Abstract
Mesenchymal stromal/stem cells (MSCs) are currently being used in clinical trials as proposed treatments for a large range of genetic, immunological, orthopaedic, cardiovascular, endocrine and neurological disorders. MSCs are potent anti-inflammatory mediators which are considered immune evasive and employ a large range of secreted vesicles to communicate and repair damaged tissue. Despite their prolific use in therapy, sex specific mechanism of action is rarely considered as a potential confounding factor for use. The purpose of this study was to examine the potency and functionality of both female and male adipose derived MSCs in order to gain further insights into donor selection. Methods MSC were expanded to passage 4, secretome was harvested and stored at − 80c. To assess potency MSC were also primed and assessed via functional immune assays, ELISA, multiplex and immunophenotyping. Results Female MSCs (fMSC), consistently suppressed Peripheral blood mononuclear cell (PBMC) proliferation significantly (p < 0.0001) more than male MSC (mMSC). In co-culture mPBMCs, showed 60.7 ± 15.6% suppression with fMSCs compared with 22.5 ± 13.6% suppression with mMSCs. Similarly, fPBMCs were suppressed by 67.9 ± 10.4% with fMSCs compared to 29.4 ± 9.3% with mMSCs. The enhanced immunosuppression of fMSCs was attributed to the production of higher concentrations of the anti-inflammatory mediators such as IDO1 (3301 pg/mL vs 1699 pg/mL) and perhaps others including IL-1RA (1025 pg/mL vs 701 pg/mL), PGE-2 (6142 pg/mL vs 2448 pg/mL) and prolonged expression of VCAM-1 post activation relative to mMSCs. In contrast, mMSCs produces more inflammatory G-CSF than fMSCs (806 pg/mL vs 503 pg/mL). Moreover, IDO1 expression was correlated to immune suppression and fMSCs, but not mMSCs induced downregulation of the IL-2 receptor and sustained expression of the early T cell activation marker, CD69 in PBMCs further highlighting the differences in immunomodulation potentials between the sexes. Conclusion In conclusion, our data shows that female MSC are more potent in vitro than their male counterparts. The inability of male MSC to match female MSC driven immunomodulation and to use the inflammatory microenvironment to their advantage is evident and is likely a red flag when using allogeneic male MSC as a therapeutic for disease states.
Subject terms: Mesenchymal stem cells, Stem-cell biotechnology, Stem-cell therapies, Stem-cell research
Introduction
Background
Mesenchymal stem/stromal cells (MSC) are a multi-potent, multifunctional cell type that are defined by their capability to proliferate, renew, differentiate and regenerate1. Tissue specific differences in MSC function and gene expression have been determined when comparing MSCs derived from adipose tissue, bone marrow, dental pulp, umbilical cord and more2,3 regardless of their origin, MSCs are recognised as immunomodulatory, angiogenic, anti-fibrotic, chondrogenic, and anti-bacterial4. The applications for MSC and their secretome within research and industry is broad due to their diverse functionality5–7 and with the expanding use of MSCs, like many other therapies, close consideration should be given to donor and recipient interaction.
The most well documented function of MSC is their immunosuppressive and immunomodulatory characteristics which, when administered as treatment have been shown to provide safe and acute relief and can potentially have long lasting effects. Clinical efficacy however, has been challenging with inconsistent results and uncommon proposed mechanism of action halting a widespread acknowledgement of the benefit of MSC therapy8.
Among others the International Society Cell and Gene Therapy have attempted to guide researchers and clinicians to a more harmonistic approach to MSC therapy by defining what an MSC should present9–12. However, the challenges associated with in vivo MSC efficacy potentially stem from a deeper set of characteristics such as donor and recipient age, secretion profile, homing capability, clearance rates, culture conditions, cryopreservation techniques and perhaps a rarely noted link to efficacy, donor and recipient sex13–16
Immune modulation
MSC driven immunomodulation is a multi-pathway and multi-cellular strategy involving MSC immunogenicity and a synergy of secreted and surface communication molecules17. Immunogenicity or “immune-evasiveness” meaning MSCs lack immune system identifiers including Major Histocompatibility Complex II (MHC-II) and co-stimulatory molecules18, is key to MSC function. This characteristic allows MSCs to home to target tissue without immediate immune detection and mediating inflammation.
MSCs rely on co-ordinated cellular communication to alter inflammation. The molecules involved in MSC immunomodulation include messenger particles like extracellular vesicles19, soluble factors like cytokines and chemokines and also cell surface markers involved in homing and cell-to-cell contact which allow for more potent paracrine effects20. Many of these molecules are regulated by an inflammatory microenvironment stimulated by tissue injury and subsequent infiltrating immune cells which the MSCs are exposed to once administered21. Immune cells and subsequent secretion of proinflammatory factors like TNF-α, IL-1 and IFN-γ along with various other chemokines and free radicals22,23 then alter the MSC to a more potent cell type increasing the expression of immunomodulatory molecules such as Indoleamine 2,3, Dioxygenase (IDO), Prostaglandin E2 (PGE-2), Hepatocyte Growth Factor (HGF), Transforming Growth Factor (TGF-B1) and Interleukin-10 (IL-10)24–26 and upregulating immunomodulatory cell surface markers such as Intercellular adhesion molecule (iCAM), Vascular Adhesion molecule (VCAM), Programmed Death Ligand-1 (PD-L1) and Major and Minor Histocompatibility complexes (MHC) also known as Human Leukocyte antigen (HLA)27. These molecules alter immune cell differentiation, proliferation and maturation and shift the microenvironment from pro-inflammatory to anti-inflammatory17,28,29. Considering the cascade of events and the well-known disparities between the sexes, it seems ignorant to apply a one size fits all approach to MSC therapy.
Sex considerations
Recipient
It has long been recognised that sexual dimorphisms exist and male and females show a sex specific susceptibility to certain diseases30–32. Fundamental differences in adipose tissue itself, a major source of MSC have been explored. Like other organs in the body, adipose tissue is receptive to a variety of factors which influence distribution and function and could affect the health and potency of MSCs derived from this tissue. Generally seen to be driven by sex hormones, oestrogens and androgens, this MSC starting material has shown major differences in receptor activity, metabolism, proliferation, fibrosis, gene expression and inflammation between male and females33.
The immune system is an extremely complex and responsive system which dictates almost every aspect of an animal’s ability to survive and function properly. Although sex disparities have been thoroughly researched, it has not been fully explored in biological therapies where sex matching may be advantageous. Females have more alert immune systems than males and better capabilities to produce antibodies which can make them more resistant to certain infections34. This has largely been attributed to oestrogen signalling which promotes inflammatory cytokines and Toll-like receptor (TLR) expression leading to increased activity of T cells35. The expression of specific TLRs differs between the sexes, with TLR3,7 and 9 expressed greater in females and TLR2 and 4 expressed higher in males indicating a fundamental disparity34. This alternate activation gives rise to differences in pathogen response and activation of downstream effector molecules34,36,37. The opposite is the case for males, where testosterone inhibits proinflammatory cytokine production, decreases TLR expression and subsequently decreases T cell activation by male Antigen Presenting Cells (APCs), resulting in lower rates of rejection of pathogens34,35.
Donor
The expansive capabilities of MSCs come with inherent risks and have posed considerable questions in the scientific community15 therefore, obtaining the most suitable starting material for the proposed indication and individual needs should be carefully considered.
This research focused on key MSC potency markers and subsequent immune modulation to determine if sex, not only of the starting material but recipient tissue had an effect on MSC efficacy. To do this we mimicked an inflammatory microenvironment using cytokines and Peripheral Blood Mononuclear cells (PBMCs) to assess MSC donor potency and probable efficacy.
Materials and methods
Ethics statement
Ethical approval for this study was provided by Northern Sydney Local Health District Human Research Ethics Committee for the use of Mesenchymal stem cells in the analysis of sex differences in Osteoarthritis (Regis 2019_ETH00501). All research was performed in accordance with relevant guidelines and regulations38.
Media preparation
This research used multiple media types including; Stromal Vascular Fraction (SVF) growth media [used for Passage 0 MSCs] Alpha Modified Essential Medium (αMEM) (Lonza, Australia) with 10% Platelet lysate (PLT) (Cook Regentec, United States) and 1% Antibiotic–Antimycotic (ABAM -ThermoFisher, Australia), MSC growth media [used for Passage 1 to Passage 4 MSCs] αMEM with 10% PLT, Priming Media [used to activate MSCs] αMEM with 10% PLT supplemented with 10 ng/mL Tumour Necrosis Factor (TNF-α) (Stem cell technologies, Australia) and 100 ng/mL Interferon Gamma (IFN-γ) (Stem cell technologies, Australia) and PBMC media Roswell Park Memorial Institute Media (RPMI)(Sigma, Australia), 1% ABAM and 10% Foetal Bovine Serum (FBS).
MSC preparation
Processing adipose tissue
MSCs were isolated from Stromal Vascular Fraction (SVF) after autologous stem cell therapy by Stem Cell company, Regeneus Ltd. Stromal vascular fraction is a heterogenous population of cells obtained from adipose tissue which contains a small population of MSCs. All patients gave written informed consent for cells to be used in future research.
SVF was isolated according to Zuk et al.39. Briefly, lipoaspirate samples were digested using 0.05% wt./vol collagenase (Sigma, Australia), washed using Dulbecco’s Modified Eagle Medium (ThermoFisher, Australia) to remove collagenase and pelleted via centrifugation. The resultant SVF were counted and viability determined via FACSCalibur (data not shown). SVF was then cryopreserved in Cryostat (CS10) (Stem cell technologies, Australia) in LN2 at a concentration of ~ 1 × 107 cells/mL.
Cell expansion
Adipose derived MSCs were isolated from stored SVF samples. Frozen SVF was thawed at 37 °C in a water bath for 2 min and cultured at 12,000 cells/cm2 in SVF growth media in cell culture treated flasks. When cells reached ~ 90% confluence cells were harvested and counted. Further MSC expansion was achieved in MSC growth media at 37 °C in 5% CO2. Once MSCs had been passaged four times (P4) they were upscaled into a 2-layer cell factory (ThermoFisher, Australia) to obtain ~ 1 × 108 cells per donor. MSC secretome (MSC-S) was harvested by decanting, centrifuged and frozen at − 80 °C. MSC were harvested when confluence reached 90–100%. Cells were counted and viability determined using the FACSCalibur after each harvest and stored at 2 × 106 cells per aliquots in CS10 (Stem cell technologies, Australia) at − 80 °C then transferred to LN2 12 h later.
FACS cell count and viability
To determine cell count and viability (CCV), cells were stained with Propidium Iodide (PI) (Sigma, Australia) and SYTO 11 (ThermoFisher, Australia) in Trucount tubes (BD Biosciences, Australia) and analysed via FACSCalibur.
Cell characterisation
Donors
Age and health status of all MSC donors was seen to be matched for both sexes.. Four female MSC donors average age 45 and four male MSC donors average age 46 (supplementary table S1) were cultured to passage 4 using MSC growth media and assessed for successful population doubling (> 2 per passage), protein analysis, and immunophenotyping. They were further exposed to inflammatory cytokines (MSC priming media) and Peripheral Blood Mononuclear cells. Three Male and three female PBMCs donors aged between 41 and 61 years old were purchased from Lonza (United States), thawed, washed and aliquoted into 5 × 106 cells/vial in RPMI, 10% FBS, 10% DMSO for use in subsequent PBMC assays.
Morphology
Cells were monitored at each passage via microscopic imaging (Dino-lite, Australia).
Phenotyping
To determine the cell phenotype, MSC or PBMC aliquots (1 × 105/test) were thawed, washed in PBS (Life Technologies, Australia) and subsequently washed with 1.0 mL Flow cytometry Buffer (ThermoFisher, Australia) by centrifuging at room temperature for 5 min at 500 g. Wash supernatant was discarded and 5 µL of appropriate conjugated flow cytometry Ab was added to the tube and incubated at 4 °C for 30 min. Cells were then washed and centrifuged twice, fixed using 200 µL BD FACS™ Lysing Solution (BD Bioscience, Australia) and stored at 4 °C overnight. Cells were run on a FACSCalibur and analysed using CellQuest.
Antibodies used for phenotyping
For Adipose derived MSC characterisation cells were labelled with CD90—Fluorescein isothiocyanate (FITC)(Thy-1), CD105 FITC (Endoglin), CD73 FITC (lymphocyte-vascular adhesion protein 2/ 5′-nucleotidase/NT5E), CD13 FITC, CD29 FITC, CD44 FITC, CD34 FITC (hematopoietic stem cells and endothelial cells markers), CD271/NGFR FITC, Human Leukocyte Antigen (HLA)-DR FITC, CD45—(leukocyte marker) phycoerythrin (PE), CD19 PE, CD106 PE (VCAM), CD11b PE (integrin α M), CD166 PE, CD31 PE (R&D systems). MSC were also labelled with IgG1K antibodies against VCAM, iCAM (CD54 PE), PD-L1 (CD274 PE), HLA-ABC PE, HLA-G PE, HLA-E PE and IgG2bK antibody HLA-DR FITC (R&D systems, United States) to assess homing capabilities after priming. Cell surface markers for PBMCs assessed T cell markers CD3 PE, T helper CD4 FITC, T cytotoxic CD8 PE, B cell CD19 PE, CD25 PE and early activation marker CD69 FITC antibodies (R&D systems, United States). All samples were run on FACSCalibur and analysed using CellQuest.
MSC-S characterisation
To assess the characteristics of donor MSC secreted protein, MSC-S was assessed under normal culture conditions and after 24 h priming with inflammatory cytokines. Analytes were measured and data was pooled using the Bio-plex Pro Human Cytokine 27-plex assay (IFN-y, IL-1b, IL-5, IL-9, IL-12, IL-15, 1L-17, TNF-α, IL-1ra, IL-4, IL-10, IL-13, Eotaxin, MCP-1, MIP-1α, MIP-1β, RANTES, FGFbasic, G-CSF, GM-CSF, IL-17, IP-10, PDGF-b, VEGF-a, IL-2, IL-6) (Bio-Rad, Australia), PGE-2 ELISA (ABCAM, Australia) and Custom ProcartaPlex Multiplex immunoassays (Thermo Scientific, Australia) (G-CSF, IDO, IFN-y, IL-1Ra, IL-6, IL-8, IP-10, MCP-1, TNF-α, VEGF-A, IL-2, IL-10, HGF, TGF-β1). Bioplex and ProcartaPlex are both Luminex based multiplex assays that utilise magnetic beads with antibodies directed against distinct analytes in the one assay, enabling measurement of many analytes at one time. Assays were performed as per manufactures instruction. Briefly, MSC-S was defrosted and spun at 10,000 g for 2 min, spun sample supernatant was bound, detection Ab conjugated with Streptavidin-PE were added and read on a MAGPIX 200 in the case of ProcartaPlex or Bioplex 200 for Bioplex.
MSC priming assay
To assess functionality, MSCs were primed by exposure to inflammatory cytokines and assessed for known functional markers via FACS. ELISA and ProcartaPlex were used for assessment of secreted molecules and immune modulation was analysed via a PBMC assay.
MSC priming
MSC were thawed at 37 °C in a water bath for 2 min and seeded into 0.4 µm transwell plates (Corning) at 12,000 cells/cm2 in 1.0 mL MSC culture media. MSCs were allowed to recover for 2 days at 37 °C in 5% CO2 or until they reached 50% confluence (48–72 h). At 50% confluence, MSCs were primed by adding Priming Media to sample wells and incubated. MSCs and MSC-S were then either (1) harvested and frozen to assess MSC immunomodulatory characteristics after the 24 h incubation, (2) media changed to fresh MSC culture media and incubated for a further 24 h to analyse any sustained expression of immunomodulatory markers once removed from inflammation, harvested and frozen or (3) were utilised in the PBMC proliferation assay to assess their ability to suppress PBMC. Control MSC wells were unprimed.
MSC co-culture
MSC priming
MSC mediated immunosuppression was analysed using a PBMC proliferation assay. Primed MSCs were seeded in the bottom chamber of a Transwell system (Corning, Australia) with 0.4uM pore size as to only allow soluble factors to pass and exposed to stained and activated PBMC.
PBMC staining and activation
PBMC aliquots were thawed and cultured overnight in PBMC media. Cells were then harvested and counted for CCV and stained with 2.5 µM CellTrace™ Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) (ThermoFisher, Australia) for 20 min at Room temperature in the dark. Cells were then quenched for excess stain by adding 15.0 mL PBMC media and incubated at room temperature for a further 10 min. PBMCs were then centrifuged for 7 min at 400 g and resuspended in 500uL prewarmed PBMC media prior to stimulation. PBMC were then induced to proliferate using the T cell Activation/Expansion kit (Miltenyi, Australia) which consists of Anti-Biotin MACSiBead Particles and biotinylated antibodies against human CD2, CD3, and CD28. Briefly, 2 × 106 PBMC/assay were exposed to T-cell activation/expansion kit in PBMC media prior to co-culturing with primed MSCs. PBMC’s (~ 2 × 105 cells) were added to the top layer of a transwell culture system with pre-primed MSCs (~ 5 × 104 cells) in the bottom chamber. The transwells were cultured in PBMC media. Untreated PBMCs were used as non-activated controls. Proliferation was assessed using FACScan and further analysis was performed using Flow logic.
PBMC proliferation
PBMC proliferation was assessed via FACSCalibur Flow cytometry. CFSE fluorescence was determined using dot plot and histogram analysis of dye dilution of PBMC generations. CFSE fluorescence of PBMC was compared to the untreated control. Untreated PBMCs show a high Mean Florescence intensity(MFI) reading and activated PBMCs show a low MFI reading. The MFI is measured as the mean intensity level of the CFSE dye, proliferating cells will show a lighter mean CFSE intensity (lower MFI). When primed MSC are included in co-culture with activated PBMCs the MFI of the PBMC was expected to be greater than untreated and less than activated control MFI readings and suppression was presented as a percentage according to the following equation.
Suppression rate
Blocking IDO1
To test the effect of IDO1 on PBMC suppression we added the IDO inhibitor Epacadostat (EPA) (Selleck chemicals, United States) to the PBMC suppression assay. EPA was added at increasing concentrations at the time of priming and the effect on fMSC mediated immune suppression was assessed. Effective IDO1 suppression was demonstrated using the ProcartaPlex (ThermoFisher, Australia).
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical significance was determined without correction for multiple comparisons using the Mann Whitney test, with p < 0.05 were considered as statistically significant and significance level is represented by * = p < 0.05, ** = p < 0.01, ***p < 0.001.
Results
Male and female MSCs have similar growth and phenotypical characteristics
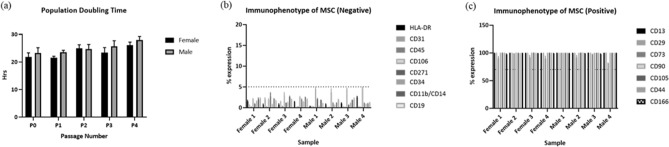
MSC doubling per day and total doublings showed no significant differences between the donors up to passage 4 (Fig. 1a) and all MSCs showed a fibroblastic like morphology (supplementary figure S1).
Figure 1.
(a) Both male and female MSCs showed similar growth population doubling times (h), (b) did not express HLA-DR, CD31, CD45, CD106, CD271, CD34, CD11b/CD14 and CD19 and (c) were positive for CD13, CD29, CD73, CD90, CD105, CD44 and CDCD166.
Additionally, male and female showed cell surface expression patterns consistent with MSC characterisation9,11 negative for CD45, CD34, CD11b, HLA-DR and CD19 and Positive (≥ 70%) for classical MSC markers CD90, CD105, CD73. In addition, in an extended MSC characterisation panel all MSC were negative (≤ 5%) for CD31 and CD271 and positive for CD166, CD44, CD13 and CD29 and negative (≤ 5%) for CD31 and CD271 via FACS (Fig. 1b,c).
Female MSCs have greater immunosuppressive properties than male MSCs
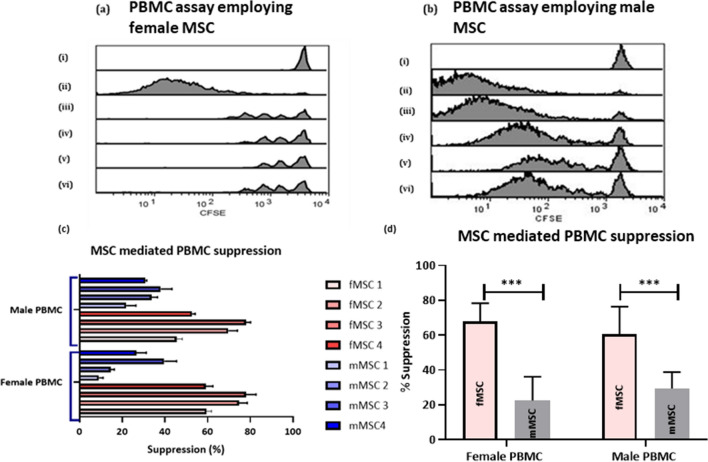
To test for difference in the immunosuppressive properties of MSCs derived from males (mMSC) and females (fMSC), MSCs were used to alter PBMC proliferation. Isolated male and female PBMC were stained with CellTrace CFSE and subsequently stimulated with MACSiBead anti-CD2, anti-CD3, anti-CD28 microparticles. PBMC from both male and females were cultured with primed male and female MSCs and were analysed on FACSCalibur for immune suppression. Figure 2 shows representative histograms showing the effect of fMSC (Fig. 2a) and mMSC (Fig. 2b) on PBMCs. The greater the loss of fluorescence, the greater the amount of proliferation. The suppression of PBMCs proliferation is represented by a shift in fluorescent intensity toward the unstimulated control. All fMSCs showed a more pronounced shift toward unstimulated controls when combined with PBMCs relative to mMSCs (Fig. 2a,b). Moreover, fMSC donors consistently presented a higher suppression rate than mMSC (Fig. 2c). The suppression rates were significantly higher (p < 0.0001) when fMSC were used to suppress both male and female PBMC (60.7 ± 15.6 and 67.9 ± 10.4), relative to mMSC (22.5 ± 13.6 and 29.4 ± 9.3) (Fig. 2d). This data suggests increased MSC mediated suppression of PBMCs by fMSC compared to mMSC is entirely due to the competency of fMSC.
Figure 2.
Example of FACS analysis of the PBMC proliferation assay using the FL-1 channel (a) PBMC proliferation assay using fMSC (i) control unstimulated fPBMC, (ii) control activated fPBMC, (iii) activated fPBMC + primedc fMSC donor 1, (iv) activated fPBMC + primed fMSC donor 2, (v) activated fPBMC + primed fMSC donor 3, (vi) activated fPBMC + primed fMSC donor 4 compared to (b) PBMC assay using mMSC (i) control unstimulated fPBMC, (ii) control activated fPBMC, (iii) activated fPBMC + primed mMSC donor 1, (iv) activated fPBMC + primed mMSC donor 2, (v) activated fPBMC + primed mMSC donor 3, (vi) activated fPBMC + primed mMSC donor 4. (c) Individual MSC mediated immune suppression of both male and female PBMC expressed as a percentage indicatiing the suppression rate and (d)suppression (mean ± SEM) of fPBMC (n = 3) and mPBMC (n = 3) against both fMSC (n = 4) and mMSC (n = 4).
Male and female MSCs respond similarly to inflammatory mediators
In addition to T-cell inhibition via paracrine mediators, it has long been recognised that cell surface markers including iCAM-1, VCAM-1 and PD-L1 play essential roles in MSC potency through their role in mediating cell-to cell contact40–44. Since primed fMSC have been shown to have a greater immune suppression than primed mMSC, this prompted us to ask whether the MSC response to inflammation was sex specific. To test this, we assessed the expression of iCAM-1, VCAM-1 and PD-L1 together with Interferon Gamma Receptor 1 (IFGR1) HLA-DR, HLA-ABC, HLA-G and HLA-E.
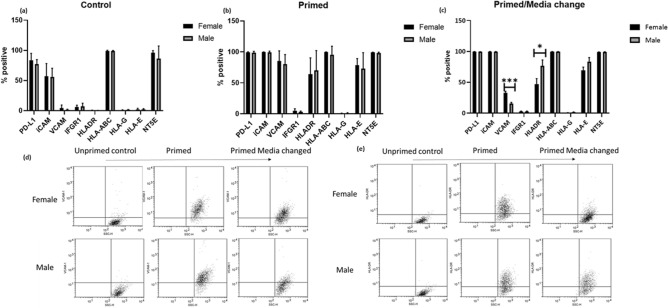
When MSC were not exposed (unprimed control) to inflammatory cytokines, there was no difference in the level of expression of iCAM, NT5E, HLA-ABC, PD-L1, HLA -DR, HLA-G, HLA-E or VCAM-1 (Fig. 3a).
Figure 3.
MSC surface marker expression (mean ± SEM) of (a) unprimed (no addition of IFN-γ/TNF-α) (b) pimed (100 ng/mL IFN-γ, 10 ng/mL TNF-α). Both fMSC and mMSC show similar cell surface characteristics when functional markers PD-L1, iCAM, VCAM, IFGR1, NT52 and HLA markers DR, ABC, G were analysed. (c) Cell surface markers were analysed 24 h post priming. (d,e) Dot plots for significance differences (p > 0.05) after media change for (d) VCAM and (e) HLA-DR (*p < 0.05, **p < 0.01, ***p < 0.001).
When MSC were primed by exposing them to inflammatory cytokines, IFN-γ and TNF-α, (Fig. 3b) all donors upregulated VCAM-1 (fMSC 85.5 ± 16, mMSC 80.27 ± 15.4), iCAM-1 (fMSC 99.77 ± 0.2, mMSC 99.14 ± 2.1), PD-L1 (fMSC 99.3 ± 0.85, mMSC 98.8 ± 2.05), HLA-DR (fMSC 64.1 ± 26.2, mMSC 70.1 ± 32), HLA-E (fMSC 78.8 ± 10.4, mMSC 73.04 ± 25.7) and NT5E (fMSC 95.9% ± 3.8. mMSC 93.1 ± 3.6). In contrast, there was no effect on the expression level of HLA-G and IFNGR1. HLA-G remains virtually undetected (< 5%) across all samples tested.
This indicates that all MSC donors irrespective of sex, possess similar ability to regulate cell surface markers when presented with an inflammatory microenvironment.
Post primed female MSCs sustain VCAM-1 while male MSCs sustain HLA-DR
Since there was no difference in the induction of cell surface receptor expression between male and female MSC, this prompted us to investigate how mMSC and fMSC respond post inflammation. MSCs were primed for 24 h and cultured for a further 24 h in MSC culture media. MSCs were assessed for immunoregulatory cell surface markers. Figure 3c–e shows fMSC sustain the presence of VCAM-1 (p = 0.002) more so than mMSC, while mMSC sustain HLA-DR expression more than fMSC (p = 0.04). There was no significant difference in the expression of PD-L1, iCAM, IFNGR1, HLA-ABC, HLA-G, HLA-E and NT5E 24 h post priming.
Male and female MSCs selectively regulate CD8, CD25 and CD69 expression in activated PBMCs
It is well known that MSC interaction with CD4+ T cells is imperative to immunomodulation. MSCs alter T-cell phenotypes from an inflammatory to anti-inflammatory phenotype45,46. To investigate whether inherent differences exist between male and female PBMCs and/or how MSCs interact with PBMCs and alter their phenotype, we assessed cell surface markers of PBMCs alone (Resting and Activated) and after 6 days in co-culture with primed MSCs.
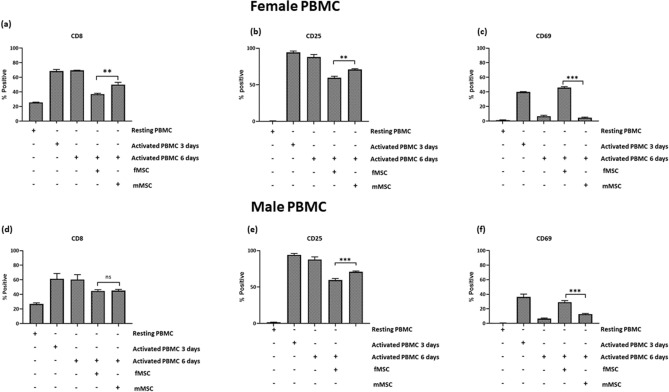
Analysis of the lymphocyte population showed no differences in the level of expression of CD3, CD4, CD8, IL-2 receptor CD25 or the early activation marker CD69 between male and female PBMCs when resting, activated with MACSiBead microparticles for 3 days (early activation) or after 6 days stimulation (supplementary Figure S3). CD8 and CD25 were upregulated in response to MACSiBead microparticle stimulation. CD8 and CD25 were increased after 3 days and remained high at day 6 (Fig. 4). In contrast, CD69 expression showed an early activation peak which declined by day 6 (resting 0.8%, 3 days activated 38.1% and 6 days activated 6.7%, Fig. 4).There was no change to CD19+ B cell numbers in response to MACSiBead microparticles (data not shown).
Figure 4.
Female PBMC (resting, activated for 3 days, activated for 6 days, activated for 6 days and co-cultured with fMSC or mMSC) and analysed for the expression of (a) CD8, (b) CD25 and (c) CD69.Male PBMC (resting, activated for 3 days, activated for 6 days, activated for 6 days and co-cultured with fMSC or mMSC) were also analysed for the expression of (d) CD8, (e) CD25 and (f) CD69 (*p < 0.05, **p < 0.01, ***p < 0.001).
When activated PBMC were co-cultured with either mMSC and fMSC for 6 days , the expression of CD8 (Fig. 4a,d) and CD25 (Fig. 4b,e) was downregulated relative to activated PBMC without MSC. The sex of the MSCs dictated the level of suppression, mMSC were less effective at downregulating CD8 in female and CD25 in male and female PBMCs than fMSC (p < 0.05). More over, in the presence of fMSCs, but not mMSCs, CD69 expression in PBMC from both sexes was sustained for 6 days (Fig. 4c,f), indicating an MSC sex related difference in mediating PBMC cell surface marker regulation.
Primed female MSCs produce higher levels of IDO, PGE-2 and IL-1RA but lower levels of G-CSF than male MSCs
To further examine the immunomodulatory capabilities of male and female MSC, we compared the secretion characteristics of both sexes post culture.
Positive analytes
There were no significant differences for Unprimed MSC-S from all donors for MCP-1, TGF-B/LAP, IL-8, IL-6, VEGF-A, Eotaxin, RANTES (supplementary Figure S3).
Induced analytes
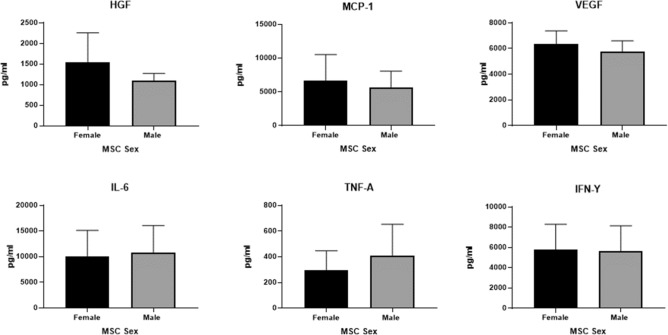
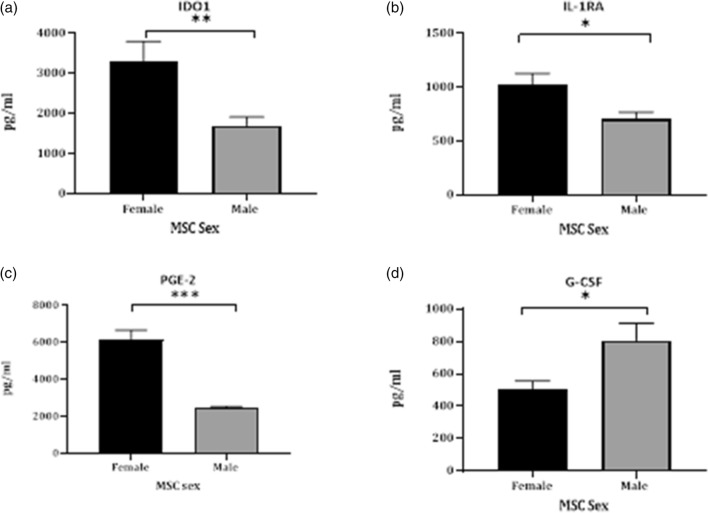
To gain further insight into the paracrine mechanisms of MSC immunomodulation, we also harvested the MSC-S 24 h post priming and assessed protein expression. MSC-S from all donor’s post priming showed increased expression of MCP-1, TGF-B/LAP, IL-8, IL-6, VEGF-A, HGF, IP-10, MIP-1α and MIP-1β (data not shown). There was no significant difference in the level of HGF, MCP-1, VEGF-A, IL-6, TNF-α or IFN-y in the secretome from male and female MSCs (Fig. 5). In contrast, MSCs from all female donors secreted higher concentrations of IDO1 than their male counterparts (Fig. 6a 3301 pg/mL vs 1699 pg/mL respectively). Female activated MSC also showed increased expression of PGE-2 (Fig. 6c fMSC 6142 pg/mL vs mMSC 2448 pg/mL) and the “IL-1 inhibitor”, IL-1RA (Fig. 6b fMSC 1025 pg/mL vs mMSC 701 pg/mL) whereas the opposite was seen for G-CSF (Fig. 6d fMSC 503 pg/mL vs mMSC 806 pg/mL). Analytes that were assessed but not detectable are indicated in the Supplementary section.
Figure 5.
Analytes measured in the secretome of male and female MSCs primed for 24 h using Procartaplex (a) Hepatocyte growth factor (HGF), (b) Monocyte chemoattractant-1 (MCP-1), (c) vascular endothelial growth factor (VEGF-A), (d) interleukin-6 (IL-6), (e) tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ).
Figure 6.
Analysis of secreted analytes from primed MSCs using the ProcartaPlex. (a) IDO1, (b) IL-1RA and (c) PGE-2 and whereas mMSC secrete higher levels of (d) G-CSF when primed with TNF-α and IFN-γ (*p < 0.05, **p < 0.01, ***p < 0.001).
Inhibition of IDO function completely ablates the suppressive function of female MSCs
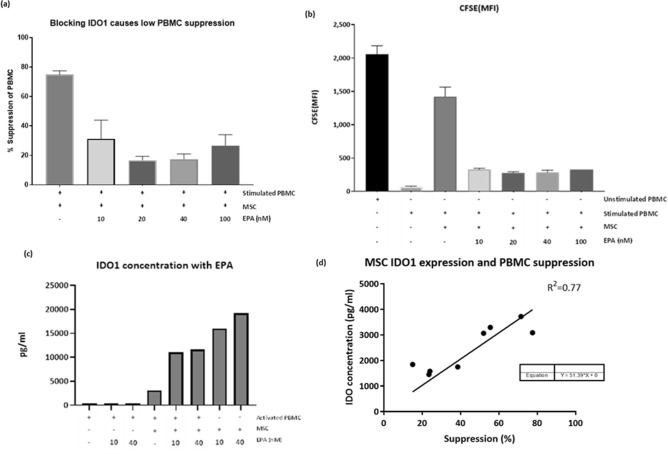
IDO1 is responsible for the catabolism of Tryptophan, which is an essential part of T-cell activation and proliferation. Our data shows fMSCs secrete greater quantities of IDO1 than mMSCs. To determine whether IDO contributed to the efficacy of fMSCs, IDO was inhibited from fMSCs using Epacadostat/INCB24360 (EPA) a known inhibitor of IDO147. CFSE stained female PBMC were co-cultured with fMSC with and without EPA treatment and the level of suppression determined.
Unstimulated PBMCs had a CFSE binding mean fluorescence intensity (MFI) of 2059 ± 249.5. Post stimulation, the PBMC MFI dropped to 70.52 ± 8.2, representing cell division. When Primed MSC were added to the culture MFI readings were closer to an unstimulated state (1421 ± 248.8) indicative of immune suppression, or impaired daughter cell division. However, when MSC were co-cultured with activated PBMC in the presence of EPA, the level of immune suppression was reduced from ~ 75 to 17% (Fig. 7a,b). Moreover, IDO1 expression by MSCs was not inhibited by EPA (Fig. 7c) rather EPA blocked IDO1 binding and inhibited the enzymatic activity necessary for T-cell activation.
Figure 7.
Assessment of fMSC and fPBMC whilst blocking the activity of IDO1 with (EPA) (a) effects of EPA on suppression PBMC (% suppression), (b) EPA effects on MSC mediated PBMC mean fluorescence intensity (MFI) and (c) effect of MSC IDO1 secretion by adding EPA to a co-culture. (d) Mean IDO1 secretion (n = 8) can be correlated (R2 = 0.77) to MSC mediated immune suppression (n = 8) and can be predicted by the equation (+) = added to culure, (−) = not added to culture.
To determine whether the expression of IDO1 correlated with PBMC suppression we carried out linear regression analysis of mean IDO1 levels from MSCs from males (n = 4) and females (n = 4) with their mean immunosuppression ability on activated PBMC. MSC immunosuppressive capacity significantly correlated with IDO1 expression levels (R2 = 0.77, Fig. 7d). Potentially providing a method for determining MSC efficacy.
Discussion
Allogeneic donor choice in cell therapy has potential clinical implications. While choices mainly focus on donor age, MSC proliferative capacity, in-vitro potency, differentiation and cell surface biomarker characterisation, little consideration has been given to donor sex and therapeutic advantage. Haemopoietic Stem Cell research for GvHD treatment have shown benefits to sex matching, making sex a highly relevant characteristic for cellular therapy including the use of Adipose derived MSC48. Despite searching for improved efficacy of MSC, donor sex is often disregarded. Indeed, data is often pooled from individual donors resulting in potentially misleading claims. The sex bias associated with disease susceptibility is well recognised30. This study shows significant differences in the efficacy of male and female MSCs in vitro and demonstrates that female MSCs are more immunosuppressive than male MSCs.
The immune suppressive and anti-inflammatory properties of MSCs are now very well established and it is clear the disease-associated activity of various immune phenotypes is central to MSCs ability to repair injured sites. In an attempt to source efficacious MSC donors, we found Female MSC are more immunosuppressive than male MSC which we believe is a result of their response to the inflammatory microenvironment and downstream immunomodulatory protein expression which may be due to inherent hormonal differences or perhaps disparities in activation pathways.
With allogeneic MSC therapy being applied to an ever-expanding range of conditions, it is a significant problem that donor and recipient interaction and matching via sex is poorly understood and not routinely considered which probed us to investigate. Examples such as the association of the “male antigen”, H-Y when assessing male to female donor/recipient response to attenuate Graft Versus Host Disease (GVHD) are overlooked where results clearly showed a sex related antibody response49–52. Although not significant, we showed a trend indicating suppressive effect of fMSCs was greater on fPBMCs than mPBMCs and similarly mMSCs showed a greater suppressive effect on mPBMCs than fPBMCs (Fig. 2d). Although further research is required to determine if sex-matching MSC therapy has the potential to provide any benefits the data presented here suggests that in-vitro, the use of fMSCs outweighs the need for sex matching.
To function, MSCs interact with a myriad of tissues each of which are phenotypically different. As such MSC full mode of action is not yet understood. MSCs are attracted to the site of injury via inflammatory signals from activated Macrophages and T cells via mediators including TNF-α, IL-1 and IFN-y and express adhesion molecules including iCAM-1, VCAM-1 and Very Late antigen-4 (VLA-4)53,54. The expression of cell surface molecules both on target and administered cells is critically important when considering the cellular therapy as a exogenous injectable43. Their role in adhesion of immune cells and homing MSCs to the site of injury is thought to be integral in allowing MSCs to perform other functions including potent immunomodulatory paracrine effects42,44,55–57. However, in our in-vitro study we showed no significant differences between the expression of these markers in MSC donors despite significant differences in immunosuppressive properties. This suggests that expression of these markers when exposed to inflammation and immune suppression may be less informative of MSC potency compared to that of secreted molecules.
To gain further information on how male and female MSC respond post-acute14, we removed the MSCs from the direct inflammation and assessed the changes in cell surface markers. HLA-DR expression was sustained in mMSC whereas fMSC continue to highly express VCAM-1. The MHC- class II complex, HLA-DR is the master behind allorecognition and is imperative in adaptive and innate immunity and therefore transplantation. Acute immune cell responses to MSC administration involves HLA, probing us to investigate its role in MSC immunomodulation58,59. Serving as a call to immune action and required for antigen presentation to CD4+ T-cells60, HLA-DR plays an essential role in inflammatory diseases. Although MHC-II presentation to CD4+ T-cells may increase the likely-hood of MSC immunomodulation acutely, sustained expression may in turn hinder suppression58. Thus, mMSC sustained expression suggests it’s a potential target for immune recognition and clearance and therefore mMSC may not be functional immune suppressors.
In contrast the adhesion molecule, VCAM-1 is sustained in fMSC and may further allow MSC to adhere to immune cells thereby allowing secondary soluble mediators to take effect. The sustained expression of different cell surface markers VCAM-1 and HLA-DR, indicates potential alternate pathways to MSC response to inflammation and may prove to play a large part in overall sustained immuno modulatory variations between the sexes.
The paracrine mechanisms of MSC mediated immune modulation are well researched. IDO1, IL-1RA, PGE-2, VEGF-A IL-6, MCP-1 and HGF are all known to play roles in immune suppression and subsequent disease modification. The correlation between MSC immunosuppression and IDO1 secretion has been extensively researched and it is often reported as one of the key potency markers for MSCs61. IFN-y mediated IDO1 secretion from MSCs has been linked with T cell suppression as well as differentiation of monocytes into M2 macrophages62. Regulated mainly by the Janus Kinase and Signal Transducer and activator of transcription (JAK/STAT) pathway63, IFN-γ mediated IDO expression not only plays direct immunosuppressive roles but plays downstream effector secondary roles with the induction of IL-10 by anti-inflammatory Macrophages (M2) and assists in regulation of other potent immunoregulatory molecules like TNF-α stimulated gene 6 (TSG-6)62,64. Our data confirms these previous reports. We have shown that not only do fMSCs secrete significantly higher levels of IDO1 than mMSCs but that blocking IDO1 almost completely ablates the suppressive effect of MSCs on PBMC proliferation. This difference in IDO1 expression likely contributes towards fMSCs showing greater efficacy in modulating PBMCs proliferation.
Links between immune modulation via IDO1, IFN-γ secretion and the female hormone, oestrogen have been previously established65,66. It is likely that the female microenvironment intrinsically commits fMSCs toward a more suppressive phenotype. Irrespective of the mechanisms that results in IDO1 expression being higher in fMSCs than mMSCs, we showed a positive correlation between MSC expression of IDO1 and immune suppression potential. This suggests that priming MSCs and measuring IDO1 may provide a method of predicting MSC potency, putting IDO1 at the forefront of a potential potency markers for MSC related immunomodulation, a cost effective and reproducible means of determining function.
Although IDO1 represents the most significant mediator of immunosuppression, it does not work in isolation as the correlation between expression and suppression levels was less than R = 1. MSC are referred to as multi-modal or-potent and yet which molecules are required in synergy to mediate their effect is yet to be determined. Another molecule produced at higher levels in fMSC than mMSCs was Prostaglandin E-2 (PGE-2) which plays functional roles across multiple body systems including immunity, gastrointestinal, neuroendocrine and central nervous systems and its expression in-vivo is believed to be crucial to cell therapy67. Cyclooxygenase 2 (COX 2) derived PGE-2 is directly involved in immunosuppression and pain67. Once activated by inflammatory signals, PGE-2 from MSCs is believed to exert a range of regulatory influence on the activation status, proliferation, differentiation and function of immune cells from adaptive and innate immunity68. Acting by a contact or paracrine manner, PGE2 has a systemic anti-inflammatory effect of reducing TNF-α, IL-6 and vascular permeability69. We were able to show that female MSC secrete significantly more PGE-2 in primed MSC-S than males. Research has indicated that sex hormones play roles in controlling the presence and function of PGE-2 and may work via a feedback loop in female adipose tissue70,71, a possible mechanism responsible for the elevated PGE-2 response to inflammatory stimuli in fMSC and subsequent immune regulation.
Additionally, we have shown that that fMSCs expressed significantly higher levels of IL-RA than mMSC. IL-1 is an important contributor in the development of OA and other inflammatory disorders and IL-1RA is a direct antagonist to IL-1. Our data is consistent with Bessler et.al (2007) who showed that fMSC express more IL-1RA than male MSCs and that males expressed higher levels of IL-1 indicating a less suppressive phenotype72. Similarly, we have shown that G-CSF which plays a central role in inflammatory arthritis was increased in mMSCs compared to fMSCs. Given that blockade of the G-CSF receptor in inflammatory arthritis models has shown positive results and is now considered an efficacious target for therapy73 highlights that mMSCs have a less immunosuppressive phenotype than fMSCs.
The assays used, deliberately investigate how the MSC respond to inflammation relatively acutely and may not attest longevity of donor potency. However, it is widely believed that the MSC have a so-called hit and run effect from their paracrine activity on inflammatory cells, which if true indicates that fMSC should be a first port of call-in cellular therapy. The poor response to inflammatory cytokines and subsequent immune modulation seen with some mMSC may in turn be due to the regulation of Inflammatory markers like TNF-α and G-CSF and also the switching of HLA markers in response to inflammatory stimuli.
Conclusion
Given that sex-matching in cellular therapy is gaining traction and has been shown to be of significance, donor selection for any clinical, commercial and preclinical cellular product should consider sex. Our research indicates that female adipose derived MSCs have far more potent immunomodulatory characteristics than their male counterparts and that the benefits of using fMSC as the MSC donor outweigh the potential benefits of sex-matching in MSC therapy. There is a need for further in-vitro and animal studies to confirm the short- and long-term advantages of sex bias, but given that MSC therapy is already happening in humans and is regarded as safe and efficacious, the groups practicing it should be mindful to consider donor/recipient sex as a means of furthering efficacy.
Supplementary Information
Acknowledgements
The author would like to thank the team at Regeneus LTD.
Abbreviations
- Ab
Antibody
- ABAM
Antibiotic antimycotic
- CCV
Cell count & viability
- CD
Cluster of differentiation
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- EPA
Epacadostat
- FBS
Foetal bovine serum
- FGFbasic
Fibroblastic growth factor
- FITC
Fluorescein isothiocyanate
- fMSC
Female Mesenchymal Stem Cells
- G-CSF
Granulocyte colony stimulating factor
- GM-CSF
Granulocyte macrophage colony stimulating factor
- HGF
Hepatocyte growth factor
- HLA
Human leukocyte antigen
- iCAM-1
Intercellular Adhesion Molecule-1
- IDO1
Indoleamine 2,3 dioxygenase
- IFGR1
Interferon gamma receptor 1
- IFN-y
Interferon gamma
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- IL-13
Interleukin 13
- IL-15
Interleukin 15
- IL-17
Interleukin 17
- IL-1Ra
Interleukin 1 receptor antagonist
- IL-1β
Interleukin 1 beta
- IL-2
Interleukin 2
- IL-4
Interleukin 4
- IL-5
Interleukin 5
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- IL-9
Interleukin 9
- IP-10/CXCL10
Interferon-γ-inducible protein 10/C-X-C motif chemokine 10
- MCP-1/CCL-2
Monocyte chemoattractant protein-1/ C-C motif chemokine 2
- MFI
Mean fluorescence intensity
- MHC-I
Major histocompatibility class I
- MHC-II
Major histocompatibility class II
- MIP-1α/CCL3
Macrophage inflammatory protein 1 alpha/C-C motif chemokine 3
- MIP-1β/CCL4
Macrophage inflammatory protein 1 beta/C-C motif chemokine 4
- mMSC
Male mesenchymal stem cells
- MSC
Mesenchymal stem cell
- MSC-S
Mesenchymal stem cell secretome
- NT5E
5′-Nucleotidase ecto
- OA
Osteoarthritis
- PBMC
Peripheral blood mononuclear cells
- PDGF-b
Platelet derived growth factor
- PD-L1
Programmed death ligand-1
- PE
Phycoerythrin
- PGE-2
Prostaglandin E-2
- PI
Propidium Iodide
- PLT
Platelet Lysate
- RANTES
Regulated upon activation, normal T cell expressed and presumably secreted
- RPMI
Roswell Park Memorial Institute
- SVF
Stromal vascular fraction
- TGF-β1
Transforming growth factor beta-1
- TLR
Toll-like receptor
- TNF-α
Tumour necrosis factor alpha
- VCAM-1
Vascular adhesion molecule-1
- VEGF-A
Vascular endothelial growth factor
- αMEM
Alpha modified eagle medium
Author contributions
F.M. analysed and interpreted all data in this manuscript and was the major contributor to the writing of this manuscript. S.M., B.H. were involved in project development and progression and S.M., B.H. and G.V. all contributed to manuscript preparation. All authors read and approve the final manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91870-4.
References
- 1.Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop. J. 2011;5(Suppl 2):242–248. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Mazini, L., Rochette, L., Amine, M., & Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci.20(10), 2523. 10.3390/ijms20102523 (2019). [DOI] [PMC free article] [PubMed]
- 4.Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial activity of mesenchymal stem cells: Current status and new perspectives of antimicrobial peptide-based therapies. Front. Immunol. 2017;8:339. doi: 10.3389/fimmu.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joo HJ, Kim J-H, Hong SJ. Adipose tissue-derived stem cells for myocardial regeneration. Korean Circ. J. 2017;47:151–159. doi: 10.4070/kcj.2016.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franck CL, Senegaglia AC, Leite LMB, de Moura SAB, Francisco NF, Ribas Filho JM. Influence of adipose tissue-derived stem cells on the burn wound healing process. Stem Cells Int. 2019;2019:1–10. doi: 10.1155/2019/2340725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravery CA, Carmen J, Fong T, Oprea W, Hoogendoorn KH, Woda J, et al. Potency assay development for cellular therapy products: An ISCT review of the requirements and experiences in the industry. Cytotherapy. 2013;15:9–19. doi: 10.1016/j.jcyt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016 doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2015;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galipeau J, Sensébé L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:1–20. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012 doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:1–10. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. No identical “mesenchymal stem cells” at different times and sites: Human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011 doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. (Lond.). 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019 doi: 10.1155/2019/5126156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyurkchiev D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014 doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012 doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2013;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren G, Zhao X, Zhang L, Zhang J, Ling W, Ren G, Zhao X, Zhang L, Zhang J, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023.Inflammatory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Jyothi PS. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2-dependent mechanism. Sci. Rep. 2016;6:1–17. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. 2019;8:467. doi: 10.3390/cells8050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015;168:140–146. doi: 10.1016/j.imlet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed. Res. Int. 2014 doi: 10.1155/2014/216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Castro, L. L., Lopes-Pacheco, M., Weiss, D. J., Cruz, F. F., & Rocco, P. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. (Berlin, Germany)97(5), 605–618. 10.1007/s00109-019-01776-y (2019). [DOI] [PubMed]
- 30.Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012;13:596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J. Autoimmun. 2012;38:J177–J186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oertelt-Prigione S, Regitz-Zagrosek V. Sex and gender aspects in clinical medicine. Sex Gend. Asp. Clin. Med. 2013 doi: 10.1007/978-0-85729-832-4. [DOI] [Google Scholar]
- 33.Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Current Diabetes Rep. 2018 doi: 10.1007/s11892-018-1031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau A, West L, Tullius SG. The impact of sex on alloimmunity. Trends Immunol. 2018 doi: 10.1016/j.it.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 37.Ono, S., Tsujimoto, H., Hiraki, S., Takahata, R., Kinoshita, M., & Mochizuki, H. Sex differences in cytokine production and surface antigen expression of peripheral blood mononuclear cells after surgery. Am. J. Surg.190(3), 439–444. 10.1016/j.amjsurg.2005.03.031 (2005). [DOI] [PubMed]
- 38.National Health and Medical Research Council. The National Statement on Ethical Conduct in Human Research. [cited 2015 Feb 5] Available from: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e72.pdf (2007).
- 39.Zuk PA, Zhu M, Mizuno H, Huang JI, Futrell WJ, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 40.Ren G, Roberts AI, Shi Y. Adhesion molecules: Key players in mesenchymal stem cell-mediated immunosuppression. Cell Adh. Migr. 2011 doi: 10.4161/cam.5.1.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi S, Shao C, Shi Andrew LYD, Ling W, Roberts AI, Guangwen Ren A, et al. Immunosuppression mesenchymal stem cells are critical for vascular cell adhesion molecule-1 in intercellular adhesion molecule-1 and inflammatory cytokine-induced inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2018;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren G, Roberts AI, Shi Y. Adhesion molecules: Key players in mesenchymal stem cell-mediated immunosuppression. Cell Adhes. Migr. 2011;5:20–22. doi: 10.4161/cam.5.1.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, Z. X., Han, Z. B., Ji, Y. R., Wang, Y. W., Liang, L., Chi, Y., Yang, S. G., Li, L. N., Luo, W. F., Li, J. P., Chen, D. D., Du, W. J., Cao, X. C., Zhuo, G. S., Wang, T., & Han, Z. C. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PloS one8(3), e59354. 10.1371/journal.pone.0059354 (2013). [DOI] [PMC free article] [PubMed]
- 45.Lysák D, Koutová L, Holubová M, Vlas T, Miklíková M, Jindra P. The quality control of mesenchymal stromal cells by in vitro testing of their immunomodulatory effect on allogeneic lymphocytes. Folia Biol. 2016;62:120–130. doi: 10.14712/fb2016062030120. [DOI] [PubMed] [Google Scholar]
- 46.Castro-Manrreza, M. E., & Montesinos, J. J. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J. Immunol. Res.2015, 394917. 10.1155/2015/394917 (2015). [DOI] [PMC free article] [PubMed]
- 47.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: From bench to bedside. Cancer Res. 2017;77:6795–6811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern M, Passweg JR, Locasciulli A, Socié G, Schrezenmeier H, Békássy AN, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–226. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- 49.Sahaf B, Yang Y, Arai S, Herzenberg LA, Herzenberg LA, Miklos DB, et al. H-Y antigen-binding B cells develop in male recipients of female hematopoietic cells and associate with chronic graft vs. host disease. Proc. Natl. Acad. Sci. USA. 2013;110:3005–3010. doi: 10.1073/pnas.1222900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson E. The role of H-Y as a minor transplantation antigen. Immunol. Today. 1982 doi: 10.1016/S0167-5699(82)80025-X. [DOI] [PubMed] [Google Scholar]
- 51.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005 doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popli R, Sahaf B, Nakasone H, Lee JYY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol. Res. 2014 doi: 10.1007/s12026-014-8514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zachar L, Bačenková D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez-Puerta GJ, Marchal JA, López-Ruiz E, Gálvez-Martín P. Role of mesenchymal stromal cells as therapeutic agents: Potential mechanisms of action and implications in their clinical use. J. Clin. Med. 2020;9:445. doi: 10.3390/jcm9020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayegh MH, Turka LA, Clarkson MR, Habicht A, Najafian N, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in Vivo. J. Immunol. 2014;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 57.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Megen KM, van’t Wout EJT, Lages Motta J, Dekker B, Nikolic T, Roep BO. Activated mesenchymal stromal cells process and present antigens regulating adaptive immunity. Front. Immunol. 2019;10:694. doi: 10.3389/fimmu.2019.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ayala García MA, González Yebra B, López Flores AL, Guaní GE. The major histocompatibility complex in transplantation. J. Transplant. 2012;2012:1–7. doi: 10.1155/2012/842141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 61.Chinnadurai R, Rajan D, Qayed M, Anderson LJ, Gibson G, Correspondence JG. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22:2504–2517. doi: 10.1016/j.celrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 63.Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karpuzoglu, E., Fenaux, J. B., Phillips, R. A., Lengi, A. J., Elvinger, F., & Ansar Ahmed, S. Estrogen up-regulates inducible nitric oxide synthase, nitric oxide, and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon-gamma. Endocrinology147(2), 662–671. 10.1210/en.2005-0829 (2006). [DOI] [PubMed]
- 66.Manukyan, M. C., Weil, B. R., Wang, Y., Abarbanell, A. M., Herrmann, J. L., Poynter, J. A., Brewster, B. D., & Meldrum, D. R. Female stem cells are superior to males in preserving myocardial function following endotoxemia. Am. J. Physiol. Regul. Integr. Comp. Physiol.300(6), R1506–R1514. 10.1152/ajpregu.00518.2010 (2011). [DOI] [PMC free article] [PubMed]
- 67.Brenneis C, Maier TJ, Schmidt R, Hofacker A, Zulauf L, Jakobsson P-J, et al. Inhibition of prostaglandin E 2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition. FASEB J. 2006;20:1352–1360. doi: 10.1096/fj.05-5346com. [DOI] [PubMed] [Google Scholar]
- 68.Kyurkchiev D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen K, Wang D, Du WT, Han Z-B, Ren H, Chi Y, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin. Immunol. 2010;135:448–458. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Kim, S., Campbell, J., Yoo, W., Taylor, J. A., & Sandler, D. P. Systemic Levels of Estrogens and PGE2 Synthesis in Relation to Postmenopausal Breast Cancer Risk. Cancer Epidemiol. Biomarkers Prev.26(3), 383–388. 10.1158/1055-9965.EPI-16-0556 (2017). [DOI] [PMC free article] [PubMed]
- 71.Sugimoto Y, Inazumi T, Tsuchiya S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015;157:73–80. doi: 10.1093/jb/mvu081. [DOI] [PubMed] [Google Scholar]
- 72.Bessler H, Osovsky M, Beilin B, Alcalay Y, Sirota L. The existence of gender difference in IL-1Ra gene polymorphism. J. Interf. Cytokine Res. 2007;27:931–935. doi: 10.1089/jir.2007.0029. [DOI] [PubMed] [Google Scholar]
- 73.Campbell IK, Leong D, Edwards KM, Rayzman V, Ng M, Goldberg GL, et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. J. Immunol. 2016;197:4392–4402. doi: 10.4049/jimmunol.1600121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.