Abstract
OBJECTIVES
Neurodevelopmental injury after cardiac surgery using cardiopulmonary bypass (CPB) for congenital heart defects is common, but the mechanism behind this injury is unclear. This study examines the impact of CPB on cerebral mitochondrial reactive oxygen species (ROS) generation and mitochondrial bioenergetics.
METHODS
Twenty-three piglets (mean weight 4.2 ± 0.5 kg) were placed on CPB for either 1, 2, 3 or 4 h (n = 5 per group) or underwent anaesthesia without CPB (sham, n = 3). Microdialysis was used to measure metabolic markers of ischaemia. At the conclusion of CPB or 4 h of sham, brain tissue was harvested. Utilizing high-resolution respirometry, with simultaneous fluorometric analysis, mitochondrial respiration and ROS were measured.
RESULTS
There were no significant differences in markers of ischaemia between sham and experimental groups. Sham animals had significantly higher mitochondrial respiration than experimental animals, including maximal oxidative phosphorylation capacity of complex I (OXPHOSCI) (3.25 ± 0.18 vs 4-h CPB: 1.68 ± 0.10, P < 0.001) and maximal phosphorylating respiration capacity via convergent input through complexes I and II (OXPHOSCI+CII) (7.40 ± 0.24 vs 4-h CPB: 3.91 ± 0.20, P < 0.0001). At 4-h, experimental animals had significantly higher ROS related to non-phosphorylating respiration through complexes I and II (ETSCI+CII) than shams (1.08 ± 0.13 vs 0.64 ± 0.04, P = 0.026).
CONCLUSIONS
Even in the absence of local markers of ischaemia, CPB is associated with decreased mitochondrial respiration relative to shams irrespective of duration. Exposure to 4 h of CPB resulted in a significant increase in cerebral mitochondrial ROS formation compared to shorter durations. Further study is needed to improve the understanding of cerebral mitochondrial health and its effects on the pathophysiology of neurological injury following exposure to CPB.
Keywords: Cardiopulmonary bypass, Mitochondria, Congenital heart disease, Reactive oxygen species
INTRODUCTION
Advances in neonatal cardiac surgical techniques and perioperative management have led to decreased mortality. Despite the survival improvement, abnormal neurodevelopment remains a common long-term adverse outcome in children with complex congenital cardiac abnormalities [1]. The mechanisms of neurological injury in neonatal patients undergoing cardiac surgery are multifactorial and incompletely understood [2–5]. Mitochondrial injury has increasingly been implicated as the critical point of cellular injury in models of critical illness including cardiac arrest and traumatic brain injury [6–10]. Furthermore, increasing levels of mitochondrial dysfunction have been correlated with worse neurological dysfunction [6, 10].
The use of cardiopulmonary bypass (CPB) is a risk factor for neurological injury in children undergoing heart surgery [11, 12], and longer CPB durations are associated with increased complications and hospital mortality [13, 14]. There are a number of recognized, albeit incompletely understood, pathways for neurological injury in patients undergoing CPB including thrombo-emboli, hypoxia–ischaemia and inflammation [2–5]. Furthermore, there are several theories for why increasing exposure time to cardiopulmonary bypass leads to injury, including the non-pulsatile nature of circulation, the exposure to immunogenic surfaces in the perfusion circuit and the potential for the disruption of normal autoregulation that maintains adequate perfusion to vital organs [15–17].
Our previous experiments have demonstrated that animals exposed to deep hypothermic circulatory arrest had greater mitochondrial injury than did those exposed to continuous CPB [18] but did not address if CPB alone results in decreased mitochondrial function or increased ROS generation. Thus, the goal of the current study is to determine how CPB, without circulatory arrest or deep hypothermia, affects cerebral mitochondrial bioenergetics and to characterize how that injury may change with increased CPB duration. We hypothesized that animals exposed to prolonged periods of CPB would exhibit significant damage to the mitochondrial electron transport system (ETS) and would have increased production of mitochondrial ROS compared to animals that did not undergo CPB.
MATERIALS AND METHODS
Animals and study design
All procedures were approved by our Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Five- to seven-day-old female piglets (n = 23) were premedicated with 30 mg/kg ketamine, followed by inhaled isoflurane in 100% oxygen via a snout mask. The piglets were intubated and oxygen was weaned to room air. Animals were randomized to either sham (n = 3) without cannulation or CPB, or 1 h (n = 5), 2 h (n = 5), 3 h (n = 5) or 4 h (n = 5) of CPB.
Perioperative care and monitoring
The following ventilator settings were used: tidal volume 10 ml/kg, positive end-expiratory pressure 5 cmH2O and minute ventilation titrated to end-tidal carbon dioxide 35–45 mmHg. The right femoral artery and vein were cannulated for arterial pressure monitoring and central venous access. Intravenous infusions of fentanyl (25–200 µg/kg/min) and dexmedetomidine (0.5–2 µg/kg/min) were initiated and titrated. Isoflurane was weaned to ∼0.5%.
Operative methods and experimental protocol
A heparin bolus of 4000 IU was given and CPB was initiated once the activated coagulation time reached >480 s. Cervical cannulation for CPB was performed using an 8-Fr arterial cannula placed into the right carotid artery and a 12-Fr venous cannula placed into the right external jugular vein. The CPB circuit and priming methods used in this experiment have been described previously [18]. Target CPB flow rates were 150 ml/kg/min, which represents full flow in these animals with complete right heart decompression as measured in our prior experiments with central cannulation and direct right atrial pressure monitoring [18]. The following was maintained: carbon dioxide levels within 35–45 mmHg, mean arterial pressure >35 mmHg, activated coagulation time >480 s and partial pressure of arterial oxygen >250 mmHg. Additional donor blood was added to the circuit when needed to achieve target haematocrit >28%. The target pulse pressure was <10 mmHg while on CPB. Subjects were cooled to 34°C at a rate no greater than 1°C/min. Consistent with clinical practice at our institution, alpha-stat was used as piglets were at mild hypothermia. Ten minutes prior to conclusion of the experiment, the animals were rewarmed to 37°C at a rate no greater than 1°C/min. Sham animals did not undergo cannulation or CPB but did undergo 4 h of anaesthesia. Sham animals were cooled to a target of 34°C using a cooling blanket.
Measurement of cerebral microdialysis
For all animals, cerebral microdialysis probes were inserted in the right frontal cortex (CMA 71 Elite, mDialysis, Sweden). Probes were placed 0.5-cm deep in the brain parenchyma, at the grey–white junction. Sterile saline was perfused at 1 µl/min, and after a 30-min calibration period, samples were collected in 20–30 min time intervals. Samples were immediately frozen at −80°C. Pyruvate, lactate, glycerol and glucose levels were analysed in a blinded fashion using the automated ISCUS Flex™ Microdialysis Analyzer (mDialysis) and data were processed using the LABpilot software (mDialysis).
Tissue extraction and preparation
At the conclusion of the assigned period of CPB or 4-h of sham, craniectomy was performed to expose the brain while perfusion was maintained. Per protocol, there was no attempt to wean animals from CPB. Our tissue preparation method has been previously described [6, 9]. Cortical tissue sections were rapidly extracted and placed in ice-cold buffer (320 mM sucrose, 10 mM Trizma base and 2 mM ethylene glycol tetraacetic acid). Blood and vasculature was dissected and 1 mg of wet weight tissue was gently homogenized on ice (MiR05 buffer: 110 mM sucrose, 0.5 mM EGTA, 3.0 mM MgCl2, 60 mM K-lactobinoate, 10 mM KH2PO4, 20 mM taurine, 20 mM HEPES and 1.0 g/1 fatty acid-free BSA) using a 5-ml Potter-Elvehjem Teflon-glass homogenizer to a concentration of 1 mg wet weight tissue/10 µl miR05 buffer. Only the left hemisphere, opposite from cervical cannulation, was used for the mitochondrial and ROS analysis as the right side had been instrumented.
Measurement of mitochondrial respiration
Citrate synthase (CS) was measured as a marker of mitochondrial content and used in addition to tissue weight for the normalization of brain tissue as previously described [19]. Samples following high-resolution respirometry measurements were frozen for subsequent CS activity measurements using a commercially available assay (CS0720; Sigma-Aldrich, St. Louis, MO, USA).
Mitochondrial respiration was analysed ex vivo in brain cortex homogenates using high-resolution respirometry (Oxygraph-2k; Oroboros Instruments, Innsbrunk, Austria) using a substrate–uncoupler–inhibitor titration protocol as described previously [6, 8, 9]. The substrate–uncoupler–inhibitor titration protocol provides the assessment of respiratory capacities of the individual components of the ETS using the sequential addition of specific substrates and inhibitors. Sequential additions included the addition of malate (5 mM) and pyruvate (5 mM), followed by adenosine diphosphate (ADP) (1 mM) and glutamate, measuring oxidative phosphorylation capacity of complex I (OXPHOSCI), driven by the nicotinamide adenine dinucleotide phosphate (NADP)-related substrates. Succinate (10 mM) was added to stimulate maximal phosphorylating respiration capacity via convergent input through complexes I and II (OXPHOSCI+CII).
Oxidative phosphorylation produces adenosine triphosphate (ATP), the primary fuel for basic cellular functions. During times of stress and injury when energy requirements are highest, increased maximal oxidative phosphorylation is necessary for neuronal salvage and repair. Oligomycin, an inhibitor of ATP synthase, induces uncoupled respiration without ATP-synthase activity (LEAK) respiration (LEAKCI+CII). If LEAK respiration is increased, the electrochemical gradient across the mitochondrial membrane is uncoupled, resulting in the production of sufficient ATP. Maximal convergent non-phosphorylating respiration of the ETS (ETSCI+CII), a marker of mitochondrial reserve and maximal output, is evaluated by titrating the protonophore, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone until no further increase in respiration was detected.
Measurement of non-phosphorylating respiration through complex II (ETSCII) was achieved through the addition of rotenone (2 mM). Antimycin-A (1 µg/ml), a complex III inhibitor, was added to measure the residual oxygen consumption, and this value was subtracted from each of the previously measured respiratory states. Complex IV activity was measured by the addition of N,N,N′,N′-tetramethyl-phenylenediamine (0.5 mM) and ascorbate (0.8 mM). The complex IV inhibitor sodium azide (10 mM) was added to reveal the chemical background that was subtracted from the N,N,N′,N′-tetramethyl-phenylenediamine-induced oxygen consumption rate.
Measurement of reactive oxygen species
Mitochondrial ROS generation is the predominate source of ROS, leads to alterations in redox signalling, oxidative damage to proteins and lipids and additional mitochondrial dysfunction and is ultimately a major cause of secondary brain injury. Cerebral ROS generation was measured using the O2k-Fluorescence LED2-Module (Oxygraph-2k). Simultaneous measurement of hydrogen peroxide (H2O2) production and mitochondrial respiration using ex vivo in brain cortex homogenates was accomplished using an Amplex UtraRed assay as previously described [20]. Amplex UtraRed (N-acetyl-3,7 dihydroxyphenoxazine) (5 mM), in the presence of horseradish peroxidase(1 U/ml), reacts with H2O2 to produce the fluorescent compound resorufin. The addition of superoxide dismutase (10 U/ml) ensures that all superoxide is converted into H2O2. Calibration of the fluorometric signal was done prior to each measurement by the addition of 100 nM H2O2. ROS was normalized to mitochondrial respiration.
Statistics and data analysis
Statistical analysis was carried out using GraphPad Prism (GraphPad, San Diego, CA, USA). Descriptive statistics were used to characterize baseline variables. A skewness–kurtosis test was performed to assess the normality of continuous variables. Differences between groups were compared with Student’s t-test or analysis of variance (ANOVA) for parametric tests and Kruskall–Wallis with Dunn’s multiple comparison tests for non-parametric and expressed as mean ± standard error of mean (SEM), except where noted. Differences were considered statistically significant if P < 0.05. This study was designed as an exploratory study and as such no correction for multiple testing was performed.
RESULTS
Baseline and blood gas chemistry variables
Sham animals were slightly smaller than those in the 2-, 3- and 4-h groups [3.7 ± 0.2 vs 4.6 ± 0.6 kg (P = 0.043), 4.4 ± 0.4 kg (P = 0.039) and 4.3 ± 0.3 kg (P = 0.025), respectively]. There were no baseline differences in haematocrit, pH, PaO2, PaCO2 or lactate between any of the groups. At the conclusion of the experiment, shams had significantly lower haematocrit, PaO2 and lactate than all experimental groups (P < 0.04, P < 0.05 and P < 0.02, respectively) (Table 1).
Table 1:
Blood gas variables at conclusion of experiment
| Sham (n = 3) | 1 h (n = 5) | 2 h (n = 5) | 3 h (n = 5) | 4-h (n = 5) | |
|---|---|---|---|---|---|
| Haematocrit (%) | 23.0 (2.6) | 35.4 (5.9)* | 32.8 (5.3)* | 31.4 (3.1)* | 31.2 (4.5)* |
| pH | 7.4 (0.07) | 7.42 (0.09) | 7.44 (0.08) | 7.47 (0.03) | 7.45 (0.10) |
| PaO2 (mmHg) | 81.7 (5.1) | 165.0 (107.2)* | 250.6 (34.8)* | 250.8 (17.3)* | 268.0 (14.8)* |
| PaCO2 (mmHg) | 48.7 (6.8) | 43.8 (7.3) | 43.0 (3.9) | 45.8 (5.8) | 40.0 (5.8) |
| Lactate | 0.6 (0.1) | 2.5 (0.5)* | 2.9 (0.9)* | 2.7 (1.1)* | 2.8 (1.6)* |
Values are represented in mean (SD).
P < 0.05 relative to sham.
PaCO2: partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen.
Microdialysis
There were no statistically significant differences between the sham group and the experimental groups for extracellular lactate/pyruvate ratio, glucose or glycerol at any of the hourly time points (P > 0.1 for all) (Table 2). The lack of significant changes over the study period indicates adequate cerebral perfusion and drainage.
Table 2:
Cerebral microdialysis
| 1 h (experimental n = 16, sham n = 3) | 2 h (experimental n = 13, sham n = 3) | 3 h (experimental n = 7, sham n = 3) | 4 h (experimental n = 4, sham n = 2) | |
|---|---|---|---|---|
| Lactate/pyruvate ratio | ||||
| CPB | 71.06 ± 8.06 | 77.98 ± 8.94 | 60.76 ± 6.42 | 58.54 ± 11.84 |
| Sham | 88.09 ± 31.68 | 90.43 ± 24.18 | 86.53 ± 28.12 | 87.22 ± 38.64 |
| Glucose (ml/dl) | ||||
| CPB | 12.25 ± 2.59 | 12.14 ± 2.87 | 10.41 ± 3.11 | 16.66 ± 3.88 |
| Sham | 21.02 ± 7.94 | 17.03 ± 9.27 | 20.07 ± 5.92 | 26.99 ± 10.09 |
| Glycerol (µM) | ||||
| CPB | 19.32 ± 2.60 | 19.75 ± 2.75 | 22.05 ± 6.93 | 29.96 ± 9.47 |
| Sham | 20.24 ± 2.89 | 11.63 ± 1.75 | 16.03 ± 0.53 | 14.93 ± 3.52 |
Values are represented in mean ± SEM. Group totals may not equal animal number as microdialysis was not successfully collected for each animal. There were no statistically significant differences between experimental groups and sham (P < 0.05).
CPB: cardiopulmonary bypass.
Cerebral mitochondrial respiration
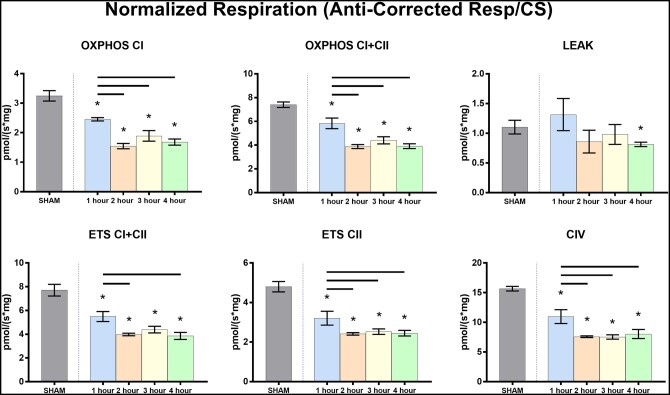
There was a significant increase in CS activity relative to shams for each experimental group and between the 1-h group and the 2-, 3- and 4-h groups (Fig. 1). A summary of the mitochondrial respiration results is found in Table 3 and represented graphically in Fig. 2. The OXPHOSCI and OXPHOSCI+CII were significantly higher in the sham group relative to all 4 CPB groups (versus 1 h: P = 0.054 and 0.025, respectively, for all others P < 0.001). The sham group had significantly higher mitochondrial LEAKCI+CII than 4-h group (P = 0.021). ETSCII, ETSCI+CII and complex IV activity were significantly lower in the CPB groups than in the sham group (versus 1-h group P = 0.003, P = 0.001 and P = 0.005, respectively, versus all others P < 0.001). These data demonstrate preserved mitochondrial function in the sham animals compared to any duration of CPB.
Figure 1:

Citrate synthase activity by cardiopulmonary bypass duration.
Table 3:
Mitochondrial respiration (pmol O2/s mg) normalized to citrate synthase activity (µmol/ml/min)
| Sham (n = 3) | 1 h (n = 5) | 2 h (n = 5) | 3 h (n = 5) | 4 h (n = 5) | |
|---|---|---|---|---|---|
| CS activity | 9.46 ± 0.11 | 12.6 ± 0.87* | 16.0 ± 1.66*,a | 17.7 ± 3.10*,a | 15.9 ± 1.52*,a |
| OXPHOSCI | 3.25 ± 0.18 | 2.45 ± 0.06* | 1.55 ± 0.09*,a | 1.89 ± 0.18*,a | 1.68 ± 0.10*,a |
| OXPHOSCI+CII | 7.40 ± 0.24 | 5.83 ± 0.44* | 3.88 ± 0.16*,a | 4.40 ± 0.30*,a | 3.91 ± 0.20*,a |
| LEAKCI+CII | 1.10 ± 0.12 | 1.31 ± 0.27 | 0.86 ± 0.19 | 0.98 ± 0.17 | 0.81 ± 0.04* |
| ETSCI+CII | 7.71 ± 0.49 | 5.49 ± 0.42* | 3.98 ± 0.11*,a | 4.39 ± 0.28* | 3.86 ± 0.29*,a |
| ETSCII | 4.80 ± 0.26 | 3.21 ± 0.35* | 2.41 ± 0.06*,a | 2.53 ± 0.14*,a | 2.45 ± 0.14*,a |
| CIV | 15.67 ± 0.38 | 10.97 ± 1.17* | 7.58 ± 0.14*,a | 7.54 ± 0.36*,a | 8.05 ± 0.77*,a |
Values are represented in mean ± SEM.
P < 0.05 relative to sham.
P < 0.05 relative to 1 h.
CS: citrate synthase; CIV: complex IV activity; ETS: electron transport system.
Figure 2:
Mitochondrial respiration by cardiopulmonary bypass duration normalized to citrate synthase.
Cerebral mitochondrial reactive oxygen species
A summary of the mitochondrial ROS results is found in Table 4 and Fig. 3. Experimental animals who had undergone 4 h of CPB had significantly higher rates of generated ROS than animals who underwent 4 h of sham procedure at both ETSCI+CII and ETSCII (P = 0.005 and P = 0.009, respectively). There was a trend towards an initial increase in ROS, followed by a decrease at 2 h and finally a trend towards increasing ROS with increasing duration of CPB for ROS measured at all points in the ETS as illustrated in Fig. 3.
Table 4:
Mitochondrial reactive oxygen species [H2O2/(pmol O2/s mg)] normalized to mitochondrial respiration
| Sham (n = 3) | 1 h (n = 5) | 2 h (n = 5) | 3 h (n = 5) | 4 h (n = 5) | |
|---|---|---|---|---|---|
| OXPHOSCI | 1.04 ± 0.23 | 0.81 ± 0.04 | 0.67 ± 0.11 | 0.79 ± 0.09 | 1.10 ± 0.10a,b,c |
| OXPHOSCI+CII | 0.42 ± 0.05 | 0.36 ± 0.03 | 0.28 ± 0.02* | 0.35 ± 0.04 | 0.49 ± 0.05a,b,c |
| LEAKCI+CII | 5.17 ± 0.80 | 3.95 ± 0.69 | 3.96 ± 0.86 | 4.57 ± 1.00 | 3.78 ± 0.70 |
| ETSCI+CII | 0.64 ± 0.04 | 0.89 ± 0.23 | 0.51 ± 0.07a | 0.74 ± 0.10b | 1.08 ± 0.13*,b,c |
| ETSCII | 0.61 ± 0.03 | 0.81 ± 0.12 | 0.55 ± 0.03a | 0.75 ± 0.09b | 0.95 ± 0.06*,b |
Values are represented in mean ± SEM.
P < 0.05 relative to sham.
P < 0.05 relative to 1 h.
P < 0.05 relative to 2 h.
P < 0.05 relative to 3 h.
ETS: electron transport system.
Figure 3:
Reactive oxygen species generation by cardiopulmonary bypass duration normalized to mitochondrial respiration.
DISCUSSION
In this study, animals exposed to CPB had significant alterations in mitochondrial bioenergetics relative to sham animals. For all durations of CPB, there was decreased respiration at complexes I, II and IV. Our microdialysis results showed no evidence of decreased cerebral perfusion or oxygenation across any of the animal groups, which indicates that mitochondrial disruption occurs irrespective of these., This finding motivates continued examination of alternative mechanisms of injury associated with CPB that may cause the altered mitochondrial bioenergetics observed in this study, such as inflammatory cascades that result from the introduction of prime blood or contact with the circuit surfaces, potential loss of cerebral autoregulation or laminar versus pulsatile flow.
The effect that CPB has, without periods of deep hypothermia or circulatory arrest, on cerebral mitochondrial function has not hitherto been assessed. CPB is thought to offer some protection compared to alternative perfusion strategies since it minimizes the ischaemic insult that is present in deep hypothermic circulatory arrest, but its use is also associated with neurological injury [11, 12]. Furthermore, a longer duration of CPB has been associated with increased mortality [13] and worse neuro-developmental outcomes [14]. The mechanisms of such neurological injury are incompletely understood and are vital to understanding how best to prevent such complications in children undergoing surgery for congenital cardiac defects. This study was designed to examine the alterations in cerebral mitochondrial bioenergetics, cerebral metabolism and markers of neuronal injury with increasing durations of CPB.
Damage to the ETS and the resulting dysfunction of oxidative phosphorylation generate ROS, which in toxic thresholds will lead to oxidative damage, cellular dysfunction and cellular death. The brain is especially vulnerable to mitochondrial injury due to its high oxidative metabolic demands [9, 21]. CPB has been identified as a risk factor for ROS generation, but the specific effect that CPB has on cerebral mitochondrial ROS generation has not been assessed [22]. This study found an increase in mitochondrial ROS with increased time on CPB after 1 h and significantly increased ROS at 4 h for non-phosphorylating respiration through complexes I and II in the experimental group relative to sham animals. The accumulation of ROS with prolonged CPB provides a possible explanation for the previously demonstrated association between increased duration of CPB and increased morbidity and mortality in congenital cardiac surgical patients [13]. Since the majority of pathological mitochondrial ROS is generated by complex I dysfunction, therapeutics targeting this complex are of particular interest. One such potential therapeutic could make use of prodrugs that bypass complex I [23]. Preventing such increases in cerebral mitochondrial ROS may be an important neuroprotective measure as our understanding of this complicated pathophysiological process improves.
It is important to address the initial increase in ROS that occurs in the 1-h group. We can only speculate on the causes of the observed mitochondrial dysfunction. This initial increase in ROS may represent an acute response to the insult of CPB initiation. The onset of CPB is a known inflammatory insult [24]. This period results in several physiological derangements, such as the transition from pulsatile to non-pulsatile flow and disruptions in biological regulatory feedback mechanisms. There is also an initial inflammatory response to the donor blood used to prime the CPB circuit, which would only exist at the initiation of CPB. While it is not possible to determine with certainty, this may also be evidence of ROS-induced ROS release where excessive oxidative stress triggers opening of mitochondrial channels and a transient increase in ROS generation occurs [25]. The subsequent improvement may be evidence of the cells adjusting to this increased stress and the conclusion of the ROS-induced ROS release pathway. It is also possible that the improvement in ROS generation seen with the 2-h animals demonstrates a period of recovery when ROS have been scavenged efficiently. ROS was significantly increased in the 4-h group compared to the 2-h group for all points except for LEAK. The secondary increase in ROS beyond the 2-h period may be 1 of the first indications that mitochondria’s ability to scavenge free radicals is becoming overwhelmed and that their efficiency is decreased. ROS generation is intimately tied to mitochondrial health and adds further evidence on how extended periods of CPB may lead to compromised neurological outcomes through mitochondrial dysfunction.
Despite a difference in systemic lactate, there were no significant differences found in microdialysis results for lactate/pyruvate, glucose or glycerol concentrations between any of the CPB groups and the sham animals. Microdialysis examines local cellular metabolism and this finding is consistent with a lack of ischaemia. The detection of decreased mitochondrial respiration and increased ROS for experimental animals relative to shams despite similar microdialysis findings highlights that mitochondrial derangement still occurs even in the absence of local ischaemia and that other mechanisms are likely underlying the changes seen in this study. Several other mechanisms have been suggested for injury pathways with CPB most notably the inflammatory response and the compensatory anti-inflammatory response [24].
Our findings are consistent with extant literature describing the cellular and clinical manifestations of CPB’s deleterious neurological effects. The mechanism underlying the adverse effect on mitochondrial function does not appear to be related to cerebral ischaemia, and the exact cause of this injury is unknown. By highlighting the specific pathways in the ETS that are impacted by CPB, this study provides potential mechanistic targets for future diagnostic and therapeutic interventions to preserve mitochondrial function. This study has demonstrated that CPB negatively impacts the bioenergetics of complex I. Prior work from our group and others has shown disruption in ETS function, particularly mitochondrial complex I with other perfusion strategies and cardiac arrest [9, 18, 26]. While complex I has previously been targeted, our results provide support to targeting other points in the ETS as well.
The results of this study should be viewed in the light of several limitations. It is notable that due to the haemoconcentration in the CPB prime circuit, all CPB intervention groups had a higher haematocrit than the sham animals. There is evidence that lower haematocrit during CPB is associated with lower psychomotor developmental index scores at 1 year in human neonates [27] and that this increase in oxygen carrying capacity of the blood decreased any potential ischaemic insult. Therefore, this haematocrit difference may have diminished the potentially deleterious effects of CPB on mitochondrial bioenergetics relative to sham animals. The CPB duration groups were chosen to mimic expected CPB durations in clinical practice [13, 28]. By standardizing CPB parameters, our study does not address other questions including how altering these perfusion parameters may impact cerebral health. Another potential limitation of this experiment is the use of cervical cannulation. While cervical cannulation is used clinically for both extracorporeal membrane oxygenation (ECMO) and for CPB in neonates and children, it is not the most commonly use cannulation strategy in elective cardiac surgery procedures. This cannulation method was chosen to lay the groundwork for future survival studies, as quadrupeds cannot humanely recover from a sternotomy. We were able to achieve comparable flows as we have previously demonstrated with central cannulation and, furthermore, to mitigate any potential confounding effects of cervical cannulation, we used the cerebral samples obtained from the opposite side of cannulation for mitochondrial analysis. It is unlikely that there was significant hemispheric ischaemia, as there were no metabolic differences seen in the microdialysis results between the experimental and sham animals. Finally, we would be remiss if we did not acknowledge that this study is only able to assess mitochondrial function during the intraoperative period and that the changes seen here, while significant, may be transient. Further study is required to determine if there the derangements seen here extend beyond the intraoperative period and the impact that these alternations have on functional neuro-developmental outcomes.
CONCLUSION
Despite CPB’s ability to provide circulation, maintain blood pressure and remove carbon dioxide, it still has a deleterious effect on cerebral mitochondrial respiratory function and results in the accumulation of ROS. Our findings suggest a possible mechanism behind the neurological morbidities seen following extended durations of CPB and offer possible therapeutic targets to mitigate such effects. We suspect that the full effect of these cellular derangements on mitochondria extends beyond the intraoperative period. Future work will examine the effects of CPB on mitochondria and other markers of neuronal health throughout the immediate postoperative period to elucidate the time course and evolution of cerebral mitochondrial injury patterns. Such research will be crucial to help diagnose and assess the critical point at which mitochondrial stress irreversibly leads to cellular death to ensure the best possible outcome for children with congenital heart disease.
ACKNOWLEDGEMENT
The authors would like to thank the veterinary staff at the Children’s Hospital of Philadelphia.
Funding
This work was supported by grants from the National Institutes of Health (grant numbers T32-HL007915 and P41-EB015893). Institutional support was provided by the Children’s Hospital of Philadelphia, Department of Anesthesiology and Critical Care and funds from the Alice Langdon Warner and Daniel S. Tabas Endowed Chairs in Paediatric Cardiac Surgery at the Children’s Hospital of Philadelphia.
Conflict of interest: none declared.
ABBREVIATIONS
- ATP
Adenosine triphosphate
- CPB
Cardiopulmonary bypass
- CS
Citrate synthase
- ETS
Electron transport system
- ROS
Reactive oxygen species
Presented at the 56th Society of Thoracic Surgeons Annual Meeting, New Orleans, LA, USA. 25–28 January 2020.
Author contributions
Lindsay E. Volk: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Constantine D. Mavroudis: Conceptualization; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Tiffany Ko: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing. Thomas Hallowell: Investigation; Project administration; Writing—review & editing. Nile Delso: Investigation; Project administration; Visualization. Anna L. Roberts: Investigation; Resources. Jonathan Starr: Formal analysis; Investigation. William Landis: Investigation; Resources; Writing—review & editing. Yuxi Lin: Investigation; Resources. Marco Hefti: Conceptualization; Formal analysis; Writing—review & editing. Ryan W. Morgan: Conceptualization; Investigation; Supervision. Richard W. Melchior: Conceptualization; Investigation; Methodology; Project administration. Tami M. Rosenthal: Conceptualization; Supervision. Alexander Chappell: CCP Investigation; Methodology. Douglas Fisher: Investigation; Methodology. Molly Dreher: Investigation; Methodology. Daniel J. Licht: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Writing—review & editing. Jonathan Chen: Funding acquisition; Supervision; Writing—review & editing. J. William Gaynor: Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Writing—original draft; Writing—review & editing. Christopher E. Mascio: Conceptualization; Data curation; Formal analysis; Funding acquisition; Writing—review & editing. Todd J. Kilbaugh: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Katrien Francois and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Wernovsky G, Shillingford AJ, Gaynor JW.. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol 2005;20:94–9. [DOI] [PubMed] [Google Scholar]
- 2. Du Plessis AJ. Mechanisms of brain injury during infant cardiac surgery. Semin Pediatr Neurol 1999;6:32–47. [DOI] [PubMed] [Google Scholar]
- 3. Lynch JM, Ko T, Busch DR, Newland JJ, Winters ME, Mensah-Brown K. et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J Thorac Cardiovasc Surg 2018;156:1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch JC, Jacobs ML, Andropoulos D, Austin EH, Jacobs JP, Licht DJ. et al. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg 2012;94:1365–73. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wernovsky G, Licht DJ.. Neurodevelopmental outcomes in children with congenital heart disease-what can we impact? Pediatr Crit Care Med 2016;17:S232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kilbaugh TJ, Bhandare S, Lorom DH, Saraswati M, Robertson CL, Margulies SS.. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J Neurotrauma 2011;28:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kilbaugh TJ, Karlsson M, Byro M, Bebee A, Ralston J, Sullivan S. et al. Mitochondrial bioenergetic alterations after focal traumatic brain injury in the immature brain. Exp Neurol 2015;271:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilbaugh TJ, Karlsson M, Duhaime AC, Hansson MJ, Elmer E, Margulies SS.. Mitochondrial response in a toddler-aged swine model following diffuse non-impact traumatic brain injury. Mitochondrion 2016;26:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kilbaugh TJ, Sutton RM, Karlsson M, Hansson MJ, Naim MY, Morgan RW. et al. Persistently altered brain mitochondrial bioenergetics after apparently successful resuscitation from cardiac arrest. J Am Heart Assoc 2015;4:e002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lautz AJ, Morgan RW, Karlsson M, Mavroudis CD, Ko TS, Licht DJ. et al. Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit Care Med 2019;47:e241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM; for the International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015;135:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G. et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston circulatory arrest trial. J Thorac Cardiovasc Surg 2003;126:1385–96. [DOI] [PubMed] [Google Scholar]
- 13. Kansy A, Tobota Z, Maruszewski P, Maruszewski B.. Analysis of 14,843 neonatal congenital heart surgical procedures in the European Association for Cardiothoracic Surgery Congenital Database. Ann Thorac Surg 2010;89:1255–9. [DOI] [PubMed] [Google Scholar]
- 14. International Cardiac Collaborative On Neurodevelopment I. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg 2016;102:843–9. [DOI] [PubMed] [Google Scholar]
- 15. Hogue CW, Gottesman RF, Stearns J.. Mechanisms of cerebral injury from cardiac surgery. Crit Care Clin 2008;24:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med 1987;13:89–95. [DOI] [PubMed] [Google Scholar]
- 17. Taylor KM. Central nervous system effects of cardiopulmonary bypass. Ann Thorac Surg 1998;66:S20–4. discussion S25-8. [DOI] [PubMed] [Google Scholar]
- 18. Mavroudis CD, Karlsson M, Ko T, Hefti M, Gentile JI, Morgan RW. et al. Cerebral mitochondrial dysfunction associated with deep hypothermic circulatory arrest in neonatal swine. Eur J Cardiothorac Surg 2018;54:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D. et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–31. [DOI] [PubMed] [Google Scholar]
- 20. Karlsson M, Hara N, Morata S, Sjovall F, Kilbaugh T, Hansson MJ. et al. Diverse and tissue-specific mitochondrial respiratory response in a mouse model of sepsis-induced multiple organ failure. Shock 2016;45:404–10. [DOI] [PubMed] [Google Scholar]
- 21. Ayoub IM, Radhakrishnan J, Gazmuri RJ.. Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med 2008;36:S440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhen R, Wenxiang D, Zhaokang S, Xinling G, Huiming H, Jingfeng L. et al. Mechanisms of brain injury with deep hypothermic circulatory arrest and protective effects of coenzyme Q10. J Thorac Cardiovasc Surg 1994;108:126–33. [PubMed] [Google Scholar]
- 23. Ehinger JK, Piel S, Ford R, Karlsson M, Sjovall F, Frostner EA. et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 2016;7:12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bronicki RA, Hall M.. Cardiopulmonary bypass-induced inflammatory response. Pediatric Critical Care Medicine 2016;17:S272–8. [DOI] [PubMed] [Google Scholar]
- 25. Zorov DB, Juhaszova M, Sollott SJ.. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 2006;1757:509–17. [DOI] [PubMed] [Google Scholar]
- 26. Han F, Da T, Riobo NA, Becker LB.. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Crit Care Med 2008;36:S447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wypij D, Jonas RA, Bellinger DC, Del Nido PJ, Mayer JE Jr, Bacha EA. et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg 2008;135:355–60. [DOI] [PubMed] [Google Scholar]
- 28. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E. et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2007;133:1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]