Abstract
Gastric cancer (GC) is one of the most common cancers globally, threatening global health. The deregulation of the Hippo signaling pathway has been discovered in GC and may be related to cancer development, proliferation, metastasis, and drug resistance. Yes-associated protein (YAP), as a downstream effector of the Hippo signaling pathway and a crucial co-transcription factor in the nucleus, is a promising and vital potential drug target for the treatment of GC. A series of drugs or compounds that inhibit YAP has been developed or confirmed. Therefore, this review will focus on summarizing the drugs and small-molecule inhibitors that have been reported to inhibit YAP and discuss the clinical prospects of YAP inhibitors in GC.
Keywords: gastric adenocarcinoma, Yes-associated protein, Hippo pathway, small molecule inhibitor, targeted therapy
Introduction
According to the statistics of GLOBOCAN 2020,1 gastric cancer (GC) is the fifth most frequently diagnosed cancer (5.6% of the total cases) and the fourth most common cause of cancer death (7.7% of the total cancer deaths), which seriously threatens the health of humans worldwide.
Recently, the deregulation of the Hippo signaling pathway has been shown to occur in various solid tumors, including GC, and is related to cancer development, proliferation, metastasis, and drug resistance.2–7 The Hippo pathway was initially discovered in Drosophila and later proved to be highly conserved in humans. It is an essential regulatory mechanism for regulating organ size and plays a crucial role in controlling cell proliferation and apoptosis.5,8,9 The core axis of the canonical Hippo pathway is composed of the phosphorylation cascade of MST1/2-LATS1/2-YAP/TAZ, which can be activated by a variety of extracellular signals, such as cell-to-cell contact, mechanical force, and hormones.10,11 The activation of the Hippo pathway will eventually lead to phosphorylation on multiple serine residues of Yes-associated protein (YAP) (S61, S109, S127, S164, and S397). Phosphorylated YAP can be sequestered in the cytoplasm by interacting with 14-3-3 protein and then degraded by the ubiquitin-proteasome system. Dephosphorylated YAP/TAZ enters the nucleus and promotes the transcription of genes that mediate proliferation and migration.11 As a downstream effector of the Hippo signaling pathway,10 YAP plays a crucial role in controlling gastric epithelial cell proliferation.
Therapy resistance is a major challenge in GC cancer treatment. Emerging evidence indicates that YAP may be a pivotal target of resistance to various targeted and chemotherapies. As the downstream of YAP, multiple transcription targets may contribute to chemoresistance downstream of YAP/TAZ.12,13 A recent study found that receptor tyrosine kinase (RTK) erythropoietin-producing hepatocellular receptor A2 (EphA2) interacts with phosphorylates YAP, contributing to stabilization, nuclear translocation, and activation of YAP in GC cells. EphA2 mediates chemotherapy resistance through increasing YAP stability and nuclear accumulation.14 Besides, Annexin A6 in Extracellular vesicles (EV) from cancer-associated fibroblasts (CAF) plays a critical role in drug resistance of GC in the extracellular matrix (ECM) via activation of β1 integrin-focal adhesion kinase (FAK)-YAP. The inhibition of YAP significantly attenuated gastric cancer drug resistance in vitro and in vivo.15
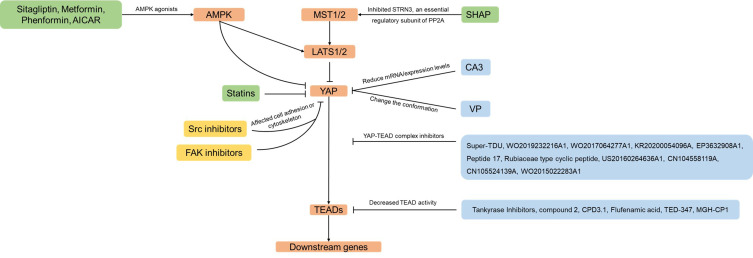
Only moderate YAP expression was observed in the hyperplasia area of the normal gastric epithelium, while the YAP expression and nuclear localization were continuously observed to increase significantly in both primary and metastatic GC.16–19 In various GC cell models, silencing YAP can inhibit GC cell proliferation, colony formation, and metastasis.16,20,21 More and more studies showed that YAP is a promising and vital potential drug target for GC treatment, and a series of drugs or compounds that inhibit YAP has been developed or confirmed. Therefore, we will summarize the drugs and small molecule inhibitors reported to inhibit YAP and discuss the clinical prospects of YAP inhibitors in GC (Figure 1).
Figure 1.
Drugs that inhibit YAP in this review. Arrows and blunt ends indicate activation and inhibition. These include various mechanisms that would result in YAP inhibition, such as inhibitors of YAP-TEAD complex (shown in blue boxes), inhibitors of YAP nuclear localization (shown in green boxes) or inhibitors of cell adhesion or cytoskeleton (shown in yellow boxes).
The Core Pharmacological Action of YAP Inhibitors and Representative Drugs
Disruption of YAP-TEAD Complex
YAP protein does not contain intrinsic DNA-binding domains. Thus, YAP binds to target gene promoters by interacting with DNA-binding transcription factors, such as TEADs. In human cells, there are four TEAD proteins, namely TEAD1-4. Four TEADs all contain the TEA domain that binds to DNA at the N-terminus, and the YAP binding domain (YBD) binds to YAP at the C-terminus.22 When the Hippo pathway is inactive, dephosphorylated YAP enters the nucleus, binding to TEAD protein. After forming a complex, it triggers the transcription of the downstream gene that promotes cell proliferation, EMT occurrence, and maintenance of stemness.23 Compared to the inhibition of the Hippo pathway’s upstream proteins, which are more interconnected with other signaling networks, the YAP-TEAD complex, the last step of the Hippo/YAP pathway, emerges as the better candidate target for modulating the Hippo/YAP signaling.
Furthermore, the possible potential side effects should be less than those of upstream protein inhibitors. The following are representative inhibitors that block the YAP-TEAD complex: Veteprofin, CA3, Super-TDU (shown in Tables 1 and 2). Besides, Tables 1 and 2 also summarize the novel inhibitors and patients we have found in the literature.
Table 1.
Compounds Inhibit the YAP-TEAD Interactions
| Ref | Name/Patent Number | Structure | Mechanism |
|---|---|---|---|
| [24–26] | Verteporfin |
 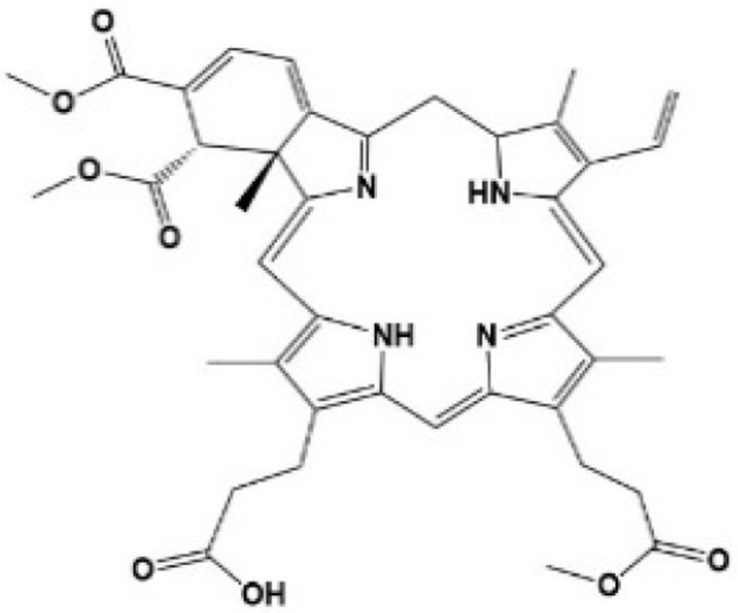
|
Inhibited the transcription and protein levels of YAP, selectively bound YAP, changed the conformation of YAP, and hindered the interaction between YAP and TEAD |
| [38] | CA3 | 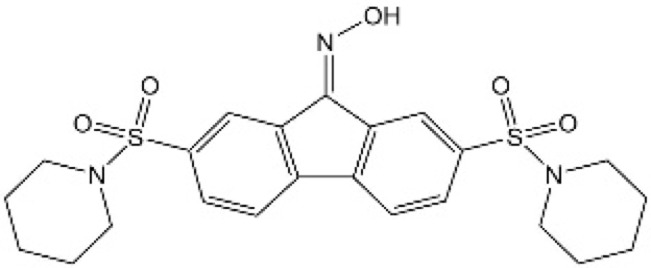 |
Inhibited the transcription and protein levels of YAP1 and hindered YAP-TEAD transcription activity |
| [39] | Compound 2 | 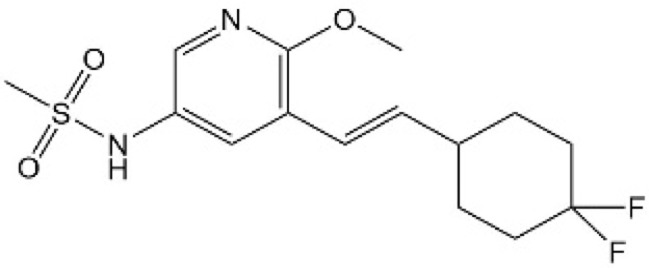 |
Bound the TEAD lipid pocket and disrupted TEAD S-palmitoylation to inhibit YAP-TEAD interaction. |
| [40] | CPD3.1 | 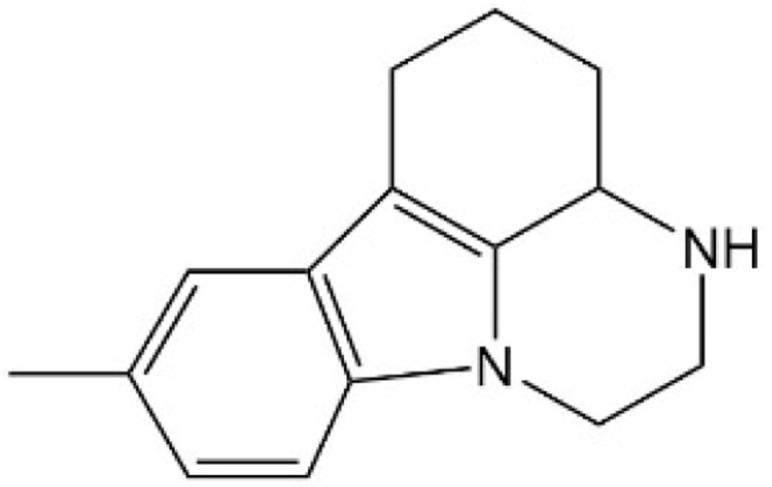 |
Disrupted YAP-TEAD interaction and inhibited TEAD activity. |
| [41] | Flufenamic acid | 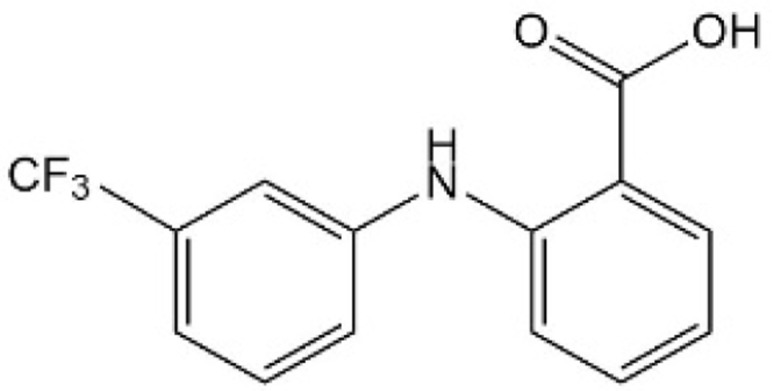 |
Bound to the central pocket of YAP binding domain in TEAD2 and inhibited YAP-TEAD dependent transcription |
| [42] | TED-347 | 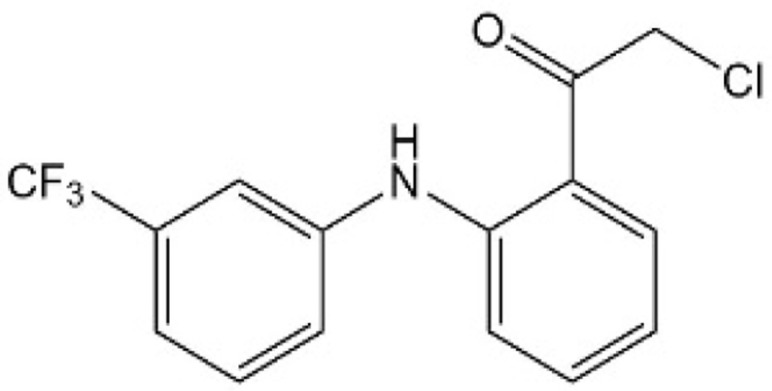 |
Formed a covalent bond with a conserved cysteine within the palmitate binding pocket of TEADs to inhibit the YAP-TEAD interaction |
| [43] | MGH-CP1 | 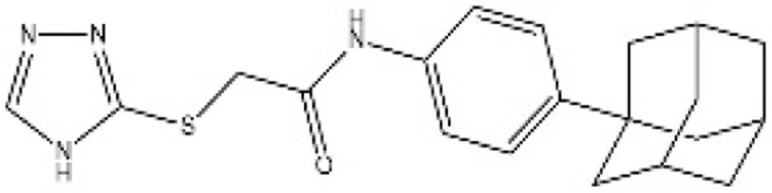 |
Inhibited TEAD2 autopalmitoylation and TEAD1 palmitoylation to disrupt YAP-TEAD interaction |
| [44] | WO2019232216A1 | 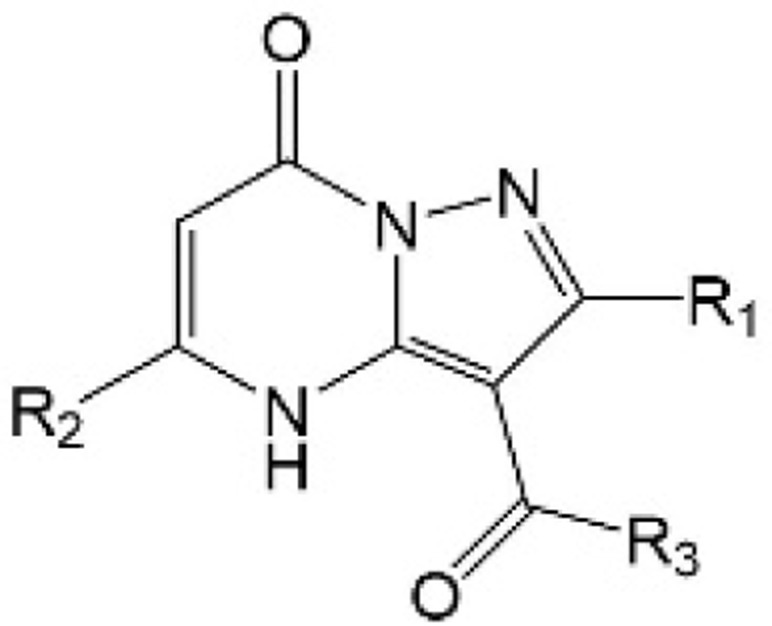 |
Inhibited the YAP-TEAD protein interaction. |
| [45] | WO2017064277A1 | 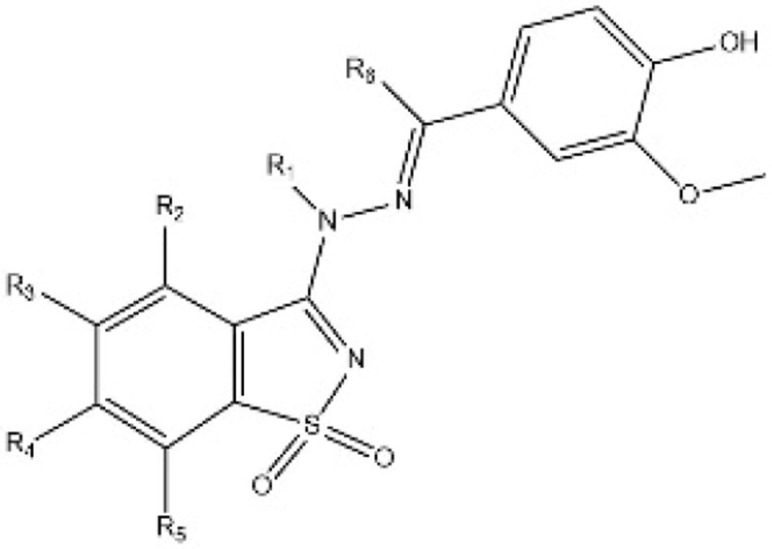 |
Inhibited the YAP-TEAD protein interaction. |
| [46] | KR20200054096A | 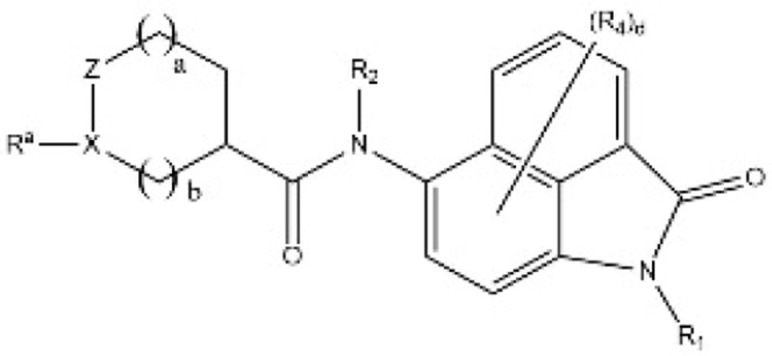 |
Inhibited the YAP-TEAD protein interaction. |
| [47] | EP3632908A1 | 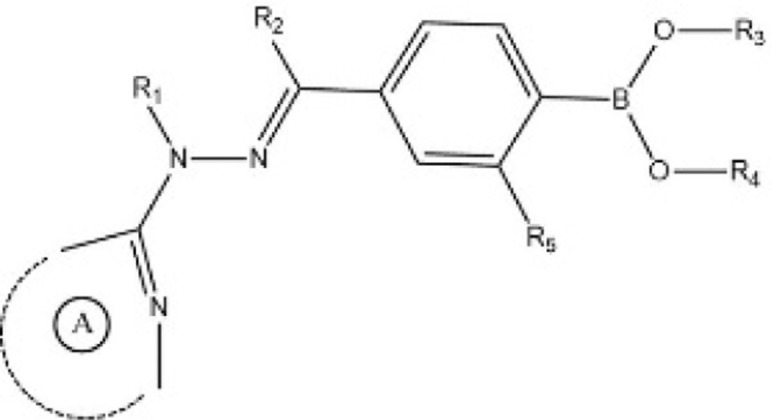 |
Inhibited the YAP-TEAD protein interaction. |
Table 2.
Peptide Inhibitors Block the YAP-TEAD Interactions
| Ref | Name/Patent Number | Mechanism |
|---|---|---|
| [35] | Super-TDU | Directly targeted TEADs and reduced the YAP-TEAD interaction |
| [48] | Peptide 17 | Potent cyclic peptide inhibitors of the YAP-TEAD interaction |
| [49] | Rubiaceae type cyclic peptide | Inhibited YAP-TEAD transcription activity |
| [50] | US20160264636A1 | A derived chimeric peptide linked to a cell-penetrating peptide inhibiting the interaction between the TEAD and YAP or TAZ proteins |
| [51] | CN104558119A | Inhibited the bonding activity of the YAP protein and TEAD |
| [52] | CN105524139A | Inhibited the bonding activity of the YAP protein and TEAD |
| [53] | WO2015022283A1 | Inhibited the bonding activity of the YAP protein and TEAD |
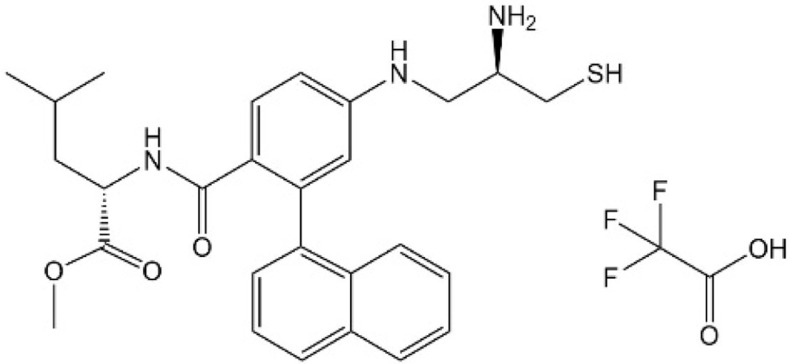
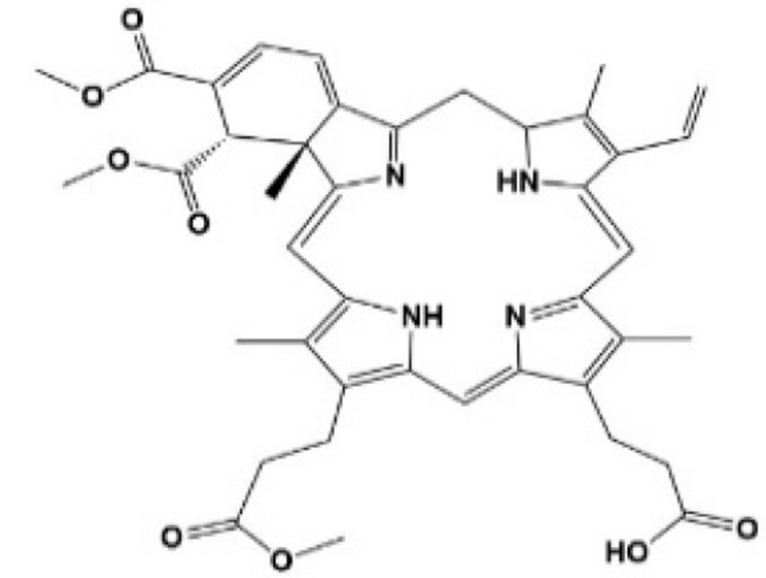
Verteporfin
Verteporfin (VP) is a benzoporphyrin derivative compound. Initially, it was thought to be a photosensitizer activated by laser to produce reactive oxygen radicals that can eliminate the abnormal blood vessels. Clinically, it is usually used for photodynamic therapy to treat ocular diseases such as macular vascular degeneration. Some recent studies showed that VP also could selectively bind YAP without laser activation, change the conformation of YAP, hinder the interaction between YAP and TEAD, and prevent YAP-induced overgrowth of tumor cells.24,25
Giraud et al found that after VP treated MKN45 and MKN74 GC cell lines for 48 hours, cell growth and proliferation were inhibited. Most of the cells were in the G0/G1 phase, and the number of cells in the S phase decreased. At the same time, the mRNA levels of the YAP-TEAD downstream genes CTGF and CYR61 decreased.
Moreover, in a xenograft model derived from GC patients, VP reduces YAP-TEAD transcription activity, inhibits the proliferation of gastric cancer stem cells (CSC), and inhibits the growth of GC in vivo. Interestingly, it was found that YAP and TEAD1 mRNA levels were also down-regulated after VP treatment, indicating that these effectors might be their target genes.26 As previously reported in lung cancer, the VP can also decrease the YAP1 protein level.27 The possible mechanism for YAP1 protein reduction is that VP increases the level of 14-3-3σ, promotes the translocation of YAP from the nucleus to the cytoplasm, targeting its degradation in the proteasome.28
Super-TDU
Similar to YAP, the vestigial like family member 4 (VGLL1-4) does not contain a DNA-binding domain. It also exerts its transcriptional regulatory functions through pairing with TEADs via Tondu (TDU) domain at the C-terminus.29–33 Studies have confirmed that VGLL4 and YAP directly compete with TEAD and form a complex with TEAD through two TDU domains, thereby inhibiting the cancer cell growth and progression.34,35 Super-TDU, a VGLL4 mimetic peptide, directly targeted TEADs and reduced the YAP-TEAD interaction, thereby down-regulating the expression of YAP downstream genes CTGF, CYR61, and CDX2 dose-dependently in GC.35 In vitro, the Super-TDU inhibited cell viability and colony formation of GC cell lines. A preclinical study showed that the sizes and weights of tumors and YAP target genes were markedly decreased for the Super-TDU treated animals in a dose-dependent manner. Notably, Super-TDU may be specific to tumors with an elevated YAP/VGLL4 ratio suggesting that increased YAP/VGLL4 ratio was possibly a potential biomarker for selecting the patients who might benefit from Super-TDU drugs. Jiao et al also found that after the treatment of Super-TDU, the number of tumors in H. pylori-infected GC mouse is significantly reduced, compared with those in the control group, indicating an apparent therapeutic effect of the Super-TDU on gastric carcinogenesis.35
It is worth mentioning that Interferon regulatory factor 2 binding protein 2 (IRF2BP2) can bind to VGLL4, resulting in enhanced interaction between YAP1 and TEAD4 and promoting the transcription of the downstream gene CTGF of YAP1.36 Whereas, knockdown of IRF2BP2 can increase the binding of TEAD4 to VGLL4, thereby reducing the binding of TEAD4 to YAP1. The combination of IRF2BP2 and VGLL4 may also promote PD-L1 expression through IRF2 inhibition and induce immune escape.37 These studies suggest that VGLL4 is a potential therapeutic target for GC, and VGLL4 mimic peptide may also be a potential targeted drug (Tables 1–2).
Tankyrase Inhibitors
Researchers screened compound libraries of novel kinase inhibitors and commercially available inhibitors and found that XAV-939, a tankyrase (TNKS) inhibitor decreased TEAD and YAP-TEAD reporter activity in HEK293T cells.54,55 Besides, MN-64 and IWR1, two other TNKS inhibitors, were able to inhibit TEAD reporter activity and TEAD-mediated transcription similarly to XAV-939 in HEK293T cells.54 Its primary mechanism is to inhibit the function of YAP by inducing the translocation of YAP from the nucleus to the cytoplasm, independent of the phosphorylation status of YAP.54,55 Notably, in non-small cell lung cancer, XAV-939 has also been found to enhance the inhibitory activity of EGFR inhibitors by inhibiting the YAP signaling pathway.56 Furthermore, the TNKS inhibitor G007-LK was found to inhibit WNT/β-catenin and YAP signaling in a homologous melanoma mouse model, making the cancer cell more sensitive to PD-1 immune checkpoint therapy.57 These indicate that TNKS inhibition may be a new strategy to suppress YAP signaling for combined targeted treatment. Although whether the effects of XAV-939 have not been confirmed in GC, based on its current research results, we still believe that it is a novel YAP inhibitor worth studying in GC.
Inhibition of the Nuclear Localization
Dephosphorylated YAP can only play its role after entering the nucleus and forming a complex with other transcription factors, while the phosphorylated YAP interacting with 14-3-3 protein is usually located in the cytoplasm and degraded by the ubiquitin-proteasome system. Therefore, some drugs suppress the biological function of YAP by preventing YAP nucleus translocation and promoting YAP phosphorylation.
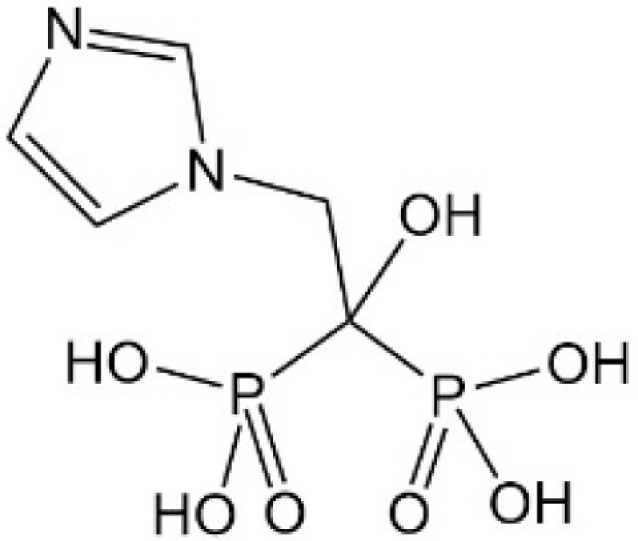
Sitagliptin
Sitagliptin, a dipeptidyl peptidase-IV (DPP4) inhibitor, is widely used in many countries to treat hyperglycemia. Recently, it was found that sitagliptin inhibited YAP nuclear translocation by introducing YAP phosphorylation (Ser 127) and reduced YAP expression in the nucleus of GC cells by regulating AMP-activated protein kinase (AMPK).58 More importantly, besides LATS1/2- dependent YAP phosphorylation, AMPK can also directly phosphorylate YAP at multiple sites and disrupt its interaction with TEAD to inhibit the transcriptional activity of YAP, thereby regulating energy homeostasis. Using AMP-activated protein kinase (AMPK) agonists, such as metformin, phenformin, 5-aminoimidazole-4-carboxamide-1-β-riboside (AICAR), similar phenomena were also found.59–61 However, this mode of action of AMPK and related drugs has not been confirmed in GC and needs to be further validated. Drugs that inhibit YAP by regulating AMPK are listed in Table 3.
Table 3.
Drugs That Inhibit YAP by Regulating the AMPK Pathway
| Ref | Name | Structure | Mechanism |
|---|---|---|---|
| [58] | Sitagliptin | 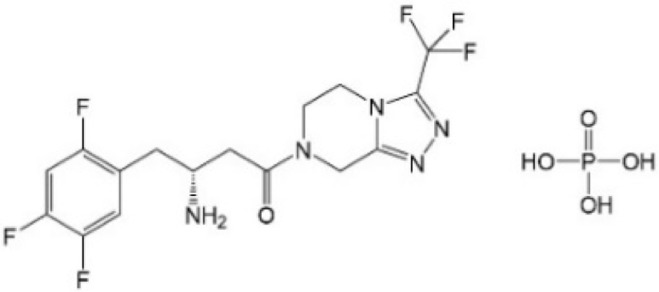 |
Inhibited YAP nuclear localization and reduced YAP expression |
| [59] | Metformin | 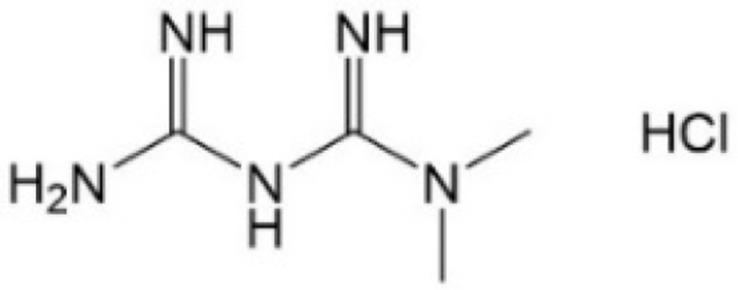 |
Inhibited YAP nuclear localization and activate AMPK (have not confirmed in GC) |
| [59] | Phenformin | 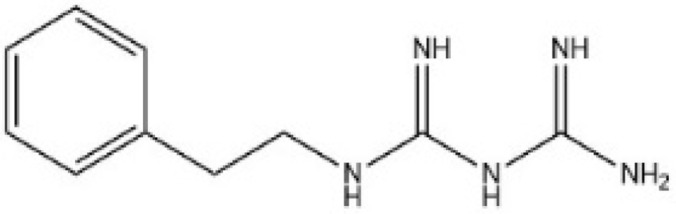 |
Inhibits YAP nuclear localization and activate AMPK (have not confirmed in GC) |
| [59] | AICAR | 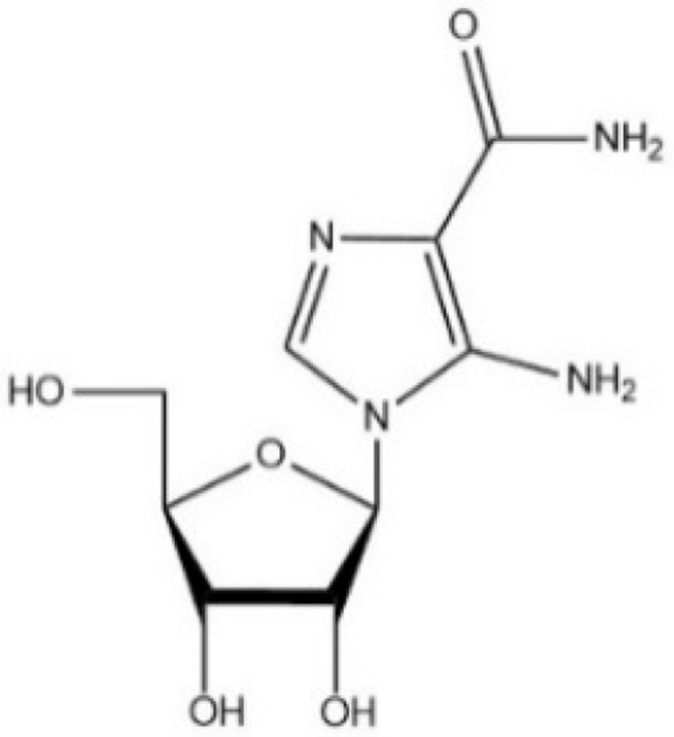 |
Direct activator of AMPK and YAP cytoplasmic retention. AICAR also potently inhibits YAP nuclear localization (have not confirmed in GC) |
Statins
The researchers used the FDA-approved drug library to conduct high-throughput screening based on fluorescence microscopy to identify drugs that significantly induced cytoplasmic relocalization of YAP. From 640 clinically used drugs with known biological activity and safety, they found that statins have the most potent inhibitory effect on YAP. Statins, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), is a hypolipidemic drug used for primary and secondary prevention of cardiovascular disease. Several observational studies have shown a significant reduction in GC risk with statin use. However, the possible cause and mechanism were still controversial and unclear.62,63
Cerivastatin and other drugs regulating the mevalonate (MVA) pathway (shown in Table 4) inhibited the mevalonate pathway and caused a marked accumulation of YAP/TAZ in the cytoplasm of multiple cell lines.64 Later, this effect of statins on YAP inhibition was also confirmed in GC. Simvastatin increased LATS1 and phosphorylated YAP (S127) protein levels and decreased CYR61 expression, but the YAP protein level hardly changed.65 In multiple cancer cell lines (including GC), this inhibitory effect on YAP may be achieved by inhibiting the activity of Rho through the MVA pathway,64–66 and Rho can inhibit the phosphorylation of YAP through LATS1/2.67 These results indicate that the MVA pathway-Rho-YAP/TAZ axis may affect the progression and metastasis of GC.
Table 4.
Drugs That Inhibit YAP by Regulating the MVA Pathway
STRN3-Derived Hippo-Activating Peptide
Tang and colleagues developed an STRN3-derived Hippo-activating peptide (SHAP), the highly selective peptide inhibitor, which has promising anti-cancer effects by inhibiting STRN3, an essential regulatory subunit of phosphatase 2A (PP2A) that recruits MST1/2 and promotes its dephosphorylation, reactivating the tumor suppressor MST1/2 to inhibit YAP activation. SHAP showed the best killing effect at low concentrations among verteporfin, super-TDU, simvastatin, and metformin. In terms of physical properties and toxicity, SHAP not only offers stability similar to verteporfin and statins but also shows water solubility and low toxicity. Comparing the therapeutic effects of SHAP and verteporfin in the PDX model, the results showed that the tumors in the SHAP-treated mice showed almost complete regression, much smaller than those in the control group or verteporfin-treated mice. In addition, SHAP showed a robust therapeutic effect on refractory GC. Simultaneously, although a higher dose (50 mg/kg) of SHAP will cause moderate toxicity in the liver, SHAP has no apparent adverse subacute toxicity in mice at a therapeutic dose. Another advantage of SHAP is that compared to VP or statins with multiple targets, SHAP has strong targeting specificity for PP2A.70 However, the therapeutic effect of SHAP on GC still needs more experimental results to confirm. At the same time, there is still urgent to develop more specific small molecule inhibitors targeting YAP.
Inhibit YAP by Affecting Cell Adhesion or Cytoskeleton
The Hippo/YAP signaling can be affected by many factors, including cell polarity, adherens junctions (AJs), cytoskeleton, mechanical forces, GPCR ligands, and some stress signals.71 Additionally, YAP activity was also affected by cell-cell contact and cell-extracellular matrix (ECM) adhesion. Therefore, the inhibitors of proteins related to those factors might regulate the function of YAP.
Src Inhibitors
Focal adhesion can be described as cell attachment to the extracellular matrix by integrins or intercellular transmembrane receptors, which connect with multiple proteins, including GTPases, actin cytoskeleton, and focal adhesion kinase (FAK). Dasatinib, a small molecule multi-target kinase inhibitor approved by the FDA for the first-line treatment of chronic myeloid leukemia, effectively targeted the proto-oncogene tyrosine-protein kinase Src, promote cell adhesion, and significantly inhibit the invasion and migration of GC cells and was predicted as an effective strategy to inhibit YAP/TAZ in cancer cells potently.72,73 Nevertheless, the precise mechanism of dasatinib in GC remains unclear. In renal cell carcinoma, dasatinib demonstrated that by inhibiting the Src-LATS pathway, inhibiting YAP activity significantly up-regulated YAP phosphorylation, promoting YAP retention in the cytoplasm, reducing YAP-TEAD binding, and further weakening the transcriptional activity of YAP.74 Moreover, selective Src inhibitors PP2 and AZD0530 have been shown to inhibit YAP expression and reduce YAP in the nucleus in colorectal cancer cells in vitro.75,76
FAK Inhibitors
Focal adhesion kinase (FAK) is a central protein of focal adhesions and regulates several cytoskeletal and other focal adhesion proteins. FAK is activated in gastric cancer, responsible for the development and metastasis of cancers by promoting cancer cell proliferation, migration, invasion, adhesion, etc.77,78 PF-573228 and defactinib, FAK inhibitors, attenuated YAP protein levels in the intestinal gastric cancer cell line SNU-719.79 In vitro experiments of colorectal cancer, FAK inhibitors increase the phosphorylated YAP protein, and the mRNA levels and protein levels of cancer stemness markers CD133, ALDH1, and Lgr-5 reduced as well.80 Besides, the FAK inhibitor PF573228 can effectively prevent YAP nuclear accumulation in the transit-amplifying (TA) cells.76 Because FAK plays a vital role in cell adhesion, and studies have shown that whether cells attach to ECM affects YAP phosphorylation status,81 FAK inhibitors are likely to regulate Hippo/YAP signaling pathways by influencing cell adhesion.
Discussion
Recently, studies have shown that over-activation of YAP may increase the therapy resistance of cancer, including GC, to several therapies, including chemotherapy, radiation therapy, EGFR inhibitors, and BRAF or MEK inhibitors.82–87 In vitro studies, YAP enhances gastric cancer cell proliferation and impairs sensitivity to cisplatin potentially via its regulation of EGFR expression, revealing its possible implications for combination therapy.88 Indeed, in other solid tumors, VP can mimic the effect of YAP being knocked down to overcome resistance to RAF inhibitors, tyrosine kinase inhibitors, or chemical drugs.84,85,89,90 Statins targeting HMG-CoA reductase can also inhibit YAP activity by inhibiting YAP nuclear translocation, making cells more sensitive to TKI or MAPK inhibitors.89,91–93 In this case, when used in combination with other therapies used in the treatment of GC, YAP inhibitors may inhibit the Hippo pathway and may reduce the resistance of existing drugs to improve the treatment effect of GC patients. Nevertheless, verteporfin and statins have their drawbacks in clinical applications. Both of them are non-specific YAP inhibitors and need to be in higher molar concentrations to inhibit YAP.93–96 CA3, a novel synthetic compound, had been found remarkable inhibitory activity on YAP1-TEAD transcriptional activity. The study of esophageal adenocarcinoma showed that CA3 was more effective than verteporfin even at one mmol/L.38
The YAP-TEAD complex, the last step of the Hippo/YAP pathway, emerges as a better candidate target for modulating the Hippo/YAP signaling with small molecules, compared to the inhibition of the upstream proteins of the Hippo pathway, which are more interconnected with other signaling networks. However, the crystal structures of the YAP-TEAD complex revealed that disruption of protein–protein interactions tended to be complicated. The interaction interface between YAP-TEAD is a very extended surface.97,98 The large interface and unclear high-affinity binding sites contribute to the difficulty of developing agents that competitively inhibit the YAP-TEAD interaction. Therefore, newer, more efficient compounds still need to be developed. Researchers screened compound libraries of novel kinase inhibitors and commercially available inhibitors and found that XAV-939, a TNKS inhibitor, decreased TEAD and YAP-TEAD reporter activity in HEK293T cells.54,55 Besides, MN-64 and IWR1, two other TNKS inhibitors, were able to inhibit TEAD reporter activity and TEAD-mediated transcription similarly to XAV-939 in HEK293T cells.56 Its primary mechanism is to inhibit the function of YAP by inducing the translocation of YAP from the nucleus to the cytoplasm, independent of the phosphorylation status of YAP.54,55 Notably, in non-small cell lung cancer, XAV-939 has also been found to enhance the inhibitory activity of EGFR inhibitors by inhibiting the YAP signaling pathway.56 Furthermore, the TNKS inhibitor G007-LK was found to inhibit WNT/β-catenin and YAP signaling in a homologous melanoma mouse model, making the cancer cell more sensitive to PD-1 immune checkpoint therapy.57 These indicate that TNKS inhibition may be a new strategy to suppress YAP signaling for combined targeted therapy.
Multiple studies have identified PD-L1 as a direct transcription target of YAP and showed that YAP activation could up-regulate PD-L1 expression and promote tumor immune escape in NSCLC, mesothelioma, and melanoma cells.99–102 The regulation of PD-L1 expression by YAP means that tumors can rely on Hippo signal regulation to evade immune surveillance, and this also suggests the broad application prospects of YAP inhibitors. However, there are still many problems that have not been resolved. It is reported that the regulation of PD-L1 transcription by YAP/TAZ is human-specific,99 which makes it difficult to model this aspect of YAP/TAZ function using traditional mouse models. According to the results of TNKS inhibitors G007-LK, G007-LK inhibits YAP, making cells more sensitive to PD-1 inhibitors. Therefore, YAP inhibitors combined with PD-1 inhibitors may be more effective than combined with PD-L1 inhibitors.57 At the same time, a full understanding of the effect of YAP inhibitors on the immune system is a prerequisite to verify this hypothesis. Therefore, further research is needed to explain the relationship between YAP signaling and immunotherapy from different perspectives.
Conclusion
At present, drugs and small molecule inhibitors that have been reported to inhibit YAP have broad clinical application prospects, but further research on their effects and toxicities in GC is needed. Due to the complexity of the YAP signaling, further study is necessary to investigate the mechanism of the Hippo signal, especially to clarify how YAP enters the nucleus and regulates the downstream genes to verify the therapeutic target and possible side effects.
Funding Statement
This work was supported by the National Science Foundation of China (Liaoning Province Joint Fund) (No. U1908207).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- 3.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu F-X, Meng Z, Plouffe SW, Guan K-L. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77(1):201–227. doi: 10.1146/annurev-physiol-021014-071733 [DOI] [PubMed] [Google Scholar]
- 5.Yu F-X, Zhao B, Guan K-L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen CDK, Yi C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer. 2019;5(5):283–296. doi: 10.1016/j.trecan.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582–1589. doi: 10.1016/j.humpath.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay AM, Camargo FD. Hippo signaling in mammalian stem cells. Semin Cell Dev Biol. 2012;23(7):818–826. doi: 10.1016/j.semcdb.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Dev Dyn. 2012;241(1):3–15. doi: 10.1002/dvdy.22723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu F-X, Guan K-L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi: 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng Z, Moroishi T, Guan K-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. doi: 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Khanal P, Savage P, She YM, Cyr TD, Yang X. YAP-induced resistance of cancer cells to antitubulin drugs is modulated by a Hippo-independent pathway. Cancer Res. 2014;74(16):4493–4503. doi: 10.1158/0008-5472.CAN-13-2712 [DOI] [PubMed] [Google Scholar]
- 13.Yeung B, Khanal P, Mehta V, Trinkle-Mulcahy L, Yang X. Identification of Cdk1-LATS-Pin1 as a novel signaling axis in anti-tubulin drug response of cancer cells. Mol Cancer Res. 2018;16(6):1035–1045. doi: 10.1158/1541-7786.MCR-17-0684 [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Yuan W, Lai C, et al. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int j Cancer. 2020;146(7):1937–1949. doi: 10.1002/ijc.32609 [DOI] [PubMed] [Google Scholar]
- 15.Uchihara T, Miyake K, Yonemura A, et al. Extracellular vesicles from cancer-associated fibroblasts containing Annexin A6 induces FAK-YAP activation by stabilizing β1 integrin, enhancing drug resistance. Cancer Res. 2020;80(16):3222–3235. doi: 10.1158/0008-5472.CAN-19-3803 [DOI] [PubMed] [Google Scholar]
- 16.Kang W, Tong JHM, Chan AWH, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130. doi: 10.1158/1078-0432.CCR-10-2467 [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z, Chen WX. Expression and Yes-associated protein in gastric adenocarcinoma and inhibitory effects of its knockdown on gastric cancer cell proliferation and metastasis. Int J Immunopathol Pharmacol. 2012;25(3):583–590. doi: 10.1177/039463201202500304 [DOI] [PubMed] [Google Scholar]
- 18.Qiao Y, Lin SJ, Chen Y, et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene. 2016;35(20):2664–2674. doi: 10.1038/onc.2015.338 [DOI] [PubMed] [Google Scholar]
- 19.Da CL, Xin Y, Zhao J, Luo XD. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World j Gastroenterol. 2009;15(32):4055–4061. doi: 10.3748/wjg.15.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z, Zhu J-S, Xu Z-P. RNA interference mediated YAP gene silencing inhibits invasion and metastasis of human gastric cancer cell line SGC-7901. Hepatogastroenterology. 2011;58(112):2156–2161. doi: 10.5754/hge11234 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, Zhu J-S, Gao C-P, et al. siRNA targeting YAP gene inhibits gastric carcinoma growth and tumor metastasis in SCID mice. Oncol Lett. 2016;11(4):2806–2814. doi: 10.3892/ol.2016.4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther. 2013;14(5):390–398. doi: 10.4161/cbt.23788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diepenbruck M, Waldmeier L, Ivanek R, et al. Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. J Cell Sci. 2014;127(Pt 7):1523–1536. doi: 10.1242/jcs.139865 [DOI] [PubMed] [Google Scholar]
- 24.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi: 10.1101/gad.192856.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodowska K, Al-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giraud J, Molina-Castro S, Seeneevassen L, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int j Cancer. 2020;146(8):2255–2267. doi: 10.1002/ijc.32667 [DOI] [PubMed] [Google Scholar]
- 27.Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7(32):51922–51933. doi: 10.18632/oncotarget.10458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Zhu X, Feng W, et al. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J Cancer Res. 2016;6(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen -H-H, Mullett SJ, Stewart AFR. Vgl-4, a novel member of the vestigial-like family of transcription cofactors, regulates alpha1-adrenergic activation of gene expression in cardiac myocytes. J Biol Chem. 2004;279(29):30800–30806. doi: 10.1074/jbc.M400154200 [DOI] [PubMed] [Google Scholar]
- 30.Günther S, Mielcarek M, Krüger M, Braun T. VITO-1 is an essential cofactor of TEF1-dependent muscle-specific gene regulation. Nucleic Acids Res. 2004;32(2):791–802. doi: 10.1093/nar/gkh248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda T, Chapman DL, Stewart AFR. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J Biol Chem. 2002;277(50):48889–48898. doi: 10.1074/jbc.M206858200 [DOI] [PubMed] [Google Scholar]
- 32.Vaudin P, Delanoue R, Davidson I, Silber J, Zider A. TONDU (TDU), a novel human protein related to the product of vestigial (vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development. 1999;126(21):4807–4816. doi: 10.1242/dev.126.21.4807 [DOI] [PubMed] [Google Scholar]
- 33.Pobbati AV, Chan SW, Lee I, Song H, Hong W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure. 2012;20(7):1135–1140. doi: 10.1016/j.str.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Gao Y, Li P, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24(3):331–343. doi: 10.1038/cr.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25(2):166–180. doi: 10.1016/j.ccr.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 36.Yao Y, Wang Y, Li L, et al. Down-regulation of interferon regulatory factor 2 binding protein 2 suppresses gastric cancer progression by negatively regulating connective tissue growth factor. J Cell Mol Med. 2019;23(12):8076–8089. doi: 10.1111/jcmm.14677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu A, Wu Q, Deng Y, et al. Loss of VGLL4 suppresses tumor PD-L1 expression and immune evasion. EMBO J. 2019;38:1. doi: 10.15252/embj.201899506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song S, Xie M, Scott AW, et al. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol Cancer Ther. 2018;17(2):443–454. doi: 10.1158/1535-7163.MCT-17-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holden JK, Cunningham CN. Targeting the Hippo pathway and cancer through the TEAD family of transcription factors. Cancers. 2018;10:3. doi: 10.3390/cancers10030081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SA, Sessions RB, Shoemark DK, et al. Antiproliferative and antimigratory effects of a novel YAP-TEAD interaction inhibitor identified using in silico molecular docking. J Med Chem. 2019;62(3):1291–1305. doi: 10.1021/acs.jmedchem.8b01402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pobbati AV, Han X, Hung AW, et al. Targeting the central pocket in human transcription factor TEAD as a potential cancer therapeutic strategy. Structure. 2015;23(11):2076–2086. doi: 10.1016/j.str.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bum-Erdene K, Zhou D, Gonzalez-Gutierrez G, et al. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEAD. Yap protein-protein interaction. Cell Chem Biol. 2019;26:3. doi: 10.1016/j.chembiol.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 43.Wu X; Inventor; The General Hospital Corporation, assignee. TEAD transcription factor autopalmitoylation inhibitors. US patent US10696642B22017. Available from: https://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PG01&s1=20180215721.PGNR.
- 44.Zbieg JR, Beroza PP, Crawford JJ; Inventors; Genentech, Inc. Hoffmann-La Roche Ag, assignee. Therapeutic compounds. US patent WO2019232216A12019. Available from: http://globaldossier.uspto.gov/#/result/publication/WO/2019232216/1.
- 45.Barth M, Contal S, Montalbetti C, Spitzer L; Inventors; Inventiva, Inc., assignee. New compounds inhibitors of the yap/taz-tead interaction and their use in the treatment of malignant mesothelioma. US patent WO2017064277A12017. Available from: https://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PG01&s1=20180297964.PGNR.
- 46.Lim HJ, Park SJ, Lee CH, et al.; Inventors; Korea Research Institute of Chemical Technology, Yonsei University, assignee. Compound inhibiting YAP-TEAD interaction and pharmaceutical composition for treating or preventing cancer comprising the same as an active ingredient. US patent KR20200054096A2020. Available from: https://worldwide.espacenet.com/patent/search/family/070913463/publication/KR20200054096A?q=pn%3DKR20200054096A.
- 47.Barth M, Contal S, Junien J-L, Massardier C, Montalbetti C, Soude A; Inventors; Inventiva SA, assignee. Inhibitors of the YAP/TAZ-TEAD interaction and their use in the treatment of cancer. US patent EP3632908A12018. Available from: https://worldwide.espacenet.com/patent/search/family/063857833/publication/EP3632908A1?q=pn%3DEP3632908A1.
- 48.Zhang Z, Lin Z, Zhou Z, et al. Structure-based design and synthesis of potent cyclic peptides inhibiting the YAP-TEAD protein-protein interaction. ACS Med Chem Lett. 2014;5(9):993–998. doi: 10.1021/ml500160m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan N, Zhao B, Zhao S, Ji X, Zeng G, Li J, Inventors; Kunming Xieli Intellectual Property Agency, assignee. Rubiaceae type cyclic peptide preparation method and be used as Hippo-YAP signal pathway inhibitor. US patent CN104193808B2014. Available from: https://worldwide.espacenet.com/patent/search/family/052079204/publication/CN104193808A?q=pn%3DCN104193808B.
- 50.Rebollo Garcia A, Nemati F, Decaudin D, Inventors; Universite Pierre et Marie Curie (Paris 6), Institut Curie, assignee. Peptide inhibitors of TEAD/YAP-TAZ interaction. US patent US20160264636A12016. Available from: http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PG01&s1=20160264636.PGNR.
- 51.Huang Z; Inventor; Xu and Partners LLC, assignee. YAP protein inhibiting polypeptides as well as application thereof. US patent CN104558119A2015. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN104558119A.
- 52.Li K; Inventor; Xu and Partners LLC, assignee. High-activity tumor inhibitor and its preparation method and use. US patent CN105524139A2015. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN105524139A.
- 53.Hu T, Lin Z, Ling C, et al.; Inventors; F. Hoffmann-La Roche Ag, Hoffmann-La Roche Inc., assignee. YAP-TEAD inhibitors. US patent WO2015022283A12015. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2015022283A1. [Google Scholar]
- 54.Troilo A, Benson EK, Esposito D, et al. Angiomotin stabilization by tankyrase inhibitors antagonizes constitutive TEAD-dependent transcription and proliferation of human tumor cells with Hippo pathway core component mutations. Oncotarget. 2016;7(20):28765–28782. doi: 10.18632/oncotarget.9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Li N, Li X, Tran MK, Han X, Chen J. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 2015;13(3):524–532. doi: 10.1016/j.celrep.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Lu B, Castillo J, et al. Tankyrase inhibitor sensitizes lung cancer cells to Endothelial Growth Factor Receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting YAP signaling. J Biol Chem. 2016;291(29):15256–15266. doi: 10.1074/jbc.M116.722967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waaler J, Mygland L, Tveita A, et al. Tankyrase inhibition sensitizes melanoma to PD-1 immune checkpoint blockade in syngeneic mouse models. Commun Biol. 2020;3(1):196. doi: 10.1038/s42003-020-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Lu P, Wang T, et al. Sitagliptin affects gastric cancer cells proliferation by suppressing Melanoma-associated antigen-A3 expression through Yes-associated protein inactivation. Cancer Med. 2020;9(11):3816–3828. doi: 10.1002/cam4.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeRan M, Yang J, Shen C-H, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9(2):495–503. doi: 10.1016/j.celrep.2014.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mo JS, Meng Z, Kim YC, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500–510. doi: 10.1038/ncb3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Xiao ZD, Li X, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17(4):490–499. doi: 10.1038/ncb3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu HF, Ho SC, Chang CC, Wu TN, Yang CY. Statins are associated with a reduced risk of gastric cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(12):2098–2103. doi: 10.1038/ajg.2011.277 [DOI] [PubMed] [Google Scholar]
- 63.Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. 2013;24(7):1721–1730. doi: 10.1093/annonc/mdt150 [DOI] [PubMed] [Google Scholar]
- 64.Sorrentino G, Ruggeri N, Specchia V, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357–366. doi: 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- 65.Liu Q, Xia H, Zhou S, et al. Simvastatin inhibits the malignant behaviors of gastric cancer cells by simultaneously suppressing YAP and β-Catenin signaling. Onco Targets Ther. 2020;13:2057–2066. doi: 10.2147/OTT.S237693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Wu Y, Wang H, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA. 2014;111(1):E89–E98. doi: 10.1073/pnas.1319190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mo J-S, Yu F-X, Gong R, Brown JH, Guan K-L. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26(19):2138–2143. doi: 10.1101/gad.197582.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao D, Wu J, Zhao Y, et al. Zoledronic acid inhibits TSC2-null cell tumor growth via RhoA/YAP signaling pathway in mouse models of lymphangioleiomyomatosis. Cancer Cell Int. 2020;20:46. doi: 10.1186/s12935-020-1131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mi W, Lin Q, Childress C, et al. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34(24):3095–3106. doi: 10.1038/onc.2014.251 [DOI] [PubMed] [Google Scholar]
- 70.Tang Y, Fang G, Guo F, et al. Selective inhibition of STRN3-containing PP2A phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell. 2020;38(1). doi: 10.1016/j.ccell.2020.05.019 [DOI] [PubMed] [Google Scholar]
- 71.Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50(3):264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenbluh J, Nijhawan D, Cox AG, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi: 10.1016/j.cell.2012.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taccioli C, Sorrentino G, Zannini A, et al. MDP, a database linking drug response data to genomic information, identifies dasatinib and statins as a combinatorial strategy to inhibit YAP/TAZ in cancer cells. Oncotarget. 2015;6(36):38854–38865. doi: 10.18632/oncotarget.5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma H, Wang J, Zhao X, et al. Periostin promotes colorectal tumorigenesis through Integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell Rep. 2020;30(3):793–806.e796. doi: 10.1016/j.celrep.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 75.Taniguchi K, Wu L-W, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu JK-H, Du W, Shelton SJ, Oldham MC, DiPersio CM, Klein OD. An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell. 2017;21(1):91–106.e6. doi: 10.1016/j.stem.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao LW, Wu LL, Zhang LH, et al. MFAP2 is overexpressed in gastric cancer and promotes motility via the MFAP2/integrin α5β1/FAK/ERK pathway. Oncogenesis. 2020;9(2):17. doi: 10.1038/s41389-020-0198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeo MS, Subhash VV, Suda K, et al. FBXW5 promotes tumorigenesis and metastasis in gastric cancer via activation of the FAK-Src signaling pathway. Cancers. 2019;11:6. doi: 10.3390/cancers11060836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Schaefer A, Wang Y, et al. Gain-of-function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 2020;10(2):288–305. doi: 10.1158/2159-8290.CD-19-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan F, Huang Y, Pei Q, Liu H, Pei H, Zhu H. Matrix stiffness mediates stemness characteristics via activating the Yes-associated protein in colorectal cancer cells. J Cell Biochem. 2018. doi: 10.1002/jcb.27532 [DOI] [PubMed] [Google Scholar]
- 81.Zhao B, Li L, Wang L, Wang C-Y, Yu J, Guan K-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47(3):250–256. doi: 10.1038/ng.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghiso E, Migliore C, Ciciriello V, et al. YAP-dependent AXL overexpression mediates resistance to EGFR inhibitors in NSCLC. Neoplasia. 2017;19(12):1012–1021. doi: 10.1016/j.neo.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao X, Wang X, Fang L, et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017;402:61–70. doi: 10.1016/j.canlet.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 85.Song S, Honjo S, Jin J, et al. The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21(11):2580–2590. doi: 10.1158/1078-0432.CCR-14-2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gujral TS, Kirschner MW. Hippo pathway mediates resistance to cytotoxic drugs. Proc Natl Acad Sci USA. 2017;114(18):E3729–E3738. doi: 10.1073/pnas.1703096114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu T, Sun L, Zhu X. Yes-associated protein enhances proliferation and attenuates sensitivity to cisplatin in human gastric cancer cells. Biomed Pharmacother. 2018;105:1269–1275. doi: 10.1016/j.biopha.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 88.Lu T, Sun L, Zhu X. Yes-associated protein enhances proliferation and attenuates sensitivity to cisplatin in human gastric cancer cells. Biomed Pharmacother. 2018;105:1269–1275. [DOI] [PubMed] [Google Scholar]
- 89.Lin C-H, Pelissier FA, Zhang H, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26(22):3946–3953. doi: 10.1091/mbc.E15-07-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song J, Xie L-X, Zhang X-Y, et al. Role of YAP in lung cancer resistance to cisplatin. Oncol Lett. 2018;16(3):3949–3954. doi: 10.3892/ol.2018.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee W-Y, Chen P-C, Wu W-S, et al. Panobinostat sensitizes KRAS-mutant non-small-cell lung cancer to gefitinib by targeting TAZ. Int j Cancer. 2017;141(9):1921–1931. doi: 10.1002/ijc.30888 [DOI] [PubMed] [Google Scholar]
- 92.Liu B-S, Xia H-W, Zhou S, et al. Inhibition of YAP reverses primary resistance to EGFR inhibitors in colorectal cancer cells. Oncol Rep. 2018;40(4):2171–2182. doi: 10.3892/or.2018.6630 [DOI] [PubMed] [Google Scholar]
- 93.Oku Y, Nishiya N, Shito T, et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio. 2015;5(1):542–549. doi: 10.1016/j.fob.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dasari VR, Mazack V, Feng W, Nash J, Carey DJ, Gogoi R. Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget. 2017;8(17):28628–28640. doi: 10.18632/oncotarget.15614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matusewicz L, Meissner J, Toporkiewicz M, Sikorski AF. The effect of statins on cancer cells–review. Tumour Biol. 2015;36(7):4889–4904. doi: 10.1007/s13277-015-3551-7 [DOI] [PubMed] [Google Scholar]
- 97.Chen L, Chan SW, Zhang X, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24(3):290–300. doi: 10.1101/gad.1865310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z, Zhao B, Wang P, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24(3):235–240. doi: 10.1101/gad.1865810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janse van Rensburg HJ, Azad T, Ling M, et al. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 2018;78(6):1457–1470. doi: 10.1158/0008-5472.CAN-17-3139 [DOI] [PubMed] [Google Scholar]
- 100.Hsu P-C, Miao J, Wang Y-C, et al. Inhibition of yes-associated protein down-regulates PD-L1 (CD274) expression in human malignant pleural mesothelioma. J Cell Mol Med. 2018;22(6):3139–3148. doi: 10.1111/jcmm.13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim MH, Kim CG, Kim S-K, et al. YAP-induced PD-L1 expression drives immune evasion in BRAFi-resistant melanoma. Cancer Immunol Res. 2018;6(3):255–266. doi: 10.1158/2326-6066.CIR-17-0320 [DOI] [PubMed] [Google Scholar]
- 102.Lee BS, Park DI, Lee DH, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491(2):493–499. doi: 10.1016/j.bbrc.2017.07.007 [DOI] [PubMed] [Google Scholar]