Child linear growth impairment, particularly stunting, remains a global health challenge. Stunting is defined as a height-for-age Z-score more than two SDs below the WHO child growth standards reference median for age and sex. The number of children younger than 5 years who are stunted was 59 million (34%) in south Asia and 58 million (33%) in sub-Saharan Africa in 2018.1 Stunting is associated with poor child development, lower productivity and earnings in adulthood, and increased risk of chronic diseases later in life.2,3 In this Comment, we argue that air pollution has been largely ignored as a potentially important cause of stunting, we outline a conceptual framework for how air pollution might lead to impaired linear growth in children, and we call for additional research into these mechanisms.
Child linear growth is a complex, multifactorial process, with the highest risk of growth impairment occurring between conception and 2 years of age. Stunting can begin in utero, caused by intrauterine growth retardation, or the programming of later growth, or both.2,4 Postnatally, immediate causes of impaired growth include inadequate dietary intake and recurrent infection. Underlying causes of impaired growth include inadequate access to food, inadequate care for women and children, and unhealthy environments.5 Interventions to address specific causes have had small but consistent effects on linear growth, and more comprehensive efforts have led to much larger improvements at the population level.6,7 Given that stunting is a marker of complex systemic effects, improvements in both immediate and underlying causes are necessary to dramatically reduce its prevalence.
Poor water, sanitation, and hygiene conditions are thought to be a major cause of stunting, through repeated episodes of diarrhoea and environmental enteric dysfunction.8 However, recent, large randomised controlled trials found that water, sanitation, and hygiene interventions failed to improve child linear growth.9–11 While these results raise questions about the effectiveness of water, sanitation, and hygiene approaches generally, they highlight the need for a broader view of environmental factors that might affect child linear growth, and the complex ways in which these factors might operate, interact, and synergise.12
The potential relationship between air pollution and child linear growth has received little attention, compared with the effect of water, sanitation, and hygiene on child linear growth. Air pollution includes both ambient (outdoor) and household (indoor) air pollution. Ambient air pollution sources include agricultural and industrial processes and vehicle fuel combustion. Household air pollution is linked primarily to cooking with solid biomass fuels (eg, wood, charcoal, and dung), which are used by approximately 3 billion people globally.13 Damaging agents in air pollution include solid particles, carbon monoxide, and nitrogen oxides. Exposure is widespread; WHO reports that 98% of children younger than 5 years in low-income and middle-income countries are exposed to air pollution above the recommended concentrations.13
To date, evidence for a link between air pollution and linear growth has come from retrospective and observational studies. These studies focused mainly on prenatal exposure to ambient air pollution and adverse birth outcomes such as low birthweight and preterm birth. In 2017, a systematic review and meta-analysis showed that increased prenatal exposure (per IQR increment) to ambient air pollution, measured as fine particulate matter (PM2·5), slightly increased the risk of preterm birth (odds ratio [OR] 1·03 [95% CI 1·01–1·05]) and low birthweight at term (1·03 [1·02–1·03]).14 Low birthweight at term is a proxy for intrauterine growth restriction that is also independently associated with stunting.4 The evidence was judged to be of good quality, though the data were all from observational studies and therefore had inherent limitations.
Few studies have addressed links between air pollution and postnatal growth. A systematic review and meta-analysis of household air pollution and child survival identified four studies that reported stunting as an outcome.15 Three of these studies were secondary analyses of national survey data.15 The meta-analysis identified associations (based on adjusted estimates) between exposure to household air pollution (defined as use of solid fuel for cooking) and both moderate stunting (OR 1·27 [95% CI 1·12 to 1·43]) and severe stunting (1·55 [1·04 to 2·30]) but found the quality of the evidence to be low to very low.15 A later analysis of national data from India found that exposure to 100 μg/m³ of PM2·5 in the month of birth was inversely associated (ie, height-for-age Z-scores were decreased by 0·05 for every 100 μg/m³ increase in PM2·5) with child height-for-age Z-score at the time of the survey (change in height-for-age Z-score −0·05 [95% CI −0·01 to −0·09]).16 The authors of the analysis note that these effect sizes might be underestimated because of potential residual confounding and measurement error.16
The figure presents a conceptual framework for air pollution as a contributor to stunting. Studies investigating air pollution and intrauterine growth impairment focus primarily on effects at the cellular level, particularly on the interlinked processes of oxidative stress and epigenetics. Exposure to air pollution during pregnancy can induce reactive oxygen species, leading to mitochondrial dysfunction and reduced telomere length, inflammation, and potentially poor fetal growth.17 Reactive oxygen species are also known to affect the epigenetic state of the cell; reduced DNA methylation might be one of several epigenetic mechanisms through which air pollution leads to poor fetal growth.18 Observational studies suggest that exposure to nitrogen oxides and particulate matter in utero might modulate DNA methylation, potentially affecting fetal growth, although detailed mechanisms remain unclear.19,20 The placenta might mediate these effects through its barrier, transport, and signalling functions.21
Beginning in utero, and continuing throughout early life, poor nutrition and environmental exposure to air pollution and pathogens can adversely affect immune ontogeny.22 Defects in innate and adaptive immune function might then contribute to inter-relationships between air pollution, infection, and undernutrition, resulting in a cycle of recurrent illness and malnutrition.23,24 Specifically, air pollution might impair linear growth through repeated episodes of febrile respiratory illness, which are associated with increased risk of child stunting.25 It is likely that this association is because of immune activity leading to increased metabolic requirements, anorexia and reduced dietary intake, increased catabolism, and altered metabolism of key nutrients, such as retinol and iron, which are redirected as part of the body’s defence mechanisms.25,26 This combination can lead to a nutrient imbalance, and hence to impaired growth. Interactions between respiratory and enteric infections might be an additional factor, leading to comorbidity with acute lower respiratory tract infection and diarrhoea.27 Another indirect pathway is possible, in which households allocate incomes away from food and nutrition, and instead towards health-care expenses related to infections, leading to inadequate diets for children and impaired linear growth.
In addition to clinical infection, subclinical biological mechanisms might be similar to those thought to result from poor water, sanitation, and hygiene, in which repeated insults from enteric pathogens affect gut barrier function and trigger chronic immune activation, local and systemic inflammation, and growth hormone resistance.8,28 Repeated exposure to air pollution might affect lung structure and function, triggering a similar biological response to the effect of enteric pathogens on the gut. Intervention studies of air pollution and inflammatory biomarkers in children are rare, but observational evidence exists of chronic systemic inflammation among schoolchildren (mean age 7 years) exposed to high concentrations of ambient air pollution.29 Proinflammatory cytokines can directly regulate growth via the growth plates through local regulation of chondrocytes.26 In combination with endocrine and nutritional factors, proinflammatory cytokines can also systemically regulate growth by suppressing insulin-like growth factor 1, which mediates the effect of growth hormone on longitudinal bone growth.26
Both prenatally and postnatally, air pollution might lead to vitamin D deficiency through several pathways, with implications for immune function and bone metabolism.30 Although evidence is scarce, studies from France and India observed inverse associations between ambient air pollution (measured as PM10, nitrogen dioxide, and haze scores) and vitamin D concentrations in neonates and children aged 9–24 months.31,32 Inadequate vitamin D concentrations have been associated with increased risk of respiratory infection in young children.33 Vitamin D also plays an important role in regulating bone metabolism and growth.34
More research is needed to explore these relationships. Much of the research done so far has been observational and focused on high-income countries, rather than on low-income and middle-income countries where exposures are generally higher. One option is for researchers to use natural experiments, in which specific events might result in an acute reduction or increase in air pollution.35 However, these observational studies are likely to miss important explanatory mechanisms related to socioeconomic factors, lifestyle factors, and genetics.18 High quality randomised controlled trials are needed in low-income and middle-income countries to examine the relationships between air pollution and child linear growth, and to understand the biological mechanisms underlying the effect. Possible interventions to reduce indoor air pollution include cleaner energy sources for cooking, heating, and lighting, and improved ventilation. Strategies to reduce ambient air pollution include measures to limit crop burning, refuse burning, and emissions from vehicle exhausts and industry.
Increased attention is urgently needed to define the effect of air pollution on child linear growth during the prenatal period and early years of life. An improved understanding of these relationships is necessary for the development of new intervention strategies, which would contribute to a comprehensive approach that addresses multiple causal factors, for the prevention of stunting. Extending research efforts to include outcomes associated with stunting, such as poor child development, could have even greater implications than a focus on stunting alone. The potential to improve child linear growth and child development could provide a persuasive new argument for addressing air pollution and improving human health globally.
Figure:
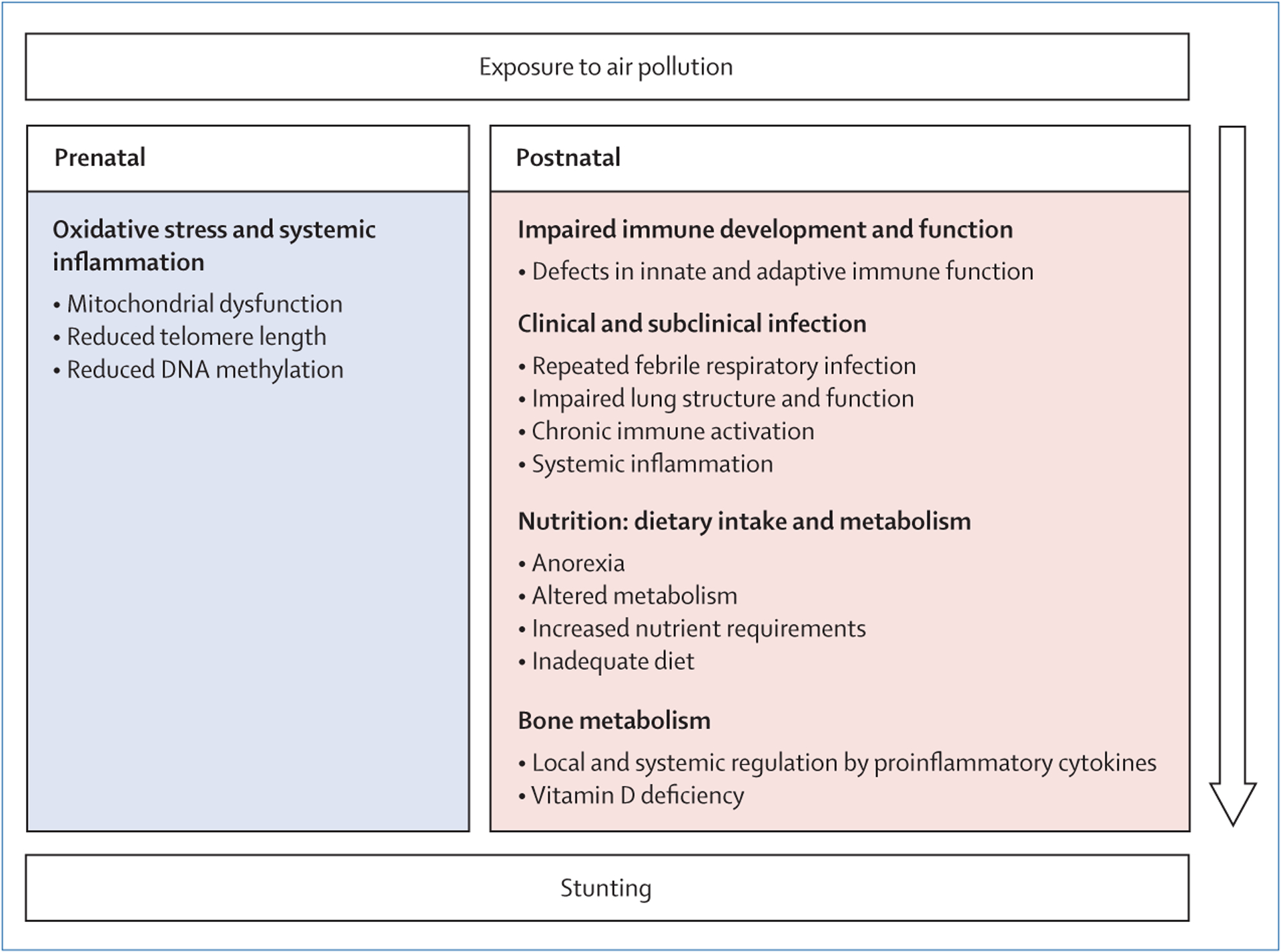
Conceptual framework of pathways from exposure to air pollution to child stunting
Footnotes
We declare no competing interests.
References
- 1.UNICEF, WHO, World Bank Group. Levels and trends in child malnutrition: joint child malnutrition estimates. Key findings of the 2019 edition. Geneva: World Health Organization, 2019. [Google Scholar]
- 2.de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr 2016; 12 (suppl 1): 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoddinott J, Behrman JR, Maluccio JA, et al. Adult consequences of growth failure in early childhood. Am J Clin Nutr 2013; 98: 1170–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol 2013; 42: 1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNICEF. Strategy for improved nutrition of children and women in developing countries. Indian J Pediatr 1991; 58: 13–24. [DOI] [PubMed] [Google Scholar]
- 6.Victora CG, Aquino EM, do Carmo Leal M, Monteiro CA, Barros FC, Szwarcwald CL. Maternal and child health in Brazil: progress and challenges. Lancet 2011; 377: 1863–76. [DOI] [PubMed] [Google Scholar]
- 7.Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013; 382: 452–77. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009; 374: 1032–35. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey JH, Mbuya MNN, Ntozini R, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health 2019; 7: e132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luby SP, Rahman M, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 2018; 6: e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Null C, Stewart CP, Pickering AJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 2018; 6: e316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cumming O, Arnold BF, Ban R, et al. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 2019; 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Air pollution and child health: prescribing clean air. Geneva: World Health Organization, 2018. [Google Scholar]
- 14.Li X, Huang S, Jiao A, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut 2017; 227: 596–605. [DOI] [PubMed] [Google Scholar]
- 15.Bruce NG, Dherani MK, Das JK, et al. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health 2013; 13 (suppl 3): S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spears D, Dey S, Chowdhury S, Scovronick N, Vyas S, Apte J. The association of early-life exposure to ambient PM2.5 and later-childhood height-for-age in India: an observational study. Environ Health 2019; 18: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iodice S, Hoxha M, Ferrari L, et al. Particulate air pollution, blood mitochondrial DNA copy number, and telomere length in mothers in the first trimester of pregnancy: effects on fetal growth. Oxid Med Cell Longev 2018; 2018: 5162905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burris HH, Baccarelli AA. Air pollution and in utero programming of poor fetal growth. Epigenomics 2017; 9: 213–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rider CF, Carlsten C. Air pollution and DNA methylation: effects of exposure in humans. Clin Epigenetics 2019; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruzieva O, Xu CJ, Yousefi P, et al. Prenatal particulate air pollution and DNA methylation in newborns: an epigenome-wide meta-analysis. Environ Health Perspect 2019; 127: 57012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luyten LJ, Saenen ND, Janssen BG, et al. Air pollution and the fetal origin of disease: A systematic review of the molecular signatures of air pollution exposure in human placenta. Environ Res 2018; 166: 310–23. [DOI] [PubMed] [Google Scholar]
- 22.Goenka A, Kollmann TR. Development of immunity in early life. J Infect 2015; 71 (suppl 1): S112–20. [DOI] [PubMed] [Google Scholar]
- 23.Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 2016; 37: 386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourke CD, Jones KDJ, Prendergast AJ. Current understanding of innate immune cell dysfunction in childhood undernutrition. Front Immunol 2019; 10: 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewey KG, Mayers DR. Early child growth: how do nutrition and infection interact? Matern Child Nutr 2011; 7 (suppl 3): 129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sederquist B, Fernandez-Vojvodich P, Zaman F, Sävendahl L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. J Mol Endocrinol 2014; 53: T35–44. [DOI] [PubMed] [Google Scholar]
- 27.Walker CL, Perin J, Katz J, Tielsch JM, Black RE. Diarrhea as a risk factor for acute lower respiratory tract infections among young children in low income settings. J Glob Health 2013; 3: 010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey JH, Jones AD, Manges A, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial: rationale, design, and methods. Clin Infect Dis 2015; 61 (suppl 7): S685–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn 2011; 77: 345–55. [DOI] [PubMed] [Google Scholar]
- 30.Mousavi SE, Amini H, Heydarpour P, Amini Chermahini F, Godderis L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: evidence and potential mechanisms. Environ Int 2019; 122: 67–90. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child 2002; 87: 111–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baïz N, Dargent-Molina P, Wark JD, Souberbielle JC, Slama R, Annesi-Maesano I. Gestational exposure to urban air pollution related to a decrease in cord blood vitamin D levels. J Clin Endocrinol Metab 2012; 97: 4087–95. [DOI] [PubMed] [Google Scholar]
- 33.Yakoob MY, Salam RA, Khan FR, Bhutta ZA. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst Rev 2016; 11: CD008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bikle DD. Vitamin D and bone. Curr Osteoporos Rep 2012; 10: 151–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich DQ. Accountability studies of air pollution and health effects: lessons learned and recommendations for future natural experiment opportunities. Environ Int 2017; 100: 62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


