issue 10, 2011 Review first published: Issue 1, 2012
Notes
We have based parts of the 'Methods' section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by Cochrane Urology.
Acknowledgements
We acknowledge the support received from the authors of included and excluded studies – Andreas Böhle, Riccardo Autorino, and Paolo Gontero – who provided additional, unpublished data.
Appendices
Appendix 1. Search strategies
| MEDLINE all segments (OvidSP) | |
| 1 | exp urinary bladder neoplasms/ |
| 2 | ((bladder* or urethra* or ureter* or urin* or urotheli*) adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or superficial or adenoma* or adenocarcinoma* or squamous* or malignan*)).tw. |
| 3 | exp carcinoma, transitional cell/ |
| 4 | (tcc or transitional cell).tw. |
| 5 | exp ureteral neoplasms/ |
| 6 | bladder neoplasms/ |
| 7 | urethral neoplasms/ |
| 8 | or/1‐7 |
| 9 | exp deoxycytidine/ |
| 10 | antimetabolites, antineoplastic/ |
| 11 | (gemc?tabin* or Gemzar*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] |
| 12 | (gem?cis or gem?cisplat or gem?carbo).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] |
| 13 | (gem adj (cis or cisplat or carbo)).mp. |
| 14 | 95058‐81‐4*.rn. |
| 15 | 103882‐84‐4*.rn. |
| 16 | B76N6SBZ8R.rn. |
| 17 | or/9‐16 |
| 18 | exp administration, intravesical/ |
| 19 | (intraves* or instill* or region* or install*).tw. |
| 20 | 18 or 19 |
| 21 | 8 and 17 and 20 |
| 22 | randomized controlled trial.pt. |
| 23 | controlled clinical trial.pt. |
| 24 | randomized.ab. |
| 25 | placebo.ab. |
| 26 | drug therapy.fs. |
| 27 | randomly.ab. |
| 28 | trial.ab. |
| 29 | groups.ab. |
| 30 | or/22‐29 |
| 31 | exp animals/ not humans.sh. |
| 32 | 30 not 31 |
| 33 | 21 and 32 |
| Embase (OvidSP) | |
| 1 | exp bladder tumor/ |
| 2 | ((bladder* or urethra* or ureter* or urin* or urotheli*) adj3 (cancer* or carcinoma* or neoplas* or tumo?r* or superficial or adenoma* or adenocarcinoma* or squamous* or malignan*)).tw. |
| 3 | exp transitional cell carcinoma/ |
| 4 | (tcc or transitional cell).tw. |
| 5 | exp ureter tumor/ |
| 6 | exp urethra tumor/ |
| 7 | or/1‐6 |
| 8 | exp deoxycytidine/ |
| 9 | exp antineoplastic antimetabolite/ |
| 10 | exp gemcitabine/ |
| 11 | (gemc?tabin* or Gemzar*).mp. |
| 12 | (gem?cis or gem?cisplat or gem?carbo).mp. |
| 13 | (gem adj (cis or cisplat or carbo)).mp. |
| 14 | 95058‐81‐4*.rn. |
| 15 | 103882‐84‐4*.rn. |
| 16 | B76N6SBZ8R.rn. |
| 17 | or/8‐16 |
| 18 | exp intravesical drug administration/ |
| 19 | (intraves* or instill* or region* or install*).tw. |
| 20 | 18 or 19 |
| 21 | 7 and 17 and 20 |
| 22 | crossover procedure/ |
| 23 | double blind procedure/ |
| 24 | randomized controlled trial/ |
| 25 | single blind procedure/ |
| 26 | (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).mp. |
| 27 | ((doubl* or singl*) adj blind*).mp. |
| 28 | or/22‐27 |
| 29 | 21 and 28 |
| The Cochrane Library (Wiley) | |
| #1 | MeSH descriptor: [Urinary Bladder Neoplasms] explode all trees |
| #2 | (bladder* or urethra* or ureter* or urin* or urotheli*) NEAR/3 (cancer* or carcinoma* or neoplas* or tumour* or tumor* or superficial or adenoma* or adenocarcinoma* or squamous* or malignan*):ti,kw,ab |
| #3 | MeSH descriptor: [Carcinoma, Transitional Cell] explode all trees |
| #4 | (tcc or transitional cell):ti,ab,kw |
| #5 | MeSH descriptor: [Ureteral Neoplasms] explode all trees |
| #6 | MeSH descriptor: [Urethral Neoplasms] explode all trees |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
| #8 | MeSH descriptor: [Deoxycytidine Kinase] explode all trees |
| #9 | MeSH descriptor: [Antimetabolites, Antineoplastic] explode all trees |
| #10 | (gemcitabin* or Gemzar*):ti,kw,ab |
| #11 | (gem NEAR (cis or cisplat or carbo)):ti,kw,ab |
| #12 | (gem?cis or gem?cisplat or gem?carbo):ti,kw,ab |
| #13 | (95058 81 4*) |
| #14 | (103882 84 4*) |
| #15 | B76N6SBZ8R |
| #16 | #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 |
| #17 | MeSH descriptor: [Administration, Intravesical] explode all trees |
| #18 | (intraves* or instill* or region* or install*):ti,kw,ab |
| #19 | #17 OR #18 |
| #20 | #7 AND #16 AND #19 |
| Web of Science Core Collection (Thomson Reuters) | |
| #1 | TS=((bladder* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan*))) |
| #2 | TS=((urethral NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan*))) |
| #3 | TS=((ureteral NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan*))) |
| #4 | TS=((urin* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan*))) |
| #5 | TS=((urotheli* NEAR/3 (cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan*))) |
| #6 | TS=(tcc OR transitional cell) |
| #7 | #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
| #8 | TS=(deoxycytidine) |
| #9 | TS=(gemc?tabin* OR gemzar*) |
| #10 | TS=(gemcis*) |
| #11 | TS=(gemcarbo*) |
| #12 | #11 OR #10 OR #9 OR #8 |
| #13 | TS=(intraves* OR instill* OR region* OR install*) |
| #14 | #13 AND #12 AND #7 |
| #15 | TS=(Clinical trial*) |
| #16 | TS=(research design*) |
| #17 | TS=(comparative stud*) |
| #18 | TS=(evaluation stud*) |
| #19 | TS=(controlled trial*) |
| #20 | TS=(follow up stud*) |
| #21 | TS=(prospective stud*) |
| #22 | TS=(random*) |
| #23 | TS=(placebo*) |
| #24 | TS=(single blind*) |
| #25 | TS=(double blind*) |
| #26 | #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 |
| #27 | #26 AND #14 |
| LILACS (Virtual Health Library) | |
| 1 | ((bladder$ or bexiga or vejiga or ureter$ or urethr$ or "transitional cell" or "célula de transición" or "célula transicional")) and ((gemcitabin$ or gemzar or gemcis or gemcisplat or gemcarbo)) |
| Scopus (Elsevier) | |
| 1 | ( ( ( TITLE‐ABS‐KEY ( ( bladder W/3 ( cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan* ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( ureteral* W/3 ( cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan* ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( urethral* W/3 ( cancer* OR carcinoma* OR neoplas* OR tumo?r* OR superficial OR adenoma* OR adenocarcinoma* OR squamous* OR malignan* ) ) ) ) OR ( TITLE‐ABS‐KEY ( ( tcc OR "transitional cell" ) ) ) ) AND ( ( TITLE‐ABS‐KEY ( ( gemc?tabin* OR gemzar OR gemcis OR gemcisplat OR gemcarbo ) ) ) OR ( CASREGNUMBER ( ( 95058‐81‐4* ) ) ) OR ( CASREGNUMBER ( ( 103882‐84‐4* ) ) ) OR ( CASREGNUMBER ( ( b76n6sbz8r ) ) ) ) AND ( TITLE‐ABS‐KEY ( ( intraves* OR instill* OR region* OR install* ) ) ) ) AND ( ( TITLE‐ABS‐KEY ( "clinical trial*" ) ) OR ( TITLE‐ABS‐KEY ( "research design*" ) ) OR ( TITLE‐ABS‐KEY ( "comparative stud*" ) ) OR ( TITLE‐ABS‐KEY ( "evaluation stud*" ) ) OR ( TITLE‐ABS‐KEY ( "controlled trial*" ) ) OR ( TITLE‐ABS‐KEY ( "follow up stud*" ) ) OR ( TITLE‐ABS‐KEY ( "prospective stud*" ) ) OR ( TITLE‐ABS‐KEY ( random* ) ) OR ( TITLE‐ABS‐KEY ( placebo* ) ) OR ( TITLE‐ABS‐KEY ( "single blind*" ) ) OR ( TITLE‐ABS‐KEY ( "double blind*" ) ) ) |
| OpenGrey (Native Interface) | |
| 1 | Bladder AND Gemcitabin* |
| ClinicalTrials.gov (US National Institute of Health) | |
| 1 |
Condition: Bladder Other Terms: Gemcitabine and Intravesical. |
| WHO International Clinical Trials Registry Search Portal (World Health Organization) | |
| 1 | Bladder AND Gemcitabin* AND Intraves* |
Appendix 2. Survey of study investigators providing information on included and excluded studies
| Study | Date study author contacted (first) | Date study author provided data (latest) | Data study author provided (short summary) |
| Böhle 2009 | 24 February 2019 | 26 February 2019 | Randomization and allocation concealment method |
| Cao 2011 | 12 February 2019 | 22 February 2019 | Study design |
| Di Lorenzo 2010 | 2 March 2019 | 2 March 2019 | Protocol existence or not |
| Gontero 2013 | 24 February 2019 | 1 March 2019 | Randomization and allocation concealment method |
Data and analyses
Comparison 1. Gemcitabine versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to recurrence | 2 | 734 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.54, 1.09] |
| 1.2 Time to progression | 2 | 654 | Hazard Ratio (IV, Random, 95% CI) | 0.96 [0.19, 4.71] |
| 1.3 Grade III–V adverse events | 2 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.58, 2.75] |
| 1.4 Time to death from bladder cancer | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Time to death from any cause | 2 | 734 | Hazard Ratio (IV, Random, 95% CI) | 0.62 [0.39, 1.00] |
| 1.6 Grade I or II adverse events | 2 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.45] |
| 1.7 Time to recurrence (subgroup analysis) | 2 | 543 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.49, 1.09] |
| 1.7.1 Low‐grade tumor | 2 | 430 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.38, 1.46] |
| 1.7.2 High‐grade tumor | 2 | 113 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.43, 1.28] |
Comparison 2. Gemcitabine versus mitomycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Time to recurrence | 1 | 109 | Hazard Ratio (IV, Random, 95% CI) | 0.36 [0.19, 0.69] |
| 2.2 Time to progression | 1 | 109 | Hazard Ratio (IV, Random, 95% CI) | 0.57 [0.32, 1.01] |
| 2.3 Grade III–V adverse events | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.13, 1.93] |
| 2.4 Grade I or II adverse events | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.37, 0.78] |
Comparison 3. Gemcitabine versus Bacillus Calmette‐Guérin (BCG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Time to recurrence | 1 | 64 | Hazard Ratio (IV, Random, 95% CI) | 10.07 [4.48, 22.63] |
| 3.2 Time to progression | 1 | 64 | Hazard Ratio (IV, Random, 95% CI) | Not estimable |
Comparison 4. Gemcitabine versus Bacillus Calmette‐Guérin [BCG] for recurrent (one‐course BCG failure) high‐risk non‐muscle invasive bladder cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Time to recurrence | 1 | 80 | Hazard Ratio (IV, Random, 95% CI) | 0.15 [0.09, 0.26] |
| 4.2 Time to progression | 1 | 80 | Hazard Ratio (IV, Random, 95% CI) | 0.45 [0.27, 0.76] |
| 4.3 Grade III–V adverse events | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.66] |
| 4.4 Time to death from bladder cancer | 1 | 80 | Hazard Ratio (IV, Random, 95% CI) | 0.04 [0.00, 2.25] |
| 4.5 Grade I or II adverse events | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.48, 1.77] |
Comparison 5. Gemcitabine versus one‐third dose Bacillus Calmette‐Guérin (BCG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Time to recurrence | 1 | 118 | Hazard Ratio (IV, Random, 95% CI) | 1.17 [0.57, 2.42] |
| 5.2 Time to progression | 1 | 118 | Hazard Ratio (IV, Random, 95% CI) | 1.63 [0.39, 6.83] |
| 5.3 Grade III–V adverse events | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.4 Grade I or II adverse events | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.46] |
| 5.5 Disease‐specific quality of life | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐1.60, 10.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Addeo 2010.
| Study characteristics | ||
| Methods |
Study design: randomized phase III trial (1:1) Setting/country: single center in Italy Dates when study was conducted: March 2003 to November 2005 |
|
| Participants |
Ethnicity: NA Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: recurrent disease Intervention: gemcitabine
Comparator: mitomycin C
|
|
| Interventions |
Intervention: gemcitabine
Comparator: mitomycin C
Follow‐up: median 36 months |
|
| Outcomes | Study did not separate the outcomes as primary and secondary Main endpoints
Subgroup analysis
|
|
| Funding Sources | Supported by grants from Lega Italiana per la Lotta contro i Tumori (LILT; Italian League for the Fight against Cancer; non‐profit organization and has oncologic prevention as its primary institutional task): organization information is available at www.lilt.it | |
| Declarations of interest | None | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Random" only. Comment: randomization stated, but no information on method used; therefore, unclear risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information given. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no information given. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: study did not investigate this outcome, but reported several progressions from all randomized participants in each arm. We approximated the effect estimates (HR) using an indirect method (Parmar 1998). Thus, low risk of bias. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available, but all objectives defined in the method section were reported. |
| Other bias | High risk |
Comment: treatment schedule differed between groups; mitomycin C was given within 2 days of TURBT followed by 4 weekly treatment, but gemcitabine was given as a 6‐week induction course. If the schedule of mitomycin C was considered as standard treatment at that time, should we rate this domain as high. |
Bendary 2011.
| Study characteristics | ||
| Methods |
Study design: randomized trial Setting/country: single center in Egypt Dates when study was conducted: January 2006 to June 2008 |
|
| Participants |
Ethnicity: NA Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: primary disease Intervention: gemcitabine
Comparator: BCG
|
|
| Interventions |
Intervention: gemcitabine
Comparator: BCG
Follow‐up: 3–18 months (mean 10.8 (SD 27) months) |
|
| Outcomes | Study did not separate the outcomes as primary and secondary. Efficacy
Safety
Subgroup analysis: NA |
|
| Funding Sources | None | |
| Declarations of interest | NA | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Random" only; abstract only. Comment: randomization stated, but no information on method used; therefore, unclear risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given; abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information given; abstract only. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no information given; abstract only. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no information given; abstract only, but objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to recurrence | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Time to progression | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Time to death from bladder cancer | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Unclear risk | Comment: no information given; abstract only. |
| Incomplete outcome data (attrition bias) Disease‐specific quality of life | Unclear risk | Comment: no information given; abstract only. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information given; abstract only. |
| Other bias | Unclear risk | Comment: no information given; abstract only |
Böhle 2009.
| Study characteristics | ||
| Methods |
Study design: randomized, double‐blind, placebo‐controlled multicenter study Setting/country: multicenter (24 centers) in Germany and Turkey Dates when study was conducted: January 2004 to June 2005 |
|
| Participants |
Ethnicity: white Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: primary and recurrent disease Intervention: gemcitabine
Comparator: saline
|
|
| Interventions |
Intervention: gemcitabine
Comparator: saline
Follow‐up: median 23.6 months (range 0–46) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Subgroup analysis; recurrence‐free survival only
|
|
| Funding Sources | The study was funded and sponsored by Eli Lilly and Company, Indianapolis, IN, USA. The sponsor assisted in the design and conduct of the study; contributed to the management, analysis, interpretation, preparation, and review of the data; and approved the manuscript. | |
| Declarations of interest | H Buttner, K Helsberg, B Lubben, V Soldatenkova, and C Stoffregen are employees of Lilly Deutschland GmbH, the German affiliate of Eli Lilly and Company, Indianapolis, IN, USA. H Buttner, K Helsberg, B Lubben, and C Stoffregen also own Eli Lilly stocks or stock options (or both). A Böhle has received honoraria for lectures from Cytochemia, Apogepha, Bard, and Hexal. | |
| Notes |
Protocol: NCT00191477 Language of publication: English Data extraction and risk of bias assessment were performed using the NCT00191477 result section (gemcitabine: n = 166, saline: n = 162 including pT2, CIS, no malignancy, pathologic specimen lost participants included). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Random" only. Comment: randomization stated and we received the author response "computer generate random number"; therefore, low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Comment: we received the author response "central randomisation"; therefore, low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote from publication: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)." Comment: participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" Comment: outcome assessor was blinded; therefore, low risk of detection bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: outcome assessor was blinded; therefore, low risk of detection bias. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: 166/179 (92.7%) in gemcitabine arm and 162/176 (92.1%) in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered this a low risk of attrition bias. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: the study did not investigate this outcome, but reported several progressions from per‐protocol participants in each arm (124/179 [69.2%]). Attrition rates were balanced in each arm. We approximated the effect estimates (HR) using an indirect method (Parmar 1998). Thus, low risk of attrition bias. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Low risk | Comment: 166/179 (92.7%) in gemcitabine arm and 162/176 (92.1%) in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered this a low risk of attrition bias. |
| Incomplete outcome data (attrition bias) Time to death from bladder cancer | Low risk | Comment: the study did not investigate this outcome, but reported several progressions from 166/179 (92.7%) in gemcitabine arm and 162/176 (92.1%) in saline arm. We approximated the effect estimates (HR) using an indirect method (Parmar 1998). Thus, low risk of incomplete outcome bias. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Low risk | Comment: the study did not investigate this outcome, but reported several progressions from 166/179 (92.7%) in gemcitabine arm and 162/176 (92.1%) in saline arm. We approximated the effect estimates (HR) using an indirect method (Parmar 1998). Thus, low risk of incomplete outcome bias. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Low risk | Comment: 166/179 (92.7%) in gemcitabine arm and 162/176 (92.1%) in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered this a low risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | Comment: the protocol (NCT00191477) was provided, but was changed during the study period due to lower events rates. All predefined outcomes were provided, but the results of primary and secondary outcomes were different between protocol and report. Owing to lower events rates (recurrence), recurrence‐free survival was calculated in subgroups as a post hoc analysis. |
| Other bias | High risk |
Quote from publication: "Concomitant BCG was used in 34 (13.7%) of efficacy eligible patients (GEM [gemcitabine]: 10.5%; PBO [placebo]: 16.9%); 14 patients (5.6%; GEM: n = 5; PBO: n = 9) received more than six BCG." Comment: possible differences in concomitant treatment instillations. Baseline imbalance: age (recalculated using MedCalc Statistical Software). |
Di Lorenzo 2010.
| Study characteristics | ||
| Methods |
Study design: multicenter, prospective, randomized, phase 2 trial Setting/country: multicenter in Italy Dates when study was conducted: June 2006 to May 2008 |
|
| Participants |
Ethnicity: NA Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: recurrent (1‐course BCG failure) high‐risk disease Intervention: gemcitabine
Comparator: BCG
|
|
| Interventions |
Intervention: gemcitabine
Comparator: BCG
Follow‐up: gemcitabine: median 15.2 months (range 6–22); BCG: median 15.8 months (range 7–21) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Subgroup analysis: none |
|
| Funding Sources | NA | |
| Declarations of interest | None | |
| Notes |
Protocol: NA Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "By using a central computer‐generated randomisation list." Comment: we considered this method of random sequence generation to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "By using a central computer‐generated randomisation list." Comment: central randomization; this method may ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: "An open label study design was used, that is, patients and investigators were not masked as to the drugs they were assigned." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote from publication: "An open label study design was used, that is, patients and investigators were not masked as to the drugs they were assigned." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: the study did not investigate this outcome, but reported several progressions from all randomized participants. We approximated the effect estimates (HR) using an indirect method (Parmar 1998). Thus, low risk of attrition bias. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Time to death from bladder cancer | Low risk | Comment: the study did not investigate this outcome, but reported several bladder cancer death from all randomized participants. We approximated the effect estimates (HR) using an indirect method (Tierney 2007). Thus, low risk of attrition bias. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol was not published (author response); time to first recurrence was not defined in the method section. |
| Other bias | High risk | Comment: treatment schedule differed between groups; gemcitabine was given twice weekly for 6 weeks, then weekly for 3 consecutive weeks at 3, 6, and 12 months; BCG was given weekly for 6 weeks, then 3 weekly instillations at 3, 6, 9, and 12 months. |
Gontero 2013.
| Study characteristics | ||
| Methods |
Study design: multicenter, prospective, randomized, phase II study Setting/country: multicenter (3 centers) in Italy, Germany, and the US Dates when study was conducted: 2006–2010 |
|
| Participants |
Ethnicity: NA Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: primary and recurrent intermediate‐risk disease Intervention: gemcitabine
Comparator: 1/3 dose BCG
|
|
| Interventions |
Intervention: gemcitabine
Comparator: 1/3 dose BCG
Follow‐up: 1 year |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Subgroup analysis: none |
|
| Funding Sources | NA | |
| Declarations of interest | NA | |
| Notes |
Protocol: NCT01697306 Language of publication: English Baseline characteristics were based on eligible participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Simple 1:1 randomisation was used." Comment: randomization stated and we received the author response "computer generate random number;" therefore, low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | Comment: we received the author response "central randomisation"; therefore, low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: in the protocol "None (open lable)" [sic: open label]. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote from publication: in the protocol "None (open lable)" [sic: open label]. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | High risk | Comment: 41/59 (69.4%) participants in gemcitabine arm and 47/59 (79.6%) participants in 1/3 dose BCG arm were included in the analysis. The reasons for attrition were reported, but attrition rates were not balanced. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Unclear risk |
Quote from publication: "At 1‐year follow up only 1 patient died of a noncancer specific cause." Comment: author reported 1 participant died due to non‐cancer cause, but they did not report the denominator. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | High risk | Comment: 41/59 (69.4%) participants in gemcitabine arm and 47/59 (79.6%) participants in 1/3 dose BCG arm were included in the analysis. The reasons for attrition were reported, but attrition rates were not balanced. |
| Incomplete outcome data (attrition bias) Disease‐specific quality of life | High risk | Comment: 41/59 (69.4%) participants in gemcitabine arm and 47/59 (79.6%) participants in 1/3 dose BCG arm were included in the analysis. The reasons for attrition were reported, but attrition rates were not balanced. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was provided (NCT01697306), but toxicity was not predefined. However, this is unlikely to introduce a bias. |
| Other bias | Low risk | Comment: treatment schedule differed between groups, but it was the same as protocol. |
Messing 2018.
| Study characteristics | ||
| Methods |
Study design: randomized double‐blind clinical trial Setting/country: multicenter (23 centers) in the US Dates when study was conducted: January 2008 to August 2012 |
|
| Participants |
Ethnicity: white: 371; black: 15; Asian: 9; American Indian: 2; unknown: 9 Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: primary and recurrent disease Intervention: gemcitabine
Comparator: saline
|
|
| Interventions |
Intervention: gemcitabine
Comparator: saline
Follow‐up: median 4 years |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Subgroup analysis: time to recurrence only
|
|
| Funding Sources | National Cancer Institute of the National Institutes of Health under award numbers CA180888, CA180819, CA180846, CA180834, CA180858, CA128567, CA180801, CA189953, CA189854, CA180830, CA180818, CA22433, CA35995, CA12644, CA68183, CA11083, CA46282, CA58416, CA46113, CA37981, and CA04919 and in part by Eli Lilly (which provided gemcitabine for the study). Role of the funders/sponsors: the National Cancer Institute approved the study design and through its grants to Southwest Oncology Group, supported the conduct of the study and collection, management, analysis, and interpretation of the data, and, through the genitourinary committee of Southwest Oncology Group, supported the preparation, review, and approval of the manuscript and the decision to submit the manuscript for publication. Eli Lilly had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or decision to submit the manuscript for publication. | |
| Declarations of interest | Dr Lerner reported receipt of personal fees for advisory board membership from BioCancell, Incyte, Nucleix, and UroGen and for consultancy from Vaxiion and clinical trial grants from UroGen, Endo, FKD, Viventia, JBL, and Roche/Genentech. Dr Koppie reported receipt of personal fees from Convergent Genomics. Dr Karsh reported receipt of personal fees for consulting or speaking or research funding to his institution (or a combination) from Astellas, Bayer, Janssen, Sanofi, Spectrum, Precision Biopsy, 3D Biopsy, Augmenix, Dendreon, Pfizer, Abbvie, Myriad Genetics, AstraZeneca, Vaxiion, and Arivan Research. No other disclosures were reported. | |
| Notes |
Protocol: NCT00445601; supplement in the original report Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: in the protocol, "Patients will be centrally randomized at the Southwest Oncology Group Statistical Center. At the time of registration, patients will be randomly assigned to either Arm 1 or Arm 2 in a blinded fashion according to a dynamic allocation scheme." Comment: we considered this method of random sequence generation to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: in the protocol, "Phone or Web registration." Comment: this method may ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote from publication: in the protocol, "The investigator, treating urologist and patient will be blinded to treatment, but the local institutional pharmacist will not." Comment: lack of blinding of the pharmacist may not introduce a bias; therefore, low risk of performance bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: in the protocol, "The investigator, treating urologist and patient will be blinded to treatment, but the local institutional pharmacist will not." Comment: lack of blinding of the pharmacist is unlikely to introduce a bias; therefore, low risk of performance bias. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: outcome assessor was blinded; therefore, low risk of detection bias. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: 201/207 (97.1%) participants in gemcitabine arm and 205/209 (98%) participants in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered risk of attrition bias to be low. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: 201/207 (97.1%) participants in gemcitabine arm and 205/209 (98%) participants in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered risk of attrition bias to be low. |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Low risk | Comment: 165/207 (79.7%) participants in gemcitabine arm and 175/209 (83.7%) participants in saline arm were included in the analysis, but attrition rates were balanced and the reasons for attrition were reported. We considered risk of attrition bias to be low. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Low risk | Comment: 201/207 (97.1%) participants in gemcitabine arm and 205/209 (98%) participants in saline arm were included in the analysis. Owing to the small number of participants excluded from the analysis and balanced in both groups, we considered risk of attrition bias to be low. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Low risk | Comment: 165/207 (79.7%) participants in gemcitabine arm and 175/209 (83.7%) participants in saline arm were included in the analysis, but attrition rates were balanced and the reasons for attrition were reported. We considered risk of attrition bias to be low. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol (NCT00445601 and supplement in the original report) was provided and all predefined analyses were reported. Post hoc analysis for 1 subgroup outcome (time to recurrence in participants with high‐grade disease) was provided, but this was unlikely to introduce bias. |
| Other bias | Low risk |
Quote from publication: "Although subsequent treatment was evenly distributed between groups but not reliably collected which might affect the tumor progression. No central pathology review." Comment: this may affect the effect estimates of progression. However, given time to progression is a secondary outcome, this is unlikely to affect the whole study results. |
Porena 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Setting/country: single center in Italy Dates when study was conducted: January 2004 to December 2006 |
|
| Participants |
Ethnicity: NA Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned
Disease type: primary high‐risk disease Intervention: gemcitabine
Comparator: BCG
|
|
| Interventions |
Intervention: gemcitabine
Comparator: BCG
Follow‐up: mean 44 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Subgroup analysis: none |
|
| Funding Sources | NA | |
| Declarations of interest | NA | |
| Notes |
Protocol: NCT00696579 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "the randomisation code was developed using a computer random number generator to select random permuted blocks." Comment: we considered this method of random sequence generation to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: "All study personnel and participants were not blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote from publication: "All study personnel and participants were not blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: objective outcomes not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to recurrence | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Time to progression | Low risk | Comment: no loss to follow‐up; all randomized participants were included in the analysis (no progression in both arms). |
| Incomplete outcome data (attrition bias) Grade III–V adverse events | Low risk | Comment: all randomized participants were included in the analysis, but no explicit description of grade III–V complications. |
| Incomplete outcome data (attrition bias) Grade I or II adverse events | Low risk | Comment: all randomized participants were included in the analysis, but no explicit description of grade I or II complications. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol (NCT00696579) and all predefined outcomes were provided. |
| Other bias | Low risk | Comment: not detected. |
BCG: Bacillus Calmette‐Guérin; CFU: colony‐forming units; CIS: carcinoma in situ; EAU: European Association of Urology; EORTC QLQ‐C30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EORTC QLQ‐BLS 24: European Organization for the Research and Treatment of Cancer Quality of Life Superficial Bladder Cancer‐Specific 24; G: tumor grade; HR: hazard ratio; IQR: interquartile range; NMIBC: non‐muscle invasive bladder cancer; NA: not available; SD: standard deviation; TCC: transitional cell carcinoma; TUR: transurethral resection; TURBT: transurethral resection of the bladder tumor; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Böhle 2010 | Commentary. |
| Brausi 2011 | Ineligible study design; no comparator. |
| Cao 2011 | Ineligible comparator; different drugs were combined as a comparator. |
| Dalbagni 2006 | Ineligible study design; no comparator. |
| Dong 2017 | Ineligible study design; not an RCT (quasi‐randomization). |
| Gantz 2018 | Ineligible study design; not an RCT. |
| Gardmark 2005 | Ineligible comparator; different dose and schedule of gemcitabine used as a comparator. |
| Lin 2016 | Ineligible study design; not an RCT. |
| Sun 2016 | Ineligible study design; not an RCT. |
| Xia 2019 | Ineligible study design; not an RCT (quasi‐randomization). |
RCT: randomized controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Xiaohong 2015.
| Methods | No information |
| Participants | 56 participants (men: 45; women: 11) |
| Interventions |
Intervention
Comparator
|
| Outcomes | No information |
| Notes | We could not obtain the full text of this study. Information about participants and interventions came from Li 2020a. |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900026643.
| Study name | The randomized controlled study for intravesical instillation of Pseudomonas aeruginosa and gemcitabine in the prevention of postoperative recurrence of non‐muscle invasive bladder cancer |
| Methods | Open‐label parallel randomized study |
| Participants |
Estimated enrollment: 80 participants Eligible ages: ages > 18 and < 75 years Eligible sexes: both Eligibility criteria
|
| Interventions |
Intervention
Comparator
|
| Outcomes | Postoperative recurrence rate |
| Starting date | 20 November 2019 Expected date of completion: 20 November 2021 |
| Contact information | Applicant: Li Jiashuo; tel: +8615779750929; email: 505684933@qq.com Study leader: Shang Panfeng; tel: +8613919785295; email: shangpf@lzu.edu.cn |
| Notes |
Funding source: subproject of the key research and development project in Gansu Province. Quote: "Regularization and diagnosis of cystatin cancer and promotion and application of different urinary diversion procedures." Sponsors and collaborators: Lanzhou University Second Hospital |
NCT00192049.
| Study name | A randomized study comparing single agent gemcitabine intravesical therapy versus mitomycin C in patients with intermediate risk superficial bladder cancer |
| Methods | Open‐label, parallel, randomized study |
| Participants |
Estimated enrollment: 90 participants Eligible ages: > 18 Eligible sexes: both Eligibility criteria
|
| Interventions |
Intervention
Comparator
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | December 2003 Expected date of completion: no date given |
| Contact information | Tel: +1‐877‐285‐4559 or +1‐317‐615‐4559 |
| Notes |
Funding source: not reported but probably Eli Lilly and Company Sponsors and Collaborators: Eli Lilly and Company |
NCT02695771.
| Study name | The bladder instillation comparison study (BIC) |
| Methods | Open‐label, parallel, randomized study |
| Participants |
Estimated enrollment: 300 participants Eligible ages: > 18 years Eligible sexes: both Eligibility criteria
|
| Interventions |
Intervention
Comparator
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | April 2016 Expected date of completion: April 2019 |
| Contact information | Contact: Susan M Engerman, BSN; tel: +1 616‐267‐8406; email: susan.engerman@spectrumhealth.org Contact: Pamela Carlon, RN; tel: +1 616‐267‐8406; email: pamela.carlon@spectrumhealth.org |
| Notes |
Funding source: not reported Sponsors and Collaborators: Spectrum Health Hospitals |
NCT04172675.
| Study name | A study of erdafitinib versus investigator choice of intravesical chemotherapy in participants who received Bacillus Calmette‐Guérin (BCG) and recurred with high risk non‐muscle‐invasive bladder cancer (NMIBC) |
| Methods | Open‐label, parallel, randomized study |
| Participants |
Estimated enrollment: 280 participants Eligible ages: > 18 years Eligible sexes: both Eligibility criteria
|
| Interventions |
Intervention: cohort 1, 2, and 3 will receive erdafitinib; orally beginning on cycle 1 day 1 until 2 years of treatment have been completed, disease recurrence, intolerable toxicity, withdrawal of consent, a decision by the investigator to discontinue treatment, or study termination, whichever occurs first. Each cycle is 28 days.
Comparator: cohort 1 will receive investigators choice of intravesical chemotherapy; gemcitabine or mitomycin C
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | February 28, 2020 Expected date of completion: 10 June 2026 |
| Contact information | Tel: +1 844‐434‐4210; email: JNJ.CT@sylogent.com |
| Notes |
Funding source: Janssen Research & Development, LLC Sponsors and Collaborators: Janssen Research & Development, LLC |
BCG: Bacillus Calmette‐Guérin; CIS: carcinoma in situ; CT: computer tomography; CTCAE: Common Terminology Criteria for Adverse Events; ECOG: Eastern Cooperative Oncology Group; G: tumor grade; NCI: National Cancer Institute; NMIBC: non‐muscle invasive bladder cancer; TUR: transurethral resection; TURBT: transurethral resection of the bladder tumor.
Differences between protocol and review
This review was based on a published protocol (Jones 2011), and was an update of a Cochrane Review first published in 2012 (Jones 2012). Major differences between the previous review and the update include the following.
We did not include Gardmark 2005 because this study compared different doses and schedules of gemcitabine.
Types of outcome measures: we renamed primary and secondary outcomes and added details in 'Method and timing of outcome measurement' for all outcomes.
We applied the GRADE approach and the Cochrane 'Risk of bias' assessment tool to assess the certainty of the evidence.
We abstracted data from included studies and re‐analyzed them in accordance with primary and secondary outcomes.
We included two new trials (Gontero 2013; Messing 2018), which allowed one comparison to include a meta‐analysis.
Although our review intended to evaluate the effect of intravesical gemcitabine in NMIBC, since Böhle 2009 (7.7%) and Messing 2018 (3.7%) included a muscle‐invasive bladder cancer in the analysis, we used this full analysis set (available analysis) in our review.
The comparisons follow the framework of the prior protocol with the exception of the analysis that gemcitabine versus BCG in patients who were previously treated with BCG since the trial participants in the control arm can be expected to have a lesser BCG response (Chang 2016) since their inclusion into an overall analysis not only contradicts clinical practice but also would have bias the results in favor of the gemcitabine arm.
Contributions of authors
MAH: drafted the review and provided GRADE methodologic input to the review.
PM: performed data abstraction and risk of bias assessments.
JHJ: provided clinical and methodologic input to the protocol and the review.
JEH: provided critical content expertise input to the review.
VN: provided critical content expertise input to the protocol and the review.
AC: created search strategies and executed the searches.
ECH: conceived, designed, and wrote the protocol and performed all aspects of data abstraction, analysis, risk of bias assessment, and certainty of evidence ratings.
PD: conceived, designed, and wrote the protocol; reviewed critical content; and gave final approval.
Sources of support
Internal sources
-
Eu Chang Hwang, Korea, South
Chonnam National University Hwasun Hospital, Hwasun, Korea, South Salary support for Eu Chang Hwang
-
Philipp Dahm, USA
Department of Urology, University of Minnesota, Minneapolis, MN, USA Salary support for PD
External sources
-
No external support received, Korea, South
None
Declarations of interest
MAH: none.
PM: none.
JHJ: none.
JEH: none.
VN: none.
AC: none.
ECH: none.
PD: serves as Co‐ordinating Editor of Cochrane Urology. However, he was not involved in the editorial processing or decision‐making for this review. Other editors of Cochrane Urology managed the editorial process, including final sign‐off for this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Addeo 2010 {published data only}
- Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. Journal of Clinical Oncology 2010;28(4):543-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Faiola V, Montella L, Addeo R, Bellini S, Miragliulo A, Cennamo G, et al. Intravesical gemcitabine versus mitomycin for recurrent superficial bladder tumours (stages pTa and pT1): a randomized prospective study. European Journal of Cancer Supplements 2008;6(14):124. [DOI: 10.1016/j.ejcsup.2008.06.049] [DOI] [Google Scholar]
- Montella L, Addeo R, Bellini S, Faiola V, Tarantino L, Pizza C, et al. Intravesical gemcitabine versus mitomycin for recurrent superficial bladder tumors (stages pTa and pT1): a randomized prospective study. Journal of Clinical Oncology 2008;26(15 Suppl):5075. [DOI: 10.1200/jco.2008.26.15_suppl.5075] [DOI] [Google Scholar]
Bendary 2011 {published data only}
- Bendary L, Khalil S, Shahin A, Nawar N. Intravesical gemcitabine versus Bacillus Calmette-Guerin (BCG) in treatment of non-muscle invasive bladder cancer: short term comparative study. Journal of Urology 2011;185(4s):e664-5. [DOI: 10.1016/j.juro.2011.02.1765] [DOI] [Google Scholar]
Böhle 2009 {published and unpublished data}
- Böhle A, Helsberg K, Soldatenkova V, Lubben B, Stoffregen C, Buttner H, S274 Study Group. Single postoperative instillation of gemcitabine in patients with superficial transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase 3 multicenter study. Journal of Urology 2009;181(4s):689. [DOI: 10.1016/S0022-5347(09)61930-3] [DOI] [PubMed] [Google Scholar]
- Böhle A, Leyh H, Frei C, Kühn M, Tschada R, Pottek T, et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. European Urology 2009;56(3):495-503. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00191477. Instillation of gemcitabine in patients with superficial bladder cancer. clinicaltrials.gov/ct2/show/NCT00191477 (first received 19 September 2005). [NCT00191477]
Di Lorenzo 2010 {published data only}
- Autorino R, Cantiello F, Damiano R, De Sio M, Di Lorenzo G, Rubino D, et al. Gemcitabine versus BCG after initial BCG failure in non muscle-invasive bladder cancer: a prospective randomized trial. European Urology Supplements 2009;8(4):283. [DOI: 10.1016/S1569-9056(09)60644-8] [DOI] [Google Scholar]
- Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus Bacille Calmette-Guerin after initial Bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010;116(8):1893-900. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gontero 2013 {published data only}
- Gontero P, Gurioli A, Mehnert A, Schmid M, Kluth L, Pappagallo G, et al. Is there a difference in quality of life between BCG and intravesical chemotherapy? Results of a randomized phase II study. European Urology Supplements 2012;11(1):e966-6a. [DOI: 10.1016/S1569-9056(12)60963-4] [DOI] [Google Scholar]
- Gontero P, Gurioli A, Oderda M, Mehnert A, Rink M, Schmid M, et al. Is there a difference in quality of life between BCG and intravesical chemotherapy? Results of a randomized phase II study. Journal of Urology 2012;187(4s):e218. [DOI: 10.1016/j.juro.2012.02.604] [DOI] [Google Scholar]
- Gontero P, Oderda M, Gurioli A, Marson F, Mehnert A, Rink M, et al. Is there a difference in quality of life between intravesical chemotherapy and BCG? Results of a randomized phase II trial. Anticancer Research 2012;32(5):1908-9. [Google Scholar]
- Gontero P, Oderda M, Mehnert A, Gurioli A, Marson F, Lucca I, et al. The impact of intravesical gemcitabine and 1/3 dose Bacillus Calmette-Guérin instillation therapy on the quality of life in patients with non-muscle invasive bladder cancer: results of a prospective, randomized, phase II trial. Journal of Urology 2013;190(3):857-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01697306. The impact of intravesical gemcitabine and 1/3 dose Bacillus Calmette-Guerin on the quality of life in superficial bladder cancer. clinicaltrials.gov/ct2/show/study/NCT01697306 (first received 2 October 2012). [NCT01697306]
Messing 2018 {published data only}
- Messing E, Tangen C, Lerner S, Sahasrabudhe D, Koppie T, Wood D, et al. A phase III blinded study of immediate post-TURBT instillation of gemcitabine versus saline in patients with newly diagnosed or occasionally recurring grade I/II non-muscle invasive bladder cancer: SWOG S0337. Journal of Urology 2017;197(4s):e914-5. [DOI: 10.1016/j.juro.2017.03.036] [DOI] [Google Scholar]
- Messing EM, Tangen CM, Lerner SP, Sahasrabudhe DM, Koppie TM, Wood DP Jr, et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA 2018;319(18):1880-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00445601. S0337, Gemcitabine after surgery in treating patients with newly diagnosed or recurrent bladder cancer. clinicaltrials.gov/ct2/show/study/NCT00445601 (first received 9 March 2007). [NCT00445601]
Porena 2010 {published data only}
- NCT00696579. Bacillus of calmette and guerin (BCG) versus gemcitabine for intravesical therapy in high risk superficial bladder cancer. clinicaltrials.gov/ct2/show/study/NCT00696579 (first received 12 June 2008). [NCT00696579]
- Porena M, Del Zingaro M, Lazzeri M, Mearini L, Giannantoni A, Bini V, et al. Bacillus Calmette-Guerin versus gemcitabine for intravesical therapy in high-risk superficial bladder cancer: a randomised prospective study. Urologia Internationalis 2010;84(1):23-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Böhle 2010 {published data only}
- Böhle A, Leyh H, Frei C, Kuhn M, Tschada R, Pottek T, et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. Journal of Urology 2010;184(3):897-8. [DOI: 10.1016/j.juro.2010.05.060] [DOI] [PubMed] [Google Scholar]
Brausi 2011 {published data only}
- Brausi MA, Gontero P, Altieri V, Colombo R, Conti I, Bono AV. Can gemcitabine instillation ablate solitary low-risk non-muscle-invasive bladder cancer? Results of a phase II marker lesion study. Urologia Internationalis 2011;87(4):470-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cao 2011 {published data only}
- Cao M, Ma CK, Ma J, Chen HG, Xue W. Evaluation of the efficacy and safety of intravesical instillation with gemcitabine after first-line intravesical chemotherapy failure in the treatment of non-muscle-invasive bladder cancer [吉西他滨膀胱灌注治疗复发性浅表性膀胱肿瘤的安全性与有效性]. Zhonghua Zhong Liu za Zhi [Chinese Journal of Oncology] 2011;33(5):385-7. [PMID: ] [PubMed] [Google Scholar]
Dalbagni 2006 {published data only}
- Dalbagni G, Russo P, Bochner B, Ben-Porat L, Sheinfeld J, Sogani P, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. Journal of Clinical Oncology 2006;24(18):2729-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dong 2017 {published data only}
- Dong X, Song L, Zhang F. Curative effect analysis of different chemotherapeutics at intravesical instillation in the prevention of tumor recurrence after TUR-Bt [TUR-Bt术后不同化疗药物膀胱灌注预防肿瘤复发的效果分析]. Zhejiang Journal of Traumatic Surgery 2017;1:109-10. [caod.oriprobe.com/articles/50688689/tur_bt_shu_hou_bu_tong_hua_liao_yao_wu_bang_zuo_gu.htm] [Google Scholar]
Gantz 2018 {published data only}
- Gantz J, Noyes E, Tangen C, Lerner S, Thompson I, Madeb R, et al. Immediate post TURB intravesical gemcitabine: a cost comparison based on SWOG S0337. Journal of Urology 2018;199(4s):e1230. [DOI: 10.1016/j.juro.2018.02.2997] [DOI] [Google Scholar]
Gardmark 2005 {published data only}
- Gardmark T, Carringer M, Beckman E, Malmstrom P U. Randomized phase II marker lesion study evaluating effect of scheduling on response to intravesical gemcitabine in recurrent stage Ta urothelial cell carcinoma of the bladder. Urology 2005;66(3):527-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin T, Sun L. Comparison of the efficacy of different chemotherapy drugs in the prevention of postoperative recurrence in superficial bladder cancer by intravesical instillation. China Foreign Medical Treatment 2016;34:118-9. [caod.oriprobe.com/articles/50430850/Different_Intravesical_Chemotherapy_Drugs_Prevention_Efficacy_of_Super.htm] [Google Scholar]
Sun 2016 {published data only}
- Sun S, Liu R, Zhao H, Wang B, Liu Y, Wen K. Comparison of intravesical perfusion of gemcitabine and mitomycin after non-muscle invasive bladder cancer [非肌层浸润性膀胱癌电切术后吉西他滨与丝裂霉素膀胱灌注疗效对比]. Journal of Medical Theory and Practice 2016;29:3238-9. [caod.oriprobe.com/articles/50444480/fei_ji_ceng_jin_run_xing_bang_zuo_ai_dian_qie_shu_hou_ji_xi_ta_bin_yu_.htm] [Google Scholar]
Xia 2019 {published data only}
- Xia H, Liang C, Yang C, Chen J. Effect of gemcitabine intravesical instillation in patients with bladder cancer and its influence on survival [吉西他滨膀胱灌注在膀胱癌患者中的疗效观察及对生存期的影响研究]. Anti-Tumor Pharmacy 2019;9(5):789-92. [DOI: 10.3969/j.issn.2095-1264.2019.05.18] [DOI] [Google Scholar]
References to studies awaiting assessment
Xiaohong 2015 {published data only}
- Xiaohong Z, Tan J, Wei Y, et al. Clinical effect of antineoplastic drug perfusion in preventing postoperative recurrence of superficial bladder cancer. J Chin Physician 2015;17:1043-5. [Google Scholar]
References to ongoing studies
ChiCTR1900026643 {unpublished data only}
- ChiCTR1900026643. The randomized controlled study for intravesical instillation of Pseudomonas aeruginosa and gemcitabine in the prevention of postoperative recurrence of non-muscle invasive bladder cancer [铜绿假单胞菌和吉西他滨膀胱灌注预防非肌层浸润性膀胱癌术后复发的随机对照研究]. chictr.org.cn/showproj.aspx?proj=44307 (first received 17 October 2019). [ChiCTR1900026643]
NCT00192049 {unpublished data only}
- NCT00192049. A randomized study comparing single agent gemcitabine intravesical therapy versus mitomycin C in patients with intermediate risk superficial bladder cancer. clinicaltrials.gov/ct2/show/NCT00192049 (first received 19 September 2005). [NCT00192049]
NCT02695771 {unpublished data only}
- NCT02695771. The bladder instillation comparison study (BIC). clinicaltrials.gov/ct2/show/NCT02695771 (first received 1 March 2016). [NCT02695771]
NCT04172675 {unpublished data only}
- NCT04172675. A study of erdafitinib versus investigator choice of intravesical chemotherapy in participants who received Bacillus Calmette-Guérin (BCG) and recurred with high risk non-muscle-invasive bladder cancer (NMIBC). clinicaltrials.gov/ct2/show/NCT04172675 (first received 20 November 2019). [NCT04172675]
- Steinberg GD, Palou-Redorta J, Gschwend JE, Tran B, Loriot Y, Daneshmand S, et al. A randomized phase II study of erdafitinib (ERDA) versus intravesical chemotherapy (IC) in patients with high-risk nonmuscle invasive bladder cancer (HR-NMIBC) with FGFR mutations or fusions, who recurred after Bacillus Calmette-Guerin (BCG) therapy. Journal of Clinical Oncology 2020;38(6 Suppl):TPS603. [DOI: 10.1200/JCO.2020.38.6_suppl.TPS603] [DOI] [Google Scholar]
Additional references
Babjuk 2019
- Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 update. European Urology 2019;76(5):639-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 2018;68(6):394-424. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burger 2008
- Burger M, Aa MN, Oers JM, Brinkmann A, Kwast TH, Steyerberg EC, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. European Urology 2008;54(4):835-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chang 2016
- Chang S S, Boorjian S A, Chou R, Clark P E, Daneshmand S, Konety B R, Pruthi R, Quale D Z, Ritch C R, Seigne J D, Skinner E C, Smith N D, McKiernan J M. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196(4):1021-9. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 18 January 2019. Melbourne, Australia: Veritas Health Innovation, 2017. Available at www.covidence.org.
Cozzi 1999
- Cozzi PJ, Bajorin DF, Tong W, Nguyen H, Scott J, Heston WD, et al. Toxicology and pharmacokinetics of intravesical gemcitabine: a preclinical study in dogs. Clinical Cancer Research 1999;5(9):2629-37. [PMID: ] [PubMed] [Google Scholar]
CTCAE
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed 17 January 2019).
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
DeGeorge 2017
- DeGeorge KC, Holt HR, Hodges SC. Bladder cancer: diagnosis and treatment. American Family Physician 2017;96(8):507-14. [PMID: ] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote. Version 7.5. Boston (MA): Clarivate Analytics, 2016. Available at: www.endnote.com/.
Epstein 1998
- Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. American Journal of Surgical Pathology 1998;22(12):1435-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffin 2013
- Griffin JG, Holzbeierlein J. Side effects of perioperative intravesical treatment and treatment strategies for these side effects. Urologic Clinics of North America 2013;40(2):197-210. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hansel 2013
- Hansel DE, Amin MB, Comperat E, Cote RJ, Knuchel R, Montironi R, et al. A contemporary update on pathology standards for bladder cancer: transurethral resection and radical cystectomy specimens. European Urology 2013;63(2):321-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Helenius 2015
- Helenius M, Brekkan E, Dahlman P, Lönnemark M, Magnusson A. Bladder cancer detection in patients with gross haematuria: computed tomography urography with enhancement-triggered scan versus flexible cystoscopy. Scandinavian Journal of Urology 2015;49(5):377-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG, Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017a
- Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2017b
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 2016
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs – Part B: prostate and bladder tumours. European Urology 2016;70(1):106-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kamat 2016
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. Bladder cancer. Lancet 2016;388(10061):2796-810. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kamat 2020
- Kamat AM, Lerner SP, O'Donnell M, Georgieva MV, Yang M, Inman BA, et al. Evidence-based assessment of current and emerging bladder-sparing therapies for non-muscle-invasive bladder cancer after Bacillus Calmette-Guerin therapy: a systematic review and meta analysis. European Urology Oncology 2020;3(3):318-40. [DOI] [PubMed] [Google Scholar]
Kilani 2002
- Kilani RT, Tamimi Y, Karmali S, Mackey J, Hanel EG, Wong KK, et al. Selective cytotoxicity of gemcitabine in bladder cancer cell lines. Anticancer Drugs 2002;13(6):557-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lamm 2014
- Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al. Defining progression in non-muscle invasive bladder cancer: it is time for a new, standard definition. Journal of Urology 2014;191(1):20-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laufer 2003
- Laufer M, Ramalingam S, Schoenberg MP, Haisfield-Wolf ME, Zuhowski EG, Trueheart IN, et al. Intravesical gemcitabine therapy for superficial transitional cell carcinoma of the bladder: a phase I and pharmacokinetic study. Journal of Clinical Oncology 2003;21(4):697-703. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2020a
- Li R, Li Y, Song J, Gao K, Chen K, Yang X, et al. Intravesical gemcitabine versus mitomycin for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized controlled trial. BMC Urology 2020;20(1):97. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020b
- Li R, Sundi D, Zhang J, Kim Y, Sylvester RJ, Spiess PE, et al. Systematic review of the therapeutic efficacy of bladder-preserving treatments for non-muscle-invasive bladder cancer following intravesical Bacillus Calmette-Guérin. European Urology 2020;78(3):387-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maffezzini 2009
- Maffezzini M, Campodonico F, Puntoni M, Martelli A, Mattioli F. Systemic absorption and pharmacokinetics of single-dose intravesical gemcitabine after transurethral resection of the bladder in non-muscle-invasive bladder cancer. Urology 2009;74(5):1078-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
MedCalc Statistical Software [Computer program]
- MedCalc Statistical Software. Version 18.11.3. Ostend, Belgium: MedCalc Software bvba, accessed 20 February 2019. Available at www.medcalc.org.
Mini 2006
- Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Annals of Oncology 2006;17 Suppl 5:v7-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCCN guideline 2019
- National Comprehensive Cancer Network. Bladder cancer, NCCN clinical practice guidelines in oncology. www.nccn.org/professionals/physician_gls/default.aspx#site (accessed 3 May 2019).
Osoba 1998
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. Journal of Clinical Oncology 1998;16(1):139-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perlis 2013
- Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. European Urology 2013;64(3):421-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peyton 2019
- Peyton CC, Chipollini J, Azizi M, Kamat AM, Gilbert SM, Spiess PE. Updates on the use of intravesical therapies for non-muscle invasive bladder cancer: how, when and what. World Journal of Urology 2019;37(10):2017-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sari 2020
- Sari Motlagh R, Pradere B, Mori K, Miura N, Abufaraj M, Shariat SF. Bladder-preserving strategies for Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer; where are we and what will be expected? Current Opinion in Urology 2020;30(4):584-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s),Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Siegel 2020
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a Cancer Journal for Clinicians 2020;70(1):7-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sternberg 2000
- Sternberg CN. Gemcitabine in bladder cancer. Seminars in Oncology 2000;27(1 Suppl 2):31-9. [PMID: ] [PubMed] [Google Scholar]
Sylvester 2006
- Sylvester RJ, Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49(3):466-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2018
- Ye Z, Chen J, Hong Y, Xin W, Yang S, Rao Y. The efficacy and safety of intravesical gemcitabine vs Bacille Calmette-Guerin for adjuvant treatment of non-muscle invasive bladder cancer: a meta-analysis. OncoTargets and Therapy 2018;11:4641-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2011
- Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non‐muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD009294. [DOI: 10.1002/14651858.CD009294] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD009294. [DOI: 10.1002/14651858.CD009294.pub2] [DOI] [PubMed] [Google Scholar]


