Abstract
Throughout the world, including the United States, men have worse outcomes from COVID-19 than women. SARS-CoV-2, the causative virus of the COVID-19 pandemic, uses angiotensin-converting enzyme 2 (ACE2) to gain cellular entry. ACE2 is a member of the renin-angiotensin system (RAS) and plays an important role in counteracting the harmful effects mediated by the angiotensin type 1 receptor. Therefore, we conducted Ovid MEDLINE and Embase database searches of basic science studies investigating the impact of the biological variable of sex on ACE2 expression and regulation from 2000, the year ACE2 was discovered, through December 31, 2020. Out of 2,131 publications, we identified 853 original research articles on ACE2 conducted in primary cells, tissues, and/or whole mammals excluding humans. The majority (68.7%) of these studies that cited the sex of the animal were conducted in males, while 11.2% were conducted solely in females; 9.26% compared ACE2 between the sexes, while 10.8% did not report the sex of the animals used. General findings are that sex differences are tissue-specific and when present, are dependent upon gonadal state. Renal, cardiac, and adipose ACE2 is increased in both sexes under experimental conditions that model co-morbidities associated with worse COVID-19 outcomes including hypertension, obesity, and renal and cardiovascular diseases; however, ACE2 protein was generally higher in the males. Studies in Ace2 knockout mice indicate ACE2 plays a greater role in protecting the female from developing hypertension than the male. Studying the biological variable of sex in ACE2 research provides an opportunity for discovery in conditions involving RAS dysfunction and will shed light on sex differences in COVID-19 severity.
Keywords: angiotensin, gonadectomy, heart, kidney, ovariectomy
INTRODUCTION
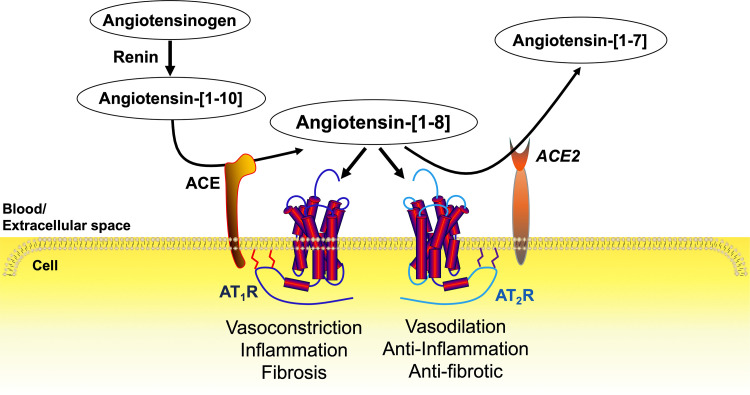
Angiotensin-converting enzyme 2 (ACE2) is a key component of the renin-angiotensin system (RAS), which controls blood pressure and water and electrolyte homeostasis in both sexes (Fig. 1). The decapeptide angiotensin (Ang)-[1–10] is cleaved from the protein angiotensinogen by renin. The dicarboxypeptidase angiotensin-converting enzyme (ACE), removes two amino acids from the carboxy terminus of the precursor peptide Ang-[1–10] to form the octapeptide hormone Ang-[1–8] (also known as ANG II). Two G protein-coupled receptors mediate the effects of Ang-[1–8]. Excessive Ang-[1–8] activity at the angiotensin type 1 receptor (AT1R) leads to hypertension, inflammation, atherogenesis, and tissue fibrosis (1, 2). The angiotensin type 2 receptor (AT2R) plays a counter-regulatory role in AT1R actions, especially in the females (3, 4).
Figure 1.
The renin-angiotensin system (RAS). The decapeptide angiotensin (Ang)-[1–10] is cleaved from the protein angiotensinogen by renin. Angiotensin-converting enzyme (ACE) removes two amino acids from the carboxy terminus of the precursor peptide Ang-[1–10] to form the octapeptide hormone Ang-[1–8] (also known as ANG II). Ang-[1–8] exerts its physiological effects by binding to the angiotensin type 1 and type 2 receptors (AT1R and AT2R). Overactivity of the Ang-[1–8]-AT1R pathway leads to hypertension, inflammation, and tissue fibrosis, which is counter regulated by the actions of the AT2R. Angiotensin-converting enzyme 2 (ACE2) attenuates Ang-[1–8] activity primarily by removing one amino acid from the carboxy terminal to form Ang-[1–7].
ACE2 is a membrane-bound monocarboxypeptidase that is expressed in most organs and blood vessels. While ACE2 can catabolize various peptides including des-Arg-bradykinin, apelins, and dynorphins, its primary substrate is Ang-[1–8] (5). ACE2 removes one amino acid from the carboxy terminal of Ang-[1–8] to form the heptapeptide Ang-[1–7] (Fig. 1).
Studies have demonstrated Ang-[1–7] has vasodilatory and anti-inflammatory effects (6–8). However, peptide concentrations used in these studies are 1,000–1,000,000 times higher than the circulating levels in rats (9) and humans (10) measured by a highly sensitive liquid chromatography-tandem mass spectrometry method (11). While there is an extensive literature attributing the MAS oncogene to be the cognate receptor for Ang-[1–7] (12), the International Union of Basic and Clinical Pharmacology determined that MAS did not “rise to the level of acceptance” as the receptor for Ang-[1–7] “because pharmacology and signaling are not rigorously established” (13). Furthermore, dynamic mass redistribution assays showed that Ang-[1–7] had no effect on MAS signaling and that radiolabeled Ang-[1–7] did not bind to human embryonic kidney-293 cells transfected with recombinant MAS (14). Other investigators have postulated that Ang-[1–7] exerts anti-Ang-[1–8] effects by acting as a biased agonist at the AT1R (15), whereas still others suggest that Ang-[1–7] exerts antihypertensive actions as an AT2R agonist (16).
In addition, ACE2 can form Ang-[1–9] from Ang-[1–10], though less efficiently than the formation of Ang-[1–7] from Ang-[1–8] (17, 18). Some investigators suggest Ang-[1–9] acts as an AT2 receptor agonist based on studies showing AT2R antagonists attenuate the effects of Ang-[1–9] administration, albeit at pharmacological doses of Ang-[1–9] (19). Clearly, more research is needed to determine the extent to which the beneficial physiological effects of ACE2 are due to reducing Ang-[1–8] through enzymatic catabolism, versus generating the hepta- and nonapeptides. Investigating the physiological and pathophysiological role of ACE2 is an essential area of research in diseases involving dysregulation of the RAS such as in hypertension, obesity, and cardiovascular and renal diseases (20–24).
The (COronoVIrus Disease-2019) (COVID-19) pandemic has generated great interest in ACE2 because this enzyme is what SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona-Virus)-2 (25, 26), the virus underlying the current pandemic, uses to enter the cells. Similar to SARS-CoV-1 (27), SARS-CoV-2 gains cellular entry through the binding of its spike protein to ACE2 (25, 26). In addition to binding ACE2 in the lungs, the spike protein also binds to ACE2 on arterial and venous endothelial cells resulting in dispersal of the virus throughout the circulatory system (28).
While the incidence of COVID-19 is similar between men and women, disease severity is worse in men (29). Therefore, understanding how ACE2 is regulated in both sexes is a critically important area of COVID-19 research. This review focuses on what is known regarding the impact of biological sex on ACE2 expression and regulation in nonhuman mammals, the gaps in knowledge and the implications for conditions and diseases involving the RAS and COVID-19.
METHODS
Literature Search
Comprehensive searches of online databases including Ovid MEDLINE and Embase were conducted in journals through December 31, 2020. All languages were included, and no start date was imposed in the search. We included all original research studies involving primary cells, tissue, and whole animals. Two separate searches were performed before Boolean logic (1) and (2) were applied: 1) (“angiotensin-converting enzyme 2” or ACE2 or “ACE 2” or “angiotensin-converting enzyme II” or “angiotensin converting enzyme two”) and 2) all mammals except humans. All systematic reviews, meta-analysis, editorials, commentaries, and letters to the editor were excluded. We also excluded candida, candidiasis, fungal, fungus, fungi, saccharomyces, yeast, yeasts, antifungal, fungicidal, and ace2p. Five individuals (B.M.S., S.M., H.J., A.M.A.dS., and K.S.) independently reviewed the original research articles and stratified these publications based on the sex of the animals studied. In addition to reviewing the methods, figure legends, and table legends for each publication, the entire article was electronically searched for the terms male, female, sex, and gender. All articles investigating both sexes were assessed for major findings (B.M.S. and S.M.).
ACE2 Enzyme Activity
ACE2 activity was measured in lung and kidney membranes obtained from male and female Long-Evans rats (2–4 mo old, Charles River Laboratories, Wilmington, MA). These animals had been used in behavioral studies with an elevated Y maze. They were also used as breeders with three of the females having given birth to and completed weaning of one litter of pups before euthanasia. Upon euthanasia with CO2, the lungs and kidneys were frozen on dry ice and stored at −80°C. A section of kidney cortex and lung were dissected from the frozen tissue and mechanically homogenized (Tissumizer) in ice-cold 100 mM NaCl, 10 µM ZnAc, 50 mM Tris-buffer, pH 7.5 at 22°C. The homogenate was centrifuged at 20,000 g for 20 min at 4°C. The pellet was resuspended in the same buffer and used to assess ACE2 activity, using a fluorescent substrate Mca-APK(dnp) (BML P163, Enzo Life Sciences, Farmingdale, NY) at a concentration of 50 µM in a volume of 50 µL in 96-well plates at 37°C (pH = 7.2), for 30 min in a Biotek Synergy plate reader. Metabolism of the fluorogenic substrate was monitored by formation of Mca-AP, measured as relative fluorescent units (RFU) at 393 nm with excitation at 328 nm. Mca-AP formation in the presence of a 1 µM concentration of the specific ACE2 inhibitor MLN-4760 (30) was subtracted from total Mca-AP formation to define ACE2 activity. The data were expressed as relative fluorescence units (RFU)/mg tissue wet weight.
Western Blot
Kidney cortex was isolated from wild-type (C67BL/6J) and Ace2 knockout mice of both sexes using isolation solution including 10 mM triethanolamine, 250 mM sucrose, and Halt Protease Inhibitor Cocktail (Cat. No. 87785, Thermo Fisher Scientific, Waltham, MA). The protein concentration was determined using the Bradford coumassie blue method (Bio-Rad Laboratories Inc., Hercules, CA). Six identical sets of protein samples (40 µg from wild-type or Ace2 knockout mice and 60 pg of recombinant mouse ACE2 protein (Cat. No. 3437-ZN-010, R&D Systems, Minneapolis, MN) were electrophoresed on two 4%–20% Criterion TGX Precast Midi Protein Gels (Bio-Rad, Cat. No. 5671094) and blotted onto polyvinylidene difluoride membranes (Trans-Blot Turbo Midi 0.2-µm PVDF Transfer Packs, Cat. No. 1704157, Bio-Rad Laboratories Inc.).
These two membranes were cut into six identical sections and each piece was incubated overnight at 4°C with one of six different sources of ACE2 antibodies including: R&D Systems anti-mouse polyclonal (Cat. No. AF3437) at 0.4 µg/mL; Abcam (Cambridge, MA) anti-mouse monoclonal (Cat. No. ab108252) at 1:1,000 dilution; Novus Biologicals (Centennial, CO) rabbit anti-human monoclonal (Cat. No. NBP2-67692) at 1:1,000 dilution; Proteintech Group, Inc. (Rosemont, IL) rabbit anti-human polyclonal (Cat. No. 21115) at 1:1,000 dilution; Cell signaling Technology (Danvers, MA) rabbit anti-human polyclonal (Cat. No. 4355) at 1:1,000 dilution; and, Santa Cruz Biotechnology (Dallas, TX) anti-mouse monoclonal (Cat. No. sc-390851) at 1:200 dilution. Note: antibody dilutions are based on company recommendations as well as pilot studies. After three washes with Tris-buffered saline with Tween 20 (Sigma Aldrich, St. Louis, MO), membranes were incubated with rabbit anti-goat (Cat. No. 14-1306) or goat anti-rabbit (Cat. No. 074-1506) antibodies 1:10,000 dilution (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD); and, mouse IgGκ light chain binding protein conjugated to horseradish peroxidase (Cat. No. sc-516102, Santa Cruz Biotechnology, Inc.) at 1:10,000 dilution.
Bands were visualized by SuperSignal West Pico PLUS Chemiluminescent Substrate (Cat. No. PI34580) and SuperSignal West Femto Maximum Sensitivity Substrate (Cat. No. PI34096) (Thermo Fisher Scientific) and quantified by densitometry (Amersham Imager 600 Systems, GE Healthcare, Chicago, IL) using recombinant mouse ACE2 (Cat. No. 3437-ZN-010, R&D Systems) as the positive control. Nonspecific binding was blocked with nonfat dry milk (5%). Membranes were stripped and then incubated with β-actin (Cat. No. A5441, Sigma Aldrich) to control for protein loading.
MALE BIAS IN ACE2 BASIC SCIENCE RESEARCH
Male bias is a common finding in basic science research. Ten years ago, an analysis of journal articles across diverse biological fields demonstrated that the majority of published research in experimental animals was conducted in males (31). This male bias has continued throughout this past decade, as evidenced by our analyses of articles published in this journal (32) as well as in the hypertension (17) and renal physiology (33) fields.
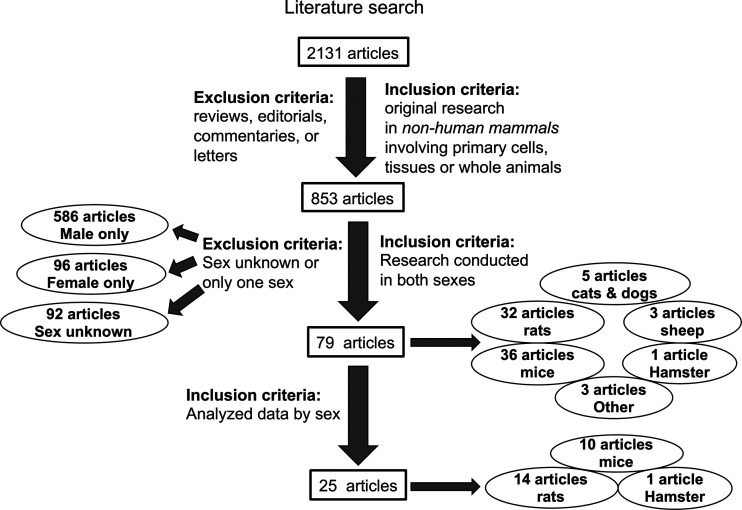
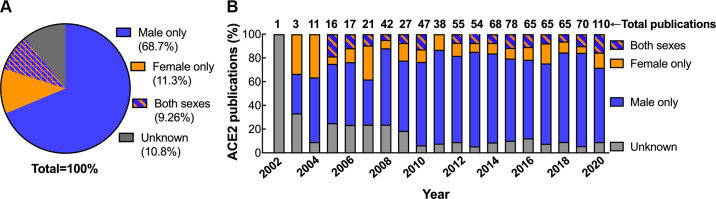
Basic science research on ACE2 exhibits a similar male bias. As of December 31, 2020, we identified 2,131 articles from Ovid MEDLINE and Embase databases published on ACE2 since its initial discovery in 2000 (34) (Fig. 2). After excluding reviews, commentaries, letters, and editorials, there were 853 original research articles on primary cells, tissues, and/or whole animal experiments from nonhuman mammals. Studies conducted solely in cells transfected with ACE2 cDNAs were excluded. Only 79 (9.26%) of these 853 articles reported data in both sexes. Only 25 (2.9%) analyzed the data by sex. The majority (68.7%) of basic science studies on ACE2 used male animals exclusively whereas 11.2% were conducted solely in females (Fig. 3A). This 6:1 ratio of male:female studies is similar to the 5:1 ratio that has been observed in the fields of neuroscience and immunology (31).
Figure 2.
Literature search on angiotensin-converting enzyme 2 (ACE2). A database search of Ovid MEDLINE and Embase led to the identification of 2,131 published articles on ACE2 through December 31, 2020. Inclusion and exclusion criteria led to the identification of 853 original research articles on nonhuman mammalian primary cells, tissues, or whole animals; studies conducted solely in cells transfected with ACE2 cDNAs were excluded. Note the original paper describing the discovery of ACE2 in 2000 (34) was not included because it did not meet our inclusion criterion. The article reported on ACE2 expression in human tissues and in cells transfected with ACE2 cDNAs. Only 79 of the 853 original articles included data in both sexes and of those, only 25 compared the data by sex. The number of articles as a function of species studied is also shown.
Figure 3.
Sex of nonhuman mammals reported in angiotensin-converting enzyme 2 (ACE2) publications over the past two decades. Shown is the proportion of original research articles on nonhuman mammalian primary cells, tissues or whole animals that are conducted in male only, female only, both sexes or studies in which the sex is unknown (i.e., not reported) as a function of the total number of articles as a pie chart (A) or number of articles published each year since ACE2 was discovered (B).
Over this past decade (2011–2020), there has been an improvement in the percentage of articles citing the sex of the animal studied (Fig. 3B). Whereas 20.4 ± 3.1% of publications did not report the sex of the animal studied in the first decade (2000–2010; n = 8), by the second decade (n = 10), this percentage dropped to 8.56 ± 0.64% (P < 0.001 vs. first decade) (Fig. 3B). There was no change, however, in the proportion of articles that included both sexes from the first to the second decade [(%/yr): first decade, 10.8 ± 4.8 vs. second decade, 9.1 ± 2.9; P = 0.4]. It is likely that these earlier studies of unreported sex were predominantly conducted in male animals. When the percentage of studies reporting the sex of the animal increased, there was also an increase in the proportion of studies conducted solely in male animals [(% of male only out of the total number of studies): first decade, 52.8 ± 12 vs. second decade, 72.6 ± 5.9; P < 0.005].
As of March 1, 2021, the COVID-19 pandemic has claimed the lives of well over 2.5 million men and women globally (https://coronavirus.jhu.edu/, accessed March 1, 2021). A review of sex-disaggregated data from New York City, as of April 11, 2020, showed there were nearly twice as many hospitalizations, admissions to the intensive care unit, and deaths due to COVID-19 in men compared with women (29). These findings of worse outcomes in men with COVID-19 compared with women are also observed globally. A recent single-arm meta-analysis of clinical characteristics, discharge rate, and fatality rate of COVID-19 from online databases including PubMed, Embase, Web of Science, WanFang Data, and CNKI from December 2019 to February 2020, showed that males had worse COVID-19 outcomes than women (35).
Given that SARS-CoV-2 uses ACE2 as the vehicle to enter the cells, this past year saw a marked increase (1.6-fold) in the number of basic science papers published on ACE2 compared with the prior year (2019) (Fig. 3B). The proportion of ACE2 basic science studies conducted solely in males compared with females, however, remained disproportionately high (Fig. 3B). The clinical findings that men have worse COVID-19 disease than women warrants the need for COVID-19 basic science research into sex differences in the receptor the virus uses to infect cells. To understand what is known and not known regarding the impact of biological sex on ACE2 expression and regulation, we reviewed the 79 articles that investigated ACE2 mRNA, protein, or enzyme activity in both sexes.
ACE2 METHODOLOGY
Not all of the 79 studies identified through the literature search used optimal conditions for measuring ACE2. Thus, results from some of these studies need to be confirmed using authenticated methods. Furthermore, it is important to consider the caveats associated with ACE2 assays in the interpretation of the data. We address these points throughout the review.
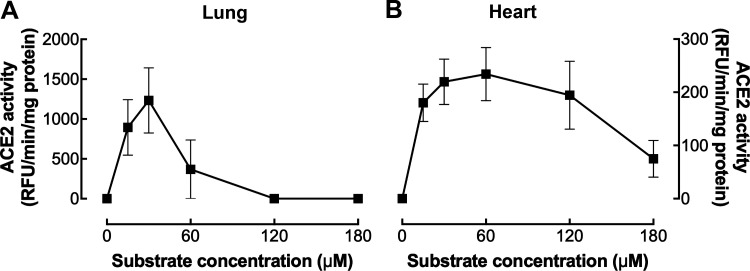
ACE2 enzyme assays, like all enzyme assays, need to be measured in the linear range of a protein concentration curve and conducted under nonsubsrate limiting conditions to ensure ACE2 activity is dependent upon the amount of sample and not inhibited by the lack of substrate. These conditions are tissue-specific. We show that nonsubstrate limiting conditions occur between 30 and 60 µM Mca-APK(dnp) for hamster heart (Fig. 4B) and 30 µM for hamster lung (Fig. 4A). Substrate inhibition must also be considered as many enzymes are inhibited by their own substrate (36). In the hamster heart, substrate inhibition starts to occur at 120 µM with significant inhibition observed at 180 µM (Fig. 4B). In comparison, at the same tissue concentration (150 ng/µL), significant substrate inhibition in the hamster lung occurs at 60 µM (Fig. 4A). Tissue-specific ACE2 substrate inhibition is an area worthy of investigation as substrate inhibition often has important physiological functions (36).
Figure 4.
Substrate dependence of angiotensin-converting enzyme 2 (ACE2) activity in the hamster lung and heart. Substrate dependence of ACE2 enzyme activity in 0.5 µg/µL of lung (n = 2) (A) and heart (n = 4) (B) tissue from male 11-wk-old hamsters. The data are presented as the means ± SE.
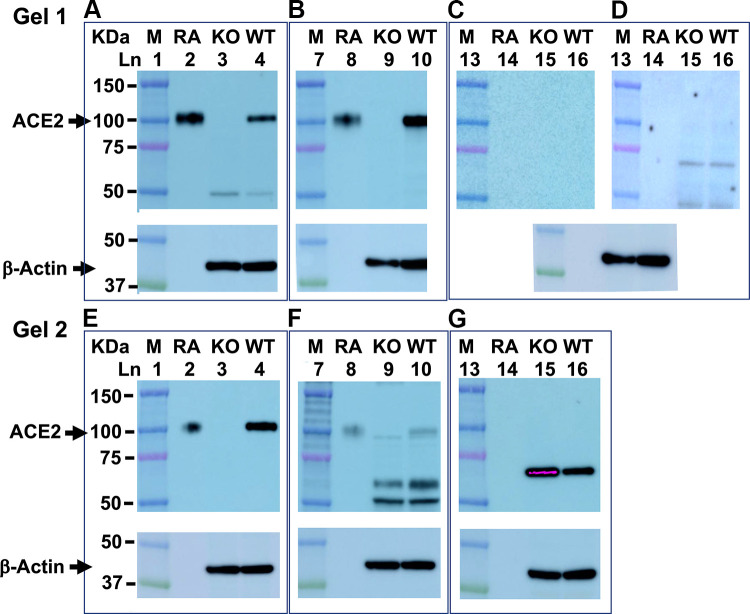
Authentication of ACE2 antibodies is another critical component of accurate ACE2 measurements by immunoblot (37). When we compared three commercially available anti-mouse ACE2 antibodies in kidney tissue from female C57BL/6 mice, we found R&D (Fig. 5A) and Abcam (Fig. 5B) recognized a band of ∼100 kDa in size, which ran with the same mobility as the positive control (mouse recombinant ACE2). Furthermore, this immuno-reactive band was specific for ACE2 because it was present in the kidney of wild-type but not ACE2 knockout mice (Fig. 5, A and B). In contrast, the antibody from Santa Cruz did not recognize mouse recombinant ACE2 with either the Pico Plus substrate (Fig. 5C) or the even more sensitive Femto Maximum-Sensitivity Substrate (Fig. 5D). Antibodies are also species-specific. Antibodies against human ACE2 poorly recognized mouse recombinant and renal ACE2 (Fig. 5, E and F), if at all (Fig. 5G).
Figure 5.
Commercial angiotensin-converting enzyme 2 (ACE2) antibody sensitivity and specificity. Shown are representative Western blots from two gels with 16 wells each. After protein transfer, the PVDF membranes contained protein markers (M; lanes 1, 6, and 13), mouse recombinant ACE2 protein (RA; lanes 2, 8, and 14), and renal tissue from ACE2 knock out (KO; lanes 3, 9, and 15) and wild-type (WT; lanes 4, 10, and 16) female C57BL/6 mice. Membranes were cut into three pieces each and blotted with either anti-mouse ACE2 antibodies from: R&D (A), Abcam (B), and Santa Cruz (C) or anti-human ACE2 antibodies from: Cell Signaling (E), Novus (F), and, Proteintech (G). Super Signal West Pico Plus substrate was used to detect ACE2 immunoreactive protein in A–C and E–G. D shows membrane exposed to the Santa Cruz antibody (C) re-probed with the Femto maximum sensitivity substrate. After probing with ACE2 antibodies, all membranes were stripped and incubated with antibodies to β-actin to control for differences in protein loading.
Ace2 mRNA is easily measured by reverse transcriptase PCR. However, it is important to note that mRNA expression does not necessarily reflect protein levels or enzyme activity due to posttranscriptional and posttranslational regulatory mechanisms. For example, Gupte et al. (20) demonstrated that adipose Ace2 mRNA is posttranscriptionally regulated by a high-fat diet in male mice. After 4 mo on a high-fat diet, Ace2 mRNA expression tripled whereas ACE2 enzyme activity was no different from the low-fat controls.
IMPACT OF BIOLOGICAL SEX ON ACE2 EXPRESSION AND REGULATION
Kidney
Many publications on ACE2 in experimental animals have focused on the kidney, where it is localized in the tunica media of the renal arterioles (38). Studies of ACE2 in male animals have shown that Ace2 mRNA expression and ACE2 protein levels and/or ACE2 enzyme activity are increased in the kidney by hypertension (21), diabetes (39, 40), and acute (41) and chronic (42) kidney injury. These studies suggest upregulation of ACE2 is a compensatory response to renal injury, reducing the Ang-[1–8]-AT1R-mediated increases in blood pressure, inflammation, and tissue fibrosis.
Less is known regarding how ACE2 is regulated in the female kidney. Our laboratory has shown that MF-1 (43) and C57BL/6 (21) female mice have half the amount of ACE2 enzyme activity than their male littermates. ACE2 protein abundance mirrors enzyme activity. Female mice have half the amount of renal ACE2 protein than male mice. Similar sex differences in renal ACE2 enzymatic activity were reported by Gupte et al. (44). They showed ACE2 activity was significantly lower in the kidneys of C57BL/6 female mice compared with male mice fed either a low-fat or high-fat diet.
Findings from our laboratory (43) and others (44) suggest the lower levels of renal ACE2 enzyme activity are due, in part, to the presence of 17β-estradiol (E2). We found ovariectomy increased renal ACE2 activity in MF-1 mice whereas orchiectomy had no effect (43). The four core genotype mouse model can discriminate sex chromosome effects from gonadal hormone effects (45). The Sry testis-determining gene was spontaneously deleted from the Y chromosome, thereby creating the XY− female mouse. By inserting the Sry gene onto an autosome, the XXSry male mouse was generated. Therefore, XX and XY− females can be compared with XXSry and XY−Sry males. Using this model, we showed ACE2 activity was downregulated by E2 independently of the sex chromosome complement (43). Administration of E2 prevented the ovariectomy-induced upregulation of renal ACE2 activity in both XX and XY− females and E2 reduced renal ACE2 activity in XXSry and XY−Sry males. If E2 can also downregulate human ACE2 regardless of the sex chromosome complement, then E2 is a potential therapeutic intervention for men and women with COVID-19. In fact, an ongoing clinical trial is investigating the ability of E2 to reduce the severity of COVID-19 symptoms in both men and women (46).
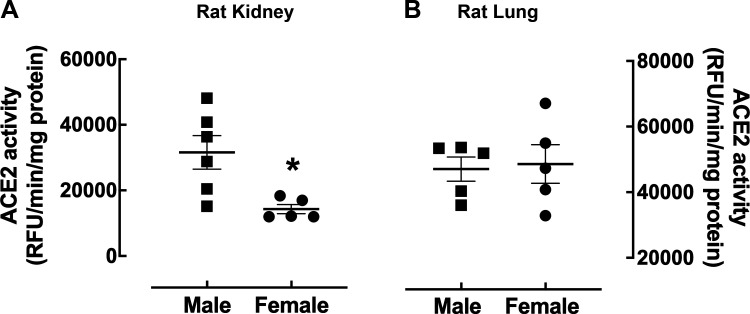
Renal ACE2 enzyme activity is also lower in the female rat kidney compared with the male. Female Long-Evans rats had half as much renal ACE2 activity than the males (Fig. 6A), which is in contrast to the fact that ACE2 activity was found to be markedly higher in the kidney of female spontaneously hypertensive rats (SHR) compared with male SHR (47). Furthermore, gonadectomy had opposite effects in the SHR kidney compared with what we observed in normotensive mice (43). Gonadectomy markedly increased renal ACE2 activity in the male SHR while reducing the activity of this enzyme in the female SHR. Thus, it will be interesting to determine if this discrepancy between Long-Evans rats and SHR in renal ACE2 expression is due to differences in blood pressure.
Figure 6.
Impact of biological sex on angiotensin-converting enzyme 2 (ACE2) activity in the rat kidney and lung. Shown is ACE2 enzyme activity in kidney (A) and lung tissue (B) from female (circle) and male (square) 2–4 mo-old Long-Evans rats at a substrate concentration of 50 µM. The data are presented as a scatter plot showing individual data points (n = 5–6) with the means and SE indicated by horizontal and vertical bars, respectively. The data were analyzed by an unpaired t test with Welch’s correction, *P < 0.05 vs. male.
Heart and Vasculature
In the heart, ACE2 is found in cardiac myocytes and in the endothelium and smooth muscle cells of most intramyocardial vessels, including capillaries, venules, and medium-sized coronary arteries and arterioles (48). We did not observe sex differences in ACE2 protein abundance or activity in whole heart homogenates from normotensive MF-1 mice (43). These findings contrast with sex differences in the kidney and illustrate how the RAS is regulated in a tissue-specific manner.
Most preclinical studies of ACE2 in cardiovascular disease have been conducted in male experimental animals (141 publications conducted in males only vs. 25 conducted exclusively in females). These studies have found that ACE2 is increased in cardiac tissue during heart failure, myocardial infarction, ischemia-reperfusion, and hypertension (23). The few studies that were conducted in both male and female animals (18 publications) suggest that biological sex impacts age effects on ACE2. ACE2 protein levels were higher in the aorta from young (2 mo) C57BL/6 mice compared with old (27 mo) female mice, whereas this effect of age was not observed in male mice (49).
Sex also impacts the pathophysiological regulation of cardiac ACE2 activity. ACE2 activity was higher in the left ventricle of male SHR compared with their female littermates (50, 51). A longitudinal study of the effect of myocardial infarction on the RAS in male and female Wistar rats showed that Ace2 mRNA was upregulated in the left ventricular penumbra in both sexes; however, the time course of changes in Ace2 mRNA were distinctly different between the sexes. Ace2 mRNA was increased 2 wk after infarction to a much greater degree in female compared with male rats, while at 3-wk postinfarction, this sex difference was reversed (52). Thus, sex differences in cardiac ACE2 regulation may be dependent upon blood pressure and time course of cardiac injury.
As observed in the normotensive mouse kidney, ovariectomy increased (1.5-fold) ACE2 activity in the left ventricle of the SHR (50, 51). However, in contrast to observing no change in the normotensive mouse kidney, orchiectomy markedly decreased the activity of ACE2 in the left ventricle by sevenfold. Gonadal regulation of left ventricular ACE2 activity differentially correlated with effects of hypertension on cardiomyocyte hypertrophy; orchiectomy decreased the degree of cardiomyocyte hypertrophy whereas ovariectomy increased the number of hypertrophied cardiomyocytes. Additional studies are needed to determine if these differences in the ability of orchiectomy to modulate ACE2 are species-dependent (mice vs. rats), tissue-dependent (kidney vs. heart), and/or blood pressure dependent (normotensive vs. hypertensive).
ACE2 also protects the vasculature. ACE2 deficiency augmented the magnitude of atherosclerosis and abdominal aortic aneurysm (AAA) formation induced by Ang-[1–8] in hypercholesterolemic mice (53). Furthermore, administration of the ACE2 activator, diminazene aceturate, markedly reduced AAA incidence and aortic diameters in these rodents (54). This protective effect of ACE2 was specific to Ang-[1–8]-dependent AAA formation. Ace2 knockout had no effect on elastase-induced AAA. The protective effects of ACE2 may, in part, be mediated by the enzyme product, Ang-[1–7]. Infusion of Ang-[1–7] at 24 µg/kg/h into mice reduced Ang-[1–8]-induced atherosclerosis (55). However, the heptapeptide dose in these mouse studies was pharmacologic rather than physiological; circulating Ang-[1–7] levels are reported in the pM range (56). These studies of ACE2 in AAA were all conducted in male mice. Therefore, it is unclear what role ACE2 plays in AAA formation in females.
Adipose Depots
Gupte et al. (20) demonstrated that 3T3-L1 adipocytes and epididymal and subcutaneous adipose tissue express Ace2 mRNA and ACE2 enzyme activity. The authors also showed that adipose ACE2 expression was regulated in a tissue-specific manner by a high-fat diet; whereas adipose ACE2 activity did not change, and plasma ACE2 activity was increased by 1.7-fold.
Adipose ACE2 regulation in the female mouse is distinctly different from the male. Gupte et al. (44) showed that ACE2 activity was higher in adipose tissue from male compared with female mice maintained on a low-fat diet. On a high-fat diet, ACE2 activity increased in adipose tissue in female mice, as did plasma Ang-[1–7] levels. The upregulation of adipose ACE2 activity was due to E2 because ovariectomy diminished the ACE2 upregulation induced by the high-fat diet whereas E2 replacement prevented this effect of ovariectomy.
The activity of the RAS contributes to and modulates metabolic syndrome and obesity, which are major risk factors for cardiovascular disease (57) and COVID-19 severity (58). Thus, investigating mechanisms of adipose ACE2 regulation in both sexes, in context of the RAS and obesity, will improve our understanding of major risk factors for cardiovascular disease and COVID-19 severity in both men and women.
Lung
ACE2 is present in type II bronchoalveolar cells in the pulmonary parenchyma, alveolar macrophages, and epithelial cells in the lungs (59, 60). Although not the highest ACE2 expressing tissue in the body (61–63), a systematic review of the histopathological observations associated with COVID-19 reveal the lungs to be the most affected tissue (64). Entry of the virus into the host cells induces a hyperimmune response, dramatically elevating inflammatory cytokine release (27, 65,66). The rapid expression of these inflammatory mediators following infection leads to acute respiratory distress with multiple organ failure.
Lung ACE2 is the portal the virus uses to gain entry and wreak havoc within cells. However, ACE2 also has a protective role in lung pathophysiology. In preclinical models of pulmonary damage, lung ACE2 defends the tissue from injury. Imai et al. (67) showed that Ace2 knockout mice had more severe lung injury induced by acid aspiration than wild-type mice. Furthermore, the addition of recombinant ACE2 rescued these knockout mice from the pulmonary injury exacerbated by Ace2 deletion. The protective effects were likely due to the ability of ACE2 to catabolize Ang-[1–8] since the lung injury observed in the Ace2 knockout mice was reversed by deleting Ace, the enzyme that synthesizes Ang-[1–8] (Fig. 1). Recombinant ACE2 also attenuated lung injury induced by lipopolysaccharide in male and female piglets (68). While the authors of these studies in mice and pigs included both male and female animals, unfortunately, neither study analyzed their data by sex. Therefore, it is unknown to what extent biological sex impacts the magnitude of these effects.
In another model of lung injury, Rey-Parra et al. (69) showed that bleomycin caused far less lung damage in female compared to male wild-type mice. While male mice exhibited marked fibrotic changes, loss in lung function, and exercise capacity, no fibrosis or loss of lung function was observed in the females. Furthermore, female Ace2 knockout mice exhibited far less bleomycin-induced architectural distortions, collagen and hydroxyproline accumulation compared with their male counterparts. While administration of recombinant ACE2 rescued male Ace2 knockout mice from the lung injury, few effects from ACE2 administration were observed in the female. This study demonstrates the value of analyzing data by sex because combining male and female data would have led to excessive variability in the results due to marked sex differences in the magnitude of injury. Furthermore, these marked sex differences in the degree of lung damage would have confounded male-female comparisons of individual genes and pathways contributing to the injury. In other words, the role of ACE2 in pulmonary dysfunction cannot be effectively assessed in the female using this model of bleomycin-induced lung injury.
Studies in other models of lung injury further support a protective role for ACE2 in pulmonary disease. Treatment of male rats with diminazene aceturate, an ACE2 activator, attenuated the development of pulmonary hypertension induced by monocrotaline administration in Sprague-Dawley rats (70). Furthermore, administration of encapsulated recombinant ACE2 was able to attenuate the progression of pulmonary hypertension in this rat model (71). Therefore, efforts to reduce lung ACE2 expression as a potential COVID-19 therapeutic must take into account the possible adverse effects of losing the pulmonary vasodilatory arm of the RAS that serves to counter the proinflammatory and fibrotic axis of the RAS (63, 72, 73).
Only 13 publications have compared lung ACE2 expression and regulation in male and female animal models. We previously showed that ACE2 enzyme activity was not significantly different in the lung of male and female MF-1 mice (43). Our recent studies show lung ACE2 enzyme activity is also similar between male and female Long-Evans rats (Fig. 6B). SARS-CoV-2 first enters the body through the lungs and swabs from the nasal pathway are most commonly used to test for SARS-CoV-2 positivity. Therefore, the lack of sex difference in lung ACE2 supports the clinical finding that the incidence of COVID-19 is similar between men and women (https://globalhealth5050.org/the-sex-gender-and-covid-19-project/, accessed March 1, 2021).
In contrast, a study of Ace2 mRNA in the lung of old (15–17 mo) hamsters showed males had over threefold higher levels of ACE2 than the old females (74). The authors also found Ace2 mRNA was 37-fold higher in old male hamsters compared with young (2–4 mo) males. It is not known if the observed sex difference in lung Ace2 mRNA was also observed at younger ages since they did not measure ACE2 in young females. Furthermore, these sex and age differences in Ace2 mRNA may not correspond to enzyme activity since ACE2 protein levels do not tightly correlate with mRNA expression due to posttranscriptional and/or posttranslational regulatory mechanisms (20, 75).
Sarver and Wong (76) reported diet-induced obesity increased lung Ace2 mRNA in male C57BL/6J mice but had no effect in females. Thus, higher lung expression of the receptor the virus uses to enter the body in obese males could contribute to why obesity and male sex are risk factors for severe COVID-19. Given that the primary pathology associated with SARS-CoV-2 occurs in the lung, it is especially important to understand the physiological and pathophysiological regulation of ACE2 in this organ in both sexes. Many questions remain and require further research to determine if biological sex impacts mechanisms of SARS-CoV-2 pathogenesis as a function of age and underlying comorbidities that are associated with worse outcomes including obesity, hypertension, cardiovascular, renal disease as well as response to treatment.
SEX-SPECIFIC EFFECTS OF ACE2 ON BLOOD PRESSURE REGULATION
ACE2 contributes to blood pressure regulation in a sex-specific manner. We found that ACE2 plays a greater role in attenuating blood pressure in female compared with male mice in the Ang-[1–8]-infusion model of hypertension (21). These studies showed ACE2 exerted greater protection in the female by lowering renal AT1Rs to compensate for the hypertension-induced by Ang-(1–8) (Fig. 1).
ACE2 also serves as a protective mechanism against obesity-associated hypertension. Shoemaker et al. (77) showed that a high-fat diet increased systolic blood pressure in male and female mice compared with low-fat-diet-fed controls; however, adipose-specific ACE2 deficiency augmented the high-fat-diet-induced elevations in systolic blood pressures only in female mice. The authors also demonstrated that administration of E2 to high-fat-diet-fed ovariectomized mice decreased day-time systolic blood pressure. Further studies in adipose-specific Ace2 knockout mice showed that this E2 depressor effect was dependent upon the presence of adipose ACE2 (77). In contrast, E2 had no effect on blood pressure in male mice fed the same diet (78). These studies indicate that upregulation of adipose ACE2 contributes to the antipressor activity of E2 in obese female mice. These findings suggest that ACE2 plays a larger role in protecting female mice from obesity-associated hypertension compared with male mice.
When diabetes was induced in the hypertensive mREN(2) Lewis rat using streptozotocin, systolic blood pressure increased to a greater extent in the males (to 224 mmHg) compared with the females (164 mmHg) (79). Under these conditions, the induction of diabetes increased circulating ACE2 activity by ninefold in the diabetic females but only threefold in the diabetic males when compared with their respective nondiabetic controls. These findings suggest that ACE2 is a more active player in combatting the diabetes pressor effect in female hypertensive rats compared with male rats.
Further evidence that ACE2 plays a greater role in controlling blood pressure in females is found in the studies of offspring from mothers subjected to in utero, undernutrition/intrauterine growth retardation. An antenatal maternal low-protein diet in pregnant mice reduced ACE2 protein levels in the lungs of female but not in male offspring (80). Furthermore, the low-protein diet increased systolic blood pressure in the female, but not in the male mice offspring. The capillary blood vessels of the lung are a major site of Ang-[1–8] production by ACE. Therefore, this research suggests that the reduction in ACE2 increased the activity of the vasoconstrictor arm of the RAS in the lung, thereby contributing to the rise in blood pressure in the female offspring.
Hypertension is a major risk factor for morbidity and mortality in COVID-19 (81). Early in the pandemic, controversy emerged over whether or not ACE inhibitors (ACEis) or AT1R blockers (ARBs) would be beneficial or harmful in patients with COVID-19 (23). This controversy arose because ACEIs and ARBs increased ACE2 in the rodent kidney (38) and heart (82, 83). Recent studies have shed light on the effects of these antihypertensives in COVID-19. A retrospective review of patients with COVID-19 and hypertension found that those receiving ACEi or ARB therapy had milder disease and lower levels of circulating inflammatory markers (84). This finding was reproduced in a larger-scale retrospective study of patients with COVID-19; the number of deaths in hospitalized patients with hypertension and coronary artery disease were less in those treated with ACEi or ARBs (85). However, these studies did not disaggregate the data by sex. Basic science findings that ACE2 plays a greater role in regulating female blood pressure have implications for COVID-19 therapeutics targeting ACE2. Not only these current and future therapeutics have different efficacies in men and women, they may also have sex-specific outcomes and side effects depending upon their interaction with ACE2.
SARS-COV-2 REGULATION OF ACE2
Research suggests that upon binding the coronavirus spike protein, ACE2 is downregulated. Prior studies with SARS-CoV-1-infected Vero E6 cells showed that the spike protein reduced membrane bound ACE2 as a result of ACE2 shedding from the cell surface through proteolytic cleavage of its extracellular domain (86). A recent report in human embryonic kidney cells cotransfected with the SARS-CoV-2 spike protein and human ACE2 showed that the spike protein can also trigger ACE2 internalization (87). Thus, it is possible that the vasoconstrictor arm of the RAS could be upregulated by SARS-CoV-2 infection through inhibition of the vasoconstrictor arm, as a result of ACE2 downregulation (Fig. 1).
This possibility was examined in a small human study, which showed no changes in circulating Ang-[1–8], Ang-[1–7] or plasma ACE2 activity in 12 patients with moderate COVID-19 compared with controls (n = 9) (88). Although this human study suggests that the circulating RAS is not affected by moderate disease, studies are needed to expand upon these findings to larger populations so that the biological variable of sex can be assessed on the course and severity of COVID-19. In a non-COVID-19 population, plasma ACE2 protein levels were measured using a mass-spectrometry-based method in 86 healthy individuals over four visits during one year. This study showed that the average plasma concentration of ACE2 protein was slightly higher in men compared with women (75).
The relationship between SARS-CoV-2 infectivity and tissue-specific regulation of ACE2 is not well understood in either sex. Although the mouth and nose are considered to be the major entry pathways of the virus into the lungs, studies now show COVID-19 pathogenesis in the heart, kidneys, and brain (6, 21, 86). The gold standard to prove the existence and cellular location of an infectious unit is electron microscopy. Therefore, it will be imperative to study tissue-specific regulation of ACE2 in the presence of SARS-CoV-2 infection along with expert diagnostic electron microscopy to avoid the growing problem of misconstruing nonviral structures as viral particles (89, 90).
SPECIES DIFFERENCES IN ACE2
ACE2 homologs in mice and rats are poor receptors for the spike protein of SARS-CoV-2, making these species resistant to COVID-19 (91). To circumvent this problem, investigators have developed mouse models that express human ACE2 (92, 93). While these mice exhibit varying degrees of COVID-19 pathology, the expressed human ACE2 gene is not under normal physiological control. Thus, investigating ACE2 regulation in the pathophysiology of SARS-CoV-2 infection is limited using these animal models.
As of December 31, 2020, only a few studies of ACE2 (13 publications) have been conducted in mammalian species susceptible to COVID-19. Although five of these publications included males and females, only one of these studies analyzed the data by sex. Rosenke et al. (94) showed that biological sex had no major impact on viral load or infectious titers in the lungs of young adult hamsters infected with SARS-CoV-2. Thus, far more research on ACE2 is needed in these COVID-19 susceptible animal models. Considering the biological variable of sex in future studies will ensure we understand the mechanisms underlying SARS-CoV-2 pathogenesis throughout all the affected tissues in the body as well as the mechanisms underlying the response to therapeutics in both males and females.
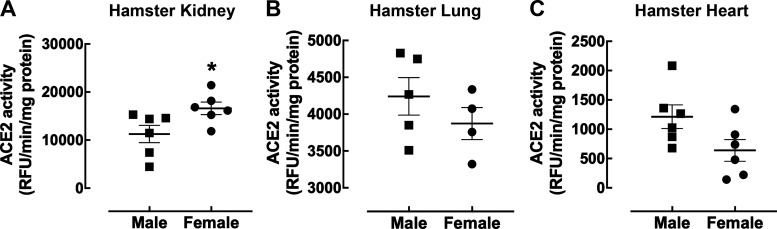
Species differences in ACE2 protein levels and enzyme activity exist. Although ACE2 protein is highly abundant in human (75) and mouse (95) kidney, this enzyme is expressed at far lower levels (only 7.3% of mouse renal activity) in the rat kidney (95). Species-specific effects of biological sex also exist. In contrast to mice and rats, female hamsters had 1.5-fold higher levels of renal ACE2 enzyme activity compared with their male counterparts (Fig. 7A). Similar to mice, no sex difference was observed in hamster ACE2 activity in the lung (Fig. 7B) and heart (Fig. 7C).
Figure 7.
Impact of biological sex on angiotensin-converting enzyme 2 (ACE2) activity in the hamster kidney, lung, and heart. Shown is ACE2 enzyme activity from 10 ng/µL of kidney tissue (A) and 50 ng/µl of lung (B) and heart (C) from female (circle) and male (square) 11-wk-old Syrian golden hamsters at 30 µM substrate concentration. The data are presented as a scatter plot showing individual data points (n = 6) with the means and SE indicated by horizontal and vertical bars, respectively. The data were analyzed by an unpaired t test with Welch’s correction, *P < 0.05 vs. male.
There are also species-specific differences in ACE2 substrate specificity. Although Ang-[1–8] is the preferred substrate for ACE2 and turnover rates are similar in both mice and humans, the mouse ACE2 turnover rate for Ang-[1–10] to Ang-[1–9] conversion is less than 10% of the respective turnover rate for human ACE2 (11). In contrast, human ACE2 can generate Ang-[1–9] at physiological peptide concentrations. This is particularly important given that inhibitors of ACE, which are widely used clinically to treat hypertension, increase the levels of Ang-[1–10] by preventing the catabolism to Ang-[1–8] (Fig. 1). Therefore, species-specific regulation of Ang peptides is an important consideration when studying ACE2 physiology and pathophysiology.
CONCLUSIONS
The 6:1 ratio of publications in male to female studies on ACE2 in preclinical animals is striking and representative of male bias in other basic science fields. It is also notable that less than 9.3% of these published studies were conducted in both sexes and that even less (2.9%) analyzed the data by sex. Of the few studies that have compared the sexes, ACE2 expression and regulation were shown to be impacted by sex in models of hypertension, obesity, cardiovascular, and renal disease. Emerging research suggests gonadal hormones rather than sex chromosomes are responsible for these sex differences though far more research is needed to fully understand the mechanisms of these sex differences in various tissues and models of disease. These observations emphasize that the inaccuracy of assuming mechanisms of ACE2 regulation elucidated in male animals occurs identically in females. Therefore, it remains imperative that basic research on ACE2 also be conducted in female models, not just males. Not appreciating the impact of sex on ACE2 expression and regulation will lead to missed opportunities for drug discovery in treating the myriad of diseases involving dysregulation of the RAS and also for COVID-19.
Perspectives and Significance
The disproportionately low level of research on ACE2 in females and the lack of substantive change in this ratio over the past two decades provides strong justification for re-examining the effectiveness of efforts by NIH (96) and others (97) to promote consideration of the biological variable of sex in basic science research. Understanding sex differences in ACE2 expression and regulation would have better positioned the field for discovering and developing therapeutic targets for COVID-19 (98). Apparently, more effective educational efforts are needed to encourage research in female animal models and to combat the false belief that physiological measurements are intrinsically more variable in females compared with males because of the estrous cycle (99). This myth is often cited as to why investigators avoid studying female animals. In addition to educating scientists, funding agencies should expand funding opportunities for research in female animal models to combat the male bias in basic science research. The dearth of studies in females contributes to irreproducibility in science and negatively impacts drug discovery, which harms both men and women through missed opportunities for unearthing novel drug targets through sex-specific findings (96, 100).
ETHICS APPROVALS
All experimental procedures were executed in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals, ARRIVE guidelines, and were approved by the Institutional Animal Care and Use Committee at Georgetown University Medical Center and Nova Southeastern University.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
GRANTS
This project was supported by the National Institutes of Health Grants R01AG019291, R21AG039779, and R01HL119380 (to K. Sandberg) and R21AG060244 (to H. Ji).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M.S. and K.S. conceived and designed research; A.M.A.d.S., X.W., D.M., and H.J. performed experiments; B.M.S., S.M., A.M.A.d.S., D.M., H.J., and R.C.S. analyzed data; B.M.S., S.M., and R.C.S. interpreted results of experiments; B.M.S., A.M.A.d.S, and H.J. prepared figures; B.M.S., S.M., H.J., R.C.S., and K.S. drafted manuscript; B.M.S., A.M.A.d.S., R.C.S., and K.S. edited and revised manuscript; B.M.S., S.M., A.M.A.d.S., X.W., D.M., H.J., R.C.S., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Daisy de la Rosa for assistance in conducting the literature review.
REFERENCES
- 1.Silva GM, Franca-Falcao MS, Calzerra NTM, Luz MS, Gadelha DDA, Balarini CM, Queiroz TM. Role of renin-angiotensin system components in atherosclerosis: focus on Ang-II, ACE2, and Ang-1–7. Front Physiol 11: 1067, 2020. doi: 10.3389/fphys.2020.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CH, Mohammadmoradi S, Chen JZ, Sawada H, Daugherty A, Lu HS. Renin-angiotensin system and cardiovascular functions. Arterioscler Thromb Vasc Biol 38: e108–e116, 2018. doi: 10.1161/ATVBAHA.118.311282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Del Borgo M, Lee HW, Baraldi D, Hirmiz B, Gaspari TA, Denton KM, Aguilar MI, Samuel CS, Widdop RE. Anti-fibrotic potential of AT2 receptor agonists. Front Pharmacol 8: 564, 2017. doi: 10.3389/fphar.2017.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Han L, Shen M, Jones ES, Spizzo I, Walton SL, Denton KM, Gaspari TA, Samuel CS, Widdop RE. Serelaxin and the AT2 receptor agonist CGP42112 evoked a similar, nonadditive, cardiac antifibrotic effect in high salt-fed mice that were refractory to candesartan cilexetil. ACS Pharmacol Transl Sci 3: 76–87, 2020. doi: 10.1021/acsptsci.9b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension 27: 523–528, 1996. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 7.Medina D, Arnold AC. Angiotensin-(1–7): translational avenues in cardiovascular control. Am J Hypertens 32: 1133–1142, 2019. doi: 10.1093/ajh/hpz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 9.de Souza AMA, West CA, de Abreu ARR, Pai AV, Mesquita LBT, Ji H, Chianca D Jr, de Menezes RCA, Sandberg K. Role of the renin angiotensin system in blood pressure allostasis-induced by severe food restriction in female fischer rats. Sci Rep 8: 10327, 2018. doi: 10.1038/s41598-018-28593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rooyen JM, Poglitsch M, Huisman HW, Mels C, Kruger R, Malan L, Botha S, Lammertyn L, Gafane L, Schutte AE. Quantification of systemic renin-angiotensin system peptides of hypertensive black and white African men established from the RAS-Fingerprint(R). J Renin Angiotensin Aldosterone Syst 17: 147032031666988, 2016. doi: 10.1177/1470320316669880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poglitsch M, Domenig O, Schwager C, Stranner S, Peball B, Janzek E, Wagner B, Jungwirth H, Loibner H, Schuster M. Recombinant expression and characterization of human and murine ACE2: species-specific activation of the alternative renin-angiotensin-system. Int J Hypertens 2012: 428950, 2012. doi: 10.1155/2012/428950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 98: 505–553, 2018. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev 67: 754–819, 2015. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaidarov I, Adams J, Frazer J, Anthony T, Chen X, Gatlin J, Semple G, Unett DJ. Angiotensin (1–7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor. Cell Signal 50: 9–24, 2018. doi: 10.1016/j.cellsig.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira LB, Parreiras ESLT, Bruder-Nascimento T, Duarte DA, Simoes SC, Costa RM, Rodriguez DY, Ferreira PAB, Silva CAA, Abrao EP, Oliveira EB, Bouvier M, Tostes RC, Costa-Neto CM. Ang-(1–7) is an endogenous beta-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Sci Rep 7: 11903, 2017. doi: 10.1038/s41598-017-12074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960–966, 2005. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 17.Pai AV, Maddox T, Sandberg K. T cells and hypertension: solved and unsolved mysteries regarding the female rat. Physiology (Bethesda) 33: 254–260, 2018. doi: 10.1152/physiol.00011.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Munoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. Angiotensin1–9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol 589: 939–951, 2011. doi: 10.1113/jphysiol.2010.203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295: R781–R788, 2008. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji H, de Souza AMA, Bajaj B, Zheng W, Wu X, Speth RC, Sandberg K. Sex-specific modulation of blood pressure and the renin-angiotensin system by ACE (angiotensin-converting enzyme) 2. Hypertension 76: 478–487, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1–7) in 17β-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 93: 648–657, 2008. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 23.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318: H1084–H1090, 2020. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia H, de Queiroz TM, Sriramula S, Feng Y, Johnson T, Mungrue IN, Lazartigues E. Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. Am J Physiol Regul Integr Comp Physiol 308: R370–R378, 2015. doi: 10.1152/ajpregu.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 94: e00127-00120, 2020. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renzi S, Landoni G, Zangrillo A, Ciceri F. MicroCLOTS pathophysiology in COVID 19. Korean J Intern Med. 2020. doi: 10.3904/kjim.2020.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein SL, Dhakal S, Ursin RL, Deshpande S, Sandberg K, Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLoS Pathog 16: e1008570, 2020. doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, Gavin JM, Hales P, Kaushik VK, Stewart M, Tummino PJ, Vickers CS, Ocain TD, Patane MA. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc 124: 11852–11853, 2002. doi: 10.1021/ja0277226. [DOI] [PubMed] [Google Scholar]
- 31.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandberg K, Verbalis JG, Yosten GL, Samson WK. Sex and basic science. A title IX position. Am J Physiol Regul Integr Comp Physiol 307: R361–R365, 2014. doi: 10.1152/ajpregu.00251.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandberg K, Pai AV, Maddox T. Sex and rigor: the TGF-β blood pressure affair. Am J Physiol Renal Physiol 313: F1087–F1088, 2017. doi: 10.1152/ajprenal.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E9, 2000. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 35.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 92: 1433, 2020. doi: 10.1002/jmv.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed MC, Lieb A, Nijhout HF. The biological significance of substrate inhibition: a mechanism with diverse functions. Bioessays 32: 422–429, 2010. doi: 10.1002/bies.200900167. [DOI] [PubMed] [Google Scholar]
- 37.McDonough AA, Veiras LC, Minas JN, Ralph DL. Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015. doi: 10.1152/ajpcell.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 296: F398–F405, 2009. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 39.Shin YH, Min JJ, Lee JH, Kim EH, Kim GE, Kim MH, Lee JJ, Ahn HJ. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels 32: 618–627, 2017. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- 40.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burchill L, Velkoska E, Dean RG, Lew RA, Smith AI, Levidiotis V, Burrell LM. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol 93: 622–630, 2008. doi: 10.1113/expphysiol.2007.040386. [DOI] [PubMed] [Google Scholar]
- 42.Tikellis C, Bernardi S, Burns WC. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens 20: 62–68, 2011. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Diff 1: 6, 2010. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392–1399, 2012. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold AP. Four Core Genotypes and XY* mouse models: update on impact on SABV research. Neurosci Biobehav Rev 119: 1–8, 2020. doi: 10.1016/j.neubiorev.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachman S. Estrogen patch for COVID-19 symptoms (Online). https://clinicaltrials.gov/ct2/show/NCT04359329 [2020 May 1].
- 47.Melo Junior AF, Dalpiaz PLM, Escouto LDS, Sousa GJ, Aires R, Oliveira ND, Carmona AK, Gava AL, Bissoli NS. Involvement of sex hormones, oxidative stress, ACE and ACE2 activity in the impairment of renal function and remodelling in SHR. Life Sci 257: 118138, 2020. doi: 10.1016/j.lfs.2020.118138. [DOI] [PubMed] [Google Scholar]
- 48.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26: 369–375, 2005. discussion 322–364. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 49.Bartova E, Legartova S, Krejci J, Arcidiacono OA. Cell differentiation and aging accompanied by depletion of the ACE2 protein. Aging (Albany NY) 12: 22495–22508, 2020. doi: 10.18632/aging.202221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalpiaz EL, Lamas AZ, Caliman IF, Ribeiro RF Jr, Abreu GR, Moyses MR, Andrade TU, Gouvea SA, Alves MF, Carmona AK, Bissoli NS. Correction: sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 10: e0133225, 2015. doi: 10.1371/journal.pone.0133225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalpiaz PL, Lamas AZ, Caliman IF, Ribeiro RF Jr, Abreu GR, Moyses MR, Andrade TU, Gouvea SA, Alves MF, Carmona AK, Bissoli NS. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 10: e0127515, 2015. doi: 10.1371/journal.pone.0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores-Monroy J, Lezama-Martinez D, Fonseca-Coronado S, Martinez-Aguilar L. Differences in the expression of the renin angiotensin system and the kallikrein-kinin system during the course of myocardial infarction in male and female Wistar rats. J Renin Angiotensin Aldosterone Syst 21: 1470320319900038, 2020. doi: 10.1177/1470320319900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thatcher SE, Zhang X, Howatt DA, Lu H, Gurley SB, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow-derived cells increases atherosclerosis in low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol 31: 758–765, 2011. doi: 10.1161/ATVBAHA.110.221614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thatcher SE, Zhang X, Howatt DA, Yiannikouris F, Gurley SB, Ennis T, Curci JA, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 34: 2617–2623, 2014. doi: 10.1161/ATVBAHA.114.304613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1–7) in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30: 1606–1613, 2010. doi: 10.1161/ATVBAHA.110.204453. [DOI] [PubMed] [Google Scholar]
- 56.Stegbauer J, Thatcher SE, Yang G, Bottermann K, Rump LC, Daugherty A, Cassis LA. Mas receptor deficiency augments angiotensin II-induced atherosclerosis and aortic aneurysm ruptures in hypercholesterolemic male mice. J Vasc Surg 70: 1658–1668.e1, 2019. doi: 10.1016/j.jvs.2018.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H1219–H1230, 2012. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 43: e72–e74, 2020. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 59.Barrantes FJ. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem Neurosci 11: 2793–2803, 2020. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- 60.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol 92: 726–730, 2020. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van GH. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 203: 740–743, 2004. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID-19: a systematic review. J Clin Pathol 74: 76–83, 2021. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 65.Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503: 535–538, 2013. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17: 613–620, 2020. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Treml B, Neu N, Kleinsasser A, Gritsch C, Finsterwalder T, Geiger R, Schuster M, Janzek E, Loibner H, Penninger J, Loeckinger A. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med 38: 596–601, 2010. doi: 10.1097/CCM.0b013e3181c03009. [DOI] [PubMed] [Google Scholar]
- 69.Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thebaud B. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med (Berl) 90: 637–647, 2012. doi: 10.1007/s00109-012-0859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rigatto K, Casali KR, Shenoy V, Katovich MJ, Raizada MK. Diminazene aceturate improves autonomic modulation in pulmonary hypertension. Eur J Pharmacol 713: 89–93, 2013. doi: 10.1016/j.ejphar.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizada MK. Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64: 1248–1259, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Ez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 182: 1065–1072, 2010. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suresh V, Parida D, Minz AP, Sethi M, Sahoo BS, Senapati S. Tissue distribution of ACE2 protein in Syrian golden hamster (Mesocricetus auratus) and its possible implications in SARS-CoV-2 related studies. Front Pharmacol 11: 579330, 2021. doi: 10.3389/fphar.2020.579330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, Song X, Wu Y, Zeng Q, Jin F, Wang Y. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 17: 1522–1531, 2020. doi: 10.7150/ijms.46695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarver DC, Wong GW. Obesity alters Ace2 and Tmprss2 expression in lung, trachea, and esophagus in a sex-dependent manner: implications for COVID-19. Biochem Biophys Res Commun 538: 92–96, 2021. doi: 10.1016/j.bbrc.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shoemaker R, Tannock LR, Su W, Gong M, Gurley SB, Thatcher SE, Yiannikouris F, Ensor CM, Cassis LA. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol Sex Diff 10: 45, 2019. doi: 10.1186/s13293-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Shoemaker R, Thatcher SE, Batifoulier-Yiannikouris F, English VL, Cassis LA. Administration of 17beta-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am J Physiol Endocrinol Metab 308: E1066–E1075, 2015. doi: 10.1152/ajpendo.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaleyeva LM, Gilliam-Davis S, Almeida I, Brosnihan KB, Lindsey SH, Chappell MC. Differential regulation of circulating and renal ACE2 and ACE in hypertensive mRen2.Lewis rats with early-onset diabetes. Am J Physiol Renal Physiol 302: F1374–F1384, 2012. doi: 10.1152/ajprenal.00656.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goyal R, Van-Wickle J, Goyal D, Longo LD. Antenatal maternal low protein diet: ACE-2 in the mouse lung and sexually dimorphic programming of hypertension. BMC Physiol 15: 2, 2015. doi: 10.1186/s12899-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 83.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970–976, 2004. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 84.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 9: 757–760, 2020. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou F, Liu Y-M, Xie J, Li H, Lei F, Yang H, Quin J-J, Cai J, Zhang X-J, Wu B, Xia M, Xian D, Yang C, Ma X, Xu Q, Lu Z, Lu H, Xia X, Wang D, Liao X, Peng G, Yang J, Huang X, Zhang B-H, Yuan Y, Wei X, Liu PP, Wang Y, Zhang P, She Z-G, Xia J, Li H. Comparative impacts of ACE (angiotensin-converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension 76: e15–e17, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15622. [DOI] [PubMed] [Google Scholar]
- 86.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pohlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 84: 1198–1205, 2010. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogunlade BO, Lazartigues E, Filipeanu CM. Angiotensin type 1 receptor-dependent internalization of SARS-CoV-2 by angiotensin converting enzyme 2. Hypertension 77: e42–e43, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kintscher U, Slagman A, Domenig O, Rohle R, Konietschke F, Poglitsch M, Mockel M. Plasma angiotensin peptide profiling and ACE (angiotensin-converting enzyme)-2 activity in COVID-19 patients treated with pharmacological blockers of the renin-angiotensin system. Hypertension 76: e34–e36, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dittmayer C, Meinhardt J, Radbruch H, Radke J, Heppner BI, Heppner FL, Stenzel W, Holland G, Laue M. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet 396: e64–e65, 2020. doi: 10.1016/S0140-6736(20)32079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet 395: e99, 2020. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, Koepfli KP, Pfenning Ar Zhao H, Genereux DP, Swofford R, Pollard KS, Ryder OA, Nweeia MT, Lindblad-Toh K, Teeling EC, Karlsson EK, Lewin HA. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA 117: 22311–22322, 2020. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johansen MD, Irving A, Montagutelli X, Tate MD, Rudloff I, Nold MF, Hansbro NG, Kim RY, Donovan C, Liu G, Faiz A, Short KR, Lyons JG, McCaughan GW, Gorrell MD, Cole A, Moreno C, Couteur D, Hesselson D, Triccas J, Neely GG, Gamble JR, Simpson SJ, Saunders BM, Oliver BG, Britton WJ, Wark PA, Nold-Petry CA, Hansbro PM. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol 13: 877–891, 2020. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, et al. Animal models for COVID-19. Nature 586: 509–515, 2020. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenke K, Meade-White K, Letko M, Clancy C, Hansen F, Liu Y, Okumura A, Tang-Huau TL, Li R, Saturday G, Feldmann F, Scott D, Wang Z, Munster V, Jarvis MA, Feldmann H. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg Microbes Infect 9: 2673–2684, 2020. doi: 10.1080/22221751.2020.1858177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277, 2005. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J 30: 519–524, 2016. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandberg K, Umans JG; Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J 29: 1646–1652, 2015. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galkina Cleary E, Beierlein JM, Khanuja NS, McNamee LM, Ledley FD. Contribution of NIH funding to new drug approvals 2010-2016. Proc Natl Acad Sci USA 115: 2329–2334, 2018. doi: 10.1073/pnas.1715368115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dayton A, Exner EC, Bukowy JD, Stodola TJ, Kurth T, Skelton M, Greene AS, Cowley AW Jr.. Breaking the cycle: estrous variation does not require increased sample size in the study of female rats. Hypertension 68: 1139–1144, 2016. doi: 10.1161/hypertensionaha.116.08207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature 505: 612–613, 2014. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.