Abstract
Inflammatory pseudotumor is a rare benign mesenchymal pediatric neoplasm, that can mimic tumoral residue or relapse at metabolic imaging with nonspecific clinical presentation and difficult diagnosis. We present the case of a 14year old male patient with fever of unknown origin and large ileal mass, diagnosed with and treated for Burkitt lymphoma, who performed several 18-fluoro-deoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) scans, during and after first line chemotherapy, showing persistent and focal uptake, while ileal mass volume decreased dramatically and the patient was clinically asymptomatic. Histopathological analysis of residual ileal mass was suggestive for xanthomatous pseudotumor, a type of inflammatory pseudotumor. No more treatment was performed and a short-term follow up with 18F-FDG PET/CT showed tracer uptake intensity decreasing progressively over the next few months. This case reports an uncommon presentation of a rare disease, inflammatory pseudotumor of the small bowel developed at the site of treated Burkitt lymphoma, underscoring the potential role of 18F-FDG PET/CT imaging in the diagnosis and management of these rare neoplasms, particularly in asymptomatic patients.
Keywords: Inflammatory pseudotumor, Burkitt lymphoma, 18F-fluorodeoxyglucose, Positron emission tomography/computed tomography, Molecular imaging
Introduction
Inflammatory pseudotumor is a rare benign mesenchymal neoplasm, also known as inflammatory myofibroblastic tumor or xanthomatous pseudotumor, and is most common in pediatric population. It is composed by inflammatory cells and myofibroblastic spindle cells, which can pose a difficult differential diagnosis with malignancies or infections [1]. Clinical presentation is quite unspecific, as fever, weight loss, malaise and/or abdominal pain may be present, along with nonspecific laboratory results. Radiological imaging can be helpful to define the anatomy of the lesion but does not help confirm the diagnosis, which is based primarily on histopathologic analysis [2]. Inflammatory pseudotumor etiopathology is still unclear although some studies suggested the involvement of abnormal inflammatory response and reparative changes due to the tumor necrosis, chemotherapy, or other inflammatory status [3]. At metabolic imaging inflammatory pseudotumor can mimic tumoral residue or relapse. Although 18-fluoro-deoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) is the most useful technique in distinguishing viable from necrotic tissue, the presence of numerous inflammatory cells, which show high 18F-FDG avidity, can cause false positive results leading to wrong diagnosis and inappropriate therapeutic protocols [4], [5], [6]. Therefore, the diagnosis and management of these tumors are challenged by the lack of defined criteria for differential diagnosis and established management guidelines.
Case report
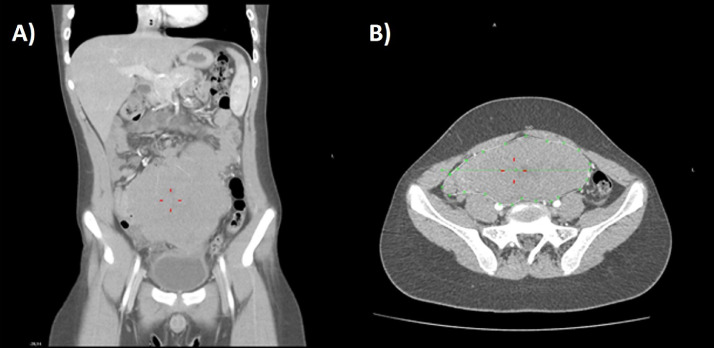
We report the case of a 14-year-old male patient, who came to clinical observation in February 2020, for persistent fever of unknown origin, constipation, abdominal and low back pain; at clinical examination, he showed a large abdominal mass extended from the right iliac region to hypogastric region. Blood tests showed an increased lactate dehydrogenase with a modest normocytic normochromic anemia. A total body CT scan with intravenous contrast media administration (Fig. 1) showed an abnormal thickening (53 mm) of small bowel wall extending for at least 170 mm along the main axis, causing stenosis of the lumen, with inhomogeneous contrast enhancement and not clearly dissociable from surrounding tissues; yet lymphadenopathies were found in mesenteric fat. Multiple video-laparoscopic tru-cut biopsies of the abdominal mass and bone marrow biopsies were also performed. Histological exam (Fig. 2) and molecular analysis of abdominal mass showed atypic lymphoid cells with high proliferation index (Ki67 = 100%), overexpression of c-Myc protein (>70%), translocation t (8;14) MYC-IgG and immunophenotypic pattern CD45+, CD20+, PAX5+, CD79a+, CD38+, CD3 −, CD5 −. Conversely, the histological and molecular exams of bone marrow resulted normal. These findings led to the diagnosis of Burkitt lymphoma (STAGE III; MDD negative). Therefore, the patient underwent 6 cycles of chemotherapy according to AIEOP LNH97 protocol and a monthly ultrasound follow-up, which showed a progressive reduction of mass volume and wall thickening over the time.
Fig. 1.
Coronal (A) and transaxial (B) CT scan after intravenous contrast media administration. In gastric meso-hypogastrium evidence of a small bowel loop with considerably thickened walls extending for 170 mm at least along the main axis of the loop, causing stenosis of the lumen, with inhomogeneous contrast enhancement (red lines). This thickening does not present a clear separation from the bladder dome caudally, with the cecum on the right, with the abdominal wall anteriorly, with the intestine cranially. Multiple lymphadenopathies are found in mesenteric fat. (Color version of figure is available online).
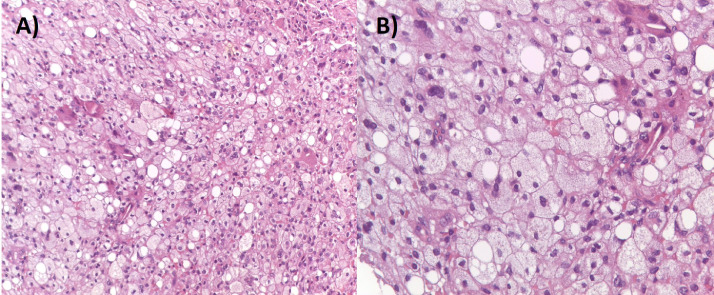
Fig. 2.
Pathology findings of Burkitt lymphoma, hematoxylin-eosin coloration, × 200 (A) and × 400 (B), showing atypic lymphoid cells with the characteristic “starry sky” appearance due to the presence of scattered histiocytes engulfing apoptotic lymphoma.
On June 2020, a 18F-FDG PET/CT scan was performed to evaluate interim response to chemotherapy and showed intense tracer uptake involving distant ileum wall, along with a significant reduction of the nodular bowel wall thickening volume (17 mm vs 170 mm) (Fig. 3). These results were consistent with a partial response to chemotherapy, as the lesion volume decreased dramatically, but the finding of intense persistent metabolic activity was confusing. A second 18F-FDG PET/CT scan was performed the next month, after completing chemotherapy protocol and showed increased tracer uptake on the stable ileal wall thickening and no other pathological findings (Fig. 4). At clinical evaluation, the patient was asymptomatic. On the light of these clinical and imaging findings, a laparoscopic biopsy of the ileal lesion was performed to rule out residual disease. Histological and immunophenotypic exam showed histiocytic elements (CD68+ CD163+) with large multi vacuolated cytoplasm, mixed with multinucleated giant cells, also incorporating cholesterin crystals, T-lymphocytes (CD3+, CD19 −, PAX5 −, CD79a −) and fibroblasts (SMA+) (Fig. 5). Furthermore, the search of translocation (t 8;14) was negative. These results were suggestive for a xanthogranulomatous reaction probably caused by chemotherapy, leading to a final diagnosis of xanthomatous pseudotumor, a type of inflammatory pseudotumor; negativity of translocation (t 8;14) also confirmed the absence of Burkitt's relapse.
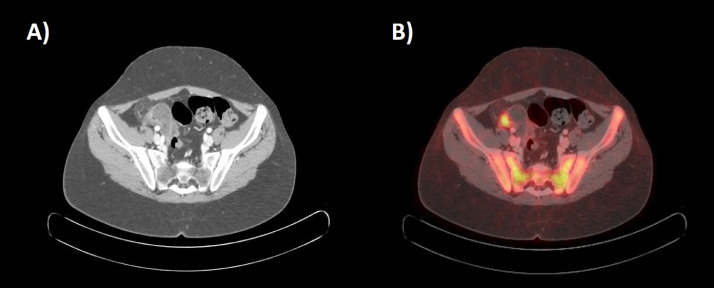
Fig. 3.
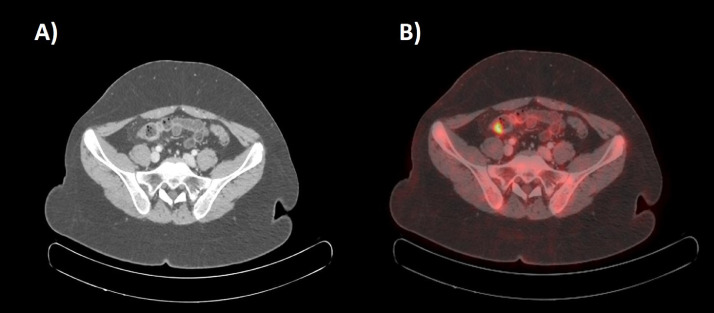
Transaxial CT scan (A) and corresponding fused 18F-FDG PET-CT images (B) showing focal thickening of nodular appearance with solid densitometric characteristics along the parietal profile of the distal segment of the ilium in right iliac region with increased focal FDG standardized uptake value (SUVmax 7).
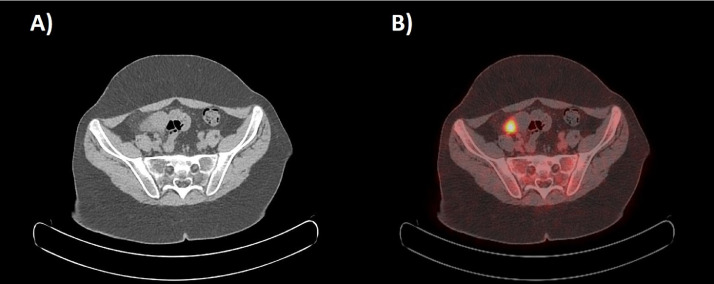
Fig. 4.
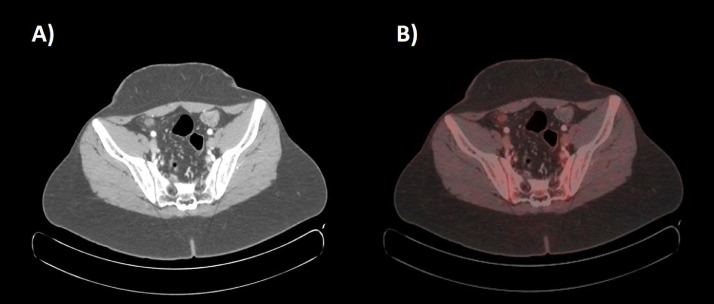
Transaxial CT scan (A) and corresponding fused 18F-FDG PET-CT images (B) showing increased focal FDG uptake (SUVmax 10.3) close to an ileal loop in the right pelvic region.
Fig. 5.
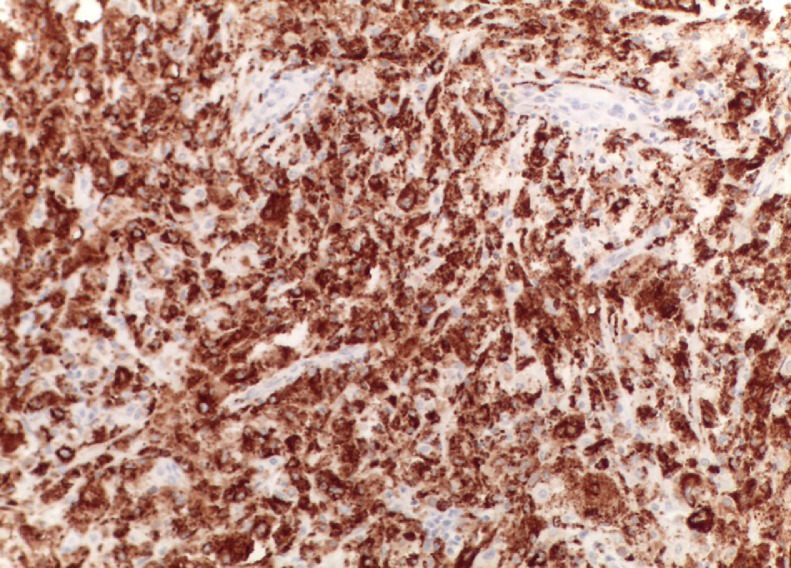
Pathology findings of xanthomatous pseudotumor: immunohistochemical stains showing CD68+ histiocytes.
In September 2020, a follow-up 18F-FDG PET/CT scan showed persistent augmentation of tracer uptake of the nodular thickening of distal ileum (Fig. 6). The patient was asymptomatic and without therapy since June 2020. In December 2020, another 18-FDG PET/CT scan showed a marked reduction in metabolic activity of the nodular ileal thickening, whose dimension resulted stable over the time (12 mm), confirming the diagnosis of inflammatory pseudotumor at the site of treated Burkitt lymphoma (Fig. 7).
Fig. 6.
Transaxial CT scan (A) and corresponding fused 18F-FDG PET-CT images (B) showing nodular wall thickening involving distal ileum with high FDG avidity (SUVmax 9).
Fig. 7.
Transaxial CT scan (A) and corresponding fused 18F-FDG PET-CT images (B) showing nodular wall thickening involving distal ileum with decreased 18F-FDG uptake (SUVmax 2) compared to prior controls.
Discussion
Inflammatory pseudotumor are rare in gastrointestinal tract and should be considered as a differential diagnosis with lymphoma, when there is only segmental bowel wall thickening and when the patient does not experience symptoms of recurrent lymphoma. Inflammatory pseudotumor have a long clinical course, ranging from weeks to several months with benign prognosis in most of the case, and a complete surgical excision is the mainstay of treatment, providing the best chance to limit recurrence. When surgery is not possible, good response can be obtained with chemotherapy and radiotherapy, although there is a lack of validated criteria and guidelines for diagnosis and management of this rare disease [5], [6], [7].
It has been reported that a few primary intestinal inflammatory pseudotumor are characterized by fever of unknown origin, which is ineffective in antibiotic therapy, as described in our case. Moreover, in a subset of patients with Burkitt lymphoma, particularly those with large bulky masses, necrotic tumor may persist as a residual mass for a variable time after therapy. The probability that this residual mass corresponds to an active residual disease or to disease relapse in asymptomatic patients (without clinical or biochemical evidence of disease) is only 10%-20% and at histological evaluation it is diagnosed as necrosis and/or fibrosis. In fact, chemotherapy can induce tumor necrosis and inflammatory response to phagocytize the dead necrotic tissue, which can increase metabolic activity in necrotic masses and cause a suspicious 18F-FDG uptake. These data underline the importance of defining these findings in the best and least invasive way possible [8], [9], [10], [11], [12].
The presented case offers several challenges in establishing the right diagnosis and management. First, the nonspecific clinical presentation with persistent fever, constipation, lower back pain and abdominal pain could be consistent both with abdominal lymphoma and abdominal inflammatory pseudotumor. Ultrasound and radiological imaging also presented nonspecific findings showing a large inhomogeneous hypoechoic mass with inhomogeneous contrast enhancement at CT imaging, suggesting both malignancies and necrotic tumor and inflammatory pseudotumor. Conversely, 18F-FDG PET/CT imaging seems to be more helpful in differential diagnosis because necrotic tissue is not metabolically active, showing no tracer uptake [13].
At first step, on the light of clinical evolution and radiological and histological results, the diagnosis was stated as stage 3 Burkitt lymphoma and an appropriate chemotherapy protocol was administrated, according to AIEOP guidelines. The dramatic response to first line chemotherapy, as shown by ultrasound and CT imaging findings, enforced the diagnosis of Burkitt lymphoma, but the finding of focal intense uptake at 18F-FDG PET/CT could suggest both a relapsed or persistent lymphoma or an inflammatory lesion, like inflammatory pseudotumor. Moreover, chemotherapy-induced inflammatory status may persist for several weeks after therapy, causing a suspicious, yet nonspecific, 18F-FDG uptake [14,15]. It must be also taken in consideration that over 80% of patients with stage III Burkitt lymphoma and no disseminated disease at diagnosis respond to therapy with achievement of complete remission after the first 3 cycles. If a tumor residue persists after the fifth cycle, as in our case, the protocol provides for a further sixth cycle of consolidation and a second surgical look (for diagnostic and possibly therapeutic purposes, when complete removal is possible) [11]. At second step, histological and molecular analysis of the residual hypermetabolic lesion, confirmed inflammatory pseudotumor diagnosis. In recurrent lymphoma 18F-FDG PET/CT imaging keeps showing persistent tracer uptake over the time, while in case of inflammatory pseudotumor 18F-FDG uptake decreases progressively. In the present case, PET/CT imaging was useful to monitor the evolution of disease, by showing decreased metabolic activity and volume over time, confirming author's diagnostic hypothesis of ileal inflammatory pseudotumor at the site of prior Burkitt lymphoma.
In conclusion, the presented case is an uncommon presentation of a rare disease, chemotherapy-induced inflammatory pseudotumor of the small bowel in an adolescent male patient with prior Burkitt lymphoma, and highlights the inflammatory pseudotumor variability in terms of clinical presentation, histopathology, and biologic behavior. This case also underscores the potential role of 18F-FDG PET/CT imaging in the diagnosis and management of these rare neoplasms, particularly in asymptomatic patients.
Patient consent
Informed consent was obtained from the patient and his legal representative.
Authors’ contributions
All authors provided clinical expertise and participated in drafting the manuscript. All authors read and approved the final manuscript.
Footnotes
Acknowledgments: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Lopes VN, Alvarez C, Dantas MJ, Freitas C, Pinto-de-Sousa J. Mesenteric inflammatory pseudotumor: a difficult diagnosis. Case report. Int J Surg Case Rep. 2017;32:1–4. doi: 10.1016/j.ijscr.2017.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalton BG, Thomas PG, Sharp NE, Manalang MA, Fisher JE, Moir CR. Inflammatory myofibroblastic tumors in children. J Pediatr Surg. 2016;51:541–544. doi: 10.1016/j.jpedsurg.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Sagar AES, Jimenez CA, Shannon VR. Clinical and histopathologic correlates and management strategies for inflammatory myofibroblastic tumor of the lung. A case series and review of the literature. Med Oncol. 2018;35:102. doi: 10.1007/s12032-018-1161-0. [DOI] [PubMed] [Google Scholar]
- 4.Dillman JR, Smith EA, Morani AC, Trout AT. Imaging of the pediatric peritoneum, mesentery and omentum. Pediatr Radiol. 2017;47:987–1000. doi: 10.1007/s00247-017-3864-3. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Terada N, Yoshida M, Shinkai M, Tanaka Y. ETV6-NTRK3-Positive inflammatory myofibroblastic tumor of the ileum: report of an infantile case and review of the differential diagnosis of pediatric intestinal polypoid lesions. AJSP. 2020;25:26–28. [Google Scholar]
- 6.Xue Q, Miao W. Inflammatory pseudotumor of intestine mimicking lymphoma on 18F-FDG PET/CT. Clin Nucl Med. 2020;45:383–384. doi: 10.1097/RLU.0000000000002981. [DOI] [PubMed] [Google Scholar]
- 7.Man I, Chao K, Fai C. Gastric inflammatory myofibroblastic tumor in a 10-year-old patient in Macao - Case report and literature review. J Canc Ther. 2019;10:345–351. [Google Scholar]
- 8.Singh AP, Murray GP, Pandey S. Benign histiocyte-rich pseudotumor developing postchemotherapy and mimicking residual disease. Case Rep Pathol. 2020;2020 doi: 10.1155/2020/4674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Qiao X, Jiang C, Liu S, Zhou Z. Texture analysis improves the value of pretreatment 18F-FDG PET/CT in predicting interim response of primary gastrointestinal diffuse large B-cell lymphoma. Contrast Media Mol Imaging. 2020;2020 doi: 10.1155/2020/2981585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod N, Lantsberg S, Anconina R, Levin D, Nalbandyan K, Levi I. Postchemotherapy histiocyte-rich pseudotumor of the spleen simulating residual disease in a patient with non-Hodgkin lymphoma on FDG PET/CT. Clin Nucl Med. 2019;44:e409–e412. doi: 10.1097/RLU.0000000000002568. [DOI] [PubMed] [Google Scholar]
- 11.Juweid ME. Utility of positron emission tomography (PET) scanning in managing patients with Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2006;1:259–265. doi: 10.1182/asheducation-2006.1.259. [DOI] [PubMed] [Google Scholar]
- 12.Ashfaq R, Timmons CF. Xanthomatous pseudotumor of the small intestine following treatment for Burkitt's lymphoma. Arch Pathol Lab Med. 1992;116:299–301. [PubMed] [Google Scholar]
- 13.Chandra P, Wen YH, Tuli S, Raphael BG, Amorosi EL, Medeiros LJ. Postchemotherapy histiocyte-rich pseudotumor involving the spleen. Am J Clin Pathol. 2009;132:342–348. doi: 10.1309/AJCPC67QOGJXCEZB. [DOI] [PubMed] [Google Scholar]
- 14.Pillon M, Di Tullio MT, Garaventa A, Cesaro S, Putti MC, Favre C. Long-term results of the first Italian Association of Pediatric Hematology and Oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92) Cancer. 2004;101:385–394. doi: 10.1002/cncr.20382. [DOI] [PubMed] [Google Scholar]
- 15.Pillon M, Mussolin L, Carraro E, Conter V, Aricò M, Vinti L. Detection of prognostic factors in children and adolescents with Burkitt and diffuse large B-Cell lymphoma treated with the AIEOP LNH-97 protocol. Br J Haematol. 2016;175:467–475. doi: 10.1111/bjh.14240. [DOI] [PubMed] [Google Scholar]