Abstract
Background:
A 57-year-old right-handed man was admitted to the Treviso Memory Clinic due to the presence of memory forgetfulness, repetition of the same questions, episodes of confusion, initial difficulties in performing complex tasks and easy distraction over the past two years, as well as recurrent and never-happened-before car accidents.
Objective:
We report a peculiar case of an early onset Alzheimer’s disease (AD) with an unusual symptomatology, apparently not fitting in any of the categorized atypical forms of AD nor being representative of a typical amnestic AD.
Methods:
The patient underwent a neuropsychological, structural, and metabolic cerebral evaluation by MRI and 18F-FDG PET, together with the search for cerebral amyloid (amyloid PET), a genetic testing for dementia related genes and the dosage of CSF protein biomarkers of neurodegenerative conditions.
Results:
We observed a convergence of predominant frontal (dysexecutive, verbal disinhibition) and posterior (visuospatial) features of cognitive impairment. Structural MRI sequences showed subarachnoid spaces of the vault enlarged in the fronto-parietal region with anterior and posterior cortical atrophy. The hippocampus appeared preserved. The 18F-FDG PET scans showed hypometabolism in the prefrontal, lateral temporal, posterior parietal, and occipital regions bilaterally. The 18F-Flutemetamol scan showed a diffused uptake of the amyloid tracer at the cerebral cortex. CSF biomarkers were compatible with Alzheimer’s disease (AD).
Conclusion:
This case report presented with clinical phenotypic aspects atypical of AD, both frontal and posterior, never described as concomitant in the most accredited criteria for atypical AD, and appeared therefore more atypical than each of the atypical AD phenotypes already reported.
Keywords: Alzheimer’s disease, frontal variant, magnetic resonance, neurodegenerative diseases, PET scan, posterior cortical atrophy, TREDEM
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative condition usually developing in middle to late life [1]. Its definition is two-fold, based on one side on cluster of cognitive modifications and on the other side on biomarkers and structural evidence of AD pathology [2, 3].
In particular, the clinical profile of AD is generally characterized by an amnestic syndrome related to hippocampal involvement with a specific episodic memory deficit, whether isolated or associated with other cognitive or behavioral symptoms [2]. Nonetheless, AD pathology can be present even in the absence of clinical features, as shown by postmortem findings of brain amyloidosis and tauopathy in asymptomatic patients [3]. In the last decades, the neuropathology of AD has been detected in vivo at any stage of the illness through amyloid-β (Aβ) positron emission tomography (PET) imaging showing an augmented concentration of Aβ present in the brain, as well as through cerebrospinal fluid (CSF) protein levels evaluations which are related to the load of amyloid plaques, neurofibrillary tangles, and neuronal degeneration [2–4]. These biological markers have shown the highest specificity correlating with the finding of AD pathology after death and represent a fundamental milestone for the diagnosis of AD during the patient’s life [4]. However, according to estimates, 6 to 14%of AD patients possessing these biological pathology features do not manifest the sort of symptomatology above mentioned to be typical of AD [4]. These cases are examples of “atypical AD”, characterized by an earlier onset of the disease, a relatively preserved episodic memory and a different clinical phenotype [2–4]. As stated by the latest standards, atypical phenotypes include the posterior variant—comprising an occipitotemporal variant and a biparietal variant, the logopenic variant, and the frontal variant of AD [4]. Specifically, the occipitotemporal posterior variant is defined by early, prevalent, and progressive difficulties in visuoperceptive functions or in visual recognition of objects, symbols, words, or faces. Instead, in the biparietal posterior variant the core impairment is in visuospatial function, possibly with features of Balint syndrome, Gerstmann syndrome, limb apraxia, or neglect [4]. Diversely, the logopenic variant is distinguished by deficits in single word retrieval and repetition of sentences. Finally, the frontal variant is characterized by behavioral changes such as an increased manifestation of primary apathy or disinhibition or predominant executive dysfunction on cognitive testing [4].
Carrying out a differential diagnosis between the different AD subtypes can be challenging, considering that no biomarker can distinguish typical from atypical forms with consistent specificity [4]. Furthermore, neither the conceptual theory underlying atypical variants and their key features have been described in depth, nor recommended tests for the diagnosis have been operationalized [4, 5]. The issue of the classification of the spectrum of atypical AD can therefore be considered as unsettled and open to changes [5]. For these reasons, the diagnostical process of the clinical phenotype of AD relies to date on the clinical history of the patient and on the cognitive and behavioral symptoms, and employs neuropsychological testing together with topographical biomarkers, such as cortical hypometabolism in Fluorodeoxyglucose (18F-FDG) PET and cortical atrophy in structural magnetic resonance imaging (MRI), to support the identification of the clinical variant [4].
The present work reports a peculiar case of an early onset AD with an unusual symptomatology, apparently not fitting in any of the categorized atypical forms of AD while also not being representative of a typical amnestic AD, thus presenting as of an “atypical” atypical AD phenotype.
Case presentation
In April 2019, a 57-year-old right-handed man was admitted to the Treviso Memory Clinic due to the presence of cognitive changes and forgetfulness over the past two years, as well as recurrent and never-happened-before car accidents which occurred from October to December 2018. These events induced the man, married and father of an adolescent, to close the express courier business company he owned with a friend, remaining unemployed for a month. After starting to work again as an employee for another haul company, his forgetfulness and difficulties in driving the van persisted, to the extent of forcing him to take a sick leave in January 2019.
No family history of neurodegenerative or psychiatric diseases was reported, while other non-neurological conditions of the patient included episodes of asthma during the first years of life and alopecia for the past 16 years. He also reported poor performance at school, making frequent spelling mistakes in writing compositions and showing little interest in school subjects. No sleep disturbances were reported. At the time of the first visit, he used to smoke about four cigarettes a day and reported no alcohol consumption. He was not under any pharmacological treatment.
The onset of neurological symptoms dated back to summer 2018, when the patient, aged 56, started exhibiting forgetfulness, repetition of the same questions, episodes of confusion, initial difficulties in performing complex tasks, and easy distraction.
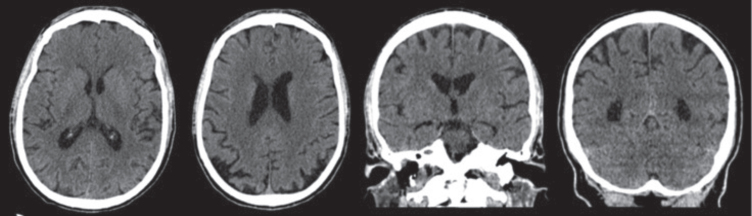
In December 2018 the patient underwent a computerized tomography (CT) scan, reporting a slight enlargement of subarachnoid spaces of the vault (Fig. 1).
Fig. 1.
CT scan (December 18, 2018), with no contrast, showing a modest enlargement of subarachnoid spaces of the vault with bilateral parietal cortical atrophy and atrophy of posterior cortex especially in the higher sections. The hippocampus and the mesial temporal area appear fairly preserved. Sagittal section not available.
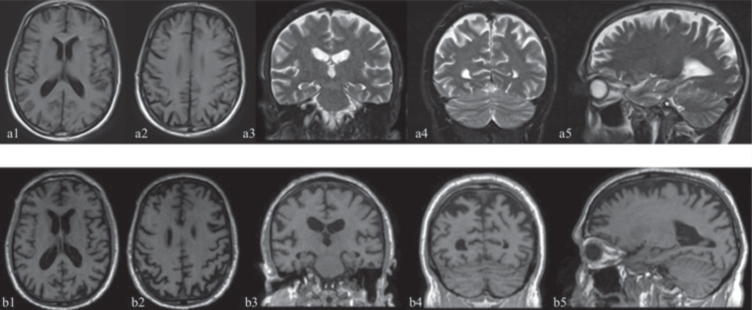
In January 2019 the patient underwent a neurological examination and was admitted to a Neurological Clinic of a Hospital near his home, where he performed a cerebral MRI, which showed enlarged subarachnoid spaces of the vault and minimal gliotic alterations within the right-hemisphere’s white matter (Fig. 2).
Fig. 2.
The first MRI study (January 12, 2019) is illustrated by the images above (a1-a5) while the second study (December 22, 2020) produced the images below (b1-b5). (a1, a2, b1, b2 axial sections; a3, a4, b3, b4 coronal sections; a5, b5 sagittal sections). Not available T1 sequences in the first study in the coronal and sagittal projections. Structural MRI T1 and T2 weighted sequences reported subarachnoid spaces of the vault enlarged in the fronto-parietal region (b1, b2, a3, b3, a5, b5), anterior (b2, a5, b5) and posterior cortical atrophy especially in the higher sections (a2, b2, a4, b4) and minimal gliotic alterations within the right-hemisphere’s white matter. The hippocampus and the mesial temporal area appear fairly preserved (a3, b3). The comparison between the two studies shows an increase in frontal (b2) and posterior (b2, b4) atrophy after 23 months.
During the same hospital admission, he completed a neuropsychological evaluation highlighting frontal executive function deficits (Frontal Assessment Battery, FAB: 9/18 [6]; Phonemic Verbal Fluency: 25.4 [7]; Cognitive Estimation Test: 4/5 [7]; Clock Drawing Test: 3/10 [7]), attention deficit (Attentive Matrices: 20.25/60 [8]), severe visuospatial deficits (Design Copy Test: 3.25/14 [8]), and memory impairment (Short-Story Memory Test: 3/16 [8]).
In January 2019, on the same hospital admission, a diagnostic lumbar puncture was performed with dosage of Aβ1–42, tau, phospho-tau, and 14-3-3 proteins.
Furthermore, in February of the same year he underwent an electroencephalogram (EEG) which revealed minimal signs of encephalic suffering, represented by inscription of theta sequences within the alpha background rhythm. The patient was discharged with the diagnosis of “Rapidly evolving cognitive decline under investigation”. No treatment followed the hospital discharge. In February 2019, the patient underwent a 18F-FDG PET scan showing a markedly reduced uptake in the parietal, lateral temporal, and prefrontal regions bilaterally, greater in the right hemisphere. The uptake was also reduced in the precuneus especially on the right and less at the level of the cingulate and in the occipital lobe.
The patient was referred to the Treviso Memory Clinic in April 2019 for a second opinion. Upon physical examination, he appeared a little anxious with hasty speech. He had no focal neurological deficits. Osteo-tendon reflexes were normal. No muscle strength deficit was observed in any body district. His walking was autonomous and normal. The patient did not perform the index-nose tests correctly: he used the thumb instead of the index finger and passed the nose with both hands. Furthermore, he showed adiadochokinesia in the prone supination of the hands. The patient was not affected by oculomotor apraxia, nor simultanagnosia, nor optic ataxia. The language was at times disinhibited.
At our first evaluation, the patient provided the images of the CT study he had previously undergone.
A careful observation of the images showed not only the enlargement of the subarachnoid spaces of the vault but also a parietal and posterior cortical atrophy especially in the higher sections. The hippocampus and the mesial temporal lobe appeared fairly preserved (Fig. 1). Blood chemistry tests showed only mild total hypercholesterolemia (222 mg/dl) and the electrocardiogram was normal.
A neuropsychological evaluation was carried out in April 2019 and showed deficits in memory, and attention, constructional apraxia, ideomotor apraxia, visual perception deficit, and executive dysfunctions (Table 1). In a second clinical evaluation in May 2019, it was possible to view images of the MRI study, that showed also posterior cortical atrophy, and of the 18FDG PET exam that showed hypometabolism in the parietal, temporal, and prefrontal regions bilaterally and at the level of the precuneus and of the cingulate, both previously performed at a different Hospital, together with CSF evaluation which showed high levels of tau protein, low ratio between Aβ1–42 and p-tau 181 protein and the absence of 14-3-3 protein.
Table 1.
Neuropsychological evaluations. The table shows the patient’s performance on tests of memory, attention, language, constructional apraxia, ideomotor apraxia, visual perception, executive functions, and the comprehensive neuropsychiatric profile (raw scores, corrected scores, and cut-off values are reported)
| Neuropsychological test | First evaluation (April 2019) | Cut-Off | Second evaluation (October 2019) | Cognitive Domain | ||
| Raw score | Corrected score | Raw score | Corrected score | |||
| Mini-Mental State Examination [7] | 19 | 18.97 | ≥23.80 | 18 | 17.97 | General Cognitive Abilities |
| Clinical Dementia Rating [6] | 1 | N.A. | 0.5 | 2 | N.A. | |
| RAVLT [9] | RI = 26; RD = 0 | RI = 26.7; RD = 0.2 | RI > 28.53; RD > 4.69 | RI = 22; RD = 0 | RI = 22.7; RD = 0.2 | Memory |
| Digit Span [8] | 6 | 6 | > 3.75 | 6 | 6 | |
| Visuo-Spatial Span [8] | 2 | 1.75 (Z = –3.32) | –1.5 < Z < 1.5 | 2 | 1.75 (Z = –3.32) | |
| Short-Story Memory Test [8] | 3 | 2.5 (Z = –2.65) | –1.5 < Z < 1.5 | 3 | 2.5 (Z = –2.65) | |
| ROCF –delayed recall [10] | 0 | –0.75 | ≥9.47 | 0 | –0.75 | |
| Attentive Matrices [13] | 12 | 7.3 (Z = –4.89) | –1.5 < Z < 1.5 | 11 | 6.5 (Z = –5) | Attention |
| Phonemic Verbal Fluency Test [13] | 24 | 26 | ≥17.35 | 19 | 21 | Language |
| Semantic Verbal Fluency Test [8] | 8.5 | 7.5 | ≥7.25 | 9.5 | 8.5 | |
| Token Test [8] | 28 | 27.25 | ≥26.5 | 19.5 | 18.75 | |
| Design Copy Test [13] | 2 | 2.3 (Z = –4.95) | –1.5 < Z < 1.5 | 2 | 2.3 (Z = –4.95) | Constructional Praxia |
| ROCF –copy [10] | 6.5 | 7 | ≥28.88 | 3.5 | 4 | |
| Ideational Apraxia Test [15] | 19/20 | N.A. | 20/20 | N.A. | Ideational Praxia | |
| Ideomotor Apraxia Test [8] | 19/20 | N.A. | 20/20 | N.A. | Ideomotor Praxia | |
| Tangled Figures Test [11] | 9 | N.A. | > 25 | 13 | N.A. | Visual Perception |
| Street’s Completion Test [8] | 4/14 | 3 (Z = –2.27) | –1.5 < Z < 1.5 | 4/14 | 3 (Z = –2.27) | |
| Silhouttes Test [16] | 44/47 | N.A. | 44/47 | N.A. | ||
| Poppelreuter-Ghent’s overlapping figures test [17] | N.E.** | N.E.* | ||||
| Clock Drawing Test [11] | 0/10 | N.A. | 0/10 | N.A. | Executive Functions | |
| Cognitive Estimation Test [11] | 5/5 | N.A. | 2/5 | N.A. | ||
| Trail Making Test –A [18] | N.E.* | < 94 sec | N.E.* | |||
| Trail Making Test –B [18] | N.E.* | < 283 sec | N.E.* | |||
| Frontal Assessment Battery (FAB) [19] | 10/18 | 10.1 (Z = –8.12) | –1.5 < Z < 1.5 | 10/18 | 10.1 (Z = –8.12) | |
| Tower of London [20] | N.E.* | N.E.* | ||||
| NPI F X G [21] | 4 | N.A. | 14 | N.A. | Neuropsychiatric Profile | |
N.A., not applicable; N.E., not executable; IR, immediate recall; DR, delayed recall; Scores outside the normal range are shown in bold. The neuropsychological assessment showed a deficit in memory (RAVLT, Visuo-Spatial Span, Short Story Memory Test, ROCF-delayed recall), attention (Attentive Matrices), constructional praxia (Design Copy Test, ROCF - copy), visual perception (Tangled Figures Test, Street’s Completion Test), and executive functions (Clock Drawing Test, FAB). *This test was not concluded because the patient could not understand the rule of the task. **This test was not executable because the patient showed a severe deficit in visual perception abilities.
As early onset AD was suspected, an amyloid Flutemetamol (18F) PET and a genetic testing for dementia related genes (APP, PSEN1, and PSEN2) were performed in June 2019.
The patient was placed on treatment with rivastigmine 4.6 mg/24 h transdermal patch and memantine starting at 10 mg/daily and progressively titrated to 20 mg/daily.
A follow-up neuropsychological evaluation was conducted in October 2019 and showed deficits of memory, attention, and language, constructional apraxia, ideomotor apraxia, visual perception deficit, and executive dysfunction (Table 1). The patient was evaluated periodically every 6 months. Due to an overall cognitive worsening in December 2020, the patient underwent a new 18FDG PET and brain MRI scan at our hospital which showed an increase in frontal and posterior cortical atrophy together with an accentuation of hypometabolism in parietal, temporal, prefrontal regions bilaterally as well as at the level of the precuneus, cingulate, and occipital lobe.
The overall clinical history, the neuropsychological and metabolic evidence, together with the amyloid Flutemetamol (18F) PET and the pathological dosage of biomarkers, oriented toward early onset AD with the distinctive occurrence of both frontal and posterior signs and symptoms seemingly pertaining to different atypical AD variants.
MATERIALS AND METHODS
Neuropsychological evaluation
A neuropsychological evaluation was carried out at admission in April 2019 and then in October 2019. Psychometric test results are included in Table 1 (raw scores, corrected scores, and cut-offs). The evaluation included the following tests: Clinical Dementia Rating (CDR) [9], Mini-Mental State Examination (MMSE) [10], Digit Span [11], Visuo-Spatial Span [8], Short Story Memory Test [8], Rey-Auditory Verbal Learning Test (RAVLT) [12], Rey-Osterrieth Complex Figure Test (ROCF)-detailed recall [13], Attentive Matrices [8], Semantic and Phonemic Verbal Fluencies [8, 7], Token Test [8], Design Copy Test [14], Rey-Osterrieth Complex Figure Test (ROCF)-copy [13], Ideational Apraxia [15], Ideomotor Apraxia [8], Tangled Figures Test [7], Street’s Completion Test [8], Silhouettes Test [16], Poppelreuter-Ghent’s overlapping figures test [17], Clock Drawing Test [7], Cognitive Estimation Test [7], Trail Making Test (TMT) A and B [18], Frontal Assessment Battery (FAB) [6], Tower of London [19], and the Neuropsychiatric Inventory (NPI) [20].
Structural and nuclear medicine imaging
The structural MRI scan was performed with 3-T Magnetom 135 mT of gradients with 64 channels (Siemens Magnetom Vida). T1, T2, and T2 FLAIR weighted sequences were extracted.
The patient underwent a 18F-FDG PET brain scan using a PET tomograph Discovery MI (General Electric Healthcare). The CT scan was used for attenuation and scatter correction with set voltage tuned to 120 kV. The scan was obtained over 15 min, starting 60 min after i.v. injection of 227 MBq. The images of the two scans were only visually assessed.
The patient also underwent a PET brain scan with 18F-Flutemetamol (Vizamil®), using a PET tomograph Discovery MI (General Electric Healthcare). The 18F-Flutemetamol study (Amyloid –β PET) was obtained over 20 min starting 90 min after intravenous injection of 198 MBq. The images of the scan were visually assessed.
Cerebrospinal fluid analyses
CSF Aβ1–42, total tau, and phosphorylated tau levels were determined by using commercially available INNOTEST ELISA kit (Fujirebio Europe N.V., Ghent, Belgium), as previously described [21]. CSF Aβ1–42, tTau, and pTau181 levels were check in duplicate. Pathological values were assumed for total tau > 350 pg/mL and Aβ1–42/pTau181 ratio < 7 [21].
The search for the 14-3-3 protein was also performed by western blot technique [22].
Genetic analysis
To investigate the presence of mutations associated with AD, including APP, PSEN1, and PSEN2, a next generation sequencing technique was performed [23].
RESULTS
Neuropsychological evaluation
The patient came to the clinical interviews accompanied by his wife. During the preliminary meeting he reported the presence of recent memory problems, difficulties in performing calculations, and difficulties in concentration. The wife reported that the patient frequently forgot his keys, his cell phone, and other personal items, and that he was responsible for several driving accidents. As a result, the patient, a former van driver for his own haul company, had to quit his job and was currently unemployed. During the interview, the patient was alert and collaborative, despite the presence of situational anxiety, easy distractibility, and loss of attention, leading to impulsive and hasty responses. His attitude was not always congruent with the context. He correctly recalled his personal data and was partially oriented in space and slightly disoriented in time. His spontaneous speech was fluent. The level of his speech comprehension was slightly impaired.
He exhibited apraxia during the execution of the drawing tests (including the Clock drawing test). At the Frontal Assessment Battery (FAB) there was a considerable sensitivity to interference and a reduced inhibitory control. There were major difficulties in both oral and written calculation. His mood was slightly low. It was not possible to administer the entire neuropsychological assessment due to difficulties in understanding the instructions necessary for carrying out some of the tests.
The first cognitive testing session (April 2019) highlighted a marked deficit in executive functions (Clock Drawing Test [7], Frontal Assessment Battery [6], Trail Making Test (TMT) A and B [18], Tower of London [19]). In addition, the patient’s executive performance worsened and, at a second evaluation (October 2019), a shortfall was evident in the Cognitive Estimation Test [7], previously scored within the normal range. The deficits in visual perception were notable and proved stable between the two evaluations (Tangled Figures Test [7], Street’s Completion Test [8]). Moreover, significant impairments were detected in the domains of memory (RAVLT [12], Visuo-Spatial Span [8], Short-Story Memory Test [8], ROCF –delayed recall [13]), attention (Attentive Matrices [8]) and of constructional praxia (Design Copy Test [14], ROCF –copy [13]), in which the patient scored below the normal threshold and slightly worsened throughout the second evaluation. A mild decline over the neuropsychiatric profile between the first and the second neuropsychological assessment was observed (NPI FxG [20]). Thus, the two evaluations signaled an overall progressive worsening of the clinical picture over six months.
Structural and nuclear medicine imaging
The CT scans (December 18, 2018) showed modest atrophy of the vault with bilateral parietal and posterior cortical atrophy especially in the higher sections. The hippocampus and the mesial temporal lobe appeared fairly preserved (Fig. 1)
Structural MRI T1 and T2 weighted sequences (January 12, 2019 and December 22, 2020), showed subarachnoid spaces of the vault enlarged (Fig. 2a3, a4; b3, b4:), posterior cortical atrophy especially in the higher sections (a2, a4; b2, b4) and minimal gliotic alterations within the right-hemisphere’s white matter. The hippocampus and the mesial temporal lobe appeared fairly preserved (a3, b3), despite modest motion artifacts. The comparison between the two studies showed an increase in frontal and posterior atrophy after 23 months.
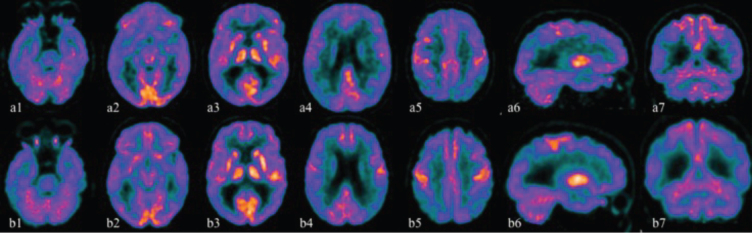
The first (February 13, 2019) and the second (December 22, 2020) 18F-FDG PET study showed a marked reduction in radiopharmaceutical uptake in the parietal (Fig. 3a2-a4; b2-b4:), lateral temporal (a1; b1) and prefrontal regions bilaterally (a5, a6; b5, b6), greater in the right hemisphere. The uptake was also significantly reduced at the level of the precuneus (a6; b6), especially on the right side, and slightly impaired at the level of the cingulate (a6; b6) and of the occipital lobe (a4, a5, a7; b4, b5, b7). The comparison between the two studies showed an increased hypometabolism in the same areas after about 22 months (b1-b8).
Fig. 3.
The first 18F-FDG PET study (February 13, 2019) is illustrated by the images above (a1-a7) while the second study (December 22, 2020) produced the images below (b1-b7). (a1-a5, b1-b5 axial sections; a6, b6 sagittal sections; a7, b7 coronal sections). 18F-FDG PET studies showed a marked reduction in radiopharmaceutical uptake in the parietal (a2-a4; b2-b4), lateral temporal (a1; b1) and prefrontal regions bilaterally (a5, a6; b5, b6), greater in the right hemisphere. The uptake was also significantly reduced at the level of the precuneus (a6; b6), especially on the right side, and slightly impaired at the level of the cingulate (a6; b6) and of the occipital lobe (a4, a5, a7; b4, b5, b7). The comparison between the two studies shows a accentuation of hypometabolism in the same areas after about 22 months (b1-b8).
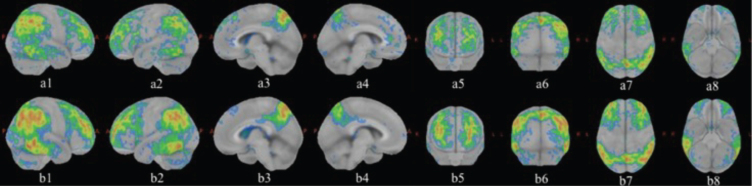
The 18F-FDG PET three-dimensional reconstruction (Fig. 4) clearly showed that the bilateral temporoparietal hypometabolism was prevalent on the right (a1, b1) together with the involvement of the precuneus (a3, b3). Some areas of the frontal (a5, b5) and of occipital cortex (a6, b6) were also clearly hypometabolic. The comparison between the two studies showed very clearly the hypometabolism of both the posterior and the frontal cortex which was accentuated after about 22 months (b1-b8).
Fig. 4.
Three-dimensional reconstruction with absorption ratio with respect to the Pons as reference region. Z score images to which a threshold of 2 SD is applied. The red and yellow colors correspond to the areas with greater hypometabolism. The first 18F-FDG PET study (February 13, 2019) is illustrated by the images above (a1-a8) while the second study (December 22, 2020) produced the images below (b1-b8). (a1, b1 right side; a2, b2 left side; a3, b3 right medial; a4, b4 left medial; a5, b5 front; a6, b6 backside; a7, b7 upper; a8, b8 bottom). The images clearly show that the bilateral temporoparietal hypometabolism was prevalent on the right (a1, b1) together with the involvement of the precuneus (a3, b3). Some areas of the frontal (a5, b5) and of occipital cortex (a6, b6) are also clearly hypometabolic. The comparison between the two studies shows a clear accentuation of hypometabolism in the same areas after about 22 months (b1-b8).
The 18F-Flutemetamol scan showed a diffused uptake of the amyloid tracer at the level of the cerebral cortex, particularly in the frontal lobes, parietal lobes, lateral temporal lobes, in the posterior cingulate, bilaterally, and in correspondence of the striate nuclei (Fig. 5).
Fig. 5.
18F-Flutemetamol PET scan highlighting a diffused uptake of the amyloid tracer at the level of the cerebral cortex, particularly in the frontal lobes, parietal lobes, lateral temporal lobes, in the posterior cingulate, bilaterally and in correspondence of the striate nuclei.
Cerebrospinal fluid analysis
The CSF analysis revealed a Aβ1–42 level of 305ng/L, a t-tau protein dosage of 957 ng/L, a p-tau protein 181 dosage of 171 ng/L, while the ratio between Aβ1–42 and p-tau 181 protein was 1.8. 14-3-3 protein in the CSF was not detected.
Genetic analysis
Mutations associated with AD (APP, PSEN1, and PSEN2) were not found.
DISCUSSION
From a neuropsychological perspective, our patient presented a very uncommon cluster of symptoms. Specifically, we observed a convergence of predominant frontal (dysexecutive, verbal disinhibition) and posterior (visuospatial) features of cognitive impairment, together with a memory loss and deficiencies in the domains of language, attention and constructional praxia. The severity of his clinical symptomatology was substantial and disabling, especially considering his young age (57 years old at the time of the first assessment in the Treviso Memory Clinic) and home situation (the patient was married and father of one kid). The loss of his job, due to the clinical manifestation of the disease, represented an additional aggravating factor, since the patient was not able to provide economical support to his family anymore. Moreover, the patient’s socialization skills were undermined by his inability to maintain sustained attention, combined with his sometimes inappropriate and disinhibited behavior. Regarding the cognitive testing sessions, the first neuropsychological evaluation (April 2019) highlighted a marked deficit in executive functions that worsened (Cognitive Estimation Test) after only six months, when the second assessment was performed. The deficits in visual perception were also conspicuous and stable between the two evaluations (Table 1). Other impairments were detected in the domains of memory, attention, and constructional praxia, the latter worsened by the second evaluation.
The patient while showing memory deficits typical of individuals with AD [2], markedly exhibited evidence of an atypical AD clinical phenotype. Furthermore, its atypical clinical features did not fulfil current AD subtype classifications [4]. We are therefore facing a two-fold atypicality, since beyond exhibiting symptoms that are representative of the posterior variant of AD (such as early, prevalent and progressive dysfunctions of visual-perceptive abilities), our patient displayed impairments in the context of executive functions and disinhibition, which are characteristics of the frontal variant of AD [4].
Given the peculiar cluster of symptoms described above, the patient presented with a variant of AD more atypical than the previously reported atypical ones [4, 24]. In our clinical case, visuospatial impairments, expression of posterior cortical damage, appear to be the prevailing deficit also with respect to compromised executive functions, manifestation of frontal cortical damage. Visuospatial disabilities strongly influenced the patient’s daily life and could also be the main cause of his car accidents, the reason that led the patient to consult a specialized center for cognitive disorders.
The increasingly widespread use of biomarkers has made it clear that the clinical presentation of symptoms does not reliably indicate the underlying neuropathology and that the same neuropathology can manifest with different clinical phenotypes [24, 25].
However, it is not yet clear why and how a certain brain network rather than another becomes selectively vulnerable to the onset of AD pathology [26].
Unlike beta amyloid protein, there would be a close association between tau pathology and regional neurodegeneration as well as clinical symptoms in atypical forms of AD, including posterior cortical atrophy [27], frontal behavioral variant of AD [28, 29], and primary progressive aphasia [30].
On the other hand, the lack in the clinical setting of a PET ligand specific for neurofibrillary tau pathology does not allow to verify the correspondence between the topographical localization of tau and the clinical phenotypic characteristics of AD [24]. Although CSF tau measurements are a validated biomarker of tau neurofibrillary pathology [25], this biomarker does not allow to study the anatomical localization of tau deposition.
We believe that, this case report has the merit of exposing different clinical phenotypic aspects of AD, both frontal and posterior in the same patient, not described together in the most accredited criteria for atypical AD [4]. A second strength is that the case has been carefully studied from a clinical, structural, metabolic, CSF and genetic point of view.
This case report also has some weaknesses.
A possible undiagnosed attention deficit at a young age could explain both patient-reported poor school performance and a more recent executive dysfunction.
Attention-deficit hyperactivity disorder (ADHD) has historically been considered a disorder of childhood and adolescence. However, it is now recognized that ADHD symptoms persist into adulthood in up to 60%of individuals [31]. The relationship between ADHD and AD is a matter of debate. Some studies have suggested an association between self-reported specific learning disabilities in childhood and an increased risk of atypical AD in adulthood and old age [32, 33].
Other evidences, instead, suggest that ADHD is a neurodevelopmental process fundamentally unrelated to mild cognitive impairment and dementia [31] and it would appear to be a confounding rather than a causal factor for dementia [34]. Future research is needed to determine the underlying mechanisms that predispose some, but not all people, with atypical neurodevelopment, to develop atypical neurodegenerative disease.
A second weakness is that the patient was not completely cooperative during the MRI scan and some sequences therefore showed modest motion artifacts.
Finally, the patient underwent the first instrumental examinations (CT, first MRI, first 18FDG PET) in a different hospital; we therefore could not check the quality of the first image examinations. We obtained the first images via CD-rom and not via our Picture Archiving and Communication System (PACS) [35]. The comparison of the same coronal and sagittal MRI sections could only take place between different sequences, T2 versus T1.
CONCLUSIONS
The case report derived from daily clinical practice, can offer an example of an undescribed clinical presentation of atypical AD.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939. [DOI] [PubMed] [Google Scholar]
- [2]. Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Scheltens P (2010) Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol 9, 1118–1127. [DOI] [PubMed] [Google Scholar]
- [3]. Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [5]. Galton CJ, Patterson K, Xuereb JH, Hodges JR (2000) Atypical and typical presentations of Alzheimer’s disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123, 484–498. [DOI] [PubMed] [Google Scholar]
- [6]. Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005) The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol Sci 26, 108–116. [DOI] [PubMed] [Google Scholar]
- [7]. Mondini S, Mapelli D, Vestri A, Arcara G, Bisiacchi PS (2011) Esame Neuropsicologico Breve 2. Una batteria di test per lo screening neuropsicologico. Raffaello Cortina Editore.
- [8]. Spinnler H, Tognoni G (1987) Standardizzazione e Taratura italiana di Test Neuropsicologici. Ital J Neurol Sci 6, 1–120. [PubMed] [Google Scholar]
- [9]. Berg L (1984) Clinical dementia rating. Br J Psychiatry 145, 339–339. [PubMed] [Google Scholar]
- [10]. Measso G, Cavarzeran F, Zappalà G, Lebowitz BD, Crook TH, Pirozzolo FJ, Amaducci LA, Massari D, Grigoletto F (1993) The Mini Mental State Examination: Normative study of an Italian random sample. Dev Neuropsychol 9, 77–95. [Google Scholar]
- [11]. Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8, 539–548. [DOI] [PubMed] [Google Scholar]
- [12]. Rey A (1964) L’examen clinique en psychologie. Presses Universitaires de France, Paris. [Google Scholar]
- [13]. Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey-Osterrieth Complex Figure: Normative values in an Italian population sample. Neurol Sci 12, 1–13. [DOI] [PubMed] [Google Scholar]
- [14]. Carlesimo GA, Caltagirone C, Gainotti G, Nocentini U e il Gruppo per la Standardizzazione della batteria per il deterioramento mentale (1995) Batteria per la valutazione del deterioramento mentale (Parte II): Standardizzazione e affidabilità diagnostica nell’identificazione dei pazienti affetti da sindrome demenziale. Arch Psicol Neurol Psichiatr 56, 471–488. [Google Scholar]
- [15]. De Renzi E, Lucchelli F (1988) Ideational apraxia. Brain 111, 1173–1185. [DOI] [PubMed] [Google Scholar]
- [16]. Warrington EK, James M (1991) The visual object and space perception battery: VOSP. Pearson, London. [Google Scholar]
- [17]. De Renzi E, Scotti E, Spinnler H (1969) Perceptual and associative disorders of visual recognition: Relationship to the site of lesion. Neurology 19, 634–642. [DOI] [PubMed] [Google Scholar]
- [18]. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail Making Test: Normative values from 287 normal adult controls. Ital J Neurol Sci 17, 305–309. [DOI] [PubMed] [Google Scholar]
- [19]. Shallice T (1982) Specific impairment of planning. Philos Trans R Soc Lond B Biol Sci 298, 199–209. [DOI] [PubMed] [Google Scholar]
- [20]. Cummings JL (1997) The Neuropsychiatric Inventory Assessing psychopathology in dementia patients. Neurology 48, 10S–16S. [DOI] [PubMed] [Google Scholar]
- [21]. Fiorini M, Bongianni M, Benedetti MD, Monaco S, Zanusso G (2018) Reappraisal of Aβ40 and Aβ42 peptides measurements in cerebrospinal fluid of patients with Alzheimer’s disease. J Alzheimers Dis 66, 219–227. [DOI] [PubMed] [Google Scholar]
- [22]. Foote M, Zhou Y (2012) 14-3-3 proteins in neurological disorders. Int J Biochem Mol Biol 3, 152–164. [PMC free article] [PubMed] [Google Scholar]
- [23]. Ghezzi L, Carandini T, Arighi A, Fenoglio C, Arcaro M, De Riz M, Pietroboni AM, Fumagalli GG, Basilico P, Calvi A, Scarioni M, Colombi A, Serpente M, Marotta G, Benti R, Scarpini E, Galimberti D (2017) Evidence of CNS β-amyloid deposition in Nasu-Hakola disease due to the TREM2 Q33X mutation. Neurology 89, 2503–2505. [DOI] [PubMed] [Google Scholar]
- [24]. Dickerson BC, McGinnis SM, Xia C, Price BH, Atri A, Murray ME, Mendez MF, Wolk DA (2017) Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr 22, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Weintraub S, Mesulam M (2009) With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain 132(Pt 11), 2906–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Hof PR, Morrison JH (1996) Hippocampal and neocortical involvement in normal brain aging and dementia: Morphological and neurochemical profile of the vulnerable circuits. J Am Geriatr Soc 44, 857–864. [DOI] [PubMed] [Google Scholar]
- [27]. Hof PR, Bouras C, Constantinidis J, Morrison JH (1989) Balint’s syndrome in Alzheimer’s disease: Specific disruption of the occipito-parietal visual pathway. Brain Res 493, 368–375. [DOI] [PubMed] [Google Scholar]
- [28]. Johnson JK, Head E, Kim R, Starr A, Cotman CW (1999) Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 56, 1233–1239. [DOI] [PubMed] [Google Scholar]
- [29]. Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ (2014) Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis 39, 63–70. [DOI] [PubMed] [Google Scholar]
- [30]. Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM (2012) Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 135(Pt 5), 1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Callahan BL, Bierstone D, Stuss DT, Black SE (2017) Adult ADHD: Risk factor for dementia or phenotypic mimic? Front Aging Neurosci 9, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Seifan A, Schelke M, Obeng-Aduasare Y, Isaacson R (2015) Early life epidemiology of Alzheimer’s disease-a critical review. Neuroepidemiology 45, 237–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Seifan A, Assuras S, Huey ED, Mez J, Tsapanou A, Caccappolo E (2015) Childhood learning disabilities and atypical dementia: A retrospective chart review. PLoS One 10, e0129919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Ivanchak N, Fletcher K, Jicha GA (2012) Attention-deficit/hyperactivity disorder in older adults: Prevalence and possible connections to mild cognitive impairment. Curr Psychiatry Rep 14, 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Ahmadi M, Mehrabi N, Sheikhtaheri A, Sadeghi M (2017) Acceptability of picture archiving and communication system (PACS) among hospital healthcare personnel based on a unified theory of acceptance and use of technology. Electron Physician 9, 5325–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]