Abstract
Background
Postoperative pain is a common consequence of surgery and can have many negative perioperative effects. It has been suggested that the administration of analgesia before a painful stimulus may improve pain control. We defined pre‐emptive nonsteroidal anti‐inflammatories (NSAIDs) as those given before surgery but not continued afterwards and preventive NSAIDs as those given before surgery and continued afterwards. These were compared to a control group given the NSAIDs after surgery instead of before surgery.
Objectives
To assess the efficacy of preventive and pre‐emptive NSAIDs for reducing postoperative pain in adults undergoing all types of surgery.
Search methods
We searched the following electronic databases: CENTRAL, MEDLINE, Embase, AMED and CINAHL (up to June 2020). In addition, we searched for unpublished studies in three clinical trial databases, conference proceedings, grey literature databases, and reference lists of retrieved articles. We did not apply any restrictions on language or date of publication.
Selection criteria
We included parallel‐group randomized controlled trials (RCTs) only. We included adult participants undergoing any type of surgery. We defined pre‐emptive NSAIDs as those given before surgery but not continued afterwards and preventive NSAIDs as those given before surgery and continued afterwards. These were compared to a control group given the NSAIDs after surgery instead of before surgery. We included studies that gave the medication by any route but not given on the skin.
Data collection and analysis
We used the standard methods expected by Cochrane, as well as a novel publication bias test developed by our research group. We used GRADE to assess the certainty of the evidence for each outcome. Outcomes included acute postoperative pain (minimal clinically important difference (MCID): 1.5 on a 0‐10 scale), adverse events of NSAIDs, nausea and vomiting, 24‐hour morphine consumption (MCID: 10 mg reduction), time to analgesic request (MCID: one hour), pruritus, sedation, patient satisfaction, chronic pain and time to first bowel movement (MCID: 12 hours).
Main results
We included 71 RCTs. Seven studies are awaiting classification. We included 45 studies that evaluated pre‐emptive NSAIDs and 26 studies that evaluated preventive NSAIDs. We considered only four studies to be at low risk of bias for most domains. The operations and NSAIDs used varied, although most studies were conducted in abdominal, orthopaedic and dental surgery. Most studies were conducted in secondary care and in low‐risk participants. Common exclusions were participants on analgesic medications prior to surgery and those with chronic pain.
Pre‐emptive NSAIDs compared to post‐incision NSAIDs
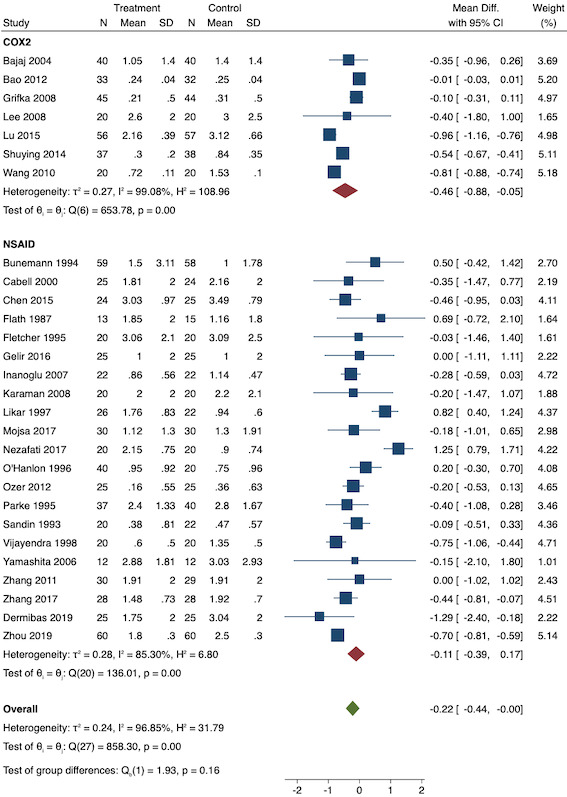
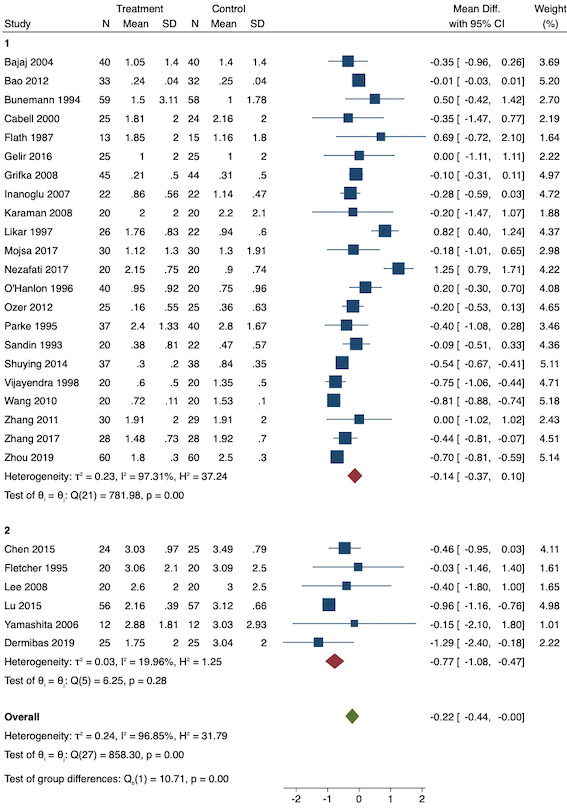
For pre‐emptive NSAIDs, there is probably a decrease in early acute postoperative pain (MD ‐0.69, 95% CI ‐0.97 to ‐0.41; studies = 36; participants = 2032; I2 = 96%; moderate‐certainty evidence). None of the included studies that reported on acute postoperative pain reported adverse events as an outcome. There may be little or no difference between the groups in short‐term (RR 1.00, 95% CI 0.34 to 2.94; studies = 2; participants = 100; I2 = 0%; low‐certainty evidence) or long‐term nausea and vomiting (RR 0.85, 95% CI 0.52 to 1.38; studies = 5; participants = 228; I2 = 29%; low‐certainty evidence). There may be a reduction in late acute postoperative pain (MD ‐0.22, 95% CI ‐0.44 to 0.00; studies = 28; participants = 1645; I2 = 97%; low‐certainty evidence). There may be a reduction in 24‐hour morphine consumption with pre‐emptive NSAIDs (MD ‐5.62 mg, 95% CI ‐9.00 mg to ‐2.24 mg; studies = 16; participants = 854; I2 = 99%; low‐certainty evidence) and an increase in the time to analgesic request (MD 17.04 minutes, 95% CI 3.77 minutes to 30.31 minutes; studies = 18; participants = 975; I2 = 95%; low‐certainty evidence). There may be little or no difference in opioid adverse events such as pruritus (RR 0.40, 95% CI 0.09 to 1.76; studies = 4; participants = 254; I2 = 0%; low‐certainty evidence) or sedation (RR 0.51, 95% CI 0.16 to 1.68; studies = 4; participants = 281; I2 = 0%; low‐certainty evidence), although the number of included studies for these outcomes was small. No study reported patient satisfaction, chronic pain or time to first bowel movement for pre‐emptive NSAIDs.
Preventive NSAIDs compared to post‐incision NSAIDs
For preventive NSAIDs, there may be little or no difference in early acute postoperative pain (MD ‐0.14, 95% CI ‐0.39 to 0.12; studies = 18; participants = 1140; I2 = 75%; low‐certainty evidence). One study reported adverse events from NSAIDs (reoperation for bleeding) although the events were low which did not allow any meaningful conclusions to be drawn (RR 1.95; 95% CI 0.18 to 20.68). There may be little or no difference in rates of short‐term (RR 1.26, 95% CI 0.49 to 3.30; studies = 1; participants = 76; low‐certainty evidence) or long‐term (RR 0.85, 95% CI 0.52 to 1.38; studies = 5; participants = 456; I2 = 29%; low‐certainty evidence) nausea and vomiting. There may be a reduction in late acute postoperative pain (MD ‐0.33, 95% CI ‐0.59 to ‐0.07; studies = 21; participants = 1441; I2 = 81%; low‐certainty evidence). There is probably a reduction in 24‐hour morphine consumption (MD ‐1.93 mg, 95% CI ‐3.55 mg to ‐0.32 mg; studies = 16; participants = 1323; I2 = 49%; moderate‐certainty evidence). It is uncertain if there is any difference in time to analgesic request (MD 8.51 minutes, 95% CI ‐31.24 minutes to 48.27 minutes; studies = 8; participants = 410; I2 = 98%; very low‐certainty evidence). As with pre‐emptive NSAIDs, there may be little or no difference in other opioid adverse events such as pruritus (RR 0.56, 95% CI 0.09 to 3.35; studies = 3; participants = 211; I2 = 0%; low‐certainty evidence) and sedation (RR 0.84, 95% CI 0.44 to 1.63; studies = 5; participants = 497; I2 = 0%; low‐certainty evidence). There is probably little or no difference in patient satisfaction (MD ‐0.42; 95% CI ‐1.09 to 0.25; studies = 1; participants = 72; moderate‐certainty evidence). No study reported on chronic pain. There is probably little or no difference in time to first bowel movement (MD 0.00; 95% CI ‐15.99 to 15.99; studies = 1; participants = 76; moderate‐certainty evidence).
Authors' conclusions
There was some evidence that pre‐emptive and preventive NSAIDs reduce both pain and morphine consumption, although this was not universal for all pain and morphine consumption outcomes. Any differences found were not clinically significant, although we cannot exclude this in more painful operations. Moreover, without any evidence of reductions in opioid adverse effects, the clinical significance of these results is questionable although few studies reported these outcomes. Only one study reported clinically significant adverse events from NSAIDs administered before surgery and, therefore, we have very few data to assess the safety of either pre‐emptive or preventive NSAIDs. Therefore, future research should aim to adhere to the highest methodology and be adequately powered to assess serious adverse events of NSAIDs and reductions in opioid adverse events.
Plain language summary
Ibuprofen‐like painkillers given before cutting the skin in surgery compared with given after cutting the skin in adults undergoing all types of surgery
We aimed to assess the effect of a single dose of a nonsteroidal anti‐inflammatory drug (NSAID: for example, ibuprofen) given before making the first cut during surgery (pre‐emptive NSAIDs) or given before the first cut and continued after surgery (preventive NSAIDs) on reducing pain in adults.
Review question We reviewed the evidence for NSAID painkillers when given before surgery compared to the same painkiller given only after the surgeon has cut the skin in adults undergoing all types of surgery.
Background Most people experience pain after surgery that requires strong opioid (similar to morphine) painkillers. These medications are associated with a number of side effects including reduced breathing, a slow heart rate, and low blood pressure, as well as vomiting, sleepiness, itching, and constipation. Reducing the amount of opioids needed after surgery can limit these side effects and improve the patient experience and outcomes. Compared to starting painkillers later, beginning painkillers before making the first cut for surgery may reduce pain sensitivity, and thus lessen the pain experienced. We wanted to find out whether giving NSAID painkillers before surgery was more effective than giving the same painkiller, at the same dose, after surgery.
Study characteristics We searched the medical literature for randomized controlled trials (a type of study in which participants are assigned to a treatment group using a random method). The evidence is current to June 2020. Patients were randomly allocated to one of two groups. One group was treated with NSAIDs before the surgeon cut the skin, whilst the other group was given the same medication after the surgeon cut the skin. We found 71 trials with patients aged 18 years or over who were undergoing many different operations. Nearly all patients were fit and healthy undergoing procedures in hospitals around the world.
Key results In 36 trials (2032 patients), use of pre‐emptive NSAIDs resulted in a small reduction in the pain experienced in the first six hours after surgery. No studies included serious side effects from NSAIDs as an outcome (bleeding, heart attacks or kidney failure). There was no difference in nausea and vomiting after surgery. In 28 studies (1645 patients), there was no difference in pain at 24 to 48 hours after surgery. In 16 studies (854 patients) there was a reduction in the amount of strong painkillers used after surgery and an increase in the time until patients needed these strong painkillers. Despite this, we found no reduction in the side effects from these strong painkillers (itching or sleepiness). No studies reported patient satisfaction, long‐term pain after surgery or the time until patients opened their bowels.
For preventive NSAIDs, in 18 studies (1140 patients), there was no difference in the pain experienced in the first six hours after surgery. One study reported bleeding after surgery requiring another operation and found no difference, although there were not enough events to be certain of this result. There was no difference in nausea and vomiting. In 21 studies (1441 patients), there was a reduction in pain at 24 to 48 hours after surgery and in 16 studies (1323 patients) a reduction in the amount of strong painkillers used after surgery. There was no difference in the time to requesting strong painkillers. There was no difference in itching, sleepiness or patient satisfaction. No study reported long‐term pain. There was no difference in time to first bowel movement.
Certainty of the evidence Although we found some differences in pain and painkiller usage, the certainty of the evidence ranged from very low to moderate. Also, any differences found were not large enough for patients to consider important. This was due to deficiencies in how the studies were conducted, the small numbers of patients recruited for some outcomes and differences in the results between studies, which means we are uncertain any differences we found are real and, therefore, future research is required.
Summary of findings
Background
This review contains text from a previous Cochrane protocol and review on pre‐emptive and preventive opioids (Doleman 2017a; Doleman 2018b). Throughout the review, we have used nonsteroidal anti‐inflammatory drugs (NSAIDs) as an umbrella term which consists of non‐selective NSAIDs (no specific enzyme activity) and cyclooxygenase‐2 (COX‐2) inhibitors (specific for COX‐2 enzyme).
Description of the condition
Postoperative pain is a common consequence of surgery that affects around 80% of patients. The severity of postoperative pain is variable, with 18% to 25% of patients suffering extreme pain (Apfelbaum 2003; Gerbershagen 2014). Pain can have deleterious effects during the postoperative period, including patient dissatisfaction (Myles 2000), interference with daily activities (Strassels 2002), pulmonary complications (Desai 1999), increases in the stress response to surgery (Desborough 2000), and an increased risk of chronic postsurgical pain (Kehlet 2006). Risk factors for severe postoperative pain include gender (Gerbershagen 2014), age (Gerbershagen 2014), the presence of preoperative pain (Gerbershagen 2014), preoperative anxiety and the type of surgery (Ip 2009). Intravenous opioids are commonly used to treat pain in the postoperative period (Benhamou 2008), however, their use is associated with many side effects such as vomiting, pruritus (itching), sedation (sleepiness) and patient concerns over addiction (Apfelbaum 2003). Therefore, alternative strategies to manage both postoperative pain and reduce postoperative opioid consumption may have important benefits for patients undergoing surgery (Frauenknecht 2019; Zhao 2004).
Description of the intervention
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are a commonly used analgesic during the peri‐operative period and include as examples: ibuprofen, naproxen, diclofenac and ketorolac. The mechanism of action of NSAIDs involves inhibition of cyclooxygenase (COX) enzymes, which are involved in the formation of hyperalgesic compounds called prostaglandins (Burian 2005). NSAIDs are effective in reducing postoperative pain, even when added to standard regimens including paracetamol (Ong 2010; Thybo 2019). Adverse events around the peri‐operative period include possible increases in bleeding (Warltier 2003), acute kidney injury and gastrointestinal ulceration (Gilron 2003). However, newer COX‐2‐specific agents that do not target gastrointestinal COX‐1 may offer lower occurrence of gastrointestinal ulceration compared with traditional NSAIDs (Jüni 2002), although studies have suggested an increased risk of cardiac events in high‐risk patients (Nussmeier 2005). Examples of these agents include celecoxib, parecoxib and rofecoxib.
Pre‐emptive analgesia involves the initiation of an analgesic agent (painkiller) prior to surgical incision (before the surgeon cuts the skin). It is thought that by initiating analgesic interventions before surgical injury, the analgesic can provide reductions in intra‐operative nociception to the central nervous system and, therefore, provide superior pain relief compared with the same analgesic given post‐incision (after the surgeon has cut the skin) (Kissin 2000). Preventive analgesia extends this definition to include increasing the intensity and duration of pre‐emptive analgesic interventions until final wound healing (Dahl 2011).
How the intervention might work
Surgical incision promotes changes in both the central and peripheral nervous system, called sensitization. Such sensitization can cause biochemical changes which manifest as hyperalgesia (the same pain stimulus causing increased pain), and allodynia (normal sensations causing pain). It is thought that by initiating analgesia before surgical incision, both peripheral and central sensitization can be reduced, resulting in reductions in intra‐operative nociception, and later, both acute and chronic postoperative pain. Preventive analgesia extends this reduction in sensitization to include the postoperative period. This enhanced definition came from an increased understanding of the development of persistent postsurgical pain, which is associated with postoperative sensitization. Postoperative sensitization may only be reduced by continuing analgesia longer into the postoperative period (Dahl 2011). As opioids are commonly used to treat pain postoperatively (Benhamou 2008), any reductions in opioid use may also result in a reduction in opioid adverse events (Doleman 2015b; Zhao 2004), and improve the patient experience.
Why it is important to do this review
Due to both its common occurrence (Apfelbaum 2003; Gerbershagen 2014), and potential deleterious effects during the postoperative period, reducing postoperative pain is an important clinical issue. A simple change in clinical practice, such as changing the timing of administration of analgesics, could have important implications for postoperative pain management. Moreover, such a change is cost‐neutral and therefore may benefit both anaesthetists in low‐income countries and those working within healthcare systems with finite resources (such as the National Health Service (NHS) in the United Kingdom).
The first review to examine the clinical effects of pre‐emptive analgesia showed pre‐emptive NSAIDs were ineffective in reducing pain scores or analgesic consumption in most of the included trials when compared to post‐incision NSAIDs (Møiniche 2002). A second review, published a few years later, demonstrated a lower analgesic consumption and delayed time to first analgesic request with pre‐emptive NSAIDs (Ong 2005). However, these reviews are now outdated and, importantly, did not evaluate reductions in opioid side effects (from reduced postoperative opioid consumption) and potential NSAID adverse events. This mandates an updated review of the evidence.
Objectives
To evaluate in adult participants undergoing all types of surgery, the effects of pre‐emptive and preventive nonsteroidal anti‐inflammatory drugs (NSAIDs) compared with post‐incision NSAIDs for reducing postoperative pain and opioid consumption.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group, randomized controlled trials (RCTs) only. We considered studies that did and did not use a double‐dummy placebo (for example, intervention group receives active drug before incision and placebo after incision; control group receives placebo before incision and active drug after incision). We excluded studies that included paediatric participants and pharmacokinetic studies not reporting any clinical outcomes.
Types of participants
Adult patients (18 years and above), both male and female, undergoing any type of surgery. We did not include studies that included both adult and paediatric participants.
Types of interventions
We compared both pre‐emptive nonsteroidal anti‐inflammatory drugs (NSAIDs) and preventive NSAIDs (intervention groups) with post‐incision NSAIDs (control group). We defined:
pre‐emptive NSAIDs as NSAIDs initiated before incision but not continued postoperatively;
preventive NSAIDs as NSAIDs initiated before surgical incision and continued postoperatively; and
post‐incision NSAIDs as the same analgesic intervention initiated after surgical incision, whether single dose (as comparator with pre‐emptive analgesia) or continued postoperatively (as comparator with preventive analgesia) (control group).
We only compared interventions if identical analgesics with identical dosages were used. In addition, we only included studies if concurrent use of other multimodal analgesic agents during the peri‐operative period were identical, in order to avoid confounding. If the studies reported multiple intervention subgroups that had comparable control groups (identical interventions), we combined these into one group using recommended methods (Higgins 2011a). We included all types of non‐selective NSAIDs and COX‐2 inhibitors, at any dose, via any route of systemic administration (oral and parenteral but not topical administration) and all types of regimen (pre‐emptive or preventive) in the analysis.
Types of outcome measures
Primary outcomes
Early acute postoperative pain (measured within six hours postoperatively using a validated pain scale; converted to a 0 to 10 scale where a 0 to 100 scale was used; and where multiple time points were reported, we included the earliest time point reported after post‐incision dosing).
Adverse events (reoperation for major bleeding within 30 days (yes/no)); acute kidney injury within 48 hours (defined using published criteria (Mehta 2007) (yes/no)); gastrointestinal ulceration or bleeding requiring endoscopy within 30 days (yes/no); myocardial infarction within 30 days (defined as two of three of the following: chest pain, electrocardiogram (ECG) changes indicating ischaemia, or > 20% rise in high‐sensitivity troponin (yes/no)). We reported these adverse events separately.
Secondary outcomes
Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic; we reported nausea and vomiting aggregated (yes/no)).
Late acute postoperative pain (measured at 24 to 48 hours postoperatively using a validated pain scale; converted to a 0 to 10 scale where a 0 to 100 scale was used; and where multiple time points were reported, we included the earliest time point reported).
24‐hour morphine consumption (mg) (if alternative opioids were used, we converted these to morphine‐equivalents using standard conversion factors (Doleman 2018a)).
Time to first analgesic request (minutes).
Pruritus (self‐reported by the patient (yes/no)).
Sedation (as defined in the individual studies (yes/no)).
Patient satisfaction (overall satisfaction self‐reported by the patient within 24 hours; converted to a 0 to 10 scale where a 0 to 100 scale was used).
Chronic pain (yes/no, measured three to six months postoperatively using a validated scale, such as the Visual Analogue Scale or the McGill Pain Questionnaire; we included the earliest time point closest to three months). We reported this outcome as a separate dichotomous and continuous outcome.
Time to first bowel movement (hours).
For the secondary outcomes where time points were not specified, we used the end point closest to two hours (one to six hours) to assess immediate short‐term effects, and the end point closest to 24 hours (six to 48 hours) to assess longer‐term effects. Outcomes did not form part of the study eligibility assessment, and so we included studies that met the participant, intervention and comparison criteria for inclusion in the review even if they reported no relevant outcomes. For the continuous outcomes, we considered the following to be minimal clinically important differences:
• Acute postoperative pain: 1.5 on a 0‐10 scale (Gallagher 2001; Myles 2017)
• 24‐hour morphine consumption: 10 mg reduction (Doleman 2015a)
• Time to analgesic request: one hour
• Time to first bowel movement: 12 hours.
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching designed to identify relevant trials as outlined in Chapter 4 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2019). We searched relevant systematic reviews for further trials (Møiniche 2002; Ong 2005). We did not apply restrictions due to language, publication status or publication year. We searched the following databases for relevant trials (Figure 1).
1.
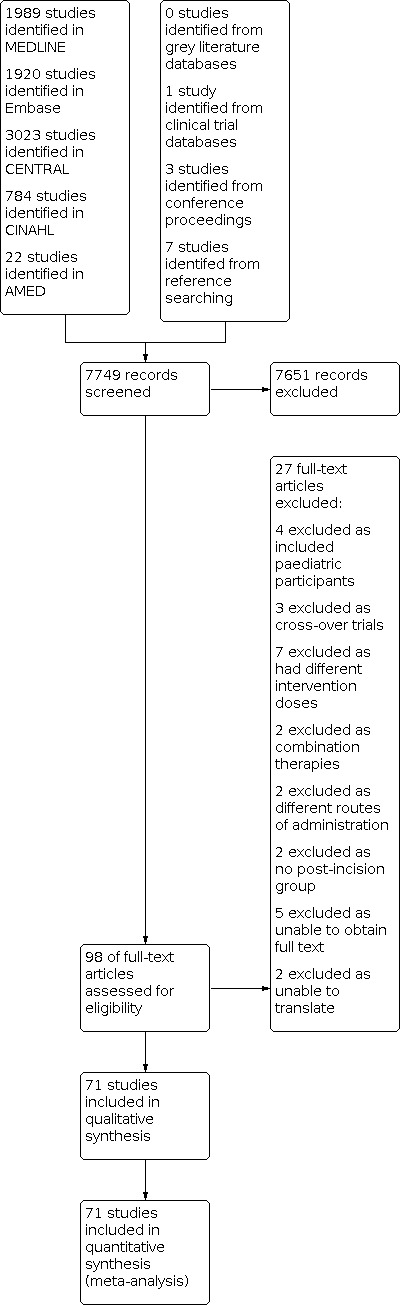
Study flow diagram.
Cochrane Central Register of Controlled Trials (CENTRAL, latest Issue) in the Cochrane Library.
MEDLINE (Ovid SP, 1946 to June 2020).
Embase (Ovid SP, 1974 to June 2020).
CINAHL (1982 to June 2020).
AMED (1985 to June 2020).
We developed a draft search strategy for MEDLINE. We used this as the basis for the search strategies in the other databases listed (Appendix 1).
We scanned the following trials registries for ongoing and unpublished trials.
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We conducted a search of the OpenSIGLE database to identify grey literature sources. We scanned the reference lists and citations of included trials and systematic reviews identified for further references to additional trials. When necessary, we contacted trial authors for additional information. In addition, we searched the following conference proceedings to identify further unpublished studies (all years considered).
World Congress on Pain (International Association for the Study of Pain).
Anaesthetic Research Society Meetings.
Association of Anaesthetists of Great Britain and Ireland Winter Symposium and Annual Congress.
American Society of Anesthesiologists Annual Meeting.
European Society of Anaesthesiologists Euroanaesthesia Conference.
The search strategy was developed in consultation with the Cochrane Anaesthesia Information Specialist.
Data collection and analysis
Selection of studies
We used two review authors (BD and JPW) to independently screen the identified studies using the inclusion criteria to assess eligibility. BD and JPW resolved any disagreements by consensus. If disagreement still existed following discussion, we consulted a third review author (JLB). BD and JPW used the information from the retrieved reports to help identify any duplicate publications, such as author name, study centre, type and dose of interventions used, and study dates. We linked any duplicate publications. We inputted details of all potentially eligible studies into PubMed to identify any retracted publications and we excluded these (Eisenach 2009).
Data extraction and management
We extracted data onto an electronic database using standardized data extraction forms (Appendix 2). We performed this independently using two review authors (BD and TH/HBC/LC), and resolved any disagreements by consensus. If disagreement still existed, we consulted a third review author (JPW). We performed the analysis using one review author (BD). Where possible, we translated non‐English language studies and extracted data following translation. If data were not contained within the original research report, we contacted the corresponding author, irrespective of the age of publication. We extracted the following information:
Bibliographic data, including date of completion/publication.
Country.
Publication status.
Source of funding.
Trial design, e.g. parallel‐group.
Study setting.
Number of participants randomized to each trial arm and number included in final analysis.
Eligibility criteria and key baseline participant data, including sex and age.
Details of treatment regimen received by each group.
Details of any co‐interventions.
Primary and secondary outcome(s) (with definitions and, where applicable, time points).
Outcome data for primary and secondary outcomes (by group).
Duration of follow‐up.
Number of withdrawals (by group) and number of withdrawals (by group) due to adverse events.
Adverse events.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using the Cochrane tool for assessing risk of bias (Higgins 2011b). Two review authors (BD and JPW) independently undertook assessment of risk of bias and reached agreement by consensus. We assessed risk of bias for the domains of sequence generation, allocation concealment, blinding of participants, study personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. We assessed each domain as being at low, unclear or high risk of bias (Higgins 2011b). We presented the results in both a risk of bias summary (Figure 2) and a risk of bias graph (Figure 3). We considered a study as being at low risk of bias if it was low risk for all domains (except selective reporting bias, as some studies were published before published protocols and clinical trial databases were standard) with no high‐risk domains and as being at high risk of bias if it was high risk in any domain. Studies were assessed as being at unclear risk if they were not classified into either low or high risk.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We presented dichotomous outcomes as risk ratios (RRs). For continuous outcomes, we presented these as mean differences (MDs). If non‐comparable scales were used across studies but still presented as continuous data, which we expected may be the case for chronic pain and patient satisfaction, we would have presented these as standardized mean differences (SMDs). We aimed to present the outcomes of time to first analgesic and time to first bowel movement as hazard ratios (HRs) (Tierney 2007). We presented the precision of effect estimates using 95% confidence intervals (CIs).
Unit of analysis issues
As we included parallel‐group RCTs only, unit of analysis issues were not a problem for the main analysis (Higgins 2011c). For the main results, we combined different dose subgroups into one treatment group, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If it was not possible to combine groups (for example, for continuous outcomes where the combined standard deviation (SD) could not be estimated), we treated these as separate studies and distributed the control group participants between these treatment groups to avoid analysing them twice (Higgins 2011c).
Dealing with missing data
We contacted corresponding authors for any data missing from the original publication, irrespective of publication date. If we did not receive a response, we extracted data from published graphs. If SDs were not reported, we attempted to calculate these from other reported statistics. If this was not possible, we estimated SDs from other studies with similar means. We estimated means from medians (equal if the assumption of normality holds) and SD from interquartile range (IQR)/1.35 (Higgins 2011a) or range (Hozo 2005). We did this as our previous review identified studies where medians were reported, and were more likely to be 'negative' than those reporting means, which could introduce bias into the results (Doleman 2018b). However, we assessed the robustness of our estimates by excluding all studies where means or SDs were estimated in a sensitivity analysis.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining study characteristics, such as the type of population, type of surgery and the intervention used. We assessed statistical heterogeneity using the I2 statistic. We used the following recommended cut‐off values in the interpretation of the I2 statistic (Deeks 2011).
> 50% may represent moderate heterogeneity.
> 85% considerable heterogeneity.
In addition to the cut‐off values, we examined the direction of the effect in the individual studies. For clinically meaningful magnitudes of the pooled effect, we explored heterogeneity using meta‐regression when the criteria set out in Subgroup analysis and investigation of heterogeneity section were fulfilled.
Assessment of reporting biases
If we included 10 or more studies in the meta‐analysis, we assessed publication bias graphically using funnel plots and quantitatively using an updated publication bias test which uses inverse sample size on the Y axis and performs better than Egger’s linear regression test (Egger 1997) for outcomes dependent on baseline risk, such as pain and morphine consumption (Doleman 2020). This is due to correlation between standard errors and mean differences with these outcomes causing artefactual funnel plot asymmetry with Egger's test, which the use of inverse sample size corrects. Due to the low power of this test, we regarded P < 0.1 as evidence of imprecise study effects and possible publication bias.
Data synthesis
We used Review Manager 5 to aggregate study data (Review Manager 2014). We conducted separate analyses for pre‐emptive and preventive interventions. We aggregated data using the adapted DerSimonian and Laird random‐effects model (for continuous and categorical outcomes), as currently available in Review Manager 5. This is because we expected the treatment effect to vary with respect to the different populations within each study, and, therefore, there was no single underlying effect to estimate, making the random‐effects model more appropriate. We attempted to aggregate reported log hazard ratios and their associated standard errors using the generic inverse variance method, although no study adequately reported this.
Subgroup analysis and investigation of heterogeneity
If there were sufficient included studies, we conducted two separate subgroup analyses for the type of NSAIDs (non‐selective NSAIDs versus COX‐2 inhibitors) and trials with different baseline pain levels (mean pain scores in the control group of < 3 (mild), 3 to 6 (moderate) and > 6 (severe)) (Moore 2013). If we included 10 studies or more in a meta‐analysis and the included studies had a sufficient number of events, we explored reasons for heterogeneity by performing a restricted maximum likelihood, random‐effects meta‐regression where covariates were entered into the model separately based on type of anaesthesia and type of surgery (univariate analysis) (Thompson 2002). For dummy variables, we used the least effective subgroup as the reference category. We presented the R2 analogue with a corresponding P value for each covariate. We used the Knapp‐Hartung method to calculate P values (as this method more appropriately uses the t‐distribution for the between‐study variance). We performed these analyses using the software STATA Version 16.1 (Stata 2020). If there was a low number of studies or events, or both, we only performed traditional subgroup analysis, and reported the P value for subgroup differences.
Sensitivity analysis
We performed a sensitivity analysis by restricting the analysis to studies at low risk of bias (defined as low risk for randomization and allocation concealment). As we judged studies that did not use a double‐dummy design as being at high risk of bias for blinding, we assessed the impact of excluding these from the analysis. We also performed a further sensitivity analysis by excluding studies where means and SDs were estimated (both where SDs were estimated from other studies and where means and/or SDs were estimated from median and IQR). As a further sensitivity analysis, we repeated analysis assuming excluded participants suffered an event to assess the robustness of the findings. Furthermore, we performed sensitivity analyses by excluding studies with a low sample size (< 50 participants).
Summary of findings and assessment of the certainty of the evidence
We presented outcomes in a summary of findings table. We produced two summary of findings tables, one for each comparison.
Pre‐emptive NSAIDs versus single dose post‐incision NSAIDs.
Preventive NSAIDs versus continuous post‐incision NSAIDs.
The outcomes presented in the summary of findings table for each comparison included: early acute postoperative pain; adverse events; nausea and vomiting; late acute postoperative pain; 24‐hour morphine consumption and time to first analgesic request. We did not include chronic pain as no studies reported this outcome. We presented these outcomes using the GRADE approach (Schünemann 2011). We downgraded the certainty of evidence from high‐certainty to moderate‐, low‐ or very low‐certainty. Downgrading was undertaken independently by two review authors (BD and JPW) and agreement reached by consensus. Characteristics of the evidence that caused downgrading included:
limitations in the design and implementation of available studies, suggesting a high likelihood of bias (for example, studies not using a double‐dummy placebo design);
indirectness of evidence (indirect population, intervention, control or outcomes);
inconsistency of results (considerable heterogeneity not explained by meta‐regression or subgroup analysis);
imprecision of results (wide confidence intervals);
evidence of publication bias from asymmetry of the funnel plot.
Results
Description of studies
Results of the search
Searching of electronic databases yielded 7749 studies (Figure 1). We found another study from clinical trial databases, a further three studies from conference proceedings and seven from reference list searches. We reviewed 98 full‐text articles and 27 were not included (Excluded studies).
Included studies
Following full‐text review, we included 71 studies (Characteristics of included studies) which satisfied our inclusion criteria.
Participants and surgery
The types of surgery included were diverse. We included 10 studies performed in dental surgery, one study included hand surgery, four in general/colorectal surgery, four in total hip arthroplasty, one in total knee arthroplasty, four in laparoscopic cholecystectomy, three in laparoscopic gynaecological surgery, five in minor orthopaedic surgery, four in laparoscopic or open gynaecological procedures, three in breast surgery, seven in hysterectomy, six in spinal surgery, one in abdominal or thoracic surgery, one in laparoscopic hernia repair, five in joint arthroscopy, one in varicocelectomy, one in laparotomy, one in lung resection, one in thoracotomy, one in mandible open reduction and internal fixation (ORIF), one in diagnostic laparoscopy, two in septo/rhinoplasty, one in spinal, breast and orthopaedic surgery, one in tonsillectomy, one in major plastic surgery and one in thyroid surgery. Seventeen studies included female participants only and one included male participants only. Most studies were conducted in low‐risk participants who were American Society of Anesthesiologists grade (ASA) 1 or 2 and seven included ASA 1‐3 participants, whilst others did not specify any exclusions on the basis of ASA grade.
Settings
Most studies were conducted in secondary care although other settings included dental clinics or dental medical schools. In terms of countries in which the trials were conducted, these included: one in Peru, four in the UK, one in Spain, four in India, 12 in China, three in France, two in Ireland, one in Denmark, six in the USA, three in Italy, one in Mexico, four in Germany, seven in Turkey, two in Romania, three in Poland, three in Austria, one in Australia, two in Japan, one in Iran, one in Canada, one in Northern Ireland, one in Greece, one in Finland, one in Sweden, one in Belgium, one in Indonesia, one in Thailand and two studies where the country was unclear.
Interventions
We included 52 studies which studied non‐selective NSAIDs and 19 which studied COX‐2 inhibitors. We included 45 studies which were pre‐emptive (single‐dose intervention not continued postoperatively) and 26 studies were preventive (intervention continued postoperatively).
Comparators
All included studies gave identical post‐incision doses as those that did not were excluded. In terms of post‐incision dose timing, 53 studies gave post‐incision doses postoperatively or at the end of surgery and 18 studies gave doses intraoperatively.
Funding
Many studies did not report sources of funding or stated no funding; overall, the total was 56 studies. Eleven studies specified that they received non‐industry funding and three studies reported industry funding. One study appeared to have authors who were pharmaceutical company employees.
Postoperative opioids and concurrent analgesia
The included studies used a diverse range of opioids for postoperative analgesia so we used conversion factors to calculate intravenous (IV) morphine equivalents. We included 16 studies which used no postoperative opioids or their use was not reported. Seventeen studies used patient controlled analgesia (PCA) morphine, one used nalbuphine, two used intramuscular (IM)/IV morphine, five used IV or IM pethidine, one used IV fentanyl and morphine, two used IV fentanyl, one used fentanyl PCA, one used buprenorphine, one used an IV morphine infusion, five used a tramadol PCA/IV, one used oral oxycodone, three used IV/PCA piritramide, two used oral or IV tramadol, one used IV and subcutaneous (SC) morphine, one used IV papaveretum, one used IV morphine and pethidine, one used IV fentanyl and codeine, one used cyclimorph and codeine, one used a diamorphine PCA, one used IV/IM oxycodone, one used dextropropoxyphene, one used sufentanil and tramadol, one used fentanyl, hydrocodone and morphine PCA, one used PCA butorphanol, one used a sufentanil PCA and one study used an unspecified analgesic.
In terms of concurrent analgesia, 52 studies reported no concurrent multimodal analgesia or did not mention any in the manuscript. Two studies used paracetamol and NSAIDs, two used NSAIDs which were different NSAIDs from the intervention, one used nurse‐controlled NSAIDs which were the same as the intervention NSAID, one used metamizole, 10 used paracetamol, one used codeine and NSAIDs, one used paracetamol, tramadol and metamizole and one used paracetamol, NSAIDs and codeine.
Excluded studies
We excluded 20 studies (Characteristics of excluded studies) which did not satisfy our inclusion criteria. Three studies were excluded because they were cross‐over trials. Seven studies included different doses of pre‐emptive or preventive versus post‐incision interventions. Two studies were excluded because they studied combination therapy rather than just NSAIDs/COX‐2 inhibitors. Two studies were excluded as they did not have any post‐incision group. Four studies were excluded as they included paediatric participants. Two studies were excluded as interventions had different routes of administration and were, therefore, not comparable.
Studies awaiting classification
There were seven studies (Characteristics of studies awaiting classification) awaiting classification. We were unable to translate two studies. We were unable to obtain the full text of another five studies via the British Library or contacting the study authors.
Risk of bias in included studies
Risk of bias assessments were conducted for the following domains.
Allocation
We included 31 studies which did not report details for randomization so were rated as having unclear risk. Nine studies used a random number chart or table, 27 used computer‐generated randomization, two used a random draw of envelopes or lots, one used block randomization and one used a random number generator so all of these studies were deemed as having low risk of bias. In terms of allocation concealment, 44 studies did not report their method of allocation concealment and four studies did not include enough details so both were deemed as having unclear risk of bias. In addition, a further nine studies reported envelopes but not enough details on envelope safeguards so were also deemed as having unclear risk of bias. Nine studies used third parties for randomization or allocation was pharmacy‐controlled so were deemed as having low risk of bias, whilst five studies reported sealed, sequentially numbered and opaque envelopes so were also deemed as having low risk of bias for allocation concealment.
Blinding
We included 14 studies which did not report the use of a double‐dummy placebo so were regarded as having high risk of bias for blinding. Fifty‐five studies used a double‐dummy placebo so were deemed as having low risk of bias. In two studies, it was unclear if a double‐dummy placebo had been used so they were considered as having unclear risk of bias for this domain.
In terms of blinding of outcome assessment, two studies were regarded as high risk from the lack of blinding of post‐incision dosing. Thirty‐seven studies used blinded outcome assessment and were deemed at low risk. Twenty‐five studies did not mention any outcome assessment blinding and were therefore deemed as having unclear risk of bias for this domain. Seven studies used participant self‐report for outcome assessment or outcomes were likely blinded from the details given, so these were deemed as having low risk for blinding of outcome assessment.
Incomplete outcome data
We included 15 studies which had low dropout rates or dropouts equal in numbers and reasons which were, therefore, regarded as low risk and 30 studies analysed all enrolled participants so were also at low risk of bias. Nine studies had unclear risk as it was unclear which to which group dropouts belonged or the study did not mention dropouts. We included 13 studies which were at high risk of bias due to a high number of dropouts which could bias results or an extreme dropout which could change results. Three studies used intention‐to‐treat analysis so were deemed as having low risk of bias.
Selective reporting
We included 53 studies which did not have a published protocol or clinical trial registration so were regarded as having unclear risk for selective outcome reporting. Ten studies did not report outcomes mentioned in the methods and were therefore deemed at high risk of bias for selective outcome reporting. One study used retrospective registration so was deemed at unclear risk for selective outcome reporting. Two studies pre‐registered the trial on a clinical trials database but did not fully report all prespecified outcomes so were deemed as having high risk of bias. Two studies with a protocol registration number that we could not locate were considered at unclear risk of bias and three studies reported all prespecified outcomes so were considered at low risk of bias.
Other potential sources of bias
We included five studies which had no details on baseline characteristics so were deemed at unclear risk of other bias and one study had industry funding but, because the extent of involvement was unclear, it was considered as having unclear risk of bias. Another 16 studies had disparities in baseline characteristics which could have affected pain so were deemed at high risk. We also judged one study as having high risk as the authors appeared to be pharmaceutical company employees. We included 48 studies which had no differences in baseline characteristics or industry funding so these were deemed at low risk of bias for other sources of bias.
Effects of interventions
Summary of findings 1. Summary of findings.
| Pre‐emptive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
|
Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: pre‐emptive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Pre‐emptive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.32 to 6.37 |
Overall MD ‐0.69 (95% CI ‐0.97 to ‐0.41) Mild pain MD ‐0.24 (95% CI ‐0.51 to 0.03) Moderate pain MD ‐1.19 (95% CI ‐1.52 to ‐0.86) Severe pain MD ‐1.44 (95% CI ‐2.28 to ‐0.59) |
2032 (36) | ⊕⊕⊕⊝ moderate1 | Results not clinically significant | |
| Adverse events | N/A | N/A | N/A | N/A | N/A | No study reported this outcome |
| Nausea and vomiting (1‐6 hours postoperatively) | 120 per 1000 | 120 per 1000 (41 to 353) |
RR 1.00 (0.34 to 2.94) | 100 (2) | ⊕⊕⊝⊝ low2 | |
| Nausea and vomiting (6‐48 hours postoperatively) | 336 per 1000 | 278 per 1000 (175 to 464) |
RR 0.85 (0.52 to 1.38) | 228 (5) | ⊕⊕⊝⊝ low2 | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.25 to 3.49 |
Overall MD ‐0.22 (95% CI ‐0.44 to 0.00) Mild pain MD ‐0.14 (95% CI ‐0.37 to 0.10) Moderate pain MD ‐0.77 (95% CI ‐1.08 to 0.47) |
1645 (28) | ⊕⊕⊝⊝ low3 | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.97 mg to 122.75 mg |
Overall MD ‐5.62 mg (95% CI ‐9.00 mg to ‐2.24 mg) Low consumption MD ‐2.66 mg (95% CI ‐4.54 mg to ‐0.79 mg) Medium consumption MD ‐5.46 mg (95% CI ‐10.73 mg to ‐0.19 mg) High consumption MD ‐15.22 mg (95% CI ‐29.67 mg to ‐0.77 mg) |
854 (16) | ⊕⊕⊝⊝ low3 | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 29.6 minutes to 1146 minutes | MD 17.04 minutes (95% CI 3.77 minutes to 30.31 minutes) | 975 (18) | ⊕⊕⊝⊝ low3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded owing to concerns over risk of bias (one level)
2Downgraded owing to concerns over risk of bias (one level) and imprecision (one level)
3Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level)
Abbreviations: CI: confidence intervals MD: mean difference mg: milligrams N/A: not applicable No: number NSAIDs: nonsteroidal anti‐inflammatory drugs RR: risk ratio
Summary of findings 2. Summary of findings.
| Preventive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
|
Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: preventive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Preventive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.18 to 6.42 |
Overall MD ‐0.14 (95% CI ‐0.39 to 0.12) Mild pain MD ‐0.04 (95% CI ‐0.31 to 0.24) Moderate pain MD ‐0.24 (95% CI ‐0.92 to 0.45) Severe pain MD ‐0.80 (95% CI ‐1.23 to ‐0.37) |
1140 (18) | ⊕⊕⊝⊝ low1 | ||
| Adverse events (Re‐operation for bleeding (reoperation for major bleeding within 30 days (yes/no))) | 25 per 1000 | 49 per 1000 (5 to 517) |
RR 1.95 (0.18 to 20.68) | 81 (1) | ⊕⊕⊝⊝ verylow2 | Study performed in tonsillectomy so unclear for other operations. Other adverse events not reported |
| Nausea and vomiting (1‐6 hours postoperatively) | 162 per 1000 | 205 per 1000 (79 to 535) |
RR 1.26 (0.49 to 3.30) | 76 (1) | ⊕⊕⊕⊝ low3 | |
| Nausea and vomiting (6‐48 hours postoperatively) | 245 per 1000 | 282 per 1000 (159 to 299) | RR 0.89 (0.65 to 1.22) | 456 (8) | ⊕⊕⊝⊝ low4 | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.42 to 4.6 |
Overall MD ‐0.33 (95% CI ‐0.59 to ‐0.07) Mild pain MD ‐0.33 (95% CI ‐0.62 to ‐0.05) Moderate pain MD ‐0.23 (95% CI ‐0.65 to 0.19) |
1441 (21) | ⊕⊕⊝⊝ low1 | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.08 mg to 42.31 mg |
Overall MD ‐1.93 mg (95% CI ‐3.55 mg to ‐0.32 mg) Low consumption MD ‐0.32 mg (95% CI ‐0.40 mg to ‐0.24 mg) Medium consumption MD ‐3.77 mg (95% CI ‐7.27 mg to ‐0.26 mg) |
1323 (16) | ⊕⊕⊕⊝ moderate5 | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 3.15 minutes to 523.1 minutes | MD 8.51 minutes (95% CI ‐31.24 minutes to 48.27 minutes) | 410 (8) | ⊕⊝⊝⊝ very low6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level)
2Downgraded owing to concerns over imprecision (two levels) and indirectness of evidence (one level)
3Downgraded owing to concerns over imprecision (one level) and indirectness of evidence (one level)
4Downgraded owing to concerns over risk of bias (one level) and imprecision (one level)
5Downgraded owing to concerns over risk of bias (one level)
6Downgradedowing to concerns over imprecision (one level), risk of bias (one level) and unexplained heterogeneity (one level)
Abbreviations: CI: confidence intervals MD: mean difference mg: milligrams N/A: not applicable No: number NSAIDs: nonsteroidal anti‐inflammatory drugs RR: risk ratio
Pre‐emptive NSAIDs versus post‐incision NSAIDs
Primary outcomes
1. Early acute postoperative pain (measured within six hours postoperatively)
Thirty‐six studies reported early acute postoperative pain for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bajaj 2004; Bao 2012; Buggy 1994; Bunemann 1994; Cabell 2000; Chen 2015; Colbert 1998; Demirbas 2019; Esparza‐Villalpando 2016; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Kaczmarzyk 2010; Karaman 2008; Lee 2008; Likar 1997; Lu 2015; Mojsa 2017; Nezafati 2017; O'Hanlon 1996; Ozer 2012; Ozyilmaz 2005; Peduto 1995; Priya 2002; Sandin 1993; Shuying 2014; Vanlersberghe 1996; Vijayendra 1998; Wang 2010; Yagar 2011; Yamashita 2006; Yan 2004; Zhang 2011; Zhang 2017; Zhou 2019). There is probably a reduction in early postoperative pain with pre‐emptive NSAIDs (MD ‐0.69, 95% CI ‐0.97 to ‐0.41; participants = 2032; I2 = 96%; Analysis 1.1). The certainty of evidence was downgraded to moderate owing to concerns over risk of bias (one level), mainly for allocation concealment. There was no evidence of publication bias both on observation of funnel plots (Figure 4) or quantitative testing (P = 0.27).
1.1. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 1: Early acute postoperative pain (within 6 hours postoperatively)
4.
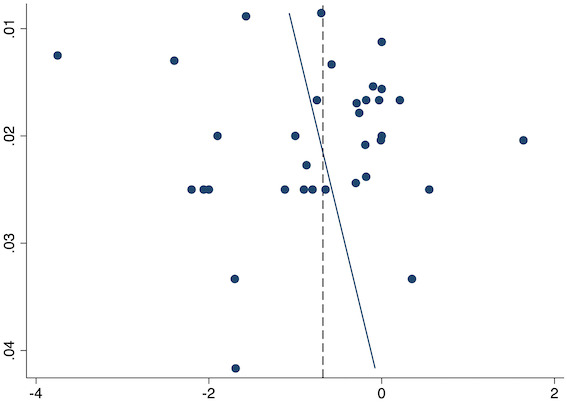
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive early acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 specific agents, there was no difference between the groups (COX‐2: MD ‐1.14; 95% CI ‐1.78 to ‐0.5 versus NSAIDs: MD ‐0.57; 95% CI ‐0.89 to ‐0.25; P = 0.12; Figure 5). However, on subgroup analysis of baseline pain level (Doleman 2018a), there were greater reductions in pain with higher baseline pain levels (mild: MD ‐0.24; 95% CI ‐0.51 to 0.03, moderate: MD ‐1.19; 95% CI ‐1.52 to ‐0.86 and severe: MD ‐1.44; 95% CI ‐2.28 to ‐0.59; P < 0.001; Figure 6), although statistical heterogeneity was still high in these subgroups (I2 = 86‐90%). On meta‐regression analysis, type of surgery (R2 = 54%; P = 0.003) and type of anaesthesia (R2 = 40%; P = 0.002) explained the majority of the between‐study heterogeneity. On sensitivity analysis, restricting analysis to studies with low risk for randomization and allocation concealment (Esparza‐Villalpando 2016; Kaczmarzyk 2010; Mojsa 2017; Shuying 2014; Zhang 2011) showed a smaller reduction in pain (MD ‐0.20, 95% CI ‐0.43 to 0.04; participants = 318; studies = 5; I2 = 13%). Restricting analysis to studies at low risk for blinding of participants and outcome assessors (Buggy 1994; Chen 2015; Esparza‐Villalpando 2016; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Kaczmarzyk 2010; Karaman 2008; Lee 2008; Likar 1997; Mojsa 2017; Ozer 2012; Pandazi 2010; Priya 2002; Shuying 2014; Zhang 2011) produced similar results to the main analysis (MD ‐0.60, 95% CI ‐0.99 to ‐0.21; participants = 888; studies = 17; I2 = 85%). Similarly, restricting analysis to studies where standard deviations were not estimated from IQR or other studies, or means were not estimated from medians (excluded studies: Bajaj 2004; Buggy 1994; Bunemann 1994; Cabell 2000; Demirbas 2019; Grifka 2008; Kaczmarzyk 2010; Lee 2008; Ozyilmaz 2005; Peduto 1995; Sandin 1993; Vanlersberghe 1996; Vijayendra 1998; Yagar 2011; Yamashita 2006; Zhang 2011) showed a similar reduction in pain to the main analysis (MD ‐0.62, 95% CI ‐0.98 to ‐0.27; participants = 1167; studies = 20; I2 = 97%). Restricting analysis to studies with more than 50 participants gave similar results to the main analysis (MD ‐0.77, 95% CI ‐1.15 to ‐0.39; participants = 1355; I2 = 97%).
5.
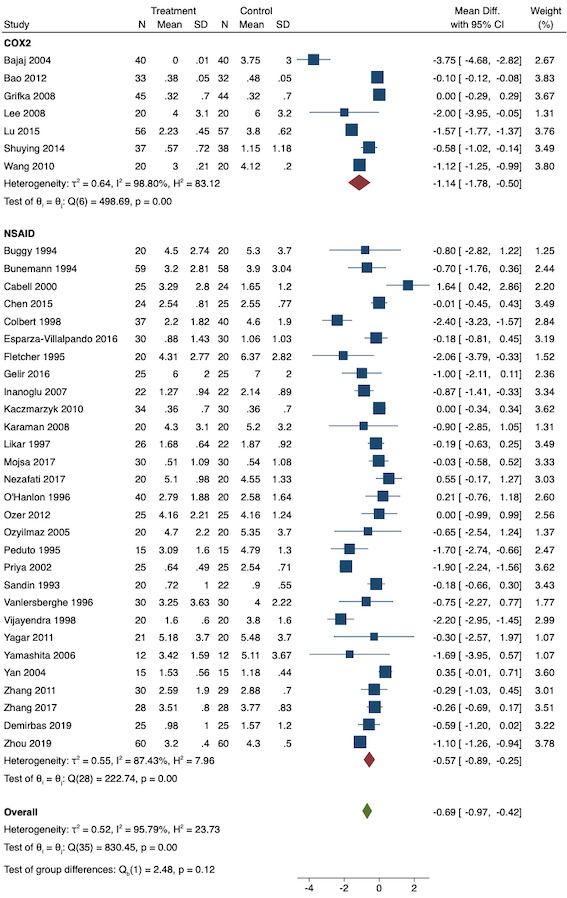
Subgroup analysis for pre‐emptive early postoperative pain (NSAID versus COX‐2 inhibitor)
6.
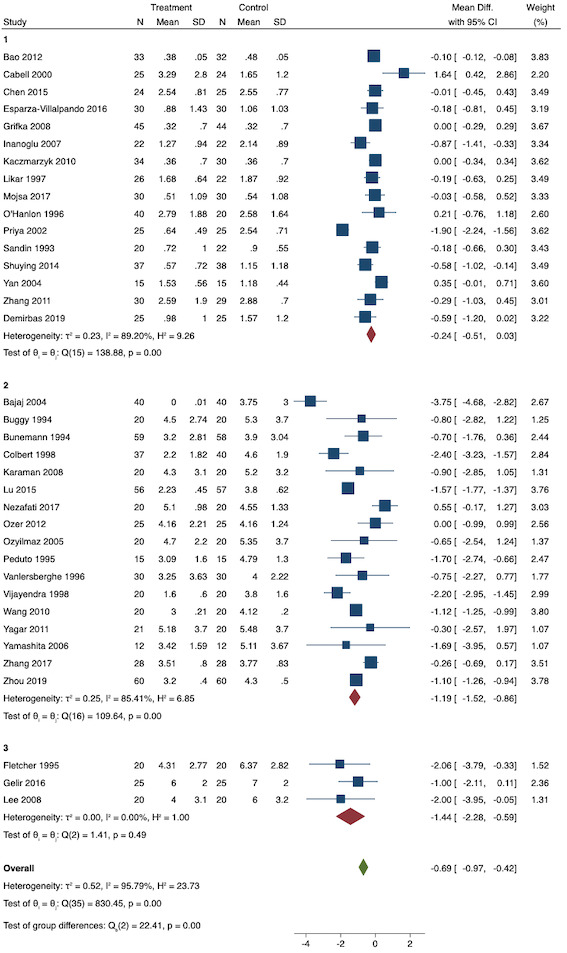
Subgroup analysis for pre‐emptive early postoperative pain (baseline pain level). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)
2. Adverse events (reoperation for major bleeding within 30 days, acute kidney injury within 48 hours, gastrointestinal ulceration or bleeding requiring endoscopy within 30 days, and myocardial infarction within 30 days)
No studies reported any adverse events for pre‐emptive NSAIDs versus post‐incision NSAIDs.
Secondary outcomes
1. Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic as composite outcome (yes/no))
Short‐term nausea and vomiting
Two studies reported short‐term nausea and vomiting (Lee 2008; Vanlersberghe 1996). Overall, there may be no difference between the pre‐emptive and post‐incision groups (RR 1.00, 95% CI 0.34 to 2.94; participants = 100; studies = 2; I2 = 0%; Analysis 1.2). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly selective outcome reporting (one level), and other bias and imprecision (one level).
1.2. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 2: Nausea and vomiting (short‐term)
We were unable to conduct assessment for publication bias or meta‐regression due to the low number of included studies. On sensitivity analysis, none of the included studies were at low risk for randomization and allocation concealment. One study was at low risk for blinding (Lee 2008) which showed similar results to the main analysis (RR 0.85, 95% CI 0.52 to 1.38). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.75, 95% CI 0.18 to 3.07; participants = 60).
Long‐term nausea and vomiting
For long‐term nausea and vomiting, five studies were included (Fletcher 1995; Karaman 2008; Lee 2008; Priya 2002; Rogers 1995). There may be no difference between the groups in the number of participants suffering from long‐term nausea and vomiting (RR 0.85, 95% CI 0.52 to 1.38; participants = 228; studies = 5; I2 = 29%; Analysis 1.3). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly selective outcome reporting (one level), and other bias and imprecision (one level).
1.3. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 3: Nausea and vomiting (long‐term)
We were unable to conduct assessment for publication bias or meta‐regression due to the low number of included studies. On sensitivity analysis, restricting analysis to one study that was at low risk of bias for randomization and allocation concealment (Rogers 1995) produced similar results to the main analysis (RR 1.28, 95% CI 0.61 to 2.72; participants = 58). All the studies were at low risk of bias for blinding so results were identical to the main analysis. Restricting analysis to studies with more than 50 participants gave similar results (RR 0.69, 95% CI 0.20 to 2.42; participants = 108; I2 = 79%). Only one study (Rogers 1995) excluded participants, although it was unclear when, as each was excluded after receiving analgesia.
2. Late acute postoperative pain (measured at 24 to 48 hours)
Twenty‐eight studies reported early acute postoperative pain for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bajaj 2004; Bao 2012; Bunemann 1994; Cabell 2000; Chen 2015; Demirbas 2019; Flath 1987; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Karaman 2008; Lee 2008; Likar 1997; Lu 2015; Mojsa 2017; Nezafati 2017; O'Hanlon 1996; Ozer 2012; Parke 1995; Sandin 1993; Shuying 2014; Vijayendra 1998; Wang 2010; Yamashita 2006; Zhang 2011; Zhang 2017; Zhou 2019). There may be a slight difference between the groups (MD ‐0.22, 95% CI ‐0.44 to 0.00; participants = 1645; I2 = 97%; Analysis 1.4). The certainty of evidence was downgraded to low due to concerns over risk of bias, mainly in allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 7) or quantitative testing (P = 0.35).
1.4. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 4: Late acute postoperative pain (24‐48 hours postoperatively)
7.
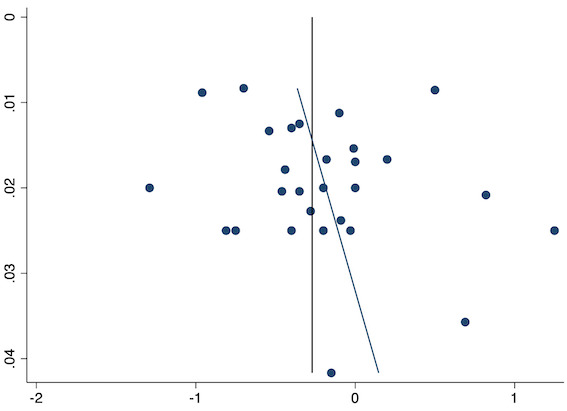
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive late acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 agents, there was no significant difference between the groups (P = 0.16; Figure 8). When conducting subgroup analysis for different baseline pain levels, there were greater reductions in pain with higher baseline pain levels (Doleman 2018a) (mild: MD ‐0.14; 95% CI ‐0.37 to 0.10, moderate: MD ‐0.77; 95% CI ‐1.08 to ‐0.47; P < 0.001; Figure 9). On meta‐regression analysis, although type of surgery explained some of the between‐study heterogeneity, the result was not statistically significant (R2 = 28%; P = 0.22). Type of anaesthesia did not predict any between‐study heterogeneity (R2 = 0%; P = 0.97). On sensitivity analysis, restricting analysis to the three studies that were at low risk of bias for randomization and allocation concealment (Mojsa 2017; Shuying 2014; Zhang 2011) showed a greater reduction in late acute postoperative pain (MD ‐0.52, 95% CI ‐0.65 to ‐0.40; participants = 194; I2 = 0%). When restricting analysis to studies at low risk for blinding, 14 studies were included (Chen 2015; Flath 1987; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Karaman 2008; Lee 2008; Likar 1997; Mojsa 2017; Ozer 2012; Parke 1995; Shuying 2014; Zhang 2011) and showed similar results to the main analysis (MD ‐0.14, 95% CI ‐0.39 to 0.11; participants = 749; I2 = 74%). When restricting analysis to studies where standard deviations were not estimated from IQR or other studies, or means were not estimated from medians (excluded studies: Bajaj 2004; Bunemann 1994; Cabell 2000; Demirbas 2019; Flath 1987; Grifka 2008; Lee 2008; Parke 1995; Sandin 1993; Vijayendra 1998; Yamashita 2006; Zhang 2011), 15 studies showed similar results to the overall analysis (MD ‐0.19, 95% CI ‐0.47 to 0.09; participants = 950; I2 = 98%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐0.16, 95% CI ‐0.41 to 0.08; participants = 999; I2 = 93%).
8.
Subgroup analysis for pre‐emptive late postoperative pain (NSAID versus COX‐2 inhibitor)
9.
Subgroup analysis for pre‐emptive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)
3. Twenty‐four‐hour morphine consumption (mg)
Sixteen studies reported 24‐hour morphine consumption for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bao 2012; Chen 2015; Coli 1993; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Ozyilmaz 2005; Parke 1995; Shuying 2014; Vijayendra 1998; Wang 2010; Yamashita 2006; Zhang 2017). There may be a reduction in 24‐hour morphine consumption with pre‐emptive versus post‐incision NSAIDs (MD ‐5.62 mg, 95% CI ‐9.00 mg to ‐2.24 mg; participants = 854; studies = 16; I2 = 99%; Analysis 1.5). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in randomization and allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 10) or quantitative testing (P = 0.61).
1.5. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 5: 24‐hour morphine consumption (mg)
10.
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive 24‐hour morphine consumption
On subgroup analysis of non‐selective NSAIDs versus COX‐2 specific agents, there was no difference between the groups (P = 0.25; Figure 11). When conducting subgroup analysis by baseline consumption of morphine (< 20 mg: low, 20‐50 mg: moderate and > 50 mg: high; Doleman 2018a), there was no evidence of a difference although subgroup results were imprecise (MD ‐2.66 mg; 95% CI ‐4.54 mg to ‐0.79 mg for low and ‐15.22 mg; 95% CI ‐29.67 mg to ‐0.77 mg for moderate; P = 0.16; Figure 12). On meta‐regression, type of surgery did not predict any of the between‐study heterogeneity (R2 = 0%; P = 0.66). Similarly, type of anaesthesia did not predict any between‐study heterogeneity (R2 = 0%; P = 0.72). On sensitivity analysis, restricting analysis to the one study that was at low risk of bias for randomization and allocation concealment (Shuying 2014) (MD ‐1.02 mg, 95% CI ‐2.16 mg to 0.12 mg; participants = 75) demonstrated a lower reduction in morphine consumption. Nine studies were at low risk of bias for blinding (Chen 2015; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Parke 1995; Shuying 2014). The results for these studies showed a smaller reduction in morphine consumption (MD ‐1.14 mg, 95% CI ‐2.29 mg to 0.01 mg; participants = 539; I2 = 62%). When restricting analysis to studies where means and standard deviations were not estimated, 12 studies remained (Bao 2012; Chen 2015; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Ozyilmaz 2005; Parke 1995; Shuying 2014; Zhang 2017). Results were similar to the main analysis (MD ‐4.76 mg, 95% CI ‐8.79 mg to ‐0.73 mg; participants = 700; I2 = 99%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐5.31 mg, 95% CI ‐8.61 mg to ‐2.02 mg; participants = 533; I2 = 93%).
11.
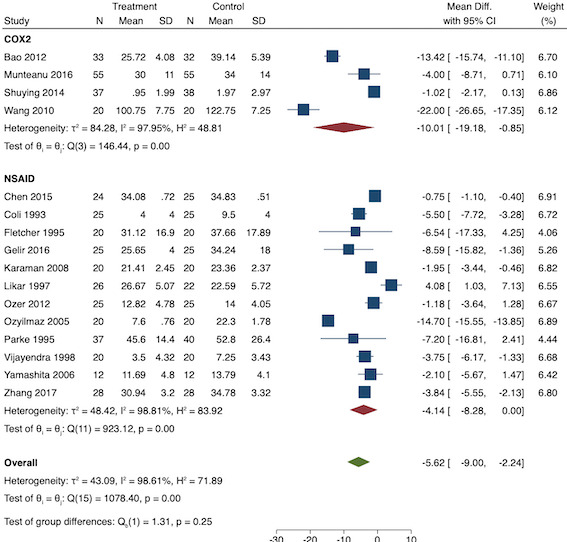
Subgroup analysis for pre‐emptive 24‐hour morphine consumption (NSAID versus COX‐2)
12.
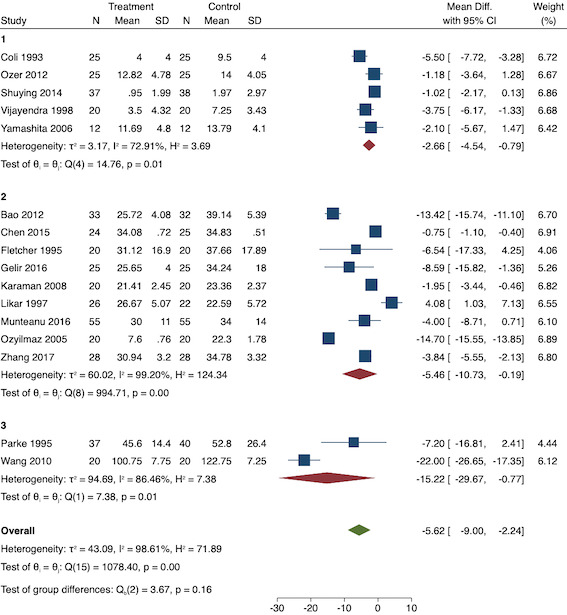
Subgroup analysis for pre‐emptive 24‐hour morphine consumption (baseline consumption). Subgroups are 1 (low), 2 (medium) and 3 (high)
4. Time to first analgesic request (minutes)
Eighteen studies reported time to analgesic request for pre‐emptive NSAIDs versus post‐incision NSAIDs (Buggy 1994; Chen 2015; Colbert 1998; Coli 1993; Esparza‐Villalpando 2016; Gelir 2016; Inanoglu 2007; Kaczmarzyk 2010; Likar 1997; Munteanu 2016; Nezafati 2017; O'Hanlon 1996; Priya 2002; Vanlersberghe 1996; Vijayendra 1998; Vogol 1992; Yagar 2011; Zhang 2017). There may be an increase in the time to analgesic request with pre‐emptive NSAIDs (MD 17.04 minutes, 95% CI 3.77 minutes to 30.31 minutes; participants = 975; studies = 18; I2 = 95%; Analysis 1.6). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 13) or quantitative testing (P = 0.52).
1.6. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 6: Time to first analgesic request (minutes)
13.
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive time to analgesic request
We did not conduct subgroup analysis as there was only one study in one of the subgroups. On meta‐regression analysis, neither type of surgery (R2 = 28%; P = 0.14) nor type of anaesthesia (R2 = 0%; P = 0.98) was a significant predictor of between‐study heterogeneity. On sensitivity analysis, restricting analysis to the two studies that were at low risk of bias for randomization and allocation concealment (Esparza‐Villalpando 2016; Kaczmarzyk 2010), results were opposite to the main analysis, with a reduction in time to analgesia (MD ‐70.25 minutes, 95% CI ‐101.28 minutes to ‐39.21 minutes; participants = 124; I2 = 0%). Nine studies were at low risk of bias for blinding (Buggy 1994; Chen 2015; Esparza‐Villalpando 2016; Gelir 2016; Inanoglu 2007; Kaczmarzyk 2010; Likar 1997; Munteanu 2016; Priya 2002) which showed a prolonged duration of time to analgesia in the pre‐emptive NSAIDs group (MD 56.84 minutes, 95% CI 13.27 minutes to 100.42 minutes; participants = 515; I2 = 97%). When restricting analysis to the five studies that did not estimate standard deviations or means (Buggy 1994; Coli 1993; Vanlersberghe 1996; Vijayendra 1998; Yagar 2011), the results were similar to the main analysis (MD 17.93 minutes, 95% CI 1.09 minutes to 34.78 minutes; participants = 744; studies = 18; I2 = 96%). Restricting analysis to studies with more than 50 participants gave broadly similar results (MD 33.66 minutes, 95% CI 14.12 minutes to 53.19 minutes; participants = 637; I2 = 97%). One study included data as time to an event (Mojsa 2017), but only reported data with reference to a control group so could not be included in the meta‐analysis.
5. Pruritus (yes/no)
Four studies included data for long‐term pruritus (Lee 2008; Likar 1997; Munteanu 2016; Zhang 2017). Overall, there may be no difference between pre‐emptive and post‐incision NSAIDs (RR 0.40, 95% CI 0.09 to 1.76; participants = 254; I2 = 0%; Analysis 1.7). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
1.7. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 7: Pruritus (long‐term)
We were unable to assess publication bias or conduct meta‐regression due to the low number of included studies. On subgroup analysis, non‐selective NSAIDs versus COX‐2 agents showed no difference between groups (P = 0.52; Figure 14). On sensitivity analysis, no study was at low risk of bias for randomization and allocation concealment. When restricting analysis to the three studies that were at low risk of bias for blinding (Lee 2008; Likar 1997; Munteanu 2016), results were similar to the main analysis (RR 0.50, 95% CI 0.09 to 2.78; participants = 198; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.25, 95% CI 0.03 to 2.25; participants = 166; I2 = 0%). Assuming those participants who dropped out from Zhang 2017 suffered an event gave similar results (RR 0.50, 95% CI 0.15 to 1.62; participants = 258; studies = 4; I2 = 0%).
14.
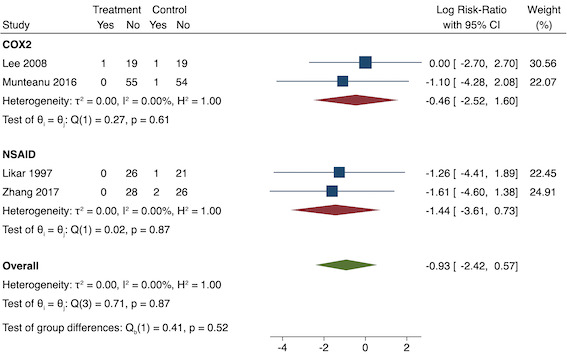
Subgroup analysis for pre‐emptive pruritus (NSAID versus COX‐2). Effect estimate presented as log risk ratio
6. Sedation (yes/no)
Four studies reported sedation (long‐term) (Fletcher 1995; Munteanu 2016; Shuying 2014; Zhang 2017). There may be no difference in sedation between the pre‐emptive and post‐incision NSAID groups (RR 0.51, 95% CI 0.16 to 1.68; participants = 281; I2 = 0%; Analysis 1.8). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
1.8. Analysis.

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 8: Sedation (long‐term)
We were unable to assess publication bias or conduct meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between the non‐selective NSAID and COX‐2 groups (P = 0.66; Figure 15). On sensitivity analysis, restricting analysis to the only study that was at low risk of bias for randomization and allocation concealment (Shuying 2014), results were similar to the main analysis (RR 0.51, 95% CI 0.05 to 5.42; participants = 75). Three studies were at low risk of bias for blinding (Fletcher 1995; Munteanu 2016; Shuying 2014) and results were again similar to the main analysis (RR 0.55, 95% CI 0.15 to 1.98; participants = 225; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.55, 95% CI 0.15 to 1.99; participants = 241; I2 = 0%). When assuming participants who dropped out suffered events from two studies (Shuying 2014; Zhang 2017), the results were similar (RR 0.75, 95% CI 0.32 to 1.78; participants = 290; I2 = 0%).
15.
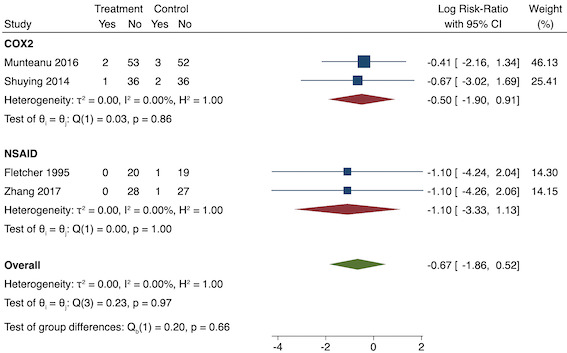
Subgroup analysis for pre‐emptive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio
7. Patient satisfaction (< 24 hours)
No studies reported patient satisfaction within 24 hours.
8. Chronic pain (yes/no)
No studies reported chronic pain.
9. Time to first bowel movement (hours)
No studies reported time to first bowel movement.
Preventive NSAIDs versus post‐incision NSAIDs
Primary outcomes
1. Early acute postoperative pain (measured within six hours postoperatively)
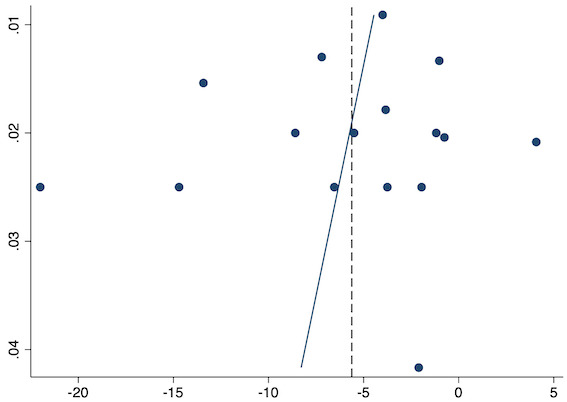
Eighteen studies reported early acute postoperative pain for preventive NSAIDs versus post‐incision NSAIDs (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Trampitsch 2003; Wnek 2004; Young 2006). There may be no reduction in early acute postoperative pain with preventive NSAIDs (MD ‐0.14, 95% CI ‐0.39 to 0.12; participants = 1140; I2 = 75%; Analysis 2.1). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in allocation concealment (one level), and unexplained heterogeneity (one level). There was some evidence of publication bias on visual inspection of funnel plots (Figure 16), although quantitative testing was not significant (P = 0.15).
2.1. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 1: Early acute postoperative pain (within 6 hours postoperatively)
16.
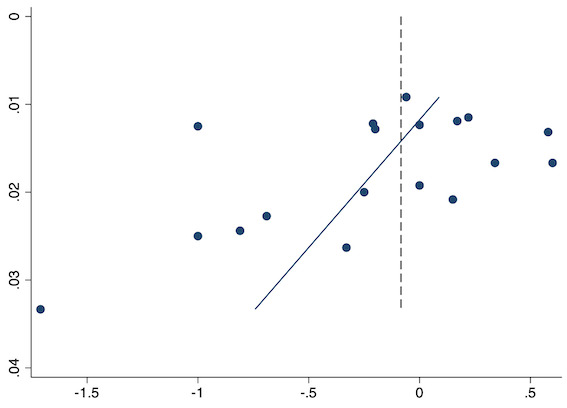
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive early acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.32; Figure 17). When comparing groups with different baseline pain levels (Doleman 2018a), there was a significant difference between the groups (P = 0.01; Figure 18) with those in the severe group having greater reductions (MD ‐0.80; 95% CI ‐1.23 to ‐0.37) than those in the moderate (MD ‐0.24; 95% CI ‐0.92 to 0.45) and mild group (MD ‐0.04; 95% CI ‐0.31 to 0.24). On meta‐regression analysis, neither type of surgery (P = 0.23) nor type of anaesthesia (P = 0.17) predicted reductions in early postoperative pain. On sensitivity analysis, when restricting analysis to the three studies that were at low risk of bias for randomization and allocation concealment (Martinez 2007; Pandazi 2010; Sun 2008), results were similar to the main analysis (MD ‐0.37, 95% CI ‐1.48 to 0.75; participants = 157; I2 = 94%). Similarly, 11 studies were at low risk of bias for blinding (Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Gramke 2006; Martinez 2007; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Wnek 2004) and showed similar results to the main analysis (MD ‐0.14, 95% CI ‐0.58 to 0.30; participants = 702; I2 = 72%). When restricting analysis to studies where SD and means were not estimated, this left nine studies (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Moonla 2018; Norris 2001; Salonen 2001; Trampitsch 2003) and results were opposite to the main analysis (MD 0.17, 95% CI ‐0.06 to 0.40; participants = 621; I2 = 10%). Restricting analysis to studies with more than 50 participants gave opposite results (MD 0.09, 95% CI ‐0.18 to 0.36; participants = 899; studies = 18; I2 = 69%).
17.
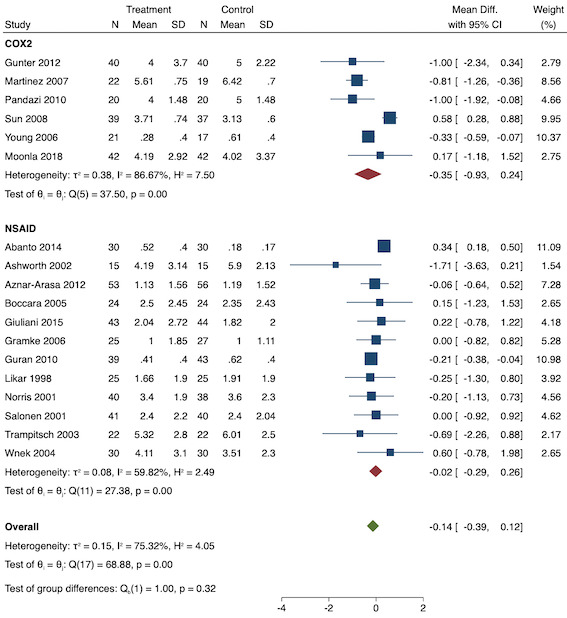
Subgroup analysis for preventive early postoperative pain (NSAID versus COX‐2)
18.
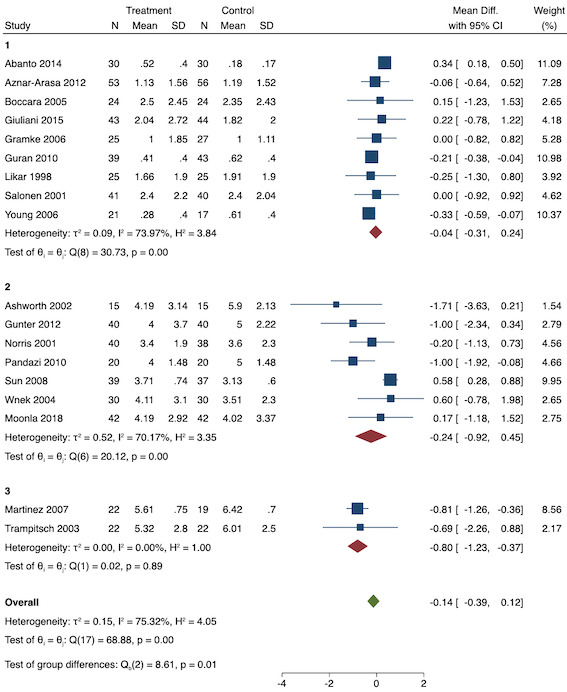
Subgroup analysis for preventive early postoperative pain (baseline pain). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)
2. Adverse events (reoperation for major bleeding within 30 days, acute kidney injury within 48 hours, gastrointestinal ulceration or bleeding requiring endoscopy within 30 days and myocardial infarction within 30 days)
No studies reported acute kidney injury, gastrointestinal ulceration or myocardial infarction for preventive NSAIDs versus post‐incision NSAIDs. One study reported reoperation for bleeding following tonsillectomy (Salonen 2001). There may be no difference between preventive or post‐incision NSAIDs (RR 1.95, 95% CI 0.18 to 20.68; participants = 81). The certainty of evidence was very low owing to concerns over imprecision, due to a low number of events (two levels), and indirectness of evidence (one level), as it was conducted in tonsillectomy only. Due to the inclusion of only one study, we could not conduct analysis for publication bias, investigation of heterogeneity or sensitivity analysis. No participants were excluded from this study (Salonen 2001).
Secondary outcomes
1. Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic as composite outcome (yes/no))
Short‐term nausea and vomiting
One study reported short‐term nausea and vomiting (Sun 2008). There may be no difference in the number of events between preventive and post‐incision NSAID groups (RR 1.26, 95% CI 0.49 to 3.30; participants = 76). The certainty of evidence was low owing to concerns over imprecision (one level) and indirectness (one level). Due to the inclusion of only one study, we could not conduct analysis for publication bias, investigation of heterogeneity or most sensitivity analyses. When assuming excluded participants suffered an event from Sun 2008, results were similar to the main analysis (RR 1.00, 95% CI 0.44 to 2.26).
Long‐term nausea and vomiting
Eight studies reported long‐term nausea and vomiting (Boccara 2005; Gramke 2006; Gunter 2012; Martinez 2007; Moonla 2018; Nakayama 2001; Pandazi 2010; Salonen 2001). There may be no difference between preventive and post‐incision NSAIDs (RR 0.89, 95% CI 0.65 to 1.22; participants = 456; I2 = 0%; Analysis 2.4). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
2.4. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 4: Nausea and vomiting (long‐term)
There were too few studies to undertake assessment of publication bias or meta‐regression analysis. On subgroup analysis, there was no difference between non‐selective NSAIDs or COX‐2 inhibitors (P = 0.13; Figure 19). On sensitivity analysis, restricting analysis to only studies with low risk for randomization and allocation concealment left two studies (Martinez 2007; Pandazi 2010) which gave similar results to the main analysis (RR 0.76, 95% CI 0.35 to 1.63; participants = 81; I2 = 0%). Restricting analysis to only studies that were low risk for blinding left six studies (Boccara 2005; Gramke 2006; Martinez 2007; Nakayama 2001; Pandazi 2010; Salonen 2001). The results were similar to the main analysis (RR 1.02, 95% CI 0.70 to 1.48; participants = 292; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.89, 95% CI 0.54 to 1.46; participants = 297; studies = 8; I2 = 30%). In Gunter 2012, it was unclear if excluded participants were analysed and, in Martinez 2007 and Moonla 2018, it was unclear to which group exclusions belonged. Assuming excluded participants suffered an event gave similar results (RR 0.89, 95% CI 0.64 to 1.24; participants = 376; studies = 7; I2 = 0%).
19.
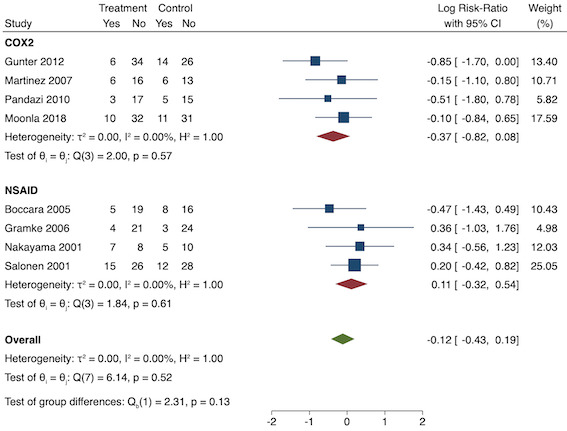
Subgroup analysis for preventive late nausea and vomiting (NSAID versus COX‐2)
2. Late acute postoperative pain (measured at 24 to 48 hours)
Twenty‐one studies reported late acute postoperative pain (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Murphy 1993; Nakayama 2001; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Trampitsch 2003; Wnek 2004; Young 2006; Yuan 2019; Zhou 2017). There may be a reduction in late acute postoperative pain with preventive NSAIDs versus post‐incision NSAIDs (MD ‐0.33, 95% CI ‐0.59 to ‐0.07; participants = 1441; I2 = 81%; Analysis 2.5). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment and blinding of outcome assessment (one level), and unexplained heterogeneity (one level). There was no evidence of funnel plot asymmetry on visual inspection (Figure 20) or on quantitative testing (P = 0.23).
2.5. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 5: Late acute postoperative pain (24‐48 hours postoperatively)
20.
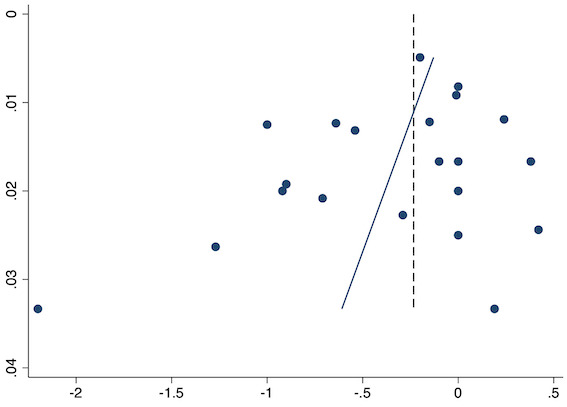
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive late acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.63; Figure 21). When comparing groups with different baseline pain levels (Doleman 2018a), there was no difference between those with mild or moderate baseline pain levels (P = 0.69; Figure 22). On meta‐regression analysis, type of anaesthesia (R2 = 29%; P = 0.11) or type of surgery (R2 = 0%; P = 0.95) did not predict between‐study heterogeneity. On sensitivity analysis, restricting analysis to only three studies (Pandazi 2010; Sun 2008; Yuan 2019) that were at low risk of bias for randomization and allocation concealment showed similar results, although these were less precise (MD ‐0.28, 95% CI ‐0.63 to 0.07; participants = 320; I2 = 64%). Eleven studies were at low risk of bias for blinding (Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Gramke 2006; Martinez 2007; Nakayama 2001; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Wnek 2004) and showed similar results (MD ‐0.35, 95% CI ‐0.61 to ‐0.09; participants = 627; I2 = 32%). When restricting analysis to studies where SD and means were not estimated, 11 studies remained in the analysis (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Moonla 2018; Murphy 1993; Nakayama 2001; Norris 2001; Salonen 2001; Trampitsch 2003; Yuan 2019). They showed similar effects, although these were less precise (MD ‐0.25, 95% CI ‐0.61 to 0.11; participants = 800; I2 = 69%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐0.28, 95% CI ‐0.56 to ‐0.01; participants = 1170; studies = 21; I2 = 76%).
21.
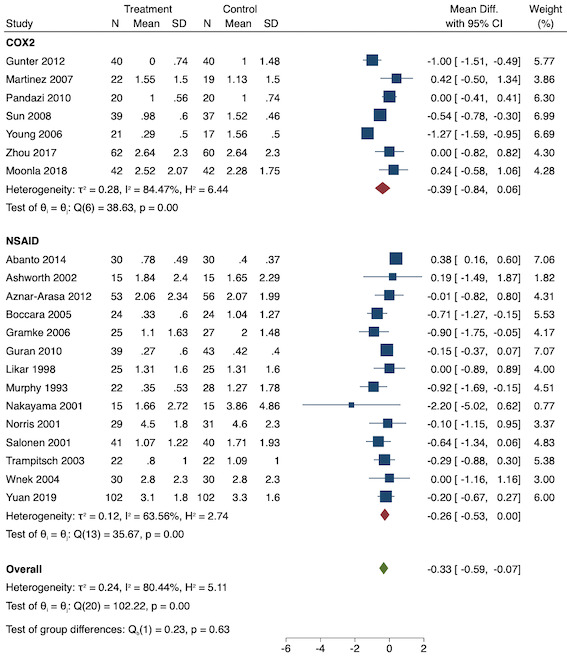
Subgroup analysis for preventive late postoperative pain (NSAID versus COX‐2)
22.
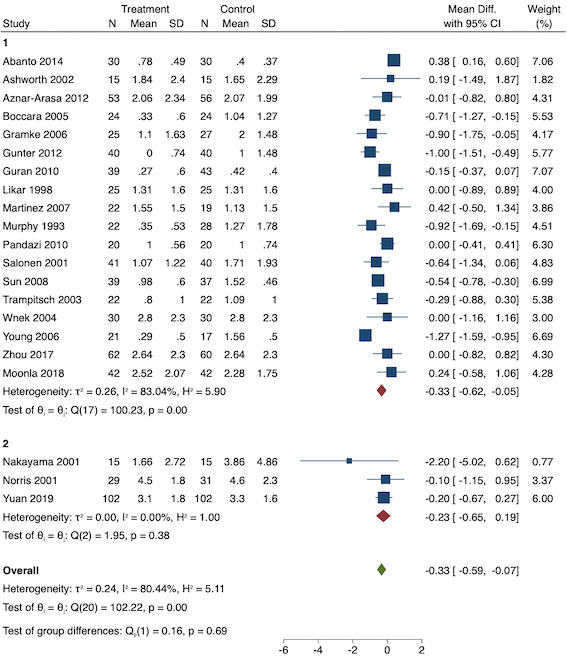
Subgroup analysis for preventive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)
3. Twenty‐four‐hour morphine consumption (mg)
Sixteen 16 studies reported 24‐hour morphine consumption (Ashworth 2002; Boccara 2005; Fleckenstein 2016; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Murphy 1993; Pandazi 2010; Riest 2006; Riest 2008; Salonen 2001; Sun 2008; Trampitsch 2003). There is probably a reduction in 24‐hour morphine consumption with preventive versus post‐incision NSAIDs (MD ‐1.93 mg, 95% CI ‐3.55 mg to ‐0.32 mg; participants = 1323; I2 = 49%; Analysis 2.6). The certainty of evidence was moderate, downgraded owing to concerns over risk of bias, mainly in allocation concealment (one level), and incomplete outcome data. There was no evidence of publication bias both on visual inspection of funnel plots (Figure 23) or quantitative testing (P = 0.18).
2.6. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 6: 24‐hour morphine consumption (mg)
23.
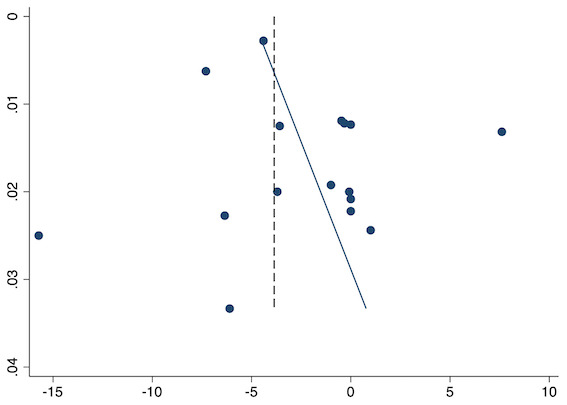
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive 24‐hour morphine consumption
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.06; Figure 24). When conducting subgroup analysis by baseline consumption of morphine (< 20 mg: low, 20‐50 mg: moderate and > 50 mg: high; Doleman 2018a), those with higher baseline consumption had greater reductions in morphine consumption (moderate: MD ‐3.77 mg; 95% CI ‐7.27 mg to ‐0.26 mg versus low: MD ‐0.32 mg; 95% CI ‐0.40 mg to ‐0.24 mg; P = 0.05; Figure 25). On meta‐regression analysis, type of surgery predicted all the between‐study heterogeneity (R2 = 100%; P = 0.1), although the number of studies in each group was limited. We could not conduct analysis for type of anaesthesia as all studies used general anaesthesia. On sensitivity analysis, restricting analysis to four studies (Fleckenstein 2016; Martinez 2007; Pandazi 2010; Riest 2006) that were low risk of bias for randomization and allocation concealment (MD ‐5.31 mg, 95% CI ‐12.36 mg to 1.73 mg; participants = 486; I2 = 68%), the results were more imprecise although differed from the main analysis with a greater reduction in morphine consumption. Nine studies were at low risk for blinding (Ashworth 2002; Boccara 2005; Fleckenstein 2016; Gramke 2006; Martinez 2007; Pandazi 2010; Riest 2006; Salonen 2001; Sun 2008) and results were similar to the main analysis although confidence intervals crossed zero (MD ‐2.13 mg, 95% CI ‐5.55 mg to 1.29 mg; participants = 773; I2 = 55%). When restricting analysis to studies that did not estimate means or SDs, there were eight studies in the analysis (Ashworth 2002; Boccara 2005; Guran 2010; Moonla 2018; Murphy 1993; Riest 2006; Riest 2008; Trampitsch 2003) and results were similar to the main analysis (MD ‐1.30 mg, 95% CI ‐2.80 mg to 0.19 mg; participants = 858; I2 = 34%) although again confidence intervals crossed zero. Restricting analysis to studies with more than 50 participants were similar although less precise (MD ‐1.07 mg, 95% CI ‐2.33 mg to 0.19 mg; participants = 1075; studies = 16; I2 = 28%).
24.
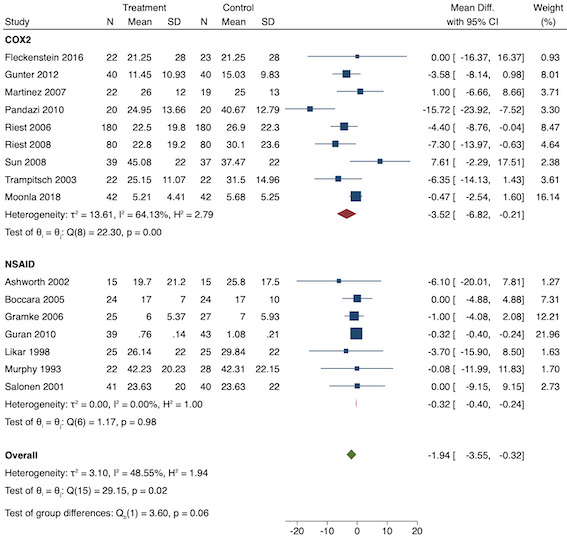
Subgroup analysis for preventive 24‐hour morphine consumption (NSAID versus COX‐2)
25.
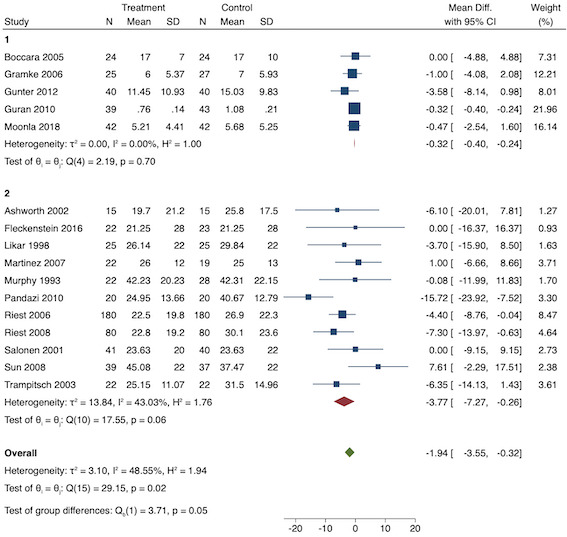
Subgroup analysis for 24‐hour morphine consumption (baseline morphine consumption). Subgroups are 1 (low) and 2 (medium)
4. Time to first analgesic request (minutes)
Eight studies reported time to analgesic request for preventive versus post‐incision NSAIDs (Boccara 2005; Gunter 2012; Likar 1998; Martinez 2007; Mishra 2012; Nakayama 2001; Sun 2008; Wnek 2004). There may be no difference between the groups in time to analgesic request (MD 8.51 minutes, 95% CI ‐31.24 minutes to 48.27 minutes; participants = 410; I2 = 98%; Analysis 2.7). The certainty of evidence was very low owing to concerns over imprecision (one level), risk of bias, mainly in allocation concealment (one level), and other bias and unexplained heterogeneity (one level).
2.7. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 7: Time to first analgesic request (minutes)
We could not conduct investigation for publication bias or meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between non‐selective NSAID and COX‐2 groups (P = 0.96; Figure 26). On sensitivity analysis, when restricting analysis to two studies that were at low risk of bias for randomization and allocation concealment (Martinez 2007; Sun 2008), it showed similar effect estimates to the main analysis (MD 7.51 minutes, 95% CI 1.21 minutes to 13.81 minutes; participants = 117; I2 = 42%). Five studies were at low risk of bias for blinding (Boccara 2005; Martinez 2007; Nakayama 2001; Sun 2008; Wnek 2004) which showed opposite effects to the main analysis (MD ‐7.94 minutes, 95% CI ‐57.28 minutes to 41.41 minutes; participants = 255; I2 = 99%). When restricting analysis to studies where means or SDs were not estimated, four studies remained (Boccara 2005; Likar 1998; Nakayama 2001; Wnek 2004) which showed opposite effects to the main analysis (MD ‐2.71 minutes, 95% CI ‐155.26 minutes to 149.83 minutes; participants = 188; I2 = 99%). Restricting analysis to studies with more than 50 participants again found opposite effects to the main analysis (MD ‐17.62 minutes, 95% CI ‐115.94 minutes to 80.70 minutes; participants = 266; I2 = 97%).
26.
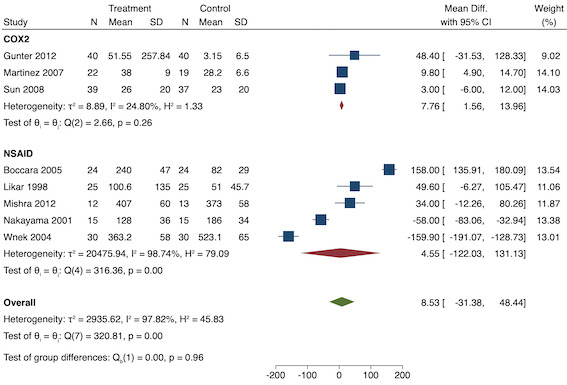
Subgroup analysis for preventive time to analgesic request (NSAID versus COX‐2)
5. Pruritus (yes/no)
No studies reported short‐term pruritus. Three studies reported long‐term pruritus for preventive versus post‐incision NSAIDs (Gunter 2012; Likar 1998; Salonen 2001). There may be no difference between the groups (RR 0.56, 95% CI 0.09 to 3.35; participants = 211; I2 = 0%; Analysis 2.8). The certainty of evidence was low owing to concerns over risk of bias (one level) and imprecision (one level).
2.8. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 8: Pruritus (long‐term)
We were unable to conduct analysis for publication bias and meta‐regression due to the low number of included studies. We did not conduct subgroup analysis as only one study used non‐selective NSAIDs. On sensitivity analysis, restricting analysis to only one study that was at low risk of bias for randomization, allocation concealment and blinding (Sun 2008), it showed different, more imprecise effects to the main analysis (RR 2.93, 95% CI 0.12 to 69.83; participants = 81). No studies had fewer than 50 participants. In Gunter 2012, it was unclear if excluded participants were analysed. In Likar 1998, it was unclear which group exclusions belonged to. The other study (Salonen 2001) had no participants lost to follow‐up.
6. Sedation (yes/no)
No studies reported short‐term sedation. Five studies reported long‐term sedation for preventive versus post‐incision NSAIDs (Boccara 2005; Guran 2010; Martinez 2007; Yuan 2019; Zhou 2017). There may be no difference between the groups (RR 0.84, 95% CI 0.44 to 1.63; participants = 497; I2 = 0%; Analysis 2.9). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
2.9. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 9: Sedation (long‐term)
We were unable to conduct analysis for publication bias and meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between non‐selective NSAID and COX‐2 agents (P = 0.86; Figure 27). On sensitivity analysis, only two studies were at low risk of bias for randomization and allocation concealment (Martinez 2007; Yuan 2019) which gave similar results to the overall analysis (RR 1.08, 95% CI 0.34 to 3.45; participants = 41). Two studies were at low risk of bias for blinding (Boccara 2005; Martinez 2007) which again gave similar results to the main analysis (RR 0.77, 95% CI 0.30 to 1.98; participants = 245; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.67, 95% CI 0.26 to 1.72; participants = 408; studies = 5; I2 = 0%). In Martinez 2007, it was unclear to which group exclusions belonged. Assuming excluded participants suffered an event in the other studies gave similar results (RR 0.95, 95% CI 0.58 to 1.58).
27.
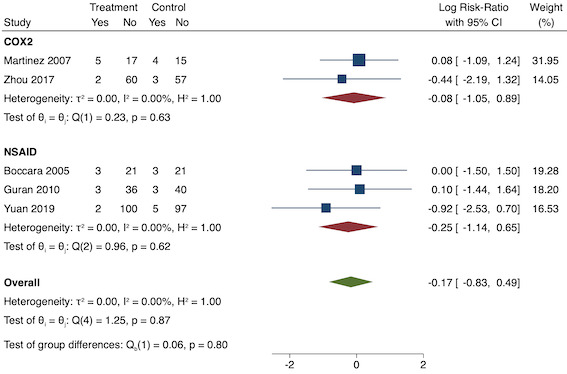
Subgroup analysis for preventive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio
7. Patient satisfaction (< 24 hours)
Only one study reported patient satisfaction for preventive versus post‐incision NSAIDs (Giuliani 2015). There is probably no difference between the groups on a 10‐point scale (MD ‐0.42, 95% CI ‐1.09 to 0.25; participants = 72; Analysis 2.10). The certainty of evidence was moderate owing to concerns over risk of bias (one level), mainly due to attrition bias. There were too few studies to undertake assessment for publication bias, investigation of heterogeneity and sensitivity analysis.
2.10. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 10: Patient satisfaction
8. Chronic pain (yes/no)
No studies reported chronic pain.
9. Time to first bowel movement (hours)
Only one study reported time to bowel movement for preventive versus post‐incision NSAIDs (Sun 2008). There is probably no difference between the groups (MD 0.00 hours, 95% CI ‐15.99 hours to 15.99 hours; participants = 76; Analysis 2.11). The certainty of evidence was moderate owing to concerns over imprecision (one level). There were too few studies to undertake assessment for publication bias, investigation of heterogeneity and sensitivity analysis.
2.11. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 11: Time to bowel movement (hours)
Discussion
Summary of main results
For pre‐emptive NSAIDs, there is probably a decrease in early acute postoperative pain, although none of the included studies reported adverse events. There may be no difference between the groups in nausea and vomiting (short‐ or long‐term). There may be a reduction in late acute postoperative pain. There may also be a reduction in 24‐hour morphine consumption with pre‐emptive NSAIDs and an increase in time to analgesic request. There may be no difference in opioid adverse events such as pruritus or sedation, although the number of included studies for these outcomes was small. No study reported patient satisfaction, chronic pain or time to first bowel movement. The certainty of evidence ranged from moderate to low mainly owing to concerns over risk of bias. However, investigation of heterogeneity showed that baseline pain or morphine consumption may result in more clinically significant reductions in agreement with previous research (Doleman 2018a).
For preventive NSAIDs, there may be no difference in early acute postoperative pain. One study (Salonen 2001) reported adverse events from NSAIDs (reoperation for bleeding) although the events were low which did not allow any meaningful conclusions to be drawn. There may be no difference in rates of nausea and vomiting (short‐ and long‐term). There may be a reduction in late acute postoperative pain. There is probably a reduction in 24‐hour morphine consumption. We are very uncertain if there is any effect on time to analgesic request. As with pre‐emptive NSAIDs, there may be no difference in other opioid adverse events such as pruritus and sedation. There is probably no difference in patient satisfaction (Giuliani 2015). No study reported chronic pain. There is probably no difference in time to first bowel movement (Sun 2008). The certainty of evidence ranged from moderate to very low and there was some evidence that higher baseline pain or morphine consumption led to larger reductions in effects (Doleman 2018a).
None of the observed differences for both pre‐emptive or preventive NSAIDs were clinically significant as defined in our protocol (1.5 reduction in pain, 10 mg reduction in morphine consumption or delay in analgesic request of one hour). However, these results need to be taken in the context of variable effects with baseline risk, that is, clinically significant effects may be observed at higher baseline risk (10 mg reduction in morphine consumption may be seen in studies with high morphine consumption in the control group; Doleman 2018a).
Overall, there may be some evidence of small, non‐clinically significant reductions in acute postoperative pain and 24‐hour morphine consumption with pre‐emptive and preventive NSAIDs, with five out of six of these outcomes showing possible benefit. In addition, there was some evidence of better efficacy with higher baseline pain levels and morphine consumption (more painful operations or higher‐risk patients; Doleman 2018a). However, only one study reported major adverse events for preventive NSAIDs (Salonen 2001). We found no difference in opioid adverse events from a limited number of included studies, further questioning the clinical significance of any observed differences.
Overall completeness and applicability of evidence
Our review had a wide ranging search strategy including electronic databases, grey literature sources, reference searches and searching of conference proceedings (Doleman 2019). This was important in order to reduce the risk of publication bias (Chong 2016). Indeed, when using more appropriate tests for publication bias, we found no evidence of publication bias (imprecise study effects). We excluded 20 studies mainly for reasons which would bias results, such as different doses in each group and different routes of administration (Characteristics of excluded studies). There are currently seven studies awaiting classification, two that we were unable to translate (Aoki 2002; Bai 1998) and five where we were unable to locate full texts despite contacting authors and the British Library (Beg 2001; Belzarena 1994; De Oliveira 1999; Jia 2011; Nicholson 2014). Of these studies, three found an improvement in pain with pre‐emptive NSAIDs (Aoki 2002; Bai 1998; Jia 2011) while another two found no difference between the groups (De Oliveira 1999; Nicholson 2014). The other two results are unknown (Beg 2001; Belzarena 1994). Depending on their results, inclusion of these studies could potentially alter the conclusions of this review. This would be less likely for outcomes that include many studies (such as pain), due to the similar sample size of the missing studies (see Characteristics of studies awaiting classification) but more likely for outcomes with few included studies (nausea and vomiting).
We chose to include a range of time points for measurement of pain outcomes such as early acute postoperative pain (0‐6 hours) and late acute postoperative pain (24‐48 hours). This is due to the fact that postoperative pain trials tend to report a variety of different time points for pain (Doleman 2018a) and exclude others. If we had included more precise time points, this would lead to the exclusion of many studies for each of these outcomes. However, we recognise that including different studies with different time points within one outcome (e.g. one study measuring pain at 0 hours versus 6 hours) could affect pain levels, as these may improve with time. From our experience, this is less of an issue with opioid consumption (Doleman 2018a). We selected early pain as our primary outcome as this is when pain is likely to be at its maximal severity. It could be argued that opioid consumption may be a more appropriate primary outcome (and opioid adverse events more so) as the aim of multimodal analgesia is to reduce opioid consumption. Also, it may not be ethical to have two groups with differences in pain. We will consider this in future review updates.
A major limitation of our previous review on preventive opioids related to the reporting of central tendency as both means and medians in the included studies (Doleman 2018b). One analysis of 24‐hour morphine consumption showed a difference on meta‐analysis of means although nearly all studies that reported median values showed no significant difference. This raises two important issues, firstly, the likelihood of this leading to false conclusions on the efficacy of preventive opioids by review consumers not appreciating this subtle fact (especially those not reading the whole review) and the issues it raises in giving false positives on publication bias assessment (by excluding a large number of 'negative' studies). The options are to include median results in a narrative synthesis (Doleman 2018b) or estimate means from medians (Higgins 2011a). For the reasons described above, the narrative synthesis option has severe disadvantages. Although estimating means from medians risks making false assumptions about the data, it helps ensure all studies contribute data to the analysis. Moreover, even if studies report data as means and SDs, this does not ensure that the underlying data is not skewed. Therefore, we felt the ideal solution was to estimate means from medians and SD from IQR as stated in the Cochrane Handbook (Higgins 2011a) or the range from published research (Hozo 2005) so that all studies could be included. We then conducted a sensitivity analysis by excluding studies where these values had been estimated or imputed. This allows both scenarios to be compared and reduces the risks described previously. In most cases, results of the sensitivity analysis were similar to the main analysis (Effects of interventions).
In terms of the applicability of the evidence, the range of operations included in this review (Description of studies) were more diverse than in our opioid review (Doleman 2018b). Although this helps with external validity, it limited the meta‐regression analysis as there were few studies in each surgical subgroup. Despite helping external validity, diverse types of surgery may raise issues surrounding clinical heterogeneity. However, our investigation of heterogeneity did not consistently identify type of surgery as a significant predictor of between‐study heterogeneity. Moreover, our previous research has demonstrated that baseline risk models (our subgroup analysis) can account for differences between different surgical subtypes (Doleman 2018a). Another issue with respect to applicability is the inclusion of mainly low‐risk participants, those without prior analgesic intake or chronic pain, and a lack of standardisation of postoperative opioids which may affect opioid consumption outcomes (Description of studies). Furthermore, some of our analyses included few studies from a limited set of surgical subtypes which may raise issues about whether these results can be applied to other operations. This also raises the issue around type of NSAIDs used and whether results from the use of certain NSAIDs can be applied to others. Despite our subgroup analysis suggesting no difference between non‐selective NSAIDs and COX‐2 inhibitors, we cannot rule out differences between agents within these classes, especially if different doses were used.
Quality of the evidence
The certainty of evidence for all outcomes ranged from moderate to very low, most commonly due to issues with risk of bias (Figure 2; Figure 3) although unexplained heterogeneity and imprecision were other common reasons. Issues with risk of bias were mainly due to a lack of reporting on allocation concealment and a lack of published protocols (unclear risk) which may result in selection bias and selective reporting bias, respectively. Moreover, 14 studies did not use a double‐dummy placebo so were at high risk of bias for blinding which may affect a subjective outcome such as pain. Our previous research has identified that bias in domains of trial conduct, such as allocation concealment, can exaggerate effects so results from this review must be treated with caution (Doleman 2018a). However, when we conducted sensitivity analysis, there was no consistent effect of risk of bias and effect estimates were similar when we included only studies that scored low risk for either domains of random allocation or blinding (Risk of bias in included studies).
Similar to our previous research, when we conducted subgroup analysis based on differences in baseline pain/morphine consumption, we found larger effects with higher baseline pain/morphine consumption which may have contributed to the heterogeneity observed with these outcomes (Doleman 2018a). This may also be the mechanism by which subgroup differences observed with type of surgery may manifest. Furthermore, unexplained heterogeneity may have contributed to imprecision as our analysis was conducted using random‐effects models where confidence intervals are wider if statistical heterogeneity exists in analyses. For our meta‐regression analysis, type of surgery and type of anaesthesia were not significant predictors of between‐study heterogeneity in most analyses. However, the limitations of such analysis should be considered when the number of included studies is 40 or less (López‐López 2014).
Potential biases in the review process
Similar to our opioid review (Doleman 2018b), none of our review authors were involved in any of the included studies. However, some authors are involved in an ongoing study on preventive paracetamol (see Declarations of interest). Our previous research has identified issues with traditional meta‐analysis when analysing outcomes dependent on baseline risk (Doleman 2018a), although we accounted for this when performing subgroup analysis and found that on most occasions this supported our previous findings. We have also conducted research that has identified type I errors when using Egger's linear regression test for this type of data using simulated meta‐analyses. With this in mind, we have used our novel test which is based on inverse sample size rather than standard error and has been found to perform better than Egger's test due to lack of dependence between effect estimates and standard errors (Doleman 2020).
Agreements and disagreements with other studies or reviews
Although now over a decade old, the two previous reviews of pre‐emptive and preventive NSAIDs found some similar and some different results to our review (Møiniche 2002; Ong 2005). The first review published in 2002 (Møiniche 2002) found that pre‐incisional NSAIDs had no effect on acute pain (MD 0 mm; 95% CI ‐2 mm to 2 mm) with many studies also showing no benefit in reducing analgesic consumption. In contrast, we found some positive evidence for pre‐emptive and preventive NSAIDs in reducing some measures of acute pain and opioid consumption, although we did find limitations in the certainty of evidence and clinical significance. A later review published in 2005 (Ong 2005) found no difference in acute pain, although it did find that pre‐incisional NSAIDs reduced analgesic consumption and prolonged time to first analgesia. We found similar results with analgesic consumption, although we did find some reductions in acute pain in contrast to this review (Ong 2005).
Authors' conclusions
Implications for practice.
We found some evidence of reductions in acute pain and analgesic consumption for both pre‐emptive and preventive NSAIDs. However, these results were not consistent, not clinically significant and the certainty of evidence ranged from moderate to very low, limiting our certainty about these results. Moreover, although the number of included studies was small, we found no difference in postoperative opioid adverse events which further limits the clinical significance of our findings. We did find some evidence of improved efficacy in studies with higher baseline pain or analgesic consumption, although the limitations of such subgroup analysis must be considered. Importantly, we found only one study (Salonen 2001) reporting serious adverse events for the use of NSAIDs (reoperation for bleeding) with a limited number of events. It remains distinctly possible that administration of NSAIDs prior to surgery could increase surgical bleeding, gastrointestinal bleeding, myocardial infarction or acute kidney injury. This, despite some potential early benefits on pain, means any findings influencing clinical practice need to consider this possibility. Due to the large number of included studies in this review, it is unlikely that the studies awaiting classification will significantly change the conclusions of this review (Characteristics of studies awaiting classification).
Implications for research.
Due to the limitations above, future research should aim to follow low risk of bias methodology to improve the certainty of evidence from these studies, particularly allocation concealment and adequate use of double‐dummy placebo. Future studies should also aim to include larger numbers of participants in order to be adequately powered to identify whether pre‐emptive or preventive NSAIDs reduce postoperative opioid adverse events and ensure they evaluate possible serious adverse events of pre‐incision NSAIDs. Future research may also wish to study pre‐emptive or preventive NSAIDs in operations with high baseline pain or analgesic consumption to improve absolute effects (Effects of interventions).
History
Protocol first published: Issue 3, 2018
Acknowledgements
The protocol was screened by the following ACE editors: Jane Cracknell, Harald Herkner, Ann Møller, Nathan Pace, Andrew Smith, Marialena Trivella, and Janne Vendt. We would like to thank Anna Lee (Content Editor), Argyro Fassoulaki and David Dickerson (Peer Reviewers) for their help and editorial advice during the preparation of this protocol for the systematic review. From Cochrane Anaesthesia, we would like to thank Stephanie Weibel (Content Editor), Nathan Pace (Statistical Editor), Argyro Fassoulaki (Peer Reviewers), Harrison Nelson (Consumer Reviewer), Janne Vendt (Cochrane Information Specialist), Liz Bickerdike (Network Associate Editor, Acute and Emergency Care Network), Teo Quay (Managing Editor), Vernon Hedge (Managing Editor) and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Search strategies
MEDLINE
1 exp Anti‐Inflammatory Agents, Non‐Steroidal/ (196911)
2 (NSAID* or non‐steroidal anti‐inflammatory or non‐steroidal antiinflammatory or cyclooxygenase enzyme* or cox or ibuprofen or ketoprofen or diclofenac or indomethacin or ketorolac or naproxen or celecoxib or parecoxib or valdecoxib).mp. (257855)
3 1 or 2 (376251)
4 exp Pain, Postoperative/ (40747)
5 exp postoperative complications/ (542898)
6 ((postoperat* or post operat* or postsurg* or post surg* or postan?esth* or post an?esth* or perioperat* or peri operat*) adj6 (pain* or recover* or analges*)).mp. (87317)
7 ((postoperat* or post operat* or postsurg* or post surg* or postan?esth* or post an?esth* or perioperat* or peri operat*) adj2 complication*).mp. (418222)
8 (((postoperat* or post operat* or postsurg* or post surg* or postan?esth* or post an?esth* or perioperat* or peri operat*) adj2 (care or period*)) and pain*).mp. (19394)
9 4 or 5 or 6 or 7 or 8 (632292)
10 Preanesthetic Medication/ (7948)
11 Premedication/ (12484)
12 (pre emptive or preemptive or preventive or preoperat* or pre operat* or preincision* or pre incision* or pre surg* or presurg* or perioperat* or peri operat* or intraoperat* or intra operat* or prophyla* or ((before or prior) adj3 (surg* or operat*))).mp. (951011)
13 10 or 11 or 12 (963150)
14 3 and 9 and 13 (5980)
15 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (exp animals/ not humans.sh.) (1224369)
16 14 and 15 (2132)
17 (exp child/ or exp infant/) not exp adult/ (1661664)
18 16 not 17 (1989)
CENTRAL
#1 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees 7551
#2 (NSAID* or (non next steroidal next anti next inflammatory) or (non next steroidal next antiinflammatory) or (cyclooxygenase next enzyme*) or cox or ibuprofen or ketoprofen or diclofenac or indomethacin or ketorolac or naproxen or celecoxib or parecoxib or valdecoxib):ti,ab,kw 38187
#3 #1 or #2 40872
#4 MeSH descriptor: [Pain, Postoperative] explode all trees 14957
#5 MeSH descriptor: [Postoperative Complications] explode all trees 39006
#6 ((postoperat* or (post next operat*) or postsurg* or (post next surg*) or postan*esth* or (post next an*esth*) or perioperat* or (peri next operat*)) near (pain* or recover* or analges*)):ti,ab,kw 42979
#7 ((postoperat* or (post next operat*) or postsurg* or (post next surg*) or postan*esth* or (post next an*esth* or perioperat* or (peri next operat*))) near/2 complication*):ti,ab,kw 36606
#8 (((postoperat* or (post next operat*) or postsurg* or (post next surg*) or postan*esth* or (post next an*esth* or perioperat* or (peri next operat*))) near/2 (care or period*)) and pain*):ti,ab,kw 10812
#9 #4 or #5 or #6 or #7 or #8 78924
#10 MeSH descriptor: [Preanesthetic Medication] explode all trees 1715
#11 MeSH descriptor: [Premedication] explode all trees 4245
#12 ((pre next emptive) or preemptive or preventive or preoperat* or (pre next operat*) or preincision* or (pre next incision*) or (pre next surg*) or presurg* or perioperat* or (peri next operat*) or intraoperat* or (intra next operat*) or prophyla* or ((before or prior) near/3 (surg* or operat*))):ti,ab,kw 128393
#13 #10 or #11 or #12 130531
#14 #3 and #9 and #13 3186
#15 ((child* or infant* or pediatric* or paediatric*) not (adult* or elder* or aged or (old next age) or geriatric*)):ti,ab,kw 114324
#16 #14 not #15 3036
#17 #16 in Trials 3023
Embase
1 exp ANTI‐INFLAMMATORY AGENTS, NON‐STEROIDAL/
2 (NSAID* OR non‐steroidal anti‐inflammatory drug* OR cyclooxygenase enzyme* OR cox OR ibuprofen OR ketoprofen OR diclofenac or indomethacin OR ketorolac OR naproxen OR celecoxib OR parecoxib OR valdecoxib).ti,ab
3 1 OR 2
4 exp PAIN, POSTOPERATIVE/
5 ((postoperati* OR post‐operati*) ADJ6 (pain* OR recover*)).ti,ab
6 4 OR 5
7 exp PREANESTHETICMEDICATION/ OR (pre‐emptive OR preemptive OR preventive OR preoperati* OR pre‐operat* OR preincision OR pre‐incision OR perioperati* OR peri‐operati* OR intraoperati* OR intra‐operati* OR prophylactic* OR ((before OR prior) ADJ3 (surg* OR operat*)))
8 3 AND 6
9 7 AND 8 [Publication types Article OR Conference Abstract OR Conference Paper OR Conference Proceeding OR Journal] [Human age groups Adult 18 to 64 years OR Aged 65+ years] [Humans] [Clinical trials Clinical Trial OR Randomized Controlled Trial OR Controlled Clinical Trial OR Multicenter Study OR Phase 1 Clinical Trial OR Phase 2 Clinical Trial OR Phase 3 Clinical Trial OR Phase 4 Clinical Trial]
CINAHL
1 (NSAID* OR non‐steroidal anti‐inflammatory drug* OR cyclooxygenase enzyme* OR cox OR ibuprofen OR ketoprofen OR diclofenac or indomethacin OR ketorolac OR naproxen OR celecoxib OR parecoxib OR valdecoxib).ti,ab
2 (pain).ti,ab
3 ((postoperati* OR post‐operati*) ADJ6 (pain* OR recover*)).ti,ab
4 (pre‐emptive OR preemptive OR preventive OR preoperati* OR pre‐operat* OR preincision OR pre‐incision OR perioperati* OR peri‐operati* OR intraoperati* OR intra‐operati* OR prophylactic* OR ((before OR prior) ADJ3 (surg* OR operat*))).ti,ab
5 2 OR 3
6 1 AND 4 AND 5
AMED
1 (NSAID* OR non‐steroidal anti‐inflammatory drug* OR cyclooxygenase enzyme* OR cox OR ibuprofen OR ketoprofen OR diclofenac or indomethacin OR ketorolac OR naproxen OR celecoxib OR parecoxib OR valdecoxib).ti,ab
2 (pain).ti,ab
3 ((postoperati* OR post‐operati*) ADJ6 (pain* OR recover*)).ti,ab
4 (pre‐emptive OR preemptive OR preventive OR preoperati* OR pre‐operat* OR preincision OR pre‐incision OR perioperati* OR peri‐operati* OR intraoperati* OR intra‐operati* OR prophylactic* OR ((before OR prior) ADJ3 (surg* OR operat*))).ti,ab
5 2 OR 3
6 1 AND 4 AND 5
Appendix 2. Data Extraction Form
Data collection form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study Eligibility
| Study Characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the protocol) |
Yes | No | Unclear |
Location in text (pg & /fig/table) |
|
| Type of study | Randomized Controlled Trial | |||||
| Controlled Clinical Trial (quasi‐randomized trial) |
||||||
| Participants | ||||||
| Types of intervention | ||||||
| Types of outcome measures | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Notes: | |||
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel‐group, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval needed/ obtained for study | Yes No Unclear | ||
| Notes: | |||
5. Risk of bias assessment
See Chapter 8 of the Cochrane Handbook
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low‐risk | High‐risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All/ | ||||
| (if required) | Outcome group: | ||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All/ | ||||
| (if required) | Outcome group: | ||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/ethnicity | ||
| Severity of illness | ||
| Comorbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention group 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Group name | ||
|
No. randomized to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
| Co‐interventions | ||
| Economic variables(i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
Continuous outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||||||
| Reanalysis possible? | Yes No Unclear | |||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Other outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear | |||||
| Reanalysis possible? | Yes No Unclear | |||||
| Reanalysed results | ||||||
| Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g.lower socioeconomic groups) | Yes No Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear | |
| Notes: | ||
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
Data and analyses
Comparison 1. Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Early acute postoperative pain (within 6 hours postoperatively) | 36 | 2032 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.97, ‐0.41] |
| 1.2 Nausea and vomiting (short‐term) | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.94] |
| 1.3 Nausea and vomiting (long‐term) | 5 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
| 1.4 Late acute postoperative pain (24‐48 hours postoperatively) | 28 | 1645 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 1.5 24‐hour morphine consumption (mg) | 16 | 854 | Mean Difference (IV, Random, 95% CI) | ‐5.62 [‐9.00, ‐2.24] |
| 1.6 Time to first analgesic request (minutes) | 18 | 975 | Mean Difference (IV, Random, 95% CI) | 17.04 [3.77, 30.31] |
| 1.7 Pruritus (long‐term) | 4 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 1.8 Sedation (long‐term) | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.68] |
Comparison 2. Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Early acute postoperative pain (within 6 hours postoperatively) | 18 | 1140 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 2.2 Re‐operation for bleeding | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3 Nausea and vomiting (short‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4 Nausea and vomiting (long‐term) | 8 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.65, 1.22] |
| 2.5 Late acute postoperative pain (24‐48 hours postoperatively) | 21 | 1441 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 2.6 24‐hour morphine consumption (mg) | 16 | 1323 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.55, ‐0.32] |
| 2.7 Time to first analgesic request (minutes) | 8 | 410 | Mean Difference (IV, Random, 95% CI) | 8.51 [‐31.24, 48.27] |
| 2.8 Pruritus (long‐term) | 3 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.35] |
| 2.9 Sedation (long‐term) | 5 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.44, 1.63] |
| 2.10 Patient satisfaction | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.11 Time to bowel movement (hours) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
2.2. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 2: Re‐operation for bleeding
2.3. Analysis.

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 3: Nausea and vomiting (short‐term)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abanto 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 60
Country: Peru
Setting: dental clinic
Dates conducted: November 2013 to July 2014
Postoperative opioid used and delivery: none Pain score collection: researcher Concurrent postoperative analgesics: paracetamol or NSAID |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Prophylactic (30 participants): naproxen sodium 550 mg PO 30 minutes before the procedure, then every 12 hours for 4 doses Group Continuous (30 participants): naproxen sodium 550 mg PO 20 minutes after the procedure, then every 12 hours for 4 doses |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: article in Spanish. Unpublished thesis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear details. Quote: "Patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of double‐dummy placebo. Quote: "Naproxen sodium 550 mg was administered orally 30 minutes before the procedure, then every 12 hours until the 4 doses". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Likely unblinded from above information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three excluded for alveolitis unlikely to bias results as this would cause confounding from increased pain. Quote: "Three people were eliminated by alveolitis". |
| Selective reporting (reporting bias) | Unclear risk | No registration or protocol. Unpublished thesis |
| Other bias | Unclear risk | No detailed information on baseline characteristics |
Ashworth 2002.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 30 Country: UK Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV morphine bolus then PCA Pain score collection: blinded nurse Concurrent postoperative analgesics: PO diclofenac |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Systemic Pre‐surgery (15 participants): 20 mg IV ketorolac before surgery and IV saline after surgery Group Systemic Post‐surgery (15 participants): IV saline before surgery and 20 mg IV ketorolac after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: data extracted from graph using software. Standard deviations calculated from reported confidence intervals. Only systemic pre‐surgery and post‐surgery compared. Included in preventive as diclofenac used postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number chart. Quote: "patients were assigned to one of three groups using a random number chart". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐dummy placebo. Quote: "each group received an intravenous injection at three stages during the operation...each injection consisted of normal saline 20 ml with or without ketorolac 20 mg according to randomization. All groups received the injection". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "the nurses in the recovery room remained blind to the treatment group". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One participant excluded for respiratory depression but unclear which group and another excluded for extended procedure. Quote: "two patients were not studied: one had an extended procedure (> 1.5 h); the other had respiratory depression while using PCA, which was therefore discontinued". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | High risk | More females in post‐incision group and more patients in preventive group received fentanyl. |
Aznar‐Arasa 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 109 Country: Spain Setting: University hospital Dates conducted: February 2008 to October 2010 Postoperative opioid used and delivery: none Pain score collection: participant self‐report Concurrent postoperative analgesics: metamizole PRN |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Experimental (53 participants): PO ibuprofen 600 mg one hour before surgery followed by placebo just after the end of the operation Group Control (56 participants): placebo one hour before surgery and PO ibuprofen 600 mg just after the end of the operation |
|
| Outcomes |
|
|
| Notes | Funding: grant from the School of Dentistry of the University of Barcelona Declarations of interest: none declared Authors contacted: no Other: early pain score taken from two hours after surgery to allow postoperative dosing to take effect. Included in preventive as PRN metamizole is thought to be an NSAID | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "sequence of random numbers in blocks (generated in www.randomization.com)" |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered envelopes although unclear if opaque and sealed. Quote: "sequentially numbered envelopes were used to conceal the allocation of patients to the two groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "In the preoperative group, patients were administered 600 mg of ibuprofen (PO) 1 hour before the surgical procedure, followed by placebo just after the end of the operation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "all patients, the statistician and the surgeons who performed the extraction and follow‐up examinations were unaware of the medication given to each participant". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of dropouts. Quote: "11 (7 in the experimental group and 4 in control group) were lost because they did not attend follow‐up visits". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics and no conflicts of interest |
Bajaj 2004.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 80 Country: India Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: unspecified rescue analgesic Pain score collection: researcher Concurrent postoperative analgesics: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre‐emptive (40 participants): 40 mg parecoxib 30‐45 minutes before surgery Group Postoperative (40 participants): 40 mg parecoxib when reported pain or awake from anaesthesia (whichever was earlier) |
|
| Outcomes |
|
|
| Notes | Funding: none reported Declarations of interest: none declared but authors appeared to be employees of Glenmark Pharmaceuticals Authors contacted: no Other: pain score taken from one hour to allow recovery from anaesthesia and postoperative dosing. Standard deviations estimated. Data extracted from graph using software | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer. Quote: "computer‐generated randomisation" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo. Quote: "to receive a single dose of 40 mg parecoxib either 30‐45 minutes prior to induction....or in the postoperative period" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...the study was assessor blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. Quote: "all patients enrolled in the study completed the trial". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | High risk | Authors appeared to be employees of Glenmark pharmaceuticals. |
Bao 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 65 Country: China Setting: secondary care Dates conducted: January 2008 to December 2011 Postoperative opioid used and delivery: morphine PCA Pain score collection: blinded staff Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre‐incisional (33 participants): 100 mL of normal saline with IV parecoxib 40 mg 30 minutes before skin incision and 100 mL normal saline IV 30 minutes after skin incision Group Post‐incisional (32 participants): 100 mL of normal saline 30 minutes before skin incision and 100 mL normal saline with IV parecoxib 40 mg 30 minutes after skin incision |
|
| Outcomes |
|
|
| Notes | Funding: none reported Declarations of interest: none declared Authors contacted: no Other: early pain score from 1 hour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how sequence generated. Quote: "by selection of sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Unclear if opaque envelopes used. Quote: "by selection of sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo and surgical teams blinded. Quote: "The preincisional group received a solution of 100 ml normal saline with parecoxib 40 mg intravenously (IV) 30 min before skin incision and 100 ml normal saline IV 30 min after skin incision". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "the postanaesthesia care unit staff were blinded to the randomization". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar groups and no conflicts of interest |
Boccara 2005.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 48 Country: France Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: nalbuphine Pain score collection: not reported Concurrent postoperative analgesics: paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group K1 (24 participants): IV ketoprofen 100 mg before induction and IV saline end of surgery and continued for 24 hours Group K2 (24 participants): IV saline before induction and IV ketoprofen 100 mg end of surgery and continued for 24 hours |
|
| Outcomes |
|
|
| Notes | Funding: none reported Declarations of interest: not reported Authors contacted: yes Other: pain score converted to 0‐10 scale, pain score data extracted from graph and taken from one hour to allow postoperative dosing to take effect. Authors contacted for raw patient satisfaction data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated random list" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "in group K1, ketoprofen was administered before induction and saline after surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "none of the patients, managing anaesthetists or nurses were aware of the randomization code". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions unlikely to bias as equal in number. Quote: "Six patients were excluded from postoperative data analysis because four needed intraoperative laparotomy (one in K1, one in K2 and two in P1) and two for incomplete data (one in K1 and one in K2)". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | High risk | More females in K2 group (Gerbershagen 2014) |
Buggy 1994.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 40 Country: Ireland Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IM morphine Pain score collection: blinded nurses Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (20 participants): IM diclofenac 75 mg as a 3 mL injection one to two hours before surgery and IM normal saline 3 mL immediately after surgery Group 2 (20 participants): IM normal saline 3 mL one to two hours before surgery and IM diclofenac 75 mg immediately after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: median and IQR converted to mean and SD, morphine consumption data not included as study follow‐up only appeared to be 6 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "patients were allocated randomly to two groups". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical double‐dummy placebo. Quote: "each patient received two identical, coded, 3 ml injections: one containing diclofenac 75 mg, the other containing normal saline". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment. Quote: "...were recorded by nursing staff who were unaware" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Bunemann 1994.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 117 Country: Denmark Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV pethidine Pain score collection: in recovery (unclear who) and 24 hour self‐report Concurrent postoperative analgesics: paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group TP (59 participants): naproxen 1100 mg one hour before surgery and placebo immediately after surgery Group PT (58 participants): placebo one hour before surgery and naproxen 1100 mg immediately after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain scores estimated from median and interquartile range; analgesic consumption not included as not just opioid consumed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "naproxen sodium 1100 mg 1 h before surgery and placebo immediately after surgery" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six exclusions, although unclear which groups. Ten missing for 24‐hour data. Quote: "six had to be excluded; three because they did not have an operation and three because they did not have general anaesthesia". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Cabell 2000.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 49
Country: USA
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: IV fentanyl and morphine Pain score collection: patient self‐report Concurrent postoperative analgesics: paracetamol, codeine and NSAIDs PRN |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (25 participants): IV ketorolac 30 mg in operating room and 1 mL saline placebo at end of surgery Group 2 (24 participants): IV ketorolac 30 mg at end of surgery and 1 mL saline placebo on entering operating room |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no contact listed Other: Although 0‐15 cm VAS, the stated text corresponded to a 0‐10 scale. SD estimated. Analgesia consumption not reported. Included in pre‐emptive as only 'several' participants received NSAIDs. Early pain score from 30 minutes to allow postoperative dosing to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information about allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "received IV ketorolac..and 1 ml IV isotonic sodium chloride" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention. Mostly patient self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two excluded, unlikely to cause bias. Quote: "2 were excluded...progressed to open laparotomy" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics although absolute values not reported |
Chan 1996.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 80
Country: UK
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: IV fentanyl Pain score collection: not reported Concurrent postoperative analgesics: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (20 participants): IM diclofenac 75 mg before surgery, IM saline and wound infiltration with 0.5% plain bupivacaine at the end of surgery Group B (20 participants): IM saline before surgery, IM diclofenac 75 mg and bupivacaine infiltration at the end of surgery Group C (20 participants): IM diclofenac 75 mg before surgery, IM saline at the end of surgery and no bupivacaine infiltration Group D (20 participants): IM saline before surgery, IM diclofenac 75 mg at the end of surgery and no bupivacaine infiltration |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: unable to use data as either not fully reported or reported as ordinal data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "patients were divided". |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "Syringes were filled by an independent anaesthetist according to a predetermined treatment schedule. Neither the patients nor the anaesthetist knew which injection was given at the aforementioned times...intramuscular diclofenac 75 mg before surgery, intramuscular saline (placebo)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropouts. Quote: "an additional 12 patients were initially enrolled in the study but failed to complete their pain relief questionnaires and have therefore been excluded from the results presented". |
| Selective reporting (reporting bias) | High risk | Satisfaction and side effects not reported |
| Other bias | Low risk | Similar baseline characteristics |
Chen 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 49 Country: China Setting: secondary care Dates conducted: July 2011 to February 2014 Postoperative opioid used and delivery: fentanyl PCA Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group P1 (24 participants): IV 50 mg flurbiprofen and 1.0 μg/kg fentanyl with 0.25 mg/kg ketamine epidurally before surgery and 5 mL normal saline IV and 10 mL normal saline epidurally after surgery Group P3 (25 participants): 5 mL IV normal saline and 1.0 μg/kg fentanyl with 0.25 mg/kg ketamine epidurally before surgery and 50 mg IV flurbiprofen and 10 mL normal saline epidurally after surgery |
|
| Outcomes |
|
|
| Notes | Funding: Wuxi Municipal Bureau of Science and Technology (government) Declarations of interest: none declared Authors contacted: no Other: fentanyl consumption converted to 70 kg weight then to morphine equivalents |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. Quote: "a random allocation number table was used for grouping the patients by two experienced chief physicians". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "the participants, care providers, those assessing outcomes were blinded after assignment to interventions". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "the participants, care providers, those assessing outcomes were blinded after assignment to interventions". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one exclusion, unlikely to cause bias. Quote: "one patient in group P1 was excluded for hypopiesia and shock during operation". |
| Selective reporting (reporting bias) | Unclear risk | ChiCTR‐IPR‐15005848, although retrospectively registered |
| Other bias | Low risk | Similar baseline characteristics and government funding |
Colbert 1998.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 77 Country: Ireland Setting: secondary care Dates conducted: July 1996 to December 1996 Postoperative opioid used and delivery: IM pethidine Pain score collection: blinded investigator Concurrent postoperative analgesics: diclofenac or paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (37 participants): 20 mg IV tenoxicam 30 minutes before surgery Group B (40 participants): 20 mg IV tenoxicam post‐incision |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: analgesic consumption not included as at 4 hours. Included as a pre‐emptive intervention as not all participants received diclofenac postoperatively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo. Quote: "The patients in group A received 20 mg tenoxicam IV 30 mins pre‐operatively and patients in group B received 20 mg tenoxicam IV post‐incision". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "outcomes were assessed by an investigator without knowledge of the timing of tenoxicam administration". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. Quote: "There were 37 patients in group A (20 mg tenoxicam and 30 min before surgery) and 40 patients in group B (20 mg tenoxicam post‐incision)". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics. Quote: "There were no differences between the groups with respect to age, duration of surgery, length of the wound or the weight of the patient". |
Coli 1993.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 50 Country: Italy Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: buprenorphine Pain score collection: not included as outcome Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (25 participants): IV sodium naproxen 550 mg immediately after induction of anaesthesia Group II (25 participants): IV sodium naproxen 550 mg at the end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: article in Italian so translated. Buprenorphine consumption converted to mg intravenous morphine. Standard deviations estimated. Time to analgesic request data extracted from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo. Quote: "group I received Naproxen sodium 550 mg intravenously immediately after induction of anesthesia; group II received sodium naproxen 550 mg intravenously at the beginning of the surgical suture". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics. Quote: "The three groups were comparative to the distribution by age, weight, sex and duration of the intervention". |
Demirbas 2019.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 50 Country: Turkey Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: none Pain score collection: not reported Concurrent postoperative analgesics: PRN paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (25 participants): 800 mg of IV ibuprofen 60 minutes before surgery and IV placebo (100 mL of saline) after surgery Group 2 (25 participants): IV placebo (100 mL of saline) 60 minutes before surgery and 800 mg of IV ibuprofen 60 minutes after surgery |
|
| Outcomes |
|
|
| Notes | Funding: "Erciyes University Scientific Research Projects Unit, Turkey (project number: TSA‐2018‐7756)" Declarations of interest: "None of the authors have any relevant financial relationship(s) with a commercial interest". Authors contacted: no Other: Pain data extracted from graphs and SD estimated from other studies. Early pain taken at 2 hours to allow post‐incision dosing to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "Patients who met the study criteria were divided randomly into equal groups using a random number generated by a computer". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "800 mg of IV ibuprofen 60 minutes before surgery and IV placebo (100 mL of saline) after surgery...Neither the surgeon nor the patient were informed of the group assignment throughout the entire study process". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention who collected data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. Quote: "No data were missing" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Non‐industry funding and similar groups. Quote: "Erciyes University Scientific Research Projects Unit, Turkey (project number: TSA‐2018‐7756)...No differences were found among the 3 groups in terms of age (P = .2) or gender (P = .13)". |
Esparza‐Villalpando 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 60 Country: Mexico Setting: secondary care Dates conducted: January to August 2015 Postoperative opioid used and delivery: none Pain score collection: self‐report Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (30 participants): 25 mg PO dexketoprofen trometamol 30 minutes before the surgery and placebo capsule immediately after the procedure Group 2 (30 participants): placebo capsule 30 minutes before the surgical intervention and 25 mg of PO dexketoprofen trometamol immediately after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: none declared Authors contacted: yes Other: pain score data 100 mm VAS as opposed to NRS due to impossible values. Authors contacted as not enough information reported to include time‐to‐event data. Pain score from one hour included to allow oral post‐incision intervention to work. Included in pre‐emptive as not all participants received postoperative NSAIDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. Quote: "randomized using software for simple random numbers" |
| Allocation concealment (selection bias) | Low risk | Blinded. Quote: "all randomization processes were performed by a separate collaborator". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study used double‐dummy placebo. Quote: "blinding was maintained by using over‐encapsulated dexketoprofen trometamol tablets in white gelatin capsules. The placebo used was an identical empty white gelatin capsule. Neither the patient, the surgeon, nor the person in charge of the administration of drugs to each group was aware of the identity of the drugs assigned". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | NCT02380001. Registration reports measuring pain for 72 hours and trial only reports data for 8 hours |
| Other bias | High risk | More participants in pre‐emptive group had more complicated surgery which is likely to be more painful |
Flath 1987.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 28
Country: USA
Setting: dental medical school
Dates conducted: not reported
Postoperative opioid used and delivery: none Pain score collection: patient self‐report Concurrent postoperative analgesics: not reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group C (13 participants): 100 mg flurbiprofen 30 minutes before procedure and placebo 3 hours after surgery Group B (15 participants): placebo 30 minutes before procedure and 100 mg flurbiprofen 3 hours after surgery |
|
| Outcomes |
|
|
| Notes | Funding: 'The Upjohn Company provided no financial support for this study’ Declarations of interest: not reported Authors contacted: no Other: pain score data extracted from graph. Three‐hour data not included as this was when postoperative administration occurred. SD estimated. Pain score data only included symptomatic preoperative patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random draw of envelopes. Quote: "By random draw" |
| Allocation concealment (selection bias) | Unclear risk | No details regarding the envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "Placebos and the flurbiprofen (The Upjohn Co Kalamazoo, MI) were compounded into identical blue tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded as patient self‐report and blinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only four dropouts, unlikely to cause bias. Quote: "...of the 120 patients enrolled in the study, 116 completed all phases". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. No industry funding |
Fleckenstein 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 45 Country: Germany Setting: secondary care Dates conducted: February 2006 to December 2011 Postoperative opioid used and delivery: morphine PCA Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (unclear number of participants): PO etoricoxib 120 mg on morning of surgery then PO etoricoxib 120 mg/day for 3 days and postoperative placebo Group 3 (unclear number of participants): placebo on morning of surgery then PO etoricoxib 120 mg/day etorocoxib for 3 days |
|
| Outcomes |
|
|
| Notes | Funding: MSD Sharp and Dome (industry) Declarations of interest: none declared Authors contacted: yes Other: standard deviation from post‐incision group estimated from preventive group. Data extracted from graph. Pain score data not reported. Participant numbers unclear but estimated from total number of participants receiving etoricoxib. Some information extracted from published protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote: "sequentially numbered envelopes containing two boxes of study medication for pre‐ and postoperative use performed by pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. The pills were similar in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "analysis of all records is performed by blinded evaluators". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients lost to follow‐up and some for high pain. Quote: "six out of the eight dropouts (75%) because of increased pain were part of the placebo group". |
| Selective reporting (reporting bias) | High risk | NCT00716833 and published protocol. Pain data not fully reported |
| Other bias | Unclear risk | No separate data to assess. Industry funding but stated not involved in study |
Fletcher 1995.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 40 Country: France Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV bolus then PCA morphine Pain score collection: blinded investigator Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (20 participants): 60 mg IV ketorolac before induction and then 2 mL IV normal saline at the end of surgery Group POST (20 participants): 2 mL IV normal saline before induction and then 60 mg IV ketorolac at the end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: some pain score and morphine consumption data extracted from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. Quote: "a random‐number table was used to generate a randomized schedule specifying the group to which each patient would be assigned upon entry into the trial". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Identical in appearance. Quote: "The pre‐operative KET group (PRE; n = 20) received 60 mg of KET and then 2 ml of NS". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "all patients and personnel involved in patient management and data collection were unaware of the group to which the patient had been assigned". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | Some adverse events not reported |
| Other bias | High risk | More females in POST group (Gerbershagen 2014) |
Gabbott 1997.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 65
Country: UK
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA morphine Pain score collection: trained nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group D (34 participants): IM ketorolac 30 mg 45‐90 minutes preoperatively and placebo at incision Group B (31 participants): IM ketorolac 30 mg at incision and placebo 45‐90 minutes preoperatively |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: unable to use any data due to different time points, lack of reporting and inability to convert the scales used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear whether a truly random process. Quote: "Randomization was achieved by allocating each patient the next numbered pair of study ampoules". |
| Allocation concealment (selection bias) | Unclear risk | Pharmacy‐controlled but in view of above unclear. Quote: "The code determining the contents of each ampoule was kept by the pharmacy department and not released to the investigators until after the study was terminated". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "All ampoules were identical and packaged in pairs labelled 'pre‐operative' and 'intra‐operative'. Each boxed pair was sequentially coded allowing the study to be carried out in a double‐blind fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large number of dropouts, although these individuals had been randomized and thus intention‐to‐treat was not followed but the intervention was assigned double‐blinded; the total dropout rate was only 14%, and there were similar numbers of dropouts between the groups (ranging from 10‐23%). Quote: "One hundred and sixty patients agreed to take part in the study. Of these, 23 were withdrawn before induction of anaesthesia. These patients were either not given the study drug or more than 90 min had elapsed between the preoperative injection and the time of arrival in the anaesthetic room". |
| Selective reporting (reporting bias) | High risk | Some outcomes not reported or not fully reported |
| Other bias | Low risk | Similar baseline characteristics. Unclear role for Selecta UK in trial conduct |
Gelir 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 50 Country: Turkey Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV morphine infusion Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (25 participants): IV infusion of 50 mg dexketoprofen in 100 mL saline solution 30 minutes before surgery and 100 mL of IV saline solution 15 minutes after the incision Group II (25 participants): 100 mL of IV saline solution 30 minutes before the operation and 50 mg of dexketoprofen in 100 mL of saline solution 15 minutes after incision |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: none declared Authors contacted: no Other: Nausea and vomiting not included as reported separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Closed envelope method but unclear if random. Quote: "the patients were randomized into two groups using the closed envelope method". |
| Allocation concealment (selection bias) | Unclear risk | Closed envelope method. Unclear if opaque and/or sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All drugs were prepared by the investigators before the operations but double‐dummy. The drugs were administered by an anaesthetist who was blinded to the patient grouping. Quote: "Group I (n 25) received an intravenous (IV) infusion of 50 mg dexketoprofen in 100 ml saline solution 30 minutes before the operation...the patients in Group I received 100 ml of IV saline solution 15 minutes after the incision was made". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative evaluations were performed by the same blinded doctor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | Not all adverse events reported |
| Other bias | Low risk | Similar baseline characteristics and no conflicts of interest. Quote: "No significant difference was found between the groups in terms of demographic data, the duration of anesthesia, the duration of the surgical procedure or ketamine consumption". |
Giuliani 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 87 Country: Italy Setting: secondary care hospital Dates conducted: February 2009 to May 2011 Postoperative opioid used and delivery: none (paracetamol) Pain score collection: medical students Concurrent postoperative analgesics: paracetamol | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (43 participants): PO Ibuprofen 400 mg 30 minutes before the operation, placebo after the procedure and Ibuprofen 400 mg every 6 hours thereafter for a total duration of 18 hours Group B (44 participants): placebo 30 minutes before the operation, PO Ibuprofen 400 mg at the end of the procedure and every 6 hours thereafter for a total duration of 18 hours |
|
| Outcomes |
|
|
| Notes | Funding: no mention Declarations of interest: "All the authors declare that there is no potential conflict of interest referring to this article". Authors contacted: yes (data received from authors) Other: unpublished data used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "AB, the pharmacist, prepared the randomization sequence using Microsoft Excel". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote: "AB, the pharmacist, prepared the randomization sequence using Microsoft Excel". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "AB prepared placebo and treatment ibuprofen capsules, enclosed them in consecutively numbered sealed envelopes in the specific sequence that each group required. AB then stored envelopes in consecutively numbered sealed boxes, each representing the therapy of one individual patient. No reference to treatment group was present on either envelopes or boxes". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition. Quote: "Thirteen were lost during follow‐up due to patient failure to complete VAS records". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Quote: "After randomization, groups were similar regarding age, sex and type of surgery, no statistically significant difference was found for these variables". |
Gramke 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 52 Country: unclear Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: tramadol PCA Pain score collection: blinded investigator or nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (25 participants): 40 mg SL piroxicam 2 hours before surgery and placebo 10 minutes postoperatively. Also received a dose of 40 mg piroxicam the morning after surgery Group POST (27 participants): placebo 2 hours before surgery and 40 mg SL piroxicam 10 minutes postoperatively. Also received a dose of 40 mg piroxicam the morning after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: data estimated from median and IQR. Data from 20 hours included in 24‐hour data. Nausea data added as stated in methods: the outcome was nausea and vomiting. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear details. Quote: "randomization was performed by sealed numbered envelopes". |
| Allocation concealment (selection bias) | Unclear risk | Unclear if opaque. Quote: "randomization was performed by sealed numbered envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "40 mg SL piroxicam 2 hours before surgery and placebo 10 minutes postoperatively" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "the scoring was performed by a blinded investigator or blinded nursing staff". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Grifka 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 89 Country: unclear Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: PO oxycodone Pain score collection: blinded investigator Concurrent postoperative analgesics: paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Preemptive (45 participants): 400 mg PO lumiracoxib one hour before the start of surgery and a placebo tablet 15 minutes after surgery Group Postoperative (44 participants): placebo tablet one hour before surgery and 400 mg PO lumiracoxib given 15 minutes after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported but some authors from pharmaceutical company Authors contacted: yes Other: data estimated from median and SD estimated from other studies. Analgesic consumption not added as included paracetamol. Although time to analgesic reported as time‐to‐event, not enough information to calculate summary statistics. Cardiac adverse events not included as not clearly defined. Not enough reported information to include satisfaction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...patients in the first group received a single dose of lumiracoxib 400 mg 1 hour before surgery and a placebo tablet 15 min after surgery (Pre‐emptive group)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "patients, investigators, persons performing the assessments, data analysts and clinical team members were blinded to the identity of the treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | High risk | More females in pre‐emptive group (Gerbershagen 2014). Industry involvement but unclear on role |
Gunter 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 80 Country: Germany Setting: secondary care Dates conducted: May 2004 to August 2007 Postoperative opioid used and delivery: IV piritramide Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (40 participants): 40 mg IV parecoxib 30 minutes before surgery and 12 and 24 hours after surgery. Placebo 30 minutes before end of surgery Group 2 (40 participants): 40 mg IV parecoxib 30 minutes before the end surgery and 12 and 24 hours after surgery. Placebo 30 minutes before surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: data estimated from median and IQR from graphs. Time to analgesic request not reported as time‐to‐event |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computerized random number generator" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo, identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Ten participants excluded, more in pre‐emptive group although unclear if these were or were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | High risk | More females in pre‐emptive group and more knee arthroscopies in post‐incision group (Gerbershagen 2014) |
Guran 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 82 Country: Romania Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV pethidine Pain score collection: not reported Concurrent postoperative analgesics: paracetamol, tramadol and metamizole |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (39 participants): IV 50 mg of dexketoprofen with normal saline up to 5 mL 30 minutes before induction and injection with 5 mL of IV saline at the time of suturing the skin Group B (43 participants): IV 50 mg of dexketoprofen with normal saline up to 5 mL at the time of suturing the skin and 5 mL of IV saline 30 minutes before surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes (response received but no further data available) Other: SD from pain score data estimated. Data extracted from graph. Nausea and vomiting not included as unclear to which group the data referred |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list. Quote: "random number list" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "the contents of the two syringes were given to a member of the team not involved in that anesthetic or in immediate postoperative follow‐up". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts unlikely to cause bias as similar number and reasons for exclusions. Quote: "three patients in group A and 2 were excluded from the group B due to technical difficulties in laparoscopy and one in each group for diagnosis of acute gallbladder". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Inanoglu 2007.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 44 Country: Turkey Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: PO tramadol Pain score collection: self‐report Concurrent postoperative analgesics: PO paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (22 participants): 8 mg IV lornoxicam in 100 mL of normal saline over 10 minutes in the preoperative room 30 minutes before skin incision and 100 mL of normal saline over 10 minutes immediately after wound closure Group 2 (22 participants): 100 mL of normal saline over 10 minutes in the preoperative room 30 minutes before skin incision and 8 mg IV lornoxicam in 100 mL of normal saline over 10 minutes immediately after wound closure |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: patient satisfaction not included as unclear what values represented. Time to analgesia not time‐to‐event |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...group 1 received 8 mg of lornoxicam diluted in 100 mL of normal saline as an intravenous infusion over a period of 10 minutes in the pre‐operative room. This infusion was completed 30 minutes before skin incision. One hundred milliliters of plain normal saline was infused over 10 minutes immediately after wound closure in this group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...investigators participating in the pain assessments and patients were blinded as to the type of medication administered". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Kaczmarzyk 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 64
Country: Poland
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: no opioid used Pain score collection: self‐report Concurrent postoperative analgesics: paracetamol PRN |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre (34 participants): 100 mg ketoprofen PO 60 minutes preoperatively, followed by 100 mg placebo PO 60 minutes postoperatively Group Post (30 participants): 100 mg placebo PO 60 minutes preoperatively, followed by 100 mg ketoprofen PO 60 minutes postoperatively |
|
| Outcomes |
|
|
| Notes | Funding: "Grant of the Jagiellonian University (K/ZDS/00519)" Declarations of interest: none declared Authors contacted: no Other: pain data extracted from graphs and SD estimated. Pain score from 2 hours to allow postoperative dosing time to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. Quote: "Each envelope contained the group assignment for one patient, which was determined in advance by a random number table". |
| Allocation concealment (selection bias) | Low risk | Sealed and opaque envelopes. Quote: "One hundred opaque, sequentially numbered envelopes were used for the concealed allocation of patients to trial groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "The patients, the statistician and the surgeon performing the qualification, operative procedure and follow‐up examination were all blinded with regard to which patients had received which form of treatment...Identical, nonmarked capsules with 100 mg ketoprofen or 100 mg placebo were prepared and coded in a professional pharmaceutical laboratory". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above as self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two dropouts from post group, unlikely to cause bias. Quote: "one hundred patients entered the trial, of whom 4 did not check‐in for the follow‐up examination. In all, complete data sets from 96 patients were statistically analysed". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Karaman 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 40 Country: Turkey Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: tramadol PCA Pain score collection: blinded anaesthesia resident Concurrent postoperative analgesics: lornoxicam if pain uncontrolled with opioid |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (20 participants): 8 mg IV lornoxicam 20 minutes before incision and IV saline after skin closure Group POST (20 participants): IV saline 20 minutes before incision and 8 mg IV lornoxicam after skin closure |
|
| Outcomes |
|
|
| Notes | Funding: none Declarations of interest: none declared Authors contacted: no Other: included as pre‐emptive as not all patients received postoperative lornoxicam. Patient satisfaction not included as not reported as continuous. Nausea and vomiting taken from anti‐emetic rescue as this was given when nausea and vomiting occurred |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "sixty participants were randomly assigned to three groups". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. None of the patients and managing anaesthetists were aware of the randomization code. Quote: "Patients in Group PRE received lornoxicam (Nycomed GmbH, Austria) IV 8 mg 20min before incision and saline IV after skin closure". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...all measurements were recorded by the same anaesthesia resident who was blinded to the study drugs administered". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | ISRCTN: 2006/34 but unable to find on trial registry |
| Other bias | Low risk | Similar baseline characteristics and no conflicts of interest |
Lee 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 40 Country: Hong Kong Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV morphine then PCA Pain score collection: blinded nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PS (20 participants): 40 mg IV parecoxib before induction of anaesthesia and IV normal saline at skin suture Group SP (20 participants): IV normal saline before induction of anaesthesia and 40 mg IV parecoxib at skin suture |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes (response received but no further data available) Other: unable to include morphine consumption data as unclear at what time points it was measured. Time to analgesic request not reported. Pain data converted from median and extracted from graph with SD estimated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated codes" |
| Allocation concealment (selection bias) | Low risk | Numbered and opaque envelopes. Quote: "maintained in sequentially numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...parecoxib was dissolved to form a clear, colourless solution and an equivalent volume of normal saline was used as the placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "the patients, investigators and clinicians who collected the data were blinded to the assigned treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | No protocol but primary outcome not reported (time to analgesic request) |
| Other bias | High risk | More females in PS group (Gerbershagen 2014) |
Likar 1997.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 48
Country: Austria
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA piritramide Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Ketoprofen Pre‐op (26 participants): 20 minutes before surgery ketoprofen 2 mg/kg IV and at the end of the operation IV placebo Group Ketoprofen Post‐op (22 participants): placebo IV before surgery and ketoprofen 2 mg/kg IV at the end of the operation (last suture) |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: translated from German. Data extracted from graphs. Nausea and vomiting not included as not composite. No data for sedation. Piritramide converted to morphine by x 0.75. Early pain score from 30 minutes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "The patients were divided into 2 groups". |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "The list on which the group allocation and patient name noted was always locked and only after completion of the study was revealed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "...the first group received ketoprofen 2 mg/kg body weight IV 20 minutes before the beginning of surgery and placebo IV at the end of surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The infusions were from a person of the nursing staff who are not involved in the anesthesia and not involved in the postoperative monitoring". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Likar 1998.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 50
Country: Austria
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA piritramide Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (25 participants): ketoprofen 100 mg IV 20 minutes before incision then saline placebo at last skin suture then ketoprofen 12 mg/hour during surgery and for 48 hours afterwards Group II (25 participants): placebo IV 20 minutes before incision and during surgery then ketoprofen 100 mg IV at last skin suture then ketoprofen 12 mg/hour for 48 hours afterwards |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: translated from German. Data extracted from graphs. SD estimated for analgesic consumption and pain score data. Nausea and vomiting not included as not composite. Piritramide converted to morphine by x 0.75. Early pain score from 30 minutes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "drawing of numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...ketoprofen 100 mg IV 20 minutes before incision then saline placebo at last skin suture" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts unlikely to cause bias. Quote: "1 patient was excluded as surgery lasted 20 minutes, 3 patients were missed because of missing documentation values, 1 patient had to go to intensive care and 1 patient had to withdraw due to compliance issues" |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Lu 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 113 Country: China Setting: secondary care Dates conducted: January 2011 to June 2013 Postoperative opioid used and delivery: none reported Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Research (56 participants): 2 mL of 40 mg IV parecoxib 30 minutes before surgery and 2 mL IV saline after surgery Group Control (57 participants): 2 mL IV saline 30 minutes before surgery and 2 mL of 40mg IV parecoxib after surgery |
|
| Outcomes |
|
|
| Notes | Funding: Health Medicine and Science Technology development plan, Shan Dong Probince, China. Project number: 2013WS0076 Declarations of interest: none declared Authors contacted: no Other: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "...were randomly divided into the research group and the control group" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "patients in the research group were treated with 2 ml parecoxib (40 mg parecoxib dissolved in 2 ml saline) IV 30 min before operation, and 2 ml saline IV after operation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two excluded from pre‐emptive group for hospital transfer. Unlikely to cause bias |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics |
Martinez 2007.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 41 Country: France Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: morphine PCA Pain score collection: blinded nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre (22 participants): 40 mg IV parecoxib at induction, placebo at wound closure and 40 mg IV parecoxib at 12 hours Group Post (19 participants): placebo at induction, 40 mg IV parecoxib at wound closure and 40 mg IV parecoxib 12 hours after surgery |
|
| Outcomes |
|
|
| Notes | Funding: NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA) and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). No financial support of Pfizer Declarations of interest: not reported Authors contacted: no Other: pain score data for 24 hours extracted from graph and SD estimated. Time to analgesic request not time‐to‐event |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated codes" |
| Allocation concealment (selection bias) | Low risk | Quote: "the randomization instructions were stored in sequentially numbered opaque envelopes opened the day of surgery before induction of anesthesia". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...all patients received three IV injections: one with anesthesia induction, a second at wound closure, and a third 12 hours after induction". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fourteen exclusions. Quote: "Eleven patients were eliminated from the study: two patients withdrew consent, one required prolonged postoperative mechanical ventilation, two were inadvertently given paracetamol (one of the exclusion criterion), four because of inadequate order of treatment attribution (third injection made instead the second), one had a surgical complication that required intervention, and one because the patient’s data were lost...Two additional patients were eliminated from the study after morphine titration in the PACU". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Low risk | Similar baseline characteristics and no drug company involvement |
Mishra 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 25
Country: India
Setting: secondary care hospital
Dates conducted: January 2010 to January 2011
Postoperative opioid used and delivery: none (ibuprofen) Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria (none reported) |
|
| Interventions |
Group Ketorolac Preoperatively (12 participants): PO 20 mg ketorolac 30 minutes preoperatively Group Ketorolac Postoperatively (13 participants): PO 20 mg ketorolac 30 minutes after surgery |
|
| Outcomes |
|
|
| Notes | Funding: none Declarations of interest: "none declared" Authors contacted: yes Other: unable to include pain as 1‐7 scale which was specific to study. SD estimated for time to analgesia. Adverse events not reported separately for ketorolac groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "Computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if double‐dummy placebo given. Quote: "Placebo was glucose powder filled in empty capsule... Both the investigator and patient were blind…Each drug was coded and packed into identical appearing packets". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics and no industry funding. Quote: "All the six patients groups were similar in terms of gender distribution, average age, the amount of local anaesthetic administered, the antibiotic coverage given, the position of the molar extracted and the duration of the procedure". |
Mojsa 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 60
Country: Poland
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: none Pain score collection: surgeon Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (30 participants): PO lornoxicam 60 mg 60 minutes before surgery and placebo 60 minutes after surgery Group B (30 participants): placebo 60 minutes before surgery and PO lornoxicam 60 mg 60 minutes after surgery |
|
| Outcomes |
|
|
| Notes | Funding: "no funding to declare" Declarations of interest: "none declared" Authors contacted: yes (data received from authors for VAS pain scores) Other: time to analgesia time‐to‐event but not enough information to extract data. Early pain score from 2 hours to allow post‐incision dose to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. Quote: "random number generator" |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. Quote: "To ensure allocation concealment, 90 identical non‐transparent sequentially numbered envelopes were used". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "Identical unmarked capsules containing either 16 mg of lornoxicam or the same weight of placebo were administered". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. Quote: "No data were missing, and all patients included in the present study attended all study visits". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics and no industry funding. Quote: "There was no difference between the three study groups in terms of age (P = 0.325) or sex distribution (P = 0.526), surgical difficulty of the procedure (P = 0.721) or duration of surgery (P = 0.919)". |
Moonla 2018.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 84
Country: Thailand
Setting: secondary care hospital
Dates conducted: February 2016 to May 2017
Postoperative opioid used and delivery: PCA morphine Pain score collection: not reported Concurrent postoperative analgesics: none |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre (42 participants): 40 mg parecoxib before skin incision and at 12 and 24 hours after the first dose Group Post (42 participants): 40 mg parecoxib at wound closure and at 12 and 24 hours after the first dose |
|
| Outcomes |
|
|
| Notes | Funding: none reported Declarations of interest: "No potential conflict of interest relevant to this article was reported". Authors contacted: no Other: data taken from graphs and SD calculated from SEM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. Quote: "...block randomization sampling method" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "This study was a double‐blind trial. Each group received six doses of intravenous solution. All solutions were colorless, and each solution was given in a 2 mL volume, prepared by a nurse anesthetist who was not involved in the assessment and patient care before, during, and after operation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of dropouts. Quote: "Because of incomplete data, 17 of 144 patients were excluded from the study; nine patients withdrew consent, five had surgical complications that required intervention, and three required prolonged postoperative mechanical ventilation". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar groups |
Munteanu 2016.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 110 Country: Romania Setting: secondary care Dates conducted: January to September 2014 Postoperative opioid used and delivery: IV and SC morphine Pain score collection: blinded staff Concurrent postoperative analgesics: IV paracetamol |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group ETORICOX‐PREOP (55 participants): etoricoxib 120 mg PO 1 hour before surgery, one placebo pill upon arrival in the postoperative care unit (PACU) and a second etoricoxib dose after 24 hours Group ETORICOX‐POSTOP (55 participants): one placebo pill 1 hour before surgery, etoricoxib 120 mg PO at the end of surgery, immediately after arrival in the PACU, and a second etoricoxib dose after 24 hours |
|
| Outcomes |
|
|
| Notes | Funding: no funding Declarations of interest: the first author is also the chair of the ethics committee that approved the study. Authors contacted: yes (response received but no further pain score data available) Other: included in pre‐emptive as second dose at 24 hours. Authors contacted for pain score data and some adverse events. Nausea and vomiting not included as reported separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "computer‐generated random number list" |
| Allocation concealment (selection bias) | Unclear risk | Unclear if opaque. Quote: "the assigned allocation group was written on a note placed inside a numbered envelope (one number corresponding to every patient enrolled); the envelope was then sealed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "to maintain the blinding conditions, medical staff had no knowledge of the randomisation of the patients or the contents of the pills...one placebo pill 1 h before surgery, etoricoxib 120 mg orally at the end of surgery, immediately after arrival in the PACU and a second etoricoxib dose after 24 h". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...to maintain the blinding conditions, medical staff had no knowledge of the randomisation of the patients or the contents of the pills". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | NCT02534610. All prespecified outcomes reported |
| Other bias | Low risk | Similar baseline characteristics and no conflicts of interest |
Murphy 1993.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 50 Country: Australia Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV infusion and boluses of papaveretum Pain score collection: nurses Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Preoperative (22 participants): indomethacin PR 200 mg commencing on the night before surgery and 100 mg BD Group Postoperative (28 participants): same indomethacin regimen after completion of surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain score data not fully reported, 14:00‐15:00 day one after surgery used for late acute pain Extracted from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "the patients were allocated randomly to one of two groups". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo used. Quote: "One group received indomethacin suppositories 200 mg commencing on the night before surgery and 100 mg twice daily thereafter. The second group commenced the same indomethacin regimen after completion of surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | Pain score data not fully reported |
| Other bias | Low risk | Similar baseline characteristics |
Nakayama 2001.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 30
Country: Japan
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: none Pain score collection: blinded author Concurrent postoperative analgesics: PRN diclofenac |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (15 participants): 1 mg/kg flurbiprofen IV 30 minutes before surgery and placebo at the end of surgery Group POST (15 participants): 1 mg/kg flurbiprofen IV at the end of surgery and placebo 30 minutes before surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: data for pain extracted from graph although unable to for early pain as unclear from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "via sealed envelope assignment" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details. Quote: "via sealed envelope assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "The PRE group received 1 mg/kg flurbiprofen IV 30 min before surgery and a placebo at the end of surgery…Both placebo and the flurbiprofen solution looked the same". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...one of the authors (MN), who was blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Nezafati 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: Iran
Setting: secondary care hospital
Dates conducted: March 2016 to January 2017
Postoperative opioid used and delivery: IV pethidine and morphine Pain score collection: blinded nurses Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (10 participants): 30 mg ketorolac IV before induction Group 2 (10 participants): 60 mg ketorolac IV before induction Group 3 (10 participants): 30 mg ketorolac IV end of surgery Group 4 (10 participants): 60 mg ketorolac IV end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: "None" Declarations of interest: "None" Authors contacted: yes Other: Group 1/2 and 3/4 combined for analysis. Analgesic consumption not included as morphine and pethidine and unclear how dose in mg was calculated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "The subjects were assigned into five study groups with the use of the Randlist (Version 1.2) by an operator blinded to the aims of the study (simple randomization method)". |
| Allocation concealment (selection bias) | Unclear risk | No details on envelope. Quote: "...was placed in a closed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...the nurses who recorded pain scores, the dose of the analgesic agents administered and the time of the first administration of the analgesic agent were blinded to the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed. Quote: "All the subjects completed the study and none was excluded from the study". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. No industry funding |
Norris 2001.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 78 Country: Canada Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: IV fentanyl then codeine Pain score collection: blinded research assistant Concurrent postoperative analgesics: PO diclofenac on discharge and paracetamol/codeine |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Preop (40 participants): 50 mg of PO diclofenac 1 hour before surgery and a placebo 30 minutes postoperatively Group Postop (38 participants): placebo 1 hour before surgery and 50 mg of PO diclofenac 30 minutes postoperatively |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: early acute pain recorded from one hour to allow postoperative dosing to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. Quote: "...random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "diclofenac and placebo (lactose) were re‐formulated into identical capsules by Toronto Western Hospital pharmacy department...the Preop group received 50 mg of potassium diclofenac PO 1 hour pre‐operatively and a placebo 30 minutes postoperatively". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...pre‐operative and postoperative assessment was done by a research assistant who was blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six exclusions but unclear to which groups they belonged |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | More females in Postop group (Gerbershagen 2014) |
O'Hanlon 1996.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 60 Country: Northern Ireland Setting: secondary care Dates conducted: not reported Postoperative opioid used and delivery: cyclimorph or co‐codamol Pain score collection: self‐report Concurrent postoperative analgesics: paracetamol PRN (as part of co‐codamol) |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 1 (20 participants): PO piroxicam 20 mg 2 hours before surgery and placebo at induction and one hour postoperatively Group 2 (20 participants): PO piroxicam 20 mg at induction and placebo 2 hours before surgery and one hour postoperatively Group 3 (20 participants): PO piroxicam 20 mg one hour postoperatively and placebo 2 hours before surgery and at induction |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain score data extracted from graph. Group 1 and 2 combined for the pre‐emptive group. Pain score at 2 hours included to allow postoperative dosing to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "...in a randomised double blind manner" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐dummy placebo used. Quote: "...in a double blind manner, either 20 mg piroxicam or placebo in the "melt" form at the following times; two hours pre‐operatively, immediately before induction of anaesthesia or one hour postoperatively" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Quote: "...patients in the three treatment groups were equally matched with respect to age, height and weight". |
Ozer 2012.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 50 Country: Turkey Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: tramadol bolus then PCA Pain score collection: no mention Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group 50/0 (25 participants): 100 mL saline containing 50 mg dexketoprofen as an infusion 30 minutes before the surgical incision and 100 mL saline 30 minutes before the end of the surgical procedure Group 0/50 (25 participants): 100 mL saline 30 minutes before the surgical incision and 100 mL saline containing 50 mg dexketoprofen as an infusion 30 minutes before the end of the surgical procedure |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: could not use data for sedation (unable to dichotomise), nausea and vomiting (unable to dichotomise) and patient satisfaction (ordinal scale) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention. Quote: "Patients were randomly divided into four groups". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo used and double‐dummy. Quote: "...patients who received 100 ml saline containing 50 mg dexketoprofen as an infusion 30 minutes before the surgical incision and received 100 ml saline 30 minutes before the end of the surgical procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The infusions of dexketoprofen and SP were prepared by a practitioner who was not involved in either application of anesthesia or postoperative evaluation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appeared to have been analysed. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial registration |
| Other bias | High risk | More females in post‐incision group (Gerbershagen 2014) |
Ozyilmaz 2005.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: Turkey
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA morphine Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (20 participants): IV lornoxicam 8 mg before surgery and saline placebo at closure Group II (20 participants): saline at induction and IV lornoxicam 8 mg before skin closure |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: pain score extracted from graph. Unable to include nausea and vomiting as not composite. Translated from Turkish. No pain at 24 hours so could not include. SD estimated for pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "...this study was carried out in a prospective, randomized, double‐blind fashion". |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "Group‐II (n=20, intraoperative group) patients received IV 2 ml saline solution before surgery and 8 mg IV lornoxicam before skin closure". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Pandazi 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 40 Country: Greece Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: morphine PCA Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (20 participants): 40 mg IV parecoxib 30 minutes before surgery and 100 mL saline 30 minutes after incision. Then parecoxib 40 mg BD for three days Group POST (20 participants): 40 mg IV parecoxib 30 minutes after incision and 100 mL saline 30 minutes before surgery. Then parecoxib 40 mg BD for three days |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain scores reported as median so used as means and IQR/1.35 to convert to SD. No events for pruritus or respiratory depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote "Computer generated randomization list run by the hospital pharmacist" |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote "Computer generated randomization list run by the hospital pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote "A trained nurse administered an intravenous (IV) solution of 100 ml normal saline with parecoxib 40 mg to group PRE (preincisional) and an IV solution of 100 ml normal saline to group POST (post‐incisional) 30 min before skin incision. The same nurse administered 100 ml normal saline with parecoxib 40 mg to group POST and 100 ml normal saline to group PRE 30 min after skin incision. The solutions were dispensed by the hospital pharmacist". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote "All participants in the study (nurse, surgeon, anesthesiologist, patients) were blinded to the perioperative intervention". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant excluded, unlikely to cause bias |
| Selective reporting (reporting bias) | Unclear risk | 24807/20‐12‐06 trial registration but unable to locate protocol |
| Other bias | Low risk | Similar baseline characteristics |
Parke 1995.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 77 Country: USA Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: morphine PCA Pain score collection: blinded investigator Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Pre‐emptive (37 participants): 30 mg ketorolac 30 minutes before incision and saline placebo at skin closure (both 1 mL) Group Postsurgical (40 participants): saline placebo 30 minutes before incision and 30 mg ketorolac at skin closure (both 1 mL) |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: median and range converted to mean and SD for pain score data (Hozo 2005) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention. Quote "Patients were randomised..." |
| Allocation concealment (selection bias) | Unclear risk | No mention. Quote "Patients were randomised..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo used and both 1 mL. Quote "...placebo injection at skin closure" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote "...by an investigator who was unaware of the group to which the patient belonged" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of dropouts, more in intervention group including two who were discharged early and therefore likely to have lower pain. Quote "...were discharged before 24 hour follow up" |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial registration |
| Other bias | High risk | More vaginal hysterectomies in post‐incision group which on regression analysis were associated with pain as an outcome |
Peduto 1995.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 30
Country: Italy
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: none Pain score collection: blinded independent observer Concurrent postoperative analgesics: none |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (15 participants): IV ketorolac 4 mg/kg 10 minutes before induction and saline placebo 5 minutes after incision Group II (15 participants): IV ketorolac 4 mg/kg 5 minutes after incision and placebo 10 minutes before induction |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain data extracted from graph and SD estimated. Side effects not included as composite |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "Patients were randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "Patients were randomly divided into two groups". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "...in the other...30 min before surgery, placebo (physiological solution of volume equal to 0.4 mg / kg of ketorolac) 10 min before intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "An independent observer, in the dark of the type of treatment performed" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Quote: "No differences were found significant between the two groups as far as concerns anthropometric data (weight, height age, sex, age) and the duration of the intervention (average 37 min, with a minimum of 23 min and a maximum of 51 min)". |
Priya 2002.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 50
Country: India
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: IV tramadol Pain score collection: blinded investigator Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (25 participants): 100 mg IV ketoprofen 30 minutes before incision and saline immediately after incision Group II (25 participants): 100 mg IV ketoprofen immediately after incision and saline placebo 30 minutes before incision |
|
| Outcomes |
|
|
| Notes | Funding: "None" Declarations of interest: "None" Authors contacted: no Other: time to analgesia reported as time‐to‐event but not enough information to extract data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "This infusion was completed 30 minutes before the surgical incision was taken. 100 ml plain normal saline was infused over 15 minutes immediately after surgical incision in this group". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...were recorded by an independent, blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | Participants excluded from pain scores once received analgesia |
Riest 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 360 Country: Germany Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: morphine PCA Pain score collection: not reported Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Perioperative (180 participants): rofecoxib 50 mg preoperatively and on postoperative days 1–3 Group Postoperative (180 participants): placebo preoperatively and rofecoxib on postoperative days 1–3 |
|
| Outcomes |
|
|
| Notes | Funding: "This work was supported financially by MSD, Germany. MSD did not participate in either generations of hypothesis, data collection, analysis or writing" up the manuscript. Declarations of interest: as above Authors contacted: no Other: unable to include pain, opioid adverse events and patient satisfaction due to 0‐4 scale. Although multiple participant exclusions, data table for morphine consumption stated data from 540 patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "Randomization of study medications was performed by the hospital’s pharmacy using a computer generated random list". |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote: "Randomization of study medications was performed by the hospital’s pharmacy using a computer generated random list". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded. Quote: "To ensure blinding the study drugs were delivered as coated tablets in blister packages not allowing identification of content". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 90 patients excluded before outcome assessment. Quote: "We secondarily excluded 90 of the 630 patients before assessment of the primary criteria for to the following reasons: cancellation of surgery after the first dose of the study medication, administration of NSAIDs, steroids or opioids other than morphine during the study period, patients’ desire to be excluded from the study before assessment of the main outcome criteria (24 h after skin closure), patients’ discharge before assessment of main criteria, and sedation for mechanical ventilation at time of assessment of the main criteria". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline characteristics. Industry funded but stated no involvement |
Riest 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 160 Country: Germany Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: morphine PCA Pain score collection: trained nurse Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Perioperative (80 participants): 40 mg IV parecoxib 45 minutes before surgery and 12 and 24 hours after surgery Group Postoperative (80 participants): placebo 45 minutes before surgery and 12 and 24 hours after surgery |
|
| Outcomes |
|
|
| Notes | Funding: "Investigator initiated trial funded by Pfizer, Germany. Pfizer did not participate in generation of the study design or interpretation of results". Declarations of interest: as above Authors contacted: no Other: unable to include opioid adverse events as not individually reported. Pain not included as average used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...randomization with a computer‐generated random list" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many patients excluded but unclear to which groups they belonged. Quote: "43 patients were excluded before assessment of the primary criteria for the following reasons..." |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Other bias | Unclear risk | No baseline characteristics table. Industry funded but stated no involvement in study |
Rogers 1995.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 58 Country: UK Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: diamorphine PCA Pain score collection: not collected Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Ketorolac before operation (30 participants): 10 mg IV ketorolac between induction and skin incision and saline placebo between closure of skin and recovery Group Ketorolac after operation (28 participants): saline placebo between induction and skin incision and 10 mg IV ketorolac between closure of skin and recovery |
|
| Outcomes |
|
|
| Notes | Funding: no mention Declarations of interest: no mention Authors contacted: no Other: anti‐emetic used for nausea and vomiting. No events for pruritus so data not entered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computer‐generated random numbers in blocks of 12" |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote: "The hospital pharmacy prepared.." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...containing either 0.9% normal saline 51 ml alone or 0.9% normal saline 50 ml with ketorolac 10 mg (1 ml). They were labelled with a code held by the pharmacy until the end of the study. Each patient was given one infusion between induction of anaesthesia and skin incision, and another between skin closure and arrival in the recovery ward". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirteen participants excluded. Quote: "...13 patients were later withdrawn; seven because a different operative procedure was performed, three because endometriosis was diagnosed at operation and three because of administrative errors in the conduct of the study". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Salonen 2001.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 81 Country: Finland Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: IM and IV oxycodone Pain score collection: nursing staff Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (41 participants): IV ketoprofen 5 minutes after induction (0.5 mg/kg) and saline in PACU then 3 mg/kg over 24 hours Group POST (40 participants): IV ketoprofen in PACU (0.5 mg/kg) and saline 5 minutes after induction then 3 mg/kg over 24 hours |
|
| Outcomes |
|
|
| Notes | Funding: no mention Declarations of interest: no mention Authors contacted: no Other: oxycodone converted to morphine and 70 kg weight. Pain score data extracted from graphs. Sedation not included as no events. Nausea and vomiting calculated from vomiting and nausea without vomiting numbers. SD estimated for opioid consumption |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "The allocation was computer generated". |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes opaque. Quote: "...sealed envelope method was used to ensure blinding". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "In the PRE ketoprofen group, patients were given ketoprofen...intravenously, mixed with 10 ml of normal saline and injected over 5 min after induction of anaesthesia but before surgical incision, and placebo (10 ml normal saline) injected over 5 min in the postanaesthesia care unit (PACU) followed by a continuous IV ketoprofen infusion of 3 mg/kg over 24 h. The drug syringes were prepared by a nurse not otherwise involved in the study, ensuring blinding". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely blinded from above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants excluded. Quote: "No patients were withdrawn from the study". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Sandin 1993.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 42 Country: Sweden Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: dextropropoxyphene (with paracetamol) Pain score collection: no details Concurrent postoperative analgesics: none reported postoperatively, preoperative epidural block | |
| Participants |
Inclusion criteria
No exclusion criteria reported |
|
| Interventions |
Group PRE (20 participants): 75 mg IM diclofenac one hour before tourniquet inflation and saline 30 minutes after start of surgery Group POST (22 participants): 75 mg IM diclofenac 30 minutes after start of surgery and one hour before tourniquet inflation |
|
| Outcomes |
|
|
| Notes | Funding: no mention Declarations of interest: "CIBA‐GEIGY AB is gratefully acknowledged for supplying the trial medicine" (unclear role in trial) Authors contacted: no Other: Pain scores extracted from graph. 24‐hour data from pain scores the next morning. Mean taken from median and IQR/1.35 used to estimate SD. Analgesic use not used as combination therapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "Randomisation was according to a computer‐manufactured list". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "All patients were given two intramuscular injections in the contralateral thigh". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One excluded but from non‐intervention group for severe pain. Quote: "One patient in group 0 was excluded from the study after the 5‐h assessment due to severe pain". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Shuying 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 75 Country: China Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: IV sufentanil and IM tramadol Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (37 participants): IV 40 mg parecoxib injected 30‐45 minutes before anaesthesia induction and 4 mL saline injected when gallbladder removed Group B (38 participants): IV 40 mg parecoxib injected when gallbladder removed and 4 mL saline injected 30‐45 minutes before anaesthesia induction |
|
| Outcomes |
|
|
| Notes | Funding: no funding Declarations of interest: no declarations Authors contacted: no Other: Pain scores extracted from graph. Analgesic consumption calculated from number requiring analgesia, tramadol and sufentanil similar conversion rate to morphine (5 mg). Unable to use Ramsey scores for sedation so taken from somnolence numbers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computer‐randomized number" |
| Allocation concealment (selection bias) | Low risk | Third party performed. Quote: "...which generated by professional statisticians" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "...40 mg parecoxib injected 30‐45 min before anesthesia induction and 4 ml saline injected when gallbladder was removed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "An anesthesiologist who gave the dugs implemented the blinding protocol and another anesthesiologist who didn’t know the regimen evaluated the postoperative indexes". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear to which groups exclusions belonged. Quote: "...7 patients were excluded eventually. The excluding reasons are described as follows: drain tube was installed after surgery in 6 patients; the LC was switched to open cholecystectomy in 1 patient". |
| Selective reporting (reporting bias) | Low risk | ChiCTR‐PRRC‐12002540. Main outcomes reported |
| Other bias | Low risk | Similar baseline characteristics |
Sun 2008.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial Sample size: 76 Country: USA Setting: secondary care hospital Dates conducted: not reported Postoperative opioid used and delivery: IV fentanyl or oral hydrocodone and acetaminophen in PACU and IV morphine PCA if admitted or oral hydrocodone and acetaminophen if discharged Pain score collection: blinded researcher Concurrent postoperative analgesics: none reported postoperatively in addition to above | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Perioperative (39 participants): 2 celecoxib 200 mg capsules 30–90 minutes before surgery and 2 placebo capsules 1 hour after surgery and celecoxib 200 mg twice daily postoperatively Group Postoperative (37 participants): 2 celecoxib 200 mg capsules 1 hour after surgery and 2 placebo capsules 30–90 minutes before surgery and celecoxib 200 mg twice daily postoperatively |
|
| Outcomes |
|
|
| Notes | Funding: non‐industry Declarations of interest: not reported Authors contacted: no Other: myocardial infarction not included as no events. Patient satisfaction not included as unclear scale used. Pain score data extracted from graph and 2 hours used for early pain as postoperative intervention given at one hour. Morphine consumption calculated as PACU plus 24 hours and extracted from graph with SD estimated. No outcomes reported as time‐to‐event. Time to bowel movement mean from median and IQR/1.35 to estimate SD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computer generated random number schedule" |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled. Quote: "The study medication was prepared by a hospital pharmacist in identical‐appearing capsules according to a computer generated random number schedule". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "The patients, nurses, surgeons, and anesthesiologists directly involved in the patients’ care were blinded as to the content of the oral study medication capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "A trained interviewer who was also blinded to the study medication contacted each patient at 24, 48, and 72 postoperatively to inquire about their maximum VRS pain score". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants excluded. Reasons unlikely to cause bias |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Non‐industry funding |
Trampitsch 2003.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 44
Country: Austria
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA morphine Pain score collection: staff not involved in the anaesthetic Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (22 participants): 8 mg IV lornoxicam 20 minutes before skin incision and saline placebo at the end of surgery then 8 mg every 8 hours postoperatively Group II (22 participants): saline placebo 20 minutes before skin incision and 8 mg IV lornoxicam at the end of surgery then 8 mg every 8 hours postoperatively |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: nausea and vomiting reported separately so not included. Other adverse events not fully reported. Values in text appeared to be SEM when compared to SD in graph. Pain scores taken from graphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not fully reported. Quote: "...randomized by means of the envelope method" |
| Allocation concealment (selection bias) | Unclear risk | Unclear envelope safeguards. Quote: "...randomized by means of the envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "at the end of surgery, before the last suture, the patients received 100 ml NaCl (placebo)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear as staff were not involved in anaesthetic but unclear if blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Low risk | Similar baseline characteristics |
Vanlersberghe 1996.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 60
Country: Belgium
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: morphine bolus in PACU Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group K (30 participants): 30 mg IV ketorolac 30 minutes before surgery and placebo in PACU Group P (30 participants): placebo 30 minutes before surgery and in PACU 30 mg IV ketorolac |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain score data taken from 45 minutes to allow post‐incision dose time to be effective. Data estimated from median and IQR/1.35 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "...allocated randomly" |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "...allocated randomly" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...30 mg ketorolac 30 mins before surgery followed by a single placebo injection" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | More females in K group. Quote: "A larger proportion of male patients were present in group P" (Gerbershagen 2014). |
Vijayendra 1998.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: India
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (20 participants): IV 60 mg ketorolac just before induction and saline placebo at the end of surgery Group II (20 participants): IV 60 mg ketorolac at the end of surgery and saline placebo before induction |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain score data taken from graph and SD estimated from other studies. Early acute postoperative pain taken from one hour to allow post‐incision dose to be effective |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention. Quote: "...the patients were divided". |
| Allocation concealment (selection bias) | Unclear risk | No mention. Quote: "...the patients were divided". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "Group I received 60 mg ketorolac...and 5cc of normal saline at the end of surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Quote: "The two groups were comparable..." |
Vogol 1992.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 36
Country: USA
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: patient self‐scoring Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I‐pretreatment (19 participants): 600 mg ibuprofen 5‐10 mins before LA and placebo after suturing Group I‐post‐treatment (17 participants): placebo 5‐10 mins before LA and 600 mg ibuprofen after suturing |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: unable to include pain and patient satisfaction due to ordinal scales |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention. Quote: "Subjects were randomly distributed..." |
| Allocation concealment (selection bias) | Unclear risk | No mention. Quote: "Subjects were randomly distributed..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...600 mg ibuprofen immediately presurgically and placebo immediately after surgery" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven participants excluded. Quote: "...7 were excluded from the efficacy analysis". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Quote: "There were no statistically significant differences..." |
Wang 2010.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: China
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: PCA butorphanol Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (20 participants): IV parecoxib 40 mg at anaesthesia induction Group B (20 participants): IV parecoxib 40 mg 30 minutes before end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: Fund Project Institute Guangdong Medical Research Fund (A2009025) Declarations of interest: not reported Authors contacted: no Other: butorphanol converted to morphine using conversion factor of 2.5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "The patients were randomly divided..." |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "The patients were randomly divided..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Table stated n = 20 but unclear if each group |
| Selective reporting (reporting bias) | High risk | Adverse events not fully reported |
| Other bias | Unclear risk | Not enough information on participant characteristics. Non‐industry funding |
Wnek 2004.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 60
Country: Poland
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: NCA ketoprofen Pain score collection: blinded doctor Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (30 participants): IV 100 mg of ketoprofen in 100 mL of normal saline 60 minutes before the induction of general anaesthesia and directly after the operation, the patients received IV 100 mL of normal saline Group POST (30 participants): IV 100 mL of normal saline 60 minutes before the induction of general anaesthesia and directly after the operation the patients received IV 100 mg of ketoprofen in 100 mL of normal saline |
|
| Outcomes |
|
|
| Notes | Funding: grant 501/KL/463/L from The Collegium Medicum, Jagiellonian University, Kraków, Poland Declarations of interest: not reported Authors contacted: no Other: classed as preventive as used NCA of ketoprofen. Data extracted from graphs. SD estimated for pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "Patients were randomly allocated into two groups..." |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "...100 mg of ketoprofen in 100 ml of 0.9% NaCl IV 60 min before the induction of general anesthesia, and directly after the operation, the patients received IV 100 ml of 0.9% NaCl". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "Pain intensity was evaluated by a physician who was unaware about the division into study groups". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One excluded, unlikely to cause bias. Quote: "One patient was excluded from the study on account of laminectomy, which is one of the reasons for postoperative opioid administration caused by insufficient ketoprofen analgesia". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics. Non‐industry funding |
Yagar 2011.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 41
Country: Turkey
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: IV fentanyl Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (21 participants): IV 40 mg tenoxicam 30 minutes before anaesthesia Group II (20 participants): IV 40 mg tenoxicam at the end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: pain score extracted from graph and SD estimated as unclear from graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "...randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "...randomly divided into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "Pain was scored by an anesthesiologist blinded to the drug allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | High risk | Adverse events not fully reported |
| Other bias | High risk | More women in Group II (Gerbershagen 2014) |
Yamashita 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 24
Country: Japan
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: morphine PCA Pain score collection: blinded anaesthetist and nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group A (12 participants): IV 1 mg/kg flurbiprofen axetil 30 minutes before surgery Group B (12 participants): IV 1 mg/kg flurbiprofen axetil after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: data extracted from graphs. Mean estimated from median. For pain data, early pain from 6 hours as unclear when postoperative dosing occurred. SD estimated from IQR for pain. For morphine consumption, 0‐6 and 6‐24 hour time points added. SD estimated from other studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. Quote: "The subjects were randomly assigned into one of three groups...". |
| Allocation concealment (selection bias) | Unclear risk | No details. Quote: "The subjects were randomly assigned into one of three groups...". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether double‐dummy placebo and how blinded. Quote: "The study was performed by three investigators in a double‐blinded manner as follows: Each solution was prepared in a syringe by the first investigator, who was responsible for subject grouping. The second investigator, who did not know the type of test solution, performed the intravenous injection. The third investigator, who was blinded to the type of test solution, evaluated postoperative morphine consumption by measuring the weight of the pump using a precision electronic balance". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded as above. Blinded nurse for pain score data. Quote: "The third investigator, who was blinded to the type of test solution, evaluated postoperative morphine consumption...". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | Longer duration of surgery in Group A |
Yan 2004.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 30
Country: China
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria (none reported) |
|
| Interventions |
Group Preoperative (15 participants): IV 8 mg lornoxicam before incision Group Intraoperative (15 participants): IV 8 mg lornoxicam 30 minutes before end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: no adverse events in study so not added to the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots. Quote: "...the patients were randomized, by drawing lots...". |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline characteristics |
Young 2006.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 38
Country: USA
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: no opioid used Pain score collection: self‐report Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group Preemptive (17 participants): valdecoxib 40 mg at least 30 minutes before initial archwire placement, placebo two hours after procedure and valdecoxib for 48 hours after surgery Group Postoperative (21 participants): placebo at least 30 minutes before initial archwire placement, valdecoxib 40 mg two hours after procedure and valdecoxib for 48 hours after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: data extracted from graphs. Mean estimated from median. Change scores used. SD estimated from other studies. Pain taken from 6 hours to allow post‐incision dosing to take effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. Quote: "...using a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo used. Quote: "The preemptive valdecoxib group received a 40 mg valdecoxib loading dose before initial archwire placement and placebo two hours later. The postoperative valdecoxib group received a placebo at least 30 minutes before initial archwire placement and 40 mg valdecoxib two hours later". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% of participants did not complete study. Quote: "Thus, 56 (80%) completed surveys were included in the statistical analysis". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | Different baseline pain levels |
Yuan 2019.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 204
Country: China
Setting: secondary care hospital
Dates conducted: January 2017 to December 2018
Postoperative opioid used and delivery: IV pethidine Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group EA (102 participants): meloxicam 15 mg oral 1 hour before surgery and 7.5 mg oral at 24 hours after surgery Group PA (102 participants): meloxicam 15 mg oral at 4 hours after surgery and 7.5 mg oral at 24 hours after surgery |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: "The authors declare that they have no competing interest". Authors contacted: no Other: Early pain not included as not measured once post‐incision dose had taken effect within 6 hours. Nausea and vomiting not reported separately so not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "A blocked randomization method was used in this study, and the randomization sequence was created using SAS 9.0". |
| Allocation concealment (selection bias) | Low risk | Third party randomization. Quote: "...by an independent analyst" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Quote: "The analysis in our study was performed according to the intention to treat (ITT) protocol". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar groups. Quote: "No difference was found regarding age and gender among the three groups, and BMI, surgery type, operative duration, pain VAS score at rest, pain VAS score at flexion as well as PGA score were also not different, (all P > 0.05)". |
Yuswono 2014.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 36
Country: Indonesia
Setting: secondary care hospital
Dates conducted: March to June 2013
Postoperative opioid used and delivery: IV tramadol Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group I (18 participants): IV parecoxib 40 mg 30 minutes before incision Group II (18 participants): IV parecoxib 40 mg at skin closure |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: no Other: unable to use pain score data as reported ordinally |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo. Quote: "Parecoxib 40 mg were given to the two groups, pre‐operative (group I) and post‐operative (group II)". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar baseline characteristics |
Zhang 2011.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 59
Country: China
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: IV tramadol Pain score collection: blinded nurse Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group C (30 participants): IV flurbiprofen axetil 50 mg 15 minutes before the cervical plexus block and a placebo at the end of the surgery Group B (29 participants): placebo 15 minutes before cervical plexus block and flurbiprofen axetil 50 mg at the end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: "This work was supported by the grants from the outstanding youth science foundation (No. JC2007716), the Science and Technique foundation (No. GC06C410) and the important research project of education bureau of Heilongjiang Province of China (NO. 1152hz33)". Declarations of interest: "no conflicts of interest declared" Authors contacted: no Other: pain score data extracted from graphs and SD estimated as unclear what error bars represented. Tramadol mg consumed not reported. Time to analgesic request not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computerized random number generator" |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes. Quote: "...sealed in numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy placebo. Quote: "The envelopes contained two 5 ml syringes, labeled pre and post, with the contents blinded to anesthesiology, surgeons, operating room staff, recovery room staff, and the patient until the study was completed". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "One ward nurse, who was blinded to group allocation.." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout unlikely to cause bias. Quote: "One patient from the group B did not have her scheduled surgery; eighty‐nine patients completed the study". |
| Selective reporting (reporting bias) | High risk | Time to analgesic request and tramadol consumption not reported |
| Other bias | Low risk | Similar baseline characteristics. No industry funding |
Zhang 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 56
Country: China
Setting: secondary care hospital
Dates conducted: February 2014 to April 2015
Postoperative opioid used and delivery: sufentanil PCA Pain score collection: blinded anaesthetist Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group F1 (28 participants): IV flurbiprofen 1 mg/kg before induction Group F2 (28 participants): IV flurbiprofen 1 mg/kg before skin closure |
|
| Outcomes |
|
|
| Notes | Funding: National Natural Science Foundation of China Declarations of interest: no conflicts of interest declared Authors contacted: no Other: unable to use sedation data as average used. Pain score data extracted from graphs. Sufentanil converted to IV morphine as 1:0.5 from 24‐hour PCA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. Quote: "...computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope but unclear in opaque. Quote: "...sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "...were blinded to randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar dropout rates and reasons |
| Selective reporting (reporting bias) | Low risk | NCT02043366. Main outcomes reported |
| Other bias | Low risk | Similar baseline characteristics |
Zhou 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 122
Country: China
Setting: secondary care hospital
Dates conducted: January 2014 to February 2017
Postoperative opioid used and delivery: IV pethidine Pain score collection: not reported Concurrent postoperative analgesics: none reported |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PEA (62 participants): Celecoxib 400 mg at 1 hour before the operation, and then 200 mg every 12 hours Group POA (60 participants): Celecoxib 400 mg at 4 hours after the operation, and then 200 mg every 12 hours |
|
| Outcomes |
|
|
| Notes | Funding: not reported Declarations of interest: "the authors of this work have nothing to disclose". Authors contacted: yes Other: unclear if pethidine consumption at 24 or 36 hours. Early pain not used as occurred at time of postoperative group dosing. SD estimated as unclear from graph. Sedation extracted from drowsiness on graph |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. Quote: "...randomization code was generated by a statistician using blocked randomization method". |
| Allocation concealment (selection bias) | Unclear risk | Unclear from description. Quote: "The randomization documents were subsequently sent to the Department of Orthopedics in Dongyang People’s Hospital kept by a doctor separately and a copy was kept in Shanghai Qeejen for backup. When a patient was eligible for the study, a unique subject identification number was provided from the randomized module and the patient was assigned to the identified group". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% of patients dropped out, some for lack of intervention efficacy. Quote: "In the POA group, there were 9 withdrawn patients (5 protocol violations, 3 insufficient efficacy, and 1 patient decision), and the remaining 60 (87%) patients completed the study. In the PEA group, there were 7 withdrawn patients, in which 4 were protocol violations, 2 insufficient efficacy, and 1 patient decision and the remaining 62 (90%) patients fulfilled the study". |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | High risk | More women in PEA group (Gerbershagen 2014) |
Zhou 2019.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 120
Country: China
Setting: secondary care hospital
Dates conducted: June 2014 to March 2016
Postoperative opioid used and delivery: no mention Pain score collection: blinded researcher Concurrent postoperative analgesics: none |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Group PRE (60 participants): IV 50 mg flurbiprofen 15 minutes before surgery Group INTRA (60 participants): IV 50 mg flurbiprofen 30 minutes before the end of surgery |
|
| Outcomes |
|
|
| Notes | Funding: "No funding was received". Declarations of interest: "The authors declare that they have no competing interests". Authors contacted: no Other: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐dummy placebo. Quote: "The PRE group received 50 mg flurbiprofen (Taide Pharmaceutical Co., Beijing, China) intravenously 15 min before surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. Quote: "A physician who was blinded to the group assignment assessed spontaneous postsurgical pain intensity at rest using a 10‐cm visual analog scale (VAS)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Low risk | Similar groups. Quote: "As shown in Table I, there were no significant differences in the three groups in terms of sex, age, weight, height, and operation time". |
ASA: American Society of Anesthesiologists grade BD: twice daily BPI: brief pain inventory CNS: central nervous system COPD: chronic obstructive pulmonary disease COX‐2: cyclooxygenase‐2 DGSS: German Society for the Study of Pain G6PD: glucose‐6‐phosphate dehydrogenase IBD: inflammatory bowel disease IL: interleukin IM: intramuscular IQR: interquartile range IV: intravenous LA: local anaesthetic mg: milligrams mcg: micrograms N/A: not applicable NaCl: sodium chloride NCA: nurse‐controlled analgesia NRS: numeric rating scale NSAID: non‐steroidal anti‐inflammatory NYHA: New York Heart Association OD: once daily ORIF: open reduction and internal fixation PACU: post‐anaesthesia care unit PADSS: Post Anaesthetic Discharge Scoring System PCA: patient controlled analgesia PEA: patient controlled epidural analgesia PGE2: prostaglandin E2 PO: oral PONV: postoperative nausea and vomiting PR: per rectum PRN: as required SC: subcutaneous SD: standard deviation SDS: opioid‐related symptom distress scale SEM: standard error of the mean SL: sublingual TDS: three times daily THR: total hip replacement TNF: tumour necrosis factor VAS: visual analogue scale VRS: verbal rating scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bridgman 1996 | Cross‐over trial |
| Castiglione 1997 | Two doses in pre‐emptive versus one in post‐incision |
| Espinet 1996 | NSAID in combination with epidural anaesthesia |
| Hill 1987 | No post‐incision group |
| Hou 2019 | Pre‐emptive group had higher dose |
| Jung 2003 | Paediatric participants included |
| Jung 2005 | Paediatric participants included |
| Lau 2009 | Cross‐over trial |
| Liu 1997 | Same drug, different transdermal locations on the body (interventions not comparable) |
| Liu 2018 | Different doses |
| Nelson 1993 | Pre‐emptive group had higher dose |
| Nordbladh 1991 | Paediatric participants included |
| Ramirez 2009 | Paediatric participants included |
| Rosaeg 2001 | Three pre‐emptive interventions studied |
| Sai 2001 | No post‐incision group |
| Settecase 2002 | Different doses |
| Turaga 2008 | Different doses |
| Wuolijoki 1987 | Different routes of administration |
| Zhu 2020 | Pre‐emptive group had higher dose |
| Zor 2014 | Cross‐over trial |
Characteristics of studies awaiting classification [ordered by study ID]
Aoki 2002.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: Japan
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: not reported |
| Participants |
Inclusion criteria
Exclusion criteria (not reported) |
| Interventions |
Group Second (20 participants): 1 mg/kg flurbiprofen IV at the end of surgery Group Third (20 participants): 1 mg/kg IV flurbiprofen before the start of surgery |
| Outcomes |
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: not included as unable to translate. Concluded "...administration of flurbiprofen before the start of surgery is more effective for peri‐operative analgesia in minor ear, neck and nose surgery". |
Bai 1998.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 30
Country: South Korea
Setting: unknown
Dates conducted: unknown
Postoperative opioid used and delivery: PCA morphine Pain score collection: unknown Concurrent postoperative analgesics: unknown |
| Participants |
Inclusion criteria
Exclusion criteria (not reported) |
| Interventions |
Group 3 (15 participants): IV ketorolac 30 mg before induction Group 2 (15 participants): IV ketorolac 30 mg at one hour after skin incision |
| Outcomes |
|
| Notes | Funding: unknown Declarations of interest: unknown Authors contacted: yes Other: unable to translate |
Beg 2001.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: unknown
Country: Bangledesh
Setting: unknown
Dates conducted: unknown
Postoperative opioid used and delivery: unknown Pain score collection: unknown Concurrent postoperative analgesics: unknown |
| Participants |
Inclusion criteria (unknown) Exclusion criteria (unknown) |
| Interventions |
Group Preemptive (unknown) Group Post‐incision (unknown) |
| Outcomes | Unknown |
| Notes | Funding: unknown Declarations of interest: unknown Authors contacted: yes Other: no abstract available. Unable to obtain full text |
Belzarena 1994.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: unknown
Country: unknown
Setting: unknown
Dates conducted: unknown
Postoperative opioid used and delivery: unknown Pain score collection: unknown Concurrent postoperative analgesics: unknown |
| Participants |
Inclusion criteria (unknown) Exclusion criteria (unknown) |
| Interventions |
Group Preemptive (unknown) Group Post‐incision (unknown) |
| Outcomes | Unknown |
| Notes | Funding: unknown Declarations of interest: unknown Authors contacted: yes Other: no abstract available. Unable to obtain full text. |
De Oliveira 1999.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 24
Country: Brazil
Setting: secondary care hospital
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: not reported |
| Participants |
Inclusion criteria
Exclusion criteria (not reported) |
| Interventions |
Group I (unknown participants): tenoxicam 20 mg IV 15 minutes before the beginning of anaesthesia Group II (unknown participants): tenoxicam 20 mg IV immediately after surgery |
| Outcomes |
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: not included as unable to obtain full text. Found no difference between groups |
Jia 2011.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 40
Country: China
Setting: not reported
Dates conducted: not reported
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: not reported |
| Participants |
Inclusion criteria
Exclusion criteria (not reported) |
| Interventions |
Group Before (20 participants): IV parecoxib sodium 40 mg before surgery Group After (20 participants): IV parecoxib sodium 40 mg after surgery |
| Outcomes |
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: not included as unable to obtain full text. "Pre‐operative application of parecoxib may be more effective". |
Nicholson 2014.
| Methods | Study design: parallel‐group randomized controlled trial
Sample size: 36
Country: not reported
Setting: not reported
Dates conducted: March 2013 to September 2013
Postoperative opioid used and delivery: not reported Pain score collection: not reported Concurrent postoperative analgesics: not reported |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Group Pre (16 participants): PR diclofenac 100 mg before surgery Group Post (20 participants): PR diclofenac 100 mg at the end of surgery |
| Outcomes |
|
| Notes | Funding: not reported Declarations of interest: not reported Authors contacted: yes Other: not included as unable to obtain full text. "Our study shows a trend towards improved post‐operative analgesia and lower post‐operative opioid requirement with administration of NSAID analgesia". |
IV: intravenous NSAID: non‐steroidal anti‐inflammatory drug PCA: patient controlled analgesia PR: per rectum VAS: visual analogue scale
Differences between protocol and review
1) Following editorial and peer review comments on the review protocol, we added sensitivity analysis by excluding studies with a low sample size (< 50 participants).
2) Nausea and vomiting was aggregated to reduce type 1 errors from multiple comparisons and following peer review feedback from our previous review on pre‐emptive and preventive opioids (Doleman 2018b).
3) We have used an updated test for publication bias due to recently published research. This test uses inverse sample size instead of standard errors to assess small study effects. In extensive simulations it performs as well as traditional tests when no baseline risk is present and reduces type 1 errors when baseline risk is present in outcomes such as pain and morphine consumption (Doleman 2020).
4) To reduce type 1 errors in investigating heterogeneity, we did not perform meta‐regression for type of NSAID as this replicated the subgroup analysis. We could not undertake meta‐regression for dose of NSAID due to a lack of ability to convert doses between agents.
5) We did not include the earliest postoperative measurement for early acute postoperative pain and, instead, included the earliest time point when post‐incision dosing was likely to be therapeutic. For example, if post‐incision dosing occurred at two hours, we included a time point at three hours rather than one hour. This is because the latter scenario is comparing pre‐emptive/preventive NSAIDs with no intervention which contradicts the objectives of the review (Doleman 2018b).
6) Sedation definition changed to include more studies for this outcome. This was previously defined on a continuous scale, although this was changed to number of events in the study.
Contributions of authors
Brett Doleman (BD), John P Williams (JPW), Jon Lund (JL), Jo Leonardi‐Bee (JLB), Thomas Heinink (TH), Laura Carrick (LC), Hannah Boyd‐Carson (HBC) and Rahil Mandalia (RM):
Conceiving the review: BD, JPW
Co‐ordinating the review: BD, JPW, JL, JLB, TH, HBC, LC, RM
Undertaking manual searches: BD
Screening search results: BD
Organizing retrieval of papers: BD
Screening retrieved papers against inclusion criteria: BD, JPW, TH, JLB
Appraising quality of papers: BD, JPW, TH, JLB
Abstracting data from papers: BD, JPW, TH, RM, HBC, LC
Writing to authors of papers for additional information: BD
Providing additional data about papers: BD
Obtaining and screening data on unpublished studies: BD
Data management for the review: BD, JPW
Entering data into Review Manager 5 (RevMan 5): BD, JPW
RevMan statistical data: BD, JPW
Other statistical analysis not using RevMan 5: BD, RM
Interpretation of data: BD, JPW, JLB, HBC, RM, LC
Statistical inferences: BD, JPW, JLB
Writing the review: BD, JPW, HBC, RM, LC, JLB, JL
Securing funding for the review: N/A
Performing previous work that was the foundation of the present study: BD, JPW, JL, TH
Guarantor for the review (one author): BD
Person responsible for reading and checking review before submission: BD, JL, JPW, JLB, TH, LC, JLB, JL
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Brett Doleman: has received a grant from Association of Anaesthetists of Great Britain and Ireland (AAGBI) for a randomized controlled trial of pre‐emptive paracetamol (ongoing) and has previously undertaken a meta‐analysis of pre‐emptive paracetamol (Doleman 2015b).
John P Williams: has received a grant from AAGBI for a randomized controlled trial of pre‐emptive paracetamol (ongoing) and has previously undertaken a meta‐analysis of pre‐emptive paracetamol (Doleman 2015b).
Jon Lund: has received a grant from AAGBI for a randomized controlled trial of pre‐emptive paracetamol (ongoing) and has previously undertaken a meta‐analysis of pre‐emptive paracetamol (Doleman 2015b).
Jo Leonardi‐Bee: no declarations of interest in relation to this review
Thomas Heinink: no declarations of interest
Hannah Boyd‐Carson: no declarations of interest
Laura Carrick: no declarations of interest
Rahil Mandalia: no declarations of interest
New
References
References to studies included in this review
Abanto 2014 {unpublished data only}
- Abanto ASA. Analgesic Effectiveness of Prophylactic Therapy and Continuous Therapy with Sodium Naproxen Post-Simple Extraction [Efectividad Analgesia Post Exodoncia Simple de la Terapia Profilactica y la Terapia Continuadad con Naproxeno Sodico] [Thesis]. 2014. [Google Scholar]
Ashworth 2002 {published data only}
- Ashworth HL, Ong C, Seed PT, Venn PJ. The influence of timing and route of administration of intravenous ketorolac on analgesia after hand surgery. Anaesthesia 2002;57(6):535-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aznar‐Arasa 2012 {published data only}
- Aznar-Arasa L, Harutunian K, Figueiredo R, Valmaseda-Castellon E, Gay-Escoda C. Effect of preoperative ibuprofen on pain and swelling after lower third molar removal: a randomized controlled trial. International Journal of Oral and Maxillofacial Surgery 2012;41(8):1005-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bajaj 2004 {published data only}
- Bajaj P, Ballary CC, Dongre NA, Baliga VP, Desai AA. Role of parecoxib in pre-emptive analgesia: comparison of the efficacy and safety of pre- and postoperative parecoxib in patients undergoing general surgery. Journal of the Indian Medical Association 2004;102(5):272-8. [PMID: ] [PubMed] [Google Scholar]
Bao 2012 {published data only}
- Bao Y, Fang J, Peng L, Yi Y, Liu K, Li W, et al. Comparison of preincisional and postincisional parecoxib administration on postoperative pain control and cytokine response after total hip replacement. Journal of International Medical Research 2012;40(5):1804-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boccara 2005 {published data only (unpublished sought but not used)}
- Boccara G, Chaumeron A, Pouzeratte Y, Mann C. The preoperative administration of ketoprofen improves analgesia after laparoscopic cholecystectomy in comparison with propacetamol or postoperative ketoprofen. British Journal of Anaesthesia 2005;94(3):347-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Buggy 1994 {published data only}
- Buggy DJ, Wall C, Carton EG. Preoperative or postoperative diclofenac for laparoscopic tubal ligation. British Journal of Anaesthesia 1994;73(6):767-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bunemann 1994 {published data only}
- Bunemann L, Thorshauge H, Herlevsen P, Iversen AD, Nielsen FB. Analgesia for outpatient surgery: placebo versus naproxen sodium (a non-steroidal anti-inflammatory drug) given before or after surgery. European Journal of Anaesthesiology 1994;11(6):461-4. [PMID: ] [PubMed] [Google Scholar]
Cabell 2000 {published data only}
- Cabell CA. Does ketorolac produce preemptive analgesic effects in laparoscopic ambulatory surgery patients? Journal of the American Association of Nurse Anesthetists 2000;68:343-9. [PMID: ] [PubMed] [Google Scholar]
Chan 1996 {published data only (unpublished sought but not used)}
- Chan A, Dore CJ, Ramachandra V. Evaluation of the effect of diclofenac given before or after surgery with or without bupivacaine infiltration. Anaesthesia 1996;51:592-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen JQ, Wu Z, Wen LY, Miao JZ, Hu YM. Preoperative and postoperative analgesic techniques in the treatment of patients undergoing transabdominal hysterectomy: a preliminary randomized trial. BMC Anesthesiology 2015;15(1):70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colbert 1998 {published data only}
- Colbert ST, McDonnell C, Keane PW, O'Hanlon DM, Given FH. Analgesia in day case breast biopsy - the value of pre-emptive tenoxicam. Canadian Journal of Anesthesia 1998;45(3):217-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coli 1993 {published data only}
- Coli A, Lari S, Vigano E, Perin S, Lari F. Evaluation of the effectiveness of NSAIDs in the prevention of postoperative pain. Comparison between pre- and postoperative administration of sodium naproxen in orthopedic surgery. Minerva Anestesiologica 1993;59(10):531-5. [PMID: ] [PubMed] [Google Scholar]
Demirbas 2019 {published data only}
- Demirbas AE, Karakaya M, Bilge S, Canpolat DG, Kütük N, Alkan A. Does single-dose preemptive intravenous ibuprofen reduce postoperative pain after third molar surgery? A prospective, randomized, double-blind clinical study. Journal of Oral and Maxillofacial Surgery 2019;77(10):1990-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Esparza‐Villalpando 2016 {published data only (unpublished sought but not used)}
- Esparza-Villalpando V, Gordillo-Moscoso A, Chavarria-Bolanos D, Masuoka-Ito D, Martinez-Rider R. Comparison of the analgesic efficacy of preoperative/postoperative oral dexketoprofen trometamol in third molar surgery: a randomized clinical trial. Journal of Cranio-Maxillofacial Surgery 2016;44(9):1350-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flath 1987 {published data only}
- Flath RK, Hicks ML, Dionne RA, Pelleu GB. Pain suppression after pulpectomy with preoperative flurbiprofen. Journal of Endodontics 1987;13:339-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fleckenstein 2016 {published data only (unpublished sought but not used)}
- Fleckenstein J, Kohls N, Evtouchenko E, Lehmeyer L, Kramer S, Lang PM, et al. No effect of the cyclooxygenase-2 inhibitor etoricoxib on pre-emptive and post-operative analgesia in visceral surgery: results of a randomized controlled trial. European Journal of Pain 2016;20(2):186-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fletcher 1995 {published data only}
- Fletcher D, Zetlaoui P, Monin S, Bombart M, Samii K. Influence of timing on the analgesic effect of intravenous ketorolac after orthopedic surgery. Pain 1995;61(2):291-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gabbott 1997 {published data only}
- Gabbott DA, Cohen AM, Mayor AH, Niemiro LAK, Thomas TAT. The influence of timing of ketorolac administration on post-operative analgesic requirements following total abdominal hysterectomy. European Journal of Anaesthesiology 1997;14(6):610-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gelir 2016 {published data only}
- Gelir IK, Gulec S, Ceyhan D. Preventive effect of dexketoprofen on postoperative pain. Agri Dergisi 2016;28(2):67-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Giuliani 2015 {published and unpublished data}
- Giuliani E, Bianchi A, Marcuzzi A, Landi A, Barbieri A. Ibuprofen timing for hand surgery in ambulatory care. Ortopedica Brasileira 2015;23(4):188-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gramke 2006 {published data only (unpublished sought but not used)}
- Gramke HF, Petry JJ, Durieux ME, Mustaki JP, Vercauteren M, Verheecke G, et al. Sublingual piroxicam for postoperative analgesia: preoperative versus postoperative administration: a randomized, double-blind study. Anesthesia and Analgesia 2006;102(3):755-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grifka 2008 {published data only (unpublished sought but not used)}
- Grifka J, Enz R, Zink J, Hugot JL, Kreiss A, Arulmani U, et al. Preemptive versus postoperative lumiracoxib for analgesia in ambulatory arthroscopic knee surgery. Journal of Pain Research 2008;1:27-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunter 2012 {unpublished data only}
- Gunter J. Preemptive Effects of Parecoxib (Dynastat®) in Trauma Surgery [Dissertation]. Ulm: Universität Ulm, 2012. [https://oparu.uni-ulm.de/xmlui/bitstream/handle/123456789/2802/vts_8266_12114.pdf?sequence=1] [Google Scholar]
Guran 2010 {published data only}
- Guran C, Dragulin E, Stelea G, Moise A, Mincu N. Preemptive or preventive? The best time for peri-operative analgesia using dexketoprofen in laparoscopic cholecystectomy. Romanian Journal of Anaesthesia and Intensive Care 2010;17(1):23-8. [Google Scholar]
Inanoglu 2007 {published data only (unpublished sought but not used)}
- Inanoglu K, Gorur S, Akkurt CO, Guven OE, Kararmaz A. The analgesic efficacy of preoperative versus postoperative lornoxicam in varicocele repair. Journal of Clinical Anesthesia 2007;19(8):587-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaczmarzyk 2010 {published data only}
- Kaczmarzyk T, Wichlinski J, Stypulkowska J, Zaleska M, Woron J. Preemptive effect of ketoprofen on postoperative pain following third molar surgery. A prospective, randomized, double-blinded clinical trial. International Journal of Oral and Maxillofacial Surgery 2010;39(7):647-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karaman 2008 {published data only}
- Karaman Y, Kebapci E, Gurkan A. The preemptive analgesic effect of lornoxicam in patients undergoing major abdominal surgery: a randomised controlled study. International Journal of Surgery 2008;6(3):193-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only (unpublished sought but not used)}
- Lee LH, Yuen MK, Irwin MG, Cheung CW, Yao TJ. Timing of intraoperative parecoxib analgesia in colorectal surgery. Acute Pain 2008;10(3):123-30. [doi.org/10.1016/j.acpain.2008.07.002] [Google Scholar]
Likar 1997 {published data only}
- Likar R, Krumpholz R, Mathiaschitz K, Pipam W, Burtscher M, Ozegovic G, et al. The preemptive action of ketoprofen. Randomized, double-blind study with gynecologic operations. Anaesthesist 1997;46(3):186-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Likar 1998 {published data only}
- Likar R, Krumpholz R, Pipam W, Sadjak A, Kapral S, Forsthuber E, et al. Randomized, double-blind study with ketoprofen in gynecologic patients. Preemptive analgesia study following the Brevik-Stubhaug design. Anaesthesist 1998;47(4):303-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lu 2015 {published data only}
- Lu J, Liu Z, Xia K, Shao C, Guo S, Wang S, et al. Effect of preemptive analgesia with parecoxib sodium in patients undergoing radical resection of lung cancer. International Journal of Clinical and Experimental Medicine 2015;8(8):14115-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Martinez 2007 {published data only}
- Martinez V, Belbachir A, Jaber A, Cherif K, Jamal A, Ozier Y, et al. The influence of timing of administration on the analgesic efficacy of parecoxib in orthopedic surgery. Anesthesia and Analgesia 2007;104(6):1521-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012 {published data only (unpublished sought but not used)}
- Mishra H, Khan FA. A double-blind, placebo-controlled randomized comparison of pre and postoperative administration of ketorolac and tramadol for dental extraction pain. Journal of Anaesthesiology Clinical Pharmacology 2012;28:221-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mojsa 2017 {published and unpublished data}
- Mojsa IM, Stypulkowska J, Novak P, Lipczynski K, Zaleska M. Pre-emptive analgesic effect of lornoxicam in mandibular third molar surgery: a prospective, randomized, double-blind clinical trial. International Journal of Oral and Maxillofacial Surgery 2017;46(5):614-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moonla 2018 {published data only}
- Moonla R, Threetipayarak A, Panwilai J. Comparison of preoperative and postoperative parecoxib administration for pain control following major spine surgery. Asian Spine Journal 2018;12:893-901. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munteanu 2016 {published data only (unpublished sought but not used)}
- Munteanu AM, Cionac Florescu S, Anastase DM, Stoica CI. Is there any analgesic benefit from preoperative vs. postoperative administration of etoricoxib in total knee arthroplasty under spinal anaesthesia? A randomised double-blind placebo-controlled trial. European Journal of Anaesthesiology 2016;33(11):840-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murphy 1993 {published data only}
- Murphy DF, Medley C. Preoperative indomethacin for pain relief after thoracotomy: comparison with postoperative indomethacin. British Journal of Anaesthesia 1993;70(3):298-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nakayama 2001 {published data only (unpublished sought but not used)}
- Nakayama M, Ichinose H, Yamamoto S, Nakabayashi KI, Satoh O. Perioperative intravenous flurbiprofen reduces postoperative pain after abdominal hysterectomy. Canadian Journal of Anesthesia 2001;48(3):234-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nezafati 2017 {published data only (unpublished sought but not used)}
- Nezafati S, Khiavi RH, Mirinejhad SS, Ammadi DA, Ghanizadeh M. Comparison of pain relief from different intravenous doses of ketorolac after reduction of mandibular fractures. Journal of Clinical and Diagnostic Research 2017;11(9):6-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norris 2001 {published data only}
- Norris A, Un V, Chung F, Thanamayooran S, Sandler A, Katz J. When should diclofenac be given in ambulatory surgery: preoperatively or postoperatively? Journal of Clinical Anesthesia 2001;13(1):11-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Hanlon 1996 {published data only}
- O'Hanlon JJ, Muldoon T, Lowry D, McCleane G. Improved postoperative analgesia with preoperative piroxicam. Canadian Journal of Anaesthesia 1996;43(2):102-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ozer 2012 {published data only}
- Ozer AB, Erhan OL, Keles E, Demirel I, Bestas A. Comparison of the effects of preoperative and intraoperative intravenous application of dexketoprofen on postoperative analgesia in septorhinoplasty patients: randomised double blind clinical trial. European Review for Medical and Pharmacological Sciences 2012;16(13):1828-33. [PMID: ] [PubMed] [Google Scholar]
Ozyilmaz 2005 {published data only (unpublished sought but not used)}
- Ozyilmaz MA, Olmez G, Gizdas Uluc D. Preemptive lornoxicam for postoperative pain relief in patients undergoing lumbar disk surgery. Anestezi Dergisi 2005;13:258-62. [Google Scholar]
Pandazi 2010 {published data only}
- Pandazi A, Kapota E, Matsota P, Paraskevopoulou P, Dervenis C, Kostopanagiotou G. Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial. World Journal of Surgery 2010;34(10):2463-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parke 1995 {published data only}
- Parke TJ, Lowson SM, Uncles DR, Daughtery MO, Sitzman BT. Pre-emptive versus post-surgical administration of ketorolac for hysterectomy. European Journal of Anaesthesiology 1995;12(6):549-53. [PMID: ] [PubMed] [Google Scholar]
Peduto 1995 {published data only}
- Peduto VA, Toscano A, D'Uva R, Piga M. Ketorolac for prevention of acute postoperative pain. Minerva Anestesiologica 1995;61(9):367-72. [PMID: ] [PubMed] [Google Scholar]
Priya 2002 {published data only}
- Priya V, Divatia JV, Sareen R, Upadhye S. Efficacy of intravenous ketoprofen for pre-emptive analgesia. Journal of Postgraduate Medicine 2002;48(2):109-12. [PMID: ] [PubMed] [Google Scholar]
Riest 2006 {published data only}
- Riest G, Peters J, Weiss M, Pospiech J, Hoffmann O, Neuhauser M, et al. Does perioperative administration of rofecoxib improve analgesia after spine, breast and orthopaedic surgery? European Journal of Anaesthesiology 2006;23(3):219-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Riest 2008 {published data only}
- Riest G, Peters J, Weiss M, Dreyer S, Klassen PD, Stegen B, et al. Preventive effects of perioperative parecoxib on post-discectomy pain. British Journal of Anaesthesia 2008;100(2):256-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rogers 1995 {published data only}
- Rogers JEG, Fleming BG, Macintosh KC, Johnston B, Morgan-Hughes JO. Effect of timing of ketorolac administration on patient-controlled opioid use. British Journal of Anaesthesia 1995;75(1):15-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salonen 2001 {published data only}
- Salonen A, Kokki H, Tuovinen K. IV ketoprofen for analgesia after tonsillectomy: comparison of pre- and post-operative administration. British Journal of Anaesthesia 2001;86(3):377-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sandin 1993 {published data only}
- Sandin R, Sternlo JE, Stam H, Brodd B, Bjorkman R. Diclofenac for pain relief after arthroscopy: a comparison of early and delayed treatment. Acta Anaesthesiologica Scandinavica 1993;37(8):747-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shuying 2014 {published data only}
- Shuying L, Xiao W, Peng L, Tao Z, Ziying L, Liang Z. Preoperative intravenous parecoxib reduces length of stay on ambulatory laparoscopic cholecystectomy. International Journal Of Surgery 2014;12(5):464-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2008 {published data only}
- Sun T, Sacan O, White PF, Coleman J, Rohrich RJ. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesthesia and Analgesia 2008;106(3):950-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Trampitsch 2003 {published data only}
- Trampitsch E, Pipam W, Moertl M, Sadjak A, Dorn C, Sittl R, et al. Preemptive randomized, double-blind study with lornoxicam in gynecological surgery. Der Schmerz 2003;17(1):4-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vanlersberghe 1996 {published data only}
- Vanlersberghe C, Lauwers MH, Camu F. Preoperative ketorolac administration has no preemptive analgesic effect for minor orthopaedic surgery. Acta Anaesthesiologica Scandinavica 1996;40(8 Pt 1):948-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vijayendra 1998 {published data only}
- Vijayendra HS, Bhardwaj N, Bala I, Chari P. Pre-emptive analgesic effect of intravenous ketorolac tromethamine in patients undergoing orthopaedic surgery. Journal of Anaesthesiology Clinical Pharmacology 1998;14:125-7. [Google Scholar]
Vogol 1992 {published data only}
- Vogel RI, Desjardins PJ, Major KV. Comparison of presurgical and immediate postsurgical ibuprofen on postoperative periodontal pain. Journal of Periodontology 1992;63(11):914-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang Q, Li Z, Wang ZP, Cui C. Preemptive analgesic effect of parecoxib sodium in patients undergoing laparoscopic colorectal surgery. Journal of Southern Medical University 2010;30(11):2556-7. [PMID: ] [PubMed] [Google Scholar]
Wnek 2004 {published data only}
- Wnek W, Zajaczkowska R, Wordliczek J, Dobrogowski J, Korbut R. Influence of pre-operative ketoprofen administration (preemptive analgesia) on analgesic requirement and the level of prostaglandins in the early postoperative period. Polish Journal of Pharmacology 2004;56:547-52. [PMID: ] [PubMed] [Google Scholar]
Yagar 2011 {published data only}
- Yağar S, Turan SK, Ayık İ, Güçlü ÇY, Koç M, Kantaroğlu S, et al. Comparative study of pre-emptive and postoperative IV tenoxicam in laparoscopic cholecystectomy. Journal of the Turkish Anaesthesiology & Intensive Care Society 2011;39:19-24. [Google Scholar]
Yamashita 2006 {published data only}
- Yamashita K, Fukusaki M, Ando Y, Fujinaga A, Tanabe T. Preoperative administration of intravenous flurbiprofen axetil reduces postoperative pain for spinal fusion surgery. Journal of Anesthesia 2006;20(2):92-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yan 2004 {published data only}
- Yan XB, Wang MA, Ouyang W, Wang XW, Shui Y. Timing of lornoxicam administration in relation to its postoperative analgesic effects. Chinese Journal of Clinical Rehabilitation 2004;8(11):2180-1. [Google Scholar]
Young 2006 {published data only}
- Young AN, Taylor RW, Buschang PH, Taylor SE, Linnebur SA. Evaluation of preemptive valdecoxib therapy on initial archwire placement discomfort in adults. Angle Orthodontist 2006;76(2):251-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yuan 2019 {published data only}
- Yuan Y, Cui D, Zhang Y. Preemptive meloxicam achieves a better effect on postoperative pain control and similar tolerance compared with postoperative meloxicam in patients receiving arthroscopic knee surgery. Inflammopharmacology 2019;27(6):1091-1100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yuswono 2014 {published data only}
- Yuswono AR, Maskoen TT, Fuadi I. Comparison of pre-operative and post-operative intravenous 40 mg parecoxib NA in gynecologic laparatomy surgery post-operative pain management. Jurnal Anestesi Perioperatif 2014;2:169-73. [Google Scholar]
Zhang 2011 {published data only}
- Zhang Z, Zhao H, Wang C, Han F, Wang G. Lack of preemptive analgesia by intravenous flurbiprofen in thyroid gland surgery: a randomized, double-blind and placebo-controlled clinical trial. International Journal of Medical Sciences 2011;8(5):433-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang L, Shu R, Zhao Q, Li Y, Wang C. Preoperative but not postoperative flurbiprofen axetil alleviates remifentanil-induced hyperalgesia after laparoscopic gynecological surgery. Clinical Journal of Pain 2017;33(5):435-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2017 {published data only (unpublished sought but not used)}
- Zhou F, Du Y, Huang W, Shan J, Xu G. The efficacy and safety of early initiation of preoperative analgesia with celecoxib in patients underwent arthroscopic knee surgery. Medicine 2017;96(42):e8234. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2019 {published data only}
- Zhou ZJ, Tang J, Li WH, Tao WD. Preoperative intravenous flurbiprofen reduces postoperative pain and inflammatory cytokines in elderly patients after hip arthroplasty. Experimental and Therapeutic Medicine 2019;17(1):354-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bridgman 1996 {published data only}
- Bridgman JB, Gillgrass TG, Zacharias M. The absence of any pre-emptive analgesic effect for non-steroidal anti-inflammatory drugs. British Journal of Oral and Maxillofacial Surgery 1996;34(5):428-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Castiglione 1997 {published data only}
- Castiglione G, Hauf ME, Panascia E, Scuderi C, Crimi G. Does the preoperative administration of ketorolac improve postoperative analgesia. Minerva Anestesiologica 1997;63:237-43. [PMID: ] [PubMed] [Google Scholar]
Espinet 1996 {published data only}
- Espinet A, Henderson DJ, Faccenda KA, Morrison LM. Does pre-incisional thoracic extradural block combined with diclofenac reduce postoperative pain after abdominal hysterectomy? British Journal of Anaesthesia 1996;76(2):209-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hill 1987 {published data only}
- Hill CM, Carroll MJ, Giles AD, Pickvance N. Ibuprofen given pre- and post-operatively for the relief of pain. International Journal of Oral & Maxillofacial Surgery 1987;16(4):420-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hou 2019 {published data only}
- Hou J, Li W, Chen Y, Yang L, Li L, Zhao L. Early preoperative versus postoperative administration of meloxicam in pain control, patient global status improvement, knee function recovery of arthroscopic knee surgery. Medicine 2019;98(40):e17133. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2003 {published data only}
- Jung YS, Kim MK, Park HS, Lee EW, Kang JW. A study on efficacy of preemptive analgesia: a comparison on efficacy of preoperative and postoperative analgesic administration. Journal of the Korean Dental Society of Anesthesiology 2003;3:10-8. [DOI: 10.17245/jkdsa.2003.3.1.10] [DOI] [Google Scholar]
Jung 2005 {published data only}
- Jung YS, Kim MK, Um YJ, Park HS, Lee EW. The effects on postoperative oral surgery pain by varying NSAID administration times: comparison on effect of preemptive analgesia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 2005;100(5):559-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lau 2009 {published data only}
- Lau SL, Chow RLK, Yeung RWK, Samman N. Pre-emptive ibuprofen arginate in third molar surgery: a double-blind randomized controlled crossover clinical trial. Australian Dental Journal 2009;54(4):355-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu K, Chia YY, Lo Y, Kan Y, Wang JJ, Ho ST. Effect of preoperative transdermal ketoprofen on post-hysterectomy pain. Chinese Medical Journal (Taipei) 1997;60(6):290-5. [PMID: ] [PubMed] [Google Scholar]
Liu 2018 {published data only}
- Liu J, Wang F. Preoperative celecoxib analgesia is more efficient and equally tolerated compared to postoperative celecoxib analgesia in knee osteoarthritis patients undergoing total knee arthroplasty: a randomized, controlled study. Medicine 2018;97(51):e13663. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1993 {published data only}
- Nelson WE, Henderson RC, Almekinders LC, DeMasi RA, Taft TN. An evaluation of pre- and postoperative nonsteroidal antiinflammatory drugs in patients undergoing knee arthroscopy. A prospective, randomized, double-blinded study. American Journal of Sports Medicine 1993;21:510-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nordbladh 1991 {published data only}
- Nordbladh I, Ohlander B, Bjorkman R. Analgesia in tonsillectomy: a double-blind study on pre and post-operative treatment with diclofenac. Clinical Otolaryngology & Allied Sciences 1991;16(6):554-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramirez 2009 {published data only}
- Ramírez V, Petuel N. Preventive Analgesia with Ketorolac in Patients Subject to Obstruction of Bilateral Tubes with Local Anaesthesia and Sedation [Thesis]. 2009. [Google Scholar]
Rosaeg 2001 {published data only}
- Rosaeg OP, Krepski B, Cicutti N, Dennehy KC, Lui AC, Johnson DH. Effect of preemptive multimodal analgesia for arthroscopic knee ligament repair. Regional Anesthesia and Pain Medicine 2001;26(2):125-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sai 2001 {published data only}
- Sai S, Fujii K, Hiranuma K, Sato T, Nemoto T. Preoperative ampiroxicam reduces postoperative pain after hand surgery. Journal of Hand Surgery 2001;26(4):377-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Settecase 2002 {published data only}
- Settecase C, Bagilet D, Bertoletti F, Laudanno C. Preoperative diclofenac does not reduce pain of laparoscopic cholecystectomy. Revista Espanola de Anestesiologia y Reanimacion 2002;49(9):455-60. [PMID: ] [PubMed] [Google Scholar]
Turaga 2008 {published data only}
- Turaga K, Wright A, Lee R, Dias WP, Destache C, Christian R, et al. A randomized trial of the peri-operative use of COX-2 inhibitors in Lichtenstein herniorrhaphy. Hernia 2008;12(5):515-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wuolijoki 1987 {published data only}
- Wuolijoki E, Oikarinen VJ, Ylipaavalniemi P, Hampf G, Tolvanen M. Effective postoperative pain control by preoperative injection of diclofenac. European Journal of Clinical Pharmacology 1987;32(3):249-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhu 2020 {published data only}
- Zhu X. Efficacy of preemptive analgesia versus postoperative analgesia of celecoxib on postoperative pain, patients' global assessment and hip function recovery in femoroacetabular impingement patients underwent hip arthroscopy surgery. Inflammopharmacology 2020;28(1):131-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zor 2014 {published data only}
- Zor ZF, Cetiner S, Isik B. Efficacy of preemptive lornoxicam on postoperative analgesia after surgical removal of mandibular third molars. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2014;117(1):27-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aoki 2002 {published data only}
- Aoki H, Satomoto M, Yamada S, Adachi Y, Higuchi H, Umeda E, et al. The effectiveness of perioperative intravenous flurbiprofen in minor ear, neck and nose surgery. Masui - Japanese Journal of Anesthesiology 2002;51(8):857-61. [PMID: ] [PubMed] [Google Scholar]
Bai 1998 {published data only (unpublished sought but not used)}
- Bai SJ, Nam SH, Lee YW, Nam YT, Kim WJ. The effects of preemptive intravenous ketorolac for total hip replacement patients. Korean Journal of Anesthesiology 1998;35:511-7. [DOI: ] [Google Scholar]
Beg 2001 {published data only}
- Beg AK, Runi FM, Iqbal KM, Ahmed S. Comparison of intravenous ketorolac tromethamine and intravenous pethedine hydrochloride in patients undergoing abdominal hysterectomy: an analysis of pre-emptive analgesic effect. Bangladesh Journal of Obstetrics and Gynecology 2001;16:22-6. [Google Scholar]
Belzarena 1994 {published data only}
- Belzarena SD. Prevention of postoperative pain after abdominal dermolipectomy with intravenous tenoxicam. Revista Brasileira de Anestesiologia 1994;44:103-7. [Google Scholar]
De Oliveira 1999 {published data only}
- De Oliveira JCC, Fenner Lyra Junior H, De Oliveira Filho GR, Dos Santos JM, Felicio F. Comparison between preoperative and postoperative tenoxican in hemorrhoidectomies. Revista Brasileira de Anestesiologia 1999;49:169-72. [Google Scholar]
Jia 2011 {published data only (unpublished sought but not used)}
- Jia DL, Han B, Wang J, Zhang LP, Guo XY. Application of parecoxib for postoperative analgesia in arthroscopic surgery of the knee. Chinese Journal of New Drugs 2011;20:440-3. [Google Scholar]
Nicholson 2014 {published data only}
- Nicholson H, Strahan S, Wines M. Does the timing of intraoperative non-steroidal anti-inflammatory analgesia affect pain outcomes in ureteroscopy? A prospective, single-blinded, randomised controlled trial. BJU International 2014;113:8. [Google Scholar]
Additional references
Apfelbaum 2003
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia and Analgesia 2003;97(2):534-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Benhamou 2008
- Benhamou D, Berti M, Brodner G, Andres JD, Draisci G, Moreno-Azcoita M, et al. Postoperative Analgesic THerapy Observational Survey (PATHOS): a practice pattern study in 7 central/southern European countries. Pain 2008;136(1-2):134-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burian 2005
- Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacology & Therapeutics 2005;107(2):139-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chong 2016
- Chong SW, Collins NF, Wu CY, Liskaser GM, Peyton PJ. The relationship between study findings and publication outcome in anesthesia research: a retrospective observational study examining publication bias. Canadian Journal of Anesthesia 2016;63:682-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dahl 2011
- Dahl JB, Kehlet H. Preventive analgesia. Current Opinion in Anesthesiology 2011;24(3):331-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Desai 1999
- Desai PM. Pain management and pulmonary dysfunction. Critical Care Clinics 1999;15(1):151-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Desborough 2000
- Desborough JP. The stress response to trauma and surgery. British Journal of Anaesthesia 2000;85(1):109-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Doleman 2015a
- Doleman B, Heinink TP, Read DJ, Faleiro RJ, Lund JN, Williams JP. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 2015;70(10):1186-204. [PMID: ] [DOI] [PubMed] [Google Scholar]
Doleman 2015b
- Doleman B, Read D, Lund JN, Williams JP. Preventive acetaminophen reduces postoperative opioid consumption, vomiting, and pain scores after surgery: systematic review and meta-analysis. Regional Anesthesia and Pain Medicine 2015;40(6):706-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Doleman 2017a
- Doleman B, Leonardi-Bee J, Heinink TP, Lund J, Williams JP. Preventive opioids for postoperative pain. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD012624. [DOI: 10.1002/14651858.CD012624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doleman 2018a
Doleman 2019
- Doleman B, Williams JP, Lund J. Why most published meta-analysis findings are false. Techniques in Coloproctology 2019;23:925-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Doleman 2020
- Doleman B, Freeman SC, Lund JN, Williams JP, Sutton AJ. Funnel plots may show asymmetry in the absence of publication bias with continuous outcomes dependent on baseline risk: presentation of a new publication bias test. Research Synthesis Methods 2020;11:522–34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenach 2009
- Eisenach JC. Data fabrication and article retraction how not to get lost in the woods. Anesthesiology 2009;110(5):955-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frauenknecht 2019
- Frauenknecht J, Kirkham KR, Jacot‐Guillarmod A, Albrecht E. Analgesic impact of intra‐operative opioids vs. opioid‐free anaesthesia: a systematic review and meta‐analysis. Anaesthesia 2019;74:651-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gallagher 2001
- Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analogue scale. Annals of Emergency Medicine 2001;38(6):633-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gerbershagen 2014
- Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, Peelen LM, Kappen TH, Van Wijck AJ, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014;120:1237-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilron 2003
- Gilron I, Milne B, Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management current evidence and future directions. Anesthesiology 2003;99(5):1198-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ip 2009
- Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009;111:657-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jüni 2002
- Jüni P, Rutjes AW, Dieppe PA. Are selective COX 2 inhibitors superior to traditional non steroidal anti-inflammatory drugs? BMJ 2002;324(7349):1287-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 2006
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367(9522):1618-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kissin 2000
- Kissin I. Preemptive analgesia. Anesthesiology 2000;93(4):1138-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:67-108. [www.training.cochrane.org/handbook] [Google Scholar]
López‐López 2014
- López‐López JA, Marín‐Martínez F, Sánchez‐Meca J, Van den Noortgate W, Viechtbauer W. Estimation of the predictive power of the model in mixed‐effects meta‐regression: a simulation study. British Journal of Mathematical and Statistical Psychology 2014;67:30-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mehta 2007
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11(2):R31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2013
- Moore R, Straube S, Aldington D. Pain measures and cut‐offs–'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Myles 2000
- Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. British Journal of Anaesthesia 2000;84(1):6-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Myles 2017
- Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. British Journal of Anaesthesia 2017;118(3):424-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Møiniche 2002
- Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 2002;96(3):725-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nussmeier 2005
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine 2005;352(11):1081-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ong 2005
- Ong CKS, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesthesia and Analgesia 2005;100(3):757-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ong 2010
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesthesia and Analgesia 2010;110(4):1170-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Stata 2020 [Computer program]
- Stata Version 16.1. Version 15. College Station, TX, USA: StataCorp, 2020. Available from www.stata.com.
Strassels 2002
- Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesthesia and Analgesia 2002;94(1):130-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine 2002;21(11):1559-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thybo 2019
- Thybo KH, Hägi-Pedersen D, Dahl JB, Wetterslev J, Nersesjan M, Jakobsen JC, et al. Effect of combination of paracetamol (acetaminophen) and ibuprofen vs either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty: the PANSAID randomized clinical trial. JAMA 2019;321:562. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warltier 2003
- Warltier DC, Marret E, Flahault A, Samama CM, Bonnet F. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy meta-analysis of randomized, controlled trials. Anesthesiology 2003;98(6):1497-502. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhao 2004
- Zhao SZ, Chung F, Hanna DB, Raymundo AL, Cheung RY, Chen C. Dose-response relationship between opioid use and adverse effects after ambulatory surgery. Journal of Pain and Symptom Management 2004;28(1):35-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Doleman 2018b
- Doleman B, Leonardi‐Bee J, Heinink TP, Bhattacharjee D, Lund JN, Williams JP. Pre-emptive and preventive opioids for postoperative pain in adults undergoing all types of surgery. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD012978. [DOI: 10.1002/14651858.CD012978] [DOI] [PMC free article] [PubMed] [Google Scholar]


