Abstract
BACKGROUND
The middle temporal gyrus (MTG) is understood to play a role in language-related tasks such as lexical comprehension and semantic cognition. However, a more specific understanding of its key white matter connections could promote the preservation of these functions during neurosurgery.
OBJECTIVE
To provide a detailed description of the underlying white matter tracts associated with the MTG to improve semantic preservation during neurosurgery.
METHODS
Tractography was performed using diffusion imaging obtained from 10 healthy adults from the Human Connectome Project. All tracts were mapped between cerebral hemispheres with a subsequent laterality index calculated based on resultant tract volumes. Ten postmortem dissections were performed for ex vivo validation of the tractography based on qualitative visual agreement.
RESULTS
We identified 2 major white matter bundles leaving the MTG: the inferior longitudinal fasciculus and superior longitudinal fasciculus. In addition to long association fibers, a unique linear sequence of U-shaped fibers was identified, possibly representing a form of visual semantic transfer down the temporal lobe.
CONCLUSION
We elucidate the underlying fiber-bundle anatomy of the MTG, an area highly involved in the brain's language network. Improved understanding of the unique, underlying white matter connections in and around this area may augment our overall understanding of language processing as well as the involvement of higher order cerebral networks like the default mode network in these functions.
Keywords: Middle temporal gyrus, MTG, DSI, Anatomy, Tractography, White matter
ABBREVIATIONS
- DMN
default mode network
- DSI
diffusion spectrum imaging
- ILF
inferior longitudinal fasciculus
- LI
laterality index
- MTG
middle temporal gyrus
- SLF
superior longitudinal fasciculus
- TPOJ
temporo-parieto-occipital junction
The middle temporal gyrus (MTG) has been implicated in numerous tasks related to lexical comprehension and semantic cognition.1,2 For example, this region is important for understanding visual2,3 and auditory messages,1,4 and is thought to utilize contextual knowledge to retrieve the relevant semantic information required for these tasks.5–7 In addition, these processes are disrupted in the MTG in several clinical disease states, such as in agraphia8 and schizophrenia.9–11
Current models of language function describe distinct dorsal and ventral pathways; however, there is disagreement regarding the anatomic pathways by which semantic language transfer occurs.12 Additionally, the role of the MTG in the relationship between the salience and default mode networks (DMN) are contentious, including whether the semantic network is a part of the DMN. There is also uncertainty over how the MTG structurally facilitates these distinct functions.
In this study, we created a cortical model of the MTG utilizing diffusion spectrum imaging (DSI)-based fiber tracking validated by cadaveric brain dissection. By analyzing the subcortical anatomy and key white matter bundle connections of the MTG, we aim to provide a more thorough understanding of the role of the MTG in language processing which can be utilized by the operating neurosurgeon for improved neuro-oncological outcomes following cerebral surgery in this area. We discuss our findings in the context of semantic language function and the anatomy of the ventral language stream.
METHODS
Defining Regions of Interest
The MTG is 1 of 3 gyri comprising the temporal lobe. It extends from the anterior temporal pole to the junction of the temporal, parietal, and occipital lobes, i.e. the temporo-parieto-occipital junction (TPOJ). In this study, we defined the MTG into 3 regions of interest (ROI): an anterior ROI extending from the temporal pole posteriorly one-third the length of the gyrus, a posterior ROI extending from the posterior point at which the superior temporal sulcus curves dorsally to terminate in the angular gyrus of the inferior parietal lobule, and a middle ROI extending between the boundaries of the anterior and posterior regions (Figure 1). Each ROI occupied approximately one-third the length of the MTG and were bounded by the superior and inferior temporal sulci.
FIGURE 1.
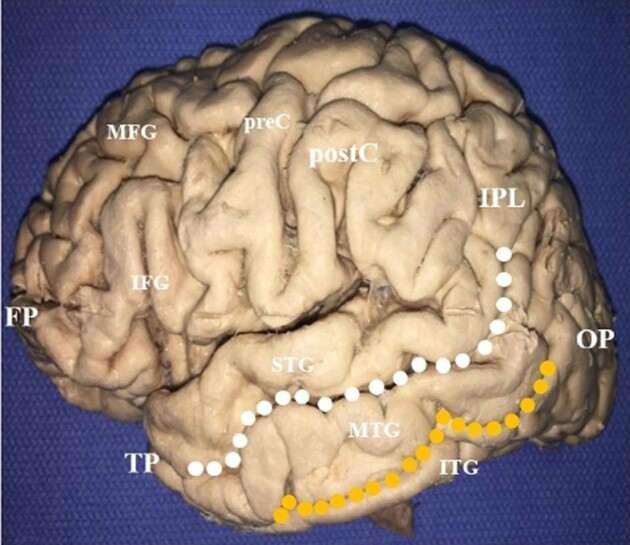
Superficial anatomy of the middle temporal gyrus (MTG). The dotted lines delineate the boundaries of the MTG. It is separated dorsally from the superior temporal gyrus by the superior temporal sulcus (white dotted line), and ventrally from the inferior temporal gyrus by the inferior temporal sulcus (orange dotted line). The boundary between the MTG and the inferior parietal lobule is marked by an ill-defined line from the terminal descending limb of the lateral fissure. MTG, middle temporal gyrus; STG, superior temporal gyrus; ITG, inferior temporal gyrus; TP, temporal pole; OP, occipital pole; IPL, inferior parietal lobule; postC, postcentral gyrus; preC, precentral gyrus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; FP, frontal pole.
Tractography
Fiber tracking analysis was completed utilizing diffusion imaging with corresponding T1-weighted images from 10 healthy, unrelated subjects obtained from the Human Connectome Project (Subjects IDs: 100307, 103414, 105115, 110411, 111312, 113619, 115320, 117112, 118730, and 118932).13 After brain registration in Montreal Neurologic Institute coordinate space,14 tractography was performed in DSI Studio (http://dsi-studio.labsolver.org) using a 2 ROI approach to initiate fiber tracking from a user-defined seed region.15,16 The tractography strategy utilized to analyze these data has been previously described elsewhere.17–20
Tractography was completed along the length of the gyrus from the anterior temporal pole to the bend of the superior temporal sulcus into the parietal lobe. Small U-shaped fibers identified during fiber tracking analysis were isolated sequentially along the length of the MTG using the same 2 ROI approach, which was used to isolate larger fiber bundles.16 All tractography was completed prior to cadaveric study. Laterality indices (LI) were calculated based on resultant tract volumes from major identified tracts, and the unpaired t-test was used to assess for significant differences between cerebral hemispheres (P ≤ .05).
Literature Review
A simulated brain image was created and marked with coordinates for the left temporal pole on multi-image analysis MANGO (Version 4.1; Research Imaging Center, UTHSCSA; http://ric.uthscsa.edu/mango/). This image was then imported into Sleuth software (Version 3.0.4) to conduct a reverse search of functional neuroimaging studies in the BrainMAP Functional database. A search strategy was created focusing only on experiments that included functional magnetic resonance imaging (fMRI) studies of the temporal pole (N = 300). Studies identified were then qualitatively analyzed by one reviewer (N.B.D.) and categorized into 1 of the 6 diagnostic and statistical manual of mental disorders, 5th edition cognitive domains: learning and memory, perceptual-motor function, language, executive function, complex attention, and social cognition. Repeated studies or studies that did not fit into one of the above domains were excluded. Results of this review are presented in Table, Supplemental Digital Content.
Postmortem Dissections
To validate the tractography results, we sought to demonstrate the location of white matter tracts connecting to the MTG with gross anatomic dissections for qualitative visual agreement.17–21 Ten specimens were used for this study from previously otherwise healthy individuals, obtained from our institution's Willed Body Program with approval from the Oklahoma State Anatomic Board. The methods utilized for postmortem dissection of the cadaveric brains and preservation of relevant white matter tracts of interest have been previously described.17–20,22
All white matter tracts were dissected in both hemispheres.
RESULTS
Long-Range White Matter Tracts of the MTG
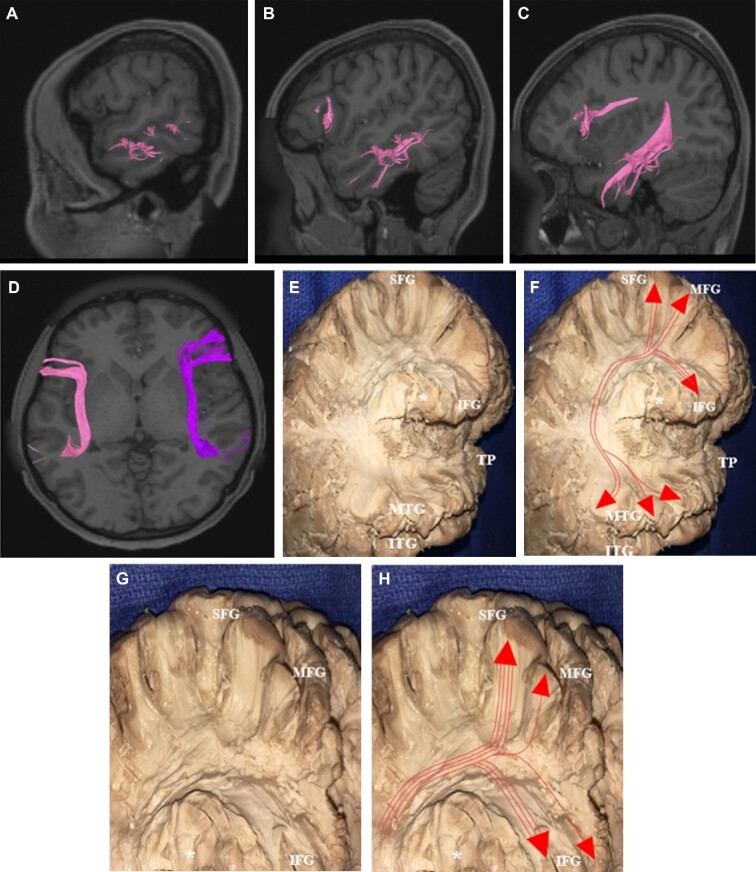
Two large fiber bundles were identified during fiber tracking analysis of the MTG: the superior longitudinal fasciculus (SLF) and the inferior longitudinal fasciculus (ILF). The SLF was found to arise predominantly within the middle and posterior thirds of the MTG, with some fibers arising within the anterior temporal pole (Figure 2). The fibers course craniocaudally within the temporal lobe before curving 180° at the level of the angular gyrus to pass anteriorly toward the frontal lobes. Coursing deep to the sensorimotor cortex, fibers from the SLF diverge at the posterior extent of the frontal lobe with terminations in the posterior aspect of the superior, middle, and inferior frontal gyri. Connections from the MTG to the inferior frontal gyrus demonstrated some variability across subjects, with fibers terminating in some combination of the pars opercularis, pars triangularis, and pars orbitalis in different brains. The SLF also projects from the posterior part of the MTG to the supramarginal and angular gyri of the inferior parietal lobule. These connections were identified bilaterally without any significant variation across subjects between right and left SLF.
FIGURE 2.
The superior longitudinal fasciculus (SLF). The SLF, pink and purple tracts on tractography A-D and depicted with and without red lines on dissection E-H, arises within the middle and posterior thirds of the MTG, and to a lesser extent from the anterior temporal pole. The fibers travel craniocaudally then curve 180° at the level of the angular gyrus to course anteriorly toward the frontal lobe, curving around the operculum (*). SLF fibers then diverge at the posterior extent of the frontal lobe and terminate in the posterior portions of the superior, middle, and inferior frontal gyri. In some subjects, fibers terminated at different regions of the operculum. MTG, middle temporal gyrus; ITG, inferior temporal gyrus; STG, superior temporal gyrus; TP, temporal pole; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus.
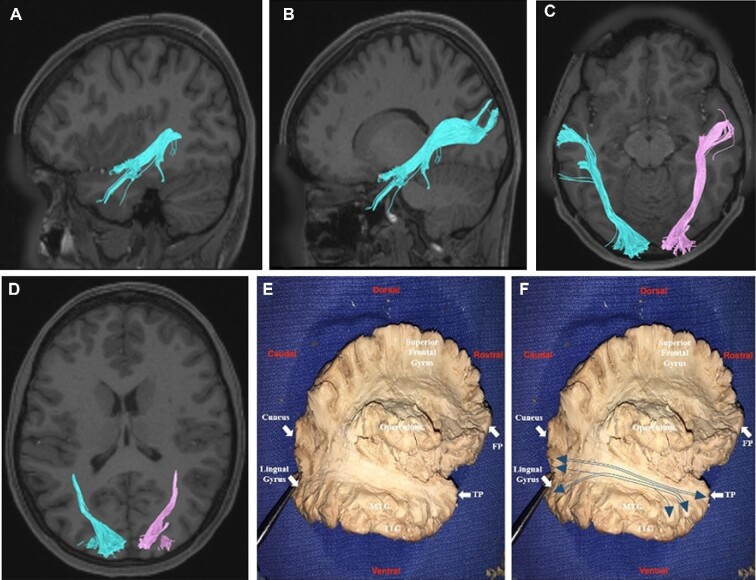
The ILF arises from the anterolateral aspect of the MTG and courses posteriorly through the subcortical white matter of the temporal lobe deep to the SLF (Figure 3). It passes deep to the gray matter of the TPOJ junction lateral to the ventricle to terminate within different parts of the occipital lobe, including the postero-superior aspect of the cuneus and the posterior part of the lingual gyrus at the occipital pole. Although there was no noted variation bilaterally between the hemispheres, terminations of the ILF demonstrated some variability across subjects. Specifically, the ILF would either terminate in both the cuneus and lingual gyrus or it would terminate in either the cuneus or lingual gyrus. Single termination tracts of the ILF were more common in the lingual gyrus at the occipital pole.
FIGURE 3.
Inferior longitudinal fasciculus (ILF). The ILF, cyan and magenta tracts on tractography A-D and depicted with and without blue lines on dissection E-F, arises from the anterolateral aspect of the middle temporal gyrus, and courses posteriorly deep to the SLF, passing deep to the gray matter of the temporo-parieto- occipital junction and lateral to the ventricle. It terminates within the parts of the occipital lobe, including the posterior part of the lingual gyrus and the postero-superior aspect of the cuneus. MTG, middle temporal gyrus; ITG, inferior temporal gyrus; TP, temporal pole; FP, frontal pole.
LIs based on the tract volumes calculated for the SLF and ILF are shown in the Table. The LI for the ILF was not statistically different bilaterally (P-value = .260). In contrast, the SLF was noted to be lateralized to the left cerebral hemisphere (P-value = .039).
TABLE.
Lateralization Indices for the Middle Temporal White Matter Tracts
| Tract | Volume/tract (left) | Volume/tract (right) | LI | P value |
|---|---|---|---|---|
| SLF | 54.7 | 35.1 | 0.215 | .039 |
| ILF | 47.0 | 36.0 | 0.103 | .260 |
Short-Range White Matter Tracts of the MTG
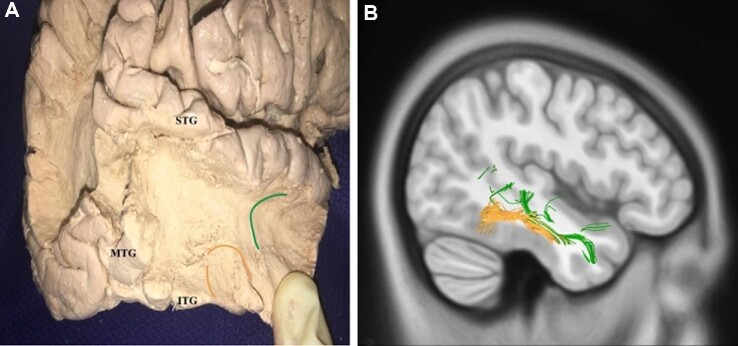
An abundance of local association fibers was identified while performing the fiber tractography and gross anatomic dissections of the MTG. These local white matter pathways have a characteristic morphology consisting of U-shaped fibers that connect adjacent areas of cortex across a sulcal structure. U-shaped fibers were identified within the MTG and between the MTG and the superior and inferior temporal gyri. Examples of the different types of U-fibers identified during fiber tractography and gross anatomic dissection are shown in Figure 4.
FIGURE 4.
Short association fibers of the middle temporal gyrus. An abundance of local U-fibers was identified on dissection A and tractography B. These fibers connect the middle temporal gyrus to the superior (green lines) and inferior (orange lines) temporal gyri.
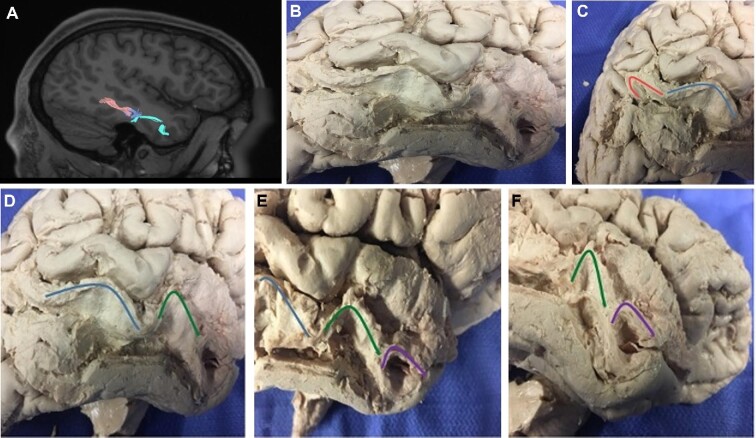
During fiber tracking analysis, we also noted a unique sequential arrangement of U-fibers coursing from the posterior MTG to the temporal pole (Figure 5). Superficial dissection of the MTG confirmed the existence of this linear sequence of short fiber bundles in both the left and right cerebral hemispheres. The fibers run in sequence along the lateral aspect of the gyrus, superficial to the SLF and ILF which run within the deep subcortex of the temporal lobe. At times, these U-shaped fibers branch to connect the MTG to parts of the superior and inferior temporal gyri.
FIGURE 5.
The unique “U”-shaped fiber sequence of the middle temporal gyrus. A sequential arrangement of U-shaped fibers from the posterior aspect of the middle temporal gyrus to the temporal pole is highlighted on tractography A and dissection B-F. These fibers are present bilaterally and run along the lateral aspect of the gyrus, superficial to the SLF and ILF. All images A-F are similarly oriented caudal-rostral.
DISCUSSION
The role of the MTG in semantic language has been well established1; however, its simultaneous involvement in the DMN has raised questions over a possible role of the DMN in language. In this study, we used diffusion tractography validated by gross anatomic dissection to describe the connections of the MTG in relation to gross anatomic structures. Although dissection allowed for qualitative understanding of tract orientation, tractography further allowed us to quantify tract volumes to understand laterality. As mentioned, the discovery of a unique U-fiber sequence helps to explain a fundamental pattern of connectivity within the MTG and the significance of the DMN in language processing.
The MTG and the Dual Stream Model of Language Function
Contemporary understanding of the human language network rests on its segmentation into 2 distinct, but interacting, language streams.23 A model by Catani24 proposes ventral semantic language transfer occurs from the posterior temporal lobe at the TPOJ down the length of the temporal lobe to the anterior temporal pole, with the uncinate then relaying the semantic information to the frontal lobe.24,25 The identification and description of a linear sequence of “U”-shaped fibers traversing the length of the MTG between the TPOJ and the anterior temporal pole in this study suggest that semantic transfer may occur according to this model. This helps to explain a fundamental pattern of resting state functional activity, namely that parcellations of the MTG are functionally linked sequentially to their anterior neighbors down the length of the gyrus. Although we did not identify the uncinate fasciculus during fiber tracking analysis of the MTG, the anterior temporal pole has been shown to connect to the frontal lobe in several other studies.25–27
Relevance of the DMN to the MTG and Semantic Retrieval
The potential relationship between the DMN and semantic function was proposed because of the role of several nodes of the network in language. Additionally, fMRI studies have shown a lack of deactivation of the DMN in semantic tasks,28,29 though this has not been consistently observed and may be dependent on task difficulty.30 The involvement of the DMN in semantic function is however consistent with the growing understanding of its important role in cognition. Recently, a distinct semantic network was proposed to underlie multimodal semantic cognition, which overlaps with areas of the extended DMN.31 Whether the semantic network is truly distinct from this system is therefore still under discussion.
The MTG is one region common to both networks; however, only the posterior MTG is primarily involved in semantic cognition,6 though fMRI studies involving language function often show activation of the entire MTG.30 The unique U-fiber sequence identified here may explain activation patterns down the length of the MTG and is also concordant with the Catani model.24,25 The internal organization of the MTG may therefore explain the integration of the DMN with the semantic network or underlie the role of the DMN in language function, as it facilitates a link between the posterior MTG and the temporal pole where different aspects of cognition may be integrated. Furthermore, when conducting a search of the literature for fMRI studies highlighting the temporal pole, the majority of research focused on the cognitive domain of learning and memory, and more specifically verbal memory, supporting this hypothesis (Supplemental Digital Content). Conversely, it may link the DMN and semantic network and form the basis of their difficult segregation. The exact role of each network in semantic function is yet to be determined, though future studies should target the MTG as a ROI when evaluating these networks.
CONCLUSION
The MTG is an important part of the cortex involved in language comprehension and contextually guided semantic retrieval. The identification and description of a linear sequence of local “U”-shaped fiber bundles down the length of the MTG may explain how semantic transfer occurs in the ventral language stream and may also underlie the DMN connectivity of the MTG. Additional studies are needed to better understand the role of the MTG in language function, and the DMN within this domain.
Funding
This work was funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnell Center for Systems Neuroscience at Washington University.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Sughrue is the Chief Medical Officer of Omniscient Neurotechnologies.
Supplementary Material
Acknowledgements
Data were provided, in part, by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657).
Contributor Information
Robert G Briggs, Department of Neurosurgery , University of Southern California, Los Angeles, California, USA.
Onur Tanglay, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Nicholas B Dadario, Rutgers Robert Wood Johnson School of Medicine, New Brunswick, New Jersey, USA.
Isabella M Young, Cingulum Health, Sydney, Australia.
R Dineth Fonseka, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Jorge Hormovas, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Vukshitha Dhanaraj, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Yueh-Hsin Lin, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Sihyong J Kim, Rutgers Robert Wood Johnson School of Medicine, New Brunswick, New Jersey, USA.
Adam Bouvette, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Arpan R Chakraborty, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Ty M Milligan, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Carol J Abraham, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Christopher D Anderson, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Daniel L O’Donoghue, Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma, USA.
Michael E Sughrue, Centre for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia.
Supplemental Digital Content. Table. Literature review. A, A reverse search of the literature was conducted using coordinates of the left temporal gyrus for fMRI studies with Sleuth software. B, Results of this search were qualitatively categorized based on their relation to the 6 key domains of cognitive function according to the diagnostic and statistical manual of mental disorders, 5th edition. Abbreviations: L_TGv, left temporal gyrus; MNI, Montreal Neurological Institute; fMRI, functional magnetic resonance imaging; ROI, region of interest.
COMMENT
The authors have presented an interesting anatomical study of the MTG and its connections using a combination of MR tractography and postmortem dissections. Through this investigation, authors identified a linear sequence of “U-shaped’ fibers traversing the length of the MTG which sheds light on its internal organization, corroborating the model proposed by Catani et al1 of ventral semantic language transfer down the temporal lobe. It also speaks to its potential role in connecting the default mode network (DMN) with language processing. It would be interesting to know whether these short fiber bundles had a hemispheric predominance since authors note that these fibers traveled bilaterally. Additionally, authors note that at times these fibers branched to parts of the superior and inferior temporal gyri. Future studies focusing on these other U-fiber connections, including laterality and whether there were any patterns to branching points along the MTG, may further enlighten our understanding of the middle temporal gyrus and its relationships and roles in language processing. Papers like this defining the different fiber tracts in the brain will result in better understanding of the neuroanatomy, safer operations, and better outcomes in neurosurgery.
Youngwon Youn
Amgad Hanna
Madison, Wisconsin, USA
REFERENCES
- 1. Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8-16. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1. Xu J, Wang J, Fan Let al. . Tractography-based parcellation of the human middle temporal gyrus. Sci Rep. 2016;5:18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122(2):250-261. [DOI] [PubMed] [Google Scholar]
- 3. Dick AS, Mok EH, Raja Beharelle A, Goldin-Meadow S, Small SL. Frontal and temporal contributions to understanding the iconic co-speech gestures that accompany speech. Hum Brain Mapp. 2014;35(3):900-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bethmann A, Brechmann A. On the definition and interpretation of voice selective activation in the temporal cortex. Front Hum Neurosci. 2014;8:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA. The semantic network at work and rest: differential connectivity of anterior temporal lobe subregions. J Neurosci. 2016;36(5):1490-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davey J, Thompson HE, Hallam Get al. . Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krieger-Redwood K, Jefferies E. TMS interferes with lexical-semantic retrieval in left inferior frontal gyrus and posterior middle temporal gyrus: evidence from cyclical picture naming. Neuropsychologia. 2014;64:24-32. [DOI] [PubMed] [Google Scholar]
- 8. Sakurai Y, Mimura I, Mannen T. Agraphia for kanji resulting from a left posterior middle temporal gyrus lesion. Behav Neurol. 2008;19(3):93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joo SW, Chon MW, Rathi Y, Shenton ME, Kubicki M, Lee J. Abnormal asymmetry of white matter tracts between ventral posterior cingulate cortex and middle temporal gyrus in recent-onset schizophrenia. Schizophr Res. 2018;192:159-166. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Li B, Wang Het al. . Decreased middle temporal gyrus connectivity in the language network in schizophrenia patients with auditory verbal hallucinations. Neurosci Lett. 2017;653:177-182. [DOI] [PubMed] [Google Scholar]
- 11. Kuroki N, Shenton ME, Salisbury DFet al. . Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163(12):2103-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(Pt 12):3529-3550. [DOI] [PubMed] [Google Scholar]
- 13. Glasser MF, Smith SM, Marcus DSet al. . The Human Connectome Project's neuroimaging approach. Nat Neurosci. 2016;19(9):1175-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans AC, Marrett S, Neelin Pet al. . Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1(1):43-53. [DOI] [PubMed] [Google Scholar]
- 15. Martino J, De Witt Hamer PC, Berger MSet al. . Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218(1):105-121. [DOI] [PubMed] [Google Scholar]
- 16. Kamali A, Sair HI, Radmanesh A, Hasan KM. Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain. Neuroscience. 2014;277:577-583. [DOI] [PubMed] [Google Scholar]
- 17. Sheets JR, Briggs RG, Bai MYet al. . Parcellation-based modeling of the dorsal premotor area. J Neurol Sci. 2020;415:116907. [DOI] [PubMed] [Google Scholar]
- 18. Burks JD, Bonney PA, Conner AKet al. . A method for safely resecting anterior butterfly gliomas: the surgical anatomy of the default mode network and the relevance of its preservation. J Neurosurg. 2017;126(6):1795-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burks JD, Boettcher LB, Conner AKet al. . White matter connections of the inferior parietal lobule: a study of surgical anatomy. Brain Behav. 2017;7(4):e00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burks JD, Conner AK, Bonney PAet al. . Anatomy and white matter connections of the orbitofrontal gyrus. J Neurosurg. 2018;128(6):1865-1872. [DOI] [PubMed] [Google Scholar]
- 21. Catani M, Dell’acqua F, Vergani Fet al. . Short frontal lobe connections of the human brain. Cortex. 2012;48(2):273-291. [DOI] [PubMed] [Google Scholar]
- 22. Koutsarnakis C, Liakos F, Kalyvas AV, Sakas DE, Stranjalis G. A laboratory manual for stepwise cerebral white matter fiber dissection. World Neurosurg. 2015;84(2):483-493. [DOI] [PubMed] [Google Scholar]
- 23. Saur D, Kreher BW, Schnell Set al. . Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105(46):18035-18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8-16. [DOI] [PubMed] [Google Scholar]
- 25. Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briggs RG, Rahimi M, Conner AKet al. . A Connectomic atlas of the human cerebrum-chapter 15: tractographic description of the uncinate fasciculus. Oper Neurosurg. 2018;15(Suppl_1):S450-S455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papagno C, Miracapillo C, Casarotti Aet al. . What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134(Pt 2):405-414. [DOI] [PubMed] [Google Scholar]
- 28. Shapira-Lichter I, Oren N, Jacob Y, Gruberger M, Hendler T. Portraying the unique contribution of the default mode network to internally driven mnemonic processes. Proc Natl Acad Sci USA. 2013;110(13):4950-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wirth M, Jann K, Dierks T, Federspiel A, Wiest R, Horn H. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage. 2011;54(4):3057-3066. [DOI] [PubMed] [Google Scholar]
- 30. Jackson RL, Cloutman LL, Lambon Ralph MA. Exploring distinct default mode and semantic networks using a systematic ICA approach. Cortex. 2019;113:279-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.