Abstract
Cardiovascular disease remains the main cause of mortality in individuals with diabetes mellitus (DM) and also results in significant morbidity. Premature and more aggressive atherosclerotic disease, coupled with an enhanced thrombotic environment, contributes to the high vascular risk in individuals with DM. This prothrombotic milieu is due to increased platelet activity together with impaired fibrinolysis secondary to quantitative and qualitative changes in coagulation factors. However, management strategies to reduce thrombosis risk remain largely similar in individuals with and without DM. The current review covers the latest in the field of antithrombotic management in DM. The role of primary vascular prevention is discussed together with options for secondary prevention following an ischaemic event in different clinical scenarios including coronary, cerebrovascular, and peripheral artery diseases. Antiplatelet therapy combinations as well as combination of antiplatelet and anticoagulant agents are examined in both the acute phase and long term, including management of individuals with sinus rhythm and those with atrial fibrillation. The difficulties in tailoring therapy according to the variable atherothrombotic risk in different individuals are emphasized, in addition to the varying risk within an individual secondary to DM duration, presence of complications and predisposition to bleeding events. This review provides the reader with an up-to-date guide for antithrombotic management of individuals with DM and highlights gaps in knowledge that represent areas for future research, aiming to improve clinical outcome in this high-risk population.
Keywords: Diabetes, Cardiovascular, Cerebrovascular, Peripheral artery disease, Antithrombotic, Antiplatelet
Graphical Abstract
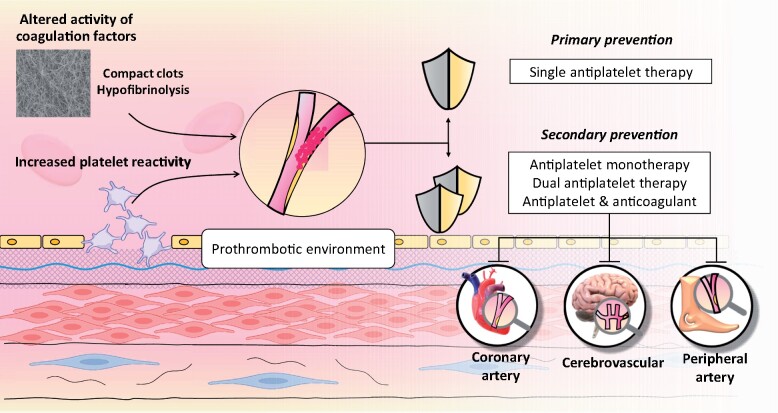
Atherothrombosis in diabetes.
Introduction
Despite advances in therapy, a diagnosis of diabetes mellitus (DM) is associated with increased morbidity and reduced lifespan, mainly due to vascular complications.1–3 Premature and more severe vascular disease, as well as a prothrombotic environment, represent key mechanisms for adverse vascular outcomes in this population.4 The prothrombotic milieu develops secondary to increased platelet reactivity coupled with hypofibrinolysis.5 , 6
Current treatment strategies to improve vascular outcomes in individuals with DM are focused on revascularization of acute atherothrombotic occlusions, where possible, together with early introduction of antithrombotic therapies, usually by inhibiting platelet function. This continues long-term coupled with multifactorial therapy targeting hypertension, dyslipidaemia and dysglycaemia in order to limit the progression of vascular pathology.
In this review, we discuss the latest in antithrombotic therapies for the management of coronary artery disease (CAD), cerebrovascular disease, and peripheral artery disease (PAD) in DM, covering therapies for primary prevention, acute vascular occlusion and long-term secondary prevention. Special emphasis is placed on the benefits and risks of antithrombotic therapy combinations, with the overall aim of providing the reader with an up-to-date guide for antithrombotic management in DM. Search strategy is detailed in the Supplementary material online, S1.
The thrombotic environment in diabetes
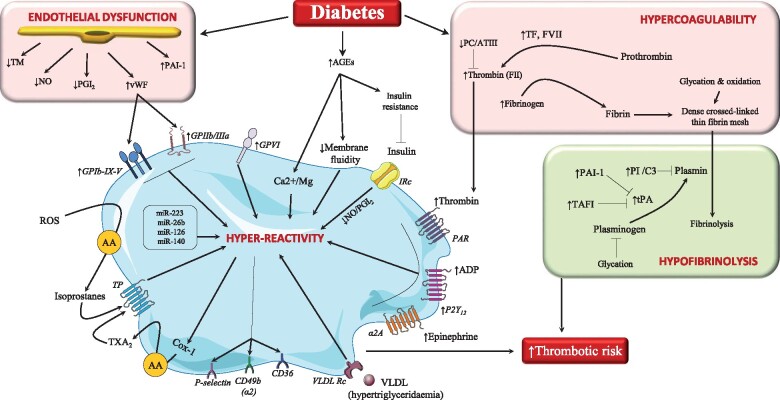
Individuals with DM are prone to both arterial and venous thrombosis.7 DM is characterized by multiple pathological processes, including hyperglycaemia, chronic inflammation, oxidative stress, and associated metabolic conditions, that damage the endothelium and increase platelet reactivity, resulting in a prothrombotic environment. Endothelial dysfunction is a consistent finding in DM patients and contributes to the prothrombotic shift (Figure 1).4
Figure 1.
Diabetes enhances the risk of thrombosis. Diabetes induces endothelial dysfunction with subsequent decline in the expression/release of molecules that can reduce platelet activation and associated thrombus formation. At a platelet level, there are several mechanisms by which diabetes could enhance platelet susceptibility to activation including: (i) a higher abundance of advanced glycation end-products (AGEs) which induces insulin resistance and alters membrane fluidity; (ii) an enhanced oxidative stress which leads to the formation of isoprostanes which in turn induce platelet activation by interacting with the thromboxane receptor (TP); (iii) a higher production of thromboxane (TXA2); and (iv) an increased expression of multiple platelet activation receptors and a higher reactivity to several platelet agonists. As for coagulation, diabetes is associated with a higher amount of tissue factor (TF), thrombin (factor II), and fibrinogen production, which, in concurrence with lower anticoagulant proteins [protein C-antithrombin III complex (PC/ATIII)], favours the formation of the fibrin mesh, which undergoes glycation and oxidative modifications, becoming more dense and resistant to fibrinolysis. Diabetes is also associated with a hypofibrinolytic state characterized by higher abundance of inhibitors of tissue plasminogen activator (tPA) such as plasminogen activator inhibitor-1 (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI), and increased incorporation of antifibrinolytic proteins into the clot [plasmin inhibitor (PI) and complement 3 (C3)] which collectively reduce the efficiency of fibrinolysis. AA: arachidonic acid; ADP: adenosine diphosphate; Cox: cyclooxygenase; GP: glycoprotein; IRc: insulin receptors; miR: microRNAs; NO: nitric oxide; PAR: protease-activated receptor; PGI2: prostacyclin; ROS: reactive oxygen species; TM: thrombomodulin; VLDL: very-low-density lipoprotein; vWF: von Willebrand factor.
An array of mechanisms operate in platelets to enhance their reactivity.8 Hyperglycaemia is associated with higher expression of platelet receptors, including glycoprotein (GP) Ibα, GPIIb/IIIa and P2Y12,9 reduced platelet membrane fluidity secondary to increased glycation, higher thromboxane (TX) A2 synthesis together with increased platelet activation markers.10–12 Diabetes-associated oxidative stress also increases production of F2-isoprostanes.13 TXA2 and F2-isoprostanes, in turn, activate the thromboxane receptors and amplify platelet activation (Figure 1).14 Platelet hyper-reactivity also results from diminished sensitivity to the inhibitory agents prostacyclin, nitric oxide, and insulin,15–17 and from changes in platelet content of miRNAs known to regulate platelet function (miR-223, miR-26b, miR-126, miR-140).18 , 19 Moreover, the imbalance in intraplatelet magnesium and calcium homeostasis renders platelets more sensitive to epinephrine, adenosine diphosphate (ADP), and thrombin.20 DM is also characterized by accelerated platelet turnover, as evidenced by release of more reactive, reticulated platelets21 , 22 that display a reduced response to antiplatelet agents.23 Finally, platelets from DM patients more easily externalize phosphatidylserine in the outer platelet membrane, thereby providing a better surface for the assembly of clotting factors and tissue factor activation.21 , 22
Other associated metabolic conditions like obesity, dyslipidaemia, and systemic inflammation also contribute to thrombosis risk.6 , 24 Circulating inflammatory molecules [tumour necrosis factor-α, interleukin (IL)-1 and IL-6, selectin, soluble CD40 ligand],17 , 25 besides enhancing platelet reactivity, favour a hypercoagulable environment. Furthermore, bone marrow transplants that created chimeras of normal rats with bone marrow cells from diabetic rats resulted in a prothrombotic phenotype similar to the donor animals, indicating the imprinting effects of DM on haematopoietic cells.22
Several prothrombotic alterations in the coagulation-fibrinolytic system also occur in DM,26 including increased levels of tissue factor, prothrombin, factor VII and fibrinogen coupled with impaired anticoagulant and fibrinolytic activity (Figure 1).27 Diabetic thrombi display compact fibrin networks with densely-packed thin fibres that are resistant to fibrinolysis.6 , 26 Furthermore, hyperglycaemia induces qualitative changes in plasminogen, hindering its fibrinolytic activity.28 Concomitantly, elevated levels of anti-fibrinolytic proteins (plasminogen activator inhibitor-1 and thrombin-activatable fibrinolysis inhibitor),29 along with increased incorporation of anti-fibrinolytic proteins (complement C3 and plasmin inhibitor) into the clot further compromise fibrinolysis.6 Despite this prothrombotic environment, DM patients have a paradoxical increased risk of bleeding, particularly following an acute coronary syndrome (ACS).30 However, data on stable patients are less clear: the dual antiplatelet therapy (DAPT) study (detailed below under ‘Secondary Prevention’) reported no increase in long-term moderate or severe bleeding events in those with DM, possibly related to excluding those with bleeding events in the first 12 months, while increased risk was documented in the REACH and CORONOR registries.31 , 32 The exact mechanisms for the increase in both thrombosis and bleeding risk in some diabetes patients are not fully understood, although renal complications may have a role.33 Also, chronic activation of platelet and coagulation proteins may ‘exhaust’ the system in some DM patients, thus increasing bleeding risk.34
Antithrombotic targets
For decades, the two main antithrombotic targets have been platelet TXA2 production and platelet P2Y12 receptor activation.
Aspirin
Low-dose aspirin irreversibly inhibits platelet cyclooxygenase-1 enzyme, preventing the conversion of arachidonic acid into bioactive prostanoid TXA2.35 Given the short half-life of aspirin and increased platelet turnover in DM, a proportion of platelets may escape 24-h inhibition by once-daily aspirin, which can be re-established by twice-daily dosing.23 , 36
P2Y12 receptor antagonists
ADP-stimulated effects on platelets are mediated primarily by Gi-coupled P2Y12 receptor activation, leading to persistent platelet aggregation, whereas P2Y1 is responsible for an initial weak, transient phase of platelet aggregation.37 There are two main classes of orally administered P2Y12 inhibitors: thienopyridines (ticlopidine, clopidogrel, and prasugrel) and non-thienopyridine agents (ticagrelor).38 Thienopyridines require conversion to an active metabolite that acts irreversibly. Ticlopidine is no longer marketed in many countries due to safety concerns.38 Ticagrelor is a direct-acting cyclopentyltriazolopyrimidine that requires no metabolism and binds reversibly to the P2Y12 receptor.38
Other antithrombotic approaches
Warfarin and other vitamin K antagonists (VKAs) require regular monitoring and this, together with high bleeding risk when combined with antiplatelet therapy, prevented widespread use.39 More modern approaches include modulation of thrombin activity either by blocking protease-activated receptor-1 on platelet membrane (vorapaxar)40 or by directly inhibiting protein function (dabigatran).41 Other non-VKA oral anticoagulants (NOAC) include inhibitors of activated factor Xa (apixaban, rivaroxaban, and edoxaban).41 Supplementary material online, Table S1 provides a summary of the main antithrombotic agents.
Antiplatelet therapy for primary prevention of ischaemic events
Primary prevention is defined as offering therapy to individuals without a history of a vascular ischaemic event. In the largest individual data meta-analysis of primary prevention trials (n = 95 000 individuals), aspirin use in DM was associated with a non-significant 12% relative risk reduction (RRR) of major adverse cardiac events (MACE), from 1.87% to 1.63% per year [hazard ratio (HR) 0.88 (0.67–1.15); Table 1].42 Although non-significant, this benefit was comparable to non-DM individuals, in whom aspirin reduced yearly MACE from 0.57% to 0.51% [HR 0.88 (0.82–0.94); P = 0.0001].42 Aspirin was associated with an increase in extracranial, mainly gastrointestinal, bleeding in both non-DM and DM populations (P = 0.20 for heterogeneity; Table 1).42 Following this meta-analysis, the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) (n = 2539)43 and prevention of progression of arterial disease and diabetes (POPADAD) (n = 1276)44 trials investigated aspirin in primary prevention but, being small and underpowered, failed to provide conclusive data. The recent ASCEND trial is the largest, longest and only adequately powered trial investigating primary prevention in DM, randomizing 15 480 DM patients without symptomatic cardiovascular disease to aspirin (100 mg daily) or placebo.45 Serious vascular events occurred in 8.5% of individuals on aspirin vs. 9.6% on placebo [RRR 12%, HR 0.88 (0.79–0.97); P = 0.01]. Major bleeding, according to the Bleeding Academic Research Consortium (BARC) 2, 3, and 5 categories,46 occurred in 4.1% and 3.2% in the aspirin and placebo arms, respectively, without significant differences in fatal or intracranial bleeding, although the absolute number of events was low (Table 1).45 Notably, >50% of major bleeding excess with aspirin was gastrointestinal. The number needed to treat (NNT)/number needed to harm (NNH) ratio was 0.8, favouring treatment. Importantly, BARC 2–5 bleeding criteria are less restrictive as compared to the Thrombolysis in Myocardial Infarction (TIMI) major criteria.47–49 Of note, there was no significant heterogeneity in the effect of aspirin according to the estimated vascular risk at baseline. A meta-analysis of 12 randomized controlled trials (RCTs) (34 227 individuals), including the ASCEND population, showed that aspirin reduces MACE by 11% compared with placebo [HR 0.89 (0.83–0.95)] (Table 1).50
Table 1.
Primary prevention in diabetes
| Study | Patients | Primary efficacy endpoint | Median follow-up | Predicted vs. observed incidence and expected benefit | Absolute and relative benefit | Absolute and relative harm | Comments |
|---|---|---|---|---|---|---|---|
| ATT meta-analysis (2009)42 | 95 000 patients from six primary prevention trials which included 4% of DM patients (n = 3818) | Stroke, MI, and CV death | NA | NA |
Overall population: Aspirin: 0.51% Control: 0.57% HR 0.88 (0.82–0.94) DM subgroup: Aspirin: 1.63% Control: 1.87% HR 0.88 (0.67–1.15) |
GI/extracranial bleed Overall population: Aspirin: 0.10% Control: 0.07% HR 1.54 (1.30–1.82) DM subgroup: Aspirin: 0.23%/year Control: 0.21%/year HR 1.10 (0.52–2.34) |
NNT/NNH ratio: 0.83 No difference in fatal bleeding |
| JPAD (2008)43 | 2539 T2DM patients without a history of atherosclerotic disease | Sudden death; death from coronary, cerebrovascular, and aortic causes; non-fatal acute MI; UA; exertional angina; non-fatal ischaemic and haemorrhagic stroke; TIA; or non-fatal aortic and PVD | 4.4 years |
Predicted: 5.2%/year vs. Observed: 1.7%/year Expected benefit: 30% RRR |
Aspirin: 5.4% Placebo: 6.7% HR 0.80 (0.58–1.10) |
Any GI bleeding: Aspirin: n = 12 Placebo: n = 4 |
Observed primary endpoint rate ∼1/3 of predicted. Expected benefit likely unrealistic based on previous data (trial largely underpowered). |
| POPADAD (2008)44 | 1276 adults aged ≥40 years with T1DM or T2DM and ABI ≤0.99 (asymptomatic) | Death from CAD or stroke, non-fatal MI or stroke, or amputation for critical limb ischaemia; and death from CAD or stroke | 6.7 years |
Predicted: 28%/year vs. observed: 2.9%/year Expected benefit: 25% RRR |
Aspirin: 18.2% Placebo: 18.3% HR 0.98 (0.76–1.26) |
Any GI bleeding: Aspirin: 4.4% Placebo: 4.9% HR 0.90 (0.53–1.52) |
Observed events were approx. 1/10 of predicted. The expected benefit was likely unrealistic based on previous data (trial was largely underpowered). |
| ASCEND (2018)45 | 15 480 Patients aged ≥40 years with DM and no evident CV disease | Non-fatal MI, non-fatal stroke (excluding confirmed ICH), TIA, or death from any vascular cause (excluding confirmed ICH) | 7.4 years |
Predicted: 1.2–1.3%/year vs. Observed: 1.3%/year Expected benefit: 15% RRR |
Aspirin: 8.5% Placebo: 9.6% HR 0.88 (0.79–0.97) |
BARC 2, 3, and 5 bleeding: Aspirin: 4.1% Placebo 3.2% HR 1.29 (1.09–1.52) ICH: Aspirin: 0.7% Placebo: 0.6% HR 1.22 (0.82–1.81) Fatal bleeding: Aspirin: 0.2% Placebo: 0.2% HR 1.18 (0.61–2.30) |
Consistency between predicted and observed incidence event rate NNT/NNH: 0.81 |
| THEMIS (2019)47 |
19 220 patients with DM, ≥50 years, stable CAD with no previous MI or stroke Randomized to ticagrelor or placebo on a background of aspirin therapy |
Stroke, MI, and CV death | 3.3 years |
Predicted benefit: 16% RRR Predicted: 2.5%/year vs. Observed: 2.5%/year |
Ticagrelor: 7.7% Placebo: 8.5% HR 0.90 (0.81–0.99) |
TIMI major bleeding: Ticagrelor: 2.2% Placebo: 1.0% HR 2.32 (1.82–2.94) BARC 3–5 bleeding: Ticagrelor: 3.7% Placebo: 1.7% HR 2.36 (1.96–2.84) ICH: Ticagrelor: 0.7% Placebo: 0.5% HR 1.71 (1.18–2.48) |
High rate of ticagrelor discontinuation: Placebo 25% vs. Ticagrelor: 35% HR 1.50 (1.42–1.58) Predicted benefit higher than observed. NNT/NNH: 1.48 (TIMI-major defined bleeding) |
|
Meta-analysis Seidu et al. (2019)50 |
34 227 participants with DM, individual patient data from 2306 participants | Stroke, MI, and CV death | 5 years | NA |
Aspirin: 8.6% Control: 9.6% HR 0.89 (0.83–0.95) |
Major bleeding: Aspirin: 4% Control: 3.5% HR 1.30 (0.92–1.82) |
Summary of primary prevention studies.
Significant differences are reported in bold.
ABI, ankle-brachial index; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; CV, cardiovascular; DM, diabetes mellitus; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial haemorrhage; MI, myocardial infarction; NA, not applicable; NNH, number needed to harm; NNT, number needed to treat; PVD, peripheral vascular disease; RRR, relative risk reduction; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack; TIMI, Thrombolysis in Myocardial Infarction; UA, unstable angina.
The THEMIS study tested intensification of antiplatelet regimen in 19 220 DM patients without previous myocardial infarction (MI) or stroke but with evidence of clinical CAD and already on low-dose aspirin therapy (Table 1).47 Individuals randomized to aspirin and ticagrelor had a modest reduction in vascular events compared with aspirin alone [7.7% and 8.5%, respectively, HR 0.90 (0.81–0.99); P = 0.04], associated with a 2.3-fold increase in TIMI major bleeding and a 1.7-fold increase in intracranial bleeding (Table 1), giving an unfavourable NNT/NNH ratio of 1.48 and arguing against routine DAPT with aspirin and ticagrelor in this population.
The recent European Society of Cardiology (ESC) guidelines indicate that those with DM and ≥1 organ damage or ≥3 major risk factors, or any risk factor and ≥10 years disease duration without organ damage, should be considered for primary prevention, in the absence of contraindications (Supplementary material online, Table S2) but routine use of aspirin for all DM individuals is not recommended.51 Overall, guidelines recommend aspirin monotherapy for DM patients with additional risk factor(s) and/or with an estimated annual risk of vascular events ≥1% (Supplementary material online, Table S2).52–54
Secondary prevention in the absence of atrial tachyarrhythmias
Following acute coronary events
ACS guidelines recommend DAPT comprising aspirin and prasugrel or ticagrelor, which applies to DM individuals.3 , 51 , 55 , 56 Patients with DM, however, exhibit enhanced platelet reactivity and reduced sensitivity to thienopyridines (but not ticagrelor),57 , 58 although the clinical significance of these biochemical observations remains unclear.
Aspirin
Guidelines recommend routine early administration of aspirin in ACS, which seems to offer similar benefits in individuals with and without DM. Based on pharmacodynamic studies in DM,23 , 59 , 60 the clinical benefit of twice-daily aspirin administration is being evaluated in the ANDAMAN trial (NCT02520921). Higher aspirin doses (300–325 mg vs. 75–100 mg) failed to reduce MACE 30 days post-ACS in the CURRENT-OASIS 7 study (23% had DM).61 The ongoing ADAPTABLE study is assessing alternative aspirin dosing, although this is not limited to the DM population.62 Moreover, ongoing studies are aiming to identify new low-dose aspirin formulations with potentially improved safety and efficacy profiles.63 , 64
P2Y12 inhibitors
In individuals with DM, prasugrel or ticagrelor combination with aspirin is preferred to clopidogrel, which shows reduced efficacy.30 , 56 , 65–67 Post hoc analysis of the DM population in the TRITON-TIMI 38 trial showed marked benefit of prasugrel over clopidogrel,66 while, in PLATO, the absolute benefit of ticagrelor over clopidogrel was greatest in patients with both DM and chronic kidney disease (CKD).68 In patients with ACS and insulin-requiring DM, ticagrelor may achieve more potent platelet inhibition than prasugrel,57 although the clinical significance is unclear. The recent ISAR-REACT 5 study, an open-label trial, demonstrated superiority of a prasugrel-based strategy over a ticagrelor-based strategy in reducing MACE in ACS patients.69 However, this was not the case in the DM subgroup: the composite primary endpoint (death, stroke, or MI) occurred in 11.2% and 13.0% in ticagrelor and prasugrel arms, respectively [HR 0.84 (0.58–1.24); P = 0.383] with treatment interaction shown for DM status (P = 0.0035).70 Bleeding complications were similar in ticagrelor- and prasugrel-treated DM individuals.
A difficulty with more potent oral P2Y12 inhibitors is the limited evidence in the older population who are at higher bleeding risk. Two smaller studies in ACS patients aged ≥70 years, of whom a third had DM, indicated that de-escalation from prasugrel or ticagrelor to clopidogrel may be safe.71 Moreover, platelet-function-guided de-escalation from prasugrel to clopidogrel at hospital discharge may be non-inferior to continued prasugrel but this strategy appears safer in those without DM.72 The TWILIGHT study (n = 7119) assessed safety of de-escalating DAPT, from ticagrelor plus aspirin to ticagrelor monotherapy, after 3 months of DAPT following high-risk percutaneous coronary intervention (PCI) for ACS or chronic coronary syndromes (CCS).73 Monotherapy reduced the primary endpoint of BARC type 2, 3, or 5 bleeding compared with DAPT at 12 months [4.0% vs. 7.1%, HR 0.56 (0.45–0.68); P < 0.001], similarly in those with and without DM, without increasing the secondary combined endpoint of death, MI, or stroke. More specifically, ticagrelor monotherapy in the DM subgroup did not increase ischaemic events compared with DAPT [4.6% vs. 5.9%; HR 0.77 (0.55–1.09); P = 0.14] but significantly decreased bleeding complications [4.5% vs. 6.7%; HR 0.65 (0.47–0.91); P = 0.012].74 The GLOBAL LEADERS trial randomized individuals undergoing PCI with drug-eluting stents for CCS or ACS to standard care (DAPT for 12 months followed by aspirin alone) or ticagrelor with aspirin for 1 month followed by ticagrelor monotherapy for 23 months. The study failed to show superiority for the intervention, although a trend towards a reduction in the primary composite endpoint of death or new Q-wave infarction was apparent [HR 0.87 (0.75–1.01); P = 0.073]. Risk of bleeding was almost identical in the two groups, regardless of DM status.75 , 76 The more recent analysis of the subgroup of DM individuals and CKD (higher risk of thrombosis and bleeding) showed no significant reduction in the primary endpoint with the intervention, although lower rates of the patient-oriented composite endpoint (POCE; death, stroke, site-reported MI/revascularization) were observed in the ticagrelor group compared with controls [20.6% vs. 25.9%, HR 0.74 (0.55–0.99)] with similar reduction in net adverse clinical events (POCE plus BARC 3 and 5 bleeding events) [22.7% vs. 28.3%, HR 0.75 (0.56–0.99)].77 Moreover, a recent meta-analysis has shown that, following PCI, monotherapy with a P2Y12 inhibitor is preferable to DAPT in older individuals and those with diabetes, CKD or multivessel disease due to reduction in bleeding risk.78 Therefore, de-escalation of DAPT may be an option in some patients, particularly when using potent P2Y12 inhibitors as monotherapy.79 However, de-escalation is perhaps best avoided in the DM population, given the high vascular risk, unless there are major concerns over bleeding risk and this remains an area for future research.
Glycoprotein IIb/IIIa inhibitors
GP inhibitor (GPI) use in ACS significantly reduced 30-day mortality, particularly in DM patients undergoing PCI, but this benefit was observed before routine P2Y12 inhibitor use.80 Abciximab reduced MACE in ACS patients undergoing PCI, even with clopidogrel pre-treatment, but the benefit in DM patients was less pronounced (abciximab has now been withdrawn in Europe).81 In contemporary practice using potent P2Y12 inhibitors, GPI therapy is mainly reserved for ‘bail-out’ in case of no-reflow or a thrombotic complication during PCI,82 although some benefit has been suggested also in opiate-treated patients undergoing emergency PCI.83
Very-low-dose non-VKA oral anticoagulant
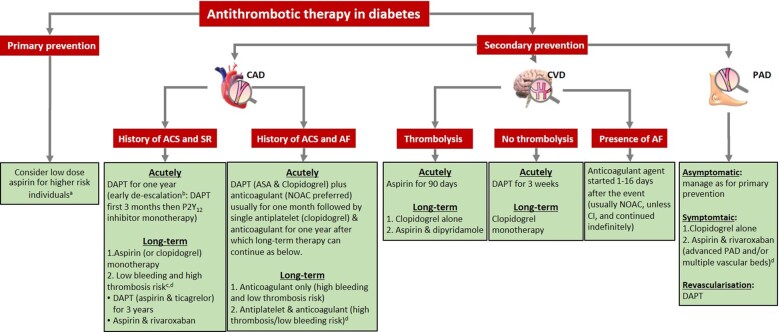
In the ATLAS ACS 2-TIMI 51 trial, the addition of very-low-dose rivaroxaban (2.5 mg twice daily) to DAPT (in the form of aspirin and clopidogrel) in ACS patients, of whom 32% had DM, significantly reduced MACE compared to placebo but the DM group appeared to derive less benefit.84 An increase in bleeding events, including intracranial, was documented and therefore this triple therapy (TT) can only be advocated for individuals at very high vascular risk with relatively low bleeding risk, accepting the challenge that ischaemic and bleeding risk factors overlap substantially.55 A summary of the agents used following ACS is given in Figure 2.
Figure 2.
Summary of antiplatelet and anticoagulant therapies in individuals with diabetes and vascular disease. ACS: acute coronary syndrome; AF: atrial fibrillation; CAD: coronary artery disease; CVD: cerebrovascular disease; DAPT: dual antiplatelet therapy; NOAC: non-vitamin K antagonist oral anticoagulants; PAD: peripheral artery disease; SR: sinus rhythm. aDiabetes mellitus individuals with ≥1 organ damage or ≥3 major risk factors, or any risk factor and ≥10-year disease duration without organ damage. bActive bleeding or comorbidities with high bleeding risk. cHistory of intracerebral haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or renal failure requiring dialysis or with estimated glomerular filtration rate <15 mL/min/1.73 m2. dDiffuse multivessel CAD with at least one of the following: diabetes mellitus requiring medication, recurrent myocardial infarction, PAD, or chronic kidney disease with estimated glomerular filtration rate 15–59 mL/min/1.73 m2.
Long-term therapy for secondary prevention
Default practice is DAPT for 1 year post-ACS followed by antiplatelet monotherapy, usually with aspirin. However, DM patients have increased long-term risk of recurrent atherothrombotic events and should be carefully considered for more intensive long-term antithrombotic therapy.85
The CAPRIE trial (n = 19 185) showed that clopidogrel for secondary prevention (75 mg daily) reduced the composite endpoint of ischaemic stroke, MI, or vascular death compared with daily 325 mg aspirin [5.32% vs. 5.83% (0.3–16.5); P = 0.043], mostly driven by PAD events, which showed a significant heterogeneity vs. MI and stroke.86 DM patients (n = 3866) showed a similar pattern (P = 0.36 for interaction) but with an amplified absolute risk reduction (15.6% vs. 17.7%; P = 0.042), without an increase in bleeding events.87 Given the high aspirin dose used, it is difficult to recommend a routine switch to clopidogrel for secondary prevention, except in special clinical scenarios, such as individuals with PAD (discussed below). The benefit of 12 vs. 30 months of DAPT, mostly consisting of aspirin and clopidogrel, was tested in the DAPT trial,88 including 9961 individuals who had not experienced ischaemic or bleeding events at 1 year post-PCI. Prolonged DAPT significantly reduced the composite of all-cause mortality, MI, or stroke [4.3% vs. 5.9%, HR 0.71 (0.59–0.85); P < 0.001] but at the expense of increased moderate/severe bleeding events [2.5% vs. 1.6%, HR 1.61 (1.21–2.16); P = 0.001]. However, in DM patients (n = 3391), this strategy did not affect the composite outcome [6.6% vs. 7.0%, HR 0.92 (0.71–1.20); P = 0.55] although the limitation of subgroup analysis should be acknowledged.89 , 90
The PEGASUS-TIMI 54 trial evaluated prolonged ticagrelor use in 21 162 patients with a history of MI 1–3 years prior to enrolment. Patients also needed to have at least one additional risk factor, which included DM requiring medication.91 Patients were randomized to either one of two doses of ticagrelor (90 mg twice daily or 60 mg twice daily) or placebo in addition to aspirin. At 3 years, the primary efficacy endpoint (cardiovascular death, MI, and stroke) was reduced with ticagrelor 60 mg twice daily compared with placebo [7.8% vs. 9.0% in placebo, HR 0.84 (0.74–0.95); P = 0.004]. TIMI major bleeding events increased [2.30% vs. 1.06% in placebo, HR 2.32 (1.68–3.21); P < 0.001], but no difference was detected in fatal or intracranial bleeding. Similar data were documented in the DM subgroup (n = 6806, 32% of study population) with a higher absolute risk reduction in the 60 mg twice daily ticagrelor arm compared with placebo (11.6% vs. 10.0% in DM subgroup and 7.8% vs. 6.7% in non-DM subgroup). TIMI major bleeding in those with DM was higher in ticagrelor-treated individuals compared with placebo [2.5% and 1.0%, HR 2.5 (1.4–4.4); P = 0.0004], an increase that was similar to the non-DM group (2.39%; P = 0.89).91
The COMPASS study in patients with either prior MI or multivessel CAD (n = 27 395; 38% with DM) showed that rivaroxaban 2.5 mg twice daily added to low-dose aspirin reduced the risk of MACE compared with aspirin alone [4.1% vs. 5.4%, HR 0.76 (0.66–0.86); P < 0.001],92 making this an option,85 , 92 , 93 particularly in DM patients who showed greater absolute net benefit.94 While the combination therapy increased bleeding risk, there was still a net clinical benefit. Vorapaxar was investigated in the TRA 2°P-TIMI 50 study, detailed in Supplementary material online given the limited clinical use of this agent.95–97
In summary, following ACS, DAPT for 1 year is the current standard of care. De-escalation of DAPT intensity or duration may be considered after 3 months if bleeding concerns prevail.98 One year post-ACS, options include switching to aspirin monotherapy or, in high ischaemic and low bleeding risk DM patients, continuation of dual antithrombotic therapy in the form of aspirin and low-dose ticagrelor (PEGASUS-TIMI 54 study) or aspirin and very-low-dose rivaroxaban (COMPASS trial).3 , 51 , 85 Studies addressing antithrombotic agents for secondary prevention are summarized in Table 2 and Supplementary material online, Figure S1.
Table 2.
Secondary prevention in diabetes
| Sample size | Population | Intervention | Control | Diabetes (%) | Primary endpoint | Duration of follow-up | Relative and absolute benefit | Relative and absolute harm | Comments | |
|---|---|---|---|---|---|---|---|---|---|---|
|
CAPRIE (1996)86 RCT 1:1 |
19 185 | Prior ischaemic stroke (within 1 week–6 months), recent MI (within 35 days), or symptomatic atherosclerotic PAD | Clopidogrel (75 mg) | Aspirin (325 mg) | 20 | Aggregate of MI, ischaemic stroke, and vascular death | 1–3 years |
5.32% vs. 5.83% RRR 8.7% (0.30–16.5%); P = 0.043 |
GI bleed 1.99% vs. 2.66%; P < 0.002 | Treatment effect by subgroup suggests heterogeneity in response with a benefit in PAD, but not is post-MI or stroke patients |
|
DAPT (2014)88 RCT 1:1 |
9961 | Prior coronary stent with DES after 12 months of DAPT (thienopyridine and aspirin) | Thienopyridine (clopidogrel 65% or prasugrel 35%) | Placebo | 30 |
(i) Stent thrombosis and (ii) MACCE (death, MI, or stroke) |
18 months |
(i) Stent thrombosis 0.4% vs. 1.4%; HR 0.29 (0.17–0.48); P < 0.001 (ii) MACCE 4.3% vs. 5.9% HR 0.71 (0.59–0.85); P < 0.001 |
Moderate–severe bleeding 2.5% vs. 1.6%; P = 0.001 | |
|
PEGASUS-TIMI 54 (2015)91 RCT 1:1:1 |
21 162 | MI 1–3 years earlier | Ticagrelor 90 mg b.i.d. | vs. placebo | 32 | Composite of CV death, MI, or stroke | 33 months |
7.85% vs. 9.04% HR 0.85 (0.75–0.96); P = 0.008 |
TIMI major bleeding 2.60% vs. 1.06%, P < 0.001 |
No difference was detected in fatal or intracranial bleeding |
| Ticagrelor 60 mg b.i.d. |
7.77% vs. 9.04% HR 0.84 (0.74–0.95); P = 0.004 |
TIMI major bleeding 2.30% vs. 1.06%; P < 0.001 |
||||||||
|
TRA 2P-TIMI 50 (2012)95 RCT 1:1 |
26 449 | History of MI, ischaemic stroke, or PAD | Vorapaxar (2.5 mg daily) | Placebo | 25 | Composite of death from CV causes, MI, or stroke | 30 months |
9.3% vs. 10.5% HR 0.87 (0.80–0.94); P < 0.001 |
Moderate or severe bleeding 4.2% vs. 2.5%, HR 1.66 (1.43–1.93); P < 0.001 ICH 1.0% vs. 0.5%; P < 0.001 |
Premature trial termination at 2 years, due to safety concerns over ICH in patients with history of stroke |
|
COMPASS (2017)92 RCT 1:1:1 |
27 395 | Stable CAD, PAD, or both | Rivaroxaban (2.5 mg b.i.d.) plus aspirin (100 mg/day) | Aspirin (100 mg/day) | 38 | Composite of CV death, stroke, or MI | 23 months |
4.1% vs. 5.4% HR 0.76 (0.66–0.86); P < 0.001 |
Any bleeding 3.1% vs. 1.9% HR 1.70 (1.40–2.05); P < 0.001 |
Major bleeding was not significantly different |
| Rivaroxaban (5 mg b.i.d.) |
4.9% vs. 5.4% HR 0.90 (0.79–1.03); P = 0.12 |
Any bleeding 2.8% vs. 1.9% HR 1.51 (1.25–1.84); P < 0.001 |
Long-term therapy for secondary prevention trials in patients with established cardiovascular disease.
This is a general guide and healthcare professionals should follow local guidelines as appropriate.
Significant differences are highlighted in bold.
b.i.d., twice daily; CAD, coronary artery disease; CV, cardiovascular; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial haemorrhage; MACE, major adverse cardiovascular or cerebrovascular events; MI, myocardial infarction; PAD, peripheral arterial disease; RCT, randomized controlled trial; RRR, relative risk reduction.
Antithrombotic therapy in the presence of atrial fibrillation
Individuals with DM have a 40% greater risk of developing atrial fibrillation (AF) compared to those without DM.99 , 100 Whilst epidemiological data suggest a causal association, the effect of confounders cannot be excluded (further discussed in Supplementary material online, S1).101–109
Antithrombotic therapy
Oral anticoagulation (OAC) is recommended for male and female AF patients with CHA2DS2-VASc score ≥2 and ≥3, respectively, but can also be considered in those with lower scores on an individual basis.51 In those without absolute indication for VKAs (e.g. mechanical valve or moderate/severe mitral stenosis), NOACs are preferred due to lower rates of severe bleeding and reduced monitoring requirements.51 , 110 In studies comparing NOACs with VKA in non-valvular AF, about a third of individuals had DM and showed similar RRR to those without DM.111 However, given the increased risk in DM, therapy with NOACs translated into a greater absolute benefit, while rates of major bleeding are similar regardless of DM status. If OAC is contraindicated, percutaneous left atrial appendage closure is an option in those with or without DM.112
Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome
Patients with AF on OAC who develop ACS and/or undergo PCI generally require combination treatment with OAC and antiplatelet therapy, with NOACs preferred over VKA.82 , 85 , 113 For TT with OAC and DAPT, clopidogrel is recommended over more potent P2Y12 inhibitors.82 , 85 , 113
After elective PCI in patients with AF who require OAC, a switch from TT (DAPT and OAC) to dual therapy (OAC and single antiplatelet therapy) should be considered in those at low risk of stent thrombosis or when bleeding risk is high. North American guidance encourages dual therapy with early aspirin cessation (i.e. by the time of hospital discharge).114 , 115 ESC guidance endorses TT for at least 1 month if stent thrombosis risk outweighs bleeding risk, followed by an antiplatelet agent (usually clopidogrel) and OAC until 12 months post-PCI, and thereafter OAC monotherapy, unless long-term dual therapy is considered due to very high ischaemic risk with low bleeding risk.82 , 85 , 102 , 113 Similar differences in international guidance exist for AF patients with ACS treated with stent implantation.55 , 56 , 116 , 117 The above applies to all individuals regardless of DM status as no specific studies have been conducted in this group. A summary of the agents used in patients with AF and ACS is provided in Figure 2.
Peripheral artery disease
PAD is thought to affect 202 million people worldwide118 and these individuals are at a higher MACE risk,119 with an even higher event rate with two or more arterial beds affected.119 , 120
Asymptomatic peripheral artery disease
Two relatively small trials have compared aspirin vs. placebo in asymptomatic PAD, the POPADAD trial44 specific to DM and the AAA trial,121 which included 3% (n = 88) of DM patients. Neither showed a difference in MACE in a combined overall cohort of 4626 patients (1256 with DM). While it can be argued these studies were underpowered, guidelines generally do not support routine antiplatelet therapy for asymptomatic lower extremity arterial disease and these individuals are best managed as per primary prevention recommendations described above.
Symptomatic peripheral artery disease
A meta-analysis of 17 000 individuals with symptomatic PAD has shown that aspirin reduced serious vascular events by 18.2% per year (P < 0.0001), marginally offset by a non-significant increase in haemorrhagic stroke.42
Subgroup analysis of 6452 patients with PAD (21% with DM) in the CAPRIE study showed potentially greater benefit with clopidogrel vs. aspirin [3.71% vs. 4.86%; RRR 24% (8.9–36.2); P = 0.0028] compared with the overall study population [5.32% vs. 5.83%; RRR 8.7% (0.3–16.5); P = 0.043].86 The EUCLID trial compared ticagrelor and clopidogrel in 13 885 patients with symptomatic PAD (38.4% with DM), finding no difference in MACE [10.8% vs. 10.6%, HR 1.02 (0.92–1.13); P = 0.65] or major bleeding over a median follow-up of 30 months, regardless of DM status.122
A subgroup analysis of PAD patients in the CHARISMA trial (n = 3096, 36.2% with DM) showed no significant difference in MACE comparing aspirin and clopidogrel therapy with aspirin and placebo [7.6% vs. 8.9%, HR 0.85 (0.66–1.08); P = 0.18], similar to the overall trial cohort [6.8% vs. 7.3%, HR 0.93 (0.83–1.05); P = 0.22].123 There was an increase in minor bleeding with DAPT [34.4% vs. 20.8%, odds ratio 1.99 (1.69–2.34); P < 0.001] but no difference in severe/moderate bleeding. The role of vorapaxar in PAD is described in the Supplementary material online.124
A systematic review and network meta-analysis reported that aspirin, ticlopidine, and ticagrelor or clopidogrel used as monotherapy (or in combination with aspirin) were effective in reducing MACE in patients with PAD, and that ticlopidine, vorapaxar, and DAPT increased bleeding risk.125 Clopidogrel had the best balance between efficacy [MACE RRR 0.72 (0.58–0.91); NNT 80] and safety profile, making it the preferred agent to use.
There were 7470 patients with PAD in the COMPASS trial, 44% of whom had DM with a median follow-up of 21 months.126 The combination of rivaroxaban and aspirin reduced MACE and major adverse limb events vs. aspirin alone to a similar degree in those with DM [8% vs. 12%, HR 0.69 (0.53–0.91)] or without [5% vs. 7%, HR 0.69 (0.50–0.94)]. This was associated with a lower incidence of major adverse limb events in the whole group [1% vs. 2%, HR 0.54 (0.35–0.84); P = 0.005], including lower incidence of major amputation [HR 0.30 (0.11–0.80); P = 0.011].
Post-revascularization
Limited evidence suggests that DAPT with aspirin and clopidogrel is beneficial after lower limb revascularization, particularly following prosthetic bypass.125 , 127 Similarly, warfarin, with or without aspirin, may improve graft patency after vein bypass.128 , 129 The VOYAGER PAD trial, assessing aspirin plus rivaroxaban vs. aspirin post-lower limb revascularization, included 6564 individuals (40% had DM) and combination therapy reduced the composite of acute limb ischaemia, major amputation, MI, ischaemic stroke, or cardiovascular death [HR 0.85 (0.76–0.96); P = 0.009]. A trend towards an increase in TIMI major bleeding, but not BARC bleeding, was documented with the combination therapy [HR 1.43 (0.97–2.10); P = 0.07].130 Results of the DM subgroup analysis are awaited. Antithrombotic management of individuals with DM and PAD is summarized in Table 3 and Supplementary material online, Figure S1.
Table 3.
Antithrombotic therapy in peripheral vascular disease
| Trial | Sample size | Population | Investigation | Control | % with diabetes | Follow-up | Outcomes | Absolute and relative benefits | Absolute and relative harms | Other comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | ||||||||||
| POPADAD (2008)44 | 1276 | Type 1 or 2 diabetes mellitus and ABPI ≤0.99 with no PAD symptoms | Aspirin 100 mg ± antioxidant | Placebo ± antioxidant | 100% |
Median 6.7 years |
MACCE or above ankle amputation for critical limb ischaemia |
18.2% vs. 18.3% HR 0.98 (0.76–1.26); P = 0.86 |
GI bleed 4.4% vs. 4.9% HR 0.90 (0.53–1.52); P = 0.69 |
|
| AAA (2010)121 | 3350 | ABPI ≤0.95; free from clinical CV disease | Aspirin 100 mg | Placebo | 3% |
Mean 8.2 years |
MACCE or revascularization |
13.7 vs. 13.3 events/1000 person-years HR 1.03 (0.84–1.27) |
Major bleed 2.0% vs. 1.2% HR 1.71 (0.99–2.97) |
|
| Symptomatic | ||||||||||
| ATT Collaboration (2009)42 | 17 000 | Meta-analysis of secondary prevention trials (not PAD specific) | Aspirin 75–500 mg | No aspirin | Not stated | NA | MACCE |
6.7% vs. 8.2% per year; HR 0.81 (0.75–0.87); P < 0.0001 |
Major extracranial bleed (incompletely reported) 23 vs. 6 events HR 2.69 (1.25–5.76) |
Non-significant increase haemorrhagic stroke, significant decrease ischaemic stroke and coronary events |
| CAPRIE (1996)86 | 6452 | Symptomatic PAD and ABPI ≤0.85; or symptomatic PAD with previous amputation or revascularization | Clopidogrel 75 mg | Aspirin 325 mg | 21% |
Mean 1.9 years |
MACCE |
3.71% vs. 4.86% per year; RR 23.8% (8.9–36.2); P = 0.0028 |
GI bleed 1.99% vs. 2.66%; P < 0.002 (for the whole group) |
No difference in amputation rate across CAPRIE cohorts; not reported specific to PAD subgroup |
| EUCLID (2017)122 | 13 885 | PAD with ABPI ≤0.8 or previous lower limb revascularization >30 days before randomization | Ticagrelor 90 mg b.i.d. | Clopidogrel 75 mg | 38% |
Median 30 months |
MACCE |
10.8% vs. 10.6% HR 1.02 (0.92–1.13); P = 0.65 |
TIMI major bleeding 1.6% vs. 1.6% HR 1.10 (0.84–1.43); P = 0.49 |
|
| Lower limb revascularization |
12.2% vs. 12.8% HR 0.95 (0.87–1.05); P = 0.30 |
|||||||||
|
CHARISMA subgroup PAD (2009)123 |
3096 (2838 symptomatic, 258 asymptomatic) |
Symptomatic PAD and ABPI ≤0.85; or symptomatic PAD with previous amputation or revascularization; asymptomatic with APBI <0.9 identified within those with other eligibility for CHARISMA study |
Aspirin 75–162 mg + clopidogrel 75 mg (DAPT) |
Aspirin 75–162 mg + placebo |
36% |
Median 28 months |
MACCE |
7.6% vs. 8.9% HR 0.85 (0.66–1.08); P = 0.183 |
Severe bleeding 1.7% vs. 1.7% HR 0.97 (0.56–1.66); P = 0.90 Minor bleeding 34.4% vs. 20.8% HR 1.99 (1.69–2.34); P < 0.001 |
Non-significant trend towards increase of fatal, intracranial, and moderate bleeding with DAPT |
| TRA2°P-TIMI 50 (2013)124 | 3787 | Symptomatic PAD and ABPI <0.85 or previous lower limb revascularization | Vorapaxar 2.5 mg | Placebo | 36% |
Median 36 months |
MACCE |
11.3% vs. 11.9% HR 0.94 (0.78–1.14); P = 0.53 |
GUSTO moderate/severe bleeding: 7.4% vs. 4.5% HR 1.62 (1.21–2.18); P = 0.001 |
|
| Acute limb ischaemia |
2.3% vs. 3.9% HR 0.58 (0.39–0.86); P = 0.006 |
|||||||||
| Revascularization |
18.4% vs. 22.2% HR 0.84 (0.73–0.97); P = 0.017 |
|||||||||
| COMPASS (2018)126 |
7470 (4129 symptomatic lower limb; 1422 asymptomatic lower limb; 1919 carotid disease) |
Previous lower limb revascularization or amputation; symptomatic PAD and ABPI <0.9 or stenosis ≥50% on arterial imaging; carotid revascularization or asymptomatic carotid artery stenosis ≥50% | Rivaroxaban 2.5 mg b.i.d + aspirin 100 mg | Aspirin 100 mg + placebo | 44% |
Median 21 months |
MACCE |
5% vs. 7% HR 0.72 (0.57–0.90); P = 0.005 |
Major bleeding 3.1% vs. 1.9% HR 1.61 (1.12–2.31); P = 0.009 |
|
| Major adverse limb event (acute/chronic ischaemia; amputation) |
1.2% vs. 2.2% HR 0.54 (0.35–0.84); P = 0.005 |
|||||||||
| Post-revascularization | ||||||||||
| CASPAR (2010)127 | 851 | Vascular bypass graft for treatment of PAD | Aspirin 75–100 mg + clopidogrel 75 mg (DAPT) | Aspirin 75–100 mg + placebo | 38% |
Median 12 months |
Graft occlusion/revascularization/amputation/death |
All grafts 35.4% vs. 35.0% HR 0.98 (0.78–1.23) Venous 23.8% vs. 20.0% HR 1.25 (0.94–1.67) Prosthetic 37.5% vs. 52.8% HR 0.65 (0.45–0.95); P = 0.025 |
Total bleeding 16.7% vs. 7.1%; P < 0.001 Severe bleeding 2.1% vs. 1.2%; P = NS |
Graft occlusion HR 0.63 (0.42–0.93) and amputation HR 0.48 (0.24–0.96) significantly reduced in prosthetic but not vein bypass |
| BOA (2000)128 | 2690 | Infrainguinal bypass graft for obstructive arterial disease | Oral anticoagulants (target INR 3.0–4.5) | Pulverized carbasalate calcium 100 mg (equivalent to aspirin 80 mg) | 26% |
Mean 21 months |
Occlusion |
23.2% vs. 24.3% HR 0.95 (0.82–1.11) |
Total bleeding 119 vs. 59 events Fatal bleeding 16 vs. 12 events Gastrointestinal bleeding 51 vs. 29 events Intracranial bleeding 18 vs. 4 events |
Fatal intracranial bleeding events were higher (8 vs. 3 events) in oral anticoagulants, whereas bleeding events in other sites were similar between groups |
| MACE plus amputation |
18.7% vs. 20.8% HR 0.89 (0.75–1.06) |
|||||||||
| Vein graft occlusion |
14.3% vs. 20.3% HR 0.69 (0.54–0.88) |
|||||||||
| Non-vein grafts occlusion |
36% vs. 30% HR 1.26 (1.03–1.55) |
|||||||||
| Sarac et al. (1998)129 | 56 | Infrainguinal bypass with autogenous vein and deemed high risk for graft occlusion (suboptimal venous conduit, poor arterial runoff or redo bypass) | Warfarin (target INR 2–3) + aspirin 325 mg | Aspirin 325 mg | 64% |
Not stated (outcomes derived from Kaplan–Meier survival curves) |
30-day graft patency | 97.3% vs. 85.2%; P = 0.07 |
Haematoma 32% vs. 4%; P = 0.004 GI bleeding 3% vs. 11%; P = NS Intracranial bleeding 3% vs. 4%; P = NS |
|
| 30-day amputation rate | 0% vs. 11.1%; P = 0.04 | |||||||||
| 3-year primary assisted patency: | 77% vs. 56%; P = 0.05 | |||||||||
| 3-year secondary patency | 81% vs. 56%; P = 0.02 | |||||||||
| VOYAGER-PAD (2020)130 | 6564 | Post-lower limb revascularization | Rivaroxaban 2.5 mg b.i.d. + aspirin 100 mg | Aspirin 100 mg + placebo | 40% | Median 28 months | MACE plus acute limb ischaemia or amputation |
15.5% vs. 17.8% HR 0.85 (0.76–0.96) |
Major bleeding 1.9% vs. 1.35% HR 1.43 (0.97–2.10); P = 0.07 |
Summary of antiplatelet and anticoagulant studies in individuals with peripheral vascular disease.
Significant differences are highlighted in bold.
ABPI, ankle brachial pressure index; b.i.d., twice daily; CV, cardiovascular; DAPT, dual antiplatelet therapy; GI, gastrointestinal; HR, hazard ratio; INR, international normalized ratio; MACE, major adverse cardiovascular or cerebrovascular events; NA, not available; PAD, peripheral artery disease.
Treatment of individuals with cerebrovascular disease
Due to lack of DM-specific studies, antithrombotic therapy in individuals sustaining a stroke is similar regardless of DM status and therefore studies are discussed accordingly.
Following acute events
In acute severe ischaemic stroke, reperfusion is attempted either through thrombolysis or endovascular thrombectomy, followed by antiplatelet monotherapy, usually with aspirin, administered 24 h later.131 , 132 Ticagrelor monotherapy showed no superiority over aspirin133 and, therefore, is only recommended if aspirin is contraindicated.132
In those with minor events [National Institute of Health Stroke Scale (NIHSS) score ≤3], high-risk transient ischaemic attack (TIA) (ABCD2 score ≥4) or TIA not requiring thrombolysis or invasive measures, antiplatelet therapy can be immediately started provided haemorrhagic stroke is excluded. DAPT (aspirin and clopidogrel) is recommended given findings from the CHANCE and POINT trials (21% and 28% of the study population had diabetes, respectively),134 , 135 starting within 24 h of the event for 21 days followed by clopidogrel only.132 While severe haemorrhagic events showed no increase in CHANCE, a doubling was noticed in POINT (Table 4 and Supplementary material online, Figure S1), although the benefit of DAPT still outweighed bleeding risk.135 The more recent THALES trial randomized 11 016 individuals (29% with diabetes), with ischaemic stroke or TIA (NIHSS score ≤5; 29% with DM) within 24 h of presentation to DAPT with ticagrelor and aspirin or aspirin alone for 30 days. The primary composite outcome of stroke or death at 30 days occurred in 5.5% in the combination group vs. 6.6% in those on aspirin alone [HR 0.83 (0.71–0.96); P = 0.02] but incidence of disability showed no difference, while DAPT was associated with increased rate of severe bleeding (0.5% vs. 0.1%; P = 0.001).136 There was no suggestion in any of the studies that the diabetes subgroup behaved differently and therefore DAPT in DM should be initiated within 24 h following acute minor stroke not requiring thrombolysis or thrombectomy and continued for 21–30 days. Future work is required to clarify the optimal duration of DAPT after acute minor stroke, or the benefit of DAPT following reperfusion therapy.132 , 137
Table 4.
Antithrombotic therapy in cerebrovascular disease
| Antiplatelet randomized trial for secondary prevention in patients with acute minor ischaemic stroke/high-risk TIA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Patients | Intervention | Control | Follow-up | Composite vascular events (stroke, MI or CVD death) | Recurrent ischaemic stroke | Intracranial haemorrhage | Major haemorrhage |
|
CHANCE (2013)134 (N = 5170; 21.1% with DM) |
Minor stroke (NIHSS <3)/high-risk TIA, onset <24 h |
Clopidogrel 300 mg loading then 75 mg/day on days 2–90 Aspirin 75–300 mg/day on days 2–21 |
Clopidogrel 75 mg/day on days 1–90 | 90 days |
8.4% vs. 11.9% HR 0.69 (0.58–0.82) |
7.9% vs. 11.4% HR 0.67 (0.56–0.81) |
0.3% vs. 0.3% HR 1.01 (0.38–2.70) (haemorrhagic stroke) |
0.2% vs. 0.2% HR 0.94 (0.24–3.79) (severe bleeding) 0.1% vs. 0.2% HR 0.73 (0.16–3.26) (moderate bleeding) |
|
POINT (2018)135 (N = 4881; 27.5% with DM) |
Minor stroke (NIHSS <3)/high-risk TIA, onset <12 h |
Clopidogrel 600 mg loading then 75 mg/day on days 2–90 Aspirin 50–325 mg/day on days 2–21 |
Aspirin 50–325 mg on days 1–90 (recommend 162 mg/day on day 1–5, then 81 mg/day afterward) |
90 days |
5.0% vs. 6.5% HR 0.75 (0.59–0.95) |
4.6% vs. 6.3% HR 0.72 (0.56–0.92) |
0.2% vs. 0.1% HR 1.68 (0.40–7.03) (haemorrhagic stroke) |
0.9% vs. 0.4% HR 2.32 (1.10–4.87) |
|
SOCRATES (2016)133 (N = 6589; 24.3% with DM) |
Non-severe stroke (NIHSS <5)/high-risk TIA, onset <24 h |
Ticagrelor 180 mg loading then 90 mg b.i.d. on days 2–90 | Aspirin 300 mg loading then 100 mg/day on days 2–90 | 90 days |
6.5% vs. 7.2% HR 0.89 (0.78–1.01) |
5.9% vs. 6.6% HR 0.87 (0.76–1.00) |
0.2% vs. 0.3% HR 0.68 (0.33–1.41) |
0.5% vs. 0.6% HR 0.83 (0.52–1.34) |
|
THALES (2020)136 (N = 11 016; 28.6% with DM) |
Mild–moderate stroke (NIHSS <5)/high-risk TIA (ABCD2 > 6), onset <24 h |
Ticagrelor 180 mg loading then 90 mg b.i.d. on days 2–30 Aspirin 300–325 mg loading then 75 to 100 mg/day on days 2–30 |
Aspirin 300–325 mg loading then 75–100 mg o.d. on days 2–30 | 30 days |
5.5% vs. 6.6% HR 0.83 (0.71–0.96) (stroke or death) |
5.0% vs. 6.3% HR 0.79 (0.68–0.93) |
0.4% vs. 0.1% HR 3.33 (1.34–8.28) |
0.5% vs. 0.1% HR 3.99 (1.74–9.14) |
| Anti-platelet randomized trials for long-term secondary prevention in patients with previous non-cardioembolic ischaemic stroke/TIA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Patient | Intervention | Control | Follow-up | Non-fatal MI, non-fatal stroke, death from vascular causes | Recurrent ischaemic stroke | Intracranial haemorrhage | Major haemorrhage |
|
CAPRIE (1996)86 (N = 19 185; 20% with DM) |
Recent MI, PAD, or stroke | Clopidogrel 75 mg/day | Aspirin 325 mg/day | 1.9 years |
5.32% vs. 5.83% HR 0.91 (0.84–1.00) |
Not reported | 0.35% vs. 0.49% |
1.38% vs. 1.55% (severe bleeding) |
|
CAPRIE (1996)86 stroke subgroup (N = 6431; 25% with DM) |
Recent stroke | Clopidogrel 75 mg/day | Aspirin 325 mg/day |
7.15% vs. 7.71% HR 0.91 (0.81–1.06) |
5.2% vs. 5.7% | Not reported | Not reported | |
|
ESPRIT (2006)140 (N = 2739; 18.7% with DM) |
Recent minor stroke/TIA (mRS <3) within 6 months |
Dipyridamole 200 mg b.i.d. plus Aspirin 30–325 mg/day (median 75 mg/day) |
Aspirin 30–325 mg/day (median 75 mg/day) |
3.5 years |
3.3% vs. 4.3% HR 0.78 (0·63–0·97) |
2.1% vs. 2.6% HR 0.84 (0.64–1.10) |
0.8% vs. 1.5% | 2.5% vs. 3.9% |
|
PRoFESS (2008)141 (N = 20 332; 28% with DM) |
Recent stroke within 90 days |
Extended release dipyridamole 200 mg b.i.d. plus Aspirin 25 mg b.i.d. |
Clopidogrel 75 mg/day | 2.5 year |
13.1% vs. 13.1% HR 0.99 (0.92–1.07) |
7.7% vs. 7.9% HR 0.97 (0.88–1.07) |
1.4% vs. 1.0% HR 1.42 (1.11–1.83) |
4.1% vs. 3.6% HR 1.15 (1.00–1.32) |
|
MATCH (2004)145 (N = 7599; 68% with DM) |
Recent stroke/TIA within 3 months plus >1 risk factors |
Clopidogrel 75 mg/day plus Aspirin 75 mg/day |
Clopidogrel 75 mg/day | 1.5 years |
16% vs. 17% HR 0.94 (0.84–1.05) |
8% vs. 9% HR 0.93 (0.80–1.09) |
1.1% vs. 0.7% |
1.9% vs. 0.6% (P < 0.001) |
|
CHARISMA (2006)142 (N = 15 603; 42.7% with DM) |
Atherosclerotic risks, CVD, stroke/TIA within 5 years |
Clopidogrel 75 mg/day plus Aspirin 75–162 mg/day |
Aspirin 75–162 mg/day | 2.3 years |
6.8% vs. 7.3% HR 0.93 (0.83–1.05) |
1.7% vs. 2.1% HR 0.81 (0.64–1.02) |
0.3% vs. 0.3% HR 0.96 (0.56–1.65) |
2.1% vs. 1.3% HR 1.62 (1.27–2.08) |
|
SPS3 (2012)143 (N = 3020; 36.5% with DM) |
Symptomatic lacuna stroke within 6 months |
Clopidogrel 75 mg/day plus Aspirin 325 mg/day |
Aspirin 325 mg/day | 3.4 years |
3.1% vs. 3.4% HR 0.89 (0.72–1.11) |
2.0% vs. 2.4% HR 0.82 (0.63–1.09) |
0.4% vs. 0.3% HR 1.65 (0.83–3.31) |
2.1% vs. 1.1% HR 1.97 (1.41–2.71) |
|
Greving et al. (2019)144 (N = 43 112; 33.3% with DM) |
Meta-analysis (6 RCTs) | Clopidogrel | Aspirin | 2.0 years | 0.88 (0.78–0.98) | 0.91 (0.81–1.02) | 0.63 (0.43–0.91) | 0.76 (0.63–0.91) |
| Aspirin/dipyridamole | 0.83 (0.74–0.94) | 0.86 (0.76–0.97) | 0.88 (0.60–1.31) | 0.86 (0.71–1.05) | ||||
| Aspirin/clopidogrel | 0.83 (0.71–0.96) | 0.83 (0.71–0.97) | 1.19 (0.68–2.08) | 1.63 (1.29–2.07) | ||||
| Aspirin/dipyridamole | Clopidogrel | 0.95 (0.85–1.06) | 0.95 (0.87–1.04) | 1.40 (1.08–1.82) | 1.14 (1.00–1.30) | |||
| Aspirin/clopidogrel | 0.94 (0.82–1.08) | 0.91 (0.80–1.04) | 1.88 (1.12–3.16) | 2.16 (1.72–2.71) | ||||
| Aspirin/clopidogrel | Aspirin/dipyridamole | 0.99 (0.84–1.17) | 0.96 (0.82–1.13) | 1.34 (0.77–2.36) | 1.89 (1.47–2.42) | |||
| Anti-coagulant randomized trials for primary and secondary preventions in patients with atrial fibrillation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Patients | Intervention | Control | Follow-up | Stroke or systemic embolic event | Ischaemic stroke | Intracranial haemorrhage | Major haemorrhage |
|
ARISTOTLE (2011)149 (N = 18 201; 25% with DM) |
Patients with AF CHADS2 >1 |
Apixaban 5 mg b.i.d. Apixaban 2.5 mg b.i.d. in age >80 years, body weight <60 kg, creatinine >1.5 mg/mL |
Warfarin (keep INR 2.0–3.0) | 2 years |
1.27% vs. 1.60% HR 0.79 (0.66–0.95) |
0.97% vs. 1.05% HR 0.92 (0.74–1.13) |
0.33% vs. 0.80% HR 0.42 (0.30–0.58) |
2.13% vs. 3.09% HR 0.69 (0.60–0.80) |
|
ARISTOTLE (2015)153 DM subgroup (N = 4547) |
DM patients were younger, more CAD, higher CHADS2 and HAS-BLED |
1.39% vs. 1.86% HR 0.75 (0.53–1.05) |
Not reported |
0.34% vs. 0.70% HR 0.49 (0.25–0.95) |
3.01% vs. 3.13% HR 0.96 (0.74–1.25) |
|||
|
RE-LY (2009)148 (N = 18 113; 23.3% with DM) |
Patients with AF CHADS2 >1 or CHA2DS2-VASc >2 for men or >3 for women |
Dabigatran 110 mg b.i.d. | Warfarin (keep INR 2.0–3.0) | 2 years |
1.53% vs. 1.69% HR 0.91 (0.74–1.11) |
1.34% vs. 1.20% HR 1.11 (0.89–1.40) |
0.23% vs. 0.74% HR 0.31 (0.20–0.47) |
2.71% vs. 3.36% HR 0.80 (0.69–0.93) |
| Dabigatran 150 mg b.i.d. |
1.11% vs. 1.69% HR 0.66 (0.53–0.82) |
0.92% vs. 1.20% HR 0.76 (0.60–0.98) |
0.3% vs. 0.74% HR 0.40 (0.27–0.60) |
3.11% vs. 3.36% HR 0.93 (0.81–1.07) |
||||
|
RE-LY (2015)152 DM subgroup (N = 4221) |
DM patients were younger, more CAD and PAD, higher CHA2DS2VASc scores | Dabigatran 110 mg b.i.d. | Warfarin (keep INR 2.0–3.0) |
1.76% vs. 2.35% HR 0.74 (0.51–1.07) |
1.62% vs. 1.65% HR 0.97 (0.64–1.40) |
0.22% vs. 0.81% HR 0.26 (0.11–0.65) |
3.81% vs. 4.19% HR 0.91 (0.70–1.19) |
|
| Dabigatran 150 mg b.i.d. |
1.46% vs. 2.35% HR 0.61 (0.41–0.91) |
1.28% vs. 1.65% HR 0.76 (0.49–1.19) |
0.47% vs. 0.81% HR 0.58 (0.29–1.16) |
4.66% vs. 4.19% HR 1.12 (0.87–1.44) |
||||
|
ROCKET AF (2011)150 (N = 14 264; 39.9% with DM) |
Patients with AF CHADS2 >2 |
Rivaroxaban 20 mg/day (15 mg/day if creatinine clearance 30–49 mL/min) |
Warfarin (keep INR 2.0–3.0) | 1.9 years |
1.7% vs. 2.2% HR 0.79 (0.66–0.96) |
2.11% vs. 2.27% HR 0.94 (0.75–1.17) |
0.8% vs. 1.2% HR 0.67 (0.47–0.93) |
5.6% vs. 5.4% HR 1.04 (0.90–1.20) |
|
ROCKET AF (2015)154 DM subgroup (N = 5695) |
DM patients were younger, more obese, higher BP, similar CHADS2 scores |
1.7% vs. 2.1% HR 0.82 (0.63–1.08) |
1.35% vs. 1.45% HR 0.94 (0.69–1.30) |
0.5% vs. 0.8% HR 0.62 (0.36–1.05) |
3.8% vs. 3.9% HR 1.00 (0.81–1.24) |
|||
|
ENGAGE AF-TIMI 48 (2013)151 (N = 21 105; 36.1% with DM) |
Patients with AF CHADS2 >2 |
Edoxaban 30 mg/day (15 mg/day if creatinine clearance 30–50 mL/min, body weight <60 kg, or concomitant use of verapamil or quinidine) |
Warfarin (keep INR 2.0–3.0) | 2.8 years |
1.61% vs. 1.50% HR 1.07 (0.87–1.31) |
1.77% vs. 1.25% HR 1.41 (1.19–1.67) |
0.26% vs. 0.85% HR 0.30 (0.21–0.43) |
1.61% vs. 3.43% HR 0.47 (0.41–0.55) |
|
Edoxaban 60 mg/day (30 mg/day if creatinine clearance 30–50 mL/min, body weight <60 kg, or concomitant use of verapamil or quinidine) |
1.18% vs. 1.50% HR 0.79 (0.63–0.99) |
1.25% vs. 1.25% HR 1.00 (0.83–1.19) |
0.39% vs. 0.85% HR 0.47 (0.34–0.63) |
2.75% vs. 3.43% HR 0.80 (0.71–0.91) |
||||
| Antiplatelet platelet and anticoagulant for secondary prevention in atrial fibrillation patients ineligible for vitamin K antagonist | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Patients | Intervention | Control | Follow-up | Stroke, systemic emboli, MI, CVD death | Ischaemic stroke | Intracranial haemorrhage | Major haemorrhage |
|
AVERROES (2011)155 (N = 5599; 19.6% with DM) |
Patients with AF Ineligible for VKA CHADS2 >1, or documented PAD |
Apixaban 5 mg b.i.d. Apixaban 2.5 mg b.i.d. in age >80 years, body weight <60 kg, creatinine >1.5 mg/mL |
Aspirin 81–324 mg/day | 1.1 years |
4.2% vs. 6.4% HR 0.66 (0.53–0.83) |
1.1% vs. 3.0% HR 0.37 (0.25–0.55) |
0.4% vs. 0.4% HR 0.85 (0.38–1.90) |
1.4% vs. 1.2% HR 1.13 (0.74–1.75) |
Antiplatelet and anticoagulant studies for secondary prevention in individuals with cerebrovascular disease.
Significant differences are highlighted in bold.
b.i.d., twice daily; CAD, coronary artery disease; CHA2DS2-VASc, score, Congestive Heart failure, hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Sex (female); CHADS2 score, Cardiac failure, Hypertension, Age, Diabetes, Stroke (doubled); CVD, cardiovascular disease; DM, diabetes mellitus; HAS-BLED score, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (65 years), drugs/alcohol concomitantly (1 point each); HR, hazard ratio; MI, myocardial infarction; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; PAD, peripheral artery disease; TIA, transient ischaemic stroke; VKA, vitamin K antagonist.
Long-term non-cardioembolic stroke prevention
The effect of aspirin on secondary prevention in stroke/TIA patients is well-established, as are the effects of clopidogrel and aspirin/dipyridamole combination.138 The benefits of aspirin (75–150 mg daily) appear to be most pronounced in the first 6–12 weeks following the event, while combination with dipyridamole may offer better longer-term protection.139 Clopidogrel is as good as aspirin/dipyridamole combination and is preferred for secondary prevention, particularly with the frequent headaches with aspirin/dipyridamole combination leading to discontinuation.140 , 141
DAPT for long-term use is discouraged as studies showed excessive bleeding without a vascular benefit.142–145
Taken together, for the management of DM individuals, aspirin is justified 24 h after an acute event requiring reperfusion therapy followed by a switch to clopidogrel 3 months later (or continue aspirin while adding dipyridamole). In those not receiving reperfusion therapy, DAPT can be immediately started (provided haemorrhagic stroke is ruled out) and continued for 21 days followed by long-term monotherapy with clopidogrel (or a combination of aspirin/dipyridamole), which applies to individuals with and without diabetes (Figure 2).139–141
Stroke prevention in association with non-valvular atrial fibrillation
As discussed above, anticoagulation is recommended for those with AF and elevated CHA2DS2-VASc score, of which DM is a component.113 , 146 , 147
Potential superior efficacy of NOACs, together with reduced bleeding events and reduced need for monitoring, offers distinct advantages over VKAs. An analysis of four large RCTs [RE-LY, ARISTOTLE, ROCKET-AF, and ENGAGE AF-TIMI 48 (23%, 25%, 40%, and 36% with DM, respectively)],148–151 enrolling over 70 000 patients with non-valvular AF with at least one additional risk factor for stroke, demonstrated that dabigatran or factor Xa inhibitors (apixaban, rivaroxaban, edoxaban) are at least as efficacious as warfarin, and in some cases superior, in preventing stroke, whilst reducing bleeding risk. Sub-analysis of DM patients in these trials showed similar anti-thrombotic benefits of NOACs but bleeding risk reduction appeared to be attenuated.152–154
In AF patients who are ineligible for VKA, apixaban (AVERROES trial; 20% with diabetes) is the only NOAC that has demonstrated superior efficacy to aspirin in preventing MACE (stroke, systemic embolism, MI, and vascular death) with similar bleeding risk in the whole study population with no subgroup analysis conducted for those with DM.155
In summary, current evidence supports OAC in DM individuals with AF with or without a history of stroke who fulfil treatment criteria and do not have excessive bleeding risk. NOACs are preferable to VKAs in eligible patients.
Timing of initiation (or therapy resumption) of OAC in AF patients suffering an acute stroke is a challenging area. Based largely on two studies, RAF and RAF-NOACs,156 , 157 the American Heart Association/American Stroke Association 2018/2019 guidelines recommend starting OAC within 4–14 days of an acute ischaemic stroke.132 The European Heart Rhythm Association-ESC guidelines give a more structured recommendation with the ‘1–3–6–12 days rule’.158 In brief, OAC should be initiated or reinstated after 1 day for TIA, 3 days for mild stroke (NIHSS score <8), 6 days for moderate stroke (NIHSS score 8–15), and 12 days for severe stroke (NIHSS score ≥16). These recommendations are based solely on expert consensus without robust RCTs supporting this approach. Of note, bridging with full-dose low-molecular-weight heparin before or together with VKA is not recommended.159 Table 4 summarizes key studies on antithrombotic agents in cerebrovascular disease.
Conclusions and future directions
While a large number of studies investigated the best antithrombotic strategy in vascular disease patients, there is still a distinct lack of DM-specific RCTs, particularly for secondary prevention. The heterogeneous vascular risk in DM patients, which can vary in the same individual according to DM duration and development of complications, adds to the complexity and prevents guidelines from making concrete recommendations. For example, advanced renal disease may alter both thrombosis and bleeding risk and may even limit the use of some antithrombotic therapies.33 The increased weight in DM individuals may also affect the response to antithrombotic agents, reviewed elsewhere.160 A key difficulty remains the lack of biomarker(s) that accurately predicts thrombotic/bleeding risk and response to therapy.
Given current knowledge, primary prevention with antiplatelet agents, mainly aspirin, may only be considered in higher-risk individuals. Following ACS, DAPT is necessary using aspirin and ticagrelor or prasugrel, usually for 12 months but also longer term with aspirin and ticagrelor in high thrombotic risk patients. In stable atherosclerotic disease, the combination of aspirin and very-low-dose rivaroxaban is useful, particularly in the presence of PAD (Figure 2).161 In individuals with stroke, the choice of antithrombotic therapy is dictated by whether the individual required reperfusion and the presence of AF (Figure 2 and Graphical Abstract).
Areas for future research include the development of reliable biomarkers and/or in silico model, able to assess thrombotic risk and response to therapy. Moreover, DM-specific studies are warranted rather than subgroup, and often post hoc, analyses of cardiovascular trials designed for the wider population (DM-specific studies are summarized in Supplementary material online, Table S3 and gaps in knowledge/future work in Supplementary material online, Table S4). This will require greater collaboration between metabolic and vascular medical disciplines to design appropriate studies aiming to reduce vascular events and improve clinical outcomes in the high-risk DM population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
N.K. is funded by Faculty of Medicine, Prince of Songkla University, Thailand. Research work in Ajjan’s laboratory is funded by the National Institute for Health Research, Diabetes UK, British Heart Foundation, Biotechnology and Biological Sciences Research Council, Abbott Diabetes Care and Avacta Life Sciences.
Conflict of interest: R.A.A. reports grants, personal fees and other from Abbott Diabetes Care, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from Menarini Pharmaceuticals, personal fees from Novo Nordisk, outside the submitted work; N.K. has nothing to disclose; L.B. reports grants from AstraZeneca, the European Union-IMI and -H2020, Carlos III Institute of Health-Spain, and CIBERCV-Spain; personal fees (scientific advisory boards/speaker fees) from AstraZeneca, Lilly, BMS/Pfizer, SANOFI, BAYER, International Aspirin Foundation, Glycardial SL, PACE and FICYE, all outside the submitted work; G.V. reports grants from AstraZeneca, outside the submitted work; D.A.G. reports personal fees and other from Astra Zeneca, grants from Bayer, personal fees from Boehringer Ingelheim, outside the submitted work; D.J.A. reports grants and personal fees from Amgen, grants and personal fees from Aralez, grants and personal fees from Bayer, grants and personal fees from Biosensors, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Chiesi, grants and personal fees from Daiichi-Sankyo, grants and personal fees from Eli Lilly, personal fees from Haemonetics, grants and personal fees from Janssen, grants and personal fees from Merck, personal fees from PhaseBio, personal fees from PLx Pharma, personal fees from Pfizer, grants and personal fees from Sanofi, personal fees from the Medicines company, grants and personal fees from CeloNova, personal fees from St Jude Medical, grants from CSL Behring, grants from Eisai, grants from Gilead, grants from Idorsia Pharmaceuticals Ltd, grants from Matsutani Chemical Industry Co., grants from Novartis, grants from Osprey Medical, grants from Renal Guard Solutions, grants from Scott R. MacKenzie Foundation, grants and personal fees from Astra Zeneca, outside the submitted work; D.R. has nothing to disclose; B.R. reports personal fees from Novartis, personal fees from Medscape, grants from Bayer AG, grants from Italian Medicines Agency, outside the submitted work; R.F.S. reports personal fees from Bayer, personal fees from Bristol-Myers Squibb/Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Thromboserin, personal fees from Haemonetics, personal fees from Amgen, grants and personal fees from Glycardial Diagnostics, personal fees from Portola, personal fees from Medscape, grants and personal fees from Cytosorbents, personal fees from Intas Pharmaceuticals, personal fees from Hengrui, personal fees from Sanofi Aventis, personal fees from Idorsia, personal fees from PhaseBio, outside the submitted work.
Supplementary Material
Contributor Information
Ramzi A Ajjan, The LIGHT Laboratories, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds LS2 7JT, UK.
Noppadol Kietsiriroje, The LIGHT Laboratories, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds LS2 7JT, UK; Endocrinology and Metabolism Unit, Internal Medicine Department, Faculty of Medicine, Prince of Songkla University, Songkhla 90110, Thailand.
Lina Badimon, Cardiovascular Program ICCC, Research Institute Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Sant Antoni M. Claret 167, 08025 Barcelona, Spain; Centro de Investigación Biomédica en Red Cardiovascular (CIBERCV), Instituto de Salud Carlos III, Sant Antoni M. Claret 167, 08025 Barcelona, Spain; Cardiovascular Research Chair, Universidad Autónoma Barcelona (UAB), Sant Antoni M. Claret 167, 08025 Barcelona, Spain.
Gemma Vilahur, Cardiovascular Program ICCC, Research Institute Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Sant Antoni M. Claret 167, 08025 Barcelona, Spain; Centro de Investigación Biomédica en Red Cardiovascular (CIBERCV), Instituto de Salud Carlos III, Sant Antoni M. Claret 167, 08025 Barcelona, Spain.
Diana A Gorog, University of Hertfordshire, College Lane Campus Hatfield, Hertfordshire AL10 9AB, UK; National Heart and Lung Institute, Guy Scadding Building, Dovehouse St, London SW3 6LY, UK.
Dominick J Angiolillo, Division of Cardiology, University of Florida College of Medicine – Jacksonville, 655 West, 8th Street, Jacksonville, FL 32209, USA.
David A Russell, The LIGHT Laboratories, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds LS2 7JT, UK; Leeds Vascular Institute, Leeds General Infirmary, Great George Street, Leeds LS1 3EX, UK.
Bianca Rocca, Institute of Pharmacology, Catholic University School of Medicine, Rome, Italy.
Robert F Storey, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Beech Hill Road, Sheffield S10 2RX, UK.
References
- 1.Emerging Risk Factors Collaboration; Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O'Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Håheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WMM, Salomaa V, Svärdsudd K, van der Harst P, Björkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr ELM, Iso H, Onat A, Trevisan M, D'Agostino RB, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJG, Leening M, Rosengren A, Dörr M, Deeg DJH, Kiechl S, Stehouwer CDA, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundström J, Woodward M, Brunner EJ, Khaw K-T, Wareham NJ, Whitsel EA, Njølstad I, Hedblad B, Wassertheil-Smoller S, Engström G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosentino F, Ceriello A, Baeres FMM, Fioretto P, Garber A, Stough WG, George JT, Grant PJ, Khunti K, Langkilde AM, Plutzky J, Ryden L, Scheen A, Standl E, Tuomilehto J, Zannad F. Addressing cardiovascular risk in type 2 diabetes mellitus: a report from the European Society of Cardiology Cardiovascular Roundtable. Eur Heart J 2019;40:2907–2919. [DOI] [PubMed] [Google Scholar]
- 4. Carrizzo A, Izzo C, Oliveti M, Alfano A, Virtuoso N, Capunzo M, Di Pietro P, Calabrese M, De Simone E, Sciarretta S, Frati G, Migliarino S, Damato A, Ambrosio M, De Caro F, Vecchione C. The main determinants of diabetes mellitus vascular complications: endothelial dysfunction and platelet hyperaggregation. Int J Mol Sci 2018;19:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivas Rios JR, Franchi F, Rollini F, Angiolillo DJ. Diabetes and antiplatelet therapy: from bench to bedside. Cardiovasc Diagn Ther 2018;8:594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol 2017;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: a PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore) 2016;95:e4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pretorius E. Platelets as potent signaling entities in type 2 diabetes mellitus. Trends Endocrinol Metab 2019;30:532–545. [DOI] [PubMed] [Google Scholar]
- 9. Soma P, Swanepoel AC, Du Plooy JN, Mqoco T, Pretorius E. Flow cytometric analysis of platelets type 2 diabetes mellitus reveals ‘angry’ platelets. Cardiovasc Diabetol 2016;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghoshal K, Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. ScientificWorldJournal 2014;2014:781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patrono C, Rocca B. Measurement of thromboxane biosynthesis in health and disease. Front Pharmacol 2019;10:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watala C, Boncer M, Golański J, Koziołkiewicz W, Trojanowski Z, Walkowiak B. Platelet membrane lipid fluidity and intraplatelet calcium mobilization in type 2 diabetes mellitus. Eur J Haematol 2009;61:319–326. [DOI] [PubMed] [Google Scholar]
- 13. Santilli F, Simeone P, Liani R, Davi G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat 2015;120:28–39. [DOI] [PubMed] [Google Scholar]
- 14. Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation 2000;101:2833–2840. [DOI] [PubMed] [Google Scholar]
- 15. Hunter RW, Hers I. Insulin/IGF-1 hybrid receptor expression on human platelets: consequences for the effect of insulin on platelet function. J Thromb Haemost 2009;7:2123–2130. [DOI] [PubMed] [Google Scholar]
- 16. Westein E, Hoefer T, Calkin AC. Thrombosis in diabetes: a shear flow effect? Clin Sci (Lond) 2017;131:1245–1260. [DOI] [PubMed] [Google Scholar]
- 17. Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001;24:1476–1485. [DOI] [PubMed] [Google Scholar]
- 18. Fejes Z, Poliska S, Czimmerer Z, Kaplar M, Penyige A, Gal Szabo G, Beke Debreceni I, Kunapuli SP, Kappelmayer J, Nagy BJ. Hyperglycaemia suppresses microRNA expression in platelets to increase P2RY12 and SELP levels in type 2 diabetes mellitus. Thromb Haemost 2017;117:529–542. [DOI] [PubMed] [Google Scholar]
- 19. Pordzik J, Jakubik D, Jarosz-Popek J, Wicik Z, Eyileten C, De Rosa S, Indolfi C, Siller-Matula JM, Czajka P, Postula M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: bioinformatic analysis and review. Cardiovasc Diabetol 2019;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gawaz M, Ott I, Reininger AJ, Neumann FJ. Effects of magnesium on platelet aggregation and adhesion. Magnesium modulates surface expression of glycoproteins on platelets in vitro and ex vivo. Thromb Haemost 1994;72:912–918. [PubMed] [Google Scholar]
- 21. Hernández Vera R, Vilahur G, Ferrer-Lorente R, Peña E, Badimon L. Platelets derived from the bone marrow of diabetic animals show dysregulated endoplasmic reticulum stress proteins that contribute to increased thrombosis. Arterioscler Thromb Vasc Biol 2012;32:2141–2148. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Beck W, Deppisch R, Marshall SM, Hoenich NA, Thompson MG. Advanced glycation end products elicit externalization of phosphatidylserine in a subpopulation of platelets via 5-HT2A/2C receptors. Am J Physiol Cell Physiol 2007;293:C328–C336. [DOI] [PubMed] [Google Scholar]
- 23. Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, Lattanzio S, Mattoscio D, Zaccardi F, Liani R, Vazzana N, Del Ponte A, Ferrante E, Martini F, Cardillo C, Morosetti R, Mirabella M, Ghirlanda G, Davi G, Patrono C. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012;10:1220–1230. [DOI] [PubMed] [Google Scholar]
- 24. Vilahur G, Ben-Aicha S, Badimon L. New insights into the role of adipose tissue in thrombosis. Cardiovasc Res 2017;113:1046–1054. [DOI] [PubMed] [Google Scholar]
- 25. Ferreiro JL, Gomez-Hospital JA, Angiolillo DJ. Platelet abnormalities in diabetes mellitus. Diab Vasc Dis Res 2010;7:251–259. [DOI] [PubMed] [Google Scholar]
- 26. Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res 2010;7:260–273. [DOI] [PubMed] [Google Scholar]
- 27. Kim HK, Kim JE, Park SH, Kim YI, Nam-Goong IS, Kim ES. High coagulation factor levels and low protein C levels contribute to enhanced thrombin generation in patients with diabetes who do not have macrovascular complications. J Diabetes Complications 2014;28:365–369. [DOI] [PubMed] [Google Scholar]
- 28. Ajjan RA, Gamlen T, Standeven KF, Mughal S, Hess K, Smith KA, Dunn EJ, Anwar MM, Rabbani N, Thornalley PJ, Philippou H, Grant PJ. Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme-specific activity. Blood 2013;122:134–142. [DOI] [PubMed] [Google Scholar]
- 29. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 2000;97:12222–12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, Maya J, Nicolau JC, Spinar J, Storey RF, Stevens SR, Wallentin L; PLATO Study Group. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2010;31:3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ducrocq G, Wallace JS, Baron G, Ravaud P, Alberts MJ, Wilson PWF, Ohman EM, Brennan DM, D'Agostino RB, Bhatt DL, Steg PG; on behalf of the REACH Investigators. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J 2010;31:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lemesle G, Meurice T, Tricot O, Lamblin N, Bauters C. Association of diabetic status and glycemic control with ischemic and bleeding outcomes in patients with stable coronary artery disease: the 5-Year CORONOR Registry. J Am Heart Assoc 2018;7:e008354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goel N, Jain D, Haddad DB, Shanbhogue D. Anticoagulation in patients with end-stage renal disease and atrial fibrillation: confusion, concerns and consequences. J Stroke 2020;22:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogami K, Muraki I, Imano H, Iso H. Risk of disseminated intravascular coagulation in patients with type 2 diabetes mellitus: retrospective cohort study. BMJ Open 2017;7:e013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373–2383. [DOI] [PubMed] [Google Scholar]
- 36. Capodanno D, Angiolillo DJ. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation 2016;134:1579–1594. [DOI] [PubMed] [Google Scholar]
- 37. Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010;74:597–607. [DOI] [PubMed] [Google Scholar]
- 38. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30–47. [DOI] [PubMed] [Google Scholar]
- 39. Andreotti F, Testa L, Biondi-Zoccai GG, Crea F. Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: an updated and comprehensive meta-analysis of 25,307 patients. Eur Heart J 2006;27:519–526. [DOI] [PubMed] [Google Scholar]
- 40. Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J 2010;31:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Angiolillo DJ, Ferreiro JL. Antiplatelet and anticoagulant therapy for atherothrombotic disease: the role of current and emerging agents. Am J Cardiovasc Drugs 2013;13:233–250. [DOI] [PubMed] [Google Scholar]
- 42. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008;300:2134–2141. [DOI] [PubMed] [Google Scholar]
- 44. Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 46. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 47. Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, Held C, Andersson M, Himmelmann A, Ridderstråle W, Leonsson-Zachrisson M, Liu Y, Opolski G, Zateyshchikov D, Ge J, Nicolau JC, Corbalán R, Cornel JH, Widimský P, Leiter LA; THEMIS Steering Committee and Investigators. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 2019;381:1309–1320. [DOI] [PubMed] [Google Scholar]
- 48. Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, Im K, Murphy SA, Held P, Braunwald E, Sabatine MS, Steg PG. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732–2740. [DOI] [PubMed] [Google Scholar]
- 49. Vranckx P, White HD, Huang Z, Mahaffey KW, Armstrong PW, Van de Werf F, Moliterno DJ, Wallentin L, Held C, Aylward PE, Cornel JH, Bode C, Huber K, Nicolau JC, Ruzyllo W, Harrington RA, Tricoci P. Validation of BARC bleeding criteria in patients with acute coronary syndromes: the TRACER trial. J Am Coll Cardiol 2016;67:2135–2144. [DOI] [PubMed] [Google Scholar]
- 50. Seidu S, Kunutsor SK, Sesso HD, Gaziano JM, Buring JE, Roncaglioni MC, Khunti K. Aspirin has potential benefits for primary prevention of cardiovascular outcomes in diabetes: updated literature-based and individual participant data meta-analyses of randomized controlled trials. Cardiovasc Diabetol 2019;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Association. 10. Cardiovascular Disease and Risk Management: standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42(Suppl 1):S103–S123. [DOI] [PubMed] [Google Scholar]
- 53. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 54. Bell AD, Roussin A, Cartier R, Chan WS, Douketis JD, Gupta A, Kraw ME, Lindsay TF, Love MP, Pannu N, Rabasa-Lhoret R, Shuaib A, Teal P, Theroux P, Turpie AG, Welsh RC, Tanguay JF. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines Executive Summary. Can J Cardiol 2011;27:208–221. [DOI] [PubMed] [Google Scholar]
- 55. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 56. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 57. Alexopoulos D, Vogiatzi C, Stavrou K, Vlassopoulou N, Perperis A, Pentara I, Xanthopoulou I. Diabetes mellitus and platelet reactivity in patients under prasugrel or ticagrelor treatment: an observational study. Cardiovasc Diabetol 2015;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thomas MR, Angiolillo DJ, Bonaca MP, Ajjan RA, Judge HM, Rollini F, Franchi F, Ahsan AJ, Bhatt DL, Kuder JF, Steg PG, Cohen M, Muthusamy R, Sabatine MS, Storey RF. Consistent platelet inhibition with ticagrelor 60 mg twice-daily following myocardial infarction regardless of diabetes status. Thromb Haemost 2017;117:940–947. [DOI] [PubMed] [Google Scholar]
- 59. Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, Tomasello SD, Capranzano P, Seecheran N, Darlington A, Tello-Montoliu A, Desai B, Bass TA, Angiolillo DJ. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011;4:180–187. [DOI] [PubMed] [Google Scholar]
- 60. Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost 2011;106:491–499. [DOI] [PubMed] [Google Scholar]
- 61. Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, Fox KA, Granger CB, Jolly S, Joyner CD, Rupprecht HJ, Widimsky P, Afzal R, Pogue J, Yusuf S; CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930–942. [DOI] [PubMed] [Google Scholar]
- 62. Johnston A, Jones WS, Hernandez AF. The ADAPTABLE trial and aspirin dosing in secondary prevention for patients with coronary artery disease. Curr Cardiol Rep 2016;18:81. [DOI] [PubMed] [Google Scholar]
- 63. Angiolillo DJ, Bhatt DL, Lanza F, Cryer B, Dong JF, Jeske W, Zimmerman RR, von Chong E, Prats J, Deliargyris EN, Marathi U. Pharmacokinetic/pharmacodynamic assessment of a novel, pharmaceutical lipid-aspirin complex: results of a randomized, crossover, bioequivalence study. J Thromb Thrombolysis 2019;48:554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bhatt DL, Grosser T, Dong J-F, Logan D, Jeske W, Angiolillo DJ, Frelinger AL, Lei L, Liang J, Moore JE, Cryer B, Marathi U. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol 2017;69:603–612. [DOI] [PubMed] [Google Scholar]
- 65. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 66. Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM; TRITON-TIMI 38 Investigators. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation 2008;118:1626–1636. [DOI] [PubMed] [Google Scholar]
- 67. Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, Ueno M, Darlington A, Desai B, Moser BA, Sugidachi A, Guzman LA, Bass TA. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 2014;64:1005–1014. [DOI] [PubMed] [Google Scholar]
- 68. Franchi F, James SK, Ghukasyan LT, Budaj AJ, Cornel JH, Katus HA, Keltai M, Kontny F, Lewis BS, Storey RF, Himmelmann A, Wallentin L, Angiolillo DJ; PLATO Investigators. Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y12 receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. J Am Heart Assoc 2019;8:e011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schupke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wohrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flugel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Mollmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schuhlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tolg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A; ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–1534. [DOI] [PubMed] [Google Scholar]
- 70. Ndrepepa G, Kastrati A, Menichelli M, Neumann FJ, Wohrle J, Bernlochner I, Richardt G, Witzenbichler B, Sibbing D, Gewalt S, Angiolillo DJ, Hamm CW, Hapfelmeier A, Trenk D, Laugwitz KL, Schunkert H, Schupke S, Mayer K. Ticagrelor or prasugrel in patients with acute coronary syndromes and diabetes mellitus. JACC Cardiovasc Interv 2020;13:2238–2247. [DOI] [PubMed] [Google Scholar]
- 71. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, Delarche N, Bellemain-Appaix A, Range G, El Mahmoud R, Carrie D, Belle L, Souteyrand G, Aubry P, Sabouret P, Du Fretay XH, Beygui F, Bonnet JL, Lattuca B, Pouillot C, Varenne O, Boueri Z, Van Belle E, Henry P, Motreff P, Elhadad S, Salem JE, Abtan J, Rousseau H, Collet JP, Vicaut E, Montalescot G; ANTARCTIC Investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015–2022. [DOI] [PubMed] [Google Scholar]
- 72. Hein R, Gross L, Aradi D, Rieber J, Hadamitzky M, Merkely B, Huczek Z, Ince H, Hummel A, Baylacher M, Massberg S, Trenk D, Sibbing D. Diabetes and outcomes following guided de-escalation of antiplatelet treatment in acute coronary syndrome patients undergoing percutaneous coronary intervention: a pre-specified analysis from the randomised TROPICAL-ACS trial. EuroIntervention 2019;15:e513–e521. [DOI] [PubMed] [Google Scholar]
- 73. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Dzavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 74. Angiolillo DJ, Baber U, Sartori S, Briguori C, Dangas G, Cohen DJ, Mehta SR, Gibson CM, Chandiramani R, Huber K, Kornowski R, Weisz G, Kunadian V, Oldroyd KG, Ya-Ling H, Kaul U, Witzenbichler B, Dudek D, Sardella G, Escaned J, Sharma S, Shlofmitz RA, Collier T, Pocock S, Mehran R. Ticagrelor with or without aspirin in high-risk patients with diabetes mellitus undergoing percutaneous coronary intervention. J Am Coll Cardiol 2020;75:2403–2413. [DOI] [PubMed] [Google Scholar]
- 75. Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Mollmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S; GLOBAL LEADERS Investigators. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–949. [DOI] [PubMed] [Google Scholar]
- 76. Chichareon P, Modolo R, Kogame N, Takahashi K, Chang C-C, Tomaniak M, Botelho R, Eeckhout E, Hofma S, Trendafilova-Lazarova D, Kőszegi Z, Iñiguez A, Wykrzykowska JJ, Piek JJ, Garg S, Hamm C, Steg PG, Jüni P, Vranckx P, Valgimigli M, Windecker S, Onuma Y, Serruys PW. Association of diabetes with outcomes in patients undergoing contemporary percutaneous coronary intervention: pre-specified subgroup analysis from the randomized GLOBAL LEADERS study. Atherosclerosis 2020;295:45–53. [DOI] [PubMed] [Google Scholar]
- 77. Gao C, Tomaniak M, Takahashi K, Kawashima H, Wang R, Hara H, Ono M, Montalescot G, Garg S, Haude M, Slagboom T, Vranckx P, Valgimigli M, Windecker S, van Geuns RJ, Hamm C, Steg PG, Onuma Y, Angiolillo DJ, Serruys PW. Ticagrelor monotherapy in patients with concomitant diabetes mellitus and chronic kidney disease: a post hoc analysis of the GLOBAL LEADERS trial. Cardiovasc Diabetol 2020;19:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Malik AH, Yandrapalli S, Shetty SS, Aronow WS, Cooper HA, Panza JA. Meta-analysis of dual antiplatelet therapy versus monotherapy with P2Y12 inhibitors in patients after percutaneous coronary intervention. Am J Cardiol 2020;127:25–29. [DOI] [PubMed] [Google Scholar]
- 79. Capodanno D, Morice MC, Angiolillo DJ, Bhatt DL, Byrne RA, Colleran R, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock SJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhovel U, Krucoff MW, Urban P, Mehran R. Trial design principles for patients at high bleeding risk undergoing PCI: JACC Scientific Expert Panel. J Am Coll Cardiol 2020;76:1468–1483. [DOI] [PubMed] [Google Scholar]
- 80. Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Heeschen C, Hamm CW, Moliterno DJ, Califf RM, White HD, Kleiman NS, Theroux P, Topol EJ. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 2001;104:2767–2771. [DOI] [PubMed] [Google Scholar]
- 81. Kastrati A, Mehilli J, Neumann FJ, Dotzer F, ten Berg J, Bollwein H, Graf I, Ibrahim M, Pache J, Seyfarth M, Schuhlen H, Dirschinger J, Berger PB, Schomig A; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006;295:1531–1538. [DOI] [PubMed] [Google Scholar]
- 82. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 83. Zwart B, Yazdani M, Ow KW, Richardson JD, Iqbal J, Gunn JP, Storey RF. Use of glycoprotein IIb/IIIa antagonists to prevent stent thrombosis in morphine-treated patients with ST-elevation myocardial infarction. Platelets 2020;31:174–178. [DOI] [PubMed] [Google Scholar]
- 84. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM; ATLAS ACS 2-TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 85. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 86.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 87. Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002;90:625–628. [DOI] [PubMed] [Google Scholar]
- 88. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Meredith IT, Tanguay JF, Kereiakes DJ, Cutlip DE, Yeh RW, Garratt KN, Lee DP, Steg PG, Weaver WD, Holmes DR Jr, Brindis RG, Trebacz J, Massaro JM, Hsieh WH, Mauri L; DAPT Study Investigators. Diabetes mellitus and prevention of late myocardial infarction after coronary stenting in the randomized dual antiplatelet therapy study. Circulation 2016;133:1772–1782. [DOI] [PubMed] [Google Scholar]
- 90. Gargiulo G, Windecker S, da Costa BR, Feres F, Hong MK, Gilard M, Kim HS, Colombo A, Bhatt DL, Kim BK, Morice MC, Park KW, Chieffo A, Palmerini T, Stone GW, Valgimigli M. Short term versus long term dual antiplatelet therapy after implantation of drug eluting stent in patients with or without diabetes: systematic review and meta-analysis of individual participant data from randomised trials. BMJ 2016;355:i5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 92. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 93. Sumaya W, Geisler T, Kristensen SD, Storey RF. Dual antiplatelet or dual antithrombotic therapy for secondary prevention in high-risk patients with stable coronary artery disease? Thromb Haemost 2019;119:1583–1589. [DOI] [PubMed] [Google Scholar]
- 94. Anand SS, Eikelboom JW, Dyal L, Bosch J, Neumann C, Widimsky P, Avezum AA, Probstfield J, Cook Bruns N, Fox KAA, Bhatt DL, Connolly SJ, Yusuf S; COMPASS Trial Investigators. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol 2019;73:3271–3280. [DOI] [PubMed] [Google Scholar]
- 95. Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA; TRA 2P-TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 96. Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wilcox R, Morrow DA; TRA 2°P-TIMI 50 Steering Committee and Investigators. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet 2012;380:1317–1324. [DOI] [PubMed] [Google Scholar]
- 97. Cavender MA, Scirica BM, Bonaca MP, Angiolillo DJ, Dalby AJ, Dellborg M, Morais J, Murphy SA, Ophuis TO, Tendera M, Braunwald E, Morrow DA. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events-TIMI 50 trial. Circulation 2015;131:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Storey RF. The long journey of individualizing antiplatelet therapy after acute coronary syndromes. Eur Heart J 2020;41:3546–3548. [DOI] [PubMed] [Google Scholar]
- 99. Pallisgaard JL, Schjerning AM, Lindhardt TB, Procida K, Hansen ML, Torp-Pedersen C, Gislason GH. Risk of atrial fibrillation in diabetes mellitus: a nationwide cohort study. Eur J Prev Cardiol 2016;23:621–627. [DOI] [PubMed] [Google Scholar]
- 100. Overvad TF, Skjoth F, Lip GY, Lane DA, Albertsen IE, Rasmussen LH, Larsen TB. Duration of diabetes mellitus and risk of thromboembolism and bleeding in atrial fibrillation: nationwide cohort study. Stroke 2015;46:2168–2174. [DOI] [PubMed] [Google Scholar]
- 101. Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010;25:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol 2019;74:1107–1115. [DOI] [PubMed] [Google Scholar]
- 103. Plitt A, McGuire DK, Giugliano RP. Atrial fibrillation, type 2 diabetes, and non-vitamin K antagonist oral anticoagulants: a review. JAMA Cardiol 2017;2:442–448. [DOI] [PubMed] [Google Scholar]
- 104. Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 2012;98:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Anselmino M, Matta M, D'Ascenzo F, Pappone C, Santinelli V, Bunch TJ, Neumann T, Schilling RJ, Hunter RJ, Noelker G, Fiala M, Frontera A, Thomas G, Katritsis D, Jais P, Weerasooriya R, Kalman JM, Gaita F. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace 2015;17:1518–1525. [DOI] [PubMed] [Google Scholar]
- 106. Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol 2014;114:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, Meng L, Li G, Liu T. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord 2017;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Budaj A, Kiss RG, Padilla F, Gause-Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation 2020;141:1227–1234. [DOI] [PubMed] [Google Scholar]
- 110. Chan YH, Lee HF, Li PR, Liu JR, Chao TF, Wu LS, Chang SH, Yeh YH, Kuo CT, See LC, Lip GYH. Effectiveness, safety, and major adverse limb events in atrial fibrillation patients with concomitant diabetes mellitus treated with non-vitamin K antagonist oral anticoagulants. Cardiovasc Diabetol 2020;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 112. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, Holmes DR Jr; PREVAIL and PROTECT AF Investigators. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 113. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 114. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American Perspective—2018 update. Circulation 2018;138:527–536. [DOI] [PubMed] [Google Scholar]
- 115. Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, Ten Berg J, Gersh BJ, Bhatt DL, Angiolillo DJ. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:83–99. [DOI] [PubMed] [Google Scholar]
- 116. Boriani G, Fauchier L, Aguinaga L, Beattie JM, Blomstrom Lundqvist C, Cohen A, Dan G-A, Genovesi S, Israel C, Joung B, Kalarus Z, Lampert R, Malavasi VL, Mansourati J, Mont L, Potpara T, Thornton A, Lip GYH, Gorenek B, Marin F, Dagres N, Ozcan EE, Lenarczyk R, Crijns HJ, Guo Y, Proietti M, Sticherling C, Huang D, Daubert JP, Pokorney SD, Cabrera Ortega M, Chin A; ESC Scientific Document Group. European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS). Europace 2019;21:7–8. [DOI] [PubMed] [Google Scholar]
- 117. Lip GYH, Collet J-P, Haude M, Byrne R, Chung EH, Fauchier L, Halvorsen S, Lau D, Lopez-Cabanillas N, Lettino M, Marin F, Obel I, Rubboli A, Storey RF, Valgimigli M, Huber K, Potpara T, Blomström Lundqvist C, Crijns H, Steffel J, Heidbüchel H, Stankovic G, Airaksinen J, Ten Berg JM, Capodanno D, James S, Bueno H, Morais J, Sibbing D, Rocca B, Hsieh M-H, Akoum N, Lockwood DJ, Gomez Flores JR, Jardine R; ESC Scientific Document Group. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019;21:192–193. [DOI] [PubMed] [Google Scholar]
- 118. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 119. Alberts MJ, Bhatt DL, Mas J-L, Ohman EM, Hirsch AT, Rother J, Salette G, Goto S, Smith SC, Liau C-S, Wilson PWF, Steg PG; for the REduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009;30:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Verma S, Bhatt DL, Bain SC, Buse JB, Mann JFE, Marso SP, Nauck MA, Poulter NR, Pratley RE, Zinman B, Michelsen MM, Monk Fries T, Rasmussen S, Leiter LA, Leader PC, On B, Of The LTI; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation 2018;137:2179–2183. [DOI] [PubMed] [Google Scholar]
- 121. Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303:841–848. [DOI] [PubMed] [Google Scholar]
- 122. Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, Blomster J, Millegard M, Reist C, Patel MR; EUCLID Trial Steering Committee and Investigators. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32–40. [DOI] [PubMed] [Google Scholar]
- 123. Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA; CHARISMA Investigators. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J 2008;30:192–201. [DOI] [PubMed] [Google Scholar]
- 124. Bonaca MP, Scirica BM, Creager MA, Olin J, Bounameaux H, Dellborg M, Lamp JM, Murphy SA, Braunwald E, Morrow DA. Vorapaxar in patients with peripheral artery disease: results from TRA2°P-TIMI 50. Circulation 2013;127:1522–1529. [DOI] [PubMed] [Google Scholar]
- 125. Katsanos K, Spiliopoulos S, Saha P, Diamantopoulos A, Karunanithy N, Krokidis M, Modarai B, Karnabatidis D. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: a systematic review and network meta-analysis. PLoS One 2015;10:e0135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 127. Belch JJ, Dormandy JCASPAR Writing CommitteeBiasi GM, Biasi BM, Cairols M, Diehm C, Eikelboom B, Golledge J, Jawien A, Lepäntalo M, Norgren L, Hiatt WR, Becquemin JP, Bergqvist D, Clement D, Baumgartner I, Minar E, Stonebridge P, Vermassen F, Matyas L, Leizorovicz A. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg 2010;52:825–833. [DOI] [PubMed] [Google Scholar]
- 128. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet 2000;355:346–351. [PubMed] [Google Scholar]
- 129. Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, Seeger JM. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg 1998;28:446–457. [DOI] [PubMed] [Google Scholar]
- 130. Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, Hess CN, Pap AF, Kittelson JM, Gudz I, Matyas L, Krievins DK, Diaz R, Brodmann M, Muehlhofer E, Haskell LP, Berkowitz SD, Hiatt WR. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020;382:1994–2004. [DOI] [PubMed] [Google Scholar]
- 131. Capodanno D, Alberts M, Angiolillo DJ. Antithrombotic therapy for secondary prevention of atherothrombotic events in cerebrovascular disease. Nat Rev Cardiol 2016;13:609–622. [DOI] [PubMed] [Google Scholar]
- 132. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: A Guideline for healthcare professionals from the American Heart Association/American Stroke Association . Stroke 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 133. Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KS; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016;375:35–43. [DOI] [PubMed] [Google Scholar]
- 134. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 135. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y; THALES Investigators. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med 2020;383:207–217. [DOI] [PubMed] [Google Scholar]
- 137. Hankey GJ. Antithrombotic therapy for stroke prevention. Circulation 2019;139:1131–1133. [DOI] [PubMed] [Google Scholar]
- 138.Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet 2016;388:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A; ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665–1673. [DOI] [PubMed] [Google Scholar]
- 141. Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 143. Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Greving JP, Diener HC, Reitsma JB, Bath PM, Csiba L, Hacke W, Kappelle LJ, Koudstaal PJ, Leys D, Mas JL, Sacco RL, Algra A; for the Cerebrovascular Antiplatelet Trialists’ Collaborative Group. Antiplatelet therapy after noncardioembolic stroke. Stroke 2019;50:1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 146. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 147. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, Liu L, Damasceno A, Grinvalds A, Nakamya J, Reilly PA, Keltai K, Van Gelder IC, Yusufali AH, Watanabe E, Wallentin L, Connolly SJ, Yusuf S; RE-LY Atrial Fibrillation Registry Investigators. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–1576. [DOI] [PubMed] [Google Scholar]
- 148. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 149. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 150. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 151. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 152. Brambatti M, Darius H, Oldgren J, Clemens A, Noack HH, Brueckmann M, Yusuf S, Wallentin L, Ezekowitz MD, Connolly SJ, Healey JS. Comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: results from the RE-LY trial. Int J Cardiol 2015;196:127–131. [DOI] [PubMed] [Google Scholar]
- 153. Ezekowitz JA, Lewis BS, Lopes RD, Wojdyla DM, McMurray JJ, Hanna M, Atar D, Cecilia Bahit M, Keltai M, Lopez-Sendon JL, Pais P, Ruzyllo W, Wallentin L, Granger CB, Alexander JH. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharmacother 2015;1:86–94. [DOI] [PubMed] [Google Scholar]
- 154. Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, Becker RC, Breithardt G, Hacke W, Hankey GJ, Nessel CC, Singer DE, Berkowitz SD, Piccini JP, Mahaffey KW, Fox KA; ROCKET AF Steering Committee and Investigators. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). Am Heart J 2015;170:675–682. [DOI] [PubMed] [Google Scholar]
- 155. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 156. Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, Rueckert C, Pezzini A, Poli L, Padovani A, Csiba L, Szabo L, Sohn SI, Tassinari T, Abdul-Rahim AH, Michel P, Cordier M, Vanacker P, Remillard S, Alberti A, Venti M, Scoditti U, Denti L, Orlandi G, Chiti A, Gialdini G, Bovi P, Carletti M, Rigatelli A, Putaala J, Tatlisumak T, Masotti L, Lorenzini G, Tassi R, Guideri F, Martini G, Tsivgoulis G, Vadikolias K, Liantinioti C, Corea F, Del Sette M, Ageno W, De Lodovici ML, Bono G, Baldi A, D'Anna S, Sacco S, Carolei A, Tiseo C, Acciarresi M, D'Amore C, Imberti D, Zabzuni D, Doronin B, Volodina V, Consoli D, Galati F, Pieroni A, Toni D, Monaco S, Baronello MM, Barlinn K, Pallesen LP, Kepplinger J, Bodechtel U, Gerber J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Mosconi MG, Bubba V, Silvestri I, Lees KR. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke 2015;46:2175–2182. [DOI] [PubMed] [Google Scholar]
- 157. Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, Chondrogianni M, Bovi P, Carletti M, Cappellari M, Zedde M, Ntaios G, Karagkiozi E, Athanasakis G, Makaritsis K, Silvestrelli G, Lanari A, Ciccone A, Putaala J, Tomppo L, Tatlisumak T, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Becattini C, Mosconi MG, Cimini LA, Soloperto R, Masotti L, Vannucchi V, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Sohn SI, Marcheselli S, Mumoli N, De Lodovici ML, Bono G, Furie KL, Tadi P, Yaghi S, Toni D, Letteri F, Tassinari T, Kargiotis O, Lotti EM, Flomin Y, Mancuso M, Maccarrone M, Giannini N, Bandini F, Pezzini A, Poli L, Padovani A, Scoditti U, Denti L, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Gourbali V, Orlandi G, Giuntini M, Chiti A, Giorli E, Gialdini G, Corea F, Ageno W, Bellesini M, Colombo G, Monaco S, Maimone BM, Karapanayiotides T, Caso V. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. J Am Heart Assoc 2017;6:e007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 159. Altavilla R, Caso V, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, Tadi P, Becattini C, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D’Amore C, Giulia Mosconi M, Anna Cimini L, Fusaro J, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn S-I, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Athanasakis G, Makaritsis K, Karagkiozi E, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Silvestrelli G, Ciccone A, Lanari A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Ulivi L, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D’Anna S, Toni D, Letteri F, Giuntini M, Maria Lotti E, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Maimone Baronello M, Csiba L, Szabó L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen L-P, Barlinn J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Paciaroni M. Anticoagulation after stroke in patients with atrial fibrillation. Stroke 2019;50:2093–2100. [DOI] [PubMed] [Google Scholar]
- 160. Rocca B, Fox KAA, Ajjan RA, Andreotti F, Baigent C, Collet JP, Grove EL, Halvorsen S, Huber K, Morais J, Patrono C, Rubboli A, Seljeflot I, Sibbing D, Siegbahn A, Ten Berg J, Vilahur G, Verheugt FWA, Wallentin L, Weiss TW, Wojta J, Storey RF. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J 2018;39:1672–1686f. [DOI] [PubMed] [Google Scholar]
- 161. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, Branch KRH, Probstfield J, Bosch J, Shestakovska O, Szarek M, Maggioni AP, Widimský P, Avezum A, Diaz R, Lewis BS, Berkowitz SD, Fox KAA, Ryden L, Yusuf S; COMPASS Steering Committee and Investigators. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS trial. Circulation 2020;141:1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.