Abstract
Cognitive decline is common among patients with low- and high-grade glioma and can significantly impact quality of life. Although cognitive outcomes have been studied after therapeutic interventions such as surgery and radiation, it is important to understand the impact of the disease process itself prior to any interventions. Neurocognitive domains of interest in this disease context include intellectual function and premorbid ability, executive function, learning and memory, attention, language function, processing speed, visuospatial function, motor function, and emotional function. Here, we review oncologic factors associated with more neurocognitive impairment, key neurocognitive tasks relevant to glioma patient assessment, as well as the relevance of the human neural connectome in understanding cognitive dysfunction in glioma patients. A contextual understanding of glioma-functional network disruption and its impact on cognition is critical in the surgical management of eloquent area tumors.
Keywords: Malignant glioma, Glioma, Cognition, Functional mapping
ABBREVIATIONS
- AED
antiepileptic drug
- CNS
central nervous system
- CVLT-II
California Verbal Learning Test – Second Edition
- D-KEFS
Delis-Kaplan Executive Function System
- IQ
intelligence quotient
- MMSE
Mini Mental Status Examination
- MoCA
Montreal Cognitive Assessment
- NART
National Adult Reading Test
- RCFT
Rey Osterrieth Complex Figure Test and Recognition Trial
- TMT
Trail Making Test
- WAIS
Wechsler Adult Intelligence Scale
- WTAR
Weschler Test of Adult Reading
Gliomas exist within a microenvironment in which central nervous system (CNS) physiology contributes to tumor invasion and proliferation.1-8 In doing so, interactions between gliomas and their neuron microenvironment can significantly impact cognitive, language, and sensorimotor processing. Surgical resection is a mainstay of treatment and combination chemoradiation is the standard of care of both high and low grade glioma,9-11 all of which may lead to further significant cognitive decline in patients compounding on that already caused by the tumor itself. Normal neurocognitive function is associated with functional independence in patients with glioma,12 yet cognitive dysfunction is common.
Broadly speaking, cognitive dysfunction is defined as an impairment in 1 or more cognitive domains which include executive function, learning and memory, perceptual-motor function, language, and attention. In a meta-analysis of cognitive impairment in glioma patients beginning at the point of diagnosis, an impairment in at least 1 cognitive domain was seen in the majority of patients.13 Cognitive impairments experienced by patients with glioma are associated with lower rates of return to work, reduced independence despite high functional scales with Karnofsky Performance Scores, and lower quality of life.14,15 Unfortunately, patients may also have poor insight into their level of impairment.16 Cognitive outcomes are often studied in a cross-sectional manner, following therapeutic interventions such as surgery, chemotherapy, and brain irradiation. However, even prior to these therapies, gliomas disrupt network dynamics with neurological consequences.17-19 The widespread application of cognition testing throughout the trajectory of disease may be difficult due to a number of obstacles; however, routine testing can be a valuable addition to patient care. The focus of this review is to provide an overview of oncological factors associated influencing cognitive dysfunction for adult glioma patients.
FACTORS ASSOCIATED WITH NEUROCOGNITIVE IMPAIRMENT PRIOR TO TREATMENT
There are a number of factors associated with neurocognitive impairment in glioma patients, including older age, tumor location, extent of peritumoral edema, and tumor size.16,19-21 In this section, we review oncologic factors as well as common medications that may impact cognition.
Impact of Antiepileptic Medications
A majority of glioma patients use antiepileptic drugs (AEDs), especially those presenting with seizures. Historically, these agents were thought to impair cognitive function22-25 with the most common domains affected including attention, psychomotor speed, and memory.24 However, some reports suggest that newer antiepileptics (eg, levetiracetam) may not be as deleterious as older agents (eg, phenytoin).22,23 For example, levetiracetam does not significantly impact cognition in epilepsy patients.26-28 De Groot et al29 studied cognition in a matched cohort of high-grade glioma patients who received older AEDs (phenytoin and valproic acid), a newer AED (levetiracetam), or no AEDs prior to chemoradiation. Neither levetiracetam nor valproic acid were associated with cognitive impairments. Interestingly, patients on levetiracetam performed better on verbal memory tests than patients not on AEDs.29 Further work, however, is needed to assess other novel AED medications, the impact of dosing on cognitive outcomes, as well as the interplay between seizure control and cognition.
Impact of Dexamethasone
Corticosteroids are a central part of symptomatic control in glioma patients. Although their association with neuropsychiatric symptoms including insomnia, mania, psychosis, and depression are well known, the interplay between dexamethasone and cognition is not well understood. In other surgical contexts, there are mixed results in regards to dexamethasone's impact on postoperative cognition. In a study by Glumac et al,30 dexamethasone reduced the incidence of postoperative cognitive dysfunction after cardiac surgery. In a meta-analysis of studies examining the effects of dexamethasone on postoperative cognitive dysfunction, there were no differences in cognitive task performance between dexamethasone-treated and placebo-treated groups 30 d after surgery.31 The generalizability of these findings to glioma patients is questionable. In practice, dexamethasone significantly improves peritumoral edema and can improve a patient's functional and cognitive status, at least in the short term. The long-term effects of corticosteroid use on cognition require further study.
Impact of Glioma Location on Cognition
As early as the 2nd century AD, many have debated a localizationist view of the brain, which assigned mental abilities to specific brain regions. It was French neurologist Paul Broca and German neurologist Carl Wernicke who first observed specific patterns of altered language following brain injury and stroke to the left frontal and temporal lobes. Awake cortical mapping with electrical stimulation, first described by Penfield and Boldrey in 1937 for epilepsy surgery, allowed for improvements in the correlation of location and function.32 Ojemann further advanced this technique in the 1970s.33,34
Presently, the neural connections in the brain underlying mental and cognitive processes remain largely unknown. However, there are distinct patterns of neurocognitive impairment across cortical locations. For example, patients with left hemispheric lesions more frequently report depressive symptoms and more difficulty with memory.35 Noll et al19 compared neurocognitive task performance between left- and right-sided temporal glioma. Left temporal gliomas frequently had impairment in verbal learning and memory, language tasks, executive function, and attention yet normal processing speed. Patients with right temporal gliomas demonstrated impairment of executive function, verbal learning and memory, processing speed, and fine motor control.19 Tucha et al16 reported on 139 patients with frontal or temporal untreated gliomas. Left-sided lesions exhibited more impairments in verbal short-term memory tasks whereas patients with bilateral and right-sided lesions more frequently demonstrated impaired visuospatial abilities.16 Hahn et al35 reported increased memory loss and poorer verbal fluency and learning in patients with left-sided tumors. Wu et al36 examined neurocognitive impairment associated with insular tumors and found lesions in this region were associated with worse visual confrontational naming. Noll et al19 reported that within the left temporal lobe, new learning appeared most impacted by tumors restricted to anterior temporal lobe. Delayed recall and recognition performance was lowest in tumors involving medial structures within the temporal lobe, suggesting site specific cognitive impairment even within a single cortical lobe.19 These results and lateralization of cognitive function appear consistent with those noted in the stroke literature.37
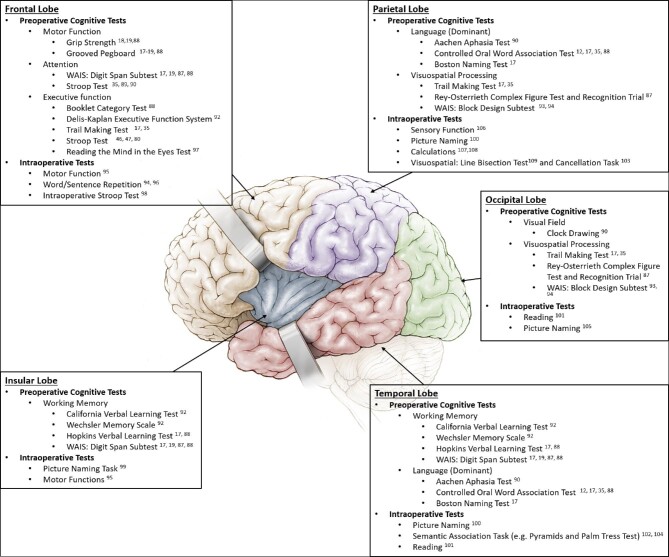
Given how cognitive dysfunction impacts quality of life, there is a need to preserve function following therapeutic interventions and this goal must be balanced with the survival benefit associated with maximal extent of resection and chemo- and radiotherapy.11,38 Although there are a lack of guidelines for patient-specific cognitive testing, the localizationist view of cognition for glioma patients leads to the idea of site-specific preoperative and postoperative cognitive testing based on glioma location (Figure).
FIGURE.
Preoperative and intraoperative cognitive tests used for assessing patient function by cortical lobe.
Influence of Growth Rate on Neurocognition
The concept of tumor rate of growth may explain differing patterns of neurological and cognitive symptom burden.39 For slower growing low-grade gliomas, there is more time for glioma-neural network functional integration resulting in topographic reorganization of functional areas. Several reports have demonstrated that faster-growing gliomas are associated with more severe neurocognitive impairment when compared with low-grade gliomas.17,18,40,41 Wefel et al17 compared the severity of neurocognitive impairment between patients with IDH1-wildtype and IDH1-mutant malignant gliomas. IDH1-wildtype gliomas which were faster growing had poorer neurocognitive function compared to IDH1-mutant gliomas despite similar tumor size. Mean task performance for patients with IDH-wildtype gliomas was lower on measures of learning and memory, processing speed, language, executive functioning, and dexterity.17 As a correlate to faster growth potential, high-grade gliomas are also associated with a higher rate of neurocognitive impairments.18,21 Noll et al18 compared neurocognitive impairment rates between patients with newly diagnosed grade II to IV gliomas. Patients with grade IV tumors suffered more frequent neurocognitive impairment when compared with grade II and III tumors.18
IMPACT OF GLIOMA ON THE HUMAN CONNECTOME
Neural network disruption results in neurocognitive impairment in glioma patients.42,43 New scientific inquiry into the mapping of cognitive functions and neurologic symptoms to specific brain regions has led to an emphasis on identifying functional networks as opposed to studies which focus on anatomic cortical correlations. This work has resulted in the development of a human brain “connectome,” which provides a map of functional connections and may explain how lesions in different locations explain specific symptomatology.44
In the context of glioma, this means that a tumor in a single location may cause global perturbance of network function. Zhang et al45 examined cerebral hemispheric functional connectivity between 20 right-handed patients with frontal lobe glioma and healthy controls. Functional connectivity between the posterior cingulate cortex and temporal-parietal junction was decreased in the glioma group. Interestingly, left hemispheric functional connectivity was negatively impacted by right frontal tumors suggesting more widespread network dysfunction remote from the primary site of a glioma.45 While long-distance inter-hemispheric connectivity may be impaired in glioma patients, local connectivity may be preserved and similar to healthy controls.46
Differences in network connectivity between glioma subtypes are also apparent. Kesler et al42 compared network organization between IDH1-mutant and IDH1-wildtype malignant astrocytomas and found that patients with IDH1-wildtype tumors had lower connectivity in medial frontal, posterior parietal, and subcortical areas. These patients also had higher rates of neurocognitive impairment, although network connectivity was inversely associated with cognitive impairment in both cohorts.42
One feature of the human connectome is the presence of “hubs” or areas of high-information throughput which may be relevant for quick processing of information.47,48 Derks et al49 found wide-spread altered connectivity changes in glioma patients with higher rates of hub-to-non-hub connectivity and lower hub-to-hub connectivity when compared to healthy controls. In terms of surgical impact on language performance, Lee et al50 found that removal of high connectivity sites that were negative for functional during intraoperative stimulation led to higher rates of transient postoperative decline on specific language tasks.
FUNCTIONAL REORGANIZATION OF NETWORKS DYNAMICS AND PRESERVATION OF NEUROCOGNITIVE FUNCTION
As gliomas grow, they alter networks dynamics and therefore cognition. However, neighboring networks may reorganize via neural plasticity allowing for functional recovery of both cognitive and language/motor activity.51 This process of functional reorganization and cortical plasticity is one reason that patient's with slow-growing diffuse low-grade gliomas in areas considered to be “eloquent” often remain neurologically intact.52 Low-grade gliomas often grow to a very large size, with minimal symptoms.53,54 This is in contrast to glioblastoma, which grows rapidly and often presents with neurological deficits. Likewise, an ischemic stroke, usually results in some degree of neurological impairment, even though functional reorganization can still occur in a delayed fashion. The observation that cortical plasticity can occur in the setting of glioma infiltration has been confirmed intraoperatively with direct electrocortical stimulation.55-57
There are a few hypotheses for how functional network reorganization occurs in the setting of oncologic growth.58 First, function might be preserved within the original network despite the tumor infiltration. This concept of functional integration may make achieving a gross total resection difficult without creating a lasting postoperative neurological deficit. Second, function may be redistributed to the adjacent nontumor cortical and subcortical networks. In this setting, an aggressive surgical resection can occur. Finally, areas remote from the lesional tissue could compensate for lost function. Some authors have argued that extent of subcortical violation limits functional connectivity and is therefore the biggest limiting factor to neuroplasticity.55 As a result, when gliomas infiltrate the subcortical white matter, it may be critical to perform subcortical mapping or resect the tumor in a subtotal fashion.59 In taking this approach, the surgeon can “stage” the resection of a slower growing low-grade glioma and return for repeat resection at a later date to remap the area and determine if function still remains.60 Lesions initially deemed unresectable due to stimulation-confirmed involvement of functional tissue may later become resectable once functional networks have been re-established elsewhere.61,62 The exact timing of this process and the underlying mechanisms remain unknown.
NEUROCOGNITIVE TESTING DOMAINS
Neurocognitive testing aides in assessing domains of cognitive impairment in patients with gliomas. Testing is performed by specialized neuropsychologists, psychometrists, speech pathologists, or intraoperative physiologists and is carried out with the patient alone in a noise-controlled, comfortable environment which is free of distractions. Cognitive impairment interferes with an individual's independence in completing everyday activities and is typically diagnosed when substantial decline is observed in one or usually more cognitive domains.63 Global measures of cognitive impairment, such as the Folstein Mini Mental Status Examination (MMSE)64 and the Montreal Cognitive Assessment (MoCA),65 are commonly used screening tools. The MMSE and MoCA are both 10-min screening tools. A score below 20 on the MMSE and a score below 26 on the MoCA indicates cognitive impairment.64,65 These assessments have poor domain-specific sensitivity and specificity.66
Utilizing a comprehensive neurocognitive testing battery can provide domain-specific information. A testing battery typically assesses the following domains in glioma patients: premorbid ability, general intellectual level, attention, memory, executive functioning, language, visual spatial functioning, motor functioning, and emotional functioning.67 Tests are chosen depending on the referral question, the patient or caregivers’ complaints and symptoms, and information collected during the clinical interview. There is no consensus regarding which tasks to administer for glioma patients limiting interpretation of comparative studies.68 Furthermore, normative standards and cut-off limits to define impairment are variable. The following domains and their corresponding measures have been commonly used in the assessment of adult glioma patients (reviewed in Table).67
TABLE.
Neuropsychological Tests for Assessing Neurocognitive Domains in Glioma
| Neurocognitive domains | Test | Functions and cognitive abilities | Example references |
|---|---|---|---|
| Attention | Digit span | Auditory attention | 17,19,87,88 |
| Stroop test | Mental speed; selective attention; inhibitory control | 35,89,90 | |
| TMT-A | Selective and divided attention; visual search speed; scanning | 17,35,88,91 | |
| Executive functions | BCT | Concept formation; abstract reasoning; problem-solving | 88 |
| COWA | Verbal fluency | 12,17,35,88 | |
| D-KEFS | Problem-solving; flexible thinking; verbal and spatial fluency; concept formation; deductive reasoning; abstract thinking | 92 | |
| TMT-B | Cognitive flexibility; visuomotor speed | 17,35,90 | |
| Stroop test | Cognitive flexibility; resistance to interference; suppression of dominant verbal response | 35,89,90 | |
| Learning and memory | CVLT-II | Verbal learning; information on acquisition, recall; retention, and retrieval of verbal information | 92 |
| HVLT-R | Verbal memory; information on acquisition, recall; retention, recognition of verbal information | 17,88 | |
| RCFT (delayed recall) | Implicit visual-spatial memory | 87 | |
| WMS-IIIa | Auditory memory; visual memory; attention; working memory | 92 | |
| Digit span | Short-term auditory memory; working memory | 87 | |
| Language | BNT | Category fluency; letter fluency; | 17 |
| Subtests of AAT | |||
| Token test | Verbal comprehension; receptive language | 17,88,90 | |
| Naming | Word finding; expressive language | 90 | |
| Written language | Verbal academic skills | 90 | |
| Language comprehension | Auditory and reading comprehension | 90 | |
| Lexical word fluency | Phonological fluency | 90 | |
| Visuospatial | RCFT (copy) | Visuoconstructional ability | 87 |
| Block design | Spatial component in perception and motor execution | 17,19,88 | |
| Clock drawing | Visuo-spatial and praxis abilities; visuospatial; Construction; abstract conceptualization | 90 | |
| Processing speed | TMT-A | Visual search speed; scanning | 17,35,88,91 |
| Motor | Grip strength | Hand strength | 18,19,88 |
| Grooved pegboard | Manual dexterity; complex visual motor coordination | 17-19,88 | |
| Intelligence | WAIS-IIIb | IQ; verbal comprehension; perceptual reasoning; processing speed; working memory | 93,94 |
| American Nelson Test | Premorbid functioning | 35 |
TMT = Trail Making Test; BCT = Booklet Category Test; COWA = Controlled Oral Word Association Test; D-KEFS = Delis-Kaplan Executive Function System; CVLT-II = California Verbal Learning Test, Second Edition; HVLT-R = Hopkins Verbal Learning Test – Revised; RAVLT = Rey Auditory Verbal Learning Test; WMS-III = Wechsler Memory Scale – Third Edition; AAT = Aachen Aphasia Test; BNT = Boston Naming Test – 2; RCFT = Rey Complex Figure and Recognition Trial; WAIS-III = Wechsler Adult Intelligence Scale, Third Edition; WTAR = Weschler Test of Adult Reading.
aReferences used WMS-III. The WMS-IV is the most up-to-date version of this test.
bReferences used WAIS-III. The WAIS-IV is the most up-to-date version of this test.
Intellectual Function and Premorbid Ability
The most widely used and established test of intelligence for adults is the Wechsler Adult Intelligence Scale (WAIS)-IV. It consists of 10 subtests measuring 4 cognitive domains to produce a full-scale intelligence quotient (IQ).69 The following domains are assessed: verbal comprehension, working memory, perceptual reasoning, and processing speed. Approximately 60 to 90 min are needed for completion. Verbal comprehension is assessed through the following subtests: similarities (describing how 2 words or concepts are similar), vocabulary (defining words presented), and information (general knowledge questions). Perceptual reasoning is assessed through block design (putting together blocks in a pattern according to a displayed model), matrix reasoning (viewing an array of pictures with a missing square and selecting a picture that fits the array from 5 options), and visual puzzles (viewing puzzles from a stimulus book and choosing from pieces of which 3 could construct the puzzle). Working memory is assessed through the digit span subtest (listening to a sequence of numbers and repeating them as heard in reverse order and ascending order) and the arithmetic subtest (orally administered arithmetic word problems that are timed). Processing speed is assessed through timed tasks, such as the symbol search subtest (viewing rows of symbols and target symbols and marking whether or not target symbols appear in each row) and the coding subtest (transcribing a digit-symbol code using a key).69
The WAIS IQ is highly correlated with tests of premorbid functioning when controlled for education,70 and patients with gliomas are typically assessed as a baseline for future comparison. The National Adult Reading Test, Second Edition (NART-2)67,71 and the Weschler Test of Adult Reading (WTAR)72 are 2 commonly used measures of premorbid functioning that correlate with WAIS IQ scores. The NART-2 is used to estimate premorbid intellectual ability in adults ages 16 to 70 yr. The test requires the patient to read aloud 50 irregularly spelled words, with the accuracy of pronunciation used to predict IQ. Because the words are irregular, intelligent guesswork will not provide the correct pronunciation and performance therefore depends more on previous knowledge than on current cognitive capacity.73 Similarly, the WTAR is used to assess premorbid intellectual functioning in individuals aged 16 to 89 yr. The test takes 5 to 10 min and involves reading 50 irregular words.72 Each item is individually presented on a word card, and examinees are asked to pronounce each word.72
Attention and Processing Speed
This domain assesses a patient's ability to focus, divide, and sustain attention, as well as resist distraction.67 The digit span subtest of the WAIS-IV has been found to be a valid and reliable measure of auditory attention, short-term memory, and mental manipulation.69 The subtest involves: digit span forward, digit span backward, and digit span sequencing. A series of digits is read to the patient, who is required to then repeat the digits in the order they were presented (digit span forward).69 The patient is then asked to recall digits presented in the reverse order (digit span backward), and lastly, the patient is required to repeat digits that are presented in sequential order from lowest to highest (digit span sequencing).69 Processing speed, and visual and focused attention, is typically measured through the Trail Making Test (TMT).74 The test usually takes less than 10 min to complete and involves 2 parts: Trail Making A (involves number sequencing) and Trail Making B (requires sequencing between letters and numbers).5 The test asks patients to connect circles numbered from 1 to 25 in order as quickly as possible. Scoring is based on the time it takes in seconds to complete the test.74 The Stroop Color Word Test is another common assessment used in assessing attention as well as executive functioning. It involves 3 tasks: word reading, color naming, and color word naming.75 In the last task, a patient is required to name as quickly as possible the ink color which is conflicting with the actual word. The task measures cognitive flexibility, resistance to interference from outside stimuli, and the ability to suppress a dominant verbal response.
Memory
Memory tests assess visuo-spatial episodic memory, verbal memory, short-term memory, and working memory. A comprehensive assessment of memory is the Wechsler Memory Scale – Fourth Edition.76 The test is co-normed with the WAIS-IV and provides 5 summary scores that assess the patient's auditory, visual, immediate, delayed, and visual working memory.76 Auditory memory subtests (Logical Memory I and II and Verbal Paired Associates I and II) assess narrative memory both from free recall and a delayed recall to assess both short- and long-term narrative memory. The patient is presented with short stories and is asked to recall each story from memory immediately and after a delay period. This domain also assesses verbal memory for associated word pairs; the examiner reads word pairs to the patient and asks the patient to provide the corresponding word after reading the first word of each pair. A delayed condition to assess long-term recall is also given. The Visual Memory Index assesses memory for nonverbal stimuli by showing a series of designs to the patient and asking them to draw the design from memory immediately and after a delay period (Visual Reproduction I and II). Additional subtests in this domain assess spatial memory for unfamiliar visual material (Designs I and II) in which the patient is shown a grid with designs for several seconds and is then asked to select designs from a set of cards by placing them in a grid corresponding to the example image. The Visual Working Memory Index assesses visual-spatial working memory with the spatial addition subtest, where the patient is shown 2 grids with blue and red circles. The patient is asked to add or subtract the location of the circles based on a set of rules. Another subtest (symbol span) assesses visual working memory by briefly showing the patient a series of abstract symbols on a page and then asking the patient to select the symbols from an array of symbols in the same order they were presented in the previous page.
The California Verbal Learning Test – Second Edition (CVLT-II) is also used to assess memory.77 The CVLT-II uses a shopping list format consisting of 16 words from 4 categories presented over 5 trials. After the 5 trails, an interference list is presented, followed by short-delay recall of the first list, and then long-delay recall after 20 min.
Executive Function
This domain encompasses a set of mental skills needed to achieve a goal, such as planning, organizing, focusing attention, judgment, reasoning, abstraction, self-regulation, and initiating or inhibiting a response.67 The Delis-Kaplan Executive Function System (D-KEFS) is a comprehensive executive functioning measure that comprises of 9 tests designed to assess problem-solving, flexible thinking, verbal and spatial fluency, concept formation, planning and reasoning, verbal inhibition, hypothesis testing, deductive reasoning, and abstract thinking.78 The Tower Test and TMT are subtests of the D-KEPFS that are commonly used to assess executive functioning.67,78 In addition to the TMT that was previously described, the Tower Test requires the patient to use verbal cues to deduce the meaning of made-up words. It measures deductive reasoning, integrating multiple pieces of information, hypothesis testing, and flexibility.5
Language
Tests that measure language for gliomas assess auditory comprehension, word retrieval deficits, and verbal association fluency. Common domain includes auditory language,67 which is assessed using the Token Test.17,79,80 In this task, the patient is asked to follow simple commands (eg, “touch a circle”) which progresses toward more complex commands (eg, “before touching the yellow circle, pick up the red square”).17 Verbal fluency can be assessed by the Controlled Oral Word Association Test,74 which measures the patient's ability to maximally produce words belonging to a particular class.67 It asks the patient to generate as many words as possible beginning with the letters F, A, and S in 1-min intervals. In category fluency, the patient is required to generate as many words as possible belonging to a particular category (eg, animals and fruits). Lastly, the Boston Naming Test-2 is used to assess confrontation naming and word retrieval.81
Visual Spatial and Visual Constructional Function
This domain evaluates visual perception and visuospatial abilities, including visual construction and visual integration.67 Functioning in this domain is assessed through one of the following assessments: The Clock Test, the Block Design subtest of the WAIS-IV, or the Rey Osterrieth Complex Figure Test and Recognition Trial (RCFT). The Clock Test assesses visuospatial construction, visual perception, and abstract conceptualization.82 It involves 3 subtests including clock drawing, clock setting, and clock reading. It asks participants to draw a clock with all the numbers and set the hands of the clock to show a specific time. Following this, the patient is asked to copy a picture of a clock. The block design subtest of the WAIS-IV assesses 3-dimensional construction abilities.69 It requires the patient to use colored blocks to construct replicas of designs that are shown from a stimulus book within a specified time limit. The copy phase of the RCFT assesses a patient's visuo-constructional ability by evaluating accuracy of copying complex geometric figures.83 The test consists of 4 trials, including copy, immediate recall, delayed recall, and recognition. The patient is asked to copy a figure and to reproduce the figure 3 min later and in a delayed fashion from memory.
Motor Function
Motor functioning is assessed through evaluation of a patient's strength, coordination, and dexterity.67 Typical tests used in this assessment include the grip strength test and the grooved pegboard. The test of grip strength uses a hand dynamometer to assess the patient's strength in each hand.74 The grooved pegboard test measures manual dexterity and requires complex visual-motor coordination.11 The test involves a board that consists of 25 randomly positioned keyholes. The patient must rotate the pegs to match the holes before the peg can be inserted into the keyhole on the board.11
Emotion
Assessment of the patient's emotional functioning is important to determine the patient's emotional state during testing. Brain tumor patients have a higher level of depression and anxiety than the normal population,37,84 and these conditions are commonly evaluated to confirm the validity of cognitive testing as a highly anxious or depressive state can reduce performance.21 The Beck Depression Inventory85 – Second Edition and the Beck Anxiety Inventory86 are 2 of many common assessments that can be used in adults.
IMPLICATIONS FOR CURRENT AND FUTURE PRACTICE
Given the impact that glioma as well as oncologic therapy have on cognition, multidisciplinary cognitive rehabilitation is becoming recognized as a valuable component of care for glioma patients. There are 2 main interventional approaches underlying cognitive rehabilitation: retraining and functional compensation. Retraining involves strengthening impaired cognitive skills through repeatedly practicing cognitive tasks whereas functional compensation focuses on improving daily function through learned strategies that modify a patient's environment or their approach toward achieving a goal. The goal of cognitive rehabilitation is to improve one's autonomy, self-awareness, emotional coping, acceptance, and management of cognitive impairments. It has been proposed that cognitive retraining may facilitate functional network reorganization. This approach would permit neurosurgeons to offer “staged” procedures leaving tumor behind for later remapping after a period of network reorganization.
It must be pointed out that computerized cognitive training is a newly developing field of therapeutics for neurological and psychiatric disorders that uses frequent game like training sessions to drive CNS plasticity. It remains to be seen whether these tools impact outcomes. Furthermore, the idea of preventing treatment related cognitive impairments has gained popularity. Recently published data have begun to advocate for preoperative cognitive rehabilitation also known as “prehab,” as a means of preparing patients for oncologic interventions in an effort to reduce treatment-related cognitive impairments.85,86 More work is needed to determine if prehab may prevent cognitive impairments in glioma patients.
CONCLUSION
Low- and high-grade gliomas are characterized by neural network invasion that can lead to neurocognitive impairment prior to any therapeutic intervention. Neurocognitive testing is valuable for assessing specific domains of cognition in patients with gliomas. Cognitive tasks may be selected in a patient- and site-specific manner based on symptoms and location of a tumor. More work is needed to elucidate how altered network dynamics impact specific neurocognitive domains and how neural networks may reorganize over time in response to tumor growth and surgical intervention.
Funding
Dr Hervey-Jumper received grant 74259 from the Robert Wood Johnson Foundation and grant K08110919-01 from National Institute of Neurological Disorders and Stroke. Dr Hervey-Jumper and Dr Berger were supported by Loglio Collective.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Ramin A Morshed, Department of Neurological Surgery, University of California San Francisco, San Francisco, California.
Jacob S Young, Department of Neurological Surgery, University of California San Francisco, San Francisco, California.
Arlena A Kroliczek, Department of Neurological Surgery, University of California San Francisco, San Francisco, California.
Mitchel S Berger, Department of Neurological Surgery, University of California San Francisco, San Francisco, California.
David Brang, Department of Psychology, University of Michigan, Ann Arbor, Michigan.
Shawn L Hervey-Jumper, Department of Neurological Surgery, University of California San Francisco, San Francisco, California.
COMMENTS
These authors provide a brief introductory review cognition testing in glioma patients, that along with the entire series of manuscripts accompanying this one provides a very comprehensive review of this topic. In this manuscript, these authors argue that there may be benefit in more site-specific cognitive testing despite limited therapeutic options. Of course, the brain connectome shows widespread connections that can be impacted by focal lesions. Anticipating specific cognitive dysfunctions may be difficult for each focal lesion. Their review of specific cognitive tests is of good value to the neurosurgeon. Preoperative, intraoperative, and postoperative testing by the neurosurgical team and cognitive therapists may help guide therapeutic options and potentially modify tumor-specific therapies that are harming cognition. If anything, this manuscript emphasizes the complexity of the question at hand and illustrates the need for including cognitive therapists on our teams to care for these patients.
David Cory Adamson
Atlanta, Georgia
The authors provide a nice overview of cognitive dysfunction in adult glioma as a guide for neurosurgeon. This is an interesting topic and of some importance to neurosurgeons. I appreciate the details in the domain description section about the steps and practical considerations of each task, in order to familiarize neurosurgeons with these tools. The authors point out that cognitive tasks are proprietary and unfortunately cannot reprint the specific images/stimuli from these tasks because they do not have permission to do so. I encourage them to follow up on this work with a review of the feasibility of an abbreviated neurocognitive testing paradigm for intraoperative use.
Randy L. Jensen
Salt Lake City, Utah
REFERENCES
- 1. Gibson EM, Purger D, Mount CWet al. . Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venkatesh HS, Johung TB, Caretti Vet al. . Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161(4):803-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Venkatesh HS, Tam LT, Woo PJet al. . Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. 2017;549(7673):533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatesh HS, Morishita W, Geraghty ACet al. . Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venkataramani V, Tanev DI, Strahle Cet al. . Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532-538. [DOI] [PubMed] [Google Scholar]
- 6. Osswald M, Jung E, Sahm Fet al. . Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93-98. [DOI] [PubMed] [Google Scholar]
- 7. Zeng Q, Michael IP, Zhang Pet al. . Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573(7775):526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. John Lin C-C, Yu K, Hatcher Aet al. . Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckner JC, Shaw EG, Pugh SLet al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stupp R, Hegi ME, Mason WPet al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [DOI] [PubMed] [Google Scholar]
- 11. Molinaro AM, Hervey-Jumper S, Morshed RAet al. . Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noll KR, Bradshaw ME, Weinberg JS, Wefel JS. Neurocognitive functioning is associated with functional independence in newly diagnosed patients with temporal lobe glioma. Neuro-Oncology Pract. 2018;5(3):184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu R, Solheim K, Polley M-Yet al. . Quality of life in low-grade glioma patients receiving temozolomide. Neuro Oncol. 2009;11(1):59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aaronson NK, Taphoorn MJB, Heimans JJet al. . Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(33):4430-4435. [DOI] [PubMed] [Google Scholar]
- 16. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324-334. [DOI] [PubMed] [Google Scholar]
- 17. Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro-Oncol. 2015;17(4):580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noll KR, Ziu M, Weinberg JS, Wefel JS. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol. 2016;128(2):323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Talacchi A, Sala F, Alessandrini F, Turazzi S, Bricolo A. Assessment and surgical management of posterior fossa epidermoid tumors: report of 28 cases. Neurosurgery. 1998;42(2):242-251. [DOI] [PubMed] [Google Scholar]
- 21. Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541-549. [DOI] [PubMed] [Google Scholar]
- 22. Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17(4):413-425. [DOI] [PubMed] [Google Scholar]
- 23. Hamed SA. The aspects and mechanisms of cognitive alterations in epilepsy: the role of antiepileptic medications. CNS Neurosci Ther. 2009;15(2):134-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(8 Suppl 5):S21-S26. [DOI] [PubMed] [Google Scholar]
- 25. Klein M, Engelberts NHJ, van der Ploeg HMet al. . Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514-520. [DOI] [PubMed] [Google Scholar]
- 26. Piazzini A, Chifari R, Canevini MP, Turner K, Fontana SP, Canger R. Levetiracetam: an improvement of attention and of oral fluency in patients with partial epilepsy. Epilepsy Res. 2006;68(3):181-188. [DOI] [PubMed] [Google Scholar]
- 27. Wu T, Chen C-C, Chen T-Cet al. . Clinical efficacy and cognitive and neuropsychological effects of levetiracetam in epilepsy: an open-label multicenter study. Epilepsy Behav. 2009;16(3):468-474. [DOI] [PubMed] [Google Scholar]
- 28. Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12(2):305-310. [DOI] [PubMed] [Google Scholar]
- 29. de Groot M, Douw L, Sizoo EMet al. . Levetiracetam improves verbal memory in high-grade glioma patients. Neuro Oncol. 2013;15(2):216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34(11):776-784. [DOI] [PubMed] [Google Scholar]
- 31. Li L-Q, Wang C, Fang M-D, Xu H-Y, Lu H-L, Zhang H-Z. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. 2019;19(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penfield W, Boldrey E.. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937:60(4):389-443. [Google Scholar]
- 33. Whitaker HA, Ojemann GA.. Graded localisation of naming from electrical stimulation mapping of left cerebral cortex. Nature. 1977;270(5632):50-51. [DOI] [PubMed] [Google Scholar]
- 34. Ojemann GA, Whitaker HA. Language localization and variability. Brain Lang 1978;6(2):239-260. [DOI] [PubMed] [Google Scholar]
- 35. Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):239-260. [DOI] [PubMed] [Google Scholar]
- 36. Wu AS, Witgert ME, Lang FFet al. . Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125. [DOI] [PubMed] [Google Scholar]
- 37. Anderson SW, Damasio H, Tranel D. Neuropsychological impairments associated with lesions caused by tumor or stroke. Arch Neurol. 1990;47(4):397-405. [DOI] [PubMed] [Google Scholar]
- 38. Gabel N, Altshuler DB, Brezzell Aet al. . Health related quality of life in adult low and high-grade glioma patients using the national institutes of health patient reported outcomes measurement information system (PROMIS) and Neuro-QOL assessments. Front Neurol. 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klein M. Lesion momentum as explanation for preoperative neurocognitive function in patients with malignant glioma. Neuro Oncol. 2016;18(12):1595-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miotto EC, Junior AS, Silva CCet al. . Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arq Neuropsiquiatr. 2011;69(4):596-601. [DOI] [PubMed] [Google Scholar]
- 41. Hom J, Reitan RM.. Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 1984;6(3):309-324. [DOI] [PubMed] [Google Scholar]
- 42. Kesler SR, Noll K, Cahill DP, Rao G, Wefel JS. The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neurooncol. 2017;131(3):565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bosma I, Douw L, Bartolomei Fet al. . Synchronized brain activity and neurocognitive function in patients with low-grade glioma: a magnetoencephalography study. Neuro Oncol. 2008;10(5):734-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237-2245. [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Shi Y, Yao Cet al. . Alteration of the intra- and cross-hemisphere posterior default mode network in frontal lobe glioma patients. Sci Rep. 2016;6(1):26972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park JE, Kim HS, Kim SJ, Kim JH, Shim WH. Alteration of long-distance functional connectivity and network topology in patients with supratentorial gliomas. Neuroradiology. 2016;58(3):311-320. [DOI] [PubMed] [Google Scholar]
- 47. Crossley NA, Mechelli A, Vertes PEet al. . Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci. 2013;110(28):11583-11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crossley NA, Mechelli A, Scott Jet al. . The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137(8):2382-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Derks J, Dirkson AR, de Witt Hamer PCet al. . Connectomic profile and clinical phenotype in newly diagnosed glioma patients. NeuroImage Clin. 2017;14:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee AT, Faltermeier C, Morshed RAet al. . The impact of high functional connectivity network hub resection on language task performance in adult low- and high-grade glioma. J Neurosurg. 2020:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity–a review. J Neurooncol. 2006;79(1):77-115. [DOI] [PubMed] [Google Scholar]
- 52. Duffau H. Hodotopy, neuroplasticity and diffuse gliomas. Neurochirurgie. 2017;63(3):259-265. [DOI] [PubMed] [Google Scholar]
- 53. Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898-914. [DOI] [PubMed] [Google Scholar]
- 54. Duffau H. Diffuse Low-Grade Gliomas in Adults: Natural History, Interaction With the Brain, and New Individualized Therapeutic Strategies. London, UK: Springer-Verlag; 2014. [Google Scholar]
- 55. Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325-337. [DOI] [PubMed] [Google Scholar]
- 56. Duffau H, Capelle L, Denvil Det al. . Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74(7):901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duffau H. Acute functional reorganisation of the human motor cortex during resection of central lesions: a study using intraoperative brain mapping. J Neurol Neurosurg Psychiatry. 2001;70(4):506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duffau H. Brain plasticity and tumors. Adv Tech Stand Neurosurg. 2008;33:3-33. [DOI] [PubMed] [Google Scholar]
- 59. Duffau H. Diffuse low-grade gliomas and neuroplasticity. Diagn Interv Imaging. 2014;95(10):945-955. [DOI] [PubMed] [Google Scholar]
- 60. Duffau H, Taillandier L.. New concepts in the management of diffuse low-grade glioma: proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015;17(3):332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg. 2016;124(5):1460-1469. [DOI] [PubMed] [Google Scholar]
- 62. Duffau H, Denvil D, Capelle L. Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatry. 2002;72(4):511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 64. Folstein M, Folstein S, McHugh P. Mini-Mental State Examination: Clinical Guide. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 65. Nasreddine ZS, Phillips NA, Bédirian Vet al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 66. Milberg W. Issues in the assessment of cognitive function in dementia. Brain Cogn. 1996;31(2):114-132. [DOI] [PubMed] [Google Scholar]
- 67. Hebben N, Milberg W.. Essentials of Neuropsychological Assessment. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 68. van Loon EMP, Heijenbrok-Kal MH, van Loon WSet al. . Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47(6):481-488. [DOI] [PubMed] [Google Scholar]
- 69. Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 70. Grober E, Sliwinski M.. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933-949. [DOI] [PubMed] [Google Scholar]
- 71. Nelson H. National Adult Reading Test (NART): Test Manual. Windsor, England: NFER Nelson; 1982. [Google Scholar]
- 72. Corporation TP , ed. Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 73. Nelson HE, O’Connell A.. Dementia: the estimation of premorbid intelligence levels using the new adult reading test. Cortex. 1978;14(2):234-244. [DOI] [PubMed] [Google Scholar]
- 74. Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 75. Golden C, Freshwater S.. Stroop Color and Word Test: Revised Examiner's Manual. Wood Dale, IL: Stoelting; 2002. [Google Scholar]
- 76. Wechsler D. Wechsler Memory Scale. 4th ed. San Antonio, TX: Pearson; 2009. [Google Scholar]
- 77. Delis DC. California Verbal Learning Test. Adult Version Manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 78. Delis D, Kaplan E, Kramer J. Delis–Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 79. Benton AL, Sivan AB. Multilingual Aphasia Examination. 3rd ed. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 80. De Renzi E, Vignolo L. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85(4):665-678. [DOI] [PubMed] [Google Scholar]
- 81. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 82. Tuokko H, Hadjistavropoulos T, Miller J, Horton A, Beattle B. The Clock Test. Toronto, Canada: Multi-Health Systems; 1995. [Google Scholar]
- 83. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 84. Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46-51. [DOI] [PubMed] [Google Scholar]
- 85. Carli F, Charlebois P, Stein Bet al. . Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97(8):1187-1197. [DOI] [PubMed] [Google Scholar]
- 86. Li C, Carli F, Lee Let al. . Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072-1082. [DOI] [PubMed] [Google Scholar]
- 87. Hendriks EJ, Habets EJJ, Taphoorn MJBet al. . Linking late cognitive outcome with glioma surgery location using resection cavity maps. Hum Brain Mapp. 2018;39(5):2064-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wager M, Du Boisgueheneuc F, Pluchon Cet al. . Intraoperative monitoring of an aspect of executive functions: administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery. 2013;72(2 Suppl Operative):ons169-ons80; discussion ons180-1. [DOI] [PubMed] [Google Scholar]
- 90. Satoer D, Vork J, Visch-Brink E, Smits M, Dirven C, Vincent A. Cognitive functioning early after surgery of gliomas in eloquent areas. J Neurosurg. 2012;117(5):831-838. [DOI] [PubMed] [Google Scholar]
- 91. Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Racine CA, Li J, Molinaro AM, Butowski N, Berger MS. Neurocognitive function in newly diagnosed low-grade glioma patients undergoing surgical resection with awake mapping techniques. Neurosurgery. 2015;77(3):371-379; discussion 379. [DOI] [PubMed] [Google Scholar]
- 93. Maesawa S, Bagarinao E, Fujii Met al. . Evaluation of resting state networks in patients with gliomas: connectivity changes in the unaffected side and its relation to cognitive function. PLoS One. 2015;10(2):e0118072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Motomura K, Chalise L, Ohka Fet al. . Neurocognitive and functional outcomes in patients with diffuse frontal lower-grade gliomas undergoing intraoperative awake brain mapping. published online: May 17, 2019. J Neurosurg. (doi:10.3171/2019.3.JNS19211). [DOI] [PubMed] [Google Scholar]
- 95. Magill ST, Han SJ, Li J, Berger MS. Resection of primary motor cortex tumors: feasibility and surgical outcomes. J Neurosurg. 2018;129(4):961-972. [DOI] [PubMed] [Google Scholar]
- 96. Fujii M, Maesawa S, Motomura Ket al. . Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg. 2015;122(6):1390-1396. [DOI] [PubMed] [Google Scholar]
- 97. Nakajima R, Yordanova YN, Duffau H, Herbet G. Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face-based mentalizing network: a disconnection analysis. Neuropsychologia. 2018;115:179-187. [DOI] [PubMed] [Google Scholar]
- 98. Puglisi G, Howells H, Sciortino Tet al. . Frontal pathways in cognitive control: direct evidence from intraoperative stimulation and diffusion tractography. Brain. 2019;142(8):2451-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Morshed RA, Young JS, Han SJ, Hervey-Jumper SL, Berger MS. Perioperative outcomes following reoperation for recurrent insular gliomas. J Neurosurg. 2018;131(2):467-473. [DOI] [PubMed] [Google Scholar]
- 100. Southwell DG, Riva M, Jordan Ket al. . Language outcomes after resection of dominant inferior parietal lobule gliomas. J Neurosurg. 2017;127(4):781-789. [DOI] [PubMed] [Google Scholar]
- 101. Zemmoura I, Herbet G, Moritz-Gasser S, Duffau H. New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum Brain Mapp. 2015;36(6):2215-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ries SK, Piai V, Perry Det al. . Roles of ventral versus dorsal pathways in language production: an awake language mapping study. Brain Lang. 2019;191:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Conner AK, Glenn C, Burks JDet al. . The use of the target cancellation task to identify eloquent visuospatial regions in awake craniotomies: technical note. Cureus. 2016;8(11):e883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ricci PT, Zelkowicz BJ, Nebes RD, Meltzer CC, Mintun MA, Becker JT. Functional neuroanatomy of semantic memory: recognition of semantic associations. Neuroimage. 1999;9(1):88-96. [DOI] [PubMed] [Google Scholar]
- 105. Picart T, Duffau H.. Awake resection of a left operculo-insular low-grade glioma guided by cortico-subcortical mapping. Neurosurg Focus. 2018;45(VideoSuppl2):V1-V1. [DOI] [PubMed] [Google Scholar]
- 106. Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TPL. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg. 2002;97(6):1333-1342. [DOI] [PubMed] [Google Scholar]
- 107. Della Puppa A, De Pellegrin S, Rossetto Met al. . Intraoperative functional mapping of calculation in parietal surgery. New insights and clinical implications. Acta Neurochir (Wien). 2015;157(6):971-977. [DOI] [PubMed] [Google Scholar]
- 108. Della Puppa A, De Pellegrin S, d’Avella Eet al. . Right parietal cortex and calculation processing: intraoperative functional mapping of multiplication and addition in patients affected by a brain tumor. J Neurosurg. 2013;119(5):1107-1111. [DOI] [PubMed] [Google Scholar]
- 109. Velásquez C, Goméz E, Martino J. Mapping visuospatial and self-motion perception functions in the left parietal lobe. Neurosurg Focus. 2018;45(VideoSuppl 2):V8-V8. [DOI] [PubMed] [Google Scholar]