Abstract
BACKGROUND
Minimally invasive surgery (MIS) has been shown to decrease length of hospital stay and opioid use.
OBJECTIVE
To identify whether surgery for epilepsy mapping via MIS stereotactically placed electroencephalography (SEEG) electrodes decreased overall opioid use when compared with craniotomy for EEG grid placement (ECoG).
METHODS
Patients who underwent surgery for epilepsy mapping, either SEEG or ECoG, were identified through retrospective chart review from 2015 through 2018. The hospital stay was separated into specific time periods to distinguish opioid use immediately postoperatively, throughout the rest of the stay and at discharge. The total amount of opioids consumed during each period was calculated by transforming all types of opioids into their morphine equivalents (ME). Pain scores were also collected using a modification of the Clinically Aligned Pain Assessment (CAPA) scale. The 2 surgical groups were compared using appropriate statistical tests.
RESULTS
The study identified 43 patients who met the inclusion criteria: 36 underwent SEEG placement and 17 underwent craniotomy grid placement. There was a statistically significant difference in median opioid consumption per hospital stay between the ECoG and the SEEG placement groups, 307.8 vs 71.5 ME, respectively (P = .0011). There was also a significant difference in CAPA scales between the 2 groups (P = .0117).
CONCLUSION
Opioid use is significantly lower in patients who undergo MIS epilepsy mapping via SEEG compared with those who undergo the more invasive ECoG procedure. As part of efforts to decrease the overall opioid burden, these results should be considered by patients and surgeons when deciding on surgical methods.
Keywords: Epilepsy, Electrocorticography, Stereotactic electroencephalography, Opioid, Pain, Grid
ABBREVIATIONS
- CAPA
Clinically Aligned Pain Assessment
- CPT
Current Procedural Terminology
- DRE
drug-resistant epilepsy
- ECoG
electrocorticography
- EEG
electroencephalogram
- EZ
epileptogenic zone
- ME
morphine equivalent
- MIS
minimally invasive surgery
- MME
milligrams of morphine equivalent
- NRS
Numeric Pain Rating Scale
- SEEG
stereotactic electroencephalography
Approximately 3 million adults have epilepsy in the United States, and an estimated 30% have drug-resistant epilepsy (DRE), meaning they continue to have seizures despite treatment with ≥2 antiepileptic medications.1-3 Among patients with DRE, surgery may be curative in up to 70%.2 An estimated 2000 to 3000 patients undergo surgical procedures for epilepsy each year.2 To effectively treat epilepsy with surgery, the epileptogenic zone (EZ) must be identified and fully mapped. Laterality of the EZ can often be found by using noninvasive electroencephalogram (EEG), but because of the poor spatial resolution of scalp electrodes, intracranial placement of EEG electrodes may be required for precise anatomical EZ mapping.1,2
The 2 predominant surgical methods for placing intracranial electrodes are electrocorticography (ECoG) and stereotactic electroencephalography (SEEG).4 ECoG is accomplished by craniotomy and placement of subdural electrodes. Grid-based ECoG allows a wider and more specific description of surface electrical activity but can miss deeper EZs and requires more invasive surgery with more tissue trauma. SEEG involves stereotactically inserting electrodes through the skull into the brain via a minimally invasive approach.1,2,5 SEEG can detect EEG patterns arising from specific deep-seated targets through smaller incisions with less tissue disruption but does not easily capture a wide extent of superficial cortical activity, although it has been done well at high-volume centers. Although SEEG was previously widely adopted in Europe and Canada, over the past decade, it has become more widely used in the United States, when possible and appropriate for a patient's disease, because it is less invasive and possibly less painful.2,4-8
The United States is currently facing dramatic increases in opioid addiction and opioid-related mortalities.9,10 Between 1999 and 2017, deaths attributed to opioid overdose quadrupled from 16 849 to 70 237 deaths/year.9,11,12 The birth of the opioid epidemic can be traced to the 2001 guidelines from the Joint Commission, which bound pain management to Medicaid/Medicare reimbursements,12-14 in conjunction with aggressive promotion by pharmaceutical companies, which resulted in an increase in opioid prescriptions for pain management.12,15 The increase was especially noticeable for patients undergoing surgery, as ∼99% of adults undergoing surgery in the United States are prescribed opioids despite their marginal efficacy.12,16-18 The perioperative risk of addiction in opioid-naïve patients has been estimated to be 6% to 10%, and the risk of addiction increases significantly for those who use opioids after postoperative day 3 and most dramatically between days 5 and 31.9,12,19,20 Multiple methods have been proposed to decrease opioid use in surgical patients, including the use of nonaddictive pain medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), ketamine, and gabapentin; meditation and mindfulness techniques; and preoperative counseling.21-27
Given the addictive nature of opioids and prior studies and anecdotal experience suggesting that SEEG may be less painful than grids,28 we sought to identify the prescription patterns at our institution and evaluate the effectiveness of different pain management strategies among adult patients who underwent intracranial electrode placement via ECoG and SEEG.28 We hypothesized that opioid medications use would be lower in the SEEG cohort. We also compared the postoperative pain scores between the 2 groups to determine whether they were correlated with the amount of opioids prescribed during hospitalization. Lastly, we sought to identify the total amount of opioids given at discharge and determine whether this differed between groups.
METHODS
Approval for this nonconsecutive case series was obtained by the Institutional Review Board with a waiver of patient consent. A retrospective chart review was used to identify patients treated with SEEG (Current Procedural Terminology [CPT] code 61760) or ECoG (CPT 61533) from 2014 through 2018. The study design and proposal are registered at researchregistry.com (filing number 5971). Data collection was done between May 2019 and August 2019. The inclusion criteria were SEEG or ECoG surgery, age ≥18 yr, and diagnosis of epilepsy. The exclusion criteria were history of chronic opioid use defined as daily preoperative opioid use, complications occurring during surgery requiring additional surgery or cessation of seizure recording, and surgery that was not SEEG or ECoG. Patients were categorized by surgery type. All surgeries were performed by 2 physicians at our quaternary hospital that specialize in functional neurosurgery and had advanced command of the procedures. All patients were placed under general anesthesia; local anesthetics were used at the headfram pin sites after induction of general anesthesia to decrease postoperative pain from the headframe placement. For SEEG, planning was conducted using preoperative T1-weighted magnetic resonance imaging with contrast, the patient was fitted with a CRW frame (Integra LifeSciences, Princeton, New Jersey), and a high-resolution computed tomography scan was obtained for registration. SEEG electrodes were removed in the operating room under monitored anesthesia. For grid placement, the patient was fixed in a Mayfield head clamp (Integra LifeSciences, Princeton, New Jersey), registered for intraoperative neuronavigation, and a craniotomy was created in the desired location for the electrodes. Electrodes were removed under general anesthesia after a reopening of the craniotomy.
A power calculation was performed before the start of the study. Our calculation showed that a 30% difference in opioid use, measured as milligrams of morphine equivalents (MME), would provide a statistically meaningful difference. The sample size needed to detect a 30% difference, for an alpha of 0.05 and a power of 0.8, was 11 patients per arm.
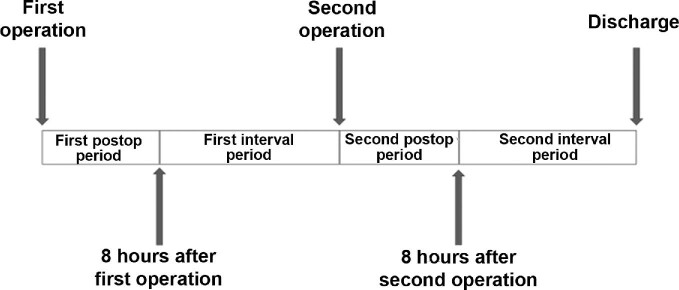
Conventionally, patients who undergo SEEG or grid-based ECoG surgery will have a second surgery to remove the electrodes. To more accurately characterize pain management patterns, we separated the hospitalization into 4 time periods: the first postoperative period, defined as the first 8 h after surgery; the first interval period, which lasted from the end of the first postoperative period until the second surgery; the second postoperative period, defined as the first 8 h after the second surgery; and the second interval period, lasting from the end of the second postoperative period until discharge (Figure 1). The types and doses of pain medications administered during these time periods for all patients were collected, and total MMEs were calculated for all opioid-based medications. Pain data from the comfort category of the Clinically Aligned Pain Assessment (CAPA) survey were also collected for each of the 4 periods.29,30 The CAPA is a verified pain assessment tool used preferentially by our institution to assess a patient's pain.29,30 The comfort category of the CAPA was the only category included because it is the most closely related to the more traditionally used Numeric Pain Rating Scale (NRS).29,30 The comfort category of the CAPA was transcribed into a numbered ordinal scale, with 0 indicating negligible pain, 1 being comfortably manageable pain, 2 being tolerable pain with discomfort, and 3 indicating intolerable pain (Table 1).29,30
FIGURE 1.
A graphical representation of the study time intervals. The first postoperative period was from the end of the first operation until 8 h postoperatively; the first interval period was from 8 h after the first surgery until the start of the second surgery; the second postoperative period was from the end of the second surgery until 8 h postoperatively; the second interval period was from 8 h after the second surgery until discharge.
TABLE 1.
The Comfort Category of the CAPA Tool
| Response | Numeric equivalent | NRS equivalent |
|---|---|---|
| Intolerable | 3 | 9-10 |
| Tolerable with discomfort | 2 | 6-8 |
| Comfortably manageable | 1 | 3-5 |
| Negligible pain | 0 | 1-2 |
The CAPA grade was transformed into a number corresponding with the pain level for the purposes of statistical interpretation. Correlation to the NRS is also shown.
The Wilcoxon rank-sum test for nonparametric data was used to compare the MMEs used between the 2 groups for the entire stay and for each interval. Spearman's correlation was used to determine correlation between the average comfort category CAPA score and the total MMEs for each interval. The demographic information of the 2 groups was evaluated to identify any confounding variables. Length of stay was significantly different between groups; thus, the total MMEs for the entire stay were divided by the length of stay for each patient to create a new variable measuring MMEs administered per day. The difference between the MMEs used per day in each surgical group was tested for significance using the Wilcoxon rank-sum test. A nonparametric equality of means test was performed to evaluate whether there was a difference in the total median MME use between periods in each surgical group. Stata IC version 15.1 (StataCorp, College Station, Texas) was used for all statistical calculations. The strengthening the reporting of observational studies in epidemiology checklist was used to ensure the study design complied with current best-practice guidelines for retrospective cohort studies.
RESULTS
Patient Cohort
This was a retrospective cohort case series study at a single academic center designed to identify whether there is a difference in opioid use between adult patients undergoing SEEG and those undergoing grid-based ECoG placement for seizure focus localization. It was powered to identify a 30% reduction in opioid use between the 2 groups. We identified a total of 53 patients who met the following inclusion criteria: 36 patients in the SEEG group and 17 patients in the ECoG group. The overall demographics of the 2 groups are shown in Table 2. The major differences between the 2 groups include the number of electrode contacts implanted with the SEEG group, which had a higher number on average, 93, when compared with the ECoG group on average, 66 (P < .002). There was also a difference in the surgical laterality, with the SEEG group having a higher proportion of bilateral surgeries than the ECoG group (80.6% vs 23.5%; P < .001). The duration of surgery was also different between the groups; the median surgical duration for the ECoG group was longer than that of the SEEG group (339 vs 260 min; P = .0245). Otherwise, the groups were fairly well matched, with no difference in the mean age, sex, type of seizures, median number of seizures recorded, median preoperative modified Rankin Scale score, mean number of antiepileptic drugs used preoperatively, and complications.
TABLE 2.
Demographic Data for the SEEG and ECoG Groups
| SEEG (n = 36) | ECoG (n = 17) | P valuea | |
|---|---|---|---|
| Mean age (yr) | 32.1 ± 8.2 | 32.9 ± 9.8 | .7514 |
| Sex (% male) | 52.8% (n = 19) | 35.3% (n = 6) | .257 |
| Type of seizure (% focal) | 47.2% (n = 17) | 58.8% (n = 10) | .130 |
| Surgical laterality | Bilateral: 80.6% (n = 29) | Bilateral: 23.5% (n = 4) | <.001 |
| Left: 11.1% (n = 4) | Left: 23.5% (n = 4) | ||
| Right: 8.3% (n = 3) | Right: 52.9% (n = 9) | ||
| Median number of seizures recorded | 5.5 ± 6.8 | 6 ± 6.8 | .977 |
| Median preoperative modified Rankin Scale score | 1 ± 0.65 | 2 ± 0.86 | .0971 |
| Mean number of antiepileptic drugs used preoperatively | 2.8 ± 1.3 | 2.8 ± 1.5 | .893 |
| Mean length of surgery (min) | 260 ± 108 | 339 ± 130 | .0245 |
| Mean number of electrode contacts implanted | 93 ± 21 | 66 ± 36 | <.002 |
| Number (%) of complications | 1 (2.8%) | 2 (11.7%) | .238 |
a P values are included to show differences between groups and not for inferential purposes.
Data are reported as mean ± SD unless otherwise indicated.
Significant values are shown in boldface type.
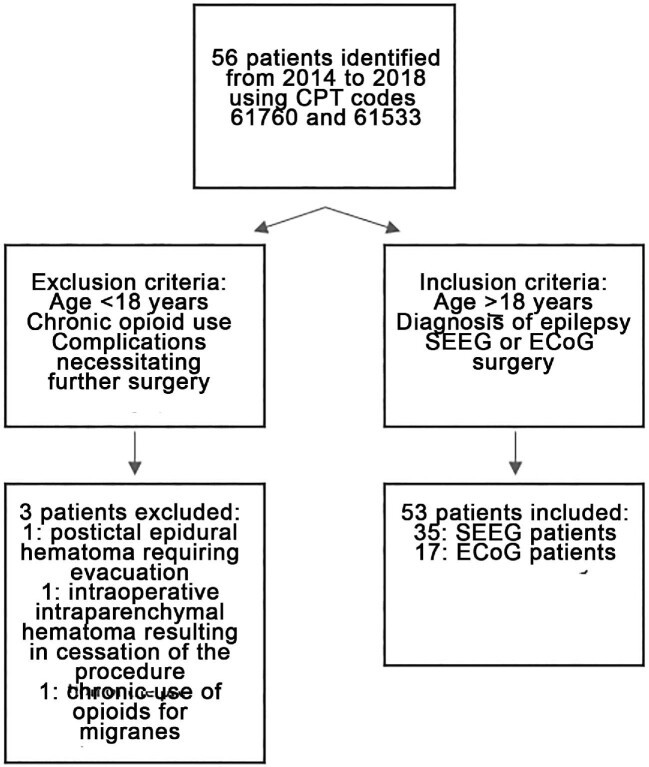
There were 3 total complications encountered in this cohort: 2 in the SEEG group and 1 in the ECoG group. The SEEG complications were 1 subarachnoid hemorrhage that was found on postoperative computed tomography head but did not result in any neurological deficits, and 1 subdural hygroma that formed after electrode placement and caused some mild right upper-extremity weakness that resolved; seizure recording was continued as planned in both cases. The one complication in the ECoG group was mild right-sided weakness postoperatively that improved; seizure recording was performed as planned. A flow diagram showing the inclusion and exclusion of patients can be found in Figure 2.
FIGURE 2.
A flow diagram of patient inclusion and exclusion. Reasons for exclusion from the study are detailed.
Three patients were excluded from the overall study cohort: one because of an epidural hematoma that occurred after a seizure that resulted in early termination of ECoG seizure recording and a unplanned evacuation of the epidural hematoma; a second because of an intraoperative hemorrhage during SEEG implantation that required the procedure to be aborted, although no further surgery was necessary; and a third because of chronic use of opioids for the treatment of migraines prior to initial hospitalization.
MME Consumption
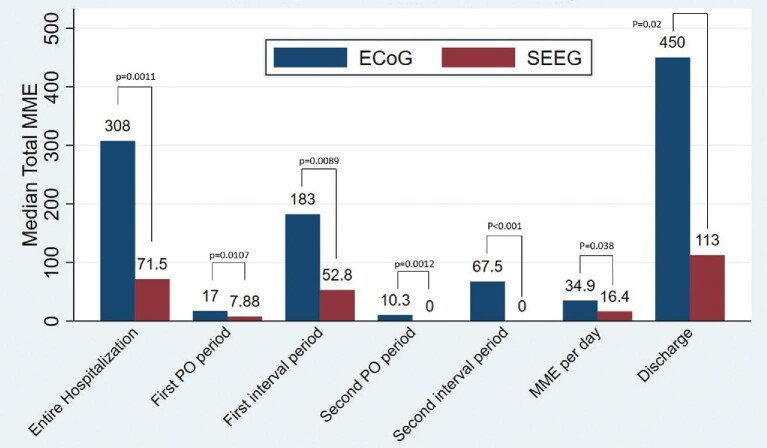
A general overview of the comparison between the 2 groups is presented in Tables 2 and 3. The median MME consumed in the SEEG cohort was significantly lower than that consumed in the ECoG cohort (71.5 vs 307.8 MME, respectively; P = .0011, Wilcoxon rank-sum test). This difference was observed across every epoch (Figure 3). To control for length of stay, MMEs per day were calculated. This measure was also significantly lower for SEEG than grids when comparing the median MME per day (16.4 vs 54.4 MME/day, respectively; P = .0377, Wilcoxon rank-sum test).
TABLE 3.
Statistical Comparisons between the SEEG and ECoG Groups
| Variable | SEEG (n = 36) | ECoG (n = 17) | P valuea |
|---|---|---|---|
| MME | |||
| Total | 71.5 ± 543.1 | 307.8 ± 296.3 | .0011 |
| First PO period | 7.9 ± 34.6 | 17 ± 15.5 | .0107 |
| First interval period | 52.72 ± 488.5 | 182.5 ± 236.3 | .0089 |
| Second PO period | 0 ± 18.2 | 10.35 ± 15.0 | .0012 |
| Second interval period | 0 ± 56.6 | 67.5 ± 84.6 | <.001 |
| Discharge total | 112.5 ± 249.7 | 450 ± 543.2 | .020 |
| MME per day | 16.4 ± 38.2 | 34.9 ± 69.4 | .0377 |
| Total NSAIDs (mg) | 0 ± 7.0 | 0 ± 1065.1 | .3823 |
| Total acetaminophen (g) | 4.9 ± 11.8 | 11.7 ± 7.8 | .0055 |
| Length of stay (d) | 6 ± 3.7 | 8 ± 8.9 | .0335 |
| Mean comfort category CAPA score | 0.8 ± 0.4 | 1.3 ± 0.6 | .0117 |
PO, postoperative.
a P values are included to show differences between groups and should not be included for inference.
Significant values are shown in boldface type.
Data are reported as median ± SD unless otherwise indicated.
FIGURE 3.
A graphical representation of the difference in median total MME used for each time period.
Non-Narcotic Pain Medication Use
The median total NSAID use among all patients in both groups was 0 mg for the entire hospitalization. When comparing the mean NSAID use in each group, the SEEG group had a lower mean of 1.7 mg throughout the hospital stay, whereas the ECOG group had a mean use of 275 mg. The median total acetaminophen use among the SEEG group was lower (4.9 g for the entire stay) when compared with ECoG (11.7 g; Table 3).
CAPA Pain Scale Score
The average comfort category of the CAPA pain scale scores for the entire stay correlated with the total MMEs used during the entire stay for both the SEEG and the ECoG groups (Spearman correlation coefficient ρ = .52, P = .0012; ρ = .56, P = .02, respectively; Table 4). The only other significant correlation was found between the comfort category CAPA scores and total MME used for the first epoch in the SEEG cohort, ie, the time period between 8 h after the first surgery and the start of the second surgery (ρ = .51, P = .002). Total MMEs prescribed at discharge were also calculated by cohort, and the median total MMEs at discharge were significantly different (SEEG 112.5 vs ECoG 450 MMEs; P = .02). There was a statistically significant difference in the comfort category CAPA score between the means of the SEEG group and ECoG group (0.8 vs 1.2; P = .0117).
TABLE 4.
Correlations Between Comfort Category CAPA Pain Score and Total MMEs Used in Each Corresponding Time Interval
| SEEG | ECoG | |||
|---|---|---|---|---|
| Time period | CAPA average | Spearman's correlation to concurrent operative intervala | CAPA average | Spearman's correlation to concurrent operative intervala |
| Overall | 0.8 | Rho = 0.5184 | 1.2 | Rho = 0.5567 |
| P = .0012 | P = .0203 | |||
| First PO | 1.3 | Rho = 0.2685 | 1.6 | Rho = 0.1102 |
| P = .1247 | P = .6958 | |||
| First interval | 1.1 | Rho = 0.5115 | 1.2 | Rho = 0.1643 |
| P = .0020 | P = .5585 | |||
| Second PO | 1.1 | Rho = 0.3286 | 1.8 | Rho = 0.1142 |
| P = .2140 | P = .7237 | |||
| Second interval | 1.4 | Rho = −0.6150 | 1.4 | Rho = 0.3189 |
| P = .1046 | P = .3391 | |||
PO, postoperative.
aSpearman's test of correlation by surgical group.
Significant values are shown in boldface type.
DISCUSSION
Surgical treatment can result in seizure freedom for patients with DRE, which leads many epilepsy patients to pursue surgical options.2,3,6 Before offering surgical resection or neuromodulation, a precise delineation of the seizure onset zone is crucial. When noninvasive workups fail or produce discordant results, intracranial monitoring is often the next step. This can be done with either penetrating depth electrodes (SEEG) or subdural epicortical electrodes (ECoG). At our institution, SEEG is typically used in cases where there is a strong network hypothesis, a hypothesized deep seizure focus, concern for bilateral foci, or the need to map within a prior craniotomy. Grids are used when there is a stronger lateral cortical hypothesis, a need to estimate the extent of a putative neocortical resection zone, or a desire to map eloquent function near a putative resection zone. ECoG appears to have a higher rate of CSF leak when compared with SEEG, 12% vs 0.3%, a higher hemorrhage rate (4% vs 1.4%), a higher infection rate (2% vs <1%), and is anecdotally more painful.28,31,32
The current study was designed to identify whether SEEG procedures led to a reduced use of opioids compared with craniotomy-based ECoG procedures during hospitalization. The primary endpoint of this study, the difference in the median total MMEs, was significantly lower in the SEEG group when compared with the ECoG group, in both the unadjusted and the adjusted length of stay calculations (Table 3). This finding implies that SEEG can significantly reduce opioid use during hospitalization for epilepsy surgery. The observed 74% median reduction in total MMEs per stay was larger than we hypothesized when designing the study. The amount of medication prescribed at discharge also differed significantly between the 2 groups, with the SEEG group receiving fewer total opioids at discharge than the ECoG group (Table 3). Another recently published study performed by Wang et al28 also demonstrated a significant difference in the opioid use between those patients undergoing SEEG and those undergoing ECoG procedures, and those findings validate our results.
We attempted to correlate the pain scores recorded during the patients’ stays with the total MMEs given during the stay and found correlations between the average overall pain score and total MMEs given for both the SEEG and ECoG cohorts, as well as during the first time period in the SEEG cohort. None of the other periods demonstrated a significant correlation between the pain scores recorded and the total MMEs, which may be due to the small sample size of the individual groups. Indeed, although this study was not powered to identify a correlation between the individual time periods, we did find a statistically significant difference in the total median MME use between the periods in each group, with more MME being used in the first interval period. This first interval typically takes place during the main portion of the patient's stay. Additionally, there was a significant difference in the comfort category CAPA score between the 2 surgical groups, with the SEEG group reporting lower pain scores overall. This finding suggests that the reason the SEEG group received a significantly lower median of opioid medication throughout their stay is that the procedure itself resulted in less pain experienced overall. This supports our expectation that the minimally invasive procedure is less painful and may be a successful opioid reduction strategy. Wang et al28 also found a significant difference in pain scores between the 2 groups and demonstrated that the ECoG group had a higher overall pain score throughout their stay, though this was measured using the NRS rather than CAPA. They also did not find any correlation between the pain scores and amount of opioids used, which may suggest that surgical pain management strategies are mostly protocol driven and are not necessarily adaptive to the needs of the patient.
The use of non-narcotic pain medications is a first-line method in opioid reduction and significantly decreases the amount of opioids used in surgical patients. Specifically, NSAIDs are a mainstay in pain management. Despite many studies demonstrating the efficacy and safety of NSAIDs in the treatment of postoperative pain management, their use in neurosurgical patients has not been widely adopted. This is likely secondary to partial platelet inhibition that occurs with NSAIDs. Our study underlines the lack of NSAID use in neurosurgical patients, with low usage in both SEEG and ECoG cohorts. In fact, the median amount of NSAIDs used was 0 mg, demonstrating this avoidance of NSAIDs. Wang et al28 described their lack of NSAID use as well, stating that they did not use any NSAIDs in their postoperative patients. As neurosurgical providers, we should reconsider our stance on NSAIDs in postoperative pain management to further decrease the use of opioids in our patients. To increase their use and potentially decrease the use of opioids, a computed tomography scan could be obtained postoperatively to confirm the lack of intraoperative hemorrhage, and then NSAIDs could be prescribed with more confidence that hemorrhagic complications would not occur or be exacerbated.
Limitations
The main limitation of this study is that it is a single-center, retrospective review that introduces institutional and selection bias. Although we powered our study to identify a 30% decrease in MME use—which we found to be significant—we lacked sufficient power to identify other differences identified in this study, including correlation between pain scores and the amount of opioids used and a difference in pain scores between the 2 groups.
CONCLUSION
The results of this study imply that the use of opioid pain medications is lower in those patients undergoing SEEG procedures for seizure localization when compared with those undergoing ECoG. Although subdural grids and SEEG are not interchangeable, the considerable reduction in postoperative pain and opioid use with SEEG should play a role in surgical decision making.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Rolston was supported by the National Institute of Neurological Disorders and Stroke (K23 NS114178 and R21 NS113031). Dr Rolston has also done prior consulting work for Medtronic Inc and NeuroPace Inc.
Contributor Information
Jonathan P Scoville, Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, USA.
Evan Joyce, Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, USA.
Joshua Hunsaker, School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Jared Reese, School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Herschel Wilde, School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Amir Arain, Department of Neurology, Clinical Neurosciences Center, University of Utah, Salt Lake City, Utah, USA.
Robert L Bollo, Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, USA.
John D Rolston, Department of Neurosurgery, Clinical Neurosciences Center, University of Utah, Salt Lake City, USA; Department of Biomedical Engineering, University of Utah, Salt Lake City, Utah, Utah, USA.
COMMENT
This retrospective study focused on periprocedural pain in a cohort of patients with epilepsy undergoing invasive monitoring for drug-refractory epilepsy. The authors succeed to objectify implanters‘ observations and anecdotal reports that stereoelectroencephalography patients fare better with respect to pain perception and need for opioids. Oftentimes regarded as ‘expected conditions’, previous studies comparing various invasive monitoring techniques insufficiently appreciated patients’ discomforts during their epilepsy monitoring unit stay. The factor ‘pain’ should be kept in mind when the epilepsy surgery group discusses their implantation strategy. However, the ultimate decision whether to implant subdural grids or use stereoelectroencephalography should primarily be made on the grounds of which technique is most suitable to delineate the putative epileptogenic zone in each individual case.
Holger Joswig
Potsdam, Germany
REFERENCES
- 1. Kaiboriboon K, Malkhachroum AM, Zrik Aet al. Epilepsy surgery in the United States: analysis of data from the National Association of Epilepsy Centers. Epilepsy Res. 2015;116:105-109. [DOI] [PubMed] [Google Scholar]
- 2. Engel J. The current place of epilepsy surgery. Curr Opin Neurol. 2018;31(2):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwan P, Arzimanoglou A, Berg ATet al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077. [DOI] [PubMed] [Google Scholar]
- 4. Abou-Al-Shaar H, Brock AA, Kundu B, Englot DJ, Rolston JD.. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J Clin Neurosci. 2018;53:132-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iida K, Otsubo H. Stereoelectroencephalography: indication and efficacy. Neurol Med Chir. 2017;57(8):375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Englot DJ. A modern epilepsy surgery treatment algorithm: incorporating traditional and emerging technologies. Epilepsy Behav. 2018;80:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiebe S, Blume WT, Girvin JP, Eliasziw M.. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 8. Joswig H, Lau JC, Abdallat Met al. Stereoelectroencephalography versus subdural strip electrode implantations: feasibility, complications, and outcomes in 500 intracranial monitoring cases for drug-resistant epilepsy. Neurosurgery. 2020;87(1):E23-E30. [DOI] [PubMed] [Google Scholar]
- 9. Singh GK, Kim IE, Girmay Met al. Opioid epidemic in the United States: empirical trends, and a literature review of social determinants and epidemiological, pain management, and treatment patterns. Int J Matern Child Health AIDS. 2019;8(2):89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinez L, Ekman E, Nakhla N.. Perioperative opioid-sparing strategies: utility of conventional NSAIDs in adults. Clin Ther. 2019;41(12):2612-2628. [DOI] [PubMed] [Google Scholar]
- 11. Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E.. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67(5):1-76. [PubMed] [Google Scholar]
- 12. Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN.. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiore K. Opioid Crisis: Scrap Pain as 5th Vital Sign? Published April 13, 2016. Accessed January 24, 2020. https://www.medpagetoday.com/publichealthpolicy/publichealth/57336. [Google Scholar]
- 14. Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, Manning MW.. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med. 2018;7(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomes T, Khuu W, Martins Det al. Contributions of prescribed and non-prescribed opioids to opioid related deaths: population based cohort study in Ontario, Canada. BMJ. 2018;362:k3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brummett CM, Waljee JF, Goesling Jet al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee L, Caplan R, Stephens Let al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659-665. [DOI] [PubMed] [Google Scholar]
- 18. Gaskell H, Derry S, Moore RA, McQuay HJ.. Single dose oral oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;2009(3):CD002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark DJ, Schumacher MA.. America's opioid epidemic: supply and demand considerations. Anesth Analg. 2017;125(5):1667-1674. [DOI] [PubMed] [Google Scholar]
- 20. Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E.. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1733-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karlow N, Schlaepfer CH, Stoll CRTet al. A systematic review and meta-analysis of ketamine as an alternative to opioids for acute pain in the emergency department. Acad Emerg Med. 2018;25(10):1086-1097. [DOI] [PubMed] [Google Scholar]
- 22. Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. [DOI] [PubMed] [Google Scholar]
- 23. McDonald EL, Daniel JN, Rogero RGet al. How does perioperative ketorolac affect opioid consumption and pain management after ankle fracture surgery? Clin Orthop Relat Res. 2020;478(1):144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepherd DM, Jahnke H, White WL, Little AS.. Randomized, double-blinded, placebo-controlled trial comparing two multimodal opioid-minimizing pain management regimens following transsphenoidal surgery. J Neurosurg. 2018;128(2):444-451. [DOI] [PubMed] [Google Scholar]
- 25. Bauer DF, Waters AM, Tubbs RSet al. Safety and utility of scheduled nonnarcotic analgesic medications in children undergoing craniotomy for brain tumor. Neurosurgery. 2010;67(2):353-356. [DOI] [PubMed] [Google Scholar]
- 26. Yi JL, Porucznik CA, Gren LHet al. The impact of preoperative mindfulness-based stress reduction on postoperative patient-reported pain, disability, quality of life, and prescription opioid use in lumbar spine degenerative disease: a pilot study. World Neurosurg. 2019;121:e786-e791. [DOI] [PubMed] [Google Scholar]
- 27. Arumugam S, Lau CS, Chamberlain RS.. Use of preoperative gabapentin significantly reduces postoperative opioid consumption: a meta-analysis. J Pain Res. 2016;9:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y-C, Grewal SS, Goyal Aet al. Comparison of narcotic pain control between stereotactic electrocorticography and subdural grid implantation. Epilepsy Behav. 2020;103(Pt A):106843. [DOI] [PubMed] [Google Scholar]
- 29. Twining J, Padula C.. Pilot testing the Clinically Aligned Pain Assessment (CAPA) measure. Pain Manage Nurs. 2019;20(5):462-467. [DOI] [PubMed] [Google Scholar]
- 30. Topham D, Drew D.. Quality improvement project: replacing the numeric rating scale with a Clinically Aligned Pain Assessment (CAPA) Tool. Pain Manage Nurs. 2017;18(6):363-371. [DOI] [PubMed] [Google Scholar]
- 31. Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA.. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia. 2013;54(5):828-839. [DOI] [PubMed] [Google Scholar]
- 32. Sacino MF, Huang SS, Schreiber J, Gaillard WD, Oluigbo CO.. Is the use of stereotactic electroencephalography safe and effective in children? A meta-analysis of the use of stereotactic electroencephalography in comparison to subdural grids for invasive epilepsy monitoring in pediatric subjects. Neurosurgery. 2019;84(6):1190-1200. [DOI] [PubMed] [Google Scholar]