Abstract
Airway oscillometry has become the de facto standard for quality assessment of lung physiology in laboratory animals and has demonstrated its usefulness in understanding diseases of small airways. Nowadays, it is seeing extensive use in daily clinical practice and research; however, a question that remains unanswered is how well physiological findings in animals and humans correlate? Methodological and device differences are obvious between animal and human studies. However, all devices deliver an oscillated airflow test signal and output respiratory impedance. In addition, despite analysis differences, there are ways to interpret animal and human oscillometry data to allow suitable comparisons. The potential with oscillometry is its ability to reveal universal features of the respiratory system across species, making translational extrapolation likely to be predictive. This means that oscillometry can thus help determine if an animal model displays the same physiological characteristics as the human disease. Perhaps more importantly, it can also be useful to determine whether an intervention is effective as well as to understand if it affects the desired region of the respiratory system, e.g., the periphery of the lung. Finally, findings in humans can also inform preclinical scientists and give indications as to what type of physiological changes should be observed in animal models to make them relevant as models of human disease. The present article will attempt to demonstrate the potential of oscillometry in respiratory research, an area where the development of novel therapies is plagued with a failure rate higher than in other disease areas.
Keywords: drug research, forced oscillation technique, lung physiology, oscillometry, translational research
THE PROBLEM
There is a need for novel therapies to treat respiratory diseases and lessen their consequences. However, drug validation is a process involving several steps, the most critical being to demonstrate efficacy in terms of improving an outcome, one of which is lung function. Despite thorough preclinical research and validation, it is common for drug candidates to fail during a late development stage. This is often due to failure to demonstrate clinical efficacy from a lack of demonstrated effects on physiological parameters or to an unexpected toxicity that can include an adverse respiratory reaction like an acute bronchoconstriction (1, 2). The cost of such failures is not only monetary. In a situation of crisis where there are urgent needs (e.g., COVID-19 pandemic), this could also mean significant time delays and loss of lives (3), as well as significant economic consequences. Physiological lung function measurements are performed for several reasons such as establishing a diagnosis or the efficacy of a therapeutic intervention, validating a drug target, monitoring disease development, or for safety pharmacology purposes, just to mention a few. The necessity for a physiological measurement is not restricted to a single species. Quantitative and reliable information about the health status of an individual is required, whether this is a mouse studied in a laboratory or a human patient seeing their doctor or participating in a clinical study. In fact, most medical research areas are developing in both laboratory animal models and patients at the same time. While there are differences in what can be practically and ethically done, it will always be desirable to be able to translate findings between the two research specialties so that they can inform one another in a meaningful way. Consequently, there is a need to understand the limitations and the differences of the laboratory models and how well they describe the human condition they are trying to emulate.
From a physiological point of view, it would make sense to use measurement techniques that closely match across species, not only in terms of readout quality, precision, and sensitivity, but also on the aspect of the lung function being assessed. In several areas, this has already been achieved (e.g., identification and quantification of cell populations, mediator release, effects on electrocardiogram, various blood parameters etc.). In lung physiology, however, there are hitherto discrepancies between what is being measured in the clinic and in the preclinical laboratory. While the standard technique used in clinical research and practice is spirometry, respiratory oscillometry has established itself as the gold standard to measure lung function in the animal laboratory over the last two decades. As it turns out, these two approaches are not similar and are based on fundamentally different principles both in terms of how the tests are done as well as how they are interpreted.
While it would be tempting to compare or correlate oscillometry outcomes with other means of assessing lung physiology, whether it is spirometry, plethysmography, nitrogen washout, exhaled nitric oxide (NO), or diffusion capacity, the primary objective of this review is to elucidate how well oscillometry compares between mice and humans. Indeed, most techniques can be justified and should probably be used in adjunct of each other for a complete picture as they assess different aspects of the respiratory system functionality and hence cannot always replace one another (4–12). Thus it was decided not to delve into a comparison between techniques but rather to focus on how oscillometry has been used in both preclinical and clinical settings and to discuss the similarities and differences that exist in terms of technology and pharmacological outcomes. The discussion will also include the possibilities and the limitations of using oscillometry in clinical and preclinical studies including the likely implications of using similar, if not identical, techniques in both settings. Finally, the second objective of this review is to address the need for education and training in oscillometry techniques among the medical and research communities. This has been identified as a barrier to the use of the technique, particularly clinical oscillometry (13). The goal here is to provide general information on oscillometry to help overcome some of the hindrances to the development and implementation of a technology having the potential to brightening the care and the well-being of patients.
OSCILLOMETRY OF THE RESPIRATORY SYSTEM
The first description of the oscillometry technique dates back to 1956, when DuBois et al. (14) applied forced oscillations to conscious human subjects in an attempt to characterize the resistive and the distensile features of the respiratory system. It has since become an active area of research that basically revolutionized preclinical studies on respiratory mechanics (Refs. 15, 16; Table 1). Its widespread use also greatly contributed to the understanding as well as to the development of the technique in general. In humans, several research reports have been published over the years all pointing to the potential of the technique to probe the lung rather than to rely upon the patient’s own breathing or efforts, as is the case with spirometry. Oscillometric measurements in animals started at about the same time as those in humans, first with subjects such as cats and dogs (14, 68). However, it was only in the 1980s that measurements in small laboratory rodents were first reported (69, 70). Although the principles of oscillometry had been known for a long time and there were several years of experience from humans or larger animals, the challenge of performing these studies was essentially one of size. The main obstacle was being able to accurately measure respiratory flows from a subject as small as a mouse (71, 72). While it was possible to estimate flow from pressure measurements through the wave-tube method, especially in stiff lungs (73, 74), oscillometry only gained real attraction in small laboratory animals from the mid-1990s and onwards with the introduction of a specialized device that was subsequently commercialized (75) and with the surge of mouse models to study human lung diseases. Having overcome the technical difficulties and understood that, like in any physiological evaluation, precise measurements can only be obtained at the expense of invasiveness (71), the detailed elucidation of the relationship between lung structure and function on a routine basis began at the preclinical level. This has had positive impacts on the understanding, interpretation, and development of oscillometry in ways not possible in human studies. Consequently, there has been a renewed interest for the technique in humans and an expansion of commercially available devices for patients.
Table 1.
A selection of published oscillometry studies in mice grouped by modeled disease studied
| Disease Type | Disease Name | Model Classification | Disease Phenotype | Ref. No. | First Author |
|---|---|---|---|---|---|
| Obstructive | |||||
| Asthma | Nonallergic | Genetic | (17) | Lofgren | |
| (18) | Wagers | ||||
| Irritant-induced | (19) | Martin | |||
| Gastric reflux | (20) | Allen | |||
| Obesity | (21) | Johnston | |||
| Virus infection | (22) | Collins | |||
| Environmental/occupational exposure | (23) | De Vooght | |||
| (24) | Vanoirbeek | ||||
| (25) | McGovern | ||||
| (26) | Sunil | ||||
| (27) | Nemmar | ||||
| Allergic | Acute allergen exposure | (28) | Wagers | ||
| (29) | Hartney | ||||
| Chronic allergen exposure | (30) | Kearley | |||
| (31) | Novali | ||||
| Recurrence | (32) | Riesenfeld | |||
| Remodeling | (30) | Kearley | |||
| (33) | Mailhot-Larouche | ||||
| (31) | Novali | ||||
| Disease-relevant antigen | (34) | Tully | |||
| Bronchoconstriction | (35) | Phillips | |||
| (36) | Li | ||||
| Airway closure | (37) | Lundblad | |||
| Exacerbation | (38) | North | |||
| Obesity | (39) | Johnson | |||
| Mechanodilation | (40) | Bates | |||
| Immunologically induced | (41) | Therien | |||
| Neonate | (42) | Saglani | |||
| COPD | Cigarette smoke-induced | Emphysema | (43) | Takubo | |
| (44) | Guerassimov | ||||
| (45) | Tam | ||||
| Genetic | Emphysema | (46) | Borel | ||
| (47) | White | ||||
| Inflammation | Inflammation | (48) | Card | ||
| Drug-induced | Emphysema | (49) | Limjunyawong | ||
| Physiological | Aging | (50) | Veldhuizen | ||
| Restrictive | |||||
| Pulmonary Fibrosis | Drug-induced | Functional changes | (51) | Manali | |
| (24) | Vanoirbeek | ||||
| (52) | Devos | ||||
| Sex differences | (53) | Voltz | |||
| Early manifestation | (54) | Headley | |||
| Persistence | (55) | Limjunyawong | |||
| Genetic | Fibrosis and emphysema | (56) | Lundblad | ||
| Occupational exposure | Novel materials | (57) | Wang | ||
| Acid-induced | (58) | Marinova | |||
| Chemical exposure | (59) | Sunil | |||
| Other | |||||
| Cystic fibrosis | Genetic | CFTR deficiency | (60) | Cohen | |
| Early manifestation | (61) | Darrah | |||
| Undetermined | E-cigarette | Subacute and long-term exposure | (62) | Glynos | |
| E-cigarette | In utero exposure | (63) | Noël | ||
| Transplant | Surgery | Lung transplant | (64) | Smirnova | |
| Infection | Influenza A virus (H1N1) | Pregnancy | (65) | Vermillion | |
| Therapeutic | (66) | Halstead | |||
| Coronavirus (SARS-CoV-2) | Functional changes | (67) | Winkler |
Measurements and Outcomes
Principles and technical aspects.
The principles behind the technique are derived from an engineering approach of characterizing systems in movement and have been the subject of a number of previous review articles and books (76–81). In brief, information about the system studied (e.g., the respiratory system) is obtained by studying the deformation of an imposed input signal going through it, the amount of change being commensurate to the overall opposition exerted by the system. Practically, the technique relies on the application of oscillatory waveforms (usually an airflow perturbation) at the subject’s airway opening and the recording of signals during the maneuver, usually pressure and flow but volume is also often recorded in commercial systems. Then, the overall opposition presented by the respiratory system to the input test signal is calculated from the ratio between the pressure (output) and flow (input) signals. This overall opposition is referred to as impedance and is abbreviated by the letter Z. There are three possible ways in which the technique can be performed, depending on the site where the input and output signals are applied or recorded. The configuration where the acquisition of the output signal is done at the same physical location (e.g., airway opening) as the application of the input one currently prevails in both preclinical and clinical research over the other techniques where the two sites differ (e.g., airway opening and chest or vice versa) (14, 71, 76, 80). The outcome obtained in this way is referred to as input impedance (abbreviated as Zin), as opposed to transfer impedance (abbreviated Ztr) in the two other configurations. Table 2 summarizes the similarities and differences as well as the strengths and limitations of the methods used to measure Zin in clinical versus preclinical settings.
Table 2.
Details of the oscillometry technique from a clinical and a preclinical viewpoint
| Clinical | Preclinical (Small Laboratory Animals) | |
|---|---|---|
| Commercial device | Available | Available |
| Current level of use | Research, clinical practice | Routine research |
| Guidelines/practical recommendations | Available | Available |
| Reference values | Available | Available |
| Subject’s state of consciousness | Unaltered | Anesthetized |
| Subject’s position | Seated, supine, upright | Supine |
| Subject’s connection to the device | Mouthpiece | Tracheotomy or oral intubation |
| Subject’s breathing pattern | Spontaneous: usually breathing at tidal volume | Mechanical ventilation |
| Subject’s lung volume at the time of assessment | Variable: spontaneous volume adopted by the subject | Standardized transpulmonary pressure |
| Subject’s airway tree | Intact, mouth to lung | Partial: lower airways only |
| Measurement type | Finite perturbations | Finite perturbations |
| Measurement duration | 20 s to 1 min | 1 to 16 s |
| Signal generator | Loudspeaker Moving mesh |
Computer-controlled piston ventilator |
| Type of input signal | Single frequency Multifrequency |
Single frequency Multifrequency Optimized ventilator waveform |
| Nature of input signal | Impulse Predefined sine waves |
Predefined sine waves |
| Frequency range | Variable: typically 5 to ∼40 Hz | Variable: typically 0.5 to 20 Hz |
| Delivery of input signal | Superimposed on subject’s breathing | During a brief apneic period |
| Aerosol administration | Via the mouth | Intratracheal |
| Output | Input impedance | Input or transfer impedance |
| Interpretation of impedance | Response at set frequencies Shape analysis of the frequency-response curves Area-under-the-curve |
Mathematical models and derived parameters |
| Strengths | Structure-function link Access to lung periphery Absolute values Sensitivity Measurement during regular quiet breathing |
Structure-function link Access to lung periphery Absolute values Sensitivity Precision: measurements made under fully controlled conditions |
| Limitations | Assessment over a limited span of the respiratory pressure, volume, and flow ranges Chest wall contribution Standardization of lung volumes |
Anesthesia Invasive measurements Variable chest wall contributions across species |
One technical challenge when assessing conscious subjects is the need to use a measurement signal containing oscillation frequencies outside of the subject’s own breathing frequency range. This is necessary to avoid contaminating the spectral content of the input signal, which is used to calculate respiratory impedance (80). In animal experiments, where the subject is deeply anesthetized and paralyzed, the control of breathing using a mechanical ventilator enables the experimenter to stop the tidal ventilation for very brief periods, during which time, the input forced oscillation signal is applied. This eliminates restrictions on the frequency content of the input signal, which can now contain a broad range of frequencies, from well below to well above the subject’s breathing rate (82). Consequently, oscillometric measurements in small preclinical subjects like mice and rats often start at a frequency as low as 0.5 Hz, allowing for an exhaustive assessment of the respiratory system (76). In human subjects, low-frequency measurements are rare since only possible under specific conditions (e.g., mechanical ventilation, paralysis, voluntary, or induced apnea) (83–86). Typically, most commercially available clinical devices are using 5 Hz as the lowest frequency (87) since measurements are done in conscious subjects and that the lowest frequency needs to be at least an order of magnitude above the spontaneous breathing frequency (0.2–0.3 Hz) (Table 2).
Impedance spectra.
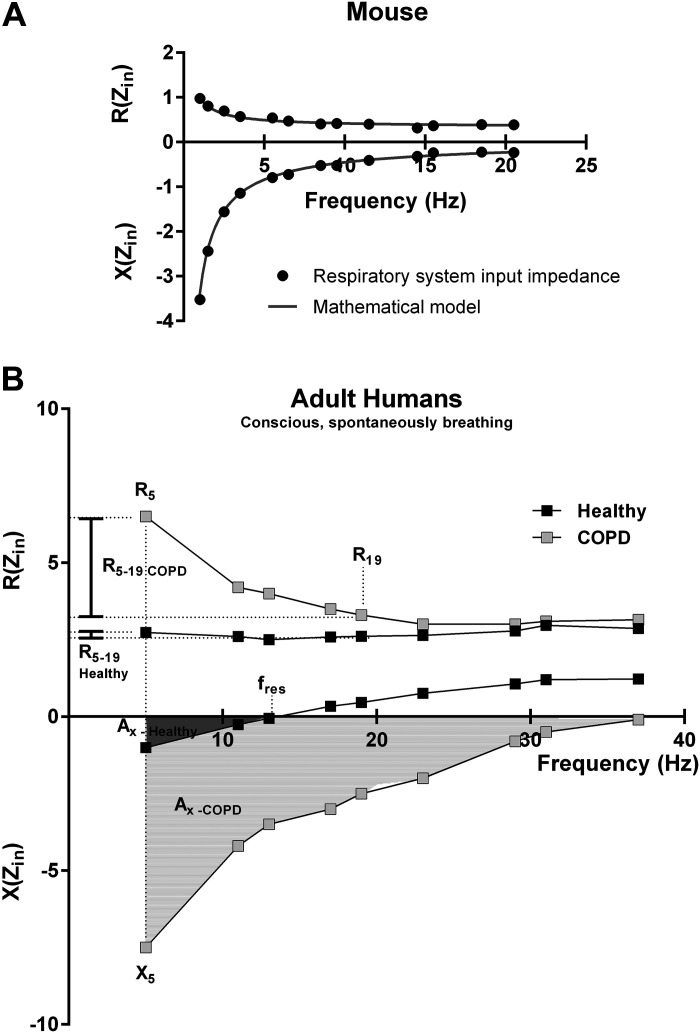
In the clinical or preclinical setting, oscillometry measurements can be conducted using either a single oscillatory frequency input signal or, alternatively, a more complex one containing a wide range of frequencies. This latter assessment evaluates the frequency-response of the respiratory system, which is typically expressed by the impedance spectra of the respiratory system (Fig. 1). The impedance is an entity originating from a mathematical calculation of the pressure-flow ratio, as indicated above. The particularity of this ratio is that it is calculated using complex numbers (76, 80). Unlike real numbers, better known in the field of physiology, complex numbers are composed of a pair of numbers which are in this case generally graphically represented in a decomposed manner in a two-part graph, expressed as a function of frequency, representing the impedance spectra (Fig. 1). The upper portion of the graph in Fig. 1, A and B, is referred to as the real part, also commonly called resistance and abbreviated with the letter R, while the lower portion of the graph Fig. 1, A and B, represents what is known as the imaginary part, or otherwise called reactance, which is abbreviated by the letter X (76, 80, 89).
Figure 1.
Interpretation of preclinical and clinical impedance spectra. A: mouse impedance spectra obtained with a flexiVent FX (SCIREQ Inc., Montréal, QC, Canada). At the preclinical level, mathematical models, such as the constant phase model (88), are often used to fit the impedance data providing parameters used to interpret respiratory impedance spectra. B: human impedance spectra from a normal subject and a chronic obstructive pulmonary disease (COPD) patient obtained with a tremoflo C-100 (Thorasys Inc., Montréal, QC, Canada). Clinical oscillometry relies on qualitative and quantitative analyses of the shape of the frequency response to interpret human impedance spectra as mathematical models are yet to be developed. Zin, input impedance; R(Zin), resistance part of input impedance; X(Zin), reactance portion of input impedance. Both parts of the impedance spectra are expressed in cmH2O·s/mL in mice and cmH2O·s/L in humans. The vertical dash line indicates the lowest frequency (5 Hz) used in a typical oscillometry measurement in a conscious, spontaneously breathing human subjects. R5, resistance at 5 Hz, considered to pertain to the entire respiratory system; R19, resistance at 19 Hz, reflecting primarily the contribution from the central airways; R5-19, difference between resistance at low and higher frequencies, a measure of airway heterogeneity; X5, reactance at 5 Hz, assessing the elastance of the respiratory system; AX, area over the reactance curve; fres, resonant frequency. Values of human COPD impedance were extracted from Peters et al. (81). Human healthy impedance was generated internally and represents unpublished data from a female subject.
Respiratory mechanics parameters: preclinical setting.
The impedance spectrum captures the condition of the respiratory system and can infer structure-function information on specific components. However, to do so and to be physiologically meaningful, it needs to be interpreted. The use of mathematical models, such as the constant phase model introduced by Hantos et al. (88) and able to fit the impedance data (Fig. 1A), has especially helped advance oscillometry by providing a parameterized approach to describe changes while contributing to the understanding of how the different compartments of the respiratory system influence the total impedance. A detailed discussion on the topic would go beyond the scope of the current review, and therefore, the reader is kindly referred to related papers and books for more details (76, 80, 88). Yet, mathematical models added great power to the technique. At the preclinical setting, it simplified the access to the lung periphery in laboratory animals as small as mice where the technical challenges of alternative approaches are significant and nontrivial (90, 91). Although many mathematical equations can fit impedance data, it is mostly the constant phase model (88) that has been used for the parameterization and interpretation of the respiratory impedance. This model has been shown to take airway and tissue properties into account with parameters like, the Newtonian resistance (RN), describing the airflow opposition to the measurement signal within the airways, and the parameters called G and H, providing a good representation of the damping and stiffness characteristics of the respiratory tissues, respectively. From these two tissue-related measures, parameter G would be sensitive to heterogenous airway obstruction and ventilation, especially in the context of small peripheral airways where opening and closure is influenced by the thoracic gas volume (56, 71, 92). Parameter H, on the other hand, would be affected by changes in stiffness and lung volume (37, 56). The constant phase model also includes a parameter describing the effect related to the movement of air within the airways or gas inertance (I). In subjects as small as mice, this effect was reported to be negligible between 0 and 20 Hz, the typical preclinical measurement range (76, 91), as a significant portion of the larger airways is bypassed by the cannula, the contribution of which is accounted for during the measurement by prior calibration steps (93). This is obviously not the case in the typical human measurement scenario where the upper airways are included. Yet, as in the preclinical measurement, the contribution from the instrument is typically determined during prior calibration and subsequently subtracted, so that only the subject’s contribution is reported. However, one must recognize that the inertance of the intrathoracic airways can play a role, especially in larger species and in disease situations. The reason for this is that inertance is proportional to the ratio of the length: cross-sectional area times the density of the air present in the airways and, as the proportions of the airways (length or cross-sectional area) change with disease, so will inertance which contribution to the reactance typically results in a decrease with disease severity. The alterations in lung pathology can be multiple in nature, and several modifications can contribute to the reactance such as restrictive processes and inhomogeneous obstructions all of which can manifest as changes in reactance (15, 76, 77, 79, 81, 89). One important point regarding the present interpretation is that while the constant phase model will provide measures of resistance and elastance, these apply to the whole respiratory system. Thus there are no specific physical structures or geographical locations that can easily be associated with the parameters from the model. However, a morphometry study conducted in rats concluded that there was concordance in the way the parameters were interpreted and the changes in airway narrowing seen (94). It has also been shown that only an increase in resistance of the airways cannot explain airway hyperresponsiveness, an additional underlying thickening of the airway wall and perturbation of the air-liquid interface leading to airway closure is also necessary (28).
Respiratory mechanics parameters: clinical setting.
In the clinical setting, the constant phase model can only fit human impedance data containing low frequencies and thus acquired under special conditions (e.g., anesthesia, mechanical ventilation) (83, 85, 86). Indeed, due to the restricted frequency range that can be applied under conscious measurement conditions, it is not yet possible to fit the constant phase model to the impedance data obtained from spontaneously breathing subjects, either from humans or experimental animals. For that reason, the interpretation of the human respiratory impedance spectra currently relies on a qualitative and quantitative analysis of the shape of the frequency-response curves (Fig. 1B). Single values from either the resistance (e.g., R5) or reactance (e.g., X5) can be extracted at specific frequencies for comparison or follow-up (Fig. 1B). At lower frequencies, essentially 5 Hz, the resistance (R5) is considered to pertain to the whole respiratory system whereas at higher frequencies, around 20 Hz (R20), it would primarily reflect the contribution from the central airways. Also, descriptive changes in the shape of the impedance curves can be used. In the healthy adult human lung, the resistance is almost independent of the oscillation frequency (approximately over the range of 5–20 Hz) and therefore will roughly display a flat line on a graph of the impedance real part against frequency (Fig. 1B) (76, 77, 79, 81, 95). In presence of airflow obstruction, however, the heterogeneity and the location of the obstructions can differ. Hence, the nature of the obstruction will alter the shape of the resistance curve. For example, centrally located or homogenous bronchoconstriction will tend to shift the resistance curve upwards at all frequencies (76, 96–99). In a disease such as chronic obstructive pulmonary disease (COPD) with peripheral airway obstruction, which tends to be heterogenous and can be understood as a distribution of pathway resistances, the resistance part of the respiratory impedance curve becomes frequency-dependent and displays an increase more toward lower frequency range (Fig. 1B). Thus the difference between resistance at low and higher frequencies (R5-20) becomes a measure of airway heterogeneity and typically increases with small airways obstruction (79, 87, 98, 100–104).
At 5 Hz, the reactance (X5) is influenced by the elastic properties of the respiratory system where it reflects the soft tissue and lung parenchyma. At higher frequencies, the reactance is dominated by the inertia of the air being oscillated but still includes a contribution from the lung tissue and the chest walls. The point where the elastic and inertive forces are equal, the resonant frequency (fres), tends to increase in both obstructive and restrictive diseases (Ref. 81; Fig. 1B). While the changes in reactance can be described using individual frequencies (e.g., X5) and fres, the integrated area over the reactance curve (AX, Fig. 1B), which has the units of elastance, is a more sensitive, less noisy, and more reproducible index of obstruction than the individual components as first suggested by Goldman (105). The way human respiratory impedance is interpreted is constantly evolving. The hope is that, similar to preclinical oscillometry, a mathematical model able to fit the impedance data from conscious and spontaneously breathing subjects will become available. In the meantime, various approaches are being evaluated and include, for example, an intrabreath analysis of resistance and reactance or similar approaches which suggest that the changes of resistance over the tidal breathing cycle could be used to identify abnormalities (106, 107). Similarly, it has been shown that changes in reactance over the breathing cycle in COPD is positive end-expiratory pressure dependent and could be used to assess the severity of the disease as a sign of flow limitation during tidal breathing (108, 109).
OSCILLOMETRY OF THE NORMAL AND DISEASED LUNG
Physiological Correlations
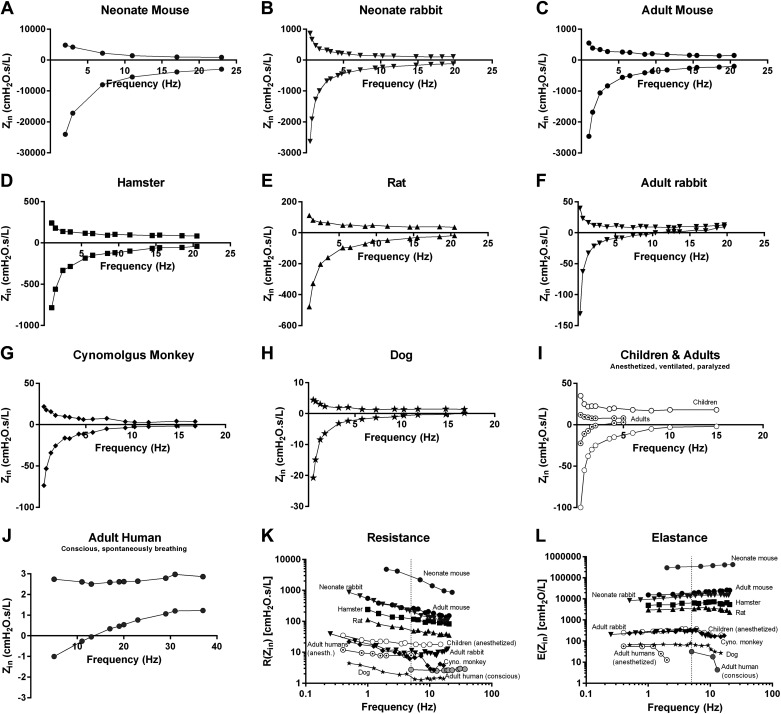
Notwithstanding the conspicuous physical differences between species, it seems that a lung from a mouse behaves in a very similar way as that from a human when subjected to a forced oscillation signal (Fig. 2). In fact, it is as if oscillometry reveals features of the respiratory system that are universal among species as the impedance spectra share many similarities between various species, with the main difference being mostly a question of scale (Fig. 2). This is rather interesting given that the lungs of a mouse and a human differ in size by at least three to four orders of magnitude as well as in their structural organization (72). In our view, this clearly highlights the potential of oscillometry as a translational tool, which is important considering the numerous mouse models that have been developed to study the pathophysiology of human lung diseases or test therapeutic candidates. Given the great degree of similarity in the impedance spectra between mammalian species (Fig. 2), it is not surprising to see that they can fold into a single relationship when expressed relative to body weight and that the parameters of constant phase model align across species, from mice to human, in an inverse correlation relative to size (77, 110). However, as mentioned above, such parameters are only available under very specific and uncommon conditions in the context of human oscillometry measurements. In addition, while preclinical and clinical (conscious) oscillometry share similarities in respiratory impedance (Fig. 2), there are differences in the way each is analyzed, at least within the commercial software programs. Descriptive analyses were once used to interpret impedance from anesthetized and mechanically ventilated laboratory animals, but this is rarely the case nowadays as mathematical models are readily available in commercially systems. However, while there are differences in the way each is analyzed, the analysis of human oscillometry provides, relatively speaking, similar information as that related by the parameters of the constant phase model at the preclinical level (76, 79). An important point to remember with oscillometry (preclinical and clinical) is that the measurements generally include a contribution from the chest walls, unless an additional pressure transducer is added to capture the intrapleural pressure or that the experimental (preclinical) conditions include an open chest. In addition, as the lung develops and grows, variations in the shape of the impedance spectra can be seen (Fig. 2), reflecting the maturation of the respiratory system, sometimes very quickly such as right after birth (111, 112). Also, as discussed in the following sections, the specific contribution of the various components of the respiratory system can vary across different health or disease conditions. Please note that for the sake of space only a few diseases were covered. Also, while only selected mouse studies have been included as examples (Table 1), many other studies have been published including some in other animal species, such as rats, guinea pigs, or rabbits, to address questions related to lung development and physiology, genetic susceptibility, or therapeutic approaches, just to mention a few (110, 111, 113–116).
Figure 2.
Input impedance (Zin) spectra of the respiratory system across various species. A: neonate mouse (data courtesy of Dr. R. Morty). B: neonate rabbit (data courtesy of Dr. J. Vanoirbeek and Dr. A. Gie). C: adult mouse. D: hamster (data courtesy of Dr. W. Mitzner). E: rat. F: adult rabbit. G: cynomolgus monkey. H: dog. I: children and adults. Impedance spectra from anesthetized, mechanically ventilated and paralyzed patients undergoing cardiac surgery extracted from Refs. 83, 85. J: adult healthy human subject, conscious and spontaneously breathing. K and L: comparison across species for resistance (K) and elastance (L). Elastance was calculated from the reactance part of the impedance. The calculation was done at every frequency up to the resonant frequency since after that point, the inertance effect dominates. The vertical dash line indicates the lowest frequency (5 Hz) used in a typical human oscillometry measurement in conscious, spontaneously breathing subjects. Zin, input impedance, expressed in the same units across species (cmH2O·s/L); R, resistance; E, elastance. Data were generated internally unless otherwise specified. Animal data were obtained with a flexiVent system and human data with a tremoflo C-100.
Asthma
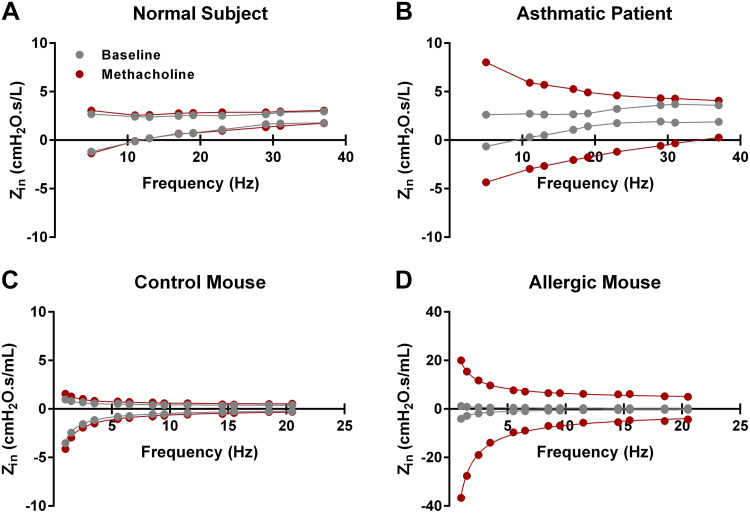
Asthma is a highly prevalent disease that comes in different presentations but is generally characterized by airway inflammation and reversible airflow obstruction that is highly variable over time (117). The disease can have both allergic and nonallergic origins; however, the final manifestations on a physiological level are similar with bronchial obstruction and peripheral airway closure (28, 37, 117). The asthmatic lung is also characterized as being hyperresponsive to various inhaled stimuli, a property that is used in a diagnostic procedure using bronchial challenges and spirometric measurements (118). It is important to emphasize that in terms of physiology the current diagnostic criteria are based on spirometry (119). Various mouse models of allergic and nonallergic asthma can be found in the literature, each displaying specific features of this multifaceted disease. Table 1 lists a selection of mouse models of asthma assessed using oscillometry and grouped according to the specific clinical features of the disease displayed. As observed in asthmatic patients, nonallergic as well as allergic mouse models frequently exhibit an augmented response to a given bronchoconstrictor agent, such as methacholine (Fig. 3). On the impedance spectra, this translates into an increase in the resistance with augmenting bronchoconstrictor challenges. In addition, the frequency-response curve generally becomes more negative in the reactance part. In both parts of the impedance spectra, the variations are more pronounced in the lower frequency range and then gradually decrease (Fig. 3), suggesting the presence of peripheral airway obstruction likely due to airway closure (37). In preclinical studies, the level of control over the experimental conditions is such that it is possible to not only synchronize the delivery of the aerosol bronchoconstrictor challenge with inspiration but also to modify various aspects related to the aerosol generation and transport such as to influence the primary site of response (120). This is obviously not as easily achieved in clinical studies. Yet, in the human lung a corresponding change in the impedance profile is observable following an inhaled methacholine challenge, such that mice and men are relatively similar in terms of their responses to an induced bronchoconstriction. In asthmatic humans the change in the impedance patterns also suggests that the airway hyperresponsiveness is dominated by peripheral airway obstruction associated with airway closure (79, 121–123). However, the increase in the R5-20 difference also indicates that the lung is becoming more heterogeneous during bronchoconstriction (123, 124). We can speculate that the heterogeneity described in asthmatic lungs by Venegas et al. (125) is just further amplified by the methacholine challenge to a point where airway narrowing, in presence of increased mucous secretions, leads to liquid bridging and ultimately airway closure. Similar closure was demonstrated in allergic mice following bronchoconstriction (28, 37). Additionally, as highlighted in Table 1, other features of human asthma have been studied using oscillometry at the preclinical level, further highlighting the power and the potential of the technique.
Figure 3.
Human and mouse input impedance spectra at baseline and following a bronchoprovocation with methacholine. A and B: impedance spectra from a normal subject (A) and an asthmatic patient (B) recorded under baseline conditions and following an inhaled methacholine challenge at 2 mg/mL (data courtesy of Dr. Y. Bossé). C and D: impedance spectra from a control mouse (C) and a chronic allergic model (D) before and after an aerosolized challenge with methacholine at 12.5 mg/mL (data generated internally). Mouse data were obtained with a flexiVent FX and human data with a tremoflo C-100. Zin, input impedance.
COPD
In COPD significant loss of lung tissue and inflammation in the smaller airways are typically observed along with irreversible airway obstruction and shortness of breath. At the preclinical level, it has been possible to observe similar tissue damage and inflammatory reaction in various models (Table 1). From a functional point of view, these changes lead to the generation of an emphysematous phenotype characterized by a change in tissue elastance (parameter H) when assessed by oscillometry. This means, as explained above, that the main changes are found in the reactance part of the impedance, as seen in human COPD patients (79, 123, 126). One important difference between the two species, however, resides in the severity of the induced lesions, which are more subtle in most mice models especially following tobacco smoke exposure and despite long exposure periods (127–129). Therefore, structural and functional alterations reflecting an advanced status of the disease as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) remains to be established at the preclinical level. While this might suggest a shortcoming of mouse models of COPD, the functional changes detected by oscillometry preclinically are representative of the structural changes, as demonstrated for the first time by Takubo et al. (43) in mice chronically exposed to tobacco smoke. In addition, an early detection of the disease or its exacerbations is clinically desirable and is, in our view, an important advantage (130). This ability to establish a sensitive and clear link between structure and function also seems to track with the GOLD stages of COPD (79, 131). It thus looks like preclinical and clinical oscillometric measurements would be useful tools in the development of intervention therapeutic approaches aiming at preventing or reducing the disease progression.
Pulmonary Fibrosis
Pulmonary fibrosis is an interstitial, restrictive lung disease. This is a group of still poorly understood and heterogeneous diseases, which, on a lung compliance spectrum, are at the opposite end of emphysematous diseases with progressive stiffening of the lung tissue over time due to inflammatory processes. The disease, which has a poor prognostic and response to available treatments, generally leads to a gradual and irreversible decline of lung function (132). Like emphysema, the functional changes are located within the lung periphery and early detection is clinically desirable to allow for lifestyle changes and therapeutic intervention. Although the diagnosis of the disease involves pulmonary function tests, this group of lung diseases has not been as extensively studied with oscillometry as asthma and COPD. Yet, the technique has the potential to measure the physiological consequences both in the clinical and preclinical context because of its ability to probe the lung periphery. It has been suggested that the reactance is particularly sensitive to changes in interstitial lung diseases whereas the resistance is less affected (133–136). In mice models of pulmonary fibrosis (Table 1), oscillometry is a technique commonly used to demonstrate a disease phenotype or a treatment effect. In some disease models, early detection (54) or disease persistence were demonstrated (55), and it has also been shown that, analogous to human studies, that lung stiffness is increased in bleomycin induced fibrosis (24, 51–53).
Other Respiratory Diseases
Cystic fibrosis.
Cystic fibrosis (CF) is a genetic disease which, as opposed to pulmonary fibrosis, presents early in life. In young children, lung function testing using spirometry is problematic as the procedure is long and requires an ability to follow instructions to adequately perform the test. For these reasons, oscillometry has been increasingly used in children, including CF patients (137, 138). The findings using oscillometry in CF have been variable with studies in children showing that oscillometry has low sensitivity detecting disease in preschoolers (139) whereas within breath assessment of impedance suggested peripheral obstruction (140). In fact, oscillometry was the tool that made it possible to demonstrate a lung phenotype in mice with a CFTR mutation (60). It was also more recently used in a mouse model to understand mechanisms associated with therapeutic options like lung transplantation and organ rejection, which are often a reality for some CF patients (64). While it is tempting to see similarities between mice and humans with regards to CF, we have to acknowledge that there are conflicting studies using oscillometry in CF patients and that CFTR-deficient species other than mice (e.g., pigs, ferrets) are being developed to study CF (139, 141–143). In addition, despite the advantages of oscillometry, very few studies, particularly interventional ones, have been done CF patients as well as in children in general. In contrast to children, oscillometry is sensitive to alterations in lung function in adults with CF and can also detect lung function improvements following exercise correlating with lung clearing index (144–146). We believe that at young age many CF patients retain a relatively functional lung which then deteriorates with time and progressing infections. This disease, as most other lung diseases, starts in the periphery and, as it progresses, it becomes measurable with oscillometry and eventually also with spirometry. Studies are underway to determine how airway clearing techniques impact different measures of lung function, and with the advent of new drugs targeting CF more studies can be expected (54, 147). Using oscillometry to monitor children with CF might be a reasonable approach to capture early signs of lung deterioration.
Infectious diseases.
Infectious airway diseases like influenza or coronavirus disease (e.g., SARS-CoV-2 causing COVID-19) have the potential of infecting a large proportion of the population and cause, in patients with increased vulnerability, a severe respiratory disease that can lead to respiratory distress and even death (3). Mice models have been developed to study these diseases as well as to evaluate the benefits of therapeutic approaches, as exemplified in Table 1. In these models, the assessment of lung function was able to demonstrate virus-induced changes in respiratory mechanics parameters and lung volumes (65, 67). In their study, Halstead et al. (66) also showed that oscillometric parameters captured the changes related to the therapeutic approach tested (granulocyte macrophage colony-stimulating factor overexpression initiated after the establishment of the infection) that positively modified the course of the disease progression. Over 40 yr ago a report from Hall et al. (148) showed that pulmonary mechanics measured with oscillometry identified early signs of small airway disease (SAD) in patients infected with H3N2 influenza and that it tracked worsening and improvement of SAD, as patients were followed for 5 wk until resolution when oscillometry normalized in most patients. During the entire period, spirometry did not change significantly. It has been argued that quiet tidal breathing is less likely to cause coughing than spirometry and hence be safer to use if there is a risk for spreading, e.g., virus. At any rate, in times of increased airways infections, there is a risk that regular visits for follow-ups are restricted and a less challenging test of lung function might be an option in this case (149, 150).
OSCILLOMETRY AND THERAPEUTIC INTERVENTIONS IN MOUSE MODELS
The recent approval by the US Food and Drug Administration of Trikafta (elexacaftor/ivacaftor/tezacaftor) for the treatment of cystic fibrosis or Dupixent (dupilumab) for moderate-to-severe asthma are examples of success stories in the development of novel pharmaceutical therapies for respiratory diseases (Table 3). However, the field in general has had poor success in the past half-century or so, with an attrition rate and a development time above that of other disease areas (151). It goes without saying that improvement is needed in the development of respiratory therapies, especially when the impact of such diseases is taken into consideration (152). The worldwide situation following the COVID-19 outbreak in the year of 2020 provides a very recent and clear example of the extent of the effect a single respiratory disease can have (153). As many preclinically validated respiratory therapy candidates fail during the late phases of development for either lack of efficacy or toxicity, there is, perhaps more than ever, a pressing need to reflect on the entire development strategy to move research more efficiently and provide preventive and therapeutic therapies to patients more rapidly. Among other elements, the use of predictable preclinical models has been previously recognized as essential to improve and speed-up the development process (151). However, this cannot be achieved without a discussion on the methodological aspects related to the assessment of lung function. Preclinical respiratory function tests are often considered as a primary study endpoint and used in the decision-making process of moving therapeutic candidates into clinical evaluation. The lack of translation seen in the development of preclinically validated novel respiratory therapies is forcing a broad reflection on the measurement methods, the strategy to assess the respiratory function at the preclinical and clinical levels, the extent of details included, as well as the alignment between the different phases of the drug developmental process. Oscillometry is already very much present at the preclinical level including in the efficacy assessment of potential therapeutic interventions. In addition, preclinical drug development strategies increasingly include a comprehensive combination of lung function measurements comprised of, for example, detailed broadband oscillometry measurements, pressure-volume loops, negative pressure-driven forced expiration, lung volumes, and/or imaging to enhance the predictability of the outcomes as well as to detect or characterize a phenotype in an exhaustive manner (30, 52, 154, 155). It thus seems that an effort is made to improve and speed-up the development of respiratory therapies by combining measurements of lung function and going beyond the classic assessments.
Table 3.
List of novel respiratory therapies approved by the US Food and Drug Administration since 2011
| Year | Name | Active Ingredient | Therapeutic Application | Class | Manufacturer |
|---|---|---|---|---|---|
| 2019 | |||||
| Trikafta | Elexacaftor/ ivacaftor/tezacaftor | Cystic fibrosis | CFTR channel potentiator | Vertex Pharmaceuticals | |
| 2018 | |||||
| Dupixent | Dupilumab | Asthma | IL4-Rα mAb | Regeneron Pharmaceuticals | |
| 2017 | |||||
| Fasenra | Benralizumab | Asthma | IL5-Rα mAb | Astra Zeneca Pharmaceuticals | |
| 2016 | |||||
| Cinqair | Reslizumab | Asthma | Humanized anti-IL5 mAb | Teva Pharmaceuticals | |
| 2015 | |||||
| Tagrisso | Osimertinib | Cancer | EGFR-blocking therapy | Astra Zeneca Pharmaceuticals | |
| Nucala | Mepolizumab | Asthma | Humanized anti-IL5 mAb | GlaxoSmithKline | |
| Okambi | Lumacaftor ivacaftor | Cystic fibrosis | CFTR channel potentiator | Vertex Pharmaceuticals | |
| 2014 | |||||
| Esbriet | Pirfenidone | IPF | Multiple pathways | InterMune | |
| Ofev | Nintedanib | IPF | Kinase inhibitor | Boehringer Ingelheim Pharmaceuticals |
|
| 2013 | |||||
| Anoro Ellipta | Umeclidinium vilanterol | COPD | Anticholinergic, long-acting β2-adrenergic agonist | GlaxoSmithKline | |
| Breo Ellipta | Fluticasone fuorate/ vilanterol | COPD | Corticosteroid, long-acting β2-adrenergic agonist | GlaxoSmithKline | |
| 2012 | |||||
| Situro | Bedaquiline | TB | Antibiotic | Janssen Therapeutics | |
| Tudorza Pressair |
Aclidinium bromide | COPD | Long-acting antimuscarinic agent | Forest Pharmaceuticals | |
| 2011 | |||||
| Arcapta Neohaler |
Indacaterol | COPD | Long-acting β2-adrenergic agonist | Novartis Pharmaceuticals | |
| Daliresp | Roflumilast | COPD | Phosphodiesterase type 4 inhibitor | Forest Pharmaceuticals |
COPD, chronic obstructive pulmonary disease; EGFR, epidermal growth factor receptor; IPF, idiopathic pulmonary fibrosis; TB, tuberculosis.
OSCILLOMETRY AND THERAPEUTIC INTERVENTIONS IN HUMAN STUDIES
Intervention with Therapeutic Drugs
In the clinical setting, oscillometry is making its way into clinical trials evaluating novel respiratory therapies in addition to spirometry (Table 4) (see also https://www.clinicaltrialsregister.eu/ and https://clinicaltrials.gov/). An important aspect of physiological measurements is the ability to accurately detect effects of treatment interventions. It would seem logical that a technique, like oscillometry, with higher sensitivity to pathological alterations, should be extensively used in this context to document drug effects (Table 4). The supply of publications currently available is likely biased toward publications with a positive outcome. The main reason might be that most published studies include traditional treatment modalities, such as bronchodilators and glucocorticosteroids (GCS), all with known beneficial effects on asthma and to some degree on COPD. While more publications using novel approaches (e.g., biologicals) are awaited, the fact that traditional therapies have effects on preclinical and clinical oscillometry parameters could be seen as testimony for the relevance of the technique and its translational potential. For example, in a study by Yamaguchi et al. (162), the ambition was to establish that newer hydrofluoroalkane beclomethasone dipropionate (HFA-BDP) inhalers had the same effect as the old, now banned, chlorofluorocarbon beclomethasone dipropionate (CFC-BDP) inhalers. Interestingly, the authors found that the HFA-BDP treatment had significant effects on the small airway indicators (R5-20 and AX), which was not the case with the CFC-BDP, a finding that can be explained by the smaller particle size generated by the HFA-BDP, which allowed the therapeutic agent to penetrate deeper into the small airways than the CFC-BDP and elicit its activity where the disease resides (162).
Table 4.
A selection of published oscillometry studies in human subjects grouped by disease and treatment studied
| Disease | Drug Type | Ref. No. | First Author |
|---|---|---|---|
| Asthma | |||
| Steroid/LABA | (156) | Kirsten | |
| (157) | Hozawa | ||
| (158) | Hozawa | ||
| (159) | Diong | ||
| (160) | Hozawa | ||
| (161) | Díaz-García | ||
| Steroid | (162) | Yamaguchi | |
| (163) | Hoshino | ||
| (164) | Gjerum | ||
| Bronchodilator | (165) | Ortiz | |
| (166) | Kahan | ||
| (167) | Manoharan | ||
| (168) | Mondal | ||
| (169) | Nair | ||
| (170) | Thamrin | ||
| (171) | Singh | ||
| (172) | Eddy | ||
| (173) | Cottee | ||
| (174) | Lipworth | ||
| Leukotriene inhibitor | (175) | Nieto | |
| IL-13 neutralizing mAb | (176) | Russell | |
| None | (177) | Lundblad | |
| COPD | |||
| Muscarinic antagonist | (178) | Houghton | |
| (179) | Borrill | ||
| (180) | Milne | ||
| (181) | Mineshita | ||
| Lung transplantation | |||
| Steroid, immunomodulator | (182) | Wu | |
| (183) | Cho | ||
| Influenza | |||
| Vaccine | (148, 184) | Hall |
LABA, long-acting beta-agonists; COPD, chronic obstructive pulmonary disease.
The combination of long-acting beta-agonists and GCS has been documented in several asthma studies. The common pattern emerging from these studies is that finer particle formulations had better effects on the parameters detecting effects in the smaller airways (R5-20 and AX) (157, 158, 160, 161). This is important because it demonstrates that targeted delivery of drugs can make a difference not only physiologically but also in patient reported outcomes and exhaled NO, which appear to correlate with the oscillometry endpoints.
In a COPD study using a triple combination of tiotropium, fluticasone, and salmeterol, it was demonstrated that oscillometry parameters detected improvements after 12 wk of treatment. The authors noted that resistance at 20 Hz was not affected by the treatment but that R5 and R5-20 tended to decrease suggesting an effect on the smaller airways (181). In this context it is important to point out that the choice of the oscillometry device might play a role because differences between devices and their sensitivity to pathological changes have been reported (87, 103, 137, 185, 186). In yet another COPD study, it was demonstrated that oscillometry parameters correlated with lung volumes both at baseline as well as 2 h postbronchodilation. The effect was again primarily documented in parameters connected with small airways (180). It is interesting to note that we also detected a strong correlation between lung volumes and elastance in mice measured with oscillometry, further illustrating the generalizability of oscillometry between species (37).
Various approaches are available in the assessment of asthma control and the outcome can be confounded by how the test is performed. Indeed, clinical pulmonary function tests can include measurements under baseline conditions, following a challenge with a bronchoconstrictor agent or other provocative substance, or even a reversibility test. While all these approaches allow for the diagnostic of the disease, it should not be a surprise that they can generate different information about the subject’s condition. In a study by Gjerum et al. (164), where both methacholine and cold air were used as bronchial challenges, the authors found that bronchial hyperresponsiveness as measured by cold air improved significantly with budesonide, whereas no improvement was found on methacholine The bronchial tone was also shown to affect the PC40 for methacholine in children measured with oscillometry and, interestingly, while there was a bronchodilator effect with salmeterol, there was no dose-response effect with doubling the dose (168). The magnitude of the response to a bronchodilator depends on the baseline lung function, and this does not change with lung diseases such as cystic fibrosis or asthma; thus it was suggested that the bronchodilator response be reported relative to the baseline (170).
Taking aim at designing a process to identify patients with poorly controlled asthma, a study by Cottee et al. (173) used oscillometry in combination with a reversibility test and also compared with spirometry. The authors reported that bronchodilation was identified more frequently by oscillometry than by spirometry (54% vs. 27% of subjects), but, perhaps more importantly, the bronchodilator effect on the reactance parameters also identified more subjects with poor asthma control than spirometry (69% vs. 41%). Of all oscillometric parameters, AX had the greatest sensitivity to detect poor asthma control (22 subjects, 69% sensitivity, specificity 75%). The authors thus conclude that the utility of a bronchodilator response in diagnosing asthma and that its presence, despite an anti-inflammatory treatment, was associated with worse asthma control, higher exacerbation rates, as well as increased mortality. Their observations point to the possibility to use the reversibility test to assess treatment efficacy and that relying on spirometry might not be sensitive enough. In a comment to the above study, it was pointed out that reversibility criteria should be used in reference to asthmatic and not healthy subjects and that changes in the frequency dependence of resistance and reactance should be part of the evaluation of a reversibility test (174).
Lung Transplantation
An interesting area where the use of oscillometry will likely become important, if not critical, is in patients with transplanted lungs. About 50% of lung transplants survive on average 5 yr with most episodes of acute rejection happening in the first year after transplantation (187). Many transplants will experience acute cellular rejection (ACR), and this is a risk factor for a condition known as chronic lung allograft dysfunction (CLAD), which remains a major hurdle limiting long-term survival postlung transplantation (187–192). The routine follow-up of transplanted patients includes spirometry and transbronchial biopsy which, while being the current gold standard to diagnose ACR, is imperfect because of the patchiness of ACR and the limited size of the sample. Indeed, the biopsies can only sample a small region of the lung and usually includes only a few small airways; hence, the disease discovery rate is only 47-57% (193–195). Transbronchial biopsy also carries associated risks, like infection and pneumothorax, which should not be ignored (196–199). Spirometry is notoriously insensitive to CLAD and studies have found that obstruction of a significant fraction of the small airways must occur before forced expiratory volume in 1 s can detect changes (200–202). In addition, it is only about 60% sensitive for detecting ACR (201). In a few recent reports, the Toronto Transplant Centre has developed an efficient protocol using oscillometry to detect early signs of rejection (182). The authors have shown that oscillometry, in particular the parameters AX and R5-19, can detect significant alterations in the smaller airways confirmed to be ACR while spirometry did not pick up any significant changes. Furthermore, oscillometry detected a positive treatment effect with augmented immunosuppression, all while spirometry did not show any differences. It is suggested that the ease by which oscillometry can be done will facilitate identification of ACR, response to treatment, and should be done in adjunct to spirometry (183). With time, it is quite likely that spirometry might become redundant in this patient category because of the distinct small airway pattern of ACR and CLAD and the absence of large airways disease.
MOVING FORWARD
Adding diversity to the way respiratory function is measured as well as alignment between the various levels of the drug development process might be important when trying to predict the translational potential of a given therapeutic candidate or to assess its clinical efficacy. Not only would there be more insight (e.g., probing the peripheral lung using oscillometry), but this might also be key to reducing the risk of late failures during drug development. The technical recommendations from the American Thoracic Society and the European Respiratory Society for the conduct of a spirometric assessment specify the importance of a fast-full inflation and a maximal expiration. While generating high inspiratory and expiratory flows is generally perceived as harmless, it can trigger the activation of protective respiratory reflexes leading to what is referred to a spirometry-induced bronchoconstriction. In addition, the dynamic response to a deep inflation was reported to differ between health and disease as well as between airways and lung parenchyma (203). Hence, the fast-deep lung inflation required for a spirometric assessment of lung function has the potential to generate an opposing reaction such that the measurement technique can negatively impact what it is intended to evaluate. As unmet medical needs in the respiratory field often involve the treatment of unstable patients with an advanced disease status, these patients are more likely to be enrolled in later phases of clinical development. They are also more likely to develop an opposing reflex response following a deep inflation, such as a transient bronchoconstriction (204). This would technically make it more difficult for any given therapeutic candidate to demonstrate a beneficial clinical effect using spirometry. Since such opposing reflex responses are unlikely to be observed in anaesthetized and paralyzed experimental animals, this could well be an important distinguishing point between the two areas of the drug development process that may have been overlooked and worth considering in future drug development strategy as the choice of measurement techniques could be key. In that sense, one translational opportunity offered by oscillometry might reside in the minimal disturbance of the respiratory system in comparison to spirometry due to the lack of effort required. The fact that oscillometry measurements are typically obtained during quiet tidal breathing might, at the same time, be perceived as a limitation of the technique given that the assessment is only performed over a small part of the pressure-volume range around the functional residual capacity of the respiratory system. This was elegantly illustrated in a recent study of obese asthmatics where impedance was measured from total lung capacity and then throughout a passive exhalation to functional residual capacity. They suggest that the airways of very obese persons are experiencing profound collapse during expiration as demonstrated by a significant increase of respiratory elastance with decreasing lung volume (205). In addition, while preclinical oscillometry measurements can easily be performed at standardized lung volumes, the same cannot be easily achieved in human patients. This may represent a confounding factor (81) and although the impedance of the chest wall can be modelled, this is not routinely done (76, 206). The fact that preclinical and clinical oscillometry are based on the same principles and that there are similarities between the impedance spectra of different species can be viewed as advantageous in eliminating methodological differences. It is impossible to predict what impact a better methodological alignment between preclinical and clinical lung function measurements will have on future attrition rates in respiratory research. However, the possibility to mutually inform both settings, in addition to testing for functional efficacy and desired site of action, may improve the process. Oscillometry is already well established at the preclinical level and the future looks promising as more and more clinical trials include it as a complement to spirometry for lung function assessment (Table 4). The large number of on-going pharmaceutical and multi-center clinical trials using oscillometry also illustrates the interest to study drug effects on small airways. With almost all novel therapies being tested aiming at pathologies affecting the lung periphery, it would not be surprising to see oscillometry displaying an edge over spirometry in most studies. Similarly, in clinical practice, it seems that oscillometry is challenging the standard technique in pulmonary care, especially in pediatric patients (112, 207). Its ease of use and the absence of a forced maneuver may also promote it in the adult population, as exemplified with ACR in lung transplantation above, where the superior sensitivity of oscillometry should make it universally adopted for monitoring lung transplant patients for diagnosis but also to confirm treatment effects (183). Since the beginning, clinical and preclinical respiratory oscillometry have evolved as techniques, not only one next to the other, but mostly one from the other. Likely, it appears that it is in a similar manner that nowadays the technique may contribute to improve the development process of future respiratory therapies. Speeding up the discovery process, making it more likely to translate into the clinical research setting and practice, advancing knowledge, objectively assessing efficiency, these are just a few examples of the potential benefits that could come from the elimination of technical problems hindering the drug development process. The COVID-19 pandemic is challenging the way clinical pulmonary function tests are being done and, thus, may provide a unique occasion to explore techniques other than spirometry (149, 150, 208), an opportunity that should not be missed.
DISCLOSURES
L. K. A. Lundblad is an employee of Thorasys Thoracic Medical Systems Inc., and A. Robichaud is an employee of SCIREQ Scientific Respiratory Equipment Inc.; both companies develop and sell equipment for respiratory function measurements.
AUTHOR CONTRIBUTIONS
L.K.A.L. and A.R. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ynuk Bossé, Université Laval (Québec, QC, Canada); Dr. André Gie and Dr. Jeroen Vanoirbeek, KU Leuven (Leuven, Belgium); Dr. Wayne Mitzner, Johns Hopkins University (Baltimore, MD); and Dr. Rory Morty, University of Giessen and Max Planck Institute for Heart and Lung Research (Germany) for sharing impedance data for the creation of figures. We also thank Dr. Bossé for constructive comments on the manuscript. No external funding supported this work.
REFERENCES
- 1.Bjermer L, Bengtsson T, Jorup C, Lötvall J. Local and systemic effects of inhaled AZD9164 compared with tiotropium in patients with COPD. Respir Med 107: 84–90, 2013. doi: 10.1016/j.rmed.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Jorup C, Bengtsson T, Strandgården K, Sjöbring U. Transient paradoxical bronchospasm associated with inhalation of the LAMA AZD9164: analysis of two Phase I, randomised, double-blind, placebo-controlled studies. BMC Pulm Med 14, 2014. doi: 10.1186/1471-2466-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med 201: 1372–1379, 2020. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alblooshi A, Alkalbani A, Albadi G, Narchi H, Hall G. Is forced oscillation technique the next respiratory function test of choice in childhood asthma. World J Methodol 2017 7: 129–138, 2017. doi: 10.5662/wjm.v7.i4.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alblooshi A, Alkalbani A, Narchi H, Al-Hamad S, Al-Houqani M, Albadi G, Souid AK, Hall GL. Respiratory function in healthy Emirati children using forced oscillations. Pediatr Pulmonol 53: 936–941, 2018. doi: 10.1002/ppul.23985. [DOI] [PubMed] [Google Scholar]
- 6.Brown NJ, Xuan W, Salome CM, Berend N, Hunter ML, Musk AW, James AL, King GG. Reference equations for respiratory system resistance and reactance in adults. Respir Physiol Neurobiol 172: 162–168, 2010. doi: 10.1016/j.resp.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Calogero C, Simpson SJ, Lombardi E, Parri N, Cuomo B, Palumbo M, Martino de M, Shackleton C, Verheggen M, Gavidia T, Franklin PJ, Kusel MM, Park J, Sly PD, Hall GL. Respiratory impedance in healthy chrildren aged 2 to 13 years. Pediatr Pulmonol 48: 707–715, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Ducharme FM, Davis GM, Ducharme GR. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest 113: 1322–1328, 1998. doi: 10.1378/chest.113.5.1322. [DOI] [PubMed] [Google Scholar]
- 9.Jabbal S, Manoharan A, Lipworth J, Lipworth B. Utility of impulse oscillometry in patients with moderate to severe persistent asthma. J Allergy Clin Immunol 138: 601–603, 2016. doi: 10.1016/j.jaci.2015.12.1336. [DOI] [PubMed] [Google Scholar]
- 10.Lipworth B, Manoharan A, Anderson W. Unlocking the quiet zone: the small airway asthma phenotype. Lancet Respir Med 2: 497–506, 2014. doi: 10.1016/S2213-2600(14)70103-1. [DOI] [PubMed] [Google Scholar]
- 11.Lipworth BJ, Jabbal S. What can we learn about COPD from impulse oscillometry? Respir Med 139: 106–109, 2018. doi: 10.1016/j.rmed.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld M, Allen J, Arets BH, Aurora P, Beydon N, Calogero C, Castile RG, Davis SD, Fuchs S, Gappa M, Gustaffson PM, Hall GL, Jones MH, Kirkby JC, Kraemer R, Lombardi E, Lum S, Mayer OH, Merkus P, Nielsen KG, Oliver C, Oostveen E, Ranganathan S, Ren CL, Robinson PD, Seddon PC, Sly PD, Sockrider MM, Sonnappa S, Stocks J, Subbarao P, Tepper RS, Vilozni D. An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc 10: S1–S11, 2013. doi: 10.1513/AnnalsATS.201301-017ST. [DOI] [PubMed] [Google Scholar]
- 13.Calverley PM, Farre R. Oscillometry: old physiology with a bright future. Eur Respi J 56: 2001815, 2020. doi: 10.1183/13993003.01815-2020. [DOI] [PubMed] [Google Scholar]
- 14.DuBois AB, Brody AW, Lewis DH, Burgess BF. Oscillation mechanics of lungs and chest in man. J Appl Physiol 8: 587–594, 1956. doi: 10.1152/jappl.1956.8.6.587. [DOI] [PubMed] [Google Scholar]
- 15.Bates JH. CORP: Measurement of lung function in small animals. J Appl Physiol 123: 1039–1046, 2017. doi: 10.1152/japplphysiol.00243.2017. [DOI] [PubMed] [Google Scholar]
- 16.Lundblad LK. Issues determining direct airways hyperresponsiveness in mice. Front Physiol 3: 408, 2012. doi: 10.3389/fphys.2012.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lofgren JL, Mazan MR, Ingenito EP, Lascola K, Seavey M, Walsh A, Hoffman AM. Restrained whole body plethysmography for measure of strain-specific and allergen-induced airway responsiveness in conscious mice. J Appl Physiol 101: 1495–1505, 2006. doi: 10.1152/japplphysiol.00464.2006. [DOI] [PubMed] [Google Scholar]
- 18.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol 102: 221–230, 2007. doi: 10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]
- 19.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 20.Allen GB, Leclair TR, von Reyn J, Larrabee YC, Cloutier ME, Irvin CG, Bates JH. Acid aspiration-induced airways hyperresponsiveness in mice. J Appl Physiol 107: 1763–1770, 2009. doi: 10.1152/japplphysiol.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 104: 1727–1735, 2008. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 22.Collins RA, Gualano RC, Zosky GR, Atkins CL, Turner DJ, Colasurdo GN, Sly PD. Hyperresponsiveness to inhaled but not intravenous methacholine during acute respiratory syncytial virus infection in mice. Respir Res 6: 142, 2005. doi: 10.1186/1465-9921-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vooght V, Vanoirbeek JA, Haenen S, Verbeken E, Nemery B, Hoet PH. Oropharyngeal aspiration: an alternative route for challenging in a mouse model of chemical-induced asthma. Toxicology 259: 84–89, 2009. doi: 10.1016/j.tox.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 25.McGovern T, Farahnak S, Chen M, Larsson K, Martin JG, Adner M. Organic dust, causing both oxidative stress and Nrf2 activation, is phagocytized by bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 317: L305–L316, 2019. doi: 10.1152/ajplung.00377.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunil VR, Vayas KN, Fang M, Zarbl H, Massa C, Gow AJ, Cervelli JA, Kipen H, Laumbach RJ, Lioy PJ, Laskin JD, Laskin DL. World Trade Center (WTC) dust exposure in mice is associated with inflammation, oxidative stress and epigenetic changes in the lung. Exp Mol Pathol 102: 50–58, 2017. doi: 10.1016/j.yexmp.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, Dhanasekaran S, Yasin J, Ali BH, Kazzam EE. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol 164: 1871–1882, 2011. doi: 10.1111/j.1476-5381.2011.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 96: 2019–2027, 2004. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 29.Hartney JM, Robichaud A. Assessment of airway hyperresponsiveness in mouse models of allergic lung disease using detailed measurements of respiratory mechanics. Methods Mol Biol 1032: 205–217, 2013. doi: 10.1007/978-1-62703-496-8_16. [DOI] [PubMed] [Google Scholar]
- 30.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med 183: 865–875, 2011. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novali M, Shalaby KH, Robichaud A, Benedetti A, Fereydoonzad L, McGovern TK, Schuessler TF, Martin JG. Mechanical consequences of allergic induced remodeling on mice airway resistance and compressibility. Respir Physiol Neurobiol 218: 11–20, 2015. doi: 10.1016/j.resp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Riesenfeld EB, Allen G, Bates JH, Poynter ME, Wu M, Aimiand S, Lundblad LK. The temporal evolution of airways hyperresponsiveness and inflammation. J Allergy Ther 1: 1–7, 2012. doi: 10.4172/2155-6121.s1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mailhot-Larouche S, Deschênes L, Gazzola M, Lortie K, Henry C, Brook BS, Morissette MC, Bossé Y. Repeated airway constrictions in mice do not alter respiratory function. J Appl Physiol 124: 1483–1490, 2018. doi: 10.1152/japplphysiol.01073.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tully JE, Hoffman SM, Lahue KG, Nolin JD, Anathy V, Lundblad LK, Daphtary N, Aliyeva M, Black KE, Dixon AE, Poynter ME, Irvin CG, Janssen-Heininger YM. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J Immunol 191: 5811–5821, 2013. doi: 10.4049/jimmunol.1301329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips JE, Peng R, Harris P, Burns L, Renteria L, Lundblad LK, Fine JS, Bauer CM, Stevenson CS. House dust mite models: will they translate clinically as a superior model of asthma? J Allergy Clin Immunol 132: 242–244, 2013. doi: 10.1016/j.jaci.2012.12.1571. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Aliyeva M, Daphtary N, Martin RA, Poynter ME, Kostin SF, van der Velden JL, Hyman AM, Stevenson CS, Phillips JE, Lundblad LK. Antigen-induced mast cell expansion and bronchoconstriction in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol 306: L196–L206, 2014. doi: 10.1152/ajplung.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 175: 768–774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, Scott JA. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir Res 12: 19, 2011. doi: 10.1186/1465-9921-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 176: 650–658, 2007. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates JH, Cojocaru A, Lundblad LK. Bronchodilatory effect of deep inspiration on the dynamics of bronchoconstriction in mice. J Appl Physiol 103: 1696–1705, 2007. doi: 10.1152/japplphysiol.00698.2007. [DOI] [PubMed] [Google Scholar]
- 41.Therien AG, Bernier V, Weicker S, Tawa P, Falgueyret JP, Mathieu MC, Honsberger J, Pomerleau V, Robichaud A, Stocco R, Dufresne L, Houshyar H, Lafleur J, Ramachandran C, O’Neill GP, Slipetz D, Tan CM. Adenovirus IL-13-induced airway disease in mice: a corticosteroid-resistant model of severe asthma. Am J Respir Cell Mol Biol 39: 26–35, 2008. doi: 10.1165/rcmb.2007-0240OC. [DOI] [PubMed] [Google Scholar]
- 42.Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol 41: 281–289, 2009. doi: 10.1165/rcmb.2008-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takubo Y, Guerassimov A, Ghezzo H, Triantafillopoulos A, Bates JH, Hoidal JR, Cosio MG. α1-antitrypsin determines the pattern of emphysema and function in tobacco smoke-exposed mice: parallels with human disease. Am J Respir Crit Care Med 166: 1596–1603, 2002. doi: 10.1164/rccm.2202001. [DOI] [PubMed] [Google Scholar]
- 44.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 45.Tam A, Bates JH, Churg A, Wright JL, Man SF, Sin DD. Sex-related differences in pulmonary function following 6 months of cigarette exposure: Implications for sexual dimorphism in mild COPD. PLoS One 11: e0164835, 2016. doi: 10.1371/journal.pone.0164835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borel F, Sun H, Zieger M, Cox A, Cardozo B, Li W, Oliveira G, Davis A, Gruntman A, Flotte TR, Brodsky MH, Hoffman AM, Elmallah MK, Mueller C. Editing out five Serpina1 paralogs to create a mouse model of genetic emphysema. Proc Natl Acad Sci USA 115: 2788–2793, 2018. doi: 10.1073/pnas.1713689115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White Z, Milad N, Tehrani AY, Lamothe J, Hogg JC, Esfandiarei M, Seidman M, Booth S, Hackett TL, Morissette MC, Bernatchez P. Sildenafil prevents marfan-associated emphysema and early pulmonary artery dilation in mice. Am J Pathol 189: 1536–1546, 2019. doi: 10.1016/j.ajpath.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limjunyawong N, Craig JM, Daniel Lagassé HA, Scott AL, Mitzner W. Experimental progressive emphysema in BALB/cJ mice as a model for chronic alveolar destruction in humans. Am J Physiol Lung Cell Mol Physiol 309: L662–L676, 2015. doi: 10.1152/ajplung.00214.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veldhuizen RA, McCaig LA, Pape C, Gill SE. The effects of aging and exercise on lung mechanics, surfactant and alveolar macrophages. Exp Lung Res 45: 113–122, 2019. doi: 10.1080/01902148.2019.1605633. [DOI] [PubMed] [Google Scholar]
- 51.Manali ED, Moschos C, Triantafillidou C, Kotanidou A, Psallidas I, Karabela SP, Roussos C, Papiris S, Armaganidis A, Stathopoulos GT, Maniatis NA. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm Med 11: 33, 2011. doi: 10.1186/1471-2466-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devos FC, Maaske A, Robichaud A, Pollaris L, Seys S, Lopez CA, Verbeken E, Tenbusch M, Lories R, Nemery B, Hoet PH, Vanoirbeek JA. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir Res 18: 1–14, 2017. doi: 10.1186/s12931-017-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voltz JW, Card JW, Carey MA, DeGraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 39: 45–52, 2008. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Headley L, Bi W, Wilson C, Collum SD, Chavez M, Darwiche T, Mertens TC, Hernandez AM, Siddiqui SR, Rosenbaum S, Johnston RA, Karmouty-Quintana H. Low-dose administration of bleomycin leads to early alterations in lung mechanics. Exp Physiol 103: 1692–1703, 2018. doi: 10.1113/EP087322. [DOI] [PubMed] [Google Scholar]
- 55.Limjunyawong N, Mitzner W, Horton MR. A mouse model of chronic idiopathic pulmonary fibrosis. Physiol Rep 2: e00249, 2014. doi: 10.1002/phy2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 171: 1363–1370, 2005. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol 8: 24, 2011. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marinova M, Solopov P, Dimitropoulou C, Colunga Biancatelli RM, Catravas JD. Acute exposure of mice to hydrochloric acid leads to the development of chronic lung injury and pulmonary fibrosis. Inhal Toxicol 31: 147–160, 2019. doi: 10.1080/08958378.2019.1624895. [DOI] [PubMed] [Google Scholar]
- 59.Sunil VR, Vayas KN, Abramova EV, Rancourt R, Cervelli JA, Malaviya R, Goedken M, Venosa A, Gow AJ, Laskin JD, Laskin DL. Lung injury, oxidative stress and fibrosis in mice following exposure to nitrogen mustard. Toxicol Appl Pharmacol 387: 114798, 2020. doi: 10.1016/j.taap.2019.114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen JC, Lundblad LK, Bates JH, Levitzky M, Larson JE. The “Goldilocks Effect” in cystic fibrosis: identification of a lung phenotype in the CFTR knockout and heterozygous mouse. BMC Genet 5: 21, 2004. doi: 10.1186/1471-2156-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darrah RJ, Mitchell AL, Campanaro CK, Barbato ES, Litman P, Sattar A, Hodges CA, Drumm ML, Jacono FJ. Early pulmonary disease manifestations in cystic fibrosis mice. J Cyst Fibros 15: 736–744, 2016. doi: 10.1016/j.jcf.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glynos C, Bibli SI, Katsaounou P, Pavlidou A, Magkou C, Karavana V, Topouzis S, Kalomenidis I, Zakynthinos S, Papapetropoulos A. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol 315: L662–L672, 2018. doi: 10.1152/ajplung.00389.2017. [DOI] [PubMed] [Google Scholar]
- 63.Noël A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, Xiao R, Penn A. In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Cell Mol Physiol 318: L705–L722, 2020. doi: 10.1152/ajplung.00408.2019. [DOI] [PubMed] [Google Scholar]
- 64.Smirnova NF, Conlon TM, Morrone C, Dorfmuller P, Humbert M, Stathopoulos G, Umkehrer S, Pfeiffer F, Yildirim A, Eickelberg O. Inhibition of B cell–dependent lymphoid follicle formation prevents lymphocytic bronchiolitis after lung transplantation. JCI Insight 4: e123971, 2019. doi: 10.1172/jci.insight.123971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vermillion MS, Nelson A, Steeg Vom L, Loube J, Mitzner W, Klein SL. Pregnancy preserves pulmonary function following influenza virus infection in c57bl/6 mice. Am J Physiol Lung Cell Mol Physiol 315: L517–L525, 2018. doi: 10.1152/ajplung.00066.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halstead ES, Umstead TM, Davies ML, Kawasawa YI, Silveyra P, Howyrlak J, Yang L, Guo W, Hu S, Hewage EK, Chroneos ZC. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization. Respir Res 19: 3, 2018. doi: 10.1186/s12931-017-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, Ritter JH, Kang LI, Dort S, Robichaud A, Head R, Holtzman MJ, Diamond MS. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21, 1327–1335, 2020. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hull WE, Long EC. Respiratory impedance and volume flow at high frequency in dogs. J Appl Physiol 16: 439–443, 1961. doi: 10.1152/jappl.1961.16.3.439. [DOI] [PubMed] [Google Scholar]
- 69.Hantos Z, Daroczy B, Suki B, Nagy S. Low-frequency respiratory mechanical impedance in the rat. J Appl Physiol 63: 36–43, 1987. doi: 10.1152/jappl.1987.63.1.36. [DOI] [PubMed] [Google Scholar]
- 70.Jackson AC, Watson JW. Oscillatory mechanics of the respiratory system in normal rats. Respir Physiol 48: 309–322, 1982. doi: 10.1016/0034-5687(82)90036-6. [DOI] [PubMed] [Google Scholar]
- 71.Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 94: 1297–1306, 2003. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- 72.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res 4: 4, 2003. doi: 10.1186/1465-9921-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bozanich EM, Collins RA, Thamrin C, Hantos Z, Sly PD, Turner DJ. Developmental changes in airway and tissue mechanics in mice. J Appl Physiol 99: 108–113, 2005. doi: 10.1152/japplphysiol.01111.2004. [DOI] [PubMed] [Google Scholar]
- 74.Fredberg JJ, Keefe DH, Glass GM, Castile RG, Frantz ID. Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J Appl Physiol Respir Environ Exerc Physiol 57: 788–800, 1984. doi: 10.1152/jappl.1984.57.3.788. [DOI] [PubMed] [Google Scholar]
- 75.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 42: 860–866, 1995. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 76.Bates JH. Lung Mechanics. An Inverse Modeling Approach. Cambridge, UK: Cambridge Univ. Press, 2009. www.cambridge.org/9780521509602. [Google Scholar]
- 77.Bates JH, Irvin CG, Farré R, Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol 1: 1233–1272, 2011. doi: 10.1002/cphy.c100058. [DOI] [PubMed] [Google Scholar]
- 78.Desiraju K, Agrawal A. Impulse oscillometry: the state-of-art for lung function testing. Lung India 33: 410–416, 2016. doi: 10.4103/0970-2113.184875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundblad LK, Siddiqui S, Bossé Y, Dandurand R. Applications of oscillometry in clinical research and practice. Can J Respir Crit Care Sleep Med 5: 54–68, 2021. doi: 10.1080/24745332.2019.1649607. [DOI] [Google Scholar]
- 80.Peslin R, Fredberg J. Oscillation mechanics of the respiratory system. In: Handbook of Physiology, edited by Fishman AP. Bethesda, MD: American Physiological Society, 1986, p. 145–178. [Google Scholar]
- 81.Peters U, Kaminsky DA, Bhatawadekar S, Lundblad L, Maksym GN. Oscillometry for lung function testing. In: Lung Function Testing in the 21st Century, edited by Ionescu C. New York: Academic Press, 1986, p. 25–47. [Google Scholar]
- 82.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp 75: e50172, 2013. doi: 10.3791/50172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Babik B, Peták F, Asztalos T, Deák ZI, Bogáts G, Hantos Z. Components of respiratory resistance monitored in mechanically ventilated patients. Eur Respir J 20: 1538–1544, 2002. doi: 10.1183/09031936.02.00000802. [DOI] [PubMed] [Google Scholar]
- 84.Lutchen KR, Yang K, Kaczka DW, Suki B. Optimal ventilation waveforms for estimating low-frequency respiratory impedance. J Appl Physiol 75: 478–488, 1993. doi: 10.1152/jappl.1993.75.1.478. [DOI] [PubMed] [Google Scholar]
- 85.Peták F, Babik B, Asztalos T, Hall GL, Deák ZI, Sly PD, Hantos Z. Airway and tissue mechanics in anesthetized paralyzed children. Pediatr Pulmonol 35: 169–176, 2003. doi: 10.1002/ppul.10252. [DOI] [PubMed] [Google Scholar]
- 86.Sly PD, Hayden MJ, Peták F, Hantos Z. Measurement of low-frequency respiratory impedance in infants. Am J Respir Crit Care Med 154: 161–166, 1996. doi: 10.1164/ajrccm.154.1.8680673. [DOI] [PubMed] [Google Scholar]
- 87.Dandurand RJ, Lavoie JP, Lands LC, Hantos Z. Comparison of oscillometry devices using active mechanical test loads. ERJ Open Res 5: 00160–02019, 2019. doi: 10.1183/23120541.00160-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 89.Kaminsky DA. What does airway resistance tell us about lung function? Respir Care 57: 85–99, 2012. doi: 10.4187/respcare.01411. [DOI] [PubMed] [Google Scholar]
- 90.Davey BL, Bates JH. Regional lung impedance from forced oscillations through alveolar capsules. Respir Physiol 91: 165–182, 1993. doi: 10.1016/0034-5687(93)90097-T. [DOI] [PubMed] [Google Scholar]
- 91.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 93: 263–270, 2002. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- 92.Lutchen KR, Greenstein JL, Suki B. How inhomogeneities and airway walls affect frequency dependence and separation of airway and tissue properties. J Appl Physiol 80: 1696–1707, 1996. doi: 10.1152/jappl.1996.80.5.1696. [DOI] [PubMed] [Google Scholar]
- 93.Bates JH, Schuessler TF, Dolman C, Eidelman DH. Temporal dynamics of acute isovolume bronchoconstriction in the rat. J Appl Physiol 82: 55–62, 1997. [DOI] [PubMed] [Google Scholar]
- 94.Siddiqui S, Tsuchiya K, Risse PA, Bullimore SR, Benedetti A, Martin JG. Site of allergic airway narrowing and the influence of exogenous surfactant in the brown norway rat. PLoS One 7: e29381, 2012. doi: 10.1371/journal.pone.0029381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michaelson ED, Grassman ED, Peters WR. Pulmonary mechanics by spectral analysis of forced random noise. J Clin Invest 56: 1210–1230, 1975. doi: 10.1172/JCI108198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bates JH, Allen GB. The estimation of lung mechanics parameters in the presence of pathology: a theoretical analysis. Ann Biomed Eng 34: 384–392, 2006. doi: 10.1007/s10439-005-9056-6. [DOI] [PubMed] [Google Scholar]
- 97.Landser FJ, Clement J, Van de Woestijne KP. Normal values of total respiratory resistance and reactance determined by forced oscillations: influence of smoking. Chest 81: 586–591, 1982. doi: 10.1378/chest.81.5.586. [DOI] [PubMed] [Google Scholar]
- 98.Otis AB, Mcherrow CB, Bartlett RA, Mead J, Mcilroy N, Ja S, Radford EP. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 8: 427–443, 1956. doi: 10.1152/jappl.1956.8.4.427. [DOI] [PubMed] [Google Scholar]
- 99.Pride NB. Forced oscillation techniques for measuring mechanical properties of the respiratory system. Thorax 47: 317–320, 1992. doi: 10.1136/thx.47.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clement J, Landser FJ, Van de Woestijne KP. Total resistance and reactance in patients with respiratory complaints with and without airways obstruction. Chest 83: 215–220, 1983. doi: 10.1378/chest.83.2.215. [DOI] [PubMed] [Google Scholar]
- 101.Foy BH, Soares M, Bordas R, Richardson M, Bell A, Singapuri A, Hargadon B, Brightling C, Burrowes K, Kay D, Owers-Bradley J, Siddiqui S. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med 200: 982–991, 2019. doi: 10.1164/rccm.201812-2322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grimby G, Takishima T, Graham W, Macklem P, Mead J. Frequency dependence of flow resistance in patients with obstructive lung disease. J Clin Invest 47: 1455–65, 1968. doi: 10.1172/JCI105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuo CR, Jabbal S, Lipworth B. I say IOS you say AOS: comparative bias in respiratory impedance measurements. Lung 197: 473–481, 2019. doi: 10.1007/s00408-019-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wouters EF, Landser FJ, Polko AH, Visser BF. Impedance measurement during air and helium-oxygen breathing before and after salbutamol in COPD patients. Clin Exp Pharmacol Physiol 19: 95–101, 1992. doi: 10.1111/j.1440-1681.1992.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 105.Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther 14: 341–350, 2001. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 106.Gray DM, Czovek D, McMillan L, Turkovic L, Stadler JA, Vanker A, Radics BL, Gingl Z, Hall GL, Sly PD, Zar HJ, Hantos Z. Intra-breath measures of respiratory mechanics in healthy African infants detect risk of respiratory illness in early life. Eur Respir J 53: 1–9, 2018. doi: 10.1183/13993003.00998-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hantos Z, Czövek D, Gyurkovits Z, Szabõ H, Maár BA, Radics B, Virág K, Makan G, Orvos H, Gingl Z, Sly PD. Assessment of respiratory mechanics with forced oscillations in healthy newborns. Pediatr Pulmonol 50: 344–352, 2015. doi: 10.1002/ppul.23103. [DOI] [PubMed] [Google Scholar]
- 108.Emanuela Z, Biswajit C, Leonardo G, Pompilio PP, Robert R, Peter MA, Dellacà RL. Detection of expiratory flow limitation by forced oscillations during noninvasive ventilation. Am J Respir Crit Care Med 200: 1063–1065, 2019. doi: 10.1164/rccm.201903-0570LE. [DOI] [PubMed] [Google Scholar]
- 109.Lorx A, Czövek D, Gingl Z, Makan G, Radics B, Bartusek D, Szigeti S, Gál J, Losonczy G, Sly PD, Hantos Z. Airway dynamics in COPD patients by within-breath impedance tracking: effects of continuous positive airway pressure. Eur Respir J 49: 1601270, 2017. doi: 10.1183/13993003.01270-2016. [DOI] [PubMed] [Google Scholar]
- 110.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol 89: 908–916, 2000. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- 111.Broussard D, Larson JE, Cohen JC, Lundblad LK. Developmental changes in respiratory mechanics in the neonatal rat. Exp Lung Res 32: 263–273, 2006. doi: 10.1080/01902140600817549. [DOI] [PubMed] [Google Scholar]
- 112.Klinger AP, Travers CP, Martin A, Kuo HC, Alishlash AS, Harris WT, Carlo WA, Ambalavanan N. Non-invasive forced oscillometry to quantify respiratory mechanics in term neonates. Pediatr Res 88: 293–299, 2020. doi: 10.1038/s41390-020-0751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adner M, Canning BJ, Meurs H, Ford W, Ramírez PR, van den Berg MP, Birrell MA, Stoffels E, Lundblad LK, Nilsson GP, Olsson HK, Belvisi MG, Dahlén SE. Back to the future: re-establishing Guinea pig in vivo asthma models. Clin Sci (Lond) 134: 1219–1242, 2020. doi: 10.1042/cs20200394. [DOI] [PubMed] [Google Scholar]
- 114.Carpe N, Mandeville I, Ribeiro L, Ponton A, Martin JG, Kho AT, Chu JH, Tantisira K, Weiss ST, Raby BA, Kaplan F. Genetic influences on asthma susceptibility in the developing lung. Am J Respir Cell Mol Biol 43: 720–730, 2010. doi: 10.1165/rcmb.2009-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gie AG, Regin Y, Salaets T, Casiraghi C, Salomone F, Deprest J, Vanoirbeek J, Toelen J. Intratracheal budesonide/surfactant attenuates hyperoxia-induced lung injury in preterm rabbits. Am J Physiol Lung Cell Mol Physiol 319: L949–L956, 2020. doi: 10.1152/ajplung.00162.2020. [DOI] [PubMed] [Google Scholar]
- 116.Südy R, Fodor GH, Dos Santos Rocha A, Schranc Á, Tolnai J, Habre W, Peták F. Different contributions from lungs and chest wall to respiratory mechanics in mice, rats, and rabbits. J Appl Physiol 127: 198–204, 2019. doi: 10.1152/japplphysiol.00048.2019. [DOI] [PubMed] [Google Scholar]
- 117.Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B. European Lung White Book (2nd ed.) (Online). Sheffield, UK: European Respiratory Society. www.erswhitebook.org [2021-Feb.]. [Google Scholar]
- 118.Coates AL, Wanger J, Cockcroft DW, Culver BH, Carlsen KH, Diamant Z, Gauvreau G, Hall GL, Hallstrand TS, Horvath I, De Jongh FH, Joos G, Kaminsky DA, Laube BL, Leuppi JD, Sterk PJ. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J 49: 1601526, 2017. doi: 10.1183/13993003.01526-2016. [DOI] [PubMed] [Google Scholar]
- 119.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 43: 343–373, 2014. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 120.Robichaud A, Fereydoonzad L, Schuessler TF. Delivered dose estimate to standardize airway hyperresponsiveness assessment in mice. Am J Physiol Lung Cell Mol Physiol 308: L837–L846, 2015. doi: 10.1152/ajplung.00343.2014. [DOI] [PubMed] [Google Scholar]
- 121.Eddy R, Maksym GN, Dandurand RJ, Parraga G. Oscillometry detects different airway abnormalities that manifest as mri ventilation heterogeneity in asthma and COPD . Am J Respir Crit Care Med A6093, 2019. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A6093. [DOI] [Google Scholar]
- 122.Eddy RL. Structure and Function of Asthma Evaluated Using Pulmonary Imaging (PhD thesis). Ontario, Canada: Western University, 2020. https://ir.lib.uwo.ca/etd/6871 [2020 Apr 20]. [Google Scholar]
- 123.Eddy RL, Westcott A, Maksym GN, Parraga G, Dandurand RJ. Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiol Rep 7: 1–11, 2019. doi: 10.14814/phy2.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young HM, Guo F, Eddy RL, Maksym G, Parraga G. Oscillometry and pulmonary MRI measurements of ventilation heterogeneity in obstructive lung disease: relationship to quality of life and disease control. J Appl Physiol 125: 73–85, 2018. doi: 10.1152/japplphysiol.01031.2017. [DOI] [PubMed] [Google Scholar]
- 125.Venegas JG, Winkler T, Musch G, Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 126.Su ZQ, Guan WJ, Li SY, Ding M, Chen Y, Jiang M, Chen XB, Zhong CH, Tang CL, Zhong NS. Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. Int J COPD 13: 3031–3044, 2018. doi: 10.2147/COPD.S172639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 294: L612–L630, 2008. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 128.Wright JL, Churg A. Animal models of cigarette smoke-induced COPD. Chest 122: 301S–306S, 2002. doi: 10.1378/chest.122.6_suppl.301S. [DOI] [PubMed] [Google Scholar]
- 129.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295: L1–L15, 2008. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zimmermann SC, Huvanandana J, Nguyen CD, Bertolin A, Watts JC, Gobbi A, Farah CS, Peters MJ, Dellacà RL, King GG, Thamrin C. Day-to-day variability of forced oscillatory mechanics for early detection of acute exacerbations in COPD. Eur Respir J 56: 1901739, 2020. doi: 10.1183/13993003.01739-2019. [DOI] [PubMed] [Google Scholar]
- 131.Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, Calverley PM. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 105: 1069–1078, 2011. doi: 10.1016/j.rmed.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 132.Wuyts WA, Wijsenbeek M, Bondue B, Bouros D, Bresser P, Robalo CC, Hilberg O, Magnusson J, Manali ED, Morais A, Papiris S, Shaker S, Veltkamp M, Bendstrup E. Idiopathic pulmonary fibrosis: best practice in monitoring and managing a relentless fibrotic disease. Respiration 99: 73–82, 2020. doi: 10.1159/000504763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dandurand R, Dandurand M, Estepar R, Bourbeau J, Eidelman D. Oscillometry in community practice ild is characterized by abnormal reactance but normal resistance. Quart J Med 109: S50, 2016. [Google Scholar]
- 134.Mori Y, Nishikiori H, Chiba H, Yamada G, Kuronuma K, Takahashi H. Respiratory reactance in forced oscillation technique reflects disease stage and predicts lung physiology deterioration in idiopathic pulmonary fibrosis. Respir Physiol Neurobiol 275: 103386, 2020. doi: 10.1016/j.resp.2020.103386. [DOI] [PubMed] [Google Scholar]
- 135.Naglaa BA, Kamal E. Role of IOS in evaluation of patients with interstitial lung diseases. Egypt J Chest Dis Tuberc 65: 791–795, 2016. doi: 10.1016/j.ejcdt.2016.05.002. [DOI] [Google Scholar]
- 136.Sugiyama A, Hattori N, Haruta Y, Nakamura I, Nakagawa M, Miyamoto S, Onari Y, Iwamoto H, Ishikawa N, Fujitaka K, Murai H, Kohno N. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med 107: 875–882, 2013. doi: 10.1016/j.rmed.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 137.Ducharme FM, Jroundi I, Jean G, Boutin GL, Lawson C, Vinet B. Interdevice agreement in respiratory resistance values by oscillometry in asthmatic children. ERJ Open Resv 5: 00138–2018, 2019. doi: 10.1183/23120541.00138-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakarya A, Uyan ZS, Baydemir C, Anık Y, Erdem E, Gokdemir Y, Karadag B, Karakoc F, Ersu R. Evaluation of children with cystic fibrosis by impulse oscillometry when stable and at exacerbation. Pediatr Pulmonol 51: 1151–1158, 2016. doi: 10.1002/ppul.23449. [DOI] [PubMed] [Google Scholar]
- 139.Ramsey KA, Ranganathan SC, Gangell CL, Turkovic L, Park J, Skoric B, Stick SM, Sly PD, Hall GL. Impact of lung disease on respiratory impedance in young children with cystic fibrosis. Eur Respir J 46: 1672–1679, 2015. doi: 10.1183/13993003.00156-2015. [DOI] [PubMed] [Google Scholar]
- 140.Zannin E, Nyilas S, Ramsey KA, Latzin P, Dellaca’ RL. Within-breath changes in respiratory system impedance in children with cystic fibrosis. Pediatr Pulmonol 54: 737–742, 2019. doi: 10.1002/ppul.24281. [DOI] [PubMed] [Google Scholar]
- 141.Buchs C, Coutier L, Vrielynck S, Jubin V, Mainguy C, Reix P. An impulse oscillometry system is less efficient than spirometry in tracking lung function improvements after intravenous antibiotic therapy in pediatric patients with cystic fibrosis. Pediatr Pulmonol 50: 1073–1081, 2015. doi: 10.1002/ppul.23301. [DOI] [PubMed] [Google Scholar]
- 142.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 17: 478–483, 2011. doi: 10.1097/mcp.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren CL, Rosenfeld M, Mayer OH, Davis SD, Kloster M, Castile RG, Hiatt PW, Hart M, Johnson R, Jones P, Brumback LC, Kerby GS. Analysis of the associations between lung function and clinical features in preschool children with cystic fibrosis. Pediatr Pulmonol 47: 574–581, 2012. doi: 10.1002/ppul.21590. [DOI] [PubMed] [Google Scholar]
- 144.Horsley A, Siddiqui S. Putting lung function and physiology into perspective: cystic fibrosis in adults. Respirology 20: 33–45, 2015. doi: 10.1111/resp.12382. [DOI] [PubMed] [Google Scholar]
- 145.Lima AN, Faria AC, Lopes AJ, Jansen JM, Melo PL. Forced oscillations and respiratory system modeling in adults with cystic fibrosis. Biomed Eng Online 14: 11, 2015. doi: 10.1186/s12938-015-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tucker MA, Crandall R, Seigler N, Rodriguez-Miguelez P, McKie KT, Forseen C, Thomas J, Harris RA. A single bout of maximal exercise improves lung function in patients with cystic fibrosis. J Cyst Fibros 16: 752–758, 2017. doi: 10.1016/j.jcf.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 147.Stanford G, Davies JC, Usmani O, Banya W, Charman S, Jones M, Simmonds NJ, Bilton D. Investigating outcome measures for assessing airway clearance techniques in adults with cystic fibrosis: Protocol of a single-centre randomised controlled crossover trial. BMJ Open Respir Res 7: e000694, 2020. doi: 10.1136/bmjresp-2020-000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall WJ, Douglas RG, Hyde RW, Roth FK, Cross AS, Speers DM. Pulmonary mechanics after uncomplicated influenza A infection. Am Rev Respir Dis 113: 141–147, 1976. doi: 10.1164/arrd.1976.113.2.141. [DOI] [PubMed] [Google Scholar]
- 149.Kouri A, Gupta S, Yadollahi A, Ryan CM, Gershon AS, To T, Tarlo SM, Goldstein RS, Chapman KR, Chow CW. Addressing reduced laboratory-based pulmonary function testing during a pandemic. Chest 158: 2502–2510, 2020. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lundblad LK, Chow C. Lung function monitoring in the era of respiratory pandemics. Clin Physiol Funct Imaging 40: 377–379, 2020. doi: 10.1111/cpf.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barnes PJ, Bonini S, Seeger W, Belvisi MG, Ward B, Holmes A. Barriers to new drug development in respiratory disease. Eur Respir J 45: 1197–1207, 2015. doi: 10.1183/09031936.00007915. [DOI] [PubMed] [Google Scholar]
- 152.Marciniuk DD, Schraufnagel DE, Ferkol T, Fong KM, Joos G, Varela VL, Zar H. Forum of International Respiratory Societies. The Global Impact of Respiratory Disease (2nd ed) (Online). Sheffield, UK: European Respiratory Society, 2017. https://www.firsnet.org/ [10 Feb. 2020]. [Google Scholar]
- 153.Giesecke J. The invisible pandemic. Lancet 395: e98, 2020. doi: 10.1016/S0140-6736(20)31035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Allinne J, Scott G, Lim WK, Birchard D, Erjefält JS, Sandén C, Ben LH, Agrawal A, Kaur N, Kim JH, Kamat V, Fury W, Huang T, Stahl N, Yancopoulos GD, Murphy AJ, Sleeman MA, Orengo JM. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol 144: 1624–1637.e10, 2019. doi: 10.1016/j.jaci.2019.08.039. [DOI] [PubMed] [Google Scholar]
- 155.Le Floc’h A, Allinne J, Nagashima K, Scott G, Birchard D, Asrat S, Bai Y, Lim WK, Martin J, Huang T, Potocky TB, Kim JH, Rafique A, Papadopoulos NJ, Stahl N, Yancopoulos GD, Murphy AJ, Sleeman MA, Orengo JM. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy Eur J Allergy Clin Immunol 75: 1188–1204, 2020. doi: 10.1111/all.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kirsten AM, Watz H, Brindicci C, Piccinno A, Magnussen H. Effects of beclomethason/formoterol and budesonide/formoterol fixed combinations on lung function and airway inflammation in patients with mild to moderate asthma–an exploratory study. Pulm Pharmacol Ther 31: 79–84, 2015. doi: 10.1016/j.pupt.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 157.Hozawa S, Terada M, Haruta Y, Hozawa M. Comparison of early effects of budesonide/formoterol maintenance and reliever therapy with fluticasone furoate/vilanterol for asthma patients requiring step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther 37: 15–23, 2016. doi: 10.1016/j.pupt.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 158.Hozawa S, Terada M, Hozawa M. Comparison of the effects of budesonide/formoterol maintenance and reliever therapy with fluticasone/salmeterol fixed-dose treatment on airway inflammation and small airway impairment in patients who need to step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther 27: 190–196, 2014. doi: 10.1016/j.pupt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Diong B, Singh K, Menendez R. Effects of two inhaled corticosteroid/long-acting beta-agonist combinations on small-airway dysfunction in mild asthmatics measured by impulse oscillometry. J Asthma Allergy: 109–116, 2013. doi: 10.2147/JAA.S48827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hozawa S, Terada M, Hozawa M. Comparison of budesonide/formoterol Turbuhaler with fluticasone/salmeterol Diskus for treatment effects on small airway impairment and airway inflammation in patients with asthma. Pulm Pharmacol Ther 24: 571–576, 2011. doi: 10.1016/j.pupt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 161.Díaz-García R, Flores-Ramírez G, Ramírez-Oseguera RT. Effect of extrafine formulation of BDP/FF inhaler on asthma control, small airway function and airway inflammation among Mexican asthmatic patients. A retrospective analysis. Respir Med 165: 105932, 2020. doi: 10.1016/j.rmed.2020.105932. [DOI] [PubMed] [Google Scholar]
- 162.Yamaguchi M, Niimi A, Ueda T, Takemura M, Matsuoka H, Jinnai M, Otsuka K, Oguma T, Takeda T, Ito I, Matsumoto H, Hirai T, Chin K, Mishima M. Effect of inhaled corticosteroids on small airways in asthma: Investigation using impulse oscillometry. Pulm Pharmacol Ther 22: 326–332, 2009. doi: 10.1016/j.pupt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 163.Hoshino M. Comparison of effectiveness in ciclesonide and fluticasone propionate on small airway function in mild asthma. Allergol Int 59: 59–66, 2010. doi: 10.2332/allergolint.09-OA-0122. [DOI] [PubMed] [Google Scholar]
- 164.Gjerum K, Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and methacholine responsiveness in 2- to 5-year-old asthmatic children. Am J Respir Crit Care Med 162: 1500–1506, 2000. doi: 10.1164/ajrccm.162.4.2002019. [DOI] [PubMed] [Google Scholar]
- 165.Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2- to 5-year-old asthmatic children as measured by impulse oscillometry. J Asthma 39: 531–536, 2002. doi: 10.1081/JAS-120004923. [DOI] [PubMed] [Google Scholar]
- 166.Kahan ES, Martin UJ, Spungen S, Ciccolella D, Criner GJ. Chronic cough and dyspnea in ice hockey players after an acute exposure to combustion products of a faulty ice resurfacer. Lung 185: 47–54, 2007. doi: 10.1007/s00408-006-0094-0. [DOI] [PubMed] [Google Scholar]
- 167.Manoharan A, Von Wilamowitz-Moellendorff A, Morrison A, Lipworth BJ. Effects of formoterol or salmeterol on impulse oscillometry in patients with persistent asthma. J Allergy Clin Immunol 137: 727–733, 2016. doi: 10.1016/j.jaci.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 168.Mondal P, Baumstein S, Prabhakaran S, Abu-Hasan M, Zeng Y, Singh S, Wang K, Ahrens RC, Hendeles L. Bioassay of salmeterol in children using methacholine challenge with impulse oscillometry. Pediatr Pulmonol 51: 570–575, 2016. doi: 10.1002/ppul.23345. [DOI] [PubMed] [Google Scholar]
- 169.Nair A, Ward J, Lipworth BJ. Comparison of bronchodilator response in patients with asthma and healthy subjects using spirometry and oscillometry. Ann Allergy Asthma Immunol 107: 317–322, 2011. doi: 10.1016/j.anai.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 170.Thamrin C, Gangell CL, Udomittipong K, Kusel MM, Patterson H, Fukushima T, Schultz A, Hall GL, Stick SM, Sly PD. Assessment of bronchodilator responsiveness in preschool children using forced oscillations. Thorax 62: 814–9, 2007. doi: 10.1136/thx.2006.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh D, Tal-Singer R, Faiferman I, Lasenby S, Henderson A, Wessels D, Goosen A, Dallow N, Vessey R, Goldman M. Plethysmography and impulse oscillometry assessment of tiotropium and ipratropium bromide; a randomized, double-blind, placebo-controlled, cross-over study in healthy subjects. Br J Clin Pharmacol 61: 398–404, 2006. doi: 10.1111/j.1365-2125.2006.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Eddy RL, Parraga G. Regional airway heterogeneity in asthma: histopathology, MRI, and CT imaging. Chest 159: 876–877, 2021. doi: 10.1016/j.chest.2020.09.090. [DOI] [PubMed] [Google Scholar]
- 173.Cottee AM, Seccombe LM, Thamrin C, King GG, Peters MJ, Farah CS. Bronchodilator response assessed using the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest 157: 1435–1441, 2020. doi: 10.1016/j.chest.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lipworth B, Chan R, Kuo CR. Criteria for airway oscillometry reversibility in asthma. Chest 158: 1282–1283, 2020. doi: 10.1016/j.chest.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 175.Nieto A, Pamies R, Oliver F, Medina A, Caballero L, Mazon A. Montelukast improves pulmonary function measured by impulse oscillometry in children with asthma (Mio study). Respir Med 100: 1180–1185, 2006. doi: 10.1016/j.rmed.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 176.Russell RJ, Chachi L, FitzGerald JM, Backer V, Olivenstein R, Titlestad IL, Ulrik CS, Harrison T, Singh D, Chaudhuri R, Leaker B, McGarvey L, Siddiqui S, Wang M, Braddock M, Nordenmark LH, Cohen D, Parikh H, Colice G, Brightling CE, Laviolette M, Skjold T, Nielsen LC, Howarth P. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med 6: 499–510, 2018. doi: 10.1016/S2213-2600(18)30201-7. [DOI] [PubMed] [Google Scholar]
- 177.Lundblad LK, Blouin N, Grudin O, Grudina L, Drapeau G, Restrepo N, Ducharme FM. Comparing lung oscillometry with a novel, portable flow interrupter device to measure lung mechanics. J Appl Physiol 130: 933–940, 2020. doi: 10.1152/japplphysiol.01072.2020. [DOI] [PubMed] [Google Scholar]
- 178.Houghton CM, Woodcock AA, Singh D. A comparison of plethysmography, spirometry and oscillometry for assessing the pulmonary effects of inhaled ipratropium bromide in healthy subjects and patients with asthma. Br J Clin Pharmacol 59: 152–159, 2005. doi: 10.1111/j.1365-2125.2004.2262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Borrill ZL, Houghton CM, Tal-Singer R, Vessey SR, Faiferman I, Langley SJ, Singh D. The use of plethysmography and oscillometry to compare long-acting bronchodilators in patients with COPD. Br J Clin Pharmacol 65: 244–252, 2008. doi: 10.1111/j.1365-2125.2007.03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Milne S, Hammans C, Watson S, Farah CS, Thamrin C, King GG. Bronchodilator responses in respiratory impedance, hyperinflation and gas trapping in COPD. COPD 15: 1–9, 2018. doi: 10.1080/15412555.2017.1421151. [DOI] [PubMed] [Google Scholar]
- 181.Mineshita M, Shikama Y, Nakajima H, Nishihira R, Komatsu S, Kubota M, Kobayashi H, Kokubu F, Shinkai M, Kaneko T, Miyazawa T. The application of impulse oscillation system for the evaluation of treatment effects in patients with COPD. Respir Physiol Neurobiol 202: 1–5, 2014. doi: 10.1016/j.resp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 182.Wu JK, DeHaas E, Nadj R, Cheung AB, Dandurand RJ, Hantos Z, Ryan CM, Chow CW. Development of quality assurance and quality control guidelines for respiratory oscillometry in clinic studies. Respir Care 65: 1687–1693, 2020. doi: 10.4187/respcare.07412. [DOI] [PubMed] [Google Scholar]
- 183.Cho E, Wu JK, Birriel DC, Matelski J, Nadj R, DeHaas E, Huang Q, Yang K, Xu T, Cheung AB, Woo LN, Day L, Cypel M, Tikkanen J, Ryan C, Chow CW. Airway oscillometry detects spirometric-silent episodes of acute cellular rejection. Am J Respir Crit Care Med 201: 1536–1544, 2020. doi: 10.1164/rccm.201908-1539OC. [DOI] [PubMed] [Google Scholar]
- 184.Hall W, Douglas RJ, Zaky D, Hyde R, Richman D, Murphy B. Attenuated influenza virus in normal adults: role of pulmonary function studies in vaccine trials. J Infect Dis 133: 145–152, 1976. [PubMed] [Google Scholar]
- 185.Kuo C, Lipworth B. Airwave oscillometry in relation to patient reported outcomes in asthma. Thorax 74: A97, 2019. [Google Scholar]
- 186.Lundblad LK, Miletic R, Piitulainen E, Wollmer P. Oscillometry in chronic obstructive lung disease: in vitro and in vivo evaluation of the impulse oscillometry and tremoflo devices. Sci Rep 9: 11618, 2019. doi: 10.1038/s41598-019-48039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gauthier JM, Hachem RR, Kreisel D. Update on chronic lung allograft dysfunction. Curr Transplant Reports 3: 185–191, 2016. doi: 10.1007/s40472-016-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation 80: 1406–1413, 2005. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 189.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 170: 1022–1026, 2004. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 190.Shino MY, Weigt SS, Li N, Derhovanessian A, Sayah DM, Huynh RH, Saggar R, Gregson AL, Ardehali A, Ross DJ, Lynch JP, Elashoff RM, Belperio JA. Impact of allograft injury time of onset on the development of chronic lung allograft dysfunction after lung transplantation. Am J Transplant 17: 1294–1303, 2017. doi: 10.1111/ajt.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Verleden SE, Vos R, Vanaudenaerde BM, Verleden GM. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis 9: 2650–2659, 2017. doi: 10.21037/jtd.2017.07.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report–2015; Focus Theme: Early Graft Failure. J Hear Lung Transplant 34: 1264–1277, 2015. doi: 10.1016/j.healun.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 193.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Hear Lung Transplant 21: 1062–1067, 2002. doi: 10.1016/S1053-2498(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 194.Trulock EP, Ettinger NA, Brunt EM, Pasque MK, Kaiser LR, Cooper JD. The role of transbronchial lung biopsy in the treatment of lung transplant recipients; An analysis of 200 consecutive procedures. Chest 102: 1049–1054, 1992. doi: 10.1378/chest.102.4.1049. [DOI] [PubMed] [Google Scholar]
- 195.Yserbyt J, Dooms C, Decramer M, Verleden GM. Acute lung allograft rejection: diagnostic role of probe-based confocal laser endomicroscopy of the respiratory tract. J Heart Lung Transplant 33: 492–8, 2014. doi: 10.1016/j.healun.2014.01.857. [DOI] [PubMed] [Google Scholar]
- 196.Chhajed PN, Tamm M, Glanville AR. Role of flexible bronchoscopy in lung transplantation. Semin Respir Crit Care Med 25: 413–423, 2004. doi: 10.1055/s-2004-832714. [DOI] [PubMed] [Google Scholar]
- 197.Glanville A. The role of surveillance bronchoscopy post-lung transplantation. Semin Respir Crit Care Med 34: 414–420, 2013. doi: 10.1055/s-0033-1348466. [DOI] [PubMed] [Google Scholar]
- 198.Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med 31: 208–221, 2010. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 199.Sandrini A, Glanville AR. The controversial role of surveillance bronchoscopy after lung transplantation. Curr Opin Organ Transplant 14: 494–498, 2009. doi: 10.1097/MOT.0b013e3283300a3b. [DOI] [PubMed] [Google Scholar]
- 200.Burgel PR, Bergeron A, de Blic J, Bonniaud P, Bourdin A, Chanez P, Chinet T, Dalphin JC, Devillier P, Deschildre A, Didier A, Kambouchner M, Knoop C, Laurent F, Nunes H, Perez T, Roche N, Tillie-Leblond I, Dusser D. Small airways diseases, excluding asthma and COPD: an overview. Eur Respir Rev 22: 131–47, 2013. doi: 10.1183/09059180.00001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 298: 1277–1281, 1978. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 202.Ochman M, Wojarski J, Wiórek A, Slezak W, Maruszewski M, Karolak W, Przybyłowski P, Krzych L, Zeglen S. Usefulness of the impulse oscillometry system in graft function monitoring in lung transplant recipients. Transplant Proc 50: 2070–2074, 2018. doi: 10.1016/j.transproceed.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 203.Brown RH, Mitzner W. Delayed distension of contracted airways with lung inflation In vivo. Am J Respir Crit Care Med 162: 2113–2116, 2000. doi: 10.1164/ajrccm.162.6.2004055. [DOI] [PubMed] [Google Scholar]
- 204.Hida W, Arai M, Shindoh C, Liu YN, Sasaki H, Takishima T. Effect of inspiratory flow rate on bronchomotor tone in normal and asthmatic subjects. Thorax 39: 86–92, 1984. doi: 10.1136/thx.39.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bates JH, Peters U, Daphtary N, MacLean ES, Hodgdon K, Kaminsky DA, Bhatawadekar S, Dixon AE. Altered airway mechanics in the context of obesity and asthma. J Appl Physiol 130: 36–47, 2020. doi: 10.1152/japplphysiol.00666.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barnas GM, Yoshino K, Stamenovic D, Kikuchi Y, Loring SH, Mead J. Chest wall impedance partitioned into rib cage and diaphragm-abdominal pathways. J Appl Physiol 66: 350–359, 1989. doi: 10.1152/jappl.1989.66.1.350. [DOI] [PubMed] [Google Scholar]
- 207.Navanandan N, Hamlington KL, Mistry RD, Szefler SJ, Liu AH. Oscillometry for acute asthma in the pediatric emergency department: a feasibility study. Ann Allerg Asthma Immunol 125: 607–609, 2020. doi: 10.1016/j.anai.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.McCormack MC, Kaminsky DA. Pulmonary Function Laboratories: Advice Regarding COVID-19 (Online). ATS Disease Related Resources. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php [8 May 2020]. [Google Scholar]