Abstract
B cells have a prominent role in the pathogenesis of systemic lupus erythematosus (SLE). They are mediators of inflammation through the production of pathogenic antibodies that augment inflammation and cause direct tissue and cell damage. Multiple therapeutic agents targeting B cells have been successfully used in mouse models of SLE; however, these preclinical studies have led to approval of only one new agent to treat patients with SLE: belimumab, a monoclonal antibody targeting B cell–activating factor (BAFF). Integrating the experience acquired from previous clinical trials with the knowledge generated by new studies about mechanisms of B cell contributions to SLE in specific groups of patients is critical to the development of new treatment strategies that will help to improve outcomes in patients with SLE. In particular, a sharper focus on B cell differentiation to plasma cells is warranted.
Introduction
Systemic lupus erythematosus (SLE) is a potentially devastating autoimmune disease. It is a leading cause of death in young women in the United States, predominantly affecting those of Hispanic and African American ancestry (1). Although recent advances in diagnosis and treatment have led to substantial improvement in prognosis, the disease still has considerable morbidity and associated costs (2, 3). The currently available treatment options are not free of complications, and it is estimated that the percentage of deaths attributed to disease activity is similar to the percentage that can be attributed to infections secondary to immunosuppressive medications (4).
Unmet needs in patients with SLE include uncontrollable disease, recurrent flares, need for long-term immunosuppressive treatment, increased rates of infections, damage accrual that impairs quality of life, and diminished long-term survival. For these reasons, we need to develop new therapeutic strategies to treat SLE. These strategies must be based on knowledge about the mechanisms that drive inflammation and damage.
Despite the fact that some patients with SLE have low titers of autoantibodies, autoantibodies, especially of the IgG isotype, are considered the main effectors of SLE inflammation and damage. Anti–double-stranded DNA (anti-dsDNA) antibodies, in particular, are present in the kidneys of patients with lupus nephritis (LN) and in the skin. Transfer of anti-dsDNA antibodies from lupus-prone mice or SLE patients to healthy animals can cause nephritis (5–7) and induce the expression of inflammatory and profibrotic genes in renal cells (8, 9). Antibodies and immune complexes can induce local inflammation in the endothelium and interstitium, which in turn contributes to the inflammatory response (10). Evidence also supports CNS pathogenicity by a subset of anti-dsDNA antibodies that cross-reacts with the NMDA receptor (NMDAR) (11, 12). Other antibodies are also pathogenic: anti-phospholipid antibodies induce thrombosis (13, 14); anti–ribosomal P antibodies contribute to CNS manifestations (15); and anti-ribonucleoprotein (anti-RNP) antibodies induce neutrophil death by NETosis, which leads to production of type I interferon (IFN) by plasmacytoid dendritic cells (pDCs) (16). Supporting the relevance of this phenomenon, an association between IFN expression, anti-RNP antibodies, and kidney disease has been described (17). Importantly, it is not clear for all these antibodies whether affinity or fine specificity is critical for pathogenicity.
Antibodies are exclusively produced by plasma cells (PCs), which are terminally differentiated B cells. Thus, B cells are an obvious therapeutic target in SLE. However, B cells and antibodies also have critical functions in normal host defense against pathogens, and some autoantibodies, especially of the IgM isotype, have a protective role against the development of autoimmunity. IgM antibodies assist in the clearance of cellular debris. In the context of complement C1q and LAIR-1 activation, they inhibit inflammatory responses (18–21).
B cell function in health and SLE
B cells have functions in addition to being the precursors of PCs. B cells are important antigen-presenting cells (APCs) and are particularly instrumental in activating autoreactive T cells by presenting novel peptides of self-antigens (22–24). B cells function as APCs to drive the activation of autoreactive T cells in many autoimmune diseases, and this is presumed to be the basis for the benefit of B cell depletion therapy in multiple sclerosis and seronegative rheumatoid arthritis (25). Lupus-prone mice expressing a mutant transgene that allows the expression of surface immunoglobulin, but blocks the secretion of antibodies, develop nephritis (26); while this observation suggests that alternative functions of B cells in addition to secretion of autoantibodies are relevant in SLE pathogenesis, there is no clear evidence that B cells are important APCs in SLE.
B cells make cytokines that can help support an immune response. For example, B cell–derived TNF is critical for the function of follicular dendritic cells in the germinal center (GC) response (27). A population of B cells with regulatory function (Bregs) has been also identified. The identification and presumably the function of these cells depend on their increased production of IL-10. Bregs can suppress inflammatory immune responses in mouse models of inflammatory arthritis, experimental allergic encephalitis, and lupus in an IL-10–dependent fashion (28, 29). The induction and suppressive activity of Bregs have been reported to be altered in SLE patients (30). However, IL-10 is considered to be pathogenic in SLE (31), and anti–IL-10 antibody has been used with success in a limited number of patients with SLE (32). Thus, the antiinflammatory role of IL-10–producing B cells and their relevance in SLE have yet to be defined.
B cell development
B cells are derived from a common lymphoid progenitor that also gives rise to T cells and NK cells. The main characteristic that differentiates B cells from other lymphoid cells is the expression in each cell of a unique immunoglobulin heavy and light chain, which allows recognition of antigen and is part of the B cell’s signaling system. The diversity of the antibody repertoire derives from VDJ (or VJ) recombination and somatic hypermutation (33). All B cells initially express IgM with or without IgD; class switch recombination causes a change in the Ig isotype to IgG, IgA, or IgE, with each isotype having different functional characteristics.
The diversity of the B cell receptor (BCR) repertoire enables recognition of numerous pathogens but also generates a large number of immature B cells that recognize self-components, termed autoreactive B cells (34). As B cells mature, the percentage of autoreactive B cells is gradually reduced. This reduction is achieved by various tolerance mechanisms, including receptor editing, deletion, and anergy. Nonetheless, the mature B cell compartment still has a considerable percentage of autoreactive B cells (35, 36). The fact that IgG anti-dsDNA or anti-RNP antibodies are not detectable in the healthy population, while IgM autoantibodies are, highlights the importance of the peripheral tolerance checkpoints that prevent these autoreactive B cells from differentiating into IgG-producing PCs (37–40).
B cell subsets.
There are different types of mature cells within the B cell lineage: B-1, marginal zone (MZ), and follicular cells. All types can differentiate into PCs, but they differ in many relevant characteristics, including their requirements for activation and differentiation and their role in the normal immune response in healthy subjects. B-1 cells are thought to represent a distinct lineage, and while they can produce autoantibodies, they are not thought to be a major contributor to SLE pathogenesis (41).
MZ and follicular B cells derive from immature B cells egressing from the bone marrow, termed transitional B cells (42). Transitional B cells are dependent for maturation on B cell–activating factor (BAFF) (43). Increased levels of BAFF allow autoreactive B cells to mature to immunocompetence. An expansion of transitional B cells has been reported in patients with SLE (44). This may relate to elevated levels of BAFF (45), which can be secondary either to disease activity or to therapy.
In mice, MZ B cells are localized within the MZ in the spleen, where they can serve as a first line of defense against antigens that arrive through the hematogenous route (46). They are highly responsive to TLR activation and costimulatory molecules, and rapidly differentiate into PCs (46–52) without a requirement for cognate T cell help. MZ B cells can also present antigen to T cells in the follicles and initiate T cell activation (53). In humans, IgM+CD27+ peripheral B cells are suggested to be a circulating population of MZ B cells (54, 55).
Follicular B cells have the most diverse repertoire among B cells and are the main contributor to the T cell–dependent responses, as well as to the GC response and memory B cell development.
B cell activation
After an encounter with antigen, follicular B cells migrate to the T-B border in lymph nodes or the spleen, where they interact with T cells (56, 57) that provide the costimulation and cytokines that contribute to B cell activation, proliferation, and differentiation. BCR engagement without costimulation induces B cell anergy. B cells receiving adequate signals become short-lived PCs in an extrafollicular response or enter into a GC response in which long-lived PCs (LLPCs) and memory cells are generated.
B cells in SLE have an increased response after BCR ligation (58, 59). This hyperresponsiveness can be intrinsic to the B cell (35), but also can be induced by the external milieu, as many molecules modify the threshold for BCR activation (60–62). Endosomal TLRs, TLR7 and TLR9, are activated by nucleic acids and enhance B cell activation. A higher expression of TLR9 in memory B cells and PCs has been observed in blood from patients with SLE and is associated with disease activity and the presence of anti-dsDNA antibodies (63–65).
Cytokines, such as IFNs, IL-21, and BAFF, can also contribute to B cell activation (66–68). Type I IFNs are considered central in SLE pathogenesis (69), and high levels of type I IFNs favor an extrafollicular over a GC response (41). BAFF and IL-21 stimulate B cell survival and proliferation and can induce IgM-to-IgG class switching (49, 70–72). Among cytokines, IL-21 is considered the strongest inducer of PC differentiation (73). IL-6 also induces PC differentiation (74); additionally, IL-6 induces IL-21 production by CD4+ T cells (75). B cells are also subject to inhibitory signals: FcγRIIb, an inhibitory receptor that modulates B cell activation, is the only Fcγ receptor expressed on B cells. Although the mechanisms are not fully understood, impaired function of FcγRIIb is associated with SLE in mouse models and in humans (76).
Memory B cells initiate the secondary immune response, which arises faster than a primary response, and leads to higher titers of IgG antibodies with greater specificity and increased affinity for the antigen (77). Many anti-dsDNA antibodies possess features of secondary response antibodies (78–81). Delayed recovery of memory B cells in SLE patients who received B cell depletion therapy has been linked to better responses (82). Recently, a subpopulation of B cells called ABCs (83) has been described. This population is increased in patients with autoimmune disease. Its origin is related to B cell activation with TLR7, IL-21, and IFN-γ (84). ABCs are reported to be enriched in autoreactivity, and some evidence suggests they are precursors of PCs in patients with SLE. The presence of high numbers of ABCs in peripheral blood is associated with LN (85, 86).
The subpopulation of B cells that are the precursors of the autoreactive PCs in patients with SLE has not been clearly defined. Using BCR sequencing to identify potential precursors of PCs, clones with similar BCRs to PCs were found in the naive, ABC, and memory compartments in patients with SLE (86, 87). There is also evidence suggesting that either the extrafollicular or the GC pathway is preferentially activated in patients with increased circulating plasmablasts (88).
Tolerance in SLE: selection versus activation defects
IgG autoantibodies in blood precede the clinical onset of SLE and are present in all patients at diagnosis. The origin and defects that lead to the production of autoantibodies have not been clearly established and may vary between patients (89). Some studies suggest an aberrant selection of B cells with defects in antigen-specific central tolerance or defective B cell anergy (38, 90–97), while other studies suggest that the major alteration in SLE is polyclonal activation and increased IgG PC differentiation (98, 99). This difference is not trivial, as the therapeutic strategies might be different in each case. Antigen-based therapies can be used in the case of selection defects; in contrast, in the case of abnormal polyclonal activation, the treatment might be focused on blocking the differentiation of B cells into PCs.
B cell–based therapeutics: approaches and experience with mouse models
Much of our understanding of SLE pathogenesis and treatment comes from mouse models. These models have been used to test therapeutic strategies prior to clinical trials. A full review of the available mouse models of SLE is beyond the scope of this article and can be found elsewhere (100, 101). The two strains that are more commonly used are NZB/NZWF1 (also known as NZB/W) and MRL/lpr. NZB/W mice develop splenomegaly and hypergammaglobulinemia with anti–nuclear antigen (ANA) and anti-dsDNA antibodies. Their clinical manifestations are immune complex glomerulonephritis and vasculitis (102). MRL/lpr mice have a complex genetic background but harbor a mutation in the FAS gene that reduces apoptosis in B and T cells and is considered a fundamental catalyst of disease. MRL/lpr mice develop prominent splenomegaly and lymphadenopathy and multiple autoantibodies (including anti-DNA, anti-SM, anti-Ro, and anti-La); their clinical manifestations are more diverse and include glomerulonephritis, arthritis, vasculitis, and skin rash (100).
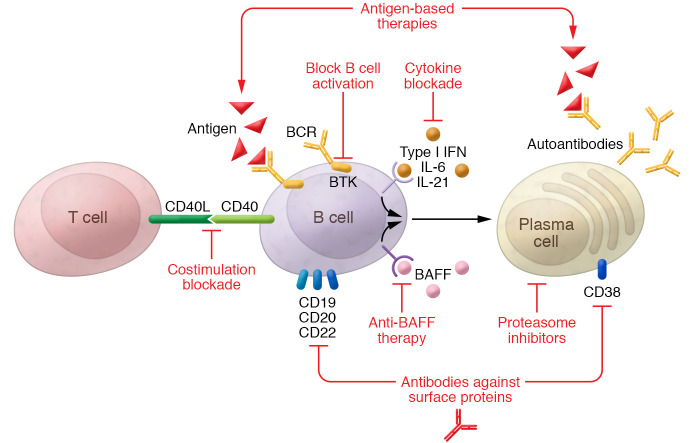
There are multiple ways to interfere with B cell inflammatory function in SLE (Figure 1). These strategies can be classified as (a) B cell depletion, (b) anti-BAFF therapy, (c) therapy directed against PCs, (d) interference with B cell costimulation and activation, and (e) antigen-based therapies, each aimed to affect B cells with pathogenic specificities.
Figure 1. Different strategies to interfere with B cell proinflammatory function in patients with SLE.
Strategies include B cell and plasma cell depletion (e.g., antibodies directed at surface proteins or proteasome inhibitors), selective depletion of autoreactive B cells (e.g., BAFF inhibition), antigen-based therapies that block pathogenic antibodies, and prevention of B cell activation (e.g., blockade of B-T cell costimulation or B cell–activating cytokines).
B cell depletion.
A straightforward, but nonspecific, way to interfere with B cell function is by directly diminishing their number. There are multiple mechanisms to induce B cell depletion. Cyclophosphamide, which has been a mainstay of SLE therapy for decades, preferentially targets B cells.
Cytotoxic antibodies directed against markers present on the B cell surface are a more recently explored approach. As they mature, B cells express different programs of cell surface markers; thus, different subpopulations of B cells will be affected according to the molecule used as a target for B cell depletion. CD20 is expressed on the surface of most mature B cells, with the exception of PCs. The exact function of CD20 has not been clearly established. This receptor is the target of rituximab, a chimeric IgG1 monoclonal antibody. In lupus-prone mice that express a transgenic human CD20, rituximab was able to induce B cell depletion and ameliorate the manifestations of disease (103). Early administration of rituximab in young led to a long-term delay in disease onset. The mechanism of action included reduction in T cell activation (104). More recently, the use of chimeric antigen receptor T cells has been proposed as an alternative method to induce B cell depletion (105).
Selective depletion of autoreactive B cells by targeting of BAFF.
BAFF is part of the TNF family. It is secreted by activated T cells, monocytes, macrophages, and dendritic cells and signals through three receptors: BAFF receptor (BAFF-R), transmembrane activator and calcium modulator interactor (TACI), and B cell maturation antigen (BCMA). The administration of exogenous BAFF increases the levels of serum immunoglobulin (68), and BAFF-transgenic mice develop an SLE-like disease (106, 107). Deletion of the BAFF gene in lupus-prone mice prevents initiation of disease, and neutralization of BAFF improves lupus manifestations (108–112).
Excess BAFF rescues self-reactive early B cells from deletion (113). In murine studies, elevated levels of BAFF promote maturation of autoreactive B cells, and reduction of BAFF levels following B cell depletion reduces the number of autoreactive cells in the reconstituted B cell repertoire (114). The efficacy of anti-BAFF therapy is independent of an intensive reduction in total B cell numbers (115). As BAFF is most relevant for protecting autoreactive B cells, anti-BAFF therapy has a certain degree of specificity against this population.
Therapy directed against PCs.
B cell depletion therapy with anti-CD20 antibodies spares LLPCs. It has been reported that the longevity of these cells is more than 10 years (116), highlighting their importance as long-term producers of antibodies or autoantibodies. Proteasome inhibitors cause accumulation of misfolded proteins within the endoplasmic reticulum, leading to apoptosis (117). Proteasome inhibitors affect predominantly the PC population, because of their extremely high rate of antibody synthesis. pDCs also have a high rate of protein synthesis and are also affected, causing a reduction in type I IFN levels. This may also be therapeutic, in part by diminishing B cell activation (118). In lupus-prone mice, proteasome inhibitors reduced the titers of autoantibodies and improved nephritis (118).
B cell activation and costimulation blockade.
Because of the relevance of the B-T cell costimulation pathways to autoantibody production, they have been considered a potential target for many years. Studies conducted 25 years ago already showed a beneficial effect of CD40/CD40L blockade therapy in lupus-prone mice (119, 120), with both strategies characterized by reduced antibody titers and improved nephritis (121). However, clinical trials with anti-CD40L antibody in lupus patients were terminated because of thromboembolic events (122). This effect was not seen in mice (123), which precluded earlier detection of the phenomenon. Second-generation molecules for CD40/CD40L blockade with low prothrombotic effect have been developed (121).
IFN is a potent stimulator of B cells. An IFN signature has been described in patients with SLE; this signature correlates with disease activity in some studies (69). In most mouse models of SLE, including the NZB/W and MRL/lpr strains, overexpression of IFN-induced genes is observed but occurs with less magnitude than in humans, with the exception of the pristane-induced model of SLE (124). In some lupus-prone strains, treatment with anti–type I IFN receptor antibody (125) or deficiency of type I IFN receptor (126–128) increased survival and improved autoimmune manifestations, including levels of autoantibodies. Interestingly, in MRL/lpr mice, deletion of the type I IFN receptor increased autoantibody titers and worsened organ damage (129).
Use of an anti–IL-21 antibody reduced antibody titers and delayed glomerulonephritis progression in lupus-prone mice. This effect was associated with a reduction in GC B cells and plasmablasts (130). Anti–IL-6 and anti–IL-6 receptor antibodies caused reduction in anti-dsDNA titers and improvement in nephritis (131, 132). Administration of synthetic oligodeoxynucleotides with immunoregulatory sequences that specifically block TLR7 or TLR7/9 activation to lupus-prone mice improved nephritis and caused reduction in the titers of autoantibodies (133, 134). Bruton’s tyrosine kinase (Btk) is an enzyme that modulates signaling downstream of the BCR and is required for BCR signaling. Btk inhibitors have shown improvement of nephritis and reduction of autoantibodies in multiple mouse models of lupus (135–138). Notably, Btk inhibitors are already approved for use in hematologic malignancies.
Antigen-based therapies.
Use of antigen conjugates may, in theory, block pathogenic autoantibodies from interacting with their target. Also, in the absence of costimulation, recognition and binding of a cognate antigen by the membrane-bound antibody molecule on the surface of B cells might induce B cell tolerance.
There are examples in animal models of successful use of “tolerizing molecules.” The administration to BXSB male lupus-prone mice of polyethylene glycol with tetrameric oligonucleotides, a molecule that mimics DNA, decreased the number of anti-dsDNA–producing cells and significantly increased survival (139). Administration of nucleosomal peptides to SNF1 mice delayed the onset of nephritis and improved survival (140); in the mechanistic analysis, an increment of regulatory T cells was shown, and a direct effect on B cells was not investigated. Finally, peptides that bind anti-DNA antibodies can prevent their pathogenicity in vivo (141, 142).
B cell–based treatment in patients with SLE
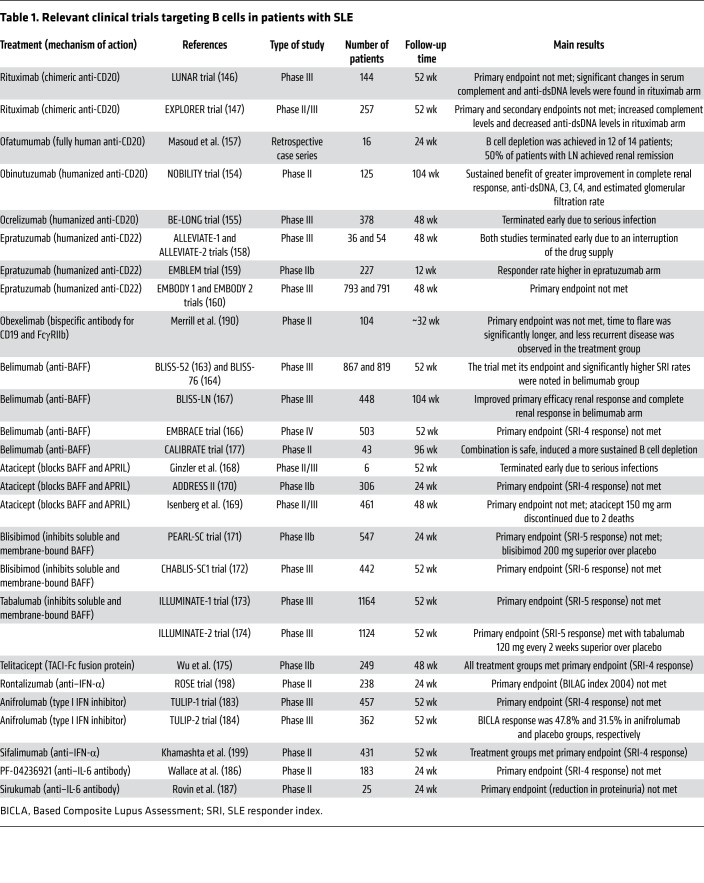
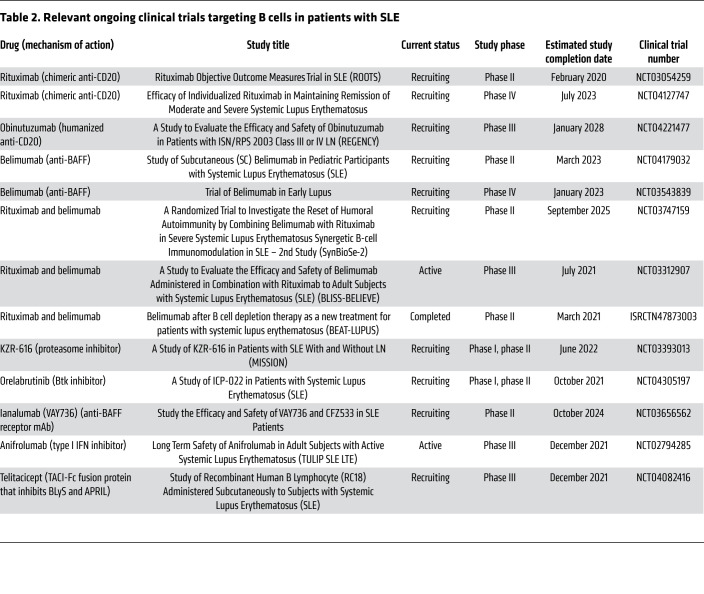
Information about relevant finished and ongoing clinical trials targeting B cells in patients with SLE is summarized in Tables 1 and 2.
Table 1. Relevant clinical trials targeting B cells in patients with SLE.
Table 2. Relevant ongoing clinical trials targeting B cells in patients with SLE.
B cell depletion.
As mentioned, one of the earliest therapies for SLE, albeit not FDA approved, is cyclophosphamide, which targets B cells as well as other lymphoid cells. There is also evidence that mycophenolate mofetil causes a reduction of circulating plasmablasts (143), among other therapeutic mechanisms.
Rituximab was approved for rheumatoid arthritis in 2006 and since then has been used off label in patients with SLE. A benefit of rituximab was suggested in multiple nonrandomized observational studies (144, 145). Two randomized, double-blind, placebo-controlled clinical trials failed to show a beneficial effect of rituximab. In the LUNAR trial, 144 patients with LN were randomized to receive rituximab or placebo with concomitant mycophenolate and steroids. Treatment with rituximab did not improve clinical efficacy, even though statistically significant changes in serum complement and anti-dsDNA levels were found in comparison with placebo (146). In the EXPLORER trial, 257 patients with moderate to severe nonrenal SLE were randomized to receive rituximab or placebo. No differences were observed in the clinical response at week 24. There were increased complement levels and decreased anti-dsDNA levels in the rituximab arm (147). Despite the findings of the EXPLORER and LUNAR trials, current guidelines from the European League Against Rheumatism and the European Renal Association, and from the American College of Rheumatology, recommend the use of rituximab as a second- or third-line option in patients with LN (148, 149).
Clinical response in patients with SLE who received rituximab correlates with the degree (150, 151) and duration (152) of B cell depletion. Thus, there has been interest in higher-affinity and higher-activity anti-CD20 antibodies. Obinutuzumab, a type 2 glycoengineered anti-CD20 monoclonal antibody, has a more potent cytotoxic effect, probably because of more efficient engagement of Fcγ receptors on NK cells and neutrophils (153). Obinutuzumab was evaluated in a placebo-controlled randomized trial including 125 patients with active class III or IV LN on background corticosteroids and mycophenolate. Patients who received obinutuzumab demonstrated a sustained benefit in renal response, anti-dsDNA titers, and C3 and C4 levels, with no unexpected safety concerns. The benefits were sustained at 104 weeks (154).
Other antibodies that target CD20 have been studied in SLE. Ocrelizumab failed to meet its primary outcome in a randomized trial of patients with LN, and raised safety concerns due to increased infection risk (155). Ofatumumab was reported to be effective in case series of patients with SLE (156, 157). Epratuzumab, an anti-CD22 antibody that engages an inhibitory receptor on B cells, showed some promising results in phase II studies that were not confirmed in a phase III clinical trial (158–160).
Anti-BAFF therapies.
In patients with SLE, serum levels of BAFF are elevated, and these levels correlate with disease severity (161, 162). Belimumab is an IgG1 human monoclonal antibody directed against soluble BAFF. It interferes with the binding of BAFF with BCMA, TACI, and BAFF-R. The efficacy of belimumab in SLE has been tested in multiple trials, and it is now approved by the FDA for treatment of both adult and pediatric SLE.
The BLISS-52 and BLISS-76 trials randomized 867 and 819 patients, respectively, to receive belimumab or placebo with background treatment. Both trials met their clinical endpoints (163, 164). There were modest reductions in autoantibody titers and reduced flares at 76 weeks, perhaps related to lower antibody titers. Belimumab efficacy was confirmed in populations from China, Japan, and South Korea (165). The EMBRACE study, conducted to evaluate the efficacy of belimumab in African American patients with SLE, did not achieve its primary endpoint; however, significant improvement was found in patients who had high disease activity (166). A recent trial, BLISS-LN, randomized 448 patients with active LN to receive belimumab or placebo, plus standard therapy. Significantly more patients in the belimumab arm had renal response at week 104 than those who received placebo (167).
A trial of atacicept, which blocks BAFF and the related molecule APRIL (168–170), was terminated because of increased infections. Blisibimod, an inhibitor of soluble and membrane-bound BAFF (171, 172), tabalumab, a human molecular antibody that binds soluble and membrane-bound BAFF (173, 174), and telitacicept, a recombinant fusion protein constructed with the extracellular domain of the TACI receptor, thereby binding both BAFF and APRIL (175), have been tested in patients with SLE without success. Their failure despite the success of belimumab may reflect both the modest effect of belimumab and differences in trial design.
Combination therapy.
Based on the observations that BAFF levels rise after induction of B cell depletion with rituximab (176), and that B cell reconstitution in a milieu of low BAFF leads to a reduction in the number of autoreactive B cells (114), a clinical trial testing the sequential administration of rituximab and belimumab was performed. The CALIBRATE study (177) included 43 patients with recurrent or refractory LN who were randomly assigned to be treated with rituximab, cyclophosphamide, and glucocorticoids or the same treatment followed by belimumab. The trial demonstrated that the combination is safe, and induced a more sustained B cell depletion. Addition of belimumab diminished the maturation of transitional to naive B cells, and enhanced the negative selection of autoreactive B cells. The trial did not find any significant clinical benefit of adding belimumab to treatment; however, the study was not powered to ascertain clinical outcome.
Theoretically, the order in which the combination of rituximab and belimumab is administered might have differential effects. Belimumab reduces the number of B cells in lymphoid tissues (178), so initial administration of belimumab might cause mobilization of memory B cells into the circulation, where they would be more susceptible to rituximab-mediated cell death. This strategy of administering belimumab followed by rituximab is being examined in a clinical trial of nonrenal SLE, the BLISS-BELIEVE study. Rituximab and belimumab combinations are also currently being evaluated in the BEAT-LUPUS and SynBioSe-2 trials.
Therapy directed against PCs.
Proteasome inhibitors have been tested in trials with small numbers of patients with SLE. Bortezomib has shown some clinical responses in patients with refractory SLE; however, a high percentage of patients developed severe adverse effects (179, 180), and for this reason, proteasome inhibitors are not part of the arsenal that is commonly used in patients with SLE. New-generation proteasome inhibitors that are relatively selective for immune cells also have significant toxicity.
Daratumumab is a human monoclonal antibody that targets CD38, a molecule expressed on PCs and plasmablasts (although not exclusive to these populations). Administration of daratumumab causes depletion of PCs and is approved for use in multiple myeloma. Successful use of daratumumab was reported in two patients with life-threatening manifestations of SLE (181). The clinical response was associated with depletion of LLPCs and reduction of type I IFN activity.
B cell activation and costimulation blockade.
Multiple trials have been performed with anti-IFN therapy in patients with SLE. While this therapy might be expected to reduce PC differentiation (182), only small reductions in autoantibodies were seen (183, 184). In the TULIP-1 study, a phase III randomized trial of anifrolumab, a human monoclonal antibody against type I IFN receptor subunit 1, the primary clinical endpoint was not met (183). A second trial of anifrolumab, in which the primary clinical endpoint was selected based on the results from the first trial, showed efficacy; however, only a modest reduction in autoantibodies was observed (184). This phenomenon might reflect stable continued autoantibody production by LLPCs.
Tocilizumab, an anti–IL-6 antibody, caused reduction of PCs and memory cells in patients with SLE (185); however, two phase II clinical trials with anti–IL-6 antibodies failed to meet their primary outcomes (186, 187). A monoclonal antibody that interferes with IL-21 activity is being tested in a phase I/II study in patients with SLE (188). Initial trials in patients with SLE showed encouraging results for CD40/CD40L blockade, including reduced number of circulating PCs and anti-dsDNA antibodies (189). After the setback caused by the increased rate of thrombotic events with the first-generation antibodies targeting this pathway, second-generation molecules without thrombotic risk are currently being tested (121).
Iberdomide, a cereblon ligand, increases the ubiquitination and subsequent degradation of the transcription factors Ikaros and Aiolos in the proteasome. The genes encoding these transcription factors are risk alleles for SLE. Ikaros is necessary for the development of B cells and pDCs, and Aiolos is necessary for PC differentiation. Iberdomide affects both total B cell number and PC differentiation. Preliminary results showed a reduction in B cell number, with the higher dose inducing a significant clinical response compared with placebo (154). Whether there is also an effect on PC differentiation is not clear.
Dual-specificity antibodies.
Obexelimab, a bispecific antibody that targets CD19 and simultaneously acts as an agonist of the inhibitory receptor FcγRIIb, was studied in a randomized phase II trial of 104 patients with SLE. In the obexelimab group, the time to flare was significantly longer and patients had less recurrent disease after treatment. The primary endpoint was not met, but a subgroup of patients with higher expression of genes associated with B cell and PC activation improved in comparison with placebo (190).
Antigen-based therapies.
The experience with these molecules in patients with SLE has been limited. In a clinical trial, abetimus (LJP-394), a molecule that contains four strands of dsDNA bound to a carrier, caused reduction of the anti-dsDNA antibody levels but did not prolong the time to renal flares (191). It is not clear whether the reduction in titer reflected B cell tolerance or the generation and subsequent removal of immune complexes.
Perspectives and future directions
Selection of B cells as a target for therapy in SLE has a solid basis according to our knowledge of the disease. It is surprising, therefore, that BAFF inhibition is the only approved therapy that targets B cells and that this strategy has been successful with only one agent, belimumab. It is possible that trial design may have contributed to some trial failures. It is important to remember that success in a clinical trial requires achieving a predetermined effect size in a predetermined number of patients.
It has not been possible to show an association between changes in autoantibodies and clinical responses in clinical trials. This highlights the fact that we do not know the extent to which autoantibody titers need to be reduced to lead to diminished disease activity or whether a reduction to a threshold level is required. Other features of the antibodies besides titers are involved in immunogenicity, such as affinity and glycosylation state (192). The determination of these characteristics is labor intensive, and they have not been explored in clinical trials in SLE; however, they might represent a mechanism by which treatment alters antibody pathogenicity and should be considered in future trials.
While therapies targeting B cells have been disappointing in clinical trials, and those targeting PCs hazardous, none of the currently available therapeutic options have focused specifically on PC differentiation. We have demonstrated that abnormal PC differentiation might represent a critical checkpoint in patients with SLE. SLE patients have a similar frequency of ANA reactivity in all B cell compartments, including PCs, when compared with healthy subjects, suggesting no defect in antigen-specific tolerance. These patients have, however, more IgG PCs. Thus, they have more autoreactive IgG PCs and higher serum titers of IgG ANAs. This suggests an increased differentiation of IgG PCs (36). Thus, targeting of pathways to reduce PC differentiation should be further explored. Some medications currently under study, such as iberdomide and Btk inhibitors, reduce PC differentiation. The hope would be that these treatments can dampen autoreactivity without causing global immunosuppression.
Furthermore, B cell activation can occur through an extrafollicular or GC pathway. Both pathways are considered to contribute to autoantibody production in patients with SLE (87, 193). Data from our laboratory suggest that SLE patients with increased circulating plasmablasts have different patterns of antigen-experienced autoreactive B cells and can be classified as having a predominant GC or a predominant extrafollicular response (88). The molecules that are differentially involved in each of these pathways are not clearly defined, but studies to identify them are currently ongoing. These molecules might represent a therapeutic tool for precision medicine.
Heterogeneity of SLE has been proposed as a major cause of failure in clinical trials. Microarray analysis and RNA sequencing (RNA-Seq) studies have allowed an interrogation of the transcriptome of immune cells in patients, and more recently, single-cell RNA-Seq has further increased the resolution of this analysis. Using these technologies, an IFN signature was described in SLE almost two decades ago (194, 195). A plasmablast signature that correlates with disease activity and a neutrophil signature that is associated with LN have also been described (196, 197) and are mutually exclusive (196). We have previously proposed that in some patients, myeloid cells are the drivers of SLE, while in others, SLE is driven by B cells. Indeed, a study of risk alleles in SLE showed that they are predominantly expressed in either myeloid cells or B cells. It may be that only those patients with a B cell–intrinsic pathway to SLE will benefit in the long term from a B cell–directed therapy. If so, clinical trials that do not select for patients with intrinsic B cell hyperresponsiveness may be underpowered for clinical efficacy. It would be a shame to discard potentially useful therapeutics because of trials that do not select for those patients with pathways of disease pathogenesis that are targeted by the therapeutic. A better understanding of SLE patient subsets is critical; in some, B cell–targeted therapies, especially those that block PC differentiation, may have long-term benefit.
Acknowledgments
The work in this manuscript was supported in part by a grant from the NIH (5U19AI144306).
Version 1. 06/15/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(12):e149095.https://doi.org/10.1172/JCI149095.
Contributor Information
Yemil Atisha-Fregoso, Email: yatishafre@northwell.edu.
Bahtiyar Toz, Email: bahtiyartoz@gmail.com.
Betty Diamond, Email: bdiamond@nshs.edu.
References
- 1.Yen EY, Singh RR. Brief report: Lupus—an unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol. 2018;70(8):1251–1255. doi: 10.1002/art.40512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber MRW, Clarke AE. Socioeconomic consequences of systemic lupus erythematosus. Curr Opin Rheumatol. 2017;29(5):480–485. doi: 10.1097/BOR.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 3.Carter EE, et al. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12(10):605–620. doi: 10.1038/nrrheum.2016.137. [DOI] [PubMed] [Google Scholar]
- 4.Tektonidou MG, et al. Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis. 2017;76(12):2009–2016. doi: 10.1136/annrheumdis-2017-211663. [DOI] [PubMed] [Google Scholar]
- 5.Tsao BP, et al. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J Immunol. 1992;149(1):350–358. [PubMed] [Google Scholar]
- 6.Vlahakos DV, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41(6):1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 7.Raz E, et al. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142(9):3076–3082. [PubMed] [Google Scholar]
- 8.Qing X, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54(7):2198–2210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- 9.Neuwirth R, et al. Evidence for immunoglobulin Fc receptor-mediated prostaglandin2 and platelet-activating factor formation by cultured rat mesangial cells. J Clin Invest. 1988;82(3):936–944. doi: 10.1172/JCI113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores-Mendoza G, et al. Mechanisms of tissue injury in lupus nephritis. Trends Mol Med. 2018;24(4):364–378. doi: 10.1016/j.molmed.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 11.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 12.Fragoso-Loyo H, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS One. 2008;3(10):e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierangeli SS, et al. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost. 1995;74(5):1361–1367. doi: 10.1055/s-0038-1649940. [DOI] [PubMed] [Google Scholar]
- 14.Piona A, et al. Placental thrombosis and fetal loss after passive transfer of mouse lupus monoclonal or human polyclonal anti-cardiolipin antibodies in pregnant naive BALB/c mice. Scand J Immunol. 1995;41(5):427–432. doi: 10.1111/j.1365-3083.1995.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi MY, et al. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun Rev. 2020;19(3):102463. doi: 10.1016/j.autrev.2020.102463. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirou KA, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 18.Vas J, et al. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64(10):3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li QZ, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115(12):3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannoor K, et al. Expression of natural autoantibodies in MRL-lpr mice protects from lupus nephritis and improves survival. J Immunol. 2012;188(8):3628–3638. doi: 10.4049/jimmunol.1102859. [DOI] [PubMed] [Google Scholar]
- 21.Son M, et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood. 2016;128(18):2218–2228. doi: 10.1182/blood-2016-05-719757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serreze DV, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161(8):3912–3918. [PubMed] [Google Scholar]
- 23.Silveira PA, et al. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol. 2002;32(12):3657–3666. doi: 10.1002/1521-4141(200212)32:12<3657::AID-IMMU3657>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Molnarfi N, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DSW, et al. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20(3):179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan OT, et al. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endres R, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med. 1999;189(1):159–168. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6(11):636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 29.Evans JG, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 30.Gao N, et al. Impaired suppressive capacity of activation-induced regulatory B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(10):2849–2861. doi: 10.1002/art.38742. [DOI] [PubMed] [Google Scholar]
- 31.Facciotti F, et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6+B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2020;117(13):7305–7316. doi: 10.1073/pnas.1917834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llorente L, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43(8):1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11(4):251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 34.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 35.Suurmond J, et al. DNA-reactive B cells in lupus. Curr Opin Immunol. 2016;43:1–7. doi: 10.1016/j.coi.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suurmond J, et al. Loss of an IgG plasma cell checkpoint in patients with lupus. J Allergy Clin Immunol. 2019;143(4):1586–1597. doi: 10.1016/j.jaci.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erikson J, et al. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349(6307):331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 38.Malkiel S, et al. Checkpoints for autoreactive B cells in the peripheral blood of lupus patients assessed by flow cytometry. Arthritis Rheumatol. 2016;68(9):2210–2220. doi: 10.1002/art.39710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noorchashm H, et al. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int Immunol. 1999;11(5):765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- 40.Mandik-Nayak L, et al. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J Exp Med. 1997;186(8):1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malkiel S, et al. Plasma cell differentiation pathways in systemic lupus erythematosus. Front Immunol. 2018;9:427. doi: 10.3389/fimmu.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsley RC, et al. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109(6):2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 43.Gross JA, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15(2):289–302. doi: 10.1016/S1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, et al. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182(7):4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 45.Landolt-Marticorena C, et al. Increased expression of B cell activation factor supports the abnormal expansion of transitional B cells in systemic lupus erythematosus. J Rheumatol. 2011;38(4):642–651. doi: 10.3899/jrheum.100214. [DOI] [PubMed] [Google Scholar]
- 46.Martin F, et al. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/S1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 47.Genestier L, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178(12):7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 48.Oliver AM, et al. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27(9):2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 49.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13(2):170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balazs M, et al. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–352. doi: 10.1016/S1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 51.Bialecki E, et al. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182(10):6105–6113. doi: 10.4049/jimmunol.0802273. [DOI] [PubMed] [Google Scholar]
- 52.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cinamon G, et al. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9(1):54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruetzmann S, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197(7):939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reif K, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416(6876):94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 57.Pereira JP, et al. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460(7259):1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flores-Borja F, et al. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52(12):3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 59.Pritchard NR, et al. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10(4):227–230. doi: 10.1016/S0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 60.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204(8):1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaturvedi A, et al. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28(6):799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Migita K, et al. Toll-like receptor expression in lupus peripheral blood mononuclear cells. J Rheumatol. 2007;34(3):493–500. [PubMed] [Google Scholar]
- 64.Papadimitraki ED, et al. Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum. 2006;54(11):3601–3611. doi: 10.1002/art.22197. [DOI] [PubMed] [Google Scholar]
- 65.Nakano S, et al. Role of pathogenic auto-antibody production by Toll-like receptor 9 of B cells in active systemic lupus erythematosus. Rheumatology (Oxford) 2008;47(2):145–149. doi: 10.1093/rheumatology/kem327. [DOI] [PubMed] [Google Scholar]
- 66.Jego G, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 67.Litinskiy MB, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 69.Pascual V, et al. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18(6):676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 70.Raval FM, et al. Long-lasting T cell-independent IgG responses require MyD88-mediated pathways and are maintained by high levels of virus persistence. mBio. 2013;4(6):e00812–e00813. doi: 10.1128/mBio.00812-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan AQ, et al. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73(1):298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pone EJ, et al. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moens L, Tangye SG. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol. 2014;5:65. doi: 10.3389/fimmu.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muraguchi A, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 76.Verbeek JS, et al. The complex association of FcγRIIb with autoimmune susceptibility. Front Immunol. 2019;10:2061. doi: 10.3389/fimmu.2019.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akkaya M, et al. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20(4):229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diamond B, et al. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 79.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 80.van Es JH, et al. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991;173(2):461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marion TN, et al. Selection of immunoglobulin variable regions in autoimmunity to DNA. Immunol Rev. 1992;128:123–149. doi: 10.1111/j.1600-065X.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 82.Anolik JH, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56(9):3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 83.Jenks SA, et al. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288(1):136–148. doi: 10.1111/imr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naradikian MS, et al. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016;269(1):118–129. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9(1):1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jenks SA, et al. Distinct effector B Cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49(4):725–739. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tipton CM, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suurmond J, et al. Patterns of ANA+ B cells for SLE patient stratification. JCI Insight. 2019;4(9):e127885. doi: 10.1172/jci.insight.127885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu D, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450(7167):299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 90.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, et al. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196(12):1543–1552. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roark JH, et al. Breakdown of B cell tolerance in a mouse model of systemic lupus erythematosus. J Exp Med. 1995;181(3):1157–1167. doi: 10.1084/jem.181.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cappione A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115(11):3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mandik-Nayak L, et al. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189(11):1799–1814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, et al. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol. 2006;176(9):5183–5190. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 96.Wellmann U, et al. Altered selection processes of B lymphocytes in autoimmune NZB/W mice, despite intact central tolerance against DNA. Eur J Immunol. 2001;31(9):2800–2810. doi: 10.1002/1521-4141(200109)31:9<2800::AID-IMMU2800>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 97.Kench JA, et al. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J Exp Med. 1998;188(5):909–917. doi: 10.1084/jem.188.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 2015;125(6):2194–2202. doi: 10.1172/JCI78084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richard ML, Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 2018;5(1):e000199. doi: 10.1136/lupus-2016-000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W, et al. An update on lupus animal models. Curr Opin Rheumatol. 2017;29(5):434–441. doi: 10.1097/BOR.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dixon FJ, et al. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21(5 suppl):S64–S67. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- 103.Ahuja A, et al. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179(5):3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 104.Bekar KW, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kansal R, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11(482):eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stohl W, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52(7):2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 108.Jacob CO, et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177(4):2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gross JA, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 110.Kayagaki N, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17(4):515–524. doi: 10.1016/S1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 111.Ramanujam M, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173(5):3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 112.Ramanujam M, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116(3):724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 114.Kawabata D, et al. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One. 2010;5(1):e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Figgett WA, et al. Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers. J Autoimmun. 2015;61:9–16. doi: 10.1016/j.jaut.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Hammarlund E, et al. Plasma cell survival in the absence of B cell memory. Nat Commun. 2017;8(1):1781. doi: 10.1038/s41467-017-01901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Obeng EA,et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ichikawa HT, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. 2012;64(2):493–503. doi: 10.1002/art.33333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohan C, et al. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154(3):1470–1480. [PubMed] [Google Scholar]
- 120.Early GS, et al. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. J Immunol. 1996;157(7):3159–3164. [PubMed] [Google Scholar]
- 121.Ramanujam M, et al. Phoenix from the flames: rediscovering the role of the CD40-CD40L pathway in systemic lupus erythematosus and lupus nephritis. Autoimmun Rev. 2020;19(11):102668. doi: 10.1016/j.autrev.2020.102668. [DOI] [PubMed] [Google Scholar]
- 122.Boumpas DT, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48(3):719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 123.Robles-Carrillo L, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185(3):1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 124.Zhuang H, et al. Animal models of interferon signature positive lupus. Front Immunol. 2015;6:291. doi: 10.3389/fimmu.2015.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baccala R, et al. Anti-IFN-α/β receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189(12):5976–5984. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197(6):777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agrawal H, et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183(9):6021–6029. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Braun D, et al. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20(1):15–25. doi: 10.1016/S0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 129.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173(3):2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 130.Choi JY, et al. Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J Immunol. 2017;198(7):2578–2588. doi: 10.4049/jimmunol.1601687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liang B, et al. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mihara M, et al. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112(3):397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pawar RD, et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18(6):1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 134.Barrat FJ, et al. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37(12):3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 135.Honigberg LA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mina-Osorio P, et al. Suppression of glomerulonephritis in lupus-prone NZB × NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase. Arthritis Rheum. 2013;65(9):2380–2391. doi: 10.1002/art.38047. [DOI] [PubMed] [Google Scholar]
- 137.Rankin AL, et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J Immunol. 2013;191(9):4540–4550. doi: 10.4049/jimmunol.1301553. [DOI] [PubMed] [Google Scholar]
- 138.Hutcheson J, et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res Ther. 2012;14(6):R243. doi: 10.1186/ar4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones DS, et al. Conjugates of double-stranded oligonucleotides with poly(ethylene glycol) and keyhole limpet hemocyanin: a model for treating systemic lupus erythematosus. Bioconjug Chem. 1994;5(5):390–399. doi: 10.1021/bc00029a003. [DOI] [PubMed] [Google Scholar]
- 140.Kaliyaperumal A, et al. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: tolerance spreading impairs pathogenic function of autoimmune T and B cells. J Immunol. 1999;162(10):5775–5783. [PubMed] [Google Scholar]
- 141.Gaynor B, et al. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci U S A. 1997;94(5):1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H, et al. ALW peptide ameliorates lupus nephritis in MRL/lpr mice. Arthritis Res Ther. 2019;21(1):261. doi: 10.1186/s13075-019-2038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fassbinder T, et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther. 2015;17(1):92. doi: 10.1186/s13075-015-0603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duxbury B, et al. Rituximab in systemic lupus erythematosus: an updated systematic review and meta-analysis. Lupus. 2013;22(14):1489–1503. doi: 10.1177/0961203313509295. [DOI] [PubMed] [Google Scholar]
- 145.Alshaiki F, et al. Outcomes of rituximab therapy in refractory lupus: a meta-analysis. Eur J Rheumatol. 2018;5(2):118–126. doi: 10.5152/eurjrheum.2018.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rovin BH, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 147.Merrill JT, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hahn BH, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64(6):797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fanouriakis A, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 150.Vital EM, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63(10):3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 151.Gomez Mendez LM, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. 2018;13(10):1502–1509. doi: 10.2215/CJN.01070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dias SS, et al. Longer duration of B cell depletion is associated with better outcome. Rheumatology (Oxford) 2015;54(10):1876–1881. doi: 10.1093/rheumatology/kev036. [DOI] [PubMed] [Google Scholar]
- 153.Reddy V, et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 2017;56(7):1227–1237. doi: 10.1093/rheumatology/kex067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.No authors listed ACR Convergence 2020 Abstract Supplement. Arthritis Rheumatol. 2020;72(suppl 10):1–4231. doi: 10.1002/art.41538. [DOI] [PubMed] [Google Scholar]
- 155.Mysler EF, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 2013;65(9):2368–2379. doi: 10.1002/art.38037. [DOI] [PubMed] [Google Scholar]
- 156.Haarhaus ML, et al. Ofatumumab treatment in lupus nephritis patients. Clin Kidney J. 2016;9(4):552–555. doi: 10.1093/ckj/sfw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Masoud S, et al. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatology (Oxford) 2018;57(7):1156–1161. doi: 10.1093/rheumatology/key042. [DOI] [PubMed] [Google Scholar]
- 158.Wallace DJ, et al. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology (Oxford) 2013;52(7):1313–1322. doi: 10.1093/rheumatology/ket129. [DOI] [PubMed] [Google Scholar]
- 159.Wallace DJ, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73(1):183–190. doi: 10.1136/annrheumdis-2012-202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clowse ME, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. 2017;69(2):362–375. doi: 10.1002/art.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheema GS, et al. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 162.Stohl W, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48(12):3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 163.Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 164.Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang F, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan, and South Korea. Ann Rheum Dis. 2018;77(3):355–363. doi: 10.1136/annrheumdis-2017-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.D’Cruz D, et al. Efficacy and safety of belimumab in patients of black race with systemic lupus erythematosus: results from the EMBRACE study. Abstract 200. Lupus Sci Med. 2019;6(suppl 1):A149–A150. [Google Scholar]
- 167.Furie R, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 168.Ginzler EM, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. 2012;14(1):R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Isenberg D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial) Ann Rheum Dis. 2015;74(11):2006–2015. doi: 10.1136/annrheumdis-2013-205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Merrill JT, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 2018;70(2):266–276. doi: 10.1002/art.40360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Furie RA, et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis. 2015;74(9):1667–1675. doi: 10.1136/annrheumdis-2013-205144. [DOI] [PubMed] [Google Scholar]
- 172.Merrill JT, et al. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(6):883–889. doi: 10.1136/annrheumdis-2018-213032. [DOI] [PubMed] [Google Scholar]
- 173.Isenberg DA, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(2):323–331. doi: 10.1136/annrheumdis-2015-207653. [DOI] [PubMed] [Google Scholar]
- 174.Merrill JT, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(2):332–340. doi: 10.1136/annrheumdis-2015-207654. [DOI] [PubMed] [Google Scholar]
- 175. Wu D, et al. A human recombinant fusion protein targeting B lymphocyte stimulator (BlyS) and a proliferation-inducing ligand (APRIL), telitacicept (RC18), in systemic lupus erythematosus (SLE): results of a phase 2b study. Abstract L18. Arthritis Rheumatol. 2019;71(suppl 10). https://acrabstracts.org/abstract/a-human-recombinant-fusion-protein-targeting-b-lymphocyte-stimulator-blys-and-a-proliferation-inducing-ligand-april-telitacicept-rc18-in-systemic-lupus-erythematosus-sle-results-of-a-phase/ Accessed May 3, 2021.
- 176.Ehrenstein MR, Wing C. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol. 2016;12(6):367–372. doi: 10.1038/nrrheum.2016.18. [DOI] [PubMed] [Google Scholar]
- 177.Atisha-Fregoso Y, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. 2021;73(1):121–131. doi: 10.1002/art.41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Halpern WG, et al. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol Sci. 2006;91(2):586–599. doi: 10.1093/toxsci/kfj148. [DOI] [PubMed] [Google Scholar]
- 179.Ishii T, et al. Multicenter double-blind randomized controlled trial to evaluate the effectiveness and safety of bortezomib as a treatment for refractory systemic lupus erythematosus. Mod Rheumatol. 2018;28(6):986–992. doi: 10.1080/14397595.2018.1432331. [DOI] [PubMed] [Google Scholar]
- 180.Segarra A, et al. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-center experience. Lupus. 2020;29(2):118–125. doi: 10.1177/0961203319896018. [DOI] [PubMed] [Google Scholar]
- 181.Ostendorf L, et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med. 2020;383(12):1149–1155. doi: 10.1056/NEJMoa2023325. [DOI] [PubMed] [Google Scholar]
- 182.Riggs JM, et al. Characterisation of anifrolumab, a fully human anti-interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus. Lupus Sci Med. 2018;5(1):e000261. doi: 10.1136/lupus-2018-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Furie RA, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1(4):e208–e219. doi: 10.1016/S2665-9913(19)30076-1. [DOI] [PubMed] [Google Scholar]
- 184.Morand EF, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382(3):211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 185.Shirota Y, et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis. 2013;72(1):118–128. doi: 10.1136/annrheumdis-2012-201310. [DOI] [PubMed] [Google Scholar]
- 186.Wallace DJ, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis. 2017;76(3):534–542. doi: 10.1136/annrheumdis-2016-209668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rovin BH, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016;68(9):2174–2183. doi: 10.1002/art.39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hussaini A, et al. A double-blind, phase I, single ascending dose study to assess the safety, pharmacokinetics, and pharmacodynamics of BOS161721 in healthy subjects. Clin Transl Sci. 2020;13(2):337–344. doi: 10.1111/cts.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grammer AC, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112(10):1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Merrill JT, et al. SAT0187 discrimination of systemic lupus (SLE) patients with clinical response to obexelimab (XMAB®5871) based on a pattern of immunologic markers. Ann Rheum Dis. 2020;79(suppl 1):1035–1036. doi: 10.1136/annrheumdis-2020-eular.2972. [DOI] [Google Scholar]
- 191.Cardiel MH, et al. Abetimus sodium for renal flare in systemic lupus erythematosus: results of a randomized, controlled phase III trial. Arthritis Rheum. 2008;58(8):2470–2480. doi: 10.1002/art.23673. [DOI] [PubMed] [Google Scholar]
- 192.Teruel M, Alarcon-Riquelme ME. The genetic basis of systemic lupus erythematosus: what are the risk factors and what have we learned. J Autoimmun. 2016;74:161–175. doi: 10.1016/j.jaut.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 193.Vinuesa CG, et al. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9(12):845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 194.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Banchereau R, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165(3):551–565. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Panousis NI, et al. Combined genetic and transcriptome analysis of patients with SLE: distinct, targetable signatures for susceptibility and severity. Ann Rheum Dis. 2019;78(8):1079–1089. doi: 10.1136/annrheumdis-2018-214379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kalunian KC, et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE) Ann Rheum Dis. 2016;75(1):196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 199.Khamashta M, et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(11):1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]