Abstract
Background/aim
In the present study we aimed to figure out the effect of metformin on the expression of AMPK-alpha, cyclin D1, and Tp53, and apoptosis in primary breast cancer cells (PBCCs).
Materials and methods
PBCCs were treated with two doses of metformin (0 mM, 25 mM). Proliferation was determined by BrdU assay. Real-time PCR was used to assess AMPK-alpha, cyclin D1, and Tp53 gene expressions; apoptotic indexes of PBCCs were analyzed using flow-cytometry.
Results
Twenty-four–hour incubation with 25 mM metformin reduced the proliferation of PBCCs. AMPK-alpha gene expression in PBCCs was not affected by 25 mM metformin treatment compared with the control group. PBCCs treated with 25 mM metformin had lower cyclin D1 expression compared with nontreated cells; however, the difference was not statistically significant. Twenty-five millimolar dose of metformin increased p53 expression significantly compared with the nontreated group. The high concentration of metformin elevated the number of annexin V-positive apoptotic cells, and the increase in the apoptotic index was statistically significant.
Conclusion
Metformin can modulate cyclin D1 and p53 expression through AMPK-alpha-independent mechanism in breast cancer cells, leading to cell proliferation inhibition and apoptosis induction.
Keywords: AMPK-alpha, cyclin D1, Tp53, breast cancer, metformin
1. Introduction
Metformin has been the most widely prescribed antihyperglycemic biguanide for treating individuals with type 2 diabetes (T2DM) for decades. It performs its glucose-lowering effect through elevated glucose use and diminished hepatic glucose production [1–3]. In the last decade, a decrease in AMP/ATP ratio was reported to induce indirectly a ubiquitously expressed enzyme—a cellular energy sensor, namely, “AMP-activated protein kinase (AMPK)”[4,5].
Metformin, nowadays, is one of the most famous anticancer drugs used in cancer treatment. The anticancer effect of metformin is considered to be partial via inhibition of cell proliferation [6–8]. Through downregulation of cyclin D1 expression and upregulation of p21, metformin, in breast, bladder, and prostate cancer cells, blocks the cell cycle in the G1 phase [9–11]. Besides, the upregulation of AMPK has been found in human breast cancer and suggested to be not only a prognostic factor but also a therapeutic target [12].
There is a general agreement that p53 is involved not only in cell metabolism but also in cell cycle control and indirectly in apoptosis and the regulation of both p53 phosphorylation and expression and is regulated by AMPK; its participation, thus, in metformin action is inevitably argued. Interestingly, accumulating through elevation pointed out that metformin without any changes in p53 status arrested cell cycle in the G0/G1” stage with a remarkable reduction of G1 cyclin expression, namely cyclin D1 [13–15]. On the other hand, p53 activity was reported to be related to metformin’s inhibitory effect on cancer cell growth [16–19]. In another valuable research about metformin’s effect on hepatocytes, Yi et al. pinpointed that while a low concentration of metformin stimulates p53-dependent senescence, a high concentration, instead, stimulates apoptotic cell death [20]. Therefore, more association studies of metformin with p53 activity would be highly desired. In the case of breast cancer progression, breast tissue cells transform into malignant ones through the dysregulation of cellular processes such as cell cycle, angiogenesis, and apoptosis [21]. Thus, to combat cancer, up-to-date treatment strategies aim at targeting these processes, more specifically apoptosis [22].
Thus, in the present study, we hypothesized that metformin could block cell cycle progression and induce cell apoptosis through the expression of AMPKα in human PBCCs and the underlying mechanism may involve the dysregulation of cyclin D1 and p53 expression.
2. Materials and methods
2.1. Cell culture and dosing
Human PBCCs were obtained from biopsies of breast tumors of five human donors in the age range of 45–55 years, who applied to the Department of General Surgery. The selection criteria were being in the postmenopausal period, as well as the positivity of both estrogen and progesterone receptors (Table 1). All study subjects were provided signed informed consent prior to the sample collection. The protocol for establishing primary human cell cultures from biopsies obtained during mastectomy was approved by “the Ethics Committee of Clinical Research Center of Cerrahpaşa School of Medicine, İstanbul University, İstanbul, Turkey (Date: 15th July 2016, Approval number: 83045809 - 604.01.02 - 257133).
Table 1.
The clinical characteristics of the patients with breast cancer.
| Patient I | Patient II | Patient III | Patient IV | Patient V | |
|---|---|---|---|---|---|
| Age (years) | 45 | 54 | 53 | 49 | 55 |
| Sex | Female | Female | Female | Female | Female |
| Diagnosis | IDC+ILC | IDC | IDC | IDC+ILC | IDC |
| Menopausal status | Post | Post | Post | Post | Post |
| Famillial breat cancer history | Negative | Negative | Negative | Negative | Negative |
| Pathology of the tumor | Malignant | Malignant | Malignant | Malignant | Malignant |
| Size of the tumor in radius (cm) | 2.1 | 2.7 | 2.4 | 3 | 2.9 |
| Histological grade | II | II | II | II | II |
| Patient follow-up | 2 years | >2 years | <1 year | 1 year | 2 years |
| Breast cancer surgery | Mastectomy | Mastectomy | Mastectomy | Mastectomy | Mastectomy |
| ER status | Positive | Positive | Positive | Positive | Positive |
| PR status | Positive | Positive | Positive | Positive | Positive |
| HER2 status | Negative | Negative | Negative | Negative | Negative |
| E-cadherin | Positive | Positive | Positive | Positive | Positive |
| %Ki 67 | 40 | 16 | 58 | 60 | 38 |
IDC: Invasive ductal carcinoma, ILC: Invasive lobular carcinoma
Breast tissues were cut into slices and cultured for 10–14 days as a monolayer in DMEM/F12 medium”(Wisent Bioproducts, Quebec, Canada)” supplemented with 10% fetal bovine serum”(Wisent Bioproducts, Quebec, Canada), 100 U/mL penicillin (Wisent Bioproducts, Quebec, Canada), and 100 µg/mL streptomycin (Wisent Bioproducts, Quebec, Canada) in a 5% CO2 containing 37 °C humidified atmosphere. PBCCs were sorted by the BD FACS Calibur system (BD Biosciences, USA), according to the protocol previously mentioned by Malecki et al. [23]. Monolayers of the cells in flasks were subcultured on each 6th–8th day using trypsin. Five of these primary cultures were included in the assay and all experiments were repeated five times. The number of cells from the 3th–6th passages was calculated as 300,000–450,000 cells/mL. The cells were then subjected to proliferation assay and flow-cytometry or immediately frozen at –20 °C for quantitative real-time polymerase chain reaction (RT-PCR). All concentrations and incubation time intervals were selected from the earlier literature [24,25].
2.2. Br-dU proliferation assay
Analysis with“5-bromo-2’-deoxyuridine (BrdU) (Sigma-Aldrich, Schnelldorf, Germany)” was performed according to the manufacturer’s protocol.”HRP/AEC (ABC) Detection IHC Kit (Abcam, ab93705, USA)”was used for immunocytochemical staining. A mouse monoclonal BrdU Antibody”(Santa Cruz Biotechnology Inc., sc-20045)”was used as the primary antibody”(1:200, overnight).”Aminoethylcarbazole (AEC) (Invitrogen, Calsbad, USA)”was used as the chromogen. BrdU was measured via visual colorimetric staining. BrdU-labeled cells were evaluated by two analysts and the proliferation index was calculated via the proliferation count equation (positively stained cell number (viable)/total cells counted (N) (viable and nonviable cells) ( N > 100 )). The proliferation count equation was applied for all doses.
2.3. Quantitative real-time PCR (qRT-PCR)
The breast cancer cells were incubated in 0 mM and 25 mM metformin (Met), and then collected and washed with PBS. Total RNA isolation was performed with RNA isolation kit”(QIAamp® RNA Blood Mini kit, QIAGEN Pty Ltd, Victoria, Australia)” according to the manufacturer’s protocol instruction. The RNA quantity and quality were determined by a spectrophotometer “(Nanospectrophotometer P300, IMPLEN, Germany)” and 1 µg total RNA was subjected to reverse transcription-PCR using”Qiagen Quantitech Reverse Transcription Kit (QIAGEN Pty Ltd, Victoria, Australia), according to manufacturer’s protocol instruction. cDNAs were then subjected to quantitative real-time PCR analysis using”Qiagen Quantitect SYBR Green PCR Kit (QIAGEN Pty Ltd, Victoria, Australia)”and self-created primer sets for each gene on a real-time PCR device”(Rotor-Gene Q-Qiagen Pty Ltd, Victoria, Australia). The values of threshold cycles (Ct) for AMPKα1 , CCND1 , and Tp53 mRNA transcripts were calculated in relation to the expression of a house-keeping gene, beta-actin (ACTB). For the used primer sets please see Table 2.
Table 2.
Specific primer sets for quantitation analysis of AMPKα1, CCND1, and Tp53 genes (and ACTB as the house-keeping gene).
| Primer | Sequence | Spanning exons |
|---|---|---|
| AMPKα1_sense | tttgagtgctcagaagaggaa | Exon 7 |
| AMPKα1_antisense | gtgtttcagcaaccaagaatg | |
| CCND1_sense | cattgaacacttcctctccaa | Exon 3 |
| CCND1_ antisense | ggtcacacttgatcactctgg | Exon 4 |
| Tp53_sense | tctacaagcagtcacagcaca | Exon 5 |
| Tp53_ antisense | gtacagtcagagccaacctca | Exon 6-7 |
| ACTB_sense | atctggcaccacaccttcta | Exon 3 |
| ACTB_ antisense | agcctggatagcaacgtaca | Exon 4 |
2.4. Apoptosis assay
Apoptosis was measured using an Annexin V-FITC apoptosis detection kit I (BMS500FI-100, eBioscience, MA USA) and analyzed by flow-cytometry. Stained cells were acquired by using MacsQuant Analyzer 10 and obtained data were analyzed by using MacsQuantity Version 2.8 flow-cytometry screening system. Briefly, primary PBCCs were treated with different concentrations of metformin”(0 mM and 25 mM)” for 24 h and the flow-cytometry procedures according to the manufacturer’s kit. The apoptotic index was calculated by means of all five repeated experiments and given as a percentage.
2.5. Statistical analysis
Experiments repeated three times were statistically analyzed using GraphPad InStat Software (Version 3.06). Results are presented as means ± standard error mean (SEM) and compared by unpaired t-test with Welch correction. P < 0.05 was accepted as statistically significant.
3. Results
3.1. Antiproliferative effects of metformin in breast cancer cells
We determined the proliferation of PBCCs treated with metformin at a concentration of “0, 5, and 10, and 25 mM” for 24 h (Figures 1a–1d). Compared to the untreated (0 mM Met) control cells, metformin inhibited the proliferation of PBCCs in a dose-dependent manner. Statistically, significant inhibition was observed at the 25 mM Met concentration compared to the 0 mM dose, although there was no conformational change in the adherence of the cells upon incubation with any of the doses (P < 0.001) (Figure 1e; Table 3). The metformin concentrations applied in this study are comparatively high, but to concentrate on the anticancer effects of metformin on PBCCs, 25 mM Met was used in the following experiments. All experiments were performed four times in quadruplicate.
Figure 1.
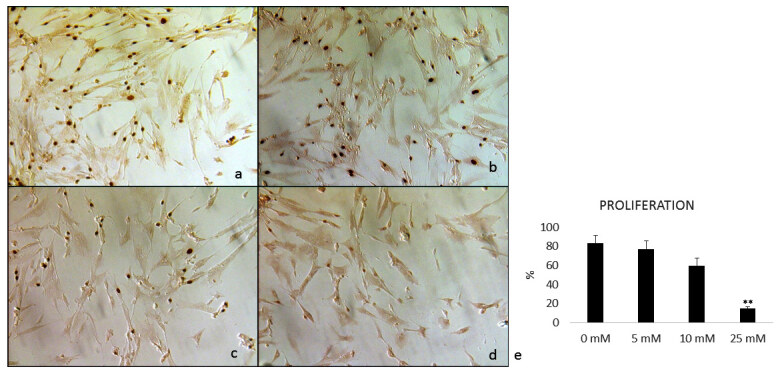
Immunocytochemical proliferation analysis (BrdU) of primary breast cancer cells treated with four concentrations of metformin: a) 0 mM metformin, b) 5 mM metformin, c) 10 mM metformin, d) 25 mM metformin, e) The graph of proliferation indexes, ***P < 0.0001 vs all groups. The number of breast cancer cells significantly decreases in the presence of increasing metformin concentration in a dose-dependent manner.
Table 3.
BrdU proliferation indexes of breast cancer cells.%0 mM Met5 mM Met10 mM Met25 mM MetMEAN ± SEM83.48 ± 8.2777.48 ± 8.5859.50 ± 8.3615.30 ± 1.89*
| % | 0 mM Met | 5 mM Met | 10 mM Met | 25 mM Met |
|---|---|---|---|---|
| MEAN ± SEM | 83.48 ± 8.27 | 77.48 ± 8.58 | 59.50 ± 8.36 | 15.30 ± 1.89* |
Met: metformin; SEM: standard error mean; *P < 0.001
3.2. Effect of metformin on AMPKα1 expression in breast cancer cultures
The expression of the AMPKα1 gene in the breast cancer cells was examined by qRT-PCR before and after 24-h treatment with metformin. Although the AMPKα1 expression reduced in the group treated with 25 mM, the change in the expression was not statistically significant (Figure 2), which suggests that the high metformin concentration used in this study did not show either inhibitory or stimulatory effect on AMPKα expression compared with the control group (P > 0.05).
Figure 2.
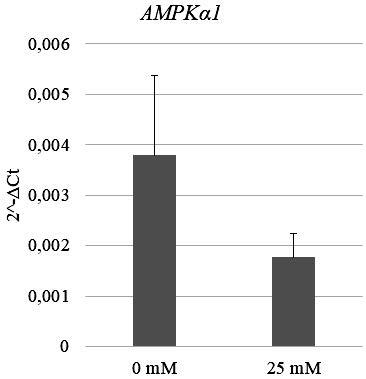
AMPKα1 expression profile of primary breast cancer cells treated with two different concentrations (0 mM and 25 mM) of metformin. The decrease in AMPKα1 expression at 25 mM metformin concentration is not statistically significant in breast cancer cells (P > 0.05).
3.3. Effects of metformin on cyclin D1 gene (CCND1) expression in breast cancer cultures
The expression of the CCND1 was also detected before and after 24-h treatment of metformin in breast cancer cells, by performing qRT-PCR analysis. According to the results, the CCND1 expression reduced in the group treated with 25 mM Met compared to the nontreated group but the decrease was not statistically significant (P = 0.64) (Figure 3).
Figure 3.
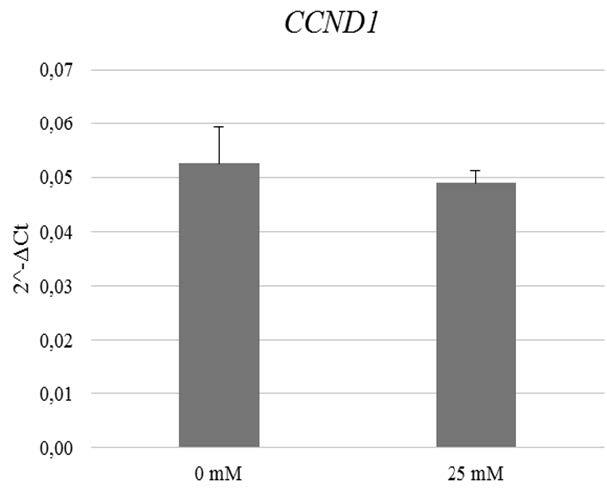
CCND1 expression profile of primary breast cancer cells treated with two different concentrations (0 mM and 25 mM) of metformin. CCND1 expression decreases at 25 mM metformin concentration but the decrease is not statistically significant (P > 0.05).
3.4. Effects of metformin on Tp53 gene expression in breast cancer cultures
To quantify the expression of the Tp53 gene in PBCCs, qRT-PCR was performed. The data revealed that the expression of Tp53 was significantly elevated by 25 mM Met application compared with 0 mM Met (P < 0.05) (Figure 4), which suggests that metformin selectively has an impact on the expression of the Tp53 gene.
Figure 4.
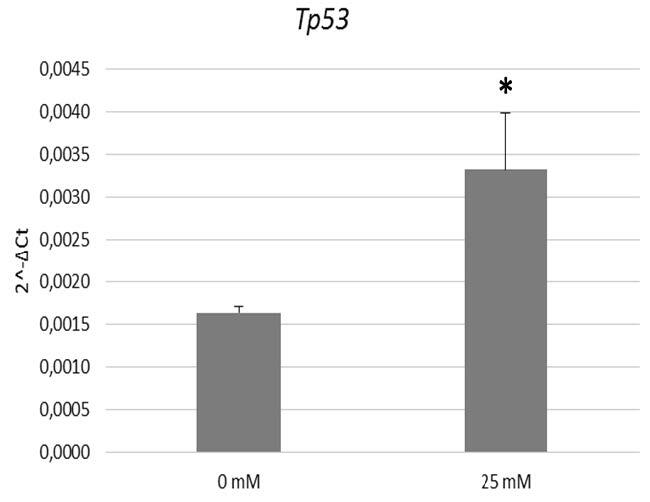
Tp53 expression profile of primary breast cancer cells treated with two different concentrations (0 mM and 25 mM) of metformin (P < 0.05). Tp53 expression is elevated at 25 mM metformin concentration in breast cancer cells.
3.5. Effects of metformin on the apoptosis levels of primary breast cancer cells
Figure 5 presents the results for the apoptotic index of PBCCs. To check the high metformin-induced apoptosis, breast cancer cells were treated with two concentrations of metformin for 24 h and followed by annexin V–FITC staining. The high concentration of metformin reduced the number of annexin V positive apoptotic cells, and the increase in the apoptotic index was statistically significant compared with 0 mM metformin-treated cells (P < 0.05).
Figure 5.
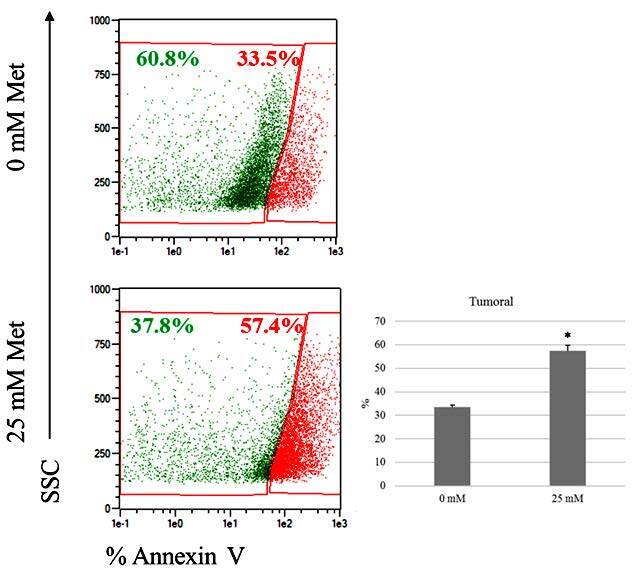
Apoptotic index of primary breast cancer cells treated with two different concentrations (0 mM and 25 mM) of metformin. Apoptosis tendency increased in the presence of 25 mM metformin treatment (P = 0.03).
4. Discussion
Nowadays, metformin, as an antitumor agent, gained momentum, leaving metformin-based cancer therapies as an afterthought. Although no conclusive data is present about the function of metformin in both cancer therapy and prevention either in diabetic or in nondiabetic populations, the possible effects are observed indirectly on systemic levels of glucose or insulin, or directly on tumor cell growth via inhibition of cell proliferation and survival via induction of cell death [10,26–30]. Metformin seems to be involved in cancer cell inhibition through AMPK and cyclin D1 downregulation leading to cell cycle arrest and apoptotic pathways activation [9,15,27,31]. AMPK appears to be the hallmark of metformin metabolism in cancer cells. However, the literature has a large controversy in AMPK pathway involvement in cancer progression; until now, no AMPK activation was observed in recent reports of lung, prostate, breast, and colorectal cancer [32]. Conversely, in the current research, we could not find any clues which support the idea that metformin suppresses the proliferation of PBCCs via upregulating AMPKα expression.
Moreover, cyclin D1, a requirement for G1/S transition of the cell cycle [33] and an indirect cell cycle player, p53 [34], was reported to be essential specifically in embryonic fibroblasts of the mouse for AMPK-induced cell cycle arrest [35]. Metformin was reported to stimulate G0/G1 cell cycle arrest, regulated through oxidative stress as well as AMPK activation [36]. In cell cycle analysis studies, S phase cell cycle accumulation with apoptosis enhancement was displayed in triple-negative breast cancer (TNBC) [26] and a pancreatic cancer cell line [37], whereas G1 phase cell cycle accumulation without any apoptosis enhancement was observed in the non-TNBC [10] and an ovarian cancer cell line [38]. Besides, dose-dependent inhibition of proliferation via a reduction in cyclin D1 levels arresting in the G0/G1 stage was observed in gastric [39] and prostate cancer cells [9]. In the present study, we found that cyclin D1 expression of PBCCS is decreased at 25 mM metformin concentration. There is a growing body of evidence suggesting that metformin is able to initiate p53 by both“AMPK-dependent”[8,14] and -independent [17,40] mechanisms to stimulate apoptosis and cell cycle arrest and hence might be essential for cancer therapy. Most importantly, not only the upregulation of AMPKα and p53 but also the downregulation of cyclin D1 are involved in the antitumor action of metformin in vivo. We observed that p53 expression of PBCCS is increased at 25 mM metformin concentration. Thus, all in all, we suggest that metformin induces cell-cycle arrest in PBCCs by downregulating cyclin D1 expression and upregulation of p53 expression through the AMPK-independent pathway.
Nonetheless, in addition to AMPK activation, metformin also induces apoptosis in breast cancer cells independently of AMPK [16]. In the current study, we found that apoptosis of PBCCS is increased at 25 mM metformin concentration correlated with p53 expression and cyclin D1 downregulation. The apparent discrepancy of the presence of AMPK activation in a trace amount of cancers in the presence of metformin uptake may be due to the dissimilarity in used medium, incubation period, and pharmacologic doses. The majority of the studies reviewed utilized DMEM (including 24 mM glucose) or DMEM/F12 (including 17.5 mM glucose) as the culture medium, and the normal plasma glucose concentration was below 7 mM. In this respect, the cytotoxic effect of metformin is proved to be potent in low-glucose media by Zhuang et al., proposing that the requirement of metformin at high concentrations in in vitro experiments is because of the masking effect glucose presents; hence, the data reported in these researches are not invalidated [41].
In conclusion, both the inhibition of tumor growth and induction of apoptosis through metformin treatment in breast cancer cells require p53 and cyclin D1 involvement (Figure 6). The current study has some limitations, albeit the promising results. First of all, the expression levels of mRNAs were not confirmed at the protein level. Secondly, the kinase activity of AMPK-a was not determined. Thus, the mechanisms on how metformin displays its inhibitory effects on cancer development and tumor growth are not fully figured out. A couple of milestones yet need to be passed according to this novel antitumor molecular approach. Firstly, the applicability of nondiabetic subjects for the anticancer effect of metformin and the appropriate safety dose should be illuminated. Secondly, in the neoplastic tissue, the adequate drug concentration of metformin should be determined, and thirdly, the benefit of metformin as a clinical marker should be defined in the future.
Figure 6.
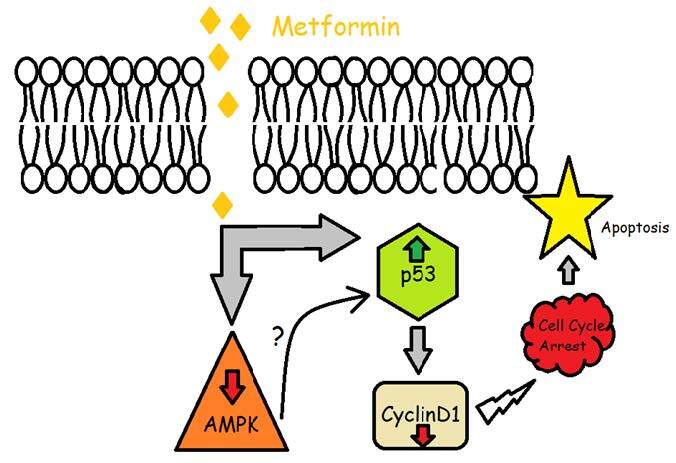
The overall process of the mechanism of metformin on cell cycle arrest and apoptosis. Metformin may regulate apoptosis via two different pathways. Firstly, metformin may decrease AMPK-alpha expression which then indirectly stimulates the cell cycle arrest (AMPK-dependent way). Secondly, metformin may trigger cell cycle arrest in primary breast cancer cells by upregulation of P53 and downregulation of cyclin D1 and which eventually results in cell cycle arrest (AMPK-independent way).
References
- Inzucchi SE Maggs DG Spollett GR Page SL Rife FS Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. The New England Journal of Medicine . 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- Musi N Hirshman MF Nygren J Svanfeldt M Bavenholm P Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes . 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Stumvoll M Nurjhan N Perriello G Dailey G Gerich JE Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. The New England Journal of Medicine . 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Metformin activates amp kinase through. [DOI] [PMC free article] [PubMed]
- inhibition of amp deaminase. Journal of Biological Chemistry . 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G Myers R Li Y Chen Y Shen X Role of amp-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation . 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y Lin B Wu J Zhang H Wu B Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncology Letters . 2014;8:1269–1274. doi: 10.3892/ol.2014.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashinuma H Takiguchi Y Kitazono S Kitazono-Saitoh M Kitamura A Antiproliferative action of metformin in human lung cancer cell lines. Oncology Reports . 2012;28:8–14. doi: 10.3892/or.2012.1763. [DOI] [PubMed] [Google Scholar]
- Storozhuk Y Hopmans SN Sanli T Barron C Tsiani E Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. British Journal of Cancer . 2013;108:2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I Laurent K Loubat A Giorgetti-Peraldi S Colosetti P The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene . 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- Alimova IN Liu B Fan Z Edgerton SM Dillon T Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle . 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- Zhang T Guo P Zhang Y Xiong H Yu X The antidiabetic drug metformin inhibits the proliferation of bladder cancer cells in vitro and in vivo. International Journal of Molecular Sciences . 2013;14:24603–24618. doi: 10.3390/ijms141224603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maghrabi J Al-Sakkaf K Qureshi IA Butt NS Damnhory L AMPK expression patterns are significantly associated with poor prognosis in breast cancer patients. Annals of Diagnostic Pathology . 2017;29:62–67. doi: 10.1016/j.anndiagpath.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Hadad SM Hardie DG Appleyard V Thompson AM Effects of metformin on breast cancer cell proliferation, the AMPK pathway and the cell cycle. Clinical and Translational Oncology . 2014;16:746–752. doi: 10.1007/s12094-013-1144-8. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I Laurent K Giuliano S Larbret F Ponzio G Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Research . 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- Zhuang Y Miskimins WK Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. Journal of Molecular Signaling . 2008;3:18–18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki A Youssef A Antidiabetic drug metformin induces apoptosis in human MCF breast cancer via targeting ERK signaling. Oncology Research . 2011;19:275–85. doi: 10.3727/096504011x13021877989838. [DOI] [PubMed] [Google Scholar]
- Janjetovic K Harhaji-Trajkovic L Misirkic-Marjanovic M Vucicevic L Stevanovic D In vitro and in vivo anti-melanoma action of metformin. European Journal of Pharmacology . 2011;668:373–382. doi: 10.1016/j.ejphar.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Nelson LE Valentine RJ Cacicedo JM Gauthier MS Ido Y A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. American Journal of Physiology-Cell Physiology . 2012;303:C4–13. doi: 10.1152/ajpcell.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M Tichet M Abbe P Ohanna M Lehraiki A Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Molecular Cancer Therapeutics . 2013;12:1605–15. doi: 10.1158/1535-7163.MCT-12-1226-T. [DOI] [PubMed] [Google Scholar]
- Yi G He Z Zhou X Xian L Yuan T Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. International Journal of Oncology . 2013;43:1503–1510. doi: 10.3892/ijo.2013.2077. [DOI] [PubMed] [Google Scholar]
- Gerl R Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis . 2005;26:263–270. doi: 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM Witzig TE Adjei AA Targeting apoptosis pathways in cancer therapy. CA: A Cancer Journal for Clinicians . 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- Malecki M Sabo C Foorohar A Tombokan X Novel paradigm for immunotherapy of breast cancer by engaging prophylactic immunity against hepatitis B. Clinical and Translational Medicine . 2016;5:32–32. doi: 10.1186/s40169-016-0111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S Samuel SM Varghese E Kubatka P Büsselberg D. High glucose represses the anti-proliferative and pro-apoptotic effect of metformin in triple negative breast cancer cells. Biomolecules . 2019;9:16–16. doi: 10.3390/biom9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena P Mancini S Benincasa M Mariani F Palumbo C Metformin induces apoptosis and alters cellular responses to oxidative stress in Ht29 colon cancer cells: preliminary findings. International Journal of Molecular Sciences . 2018;19:1478–1478. doi: 10.3390/ijms19051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B Fan Z Edgerton SM Deng XS Alimova IN Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle . 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- Kisfalvi K Eibl G Sinnett-Smith J Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Research . 2009;69:6539–6545. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ Pritchard KI Ennis M Clemons M Graham M Insulin-lowering effects of metformin in women with early breast cancer. Clinical Breast Cancer . 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- Isakovic A Harhaji L Stevanovic D Markovic Z Sumarac-Dumanovic M Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cellular and Molecular Life Sciences . 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S Furuno A Sakurai J Sakamoto A Park HR Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Research . 2009;69:4225–4234. doi: 10.1158/0008-5472.CAN-08-2689. [DOI] [PubMed] [Google Scholar]
- Kefas BA Cai Y Kerckhofs K Ling Z Martens G Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochemical Pharmacology . 2004;68:409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Yang YC Chien MH Liu HY Chang YC Chen CK Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer letters . 2018;421:28–40. doi: 10.1016/j.canlet.2018.01.075. [DOI] [PubMed] [Google Scholar]
- Baldin V Lukas J Marcote MJ Pagano M Draetta G Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes & Development . 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Vermeulen K Berneman ZN Bockstaele DRVn. Cell Proliferation . 2003;36:165–175. doi: 10.1046/j.1365-2184.2003.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG Plas DR Kubek S Buzzai M Mu J AMP-activated protein kinase induces a p53-Dependent metabolic checkpoint. Molecular Cell . 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Queiroz EA Puukila S Eichler R Sampaio SC Forsyth HL Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One . 2014;9 doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW Li ZS Zou DW Jin ZD Gao J Xu GM Metformin induces apoptosis of pancreatic cancer cells. World Journal of Gastroenterology . 2008;14:7192–7198. doi: 10.3748/wjg.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan R Giri S Hartmann LC Shridhar V Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. Journal of Cellular and Molecular Medicine . 2011;15:166–178. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K Gong J Iwama H Kitanaka A Tani J The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Molecular Cancer Therapeutics . 2012;11:549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I Regazzetti C Robert G Laurent K Le Marchand-Brustel Y Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Research . 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- Zhuang Y Chan DK Haugrud AB Miskimins WK Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PloS One . 2014;10 doi: 10.1371/journal.pone.0108444. [DOI] [PMC free article] [PubMed] [Google Scholar]


