Abstract
Although exposure to overweight and obesity at different ages is associated to a higher risk of type 2 diabetes, the effect of different patterns of exposure through life remains unclear. We aimed to characterize life-course trajectories of weight categories and estimate their impact on the incidence of type 2 diabetes. We categorized the weight of 7203 participants as lean, normal or overweight at five time-points from ages 7–55 using retrospective data. Participants were followed for an average of 19 years for the development of type 2 diabetes. We used latent class analysis to describe distinctive trajectories and estimated the risk ratio, absolute risk difference and population attributable fraction (PAF) associated to different trajectories using Poisson regression. We found five distinctive life-course trajectories. Using the stable-normal weight trajectory as reference, the stable overweight, lean increasing weight, overweight from early adulthood and overweight from late adulthood trajectories were associated to higher risk of type 2 diabetes. The estimated risk ratios and absolute risk differences were statistically significant for all trajectories, except for the risk ratio of the lean increasing trajectory group among men. Of the 981 incident cases of type 2 diabetes, 47.4% among women and 42.9% among men were attributable to exposure to any life-course trajectory different from stable normal weight. Most of the risk was attributable to trajectories including overweight or obesity at any point of life (36.8% of the cases among women and 36.7% among men). The overweight from early adulthood trajectory had the highest impact (PAF: 23.2% for woman and 28.5% for men). We described five distinctive life-course trajectories of weight that were associated to increased risk of type 2 diabetes over 19 years of follow-up. The variability of the effect of exposure to overweight and obesity on the risk of developing type 2 diabetes was largely explained by exposure to the different life-course trajectories of weight.
Subject terms: Diabetes, Obesity, Epidemiology
Introduction
The prevalence of high body weight, including overweight and obesity, has increased considerably over the last several decades, making it one of the main contributors to the global burden of disease and a pressing public health concern1, 2. Although there is a strong association between high body weight and increased risk of type 2 diabetes, the mechanisms underlying this association remain unclear3, 4, and so does the understanding of differences in the risk of type 2 diabetes between individuals5.
Weight has an important variability through life and a person’s body weight is the result of a complex combination of social, behavioral and genetic factors6, 7. Therefore, the consequences of exposure to high body weight are also heterogeneous8, 9. Although overweight and obesity considerably increase the risk of type 2 diabetes, most overweight and obese individuals never develop this complication5. From a public health perspective, there is a need for more precise identification of people whit a higher risk of type 2 diabetes.
Epidemiological evidence of the association between body weight and type 2 diabetes usually comes from studies linking weight status at a certain point in time or some measure of cumulative exposure to the occurrence of type 2 diabetes, typically several years later in life10–14. The segmented examination of the effects of high body weight comes with important limitations. Studies looking into a single time of exposure ignore the time-varying effects of changes in weight through different stages of life, while measures of cumulative exposure assume a constant effect at different ages, ignoring important physiological changes in metabolism as well as in social and behavioral factors related to age.
The use of longitudinal data and analyses has partially addressed these limitations15–17. However, longitudinal studies usually ignore the important differences observed between individuals exposed to overweight or obesity by generalizing the effect of exposure across the population. Furthermore, studies covering the whole life span, from childhood to adulthood, are scarce.
Identifying characteristics patterns of changes in weight through life by grouping individuals into more homogeneous sub-groups can lead to better-targeted and more precise public health strategies18, 19. Previous studies have examined the existence of sub-populations of individuals based on the developmental trajectories of weight at different points of life20–22, and some have investigated the association between different trajectories and the occurrence of type 2 diabetes23–26. However, little is known about the public health impact of different life-course weight trajectories.
Therefore, we aimed to ascertain characteristics life-course trajectories of weight from childhood to adulthood, estimate their association to the risk of type 2 diabetes, and to evaluate their effect at a population level.
Results
The study sample for this analysis included 7203 (4820 women and 2383 men) participants of the Stockholm Diabetes Prevention Program cohort (SDPP) who received questions about recalled weight at different ages. During the study period, 65 (0.9%) participants were lost to follow-up.
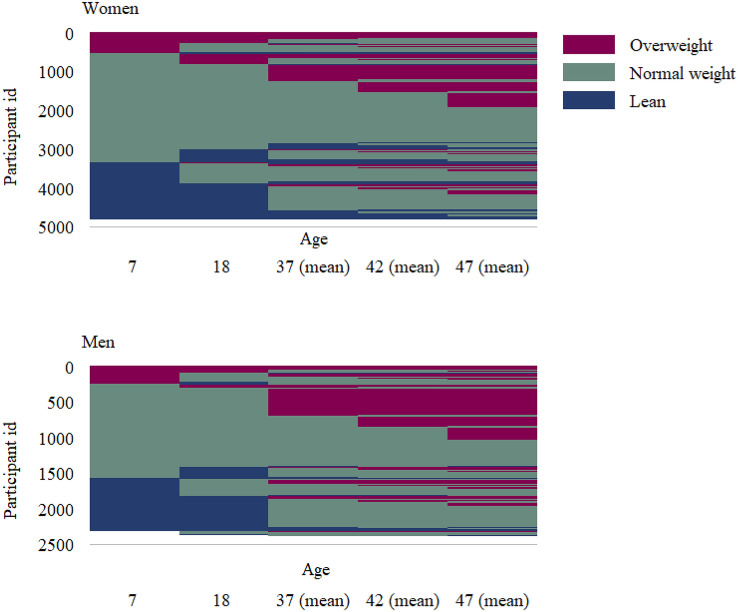
Self-reported categories of body weight at ages 7 and 18, body mass index (BMI) at ages 37 and 42, as well as measured BMI at a mean age 47 were used to ascertain the most common patterns of life-course development of weight categories. Figure 1 shows the individual level transitions between weight categories at each time point. A detailed description of recalled weight categories at each age is available online as supplemental Table S1.
Figure 1.
Individual weight categories at each of the study time-points. Categories of body weight were self-reported for childhood (7 years), adolescence (18 years), ten years before the study baseline (37 years in average) and five years before the study (42 years in average), and measured during the baseline examination (47 years in average). The individual participants are listed in the Y-axis; a change in color along time-points in the X-axis represents a change of category for an individual.
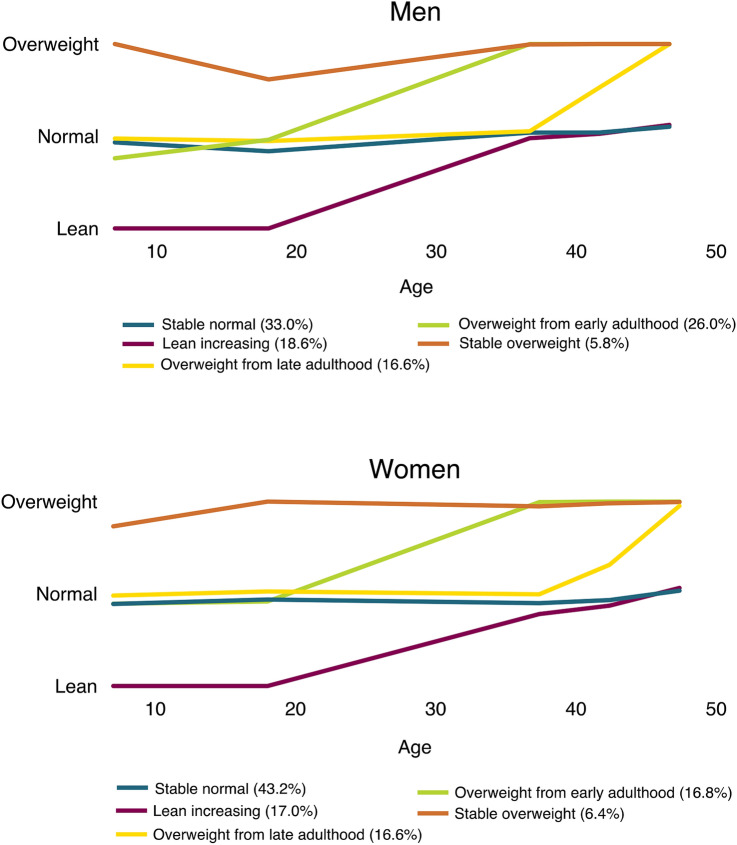
Using group-based trajectory modelling (GBTM), we found five distinct life-course weight trajectories, which exhibited similar patterns of changes for both sexes; although, there were differences in the proportion of the population within each trajectory group (Fig. 2).
Figure 2.
Life-course trajectories of weight categories by sex. The y-axis represents the probability of belonging to each weight category (lean, normal weight and overweight) estimated using group-based trajectory models. The predicted proportion of the population in each trajectory group is presented in parenthesis.
The five trajectories were labelled based on their particular patterns as: (1) a stable normal weight trajectory, with 43.2% of all women and 33.0% of all men, largely made up of those with a normal weight throughout their life-course. (2) A stable overweight trajectory, with 6.4% of all women and 5.8% of all men, characterized by overweight or obesity from childhood and continues to adulthood. (3) A lean increasing weight trajectory, including 17.0% of all women and 18.6% of all men, that largely consisted of those who were lean in childhood and increased in weight in early adulthood without ever reporting overweight or obesity. (4) An overweight from early adulthood trajectory, with 16.8% of all women and 26.0% of men who developed overweight or obesity between their 20 s and 30 s. And (5) An overweight from late adulthood trajectory, with 16.6% of all women and 16.6% of men, characterized by people developing overweight or obesity after the age of 40.
Table 1 describes the baseline characteristics of the participants in each trajectory group. All the covariates were significantly different between trajectories. In comparison to the other trajectories, participants in the stable high weight and the high weight from early adulthood trajectory groups were characterized by having higher BMIs, higher systolic and diastolic blood pressure, lower levels of physical activity and educational attainment, and worse self-reported health at the time of the baseline examination for this study. Moreover, subjects in these groups had a higher prevalence of comorbidities, and a higher proportion had a family history of diabetes, as well as of gestational diabetes among women.
Table 1.
Baseline characteristics of the study participants.
| Stable normal weight | Stable overweight | Lean increasing weight | Overweight from early adulthood | Overweight from late adulthood | |
|---|---|---|---|---|---|
| (n = 2966) | (n = 469) | (n = 1279) | (n = 1497) | (n = 992) | |
| Sex n (%) | |||||
| Female | 2158 (72.8%) | 328 (69.9%) | 833 (65.1%) | 843 (56.3%) | 658 (66.3%) |
| Male | 808 (27.2%) | 141 (30.1%) | 446 (34.9%) | 654 (43.7%) | 334 (33.7%) |
| Mean age (SD) | 47.3 (5.1) | 46.4 (4.9) | 47.3 (4.8) | 46.4 (5.3) | 47.82 (3.6) |
| Mean BMI (SD) | 23.2 (1.9) | 31.5 (4.9) | 23.2 (2.5) | 29.6 (3.4) | 27.0 (1.9) |
| Family history of diabetes n (%) | 1430 (48.2%) | 299(63.8%) | 658 (51.4%) | 862 (57.6%) | 558 (56.3%) |
| History of gestational diabetes n (%) | 58 (2.7%) | 20 (6.1%) | 28 (3.4%) | 66 (7.8%) | 20 (3.0%) |
| Self-reported general health n (%) | |||||
| Very good | 1358 (45.8%) | 156 (33.3%) | 518 (40.5%) | 529 (35.4%) | 372 (37.6%) |
| Good | 1431 (48.3%) | 264 (56.3%) | 641 (50.2%) | 803 (53.7%) | 536 (54.1%) |
| Neither good nor bad | 132 (4.5%) | 37 (7.9%) | 90 (7.0%) | 122 (8.2%) | 57 (5.8%) |
| Bad or very bad | 44 (1.5%) | 12 (2.6%) | 29 (2.3%) | 42 (2.8%) | 25 (2.5%) |
| Chronic disease n (%) | 709 (24.0%) | 151 (32.3%) | 387 (30.4%) | 455 (30.7%) | 290 (29.4%) |
| Education level n (%) | |||||
| Primary or secondary education | 863 (29.1%) | 175 (37.4%) | 391 (30.7%) | 584 (39.2%) | 365 (36.9%) |
| High school | 1065 (36.0%) | 182 (38.9%) | 460 (36.1%) | 575 (38.6%) | 370 (37.4%) |
| University or higher | 1034 (34.9%) | 111 (23.7%) | 424 (33.3%) | 331 (22.2%) | 254 (25.7%) |
| Mean systolic blood pressure (mmHg) | 118.8 (14.9) | 128.0 (16.3) | 120.9 (15.4) | 127.6 (16.0) | 125.0 (15.1) |
| Mean diastolic blood pressure (mmHg) | 74.3 (9.4) | 80.2 (10.1) | 75.6 (9.7) | 80.0 (10.0) | 78.4 (9.3) |
| Self-reported physical activity n (%) | |||||
| Much less | 100 (3.4%) | 48 (10.3%) | 54 (4.2%) | 119 (8.0%) | 63 (6.4%) |
| Somewhat less | 428 (14.5%) | 122 (26.1%) | 264 (20.7%) | 379 (25.4%) | 198 (20.0%) |
| Around the same | 1341 (45.3%) | 202 (43.2%) | 572 (44.8%) | 667 (44.7%) | 454 (45.8%) |
| Somewhat more | 835 (28.2%) | 84 (17.9%) | 316 (24.7%) | 264 (17.7%) | 232 (23.4%) |
| Much more | 257 (8.7%) | 12 (2.6%) | 72 (5.6%) | 63 (4.2%) | 44 (4.4%) |
| Smoking category n (%) | |||||
| Never smoked | 1152 (38.9%) | 176 (37.6%) | 473 (37.0%) | 540 (36.1%) | 352 (35.5%) |
| Current smoker | 1005 (33.9%) | 164 (35.0%) | 499 (39.0%) | 568 (38.0%) | 409 (41.2%) |
| Previous smoker | 807 (27.2%) | 128 (27.4%) | 306 (23.9%) | 388 (25.9%) | 231 (23.3%) |
Incidence of type 2 diabetes
Over an average follow-up of 19 years for women and 21 years for men, a total of 981 incident cases of type 2 diabetes (536 among women and 445 among men) were identified by clinical examinations, linkage to regional inpatient and outpatient health-care registers or by self-report. Self-report was the only source of diagnosis information for 2.3% (23) of the recorded cases of type 2 diabetes.
The cumulative incidence of type 2 diabetes was 13.74% and was higher among men (18.7%) than women (11.3%). A detailed description of the cumulative incidence in each trajectory group is presented in Table 2.
Table 2.
Cumulative incidence of type 2 diabetes by sex and life-course trajectories of weight.
| Total | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | Cases | Cumulative incidence (%) | Sample size | Cases | Cumulative incidence (%) | Sample size | Cases | Cumulative incidence (%) | |
| Stable normal weight | 2952 | 196 | 6.64 | 2144 | 111 | 5.18 | 808 | 85 | 10.52 |
| Stable overweight | 454 | 104 | 22.91 | 313 | 62 | 19.81 | 141 | 42 | 29.78 |
| Lean increasing weight | 1270 | 143 | 11.26 | 824 | 84 | 10.19 | 446 | 59 | 13.23 |
| Overweight from early adulthood | 1476 | 383 | 25.95 | 822 | 184 | 22.38 | 654 | 199 | 30.42 |
| Overweight from late adulthood | 986 | 155 | 15.72 | 652 | 95 | 14.57 | 334 | 60 | 17.96 |
| Total | 7138 | 981 | 13.74 | 4755 | 536 | 11.3 | 2383 | 445 | 18.7 |
The results from the regression analyses showed that, relative to the stable normal weight trajectory group, the estimated risk ratio (RR) for diabetes under the study period was higher for all the trajectory groups. This trend was statistically significant for all trajectories except the lean increasing weight trajectory in men. While adjustments for confounders resulted in a modest decrease of the point estimates, they had no influence on the overall direction of the effect.
Estimates of RR were higher among women than men. The highest RR in the fully adjusted model were found in the overweight from early adulthood trajectory with an RR of 3.43 (95% CI 2.72–4.34) among women and 2.77 (95% CI 2.17–3.37) in men, and in the stable overweight trajectory with an RR of 2.77 (95% CI 2.06–3.72) for women and 2.68 (95% CI 1.92–3.75) for men.
In the fully adjusted model, the absolute risk difference (ARD) under the study period was significantly higher in all trajectories compared to the stable normal trajectory. The highest estimates were for the overweight from early adulthood trajectory, with 7.76% (95% CI 5.36–10.17%) more cases among women and 14.05% (95% CI 10.30–17.79%) among men. Followed by the stable overweight trajectory with an estimated ARD of 7.94% (95% CI 4.65–11.24%) more cases in women and 13.11% (95% CI 6.23–19.99%) in men. All the results from the adjusted and unadjusted modified Poisson regression models are summarized in Table 3. The ARD of a given trajectory in comparison to the stable normal trajectory was, in contrast to the RR, higher in men than women. This indicates that there was a larger effect of the weight trajectories among women, but an overall higher incidence of type 2 diabetes among men.
Table 3.
Association between life-course trajectories of weight categories and cumulative incidence of type 2 diabetes.
| Risk ratios (RR) | Women | Men | ||
|---|---|---|---|---|
| Unadjusted RR (95% CI) |
Adjusted RR (95% CI) |
Unadjusted RR (95% CI) |
Adjusted RR (95% CI) |
|
| Stable normal weight | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Stable overweight | 3.69 (2.76–4.95) | 2.77 (2.06–3.72) | 2.94 (2.12–4.08) | 2.68 (1.92–3.75) |
| Lean increasing weight | 1.98 (1.50–2.60) | 1.71 (1.31–2.24) | 1.32 (0.96–1.81) | 1.35 (0.98–1.85) |
| Overweight from early adulthood | 4.07 (3.25–5.11) | 3.43 (2.72–4.34) | 3.03 (2.40–3.84) | 2.77 (2.17–3.37) |
| Overweight from late adulthood | 2.78 (2.13–3.63) | 2.27 (1.75–2.95) | 1.76 (1.29–2.40) | 1.73 (1.26–2.37) |
| Absolute risk difference (ARD) | Unadjusted ARD% (95% CI) |
Adjusted ARD% (95% CI) |
Unadjusted ARD% (95% CI) |
Adjusted ARD% (95% CI) |
|---|---|---|---|---|
| Stable normal weight | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| Stable overweight | 10.90% (6.97%-14.83%) | 7.94% (4.65%-11.24%) | 15.05% (7.89%-22.21%) | 13.11% (6.23%-19.99%) |
| Lean increasing weight | 3.35% (1.34%-5.27%) | 2.46% (0.57%-4.36%) | 3.92% (0.05%-7.36%) | 4.61% (0.95%-8.28%) |
| Overweight from early adulthood | 9.03% (6.63%-11.40%) | 7.76% (5.36%-10.17%) | 15.23% (11.52%-18.95%) | 14.05% (10.30%-17.79%) |
| Overweight from late adulthood | 5.02% (2.66%-7.38%) | 3.512% (1.36%-5.68% | 4.84% (0.85%-8.82%) | 5.23% (1.03%-9.43%) |
Models for males and females were estimated separately. Covariates included in the adjusted model for were age, family history of type 2 diabetes, general health, comorbidities, self-reported physical activity, smoking, and alcohol consumption for both sexes and history of gestational diabetes for women. Adjusted absolute risk differences were estimated using the mean of all covariates.
Population attributable fraction (PAF)
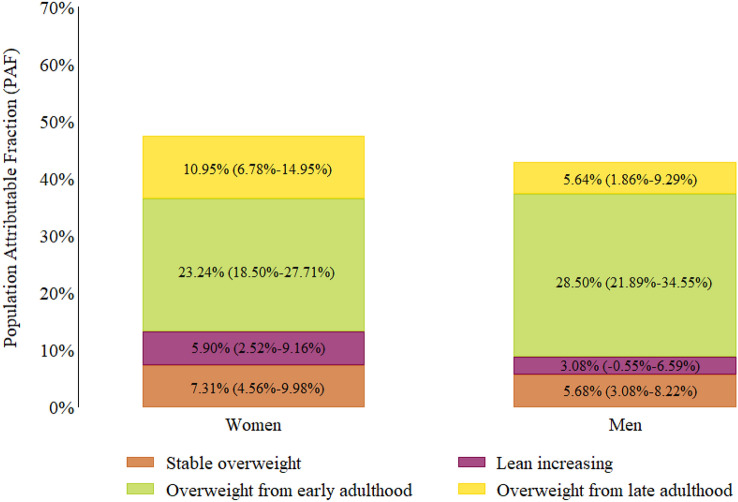
Overall, nearly half of the cases of diabetes among women (PAF: 47.40%, 95% CI 38.06%–55.34%) and men (PAF: 42.91%, 95% CI 31.47%–52.45%) were attributed to exposure to any life-course trajectory other than the stable normal. The trajectories including exposure to overweight or obesity at any point of life were associated to the highest population attributable fraction in both sexes (PAF: 36.82%, 95% CI 29.48%–43.40% for women and PAF: 36.71%, 95% CI 28.08%–44.31% for men).
Individually, the PAF was 7.31% (95% CI 4.56%–9.98%) for the stable overweight trajectory, 5.90% (95% CI 2.52%–9.16%) for the lean increasing weight trajectory, 23.24% (95% CI 18.50%–27.71%) for the overweight from early adulthood group, and 10.95% (95% CI 6.78%–14.95%) for the overweight from late adulthood trajectory group among women. In men, the PAF was 5.68% (95% CI 3.08%–8.22%) for the stable overweight trajectory, 3.08% (95% CI − 0.55%–6.59%) for the lean increasing weight trajectory, 28.50% (95% CI 21.89%–34.55%) for the overweight from early adulthood trajectory, and 5.64% (95% CI 1.86%–9.29%) for the overweight from late adulthood trajectory group (Fig. 3).
Figure 3.
Population attributable fractions (PAF) of trajectory groups by sex. Compared to the stable normal trajectory group, the overall proportion of type 2 diabetes cases attributable to any of the life-course weight trajectories was 47.40% (95% CI 38.06–55.34%) for women and 42.91% (95% CI 31.47–52.45%) for men.
Discussion
We identified five distinct life-course trajectories of weight categories using data from a large cohort spanning from ages 7–55. We then determine the association and public health impact of these trajectories with prospective risk of type 2 diabetes.
We found that the variability of the effect of exposure to overweight and obesity on the risk of developing type 2 diabetes can be largely explained by the different life-course trajectories of exposure. Around half of the cases of type 2 diabetes were attributed to exposure to any life-course trajectory other than a stable normal weight though life. The trajectories including overweight or obesity at any point through the life-course accounted for most of the attributable proportion of cases in the population.
Childhood overweight continued to adulthood was associated to a high risk of type 2 diabetes among those exposed, but a low population attributable proportion. While the trajectory of overweight or obesity developed in early adulthood was associated to both a high risk of type 2 diabetes and population attributable proportion.
We found a higher estimated risk of type 2 diabetes, although not significant among men, for the lean increasing trajectory, despite never reporting overweight or obesity. The trajectory developing overweight or obesity later in adulthood was also associated with an increased risk of type 2 diabetes, but had a rather low impact at the population level.
Strengths and limitations
The SDPP cohort study has compiled extensive clinical, self-reported and register data for a large, community-representative sample of healthy adults followed over 20 years for the development of type 2 diabetes. The use of inpatient and outpatient registries allowed us to obtain information regarding a new diagnosis of type 2 diabetes, even among participants who dropped out of the study. Loss to follow-up, an important source of bias in longitudinal studies was therefore minimized.
Another related strength was the ability to accurately estimate the incidence of type 2 diabetes. Ascertaining incidence is challenging due the long and insidious initial manifestations of the disease, and under diagnosis is common. In the SDPP, we used a combination of repeated OGTTs (the gold standard for the diagnosis of type 2 diabetes), individual level data linked to inpatient and outpatient registries, and self-reports of new diagnoses. This reduced the possibility of misclassifying the outcome, or over- or underestimating the estimated effects.
One limitation of the study is the fact that SDPP is a closed cohort and includes only participants born between 1938 and 1961. The prevalence of childhood obesity has increased importantly since then, which might affect the external generalizability of this study. Another important limitation of our study concerns the retrospective collection of data for childhood and adolescent weight categories, as well as weight 10 and 5 years previous to the study’s baseline examination. This information was, therefore, subject to recall bias. This could have led to differential misclassification of the exposure, which might have been more important among the males, who answered the question regarding childhood and adolescence weight during the 10-year follow-up and, at this stage, some of them had already developed type 2 diabetes. The women, however, answered questions regarding recalled weight at baseline, before any diagnosis of type 2 diabetes.
To use data from the entire life span, the data from recalled weight status from the baseline questionnaire, as well as recalled and measured BMIs, were categorized as lean, normal weight or overweight. This may have been an oversimplification and, therefore, it is possible that some degree of misclassification of the exposure was present in each category. Nonetheless, the prevalence of overweight and obesity during childhood reported by our participants was similar to that previously described in another Swedish study27.
There are also some limitations related to the use of GBTM28. First, although the variables used to categorize weight were ordinal, they were handled as censored normal in the statistical models. This approach has been described as reliable when dealing with longitudinal, categorical data29. In addition, classification in a certain trajectory group could have been conditional on age, as body weight normally increases with age, which would potentially explain why the higher risk groups were older at baseline. We addressed this limitation by adjusting for age at baseline. Another common issue with models based on finite mixture models is that exposure is not directly observed, but rather individuals are assigned to the different groups based on their posterior class probabilities according to the selected model. This might create bias due to misclassification of the exposure. However, the different methods used to relate latent classes to auxiliary variable, such as the three-step method used in this study, have been shown to underestimate the observed associations, which would in any case lead to a type 2 error30.
Finally, as with all generalizations, there is a trade-off between complexity and interpretability of the data. The trajectories presented in this study are a useful generalization of a complex underlying longitudinal process and attempt to describe the most characteristic patterns at the population level. At an individual level, however, several different trajectories exist. Individual level patterns within each trajectory group of a sub-sample of the participants is available online as supplemental material as Figure S1. Overall, when using these methods, it is important to be cautious in the interpretation of the trajectory groups, since the external generalizability might be limited. Comparison with previous studies is therefore important28.
Comparison with previous literature
The life-course trajectories described in this study are consistent with those previously reported in the literature. Several studies have used repeated measures of BMI, waist circumference or body shape to approximate unobserved sub-groups of developmental trajectories of weight through different periods of life and estimated their association to different outcomes, including type 2 diabetes31.
Among the studies looking into the association between the underlying trajectories of weight and risk of type 2 diabetes. One study used prospective BMI data from a large cohort of white civil servants from Britain and found three distinctive trajectories associated to a higher risk of type 2 diabetes23. Another study using retrospective BMI data from a Chinese population of adults followed for 6 years found 4 distinctive trajectories associated to higher risk of type 2 diabetes32. And a study that used retrospective data from a cohort of Australian adult women reported three trajectories of BMI that were associated to the risk of type 2 diabetes33. However, these studies did not include information from body weight during childhood or adolescence, ignoring the life-course effects of different patterns of changes in weight or body composition.
Studies covering larger periods of life also exist. A study using retrospective data from two large cohorts in the United States reported five trajectories of body shape from ages 5–55 associated to increased risk of type 2 diabetes24. Another similar study used data from a large cohort of women in France reporting five trajectories of self-reported body shapes from ages 8–40 associated to higher risk of type 2 diabetes26. And a study using prospective BMI data from Finland from ages ranging from 6 to 49 years reported six trajectories and their association to the risk of type 2 diabetes and other cardiovascular outcomes21. However, these studied estimated only relative measures of effect. Our study adds to the literature by estimating absolute measures of association (absolute risk difference and population attributable fraction), which provide information regarding the potential public health impact of the exposure to different patterns of weight categories through life.
The different life-course trajectories of exposure helped explained the variability of the effect of exposure to overweight and obesity on the risk of developing type 2 diabetes. Weight gains at different ages had a significant association to the risk and public health impact on the incidence of type 2 diabetes. The highest risk and public health impact was among those who became overweight or obese during early adulthood. Although previous studies have pointed out the importance of high body weight during early adulthood and the risk of type 2 diabetes13, 21, the public health impact of this period is often neglected. Our findings point out to early adulthood as an important period for public health interventions to reduce the burden of type 2 diabetes related to high body weight.
The stable overweight trajectory group (i.e. to overweight or obesity from childhood continued to adulthood) was associated to a high risk of type 2 diabetes among those exposed, but to a low public health impact in the population. These findings are consistent with existing literature, a recent meta-analysis of 37 studies concluded that, while there was a moderate association between childhood obesity and type 2 diabetes in adulthood, the public health relevance is limited10.
Interestingly, we found an increased risk, although not significant for men, among people who were not overweight or obese, but increased from lean to normal weight during their early adulthood. Other studies have previously pointed out the importance of weight changes on the risk of type 2 diabetes even among lean individuals34.
Implications for future research and public health
Our findings suggests at least two different underlying mechanisms of the effect of overweight on type 2 diabetes. A cumulative effect from childhood to adulthood, and a sensitive period, early adulthood, when exposure to changes in weight status, even among non-overweight or obese individuals, seems to have a greater effect.
We provide further evidence for the importance of a life-course approach in public health. Rather than investigating the effects of exposure at a single time point or stage of development, studying the effects of overweight and obesity as a continuous exposure during the life-course might help to better understand the natural history of its association to type 2 diabetes and point towards different causal mechanisms18.
Describing the determinants that differentiate these trajectories as well as their association to other health outcomes can aid in the design of more precise and targeted interventions to influence an individual´s health most efficiently18.
However, the categories presented in this study are not expected to be stable through generations. The prevalence of childhood obesity has increased in Sweden and around the world drastically during the past few decades27, 35. What is the effect of the increased prevalence of childhood obesity on the life-course trajectories of weight and on the incidence of type 2 diabetes of younger cohorts remains an important area for further research.
Research design and methods
Study population
Data for this study comes from the clinical subsample of the Stockholm Diabetes Prevention Program (SDPP), a 20-year prospective study that followed healthy individuals living in Stockholm, a detailed description of SDPP can be found elsewhere36.
In short, 16,481 healthy women and men from five selected municipalities in Stockholm, who were between 35 and 56 years old were identified between 1992 and 1998 using the National Population Registry of Sweden. A clinical subsample was created including 7948 participants, born in Sweden, with a family history of type 2 diabetes, women with a history of gestational diabetes, and a randomly selected sample of controls without a family history of type 2 diabetes.
Clinical examinations have been conducted at baseline, 10-years follow-up and 20-years follow-up and included extensive questionnaires, oral glucose tolerance test (OGTT), anthropometric and blood pressure measurements, and blood sample collection. Additionally, the personal identification number of all baseline participants was used to link individual level data to the regional data base of healthcare utilization, the Vårdanalysdatabasen (VAL) registry, which includes information about inpatient and outpatient services of Stockholm, including all diagnostics according to the International Classification of Diseases, tenth revision (ICD-10)37.
The sample for the present study includes 7203 participants (4820 women and 2383 men) who received the questionnaire including questions about their recalled weight status at ages 7 and 18. For women, this question was included in the baseline questionnaire while for men in the 10-year follow-up study.
Variables
Weight categories through life
To estimate the life course trajectories of weight categories, we used self-reported weight status at ages 7 and 18, recalled weight 10 and 5 years prior to the baseline examination and measured BMI from the baseline examination. In order to allow the analysis using all time points, the data at each time point was categorized into lean, normal weight or overweight as specified below.
Self-Reported weight status during childhood and adolescence
Participants answered questions about their weight at ages 7 and 18 during the baseline examination for women and on the 10-year follow-up questionnaire for men. The specific ages were selected in order to represent childhood and the end of adolescence. The question was the same for both sexes: “Compared to others of your same age, what was your weight status at age 7 and 18”. The answer categories were given as a Likert scale with the values: (1) very lean, (2) somewhat lean, (3) normal weight, (4) somewhat overweight, (5) very overweight. Similar recall measurements of childhood weight status have previously been validated38, 39. We categorized very lean and somewhat lean as lean, normal weight was kept as a category, and somewhat overweight and very overweight were categorized as overweight for each age.
Self-Reported weight 10 and 5 years before baseline
During the baseline examination, participants were asked to report their recalled weight 10 and 5 years prior to study inclusion. BMI was then calculated as BMI = weight (kg)/height2 (m) using measured height from the baseline examination. BMI values were categorized as lean (BMI < 19.5), normal weight (BMI ≥ 19.5 and < 25), and overweight (BMI ≥ 25)40, 41.
BMI during the baseline clinical examination
Weight and height for all participants was measured during the baseline clinical examination by trained study staff. BMI was calculated using measured weights and heights at each time point and participants were categorized as described above.
Incidence of type 2 diabetes
Incidence of type 2 diabetes was determined from three different sources: OGTTs performed during the clinical examinations, a new diagnosis of type 2 diabetes (ICD-10 code E11) in the VAL registry at any time after the baseline examination, or a self-reported new diagnosis of type 2 diabetes given by a health care professional during any of the study’s follow-up periods.
For the OGTT, we defined type 2 diabetes as a fasting plasma glucose level higher than or equal to 7 mmol/L or a 2 h, post-load plasma glucose level higher than or equal to 11 mmol/L during the OGTT42. During the 10- and 20-year follow-ups, only participants without a self-reported diagnosis of type 2 diabetes were offered a new OGTT.
Covariates
At baseline, we assessed the following possible confounders: age, educational level, self-reported general health, chronic comorbidities, family history of type 2 diabetes, physical activity, smoking status, alcohol consumption, and, for women, a history of gestational diabetes.
Age was available from the total population register of Sweden. Educational level was self-reported, categorized as primary education, upper secondary education, or university education, and higher. Self-reported general health was evaluated with the question: “How is your general state of health “and included the categories: “very good”, “good”, “neither good or bad” and “bad or very bad”. The presence of any self-reported comorbidities in the baseline questionnaire was used as a dichotomous variable. A family history of diabetes was self-reported, defined as having one first-degree relative or two second-degree relatives with the disease and was dichotomized as present or not present. Information regarding gestational diabetes was also obtained from the baseline questionnaire administered to women and was used as a dichotomous measure. Level of physical activity was assessed with the question “Compared to other men/women your age, how do you consider your physical activity in your spare time?” and categorized as “much lower”, “somewhat lower”, “about the same”, “slightly higher” and “much higher”. Smoking was self-reported and categorized as never smoked, previous smoker and current smoker, and alcohol consumption was calculated in cl per week from self-reported standard units and frequencies.
Statistical analysis
Descriptive statistics are presented as means and standard deviations (SDs) for continuous variables and as proportions for binary and categorical data.
We used Group Based Trajectory Modelling (GBTM) to ascertain the life course trajectories of the five weight categories using the Traj procedure43 in Stata 1444. GBTM is an application of finite mixture modelling that is useful to study the development of an outcome over time. The main assumption is that an observed population is composed of underlying distinctive sub-populations (or latent classes) of individuals. And that the composition and characteristics of such sub-groups can be estimated using parametric methods45.
The model selection for the GBTM was done in multiple steps to identify the best number and structure of the trajectory groups. Trajectories were estimated separately for men and women given their differences in lipid metabolism, development of adiposity, and the effect of high body weight between men and women46.
Determination of the goodness-of-fit and parsimony of the model was based on a low Bayesian information criterion, a large Bayes factor, an average posterior probability of assignment to a given trajectory group greater than 75%, and a size of more than 1% in each group47, 48. After the number of trajectory groups and parameters of the models were selected, participants were assigned to the trajectory group to which they were more likely to belong based on their individual weight categories at all the time points.
Missing data in the weight categories at any time point was managed using the full information maximum-likelihood method based on the available data points under the missing at random assumption, i.e., missing was not related to the outcome, but was related to other observed variables45. Further information on the model selection process can be found online as Supplemental Tables S2–S4.
We investigated the association between the different life-course trajectories of weight categories and incidence of type 2 diabetes using modified Poisson regression with robust error variance49. The study period for this analysis extended from the date of baseline examination to the date of the third study follow-up, or until January 1st 2018 for those participants whose outcome status was retrieved from regional inpatient or outpatient registries. The mean follow-up time was 19 years for women and 21 years for men. We reported estimated risk ratios, absolute risk differences and population attributable fractions based on the cumulative incidence during the whole study period.
The connection between the exposure to the estimated trajectory groups and incidence of type 2 diabetes was done using a three-step approach: First, the selected GBTM model was fitted using only the latent class indicator variables, i.e. the weight categories at each of the five time points. Next, individuals were assigned to the trajectory group they were most likely to belong based on the posterior probability of group membership. Lastly, the modified Poisson regression models were fitted using the inverse probability of class membership as weights to adjust for classification uncertainty30, 50.
Covariates included in the adjusted model included age at baseline, self-reported physical activity, family history of diabetes, comorbidities, self-reported general health status, educational level, smoking and alcohol use. For women, additional adjustment was done for history of gestational diabetes.
The burden of type 2 diabetes attributable to each trajectory group was calculated using the population attributable fractions (PAF), defined as the proportion of cases in the population that can be attributed to a certain exposure. In other words, the proportion of cases that would not have occurred had the exposure not been present.
We calculated the PAF for exposure to any trajectory compared to the stable normal weight trajectory group according to the general formula:
where pi represents the proportion of cases in each level of exposure (i.e. each trajectory group) and RRi represents the adjusted RR of type 2 diabetes for each trajectory group compared to the stable normal group.
All statistical analysis was done using Stata 1451. The user written commands traj43 and punaf52 were used to estimate the GBTM and PAFs, respectively.
Ethics approval
This study was carried out in accordance with the ethical principles outlined in the Declaration of Helsinki53. The SDPP study received approval by the regional ethics review board of Stockholm (2013/1982-31/2).
All participants of the SDPP study received verbal and written information about the study and provided informed consent at the time of recruitment and at each follow-up visit.
Supplementary Information
Acknowledgements
We are grateful to the participants of the Stockholm Diabetes Prevention Program study and everyone involved in the planning and conduction of the study.
Abbreviations
- BMI
Body mass index
- OGTT
Oral glucose tolerance test
- ICD-10
The International Classification of Diseases, tenth revision
- CI
Confidence interval
- ARD
Absolute risk difference
- RR
Risk ratio
- GBTM
Group based trajectory modelling
- PAF
Population attributable fraction
- SDPP
Stockholm diabetes prevention program
- VAL
Stockholm County central regional data base of healthcare utilization (Vårdanalysdatabasen)
Author contributions
D.Y.M. and A.L. designed and planned the study; H.G. and P.T. contributed to data collection and management; D.Y.M., P.T. and A.P.L. and were involved in the data analysis. D.Y.M., P.T., A.P.L., A.L., M.Z. and Y.T.L. participated in the interpretation of the data. DYM wrote the first draft of the manuscript, A.L., M.Z. and Y.T.L. provided critical revisions to previous versions of this manuscript. All authors contributed relevant intellectual content, have approved the final version, and have agreed to be accountable for the work presented in this article.
Funding
Open access funding provided by Karolinska Institute. DYM received a scholarship from the National Council for Science and Technology of Mexico (CONACyT) (Grant No. CVU:754768). YTL was funded by The Swedish Research Council for Health, Working Life and Wellfare (FORTE) (Dnr:2016–00985). The funding agencies had no role in the design, execution, analysis or interpretation of this study.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding authors upon reasonable requests. The Centre or Epidemiology and Community Medicine of Region Stockholm manages the data from the Stockholm Diabetes Prevention Program. Sensitive information is protected by the general data protection regulation (GDPR). The code used for data analysis of this manuscript is available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors. Modifications have been made to the Results section, Table 2, Table 3, the legend of Figure 3 and the Supplementary Information file. Full information regarding the corrections made can be found in the correction for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/14/2021
A Correction to this paper has been published: 10.1038/s41598-021-98091-9
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91910-z.
References
- 1.GBD Obesity collaborators health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2015;377(13–27):2017. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/s0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Censin JC, et al. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019;15:e1008405. doi: 10.1371/journal.pgen.1008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Jin C, Guan Q. Causal effects of overall and abdominal obesity on insulin resistance and the risk of type 2 diabetes mellitus: A two-sample Mendelian randomization study. Front. Genet. 2020 doi: 10.3389/fgene.2020.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel RH, et al. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? Diabetes Care. 2011;34:1424. doi: 10.2337/dc11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA. 2018;319:223–224. doi: 10.1001/jama.2017.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruby A, et al. Determinants and consequences of obesity. Am. J. Public Health. 2016;106:1656–1662. doi: 10.2105/ajph.2016.303326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeland IJ, Poirier P, Després J-P. Cardiovascular and metabolic heterogeneity of obesity. Circulation. 2018;137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faerch K, Hulman A, Solomon TP. Heterogeneity of pre-diabetes and type 2 diabetes: Implications for prediction, prevention and treatment responsiveness. Curr. Diabetes Rev. 2016;12:30–41. doi: 10.2174/1573399811666150416122903. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016;17:56–67. doi: 10.1111/obr.12316. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann E, et al. Childhood body mass index and development of type 2 diabetes throughout adult life—A large-scale danish cohort study. Obesity. 2017;25:965–971. doi: 10.1002/oby.21820. [DOI] [PubMed] [Google Scholar]
- 12.Feng C, Osgood ND, Dyck RF. Low birth weight, cumulative obesity dose, and the risk of incident type 2 diabetes. J. Diabetes Res. 2018;2018:8435762. doi: 10.1155/2018/8435762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjerregaard LG, et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 2018;378:1302–1312. doi: 10.1056/NEJMoa1713231. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson JG, Kajantie E, Lampl M, Osmond C. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. J. Intern. Med. 2015;278:219–226. doi: 10.1111/joim.12354. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson J, Forsén T, Osmond C, Barker D. Obesity from cradle to grave. Int. J. Obes. 2003;27:722–727. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- 17.Wei GS, et al. Duration and degree of weight gain and incident diabetes in younger versus middle-aged black and white adults: ARIC, CARDIA, and the Framingham heart study. Diabetes Care. 2015;38:2042–2049. doi: 10.2337/dc14-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song M. Trajectory analysis in obesity epidemiology: A promising life course approach. Curr. Opin. Endocr. Metab. Res. 2019;4:37–41. doi: 10.1016/j.coemr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Bonsdorff MB, et al. Early life body mass trajectories and mortality in older age: Findings from the Helsinki Birth Cohort Study. Ann. Med. 2015;47:34–39. doi: 10.3109/07853890.2014.963664. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, et al. Trajectories of body mass index in adulthood and all-cause and cause-specific mortality in the Melbourne Collaborative Cohort Study. BMJ Open. 2019;9:e030078. doi: 10.1136/bmjopen-2019-030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buscot M-J, et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 2018;39:2263–2270. doi: 10.1093/eurheartj/ehy161. [DOI] [PubMed] [Google Scholar]
- 22.Song M, et al. Trajectory of body shape in early and middle life and all cause and cause specific mortality: Results from two prospective US cohort studies. BMJ. 2016;353:i2195. doi: 10.1136/bmj.i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vistisen D, et al. Patterns of obesity development before the diagnosis of type 2 diabetes: The Whitehall II cohort study. PLoS Med. 2014;11:e1001602. doi: 10.1371/journal.pmed.1001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Song M, Manson JE, Giovannucci EL, Hu FB. Group-based trajectory of body shape from ages 5 to 55 years and cardiometabolic disease risk in 2 US Cohorts. Am. J. Epidemiol. 2017;186:1246–1255. doi: 10.1093/aje/kwx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nano J, et al. Trajectories of BMI before diagnosis of type 2 diabetes: The Rotterdam study. Obesity. 2020;28:1149–1156. doi: 10.1002/oby.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagherazzi G, et al. The association of body shape trajectories over the life course with type 2 diabetes risk in adulthood: A group-based modeling approach. Ann. Epidemiol. 2015;25:785–787. doi: 10.1016/j.annepidem.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Bygdell M, et al. The rise and the recent decline of childhood obesity in Swedish boys: The BEST cohort. Int. J. Obes. 2017;41:807–812. doi: 10.1038/ijo.2017.23. [DOI] [PubMed] [Google Scholar]
- 28.Herle M, et al. Identifying typical trajectories in longitudinal data: Modelling strategies and interpretations. Eur. J. Epidemiol. 2020;35:205–222. doi: 10.1007/s10654-020-00615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman BJ, Masyn KE, Conger RD. New approaches to studying problem behaviors: A comparison of methods for modeling longitudinal, categorical adolescent drinking data. Dev. Psychol. 2009;45:652–676. doi: 10.1037/a0014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamata A, Kara Y, Patarapichayatham C, Lan P. Evaluation of analysis approaches for latent class analysis with auxiliary linear growth model. Front. Psychol. 2018;9:130. doi: 10.3389/fpsyg.2018.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Rubeis V, et al. Group-based trajectory modeling of body mass index and body size over the life course: A scoping review. Obes. Sci. Pract. 2021;7:100–128. doi: 10.1002/osp4.456. [DOI] [Google Scholar]
- 32.Dai H, et al. Distinct developmental trajectories of body mass index and diabetes risk: A 5-year longitudinal study of Chinese adults. J. Diabetes Investig. 2019 doi: 10.1111/jdi.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo J, Hodge A, Hendryx M, Byles JE. Age of obesity onset, cumulative obesity exposure over early adulthood and risk of type 2 diabetes. Diabetologia. 2020;63:519–527. doi: 10.1007/s00125-019-05058-7. [DOI] [PubMed] [Google Scholar]
- 34.Oguma Y, Sesso HD, Paffenbarger RS, Jr, Lee IM. Weight change and risk of developing type 2 diabetes. Obes. Res. 2005;13:945–951. doi: 10.1038/oby.2005.109. [DOI] [PubMed] [Google Scholar]
- 35.Lager A, Berlin M, Heimerson I, Danielsson M. Young people’s health: Health in Sweden: The National Public Health Report 2012. Chapter 3. Scand. J. Public Health. 2012;40:42–71. doi: 10.1177/1403494812459459. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson AK, et al. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet. Med. 2008;25:834–842. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- 37.Svensson AC, et al. Cohort profile: The Stockholm Public Health Cohort. Int. J. Epidemiol. 2013;42:1263–1272. doi: 10.1093/ije/dys126. [DOI] [PubMed] [Google Scholar]
- 38.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am. J. Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 39.Casey VA, et al. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann. Hum. Biol. 1991;18:155–166. doi: 10.1080/03014469100001492. [DOI] [PubMed] [Google Scholar]
- 40.WHO. World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of the WHO Consultation of Obesity. (World Health Organization Geneva, 2000). [PubMed]
- 41.Flegal KM, Kit BK, Graubard BI. Body mass index categories in observational studies of weight and risk of death. Am. J. Epidemiol. 2014;180:288–296. doi: 10.1093/aje/kwu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Diabetes A. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 43.Jones BL, Nagin DS. A note on a stata plugin for estimating group-based trajectory models. Sociol. Methods Res. 2013;42:608–613. doi: 10.1177/0049124113503141. [DOI] [Google Scholar]
- 44.Stata Statistical Software . Release 15. StataCorp LLC; 2017. [Google Scholar]
- 45.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 46.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 2001;29:374–393. doi: 10.1177/0049124101029003005. [DOI] [Google Scholar]
- 48.Lennon H, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8:e020683. doi: 10.1136/bmjopen-2017-020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 50.Vermunt JK. Latent class modeling with covariates: Two improved three-step approaches. Polit. Anal. 2010;18:450–469. doi: 10.1093/pan/mpq025. [DOI] [Google Scholar]
- 51.StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
- 52.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13:672–698. doi: 10.1177/1536867X1301300402. [DOI] [Google Scholar]
- 53.World Medical Association World medical association Declaration of Helsinki: Ethical principles for medical research involving human subjectsworld medical association declaration of helsinkispecial communication. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding authors upon reasonable requests. The Centre or Epidemiology and Community Medicine of Region Stockholm manages the data from the Stockholm Diabetes Prevention Program. Sensitive information is protected by the general data protection regulation (GDPR). The code used for data analysis of this manuscript is available from the corresponding author upon reasonable request.