Abstract
Introduction
Antibiotic envelopes are being developed for cardiac implantable electronic device (CIED) wrapping to reduce the risk of infections.
Methods
Fifteen CIED infection-associated bacterial isolates of Staphylococcus aureus, Staphylococcus epidermidis and Cutibacterium acnes were used to assess in vitro biofilm formation on Hylomate® compared to titanium, silicone and polyurethane coupons pre-treated with vancomycin (400 µg/ml), bacitracin (1000 U/ml) or a combination of rifampin (80 µg/ml) plus minocycline (50 µg/ml). Scanning electron microscopy (SEM) was performed to visualize bacteria on Hylomate®.
Results
There was significantly less (p < 0.05) S. aureus and S. epidermidis on Hylomate® pre-treated with vancomycin, bacitracin or rifampin plus minocycline after 24 h of incubation (≤1.00 log10 CFU/cm2) compared with titanium, silicone or polyurethane pre-treated with vancomycin, bacitracin or rifampin plus minocycline. C. acnes biofilms were not detected (≤1.00 log10 CFU/cm2) on pre-treated Hylomate® coupons.
Conclusions
This study showed that Hylomate® coupons pre-treated with antibiotics reduced staphylococcal and C. acnes biofilm formation in vitro.
Keywords: Antibacterial envelope, Cardiac device infection, Staphylococcus aureus, Staphylococcus epidermidis, Cutibacterium acnes, Biofilm
1. Introduction
There has been a 95% rise in numbers of cardiac implantable electronic device (CIED) implantations between 1993 and 2008 [1], which has, in turn, been associated with a higher burden of device replacement, generator change-outs, and upgrade/revision surgeries. The incidence of CIED infection has increased in parallel, with infection being a particular burden among those with underlying comorbidities [2]. CIED infections carry significant morbidity and mortality. The estimated annual rate of CIED infections is 1–6%, corresponding to ~ 8,000 to 13,000 CIED-related infections in the United States yearly [3]. The average cost associated with a single CIED infection event is ~$45,000–83,000, representing a significant financial burden to the healthcare system [4], [5]. Organisms associated with CIED infections attach to and grow in biofilms on generator and/or generator lead surfaces; the most frequently involved bacteria are Staphylococcus epidermidis, Staphylococcus aureus and Cutibacterium acnes [6]. The management of CIED-related infection usually includes complete device removal, including accessory hardware, as the use of systemic antibiotics alone will typically not suffice [7]. Given risks associated with treatment, prevention of CIED infections is desirable.
Several strategies to limit CIED infection have been proposed, including proper selection of patients for CIED placement, optimization of aseptic technique, administration of antibiotics at the time of device implantation, and use of antibiotic-coated implantable devices [2]. The last is intended to reduce or eliminate bacteria accessing device surfaces during implantation surgery, and includes antibacterial envelopes designed to release antimicrobial drugs directly into the CIED generator pocket [8], [9], [10]. Currently, the only antibacterial envelope available for use with CIEDs is TYRX™ (Medtronic, Minneapolis, MN), an absorbable envelope made of polypropylene and impregnated with rifampin and minocycline.
Hylomate® is a membrane made of cellulose synthesized by Acetobacter xylinum, which has been reported to be a well-tolerated material with potential biomedical applications, such as CIED wrappings [11], corneal bandages [12], wound dressings [13], treatment of oral diseases [14], and nerve repair [15]. According to Robotti and collaborators, Hylomate® is highly hydrophilic and able to decrease tissue fibrosis around CIEDs, facilitating implant removal or revision, if required [11], [16].
The aim of this study was to evaluate biofilm formation on Hylomate® compared to other surfaces used in CIED generators and generator leads after pre-treatment of these surfaces with vancomycin, bacitracin or a combination of rifampin and minocycline.
2. Methods
Fifteen CIED infection-associated bacterial isolates, including five each of S. aureus (IDRL-9774, IDRL-11332, IDRL-11567, IDRL-11905 and IDRL-11992), S. epidermidis (IDRL-11532, IDRL-11770, IDRL-11889, IDRL-11913, and IDRL-12398), and C. acnes (IDRL-11914, IDRL-11980, IDRL-12431, IDRL-12532, and IDRL-12396), collected at Mayo Clinic, Rochester, MN from 2013 to 2020, and stored in the Infectious Diseases Research Laboratory biobank, were studied. Vancomycin minimum inhibitory concentration (MIC) values were ≤2 µg/ml for S. aureus and ≤4 µg/ml for S. epidermidis. Rifampin MICs were ≤0.5 µg/ml for S. aureus and S. epidermidis. Minocycline MICs were ≤0.5 µg/ml for all study isolates.
Ability to form biofilm after pre-treatment with antimicrobial agents was assessed on 12.7 mm diameter coupons made of Hylomate® (Hylomorph AG, Zurich, Switzerland) prepared with a biopsy punch, or of titanium, silicone, and polyurethane (Biosurface Technologies Corporation, Bozeman, MT), using an in vitro assay. Coupons received no pre-treatment (control) or pre-treatment for 15 min at room temperature with 1 ml vancomycin 400 µg/ml, bacitracin 1000 U/ml, or a combination of rifampin 80 µg/ml and minocycline 50 µg/ml [17], [18] diluted according to CLSI guidelines [19]. Coupons were rinsed in sterile saline, inoculated with 103 Colony Forming Unit (CFU)/ml of bacteria in 2 ml tryptic soy broth (TSB) for staphylococci or brain heart infusion broth (BHI) supplemented with glucose 1% for C. acnes, and incubated at 37°C on an orbital shaker (110 rpm) with staphylococci incubated aerobically, and C. acnes incubated anaerobically. Three coupons were removed at each of 2, 4, 6, and 24 h for staphylococci, and 24, 36, 48 and 60 h for C. acnes. After removal, coupons were rinsed in 2 ml saline, placed in 1 ml saline, vortexed for 30 s, sonicated for 5 min, and then vortexed again to disaggregate biofilms and create bacterial suspensions. Sonicate fluids were serially diluted in sterile saline and 100 µl of the dilutions spread on blood agar plates, and incubated at 37 °C in 5% CO2 for 24 h for staphylococci or 72 h under anaerobic conditions for C. acnes. The number of CFU per cm2 was determined and results expressed as log10 CFU/cm2. If no growth was present, results were reported log10 CFU/cm2 < 1.0.
Descriptive summaries of bacterial densities (log10 CFU/cm2) were reported as medians (minimums, maximums) by combinations of material type, isolate and treatment for each bacterial species studied. Effects of titanium, silicone and polyurethane on reductions in bacterial concentrations relative to Hylomate® after 24 h or 60 h incubation, were assessed for each species/isolate/treatment combination using Wilcoxon rank-sum tests. Non-parametric tests were used due to small sample sizes and non-normal data distributions. All statistical tests were 2-sided, with an α level of 0.05. Due to small sample sizes, no formal adjustment for multiple comparisons was performed. Analysis was performed using SAS version 9.4 software (SAS Inc, Cary, NC).
To visualize bacteria on Hylomate®, scanning electron microscopy (SEM) was performed on Hylomate® coupons incubated with bacteria overnight. After incubation, coupons were rinsed in sterile water, and then fixed in Trump's fixative solution (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.2) [20]. Coupons were rinsed for 30 min in 2 changes of 0.1 M phosphate buffer, pH 7.2. Following dehydration in progressive concentrations of ethanol to 100%, samples underwent critical point drying. Coupons were mounted on aluminum stubs and sputter coated with gold/palladium. Images were captured on an Hitachi S4700 scanning electron microscope operating at 3KV.
3. Results
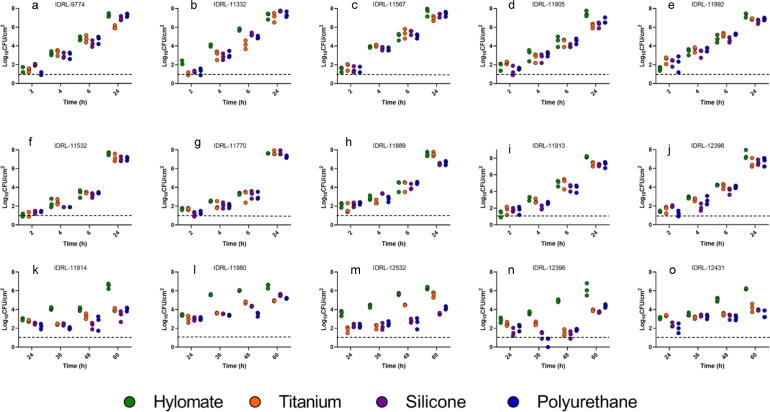
Fig. 1 shows biofilm formation on non pre-treated coupons (i.e., blank). There was less staphylococcal biofilm formed on Hylomate® in comparison to titanium, silicone and polyurethane coupons when pre-treated with vancomycin, bacitracin or rifampin plus minocycline after 24 h of incubation (p < 0.05; Fig. 2, Fig. 3, Fig. 4). The combination of rifampin and minocycline was the most active overall of all antibiotics/antibiotic combinations tested, and the only regimen that decreased biofilm formation of some isolates on titanium, silicone and polyurethane coupons.
Fig. 1.
Quantitative pre-treated with vancomycin or bacitracin culture of Staphylococcus aureus (a, b, c, d, e), Staphylococcus epidermidis (f, g, h, i, j) and Cutibacterium acnes (k, l, m, n, o) on non pre-treated (i.e., blank) Hylomate®, titanium, silicone and polyurethane coupons (controls).
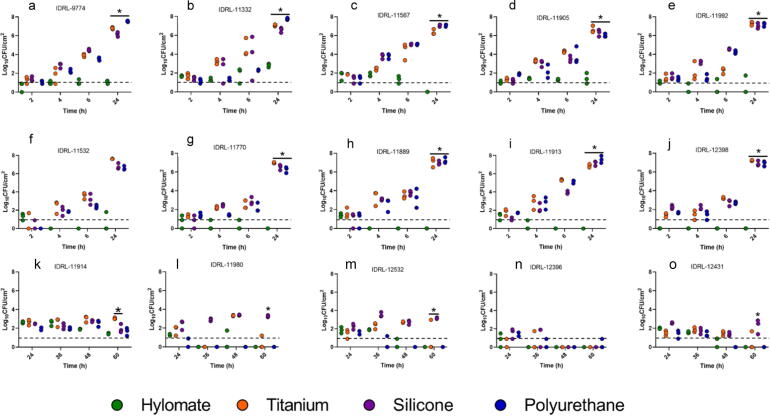
Fig. 2.
Quantitative culture of Staphylococcus aureus (a, b, c, d, e), Staphylococcus epidermidis (f, g, h, i, j) and Cutibacterium acnes (k, l, m, n, o) on Hylomate®, titanium, silicone and polyurethane coupons pre-treated with vancomycin (400 µg/ml). *Represents significant difference (p < 0.05) between Hylomate and other materials after 24 h or 60 h incubation for staphylococci and C. acnes respectively.
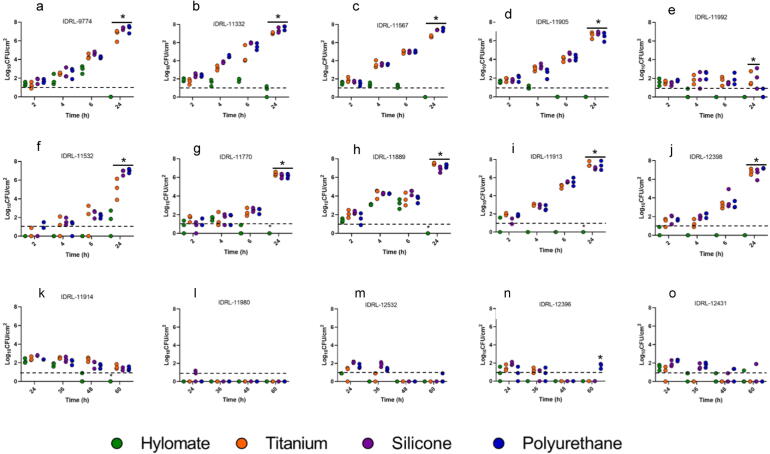
Fig. 3.
Quantitative culture of Staphylococcus aureus (a, b, c, d, e), Staphylococcus epidermidis (f, g, h, i, j) and Cutibacterium acnes (k, l, m, n, o) on Hylomate®, titanium, silicone and polyurethane pre-treated with bacitracin (1000 U/ml). *Represents significant difference (p < 0.5) between Hylomate and other materials 24 h incubation.
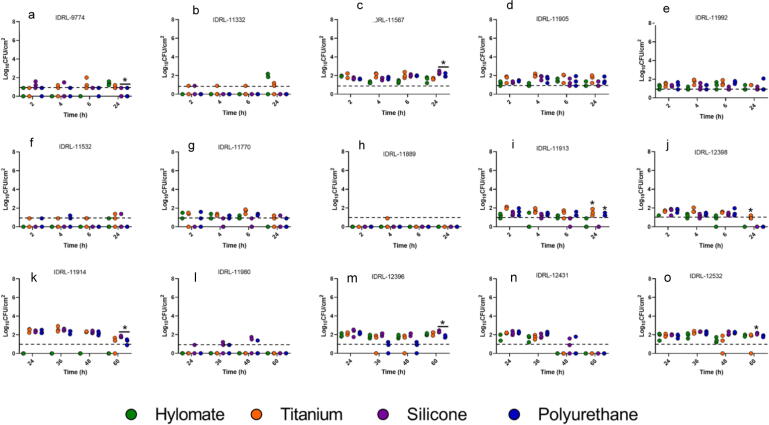
Fig. 4.
Results of quantitative culture of Staphylococcus aureus (a, b, c, d, e), Staphylococcus epidermidis (f, g, h, i, j) and Cutibacterium acnes (k, l, m, n, o) on Hylomate®, titanium, silicone and polyurethane coupons pre-treated with rifampin (80 µg/ml) plus minocycline (50 µg/ml). *Represents significant difference (p < 0.05) between Hylomate and other materials after 24 h or 60 h incubation for staphylococci and C. acnes, respectively.
The amount of S. aureus on Hylomate® pre-treated with vancomycin, bacitracin or rifampin plus minocycline after incubation for 24 h (median 1.20, ≤1.00 and 1.85 log10 CFU/cm2, respectively) was lower (p < 0.05) than on titanium pre-treated with vancomycin or bacitracin (median 6.80 and 6.66 log10 CFU/cm2, respectively), silicone pretreated with vancomycin or bacitracin pretreated with vancomycin or bacitracin (median 6.64 and 7.29 log10 CFU/cm2, respectively), or polyurethane pretreated with vancomycin or bacitracin (median 7.24 and 7.44 log10 CFU/cm2, respectively). Rifampin plus minocycline was the only pre-treatment that resulted in biofilm reductions on titanium, silicone or polyurethane coupons (median 1.37, ≤1.00, and ≤1.00 log10 CFU/cm2, respectively) after 24 h, with no significant difference between bacterial quantities on Hylomate® and the other studied substrates.
Similarly, there was a difference (p < 0.05) in the amount of S. epidermidis on Hylomate® pre-treated with vancomycin, bacitracin or rifampin plus minocycline after 24 h of incubation (≤1.00 log10 CFU/cm2) compared with titanium (median 7.20 and 6.80 log10 CFU/cm2), silicone (median 6.80 and 6.90 log10 CFU/cm2), or polyurethane (median 7.01 and 7.15 log10 CFU/cm2) pre-treated with vancomycin or bacitracin, respectively. Biofilm was not detected on titanium, silicone or polyurethane coupons pre-treated with rifampin plus minocycline (≤1.00 log10 CFU/cm2).
At 60 h, C. acnes biofilms were not detected (≤1.00 log10 CFU/cm2) on Hylomate® pre-treated with vancomycin, bacitracin or rifampin plus minocycline, polyurethane or titanium pre-treated with bacitracin or vancomycin, or silicone pre-treated with bacitracin. Only silicone pre-treated with vancomycin (median 2.50 log10 CFU/cm2), and titanium, silicone and polyurethane pre-treated with rifampin plus minocycline (median 1.37, 1.74 and 1.37 log10 CFU/cm2) had detectable biofilm, with significant differences (p < 0.05) between Hylomate® and silicone pre-treated with vancomycin, and between Hylomate® and silicone pre-treated with rifampin plus minocycline.
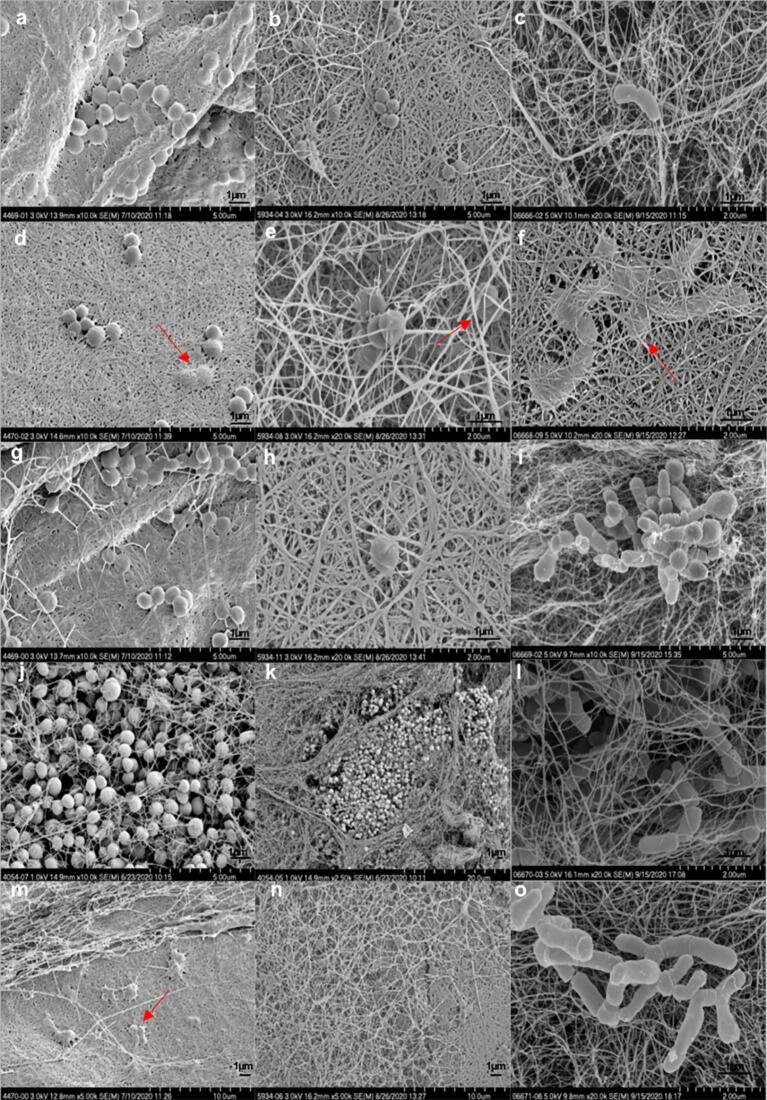
SEM images (Fig. 5) show biofilms formed on Hylomate® with no antibiotic treatment, with bacterial cells apparently penetrating Hylomate® indicated by the red arrows.
Fig. 5.
Scanning electron micrographs of Staphylococcus aureus (a, d, g, j, m), Staphylococcus epidermidis (b, e, h, k, n), and Cutibacterium acnes (c, f, i, l, o) biofilms on cellulose after 24 (S. aureus and S. epidermidis), and 60 h (C. acnes) of incubation at different magnifications. Red arrows indicate bacterial cell penetration on Hylomate®. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Results of this study demonstrate that Hylomate® pre-treated with antibiotics reduced the ability of S. aureus, S. epidermidis, and C. acnes to form biofilms. This may be facilitated by the hydrophilicity of Hylomate®, potentially enabling better absorption of antibiotics used as pre-treatments when compared with the other materials studied. We note that these are in vitro results and do not imply in vivo activity, such as with CIED implant surgery.
Several studies have been carried out to test the activity and cost effectiveness of antibacterial envelopes in various patient groups undergoing de novo CIED implantation, revisions, or upgrades. The most comprehensive CIED clinical trial was the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT), which evaluated the antibacterial envelope TYRXTM impregnated with rifampin and minocycline in 6,983 patients at high risk for infection. There was a 40% reduction in the incidence of major CIED infections within 12 months of initial procedures, in comparison to standard-of-care infection prevention strategies [9]. In a follow-up study, beneficial effects of the TYRXTM envelope on reduction of the risk of CIED infection were sustained beyond the first year post-procedure without no apparent increased risk of complications [21].
A meta-analysis review of 11,897 high-risk patients from six studies showed risk reductions of CIED infections among patients with absorbable and non-absorbable antibacterial envelopes (TYRXTM and AISGIRx®) impregnated with rifampin plus minocycline compared with those managed conventionally [22]. There was a reported trend of lower mortality in those with antibacterial envelopes, although this finding did not reach statistical significance. How rifampin- and minocycline-loaded Hylomate® might compare to TYRXTM impregnated with rifampin and minocycline is unknown.
Using an extracellular-matrix envelope derived from porcine small intestinal submucosa hydrated with gentamicin, Sohail et al. demonstrated in vitro elimination of microorganisms when envelopes were incubated with S. aureus, S. epidermidis, Escherichia coli, Pseudomonas aeruginosa, or Serratia marcescens. In the same study, the authors showed bacterial reductions when the envelope was tested in a rabbit cardiac device pocket infection model after seven days of implantation compared with controls [23].
Here, the most active pre-treatment was the combination of rifampin and minocycline. Clinical studies incorporating rifampin and minocycline into central venous catheters, cerebrospinal fluid drains, and hemodialysis catheters have demonstrated reductions in device-related infections [24], [25], [26].
5. Conclusion
This study showed that Hylomate® coupons pre-treated with antibiotics reduced staphylococcal and C. acnes biofilm formation in vitro. This suggests that antibiotic-impregnated Hylomate® should be further evaluated as a potential strategy to prevent CIED infections, including animal model and potentially human studies.
Funding
This study was supported by Hylomorph.
CRediT authorship contribution statement
Mariana Albano: Methodology, Investigation, Validation, Writing - original draft. Kerryl E. Greenwood-Quaintance: Conceptualization, Methodology, Writing - review & editing. Melissa J. Karau: Conceptualization, Methodology, Writing - review & editing. Jayawant N. Mandrekar: Formal analysis. Robin Patel: Funding acquisition, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
Dr. Patel reports grants from Merck, ContraFect, TenNor Therapeutics Limited, Hylomorph and Shionogi. Dr. Patel is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, and Qvella; monies are paid to Mayo Clinic. Dr. Patel is also a consultant to Netflix. In addition, Dr. Patel has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Dr. Patel receives an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course.
Acknowledgements
We thank Scott I. Gamb and Jon E. Charlesworth from the Microscopy and Cell Analysis Core, Mayo Clinic Rochester, for assisting with the sample processing and obtaining electron microscopy images.
References
- 1.Greenspon A.J., Patel J.D., Lau E., Ochoa J.A., Frisch D.R., Ho R.T. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011;58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Barbar T., Patel R., Thomas G., Cheung J.W. Strategies to prevent cardiac implantable electronic device infection. J. Innov. Card Rhythm. Manag. 2020;11:3949. doi: 10.19102/icrm.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohail M.R., Eby E.L., Ryan M.P., Gunnarsson C., Wright L.A., Greenspon A.J. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ: Arrhythmia Electrophysiol. 2016;9:e003929. doi: 10.1161/CIRCEP.116.003929. [DOI] [PubMed] [Google Scholar]
- 4.Greenspon A.J., Eby E.L., Petrilla A.A., Sohail M.R. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin. Electrophysiol. 2018;41:495–503. doi: 10.1111/pace.13300. [DOI] [PubMed] [Google Scholar]
- 5.Shariff N., Eby E., Adelstein E., Jain S., Shalaby A., Saba S. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J. Cardiovasc. Electrophysiol. 2015;26:783–789. doi: 10.1111/jce.12684. [DOI] [PubMed] [Google Scholar]
- 6.Esquer Garrigos Z., Sohail M.R., Greenwood-Quaintance K.E., Cunningham S.A., Vijayvargiya P., Fida M. Molecular approach to diagnosis of cardiovascular implantable electronic device infection. Clin. Infect. Dis. 2020;70:898–906. doi: 10.1093/cid/ciz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagpal A., Baddour L.M., Sohail M.R. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ: Arrhythmia Electrophysiol. 2012;5:433–441. doi: 10.1161/CIRCEP.111.962753. [DOI] [PubMed] [Google Scholar]
- 8.Ellis C.R., Kolek M.J. Rising infection rate in cardiac electronic device implantation; the role of the AIGISRx® antibacterial envelope in prophylaxis. Comb. Prod. Ther. 2011;1:003. [Google Scholar]
- 9.Tarakji K.G., Mittal S., Kennergren C., Corey R., Poole J.E., Schloss E. Antibacterial envelope to prevent cardiac implantable device infection. N. Engl. J. Med. 2019;380:1895–1905. doi: 10.1056/NEJMoa1901111. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Doshi R., Shariff M. Role of antibiotic envelopes in preventing cardiac implantable electronic device infection: a meta-analysis of 14, 859 procedures. J. Arrhythm. 2020;36:176–179. doi: 10.1002/joa3.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robotti F., Sterner I., Bottan S., Rodríguez J.M.M., Pellegrini G., Schmidt T. Microengineered biosynthesized cellulose as anti-fibrotic in vivo protection for cardiac implantable electronic devices. Biomaterials. 2020;229 doi: 10.1016/j.biomaterials.2019.119583. [DOI] [PubMed] [Google Scholar]
- 12.Anton-Sales I., D'Antin J.C., Fernández-Engroba J., Charoenrook V., Laromaine A., Roig A. Bacterial nanocellulose as a corneal bandage material: a comparison with amniotic membrane. Biomater. Sci. 2020;8:2921–2930. doi: 10.1039/d0bm00083c. [DOI] [PubMed] [Google Scholar]
- 13.Orlando I., Basnett P., Nigmatullin R., Wang W., Knowles J.C., Roy I. Chemical modification of bacterial cellulose for the development of an antibacterial wound dressing. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.557885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue B.S., Streit S., dos Santos Schneider A.L., Meier M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020;148:1098–1108. doi: 10.1016/j.ijbiomac.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Kowalska-Ludwicka K., Cala J., Grobelski B., Sygut D., Jesionek-Kupnicka D., Kolodziejczyk M. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch Med Sci. 2013;9:527. doi: 10.5114/aoms.2013.33433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemm D., Kramer F., Moritz S., Lindström T., Ankerfors M., Gray D. Nanocelluloses: a new family of nature-based materials. Angew. Chem. Int. Ed. 2011;50:5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- 17.Krahn A.D., Longtin Y., Philippon F., Birnie D.H., Manlucu J., Angaran P. Prevention of arrhythmia device infection trial: the PADIT trial. J. Am. Coll. Cardiol. 2018;72:3098–3109. doi: 10.1016/j.jacc.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 18.Biffi M. The never-ending story of CIED infection prevention: Shall we WRAP-IT and go? J. Cardiovasc. Electrophysiol. 2019;30:1191–1196. doi: 10.1111/jce.14010. [DOI] [PubMed] [Google Scholar]
- 19.CLSI . 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2020. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. [Google Scholar]
- 20.McDowell E., Trump B. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 1976;100:405–414. [PubMed] [Google Scholar]
- 21.Mittal S., Wilkoff B.L., Kennergren C., Poole J., Corey R., Bracke F. The world-wide randomized antibiotic envelope infection prevention (WRAP-IT) trial: long-term follow-up. Heart Rhythm. 2020;17:1115–1122. doi: 10.1016/j.hrthm.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Ullah W., Nadeem N., Haq S., Thelmo F.L., Jr, Abdullah H.M.A., Haas D.C. Efficacy of antibacterial envelope in prevention of cardiovascular implantable electronic device infections in high-risk patients: a systematic review and meta-analysis. Int. J. Cardiol. 2020;350:51–56. doi: 10.1016/j.ijcard.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Sohail M.R., Esquer Garrigos Z., Elayi C.S., Xiang K., Catanzaro J.N. Preclinical evaluation of efficacy and pharmacokinetics of gentamicin containing extracellular-matrix envelope. Pacing Clin. Electrophysiol. 2020;43:341–349. doi: 10.1111/pace.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzinikolaou I., Finkel K., Hanna H., Boktour M., Foringer J., Ho T. Antibiotic-coated hemodialysis catheters for the prevention of vascular catheter–related infections: a prospective, randomized study. Am. J. Med. 2003;115:352–357. doi: 10.1016/s0002-9343(03)00367-x. [DOI] [PubMed] [Google Scholar]
- 25.Hockenhull J., Dwan K., Boland A., Smith G., Bagust A., Dündar Y. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol. Assess. 2008;12 doi: 10.3310/hta12120. [DOI] [PubMed] [Google Scholar]
- 26.Zabramski J.M., Whiting D., Darouiche R.O., Horner T.G., Olson J., Robertson C. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J. Neurosurg. 2003;98:725–730. doi: 10.3171/jns.2003.98.4.0725. [DOI] [PubMed] [Google Scholar]