Abstract
Objectives
To obtain timely and detailed data on COVID-19 cases in the United States, the Centers for Disease Control and Prevention (CDC) uses 2 data sources: (1) aggregate counts for daily situational awareness and (2) person-level data for each case (case surveillance). The objective of this study was to describe the sensitivity of case ascertainment and the completeness of person-level data received by CDC through national COVID-19 case surveillance.
Methods
We compared case and death counts from case surveillance data with aggregate counts received by CDC during April 5–September 30, 2020. We analyzed case surveillance data to describe geographic and temporal trends in data completeness for selected variables, including demographic characteristics, underlying medical conditions, and outcomes.
Results
As of November 18, 2020, national COVID-19 case surveillance data received by CDC during April 5–September 30, 2020, included 4 990 629 cases and 141 935 deaths, representing 72.7% of the volume of cases (n = 6 863 251) and 71.8% of the volume of deaths (n = 197 756) in aggregate counts. Nationally, completeness in case surveillance records was highest for age (99.9%) and sex (98.8%). Data on race/ethnicity were complete for 56.9% of cases; completeness varied by region. Data completeness for each underlying medical condition assessed was <25% and generally declined during the study period. About half of case records had complete data on hospitalization and death status.
Conclusions
Incompleteness in national COVID-19 case surveillance data might limit their usefulness. Streamlining and automating surveillance processes would decrease reporting burdens on jurisdictions and likely improve completeness of national COVID-19 case surveillance data.
Keywords: COVID-19, SARS-CoV-2, case surveillance, data completeness, race/ethnicity
The COVID-19 pandemic is the largest public health crisis the nation has faced in a century. Timely and complete COVID-19 surveillance data are important to inform prevention strategies, health policies, and resource allocation for national and local responses. To obtain data on the pandemic trajectory, the Centers for Disease Control and Prevention (CDC) collaborates with local, state, tribal, and territorial public health jurisdictions (hereinafter, jurisdictions) to rapidly conduct national COVID-19 surveillance that relies on 2 data sources: (1) aggregate counts for daily situational awareness and (2) person-level data for each case (case surveillance). An aggregate count of COVID-19 cases and deaths is compiled daily by CDC response teams using data made available by jurisdictions. Although these data provide essential epidemic intelligence needed for daily situational awareness and knowledge of emerging hotspots, they lack information on individual case characteristics.
As the pandemic evolved and the need for ongoing, sustainable national case surveillance data beyond an initial acute response became clear, CDC built upon the existing National Notifiable Diseases Surveillance System (NNDSS), which has provided case surveillance data for decades. NNDSS is the national surveillance system for >120 diseases and conditions of public health concern. CDC uses this system to receive critical case notification data from jurisdictions nationwide to monitor, control, and prevent the occurrence and spread of diseases and conditions under national surveillance. 1
Ongoing analyses of national COVID-19 case surveillance data have guided the US response and identified population groups disproportionately affected by this disease. Case surveillance data have been used to monitor disease incidence, identify underlying medical conditions associated with poor outcomes, examine infections occurring among health care personnel, and highlight disparities in infection and severe outcomes among racial/ethnic groups. 2 -6 However, incomplete case surveillance data may result in a limited, noncomprehensive picture of the pandemic and have precluded the use of national COVID-19 case surveillance data to estimate the incidence and proportions of underlying health conditions, symptoms, and severe outcomes by race/ethnicity. 2
Summarizing the purpose, limitations, and completeness of national COVID-19 case surveillance data may help improve these data over time and aid in their interpretation. To describe regional variations in estimated sensitivity of case ascertainment and completeness in COVID-19 case surveillance data, we compared counts of COVID-19 cases and deaths from case surveillance data with aggregate counts of cases and deaths compiled by CDC from jurisdictions during April 5–September 30, 2020. We also analyzed case surveillance data to describe geographic and temporal trends in the proportion of cases with complete data on selected demographic characteristics, underlying medical conditions, and outcomes.
Methods
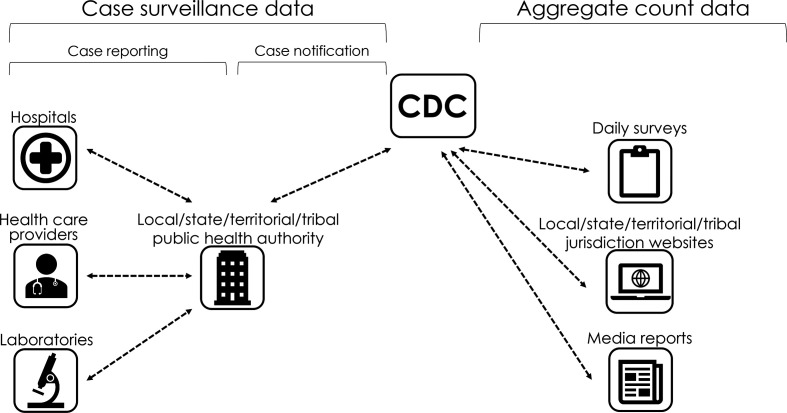
Throughout the COVID-19 pandemic, CDC has been tracking both aggregate and individual counts (case surveillance) of US cases and deaths (Figure 1). CDC collects aggregate counts from jurisdiction websites using manual and automated processes, including a daily survey of jurisdictions to validate counts. These data are used to calculate the number of COVID-19 cases and deaths occurring in each jurisdiction daily and are posted online for the public. 7
Figure 1.
Flow diagram depicting the process through which case surveillance (person-level) and aggregate count data are collected and received by the Centers for Disease Control and Prevention (CDC). Case surveillance data are transmitted to CDC by local, state, territorial, and tribal public health authorities. Aggregate count data were compiled by CDC COVID-19 response teams who gathered aggregate counts of cases and deaths reported by jurisdictions on their websites and validated data using daily surveys. Response teams reviewed media reports to corroborate substantial changes or discrepancies in case data posted on the jurisdictions’ websites.
The process for collecting case surveillance data has changed during the pandemic (supplementary figure available from authors upon request). During the early phase of the pandemic (starting January 22, 2020), CDC established an outbreak response database (called DCIPHER, or Data Collation and Integration for Public Health Emergency Response) to facilitate data collection from jurisdictions. On April 5, 2020, the Council of State and Territorial Epidemiologists published the first interim case surveillance definition, making COVID-19 a nationally notifiable condition. 8 This event marked the start of the transition from an acute infectious disease outbreak response to a national standardized surveillance approach built upon the existing surveillance infrastructure of NNDSS. On August 5, 2020, the Council of State and Territorial Epidemiologists published an updated interim case surveillance definition. 9
Case surveillance data are sent to CDC via several mechanisms that allow jurisdictions to voluntarily submit de-identified, standardized information electronically for individual COVID-19 cases. These data are provided by jurisdictions via 3 primary mechanisms: (1) direct data entry by health department staff members into DCIPHER; (2) daily comma-separated value file submission to CDC for direct electronic upload of case surveillance data into DCIPHER; and (3) existing NNDSS data transmission mechanisms (after the release of the COVID-19 interim case surveillance definition). As of November 18, 2020, national COVID-19 case data were being submitted to CDC through NNDSS notification mechanisms alone, a combination of NNDSS mechanisms and comma-separated value–uploaded files, or comma-separated value–uploaded files alone. Jurisdictions may submit updated case notifications as they gather new information about previously reported cases (eg, death occurred or change in case classification). 7
For this analysis, we analyzed aggregate count and case surveillance data received by CDC during April 5–September 30, 2020, from 56 jurisdictions: the 50 US states, New York City, the District of Columbia, Guam, Puerto Rico, the Northern Mariana Islands, and the US Virgin Islands. No person-level case data were received from American Samoa, the Federated States of Micronesia, Palau, or the Republic of Marshall Islands because these jurisdictions had no COVID-19 cases to report. We accessed case surveillance data and aggregate jurisdictional COVID-19 case and death counts on November 18, 2020. To estimate the sensitivity of case ascertainment, we calculated proportions using the number of COVID-19 cases and deaths in case surveillance compared with aggregate counts, by US Department of Health and Human Services (HHS) region. 10 The 10 regions were:
Region 1: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont
Region 2: New York State, New Jersey, New York City, Puerto Rico, and the Virgin Islands
Region 3: Delaware, District of Columbia, Maryland, Pennsylvania, Virginia, and West Virginia
Region 4: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee
Region 5: Illinois, Indiana, Michigan, Minnesota, Ohio, and Wisconsin
Region 6: Arkansas, Louisiana, New Mexico, Oklahoma, and Texas
Region 7: Iowa, Kansas, Missouri, and Nebraska
Region 8: Colorado, Montana, North Dakota, South Dakota, Utah, and Wyoming
Region 9: Arizona, California, Hawaii, Nevada, American Samoa, Commonwealth of the Northern Mariana Islands, Federated States of Micronesia, Guam, Marshall Islands, and Republic of Palau
Region 10: Alaska, Idaho, Oregon, and Washington
Because the COVID-19 public health case definition is intended to be applied uniformly across jurisdictions, we expected close alignment between the number of case notifications transmitted to case surveillance and the number of cases captured in the aggregate counts. Case surveillance counts may be lower than aggregate counts if reporting is incomplete, reporting is lagged, or only laboratory-confirmed cases were provided to case surveillance data (in contrast to both probable and confirmed cases being reported in aggregate counts). Aggregate counts may not include all cases that are in the case surveillance data, because some jurisdictions do not include probable cases in their aggregate counts but send notifications of these cases to CDC.
We analyzed case surveillance data to determine the proportion of case surveillance records with complete data for selected characteristics, including demographic characteristics, underlying medical conditions, symptomatic status (ie, presence vs absence of symptoms), and outcomes (ie, hospitalization, intensive care unit admission, and death) by month and HHS region.
To approximate the timeliness of case surveillance, we calculated the median number of days between the date of collection of first specimen with a positive test result for SARS-CoV-2 (specimen collection date) and CDC case notification date, which indicates the first day CDC was notified of a case. We used R version 3.6.3 (R Foundation) to conduct all analyses. We conducted analyses as part of public health surveillance activities, and CDC determined this project to be exempt from human subjects approval.
Results
Comparison of National COVID-19 Case and Death Counts in Case Surveillance and Aggregate Counts
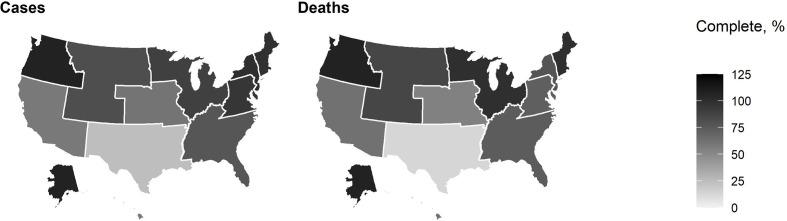
During April 5–September 30, 2020, data on 4 990 629 cases and 141 935 death notifications were received by national COVID-19 case surveillance, representing 72.7% (n = 6 863 251) of the volume of cases and 71.8% (n = 197 756) of the volume of deaths in aggregate counts (Figure 2). For 7 HHS regions (1, 2, 3, 4, 5, 8, and 10), the proportion of cases in case surveillance data to cases in aggregate counts was >75%. The proportion of cases in case surveillance data compared with aggregate counts was 50%-75% in regions 7 and 9 and <25% in region 6. In 5 regions (1, 2, 5, 8, and 10), the proportion of deaths in case surveillance to deaths in aggregate counts was >75%. The proportion of deaths in case surveillance compared with deaths in aggregate counts was 50%-75% in 4 regions (3, 4, 7, and 9) and <25% in region 6.
Figure 2.
Proportion of cases and deaths with complete data received by national COVID-19 case surveillance compared with aggregate counts, by US Department of Health and Human Services region, United States, April 5–September 30, 2020. Case surveillance counts may be lower than aggregate counts if reporting is incomplete, reporting is lagged, or only laboratory-confirmed cases were provided to case surveillance data (in contrast to both probable and confirmed cases being reported in aggregate counts). Aggregate counts may not include all cases that are in the case surveillance data because some jurisdictions do not include probable cases in their aggregate counts but send notifications of these cases to the Centers for Disease Control and Prevention; therefore, case surveillance counts may also be higher than aggregate counts.
Completeness of Person-Level Case Surveillance Data by HHS Region
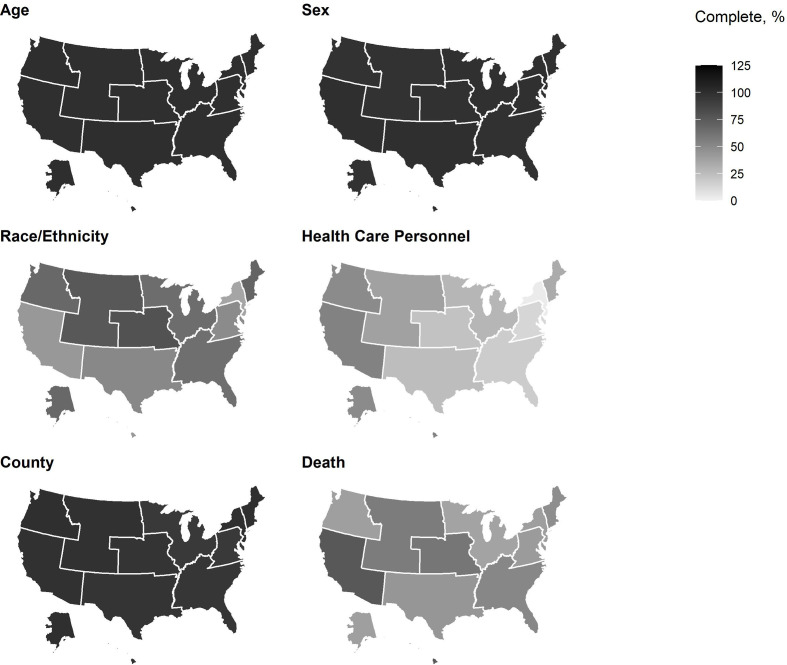
During April 5–September 30, 2020, case surveillance data on age and sex were >95% complete for all HHS regions (Figure 3). Completeness of data on race/ethnicity varied, with 2 HHS regions (7 and 8) providing complete data on >75% of cases, 5 HHS regions (1, 4, 5, 6, and 10) providing complete data on 50%-75% of cases, and 3 HHS regions (2, 3, and 9) providing data on <50% of cases. Data on county were >95% complete in all regions except regions 3 and 5. Region 9 had the highest percentage of cases with complete data on health care personnel status (53.9%), and completeness for other HHS regions was <50% (range, 3.1%-49.5%). Data on death status were provided for >75% of cases from HHS region 9; for 50%-75% of cases from HHS regions 4, 7, and 8; and for <50% of cases from the remaining regions.
Figure 3.
Completeness of data in national COVID-19 case surveillance for selected variables by US Department of Health and Human Services region, United States, April 5–September 30.
Trends in Completeness of Person-Level Case Surveillance Data
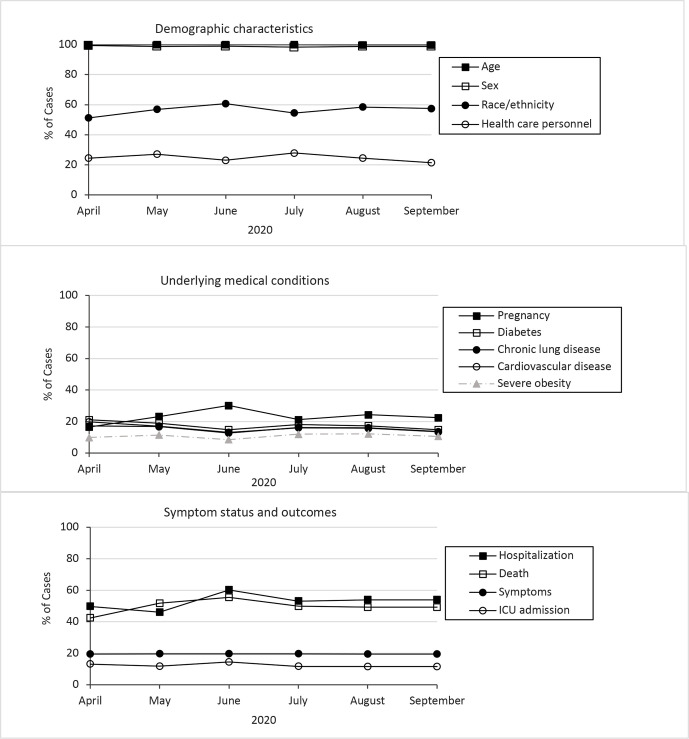
In 2020, 489 763 case notifications were sent to case surveillance during April 5-30, and 983 244 case notifications were sent during September 1-30, 2020 (Table). Data completeness was highest overall for age (99.9%), sex (98.8%), and county (96.3%), and data completeness changed for these data elements by –5.3 to 0 percentage points from April 5-30 to September 1-30. Data completeness on race/ethnicity was 56.9% overall and increased from 51.2% during April 5-30 to 57.5% during September 1-30 (Table, Figure 4).
Table.
Completeness of selected data elements for case notifications sent to national COVID-19 case surveillance, United States, April 5–September 30, 2020 a
| Characteristics | Total | Month during 2020 | |||||
|---|---|---|---|---|---|---|---|
| April 5–September 30 (n = 4 990 629) | April 5-30 (n = 489 763) | May 1-31 (n = 579 134) | June 1-30 (n = 878 662) | July 1-31 (n = 1 077 938) | August 1-31 (n = 981 888) | September 1-30 (n = 983 244) | |
| Demographic | |||||||
| Age | 99.9 | 99.8 | 99.9 | 99.9 | 99.9 | 99.8 | 99.8 |
| Sex | 98.8 | 99.4 | 99.0 | 98.9 | 98.3 | 98.8 | 98.8 |
| Race/ethnicity | 56.9 | 51.2 | 56.9 | 60.7 | 54.5 | 58.5 | 57.5 |
| County | 96.3 | 98.7 | 97.5 | 97.7 | 95.1 | 97.5 | 93.4 |
| Tribal affiliation b | <1.0 | — | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 |
| Health care personnel | 24.7 | 24.4 | 27.1 | 23.0 | 27.9 | 24.5 | 21.4 |
| Underlying medical conditions | |||||||
| Pregnancy status | 23.4 | 16.5 | 23.2 | 30.2 | 21.2 | 24.3 | 22.4 |
| Diabetes | 17.1 | 21.1 | 19.0 | 14.8 | 18.1 | 17.3 | 14.8 |
| Cardiovascular disease | 15.4 | 19.8 | 17.1 | 13.1 | 16.1 | 15.7 | 13.4 |
| Chronic lung disease | 15.2 | 17.2 | 16.8 | 12.7 | 16.3 | 16.0 | 13.6 |
| Immunocompromised condition | 14.7 | 15.5 | 16.0 | 11.3 | 16.3 | 15.8 | 13.5 |
| Renal disease | 14.5 | 16.1 | 16.2 | 11.4 | 15.9 | 15.5 | 13.2 |
| Liver disease | 13.6 | 14.3 | 14.9 | 10.1 | 15.1 | 14.7 | 12.6 |
| Severe obesity (BMI ≥40 kg/m2) b | 10.9 | — | 11.5 | 8.5 | 12.0 | 12.2 | 10.6 |
| Autoimmune condition b | 9.5 | — | 10.0 | 6.9 | 10.4 | 11.0 | 9.6 |
| Psychologic/psychiatric condition | 9.5 | 8.5 | 10.1 | 7.1 | 10.4 | 11.0 | 9.6 |
| Disability status | 6.7 | 5.7 | 7.2 | 6.8 | 7.7 | 6.7 | 5.7 |
| Symptomatic status c | 19.6 | 19.5 | 19.6 | 19.6 | 19.6 | 19.5 | 19.6 |
| Outcomes | |||||||
| Hospitalization | 52.7 | 49.8 | 46.1 | 60.2 | 53.1 | 54.0 | 49.6 |
| ICU admission | 12.0 | 13.1 | 11.8 | 14.5 | 11.7 | 11.6 | 10.1 |
| Death | 49.4 | 42.4 | 51.8 | 55.5 | 49.9 | 49.2 | 46.0 |
Abbreviations: BMI, body mass index; ICU, intensive care unit.
aData include the 50 states, New York City, the District of Columbia, Guam, Puerto Rico, the Northern Mariana Islands, and the US Virgin Islands. All data are percentages.
bData element was added to the COVID-19 case report form on May 5, 2020.
cSymptomatic status was considered known if any of the following symptoms were reported as present or absent: fever, cough, shortness of breath, wheezing, difficulty breathing, chills, rigors, myalgia, rhinorrhea, sore throat, chest pain, nausea or vomiting, abdominal pain, headache, fatigue, diarrhea (≥3 loose stools in a 24-hour period), anosmia, ageusia, or other symptom not otherwise specified on the form.
Figure 4.
Percentage of COVID-19 cases with complete data for selected data elements, United States, April 5–September 30, 2020. Abbreviation: ICU, intensive care unit.
Data for underlying medical conditions were complete for <25% of all cases received by case surveillance during April 5–September 30 (Table). Completeness of data on pregnancy status increased from 16.5% during April 5-30 to 22.4% during September 1-30. Data completeness for the other underlying medical conditions was consistent or decreased during the same period (range, –6.4 to 1.1 percentage-point change in completeness).
Symptomatic status was provided for 19.6%, hospitalization status for 52.7%, and intensive care unit admission status for 12.0% of cases during the study period; completeness for these data elements changed by <5 percentage points from April 5-30 to September 1-30 (Table). Death status was provided for 49.4% of cases during the study period, and completeness improved by 3.6 percentage points from April 5-30 to September 1-30.
Among 34.6% of cases with available information for specimen collection date, the median time from specimen collection date to date of initial notification to CDC was 2 days (interquartile range, 0-4 days).
Discussion
To obtain necessary epidemic intelligence for the ongoing COVID-19 pandemic, CDC has relied on partnerships, expertise, surveillance infrastructure, and the diligent work of health departments to collect, process, and disseminate case surveillance data. As part of this effort, CDC has continued to work with jurisdiction health departments to transition from an acute infectious disease outbreak response (starting January 22, 2020) to a national standardized surveillance approach (starting April 5, 2020) built upon the existing surveillance infrastructure of NNDSS. Our analysis, performed as part of an ongoing effort to improve the timeliness and completeness of case surveillance, showed that the level of completeness of data elements for age, sex, and race/ethnicity was stable or improved during the study period, whereas data on underlying medical conditions had low levels of completeness (<25%) that generally declined or remained stable during the study period. We also identified important regional differences in the estimated sensitivity of case ascertainment by case notifications sent to CDC, which poses a challenge when assessing the national burden of disease. Because national COVID-19 case surveillance data are available from CDC for analysis in public use data sets, 11 anyone who is planning to analyze and interpret case surveillance data should be aware of differences in data completeness in both geography and period of the pandemic.
During the analysis period, we noted a modest overall improvement in completeness of data on race/ethnicity (6 percentage points), but the completeness of data on race/ethnicity varied widely by region. Completeness of data on race/ethnicity varies by state historically. For example, an analysis of NNDSS data for 32 nationally notifiable conditions during 2006-2010 found that data on race were complete for about 70% of cases (range among jurisdictions, 29%-95%) and data on ethnicity were complete for about 49% of cases (range among jurisdictions, 15%-91%). 12 The incompleteness of case surveillance data on race/ethnicity may hinder the detection of important disparities in COVID-19 illness. Multiple studies have demonstrated disparities in COVID-19 outcomes for communities of color, 13 -19 and more complete national-level data could play an essential role in describing racial/ethnic disparities and providing data to inform allocation of resources to disproportionately affected groups.
The sensitivity of case ascertainment and completeness of data for case notifications received at CDC may be affected by multiple factors, including the source of the report, jurisdiction resource limitations, and the structure of electronic data systems that transmit case notifications from jurisdictions to CDC. State and local laws mandate that health care providers and laboratories report notifiable conditions to local public health authorities. This case reporting can represent a large amount of work for health care providers and public health agencies, particularly when case reporting requires use of paper reports or internet-based entry of reports to state health department systems, as opposed to automated data feeds. 20 In addition, nationally, the NNDSS data systems used to transmit notifications from jurisdictions to CDC may have limited interoperability, making data transmission challenging. 21 The unprecedented volume and velocity of COVID-19 cases have strained the limited resources of jurisdictions to investigate individual cases and submit all requested data elements to CDC in a timely manner, particularly when data are not reported to jurisdictions or to CDC in an automated fashion. 7
To improve the completeness and timeliness of case surveillance data and decrease the workload on jurisdictions, CDC and jurisdictional partners are working to increase the use of electronic laboratory reporting and electronic case reporting at public health departments. 22 These automated workflows can reduce the reporting burden on data providers by replacing manual reporting processes, thereby also increasing the timeliness of reporting to public health departments. 23 -26 Electronic laboratory reporting and electronic case reporting have the potential to make submission of information from health care providers and laboratories to public health departments a seamless and automated process, reduce the burden of manually reporting COVID-19 cases, increase the timeliness of reporting, and improve completeness by extracting data directly from across electronic systems that transmit notifications from jurisdictions to CDC. 7,27 Although the transition from the acute COVID-19 response phase began in early April, the timing of adoption of NNDSS mechanisms to transmit case data to CDC has varied. Scientists using these data should be aware that case surveillance data have been sent to CDC via multiple mechanisms, which have varied by jurisdiction and timing during the COVID-19 response.
In recognition that it may be difficult for overburdened jurisdictions to collect the large number of data elements (n = 72) included on the current COVID-19 case report form, 28 CDC worked with jurisdictions to prioritize specific data elements for submission to NNDSS when COVID-19 case volume is too high to permit detailed case investigations. CDC has requested that jurisdictions prioritize the submission of a core set of 26 data elements requested for all notifiable diseases, which are included in the generic case notification message mapping guide, a technical document that describes the content and message mapping specifications for data elements used to communicate case information using Health Level 7 version 2.5.1 to CDC. 29 The prioritization of reporting of core data elements might explain the slight decline in data completeness for certain underlying medical conditions observed during the study period, because underlying medical conditions are not included in the core set of data elements and obtaining data on these conditions requires medical record review or patient interview in the absence of electronic case reporting. If case volume is manageable and resources are available, health departments are encouraged to collect and transmit additional elements (n = 46) to NNDSS using the COVID-19–specific message mapping guide, which was made available to jurisdictions for implementation on June 26, 2020. 30
During the acute response phase of a response, data collection efforts are focused on trying to understand all potential exposures and disease dynamics and rely on the collection of a large number of data elements. However, as a response continues and disease dynamics are better understood, surveillance efforts can shift to focus on the collection and reporting of those core data elements that are needed to verify and count cases by high-level population characteristics, such as geography, sex, age, and race/ethnicity. This shift allows public health resources to be directed rapidly to disease control and prevention activities such as contract tracing and targeted health promotion messaging. Targeted epidemiologic studies, such as case-control, cohort, or cross-sectional studies, may be better suited than case surveillance data to study clinical details and other characteristics of people with COVID-19. CDC regularly collaborates with jurisdictions to conduct these epidemiologic studies to better understand risk factors, such as underlying conditions and disabilities, that may be associated with serious COVID-19 infection. 7
Case surveillance data compose just one aspect of CDC’s ongoing COVID-19 surveillance efforts. To obtain epidemic intelligence, CDC draws from a combination of data sources, such as existing influenza and viral respiratory disease surveillance systems, syndromic surveillance systems, laboratory reporting, and other surveillance systems designed to answer epidemiologic questions about the spread and impact of SARS-CoV-2 in specific settings and populations. 31 All these data sources, in combination with case surveillance data, create a dynamic picture of the distribution and effects of COVID-19 in the United States.
Conclusion
National COVID-19 case surveillance includes person-level data on millions of cases and has provided crucial information needed to respond to the COVID-19 pandemic; however, important gaps in person-level data on COVID-19 persist. To fill these gaps, CDC will continue to work with jurisdictional public health partners to strengthen and modernize disease surveillance activities in the United States, with an emphasis on streamlining and automating processes to minimize manual data collection and reporting burdens on health care providers, laboratories, and jurisdictional public health officials. Investment in the national public health informatics infrastructure that supports comprehensive disease surveillance will fortify the current COVID-19 response and will also provide an adaptable, interoperable, and flexible public health data supply chain and pandemic-ready systems to prepare the nation for future public health emergencies.
Acknowledgments
The authors acknowledge Lesliann Helmus and Sara Johnston from CDC for providing comments on this article.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention . National Notifiable Diseases Surveillance System (NNDSS): NNDSS supports the COVID-19 response. Accessed September 22, 2020. https://wwwn.cdc.gov/nndss
- 2. Stokes EK., Zambrano LD., Anderson KN. et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759-765. 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC COVID-19 Response Team . Characteristics of health care personnel with COVID-19—United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481. 10.15585/mmwr.mm6915e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC COVID-19 Response Team . Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. 10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC COVID-19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382-386. 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes MM., Groenewold MR., Lessem SE. et al. Update: characteristics of health care personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1364-1368. 10.15585/mmwr.mm6938a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . FAQ: COVID-19 data and surveillance: national COVID-19 case surveillance. Accessed September 22, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/faq-surveillance.html
- 8. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID-19): 2020 interim case definition, approved April 5, 2020. Accessed September 7, 2020. https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020
- 9. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID-19): interim case definition, approved August 5, 2020. Accessed September 9, 2020. https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/08/05
- 10. US Department of Health and Human Services . Regional offices. Accessed March 3, 2021. https://www.hhs.gov/about/agencies/iea/regional-offices/index.html
- 11. Centers for Disease Control and Prevention . COVID-19 case surveillance public use data. Accessed September 21, 2020. https://data.cdc.gov/Case-Surveillance/COVID-19-Case-Surveillance-Public-Use-Data/vbim-akqf
- 12. Adekoya N., Truman BI., Ajani UA. Completeness of reporting of race and ethnicity data in the Nationally Notifiable Diseases Surveillance System, United States, 2006-2010. J Public Health Manag Pract. 2015;21(2):E16-E22. 10.1097/PHH.0000000000000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gold JAW., Wong KK., Szablewski CM. et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545-550. 10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garg S., Kim L., Whitaker M. et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Price-Haywood EG., Burton J., Fort D., Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wortham JM., Lee JT., Althomsons S. et al. Characteristics of persons who died with COVID-19—United States, February 12–May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):923-929. 10.15585/mmwr.mm6928e1 [DOI] [PubMed] [Google Scholar]
- 17. Moore JT., Ricaldi JN., Rose CE. et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020—22 states, February–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122-1126. 10.15585/mmwr.mm6933e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatcher SM., Agnew-Brune C., Anderson M. et al. COVID-19 among American Indian and Alaska Native persons—23 states, January 31–July 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1166-1169. 10.15585/mmwr.mm6934e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gold JAW., Rossen LM., Ahmad FB. et al. Race, ethnicity, and age trends in persons who died from COVID-19—United States, May–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. 10.15585/mmwr.mm6942e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mac Kenzie WR., Davidson AJ., Wiesenthal A. et al. The promise of electronic case reporting. Public Health Rep. 2016;131(6):742-746. 10.1177/0033354916670871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon P., Pollock D., Foldy S. National public health informatics, United States. In: Magnuson JA., Dixon BE., eds. Public Health Informatics and Information Systems. 3rd ed. Springer International Publishing; 2020:439-458. [Google Scholar]
- 22. Richards CL., Iademarco MF., Anderson TC. A new strategy for public health surveillance at CDC: improving national surveillance activities and outcomes. Public Health Rep. 2014;129(6):472-476. 10.1177/003335491412900603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . ELR/electronic laboratory reporting. Accessed October 10, 2020. https://www.cdc.gov/elr/about.html
- 24. Centers for Disease Control and Prevention . Progress in increasing electronic reporting of laboratory results to public health agencies—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(38):797-999. [PMC free article] [PubMed] [Google Scholar]
- 25. Lamb E., Satre J., Hurd-Kundeti G. et al. Update on progress in electronic reporting of laboratory results to public health agencies—United states, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(12):328-330. [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . Electronic case reporting (eCR): what is eCR? Accessed October 10, 2020. https://www.cdc.gov/ecr/index.html
- 27. Centers for Disease Control and Prevention . Electronic case reporting (eCR): what are the benefits of eCR? Accessed October 10, 2020. https://www.cdc.gov/ecr/benefits-of-eCR.html
- 28. Centers for Disease Control and Prevention . Information for health departments on reporting cases of COVID-19. Accessed September 9, 2020. https://www.cdc.gov/coronavirus/2019-ncov/php/reporting-pui.html
- 29. Centers for Disease Control and Prevention . NNDSS modernization initiative: frequently asked questions. Accessed November 27, 2020. https://www.cdc.gov/nmi/faq.html
- 30. Centers for Disease Control and Prevention . MMGs and artifacts. Accessed September 7, 2020. https://wwwn.cdc.gov/nndss/case-notification/message-mapping-guides.html
- 31. Centers for Disease Control and Prevention . Surveillance and data analytics: the latest in COVID-19 data and surveillance. Accessed September 23, 2020. https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/surveillance-data-analytics.html