Abstract
Prescription opioid misuse is a major public health concern among children and adolescents in the USA 1. Opioids are the most commonly abused drugs and are the fastest growing drug problem among adolescents 2. In humans and animals, adolescence is a particularly sensitive period associated with an increased response to drugs of abuse 3–5. Our previous studies indicate that oxycodone exposure during adolescence increases morphine reward in adulthood 6. How early drug exposure mediates long-term changes in the brain and behavior is not known, but epigenetic regulation is a likely mechanism. To address this question, we exposed mice to oxycodone or saline during adolescence and examined epigenetic modifications at genes associated with dopamine activity during adulthood at early and late withdrawal, in the ventral tegmental area (VTA). We then compared these to alterations in the VTA of adult-treated mice following an equivalent duration of exposure and withdrawal to determine if the effects of oxycodone are age-dependent. We observed persistence of adolescent-like gene expression following adolescent oxycodone exposure relative to age-matched saline exposed controls, although dopamine related gene expression was transiently activated at 1-day of withdrawal. Following prolonged withdrawal enrichment of the repressive histone mark, H3K27me3, was maintained, consistent with inhibition of gene regulation following adolescent exposure. In contrast, mice exposed to oxycodone as adults showed loss of the repressive mark and increased gene expression following 28-days of withdrawal following oxycodone exposure. Together our findings provide evidence that adolescent oxycodone exposure has long-term epigenetic consequences in VTA of the developing brain.
Keywords: Adolescent, CART, H3K27me3, Mice, Nr4a2 Oxycodone
Introduction
The impact of prescription opioid abuse continues to be a serious public health problem. Nonmedical use of prescription opioids remains the number one cause of drug overdose 7. Current estimates indicate that 10.3 million people in the United States abuse prescription opioids, including 700 thousands afflicted individuals between the ages of 12 and 17 7. Use of prescription opioids, such as oxycodone, increase the propensity to initiate heroin use in adulthood 7,8. In humans and mouse, the association between adolescent opioid use and the propensity to initiate adult heroin use has been well documented 6,8–10. This underscores the importance of efforts to identify molecular mechanisms that contribute to the transition from prescription opioid use to heroin addiction 6,8.
Adolescence is defined by age-specific behavioral characteristics associated with the transition from childhood to adulthood 3. Neurobiological correlates to human adolescence, such as synaptic pruning and reductions in dopamine receptor expression, are conserved across the mammalian species. Compared to adult, adolescent dopamine neurons in the ventral tegmental area (VTA) fire at higher rates and release larger pools of dopamine in the NAc, which facilitate the development of drug taking in mice 11,12. Enhanced dopaminergic neuron activity during adolescence results in increased levels of dopamine in the NAc, relative to adult 13. A similar trend is observed in the expression of dopamine receptors 1 and 2 (D1 and D2) 14. These data indicate that molecular adaptions associated with adolescence may render the nervous system more sensitive to environmental stimuli, particularly in the mesolimbic dopamine system 3,15. However, knowledge regarding the molecular mechanisms that regulate functional changes in the VTA during adolescence remains limited and requires further investigation.
Adolescent structural and molecular remodeling contributes to enhanced drug sensitivity and reward 16. μ-opioid receptor agonists such as oxycodone and morphine, disinhibit dopamine neurons in the VTA, thereby increasing levels of dopamine in the nucleus accumbens (NAc) 17., Compared to the transient dopamine release caused by morphine, oxycodone has a greater potential to promote addiction-like behaviors given its long-lasting effect on dopamine release 18. We previously demonstrated that oxycodone exposure during adolescence enhances rewarding properties of opioids later in life 6. The effects of oxycodone exposure differ between adolescents and adults, including enhanced striatal dopamine release 19 and oxycodone conditioned place preference (CPP) 10, and attenuated self-administration in adolescent mice 19. In fact, prolonged exposure to oxycodone during adolescence enhances both behavioral responses to opioids in adulthood and alters gene expression 6,9,20. These data suggest that the effects of adolescent oxycodone exposure on the mesolimbic reward pathway persist well into adulthood. This long-lasting phenotype led us to hypothesize that adolescent oxycodone exposure induces a persistent adolescent-like state in gene expression and behavior 6.
There is increasing evidence that epigenetic mechanisms mediate the influence of drugs on the CNS and are likely involved in the pathology of drug abuse. Epigenetic changes, such as histone modifications, remain largely plastic during development. However, epigenetic regulation during adolescence is understudied and may underlie the behavioral responses to drugs of abuse. Histone H3 lysine 27 trimethylation (H3K27me3) is a well-studied epigenetic modification that is associated with gene repression 21. We hypothesized that H3K27me3 regulates drug induced gene expression during adolescence and early adulthood, given its critical role in the maintenance of dopaminergic neurons in the VTA 21. Given that H3K27me3 is regulated following opioid exposure, its abundance may be associated with important gene expression changes related to drug behavior.
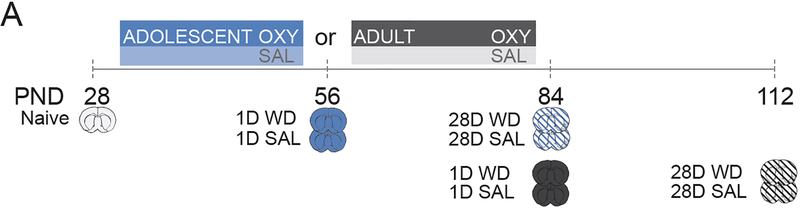
In mouse models, adolescence spans approximately from post-natal day (PND) 28–42, followed by a considerable “gray zone” approaching adulthood (PND 60+) 3,22. Therefore, we examined gene expression and the enrichment of H3K27me3 at several target genes during adulthood (PND56, PND84, and PND112) relative to the naive adolescent brain at PND 28. In addition, we exposed mice to oxycodone (or saline) during adolescence or early adulthood and examined epigenetic modifications at genes associated with the dopamine transmission during adulthood at 1- and 28-days of withdrawal 6,23. Adolescent mice were exposed to oxycodone from PND28–56 to ensure coverage of the entire period in which neurobehavioral discontinuities from younger and older mice are evident 3,24. We utilized two controls to distinguish alterations in normal development from those that occur as a result of withdrawal: (1) all oxycodone groups were compared to the naive adolescent brain (PND28) to define developmentally regulated gene expression changes; (2) oxycodone exposed mice were compared to saline exposed, age-matched controls to define effects of withdrawal and age of exposure. We hypothesized that genes associated with the development of dopamine neurons 25 and opiate reward 6,26, such as, nuclear receptor subfamily 4 group A member 2 (Nr4a2), and its downstream target genes, cocaine and amphetamine related transcript (Cartpt), tyrosine hydroxylase (Th) and the dopamine transporter (DAT), would be particularly prone to long-lasting alterations following oxycodone exposure. In sum, we identified an epigenetic mechanism specific to adolescent oxycodone exposure that is associated with the inhibition of withdrawal-induced gene expression.
Materials and Methods
Animals
C57Bl/6NTac mice (Taconic, Hudson, New York) were bred in house at the University of Pennsylvania for a minimum of two generations to eliminate the potential effects of shipping. Male offspring, used in experiments, were grouped housed (3–5/cage) with ad libitum access to food and water under a 12h light cycle. All procedures were approved by the University of Pennsylvania Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
All surgical procedures were conducted using aseptic techniques under 1–3% isoflurane/oxygen vapor. Osmotic minipumps (model 1004; Alzet, Cupertino, CA) were inserted subcutaneously at either PND 28 or 56 during adolescence or early adulthood, respectively. Minipumps were placed parallel to the thoracic vertebrae with the flow moderator directed away from the wound. Minipumps chronically delivered either oxycodone (oxycodone HCl, Spectrum Chemical, New Brunswick, NJ) dissolved in 0.9 % saline at a dosage of 3 mg/kg/day for 28 days, or saline only for saline control mice. Mice were surgerized at different developmental timepoints to measure the effects of oxycodone exposure on adolescence (PND28 – 56) or adulthood (PND 56 – 84). After 28 days of oxycodone or saline exposure, minipumps were surgically removed from all groups.
Tissue collection
To obtain sufficient numbers of mice in all treatment groups, tissues were collected from 4 different cohorts of mice. Every cohort included mice in each of the following treatment groups; adolescent saline exposure, adolescent oxycodone exposure, adult saline exposure and adult oxycodone exposure. Mice were weaned at PND21 at which time individual littermates (average # of litters per cohort: n = 5–6) were randomly assigned to each of the 9 groups; and every group contained mice from each cohort. Tissue was collected at PND28 in naïve mice or after 1- or 28-days following oxycodone/saline exposure. Mice were euthanized by cervical dislocation and brains were rapidly removed and placed in ice-cold phosphate buffered saline with protease inhibitor cocktail (Roche cOmplete Protease inhibitor). One-millimeter thick sections of the ventral tegmental area were prepared using a cold tissue matrix and regions were bilaterally micro-dissected with a 1.2-mm diameter micro-punch (Harris). Tissue was immediately frozen in liquid nitrogen and stored at −80oC for downstream analysis. During tissue processing, all samples were coded and randomized to reduce experimenter bias and batch effects.
Single Sample RNA extraction and Chromatin Immunoprecipitation (S3EQ)
S3EQ was performed on VTA 1.2 mm bilateral punches dissected as described above (1 mouse/sample), 24 hours after minipump removal or withdrawal. Tissue was homogenized in cell lysis buffer (10mM Tris Hcl pH 8.1, 10mM NaCl, 3mM MgCl2, 0.5% Np-40). Low speed centrifugation (1000xg) separated the cytosol (supernatant) from the nuclei (pellet). RNA was isolated from the supernatant using the RNeasy Mini Kit (Qiagen) according to the manufacturer instructions. RNA concentration and integrity were determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE) and the RNA NanoChip on the Bioanalyzer (Agilent), respectively. All RNA samples were of good quality (RIN above 8) and purity (1.9 +/− 0.1). cDNA was synthesized from RNA (100ng) using the iScript cDNA Synthesis Kit (Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed on the QuantStudio 7 Flex Real-Time PCR System (ThermoFisher). Ct values of the experimental group to control were analyzed using the ΔΔCt method 27. Primer sequences can be found in Supplementary Table 1.
Nuclei were mildly fixed in 1% formaldehyde for 6 minutes and the reaction was terminated with glycine and pelleted by centrifugation (5500xg). Fixed nuclei were resuspended in nuclear isolation buffer (50mM Tris pH 8.1, 5mM EDTA, 1% SDS) and chromatin was sheared using a diogenode bioruptor XL at high sonication intensity. For histone modification enrichment, chromatin was sheered for 30 min (30 s on/30 s off) and fragment size was verified at 150–300 bps with an Agilent 2100 bioanalyzer. Sheared chromatin was incubated overnight with H3K27me3 (EMD Millipore 07–449) previously bound to magnetic beads (Dynabeads M-280, Life Technologies): The Dynabeads were washed twice each with 1 ml of Low Salt Wash Buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA, 1% TritonX-100, 0.1% SDS), High Salt Wash Buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM EDTA, 1% TritonX-100, 0.1% SDS), and TE Buffer (10 mM Tris, pH 8.0, 1 mM EDTA). After reverse cross-linking and DNA purification (Qiagen Spin Column), primers were designed to amplify regions ~150–200 within gene promoter regions.
Statistics
The appropriate statistical test was determined based on the number of comparisons being conducted. We utilized two controls to distinguish alterations in normal development from those that occur as a result of withdrawal: (experiment 1 & 2) all oxycodone groups were compared to the naive adolescent brain (PND28) to define developmentally regulated gene expression changes; (experiment 3) oxycodone exposed mice were compared to saline exposed, age-matched controls to define effects of withdrawal and age of exposure. For experiment 1 (Fig 2), multiple two-tailed student ttest were used to compared saline exposed mice at developmental timepoints relative to naïve mice at PND28; we then adjusted the p value using the Two-stage Benjamini, Krieger, & Yekutieli FDR procedure to account for the multiple comparisons being made. For experiment 2 (Fig. 3 and 4), multiple two-tailed student ttest were used to compare naïve mice relative to oxycodone exposed mice at 1- and 28-days of withdrawal; we then adjusted the p value using the Two-stage Benjamini, Krieger, & Yekutieli FDR procedure to account for the multiple comparisons being made. The Two-stage Benjamini, Krieger, & Yekutieli FDR procedure has been shown to have greater power to detect true positives, while still controlling the proportion of type I errors at a specified level (5%) 28. All seven comparisons to naive control mice (PND28) were used to adjust the p value and account for multiple testing in experiments 1 and 2 (Supplementary Fig. 2). For experiment 3 (Fig. 5 and 6), two-way ANOVAs compared saline exposed mice relative to age-matched oxycodone exposed mice at 1- day or 28-days of withdrawal; when appropriate, a Holm-Sidak’s post-hoc test followed to determine significant differences across multiple comparisons. Main and interaction effects were considered significant at P < 0.05. P-values greater than 0.05 and below 0.1 were considered trends. Data are expressed as mean [+ or −] s.e.m. The Grubbs’ test was used when appropriate to identify outliers. F-tests of variance were conducted on all data sets to ensure that the data followed a normal distribution.
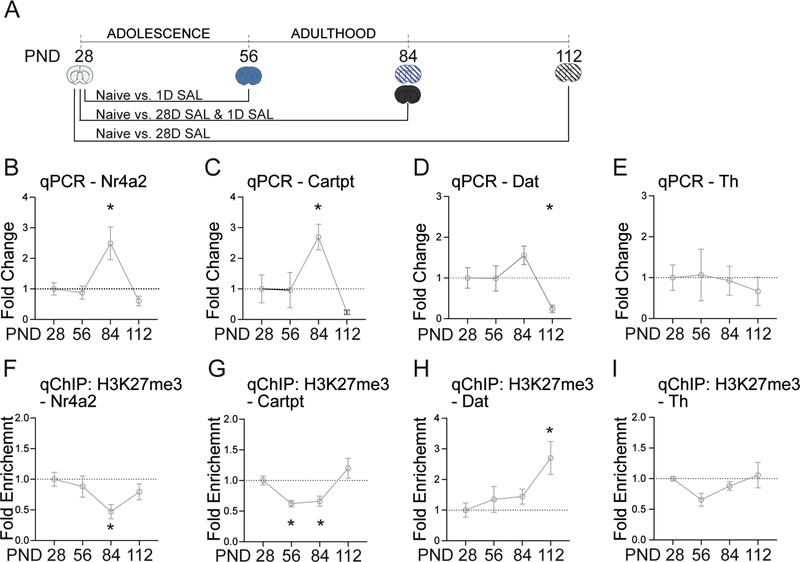
Figure 2. Epigenetic modifications at dopamine related genes across developmental time points in the VTA.
A.) Timeline of developmental time points. B.) Nr4a2 is activated at PND 84, (Students two-tailed ttest; PND 84, p=0.029, adjusted p = 0.012). C.) Cartpt is activated at PND 84 (Students two-tailed ttest; PND 84, p=0.013, adjusted p = 0.019). D.) Dat is repressed at PND 112 (Students two-tailed ttest; PND 112, p=0.015, adjusted p = 0.047). E.) No significant changes in Th. F.) H3K27me3 is depleted at the Nr4a2 promotor at PND 84 (Students two-tailed ttest; PND 84, p=0.012, adjusted p = 0.017). G.) H3K27me3 is depleted at the Cartpt promotor at PND 56 and 84 (Students two-tailed ttest; PND 56, p=0.001, adjusted p = 0.005; PND 84, p=0.002, adjusted p = 0.005). H.) H3K27me3 is enriched at the Dat promotor at PND 112 (Students two-tailed ttest; PND 112, p=0.006, adjusted p = 0.040). I.) H3K27me3 is depleted at the Th promotor at PND 56 (Students two-tailed ttest; p=0.029, adjusted p = 0.012). All data normalized to GAPDH relative to PND28. n = 5 – 12 mice per group. Error bars represent S.E.M. *p<.05 relative to PND28.
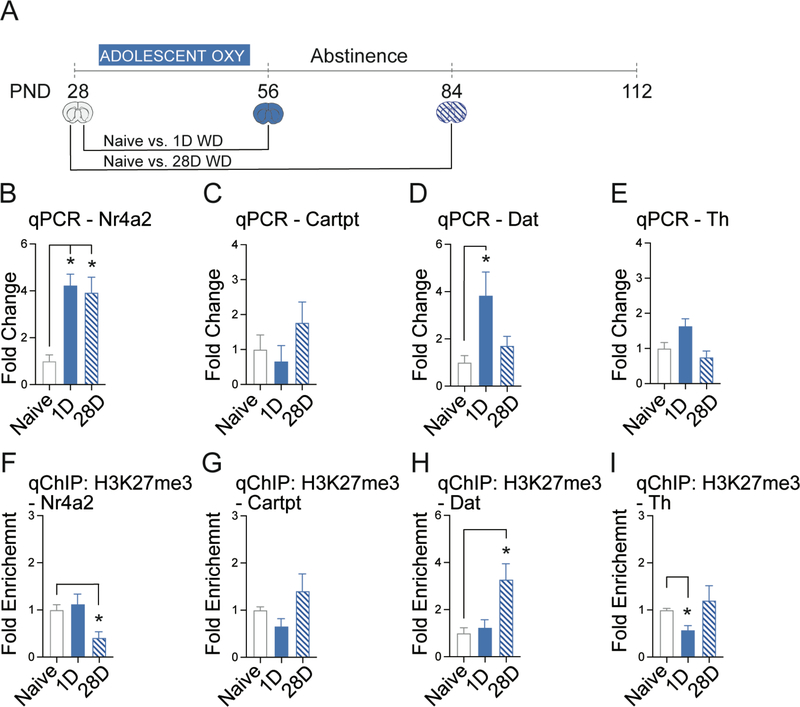
Figure 3. Adolescent oxycodone exposure alters dopamine related gene expression in the VTA.
A.) Timeline of adolescent oxycodone exposure and subsequent periods of oxycodone withdrawal (WD). B.) Adolescent oxycodone activates Nr4a2 at 1- and 28-days (Students two-tailed ttest; 1-day, p=0.01, adjusted p = 0.007, 28-days,, p=0.001, adjusted p = 0.001). C.) No significant changes in Cartpt. D.) Adolescent oxycodone activates Dat at 1-day (Students two-tailed ttest; 28-days, p=0.004, adjusted p = 0.025). E.) No significant changes in Th expression. F.) H3K27me3 is depleted at the Nr4a2 promoter at 28-days (Students two-tailed ttest; 28-days,, p=0.003, adjusted p = 0.007). G.) No significant changes in H3K27me3 enrichment at the Cartpt promoter (Students two-tailed ttest; p=0.029, adjusted p = 0.012). H.) H3K27me3 is enriched at the Dat promoter at 28-days (Students two-tailed ttest; 28-days, p=0.015, adjusted p = 0.049). I.) H3K27me3 is depleted from the Th promoter at 1-day (Students two-tailed ttest; 1-day, p=0.012, adjusted p = 0.03). All data normalized to GAPDH relative to drug naïve adolescent brain (PND28). n = 5 – 7 mice per group Error bars represent S.E.M. *p<.05 relative to drug naïve adolescent brain (PND28).
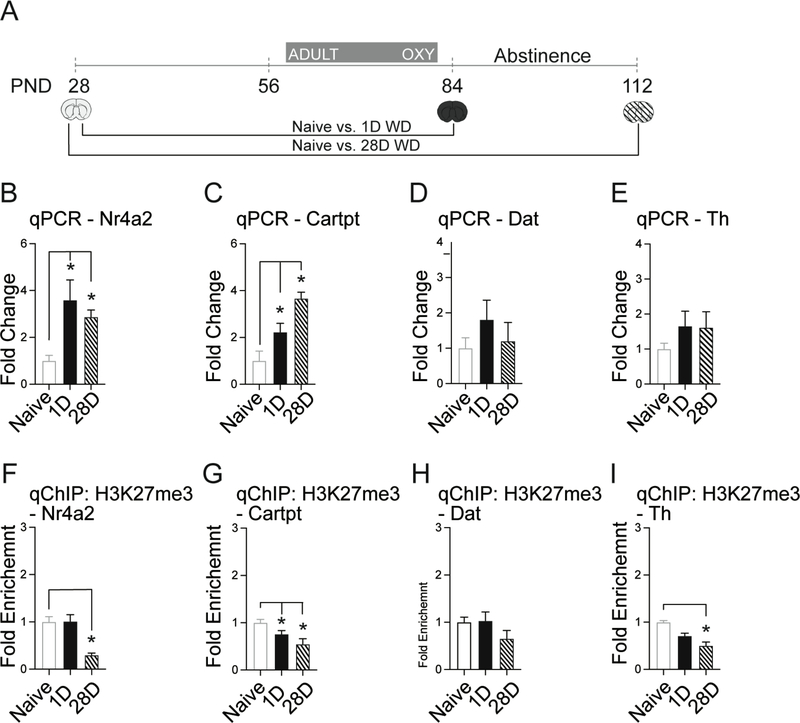
Figure 4. Adult oxycodone exposure alters dopamine related gene expression in the VTA.
A.) Timeline of adult oxycodone exposure and subsequent periods of oxycodone withdrawal (WD). B.) Adult oxycodone activates Nr4a2 at 1- and 28-days (Students two-tailed ttest; 1-day, p=0.014, adjusted p = 0.007, 28-days,, p=0.001, adjusted p = 0.001). C.) Adult oxycodone activates Cartpt at 28-days (Students two-tailed ttest; 1-day, p=0.014, adjusted p = 0.019, 28-days,, p=0.003, adjusted p = 0.001). D.) No significant changes in Dat expression. E.) No significant changes in Th expression. F.) H3K27me3 is depleted at the Nr4a2 promoter at 28-days (Students two-tailed ttest; 28-days, p=0.001, adjusted p = 0.005). G.) H3K27me3 is depleted at the Cartpt promoter at 1-day and 28-days (Students two-tailed ttest; 1-day p=0.036, adjusted p = 0.038, 28-days, p=0.003, adjusted p = 0.005). H.) No significant changes in H3K27me3 enrichment at the Dat promoter (Students two-tailed ttest; p=0.029, adjusted p = 0.012). I.) H3K27me3 is depleted at the Th promoter at 28-days (Students two-tailed ttest; 28-days, p=0.008, adjusted p = 0.03). All data normalized to GAPDH relative to naïve adolescent brain (PND28). n = 5 – 7 mice per group. Error bars represent S.E.M. *p<.05 relative to naïve adolescent brain (PND28).
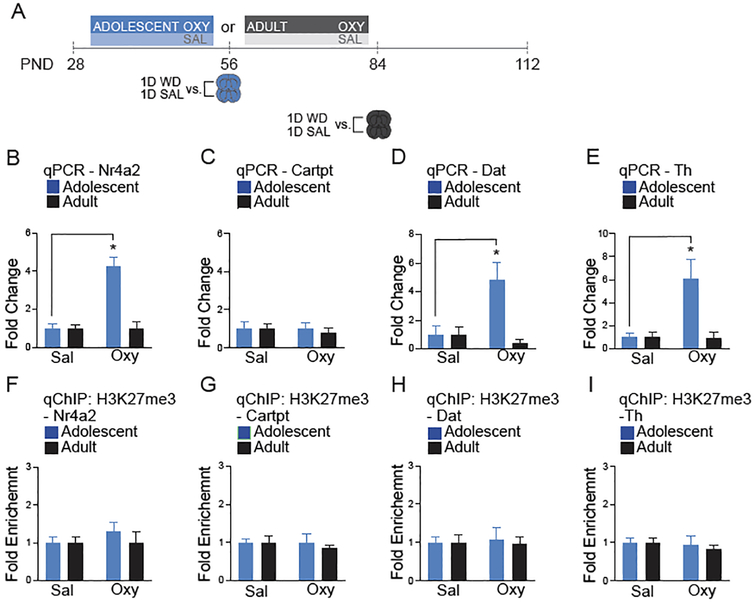
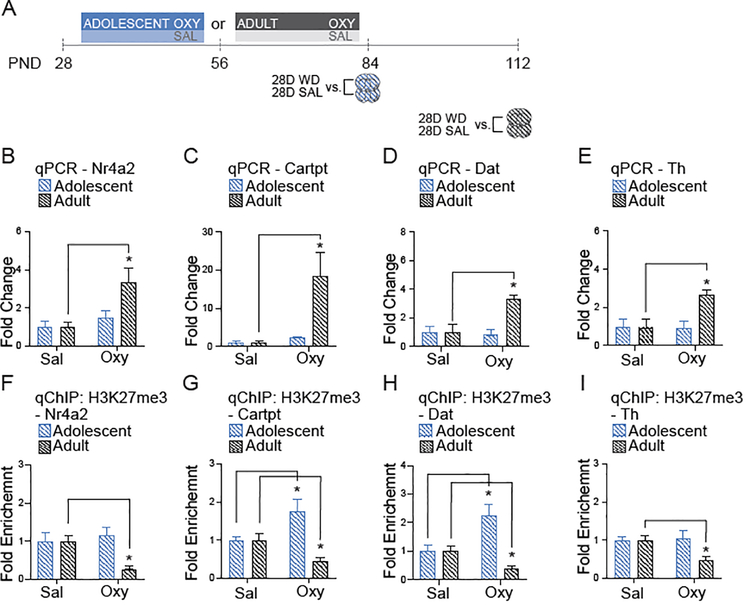
Figure 5. Adolescent oxycodone exposure regulates gene expression at 1-day of withdrawal in the VTA.
A.) Timeline of oxycodone withdrawal (WD) and tissue collection. B.) Adolescent oxycodone exposure activates Nr4a2 (Two-way ANOVA, n=6 per group, Interaction: F (1,20) = 22.98, p=0.0001; Drug: F1,20 = 18.21, p=0.0004; Age of Exposure F (1,20) = 18.21, p=0.0004; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p<0.0001; Adult Saline vs. Oxycodone, p=0.999). C.) No significant difference in Cartpt expression following adolescent or adult oxycodone exposure (Two-way ANOVA, n=6 per group Interaction: F1,20 = 0.371, p=0.549; Drug: F1,20 = 0.371, p=0.549; Age of Exposure F1,20 = 0.011, p=0.917). D.) Dat is activated following adolescent oxycodone exposure (Two-way ANOVA, Interaction: F1,19 = 8.010, p=0.017; Drug: : F1,19 = 4.175, p=0.052; Age of Exposure : F1,19 = 8.010, p=0.0176; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.004; Adult Saline vs. Oxycodone, p=0.834). E.) Th is activated following adolescent oxycodone exposure when compared to saline. (Two-way ANOVA, Interaction: : F1,19 = 7.499, p=0.0131; Drug: : F1,19 = 7.097, p=0.015; Age of Exposure : F1,19 = 7.498, p=0.013; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.002; Adult Saline vs. Oxycodone, p=0.834). F.) No significant difference in H3K27me3 enrichment at the Nr4a2 promoter following adolescent or adult oxycodone exposure (Two-way ANOVA, Interaction: F1,20 = 0.396, p=0.536; Drug: F1,20 = 0.616, p=0.447; Age of Exposure F1,20 = 0.616, p=0.447). G.) No significant difference in H3K27me3 enrichment at the Cartpt promoter following adolescent or adult oxycodone exposure (Two-way ANOVA, Interaction: F1,20 = 0.111, p=0.741; Drug: F1,20 = 0.111, p=0.741; Age of Exposure F1,20 = 0.410, p=0.523). H.) No significant difference in H3K27me3 enrichment at the Dat promoter following adolescent or adult oxycodone exposure (Two-way ANOVA, Interaction: F1,17 = 0.067, p=0.7978; Drug: F1,17 = 0.028, p=0.884; Age of Exposure F1,17 = 0.410, p=0.7971). I.) No significant difference in H3K27me3 enrichment at the Th promoter following adolescent or adult oxycodone exposure (Two-way ANOVA, Interaction: F1,19 = 1.340, p=0.7184; Drug: F1,19 = 0.444, p=0.5130; Age of Exposure: F1,19 = 0.1340, p=0.7184). All data normalized to GAPDH relative to saline age-matched controls. n = 5–6 mice per group. Error bars represent S.E.M. *p<.05 relative to saline.
Figure 6. Adult oxycodone exposure regulates gene expression at 28-days of withdrawal in the VTA.
A.) Timeline of oxycodone withdrawal (WD) and tissue collection. B.) Nr4a2 is activated following adult oxycodone exposure when compared to saline. (Two-way ANOVA, Interaction: F1,17 = 5.468, p=0.0318; Drug: F1,17 = 12.94, p=0.002; Age of Exposure F1,17 = 5.470, p=0.0318; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.7949; Adult Saline vs. Oxycodone, p=0.004). C.) Cartpt is activated following adult oxycodone exposure (Two-way ANOVA, Interaction: F1,18 = 5.221, p=0.035; Drug: F1,18 = 7.085, p=0.016; Age of Exposure F1,18 = 5.221, p=0.035; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.994; Adult Saline vs. Oxycodone, p=0.009). D.) Dat is activated following adult oxycodone exposure (Two-way ANOVA, Interaction: F1,18 = 8.290, p=0.010; Drug: F1,18 = 6.310, p=0.021; Age of Exposure F1,18 = 8.332, p=0.009; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.994; Adult Saline vs. Oxycodone, p=0.007). E.) Adult oxycodone exposure activates Th when compared to saline (Two-way ANOVA, Interaction: F1,17 = 5.938, p=0.0261; Drug: F1,17 = 5.186, p=0.036; Age of Exposure F1,17 = 5.258, p=0.049; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.9918; Adult Saline vs. Oxycodone, p=0.0272). F.) Adult oxycodone exposure depletes H3K27me3 at the Nr4a2 promoter (Two-way ANOVA, Interaction: F1,18 = 5.286, p=0.0337; Drug: F1,18 = 2.292, p=0.147; Age of Exposure F1,18 = 5.286, p=0.0337; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.809; Adult Saline vs. Oxycodone, p=0.039). G.) Adolescent oxycodone exposure enriched H3K27me3 and adult oxycodone exposure depleted H3K27me3 (Two-way ANOVA, Interaction: F1,18 = 13.57, p=0.001; Drug: F1,18 = 0.3661, p=0.5527; Age of Exposure F1,18 = 13.57, p=0.001; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.0143; Adult Saline vs. Oxycodone, p=0.0430). H.) Adolescent oxycodone exposure enriched H3K27me3 when compared to saline (Two-way ANOVA, Interaction: F1,17 = 13.91, p=0.001; Drug: F1,17 = 1.839, p=0.193; Age of Exposure F1,17 = 13.91, p=0.001; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.003; Adult Saline vs. Oxycodone, p=0.12). I.) Adult oxycodone exposure depletes H3K27me3 at the Th promoter (Two-way ANOVA, Interaction: F1,17 = 4.901, p=0.0261; Drug: F1,17 = 3.074, p=0.097; Age of Exposure F1,17 = 4.906, p=0.040; followed by Sidak’s multiple comparisons test, Adolescent Saline vs. Oxycodone, p=0.932; Adult Saline vs. Oxycodone, p=0.003). All data normalized to GAPDH relative to saline age-matched controls. n = 5 – 6 mice per group. Error bars represent S.E.M. *p<.05 relative to saline.
RESULTS
Developmental expression of genes involved in dopamine transmission
To examine molecular changes associated with the dopamine transmission across developmental time points in the VTA (Figure 1), we focused on the following genes: (1) Nr4a2 encoding the nuclear receptor NURR1 (Nuclear receptor related-1 protein) known to be important in dopamine neuron development and neuroprotection in the context of opiate exposure 29, (2) Cartpt, encoding the “cocaine and amphetamine regulated transcript’ which is a neuropeptide activated acutely by morphine in sensitized and naive animals 30, (3) Th, encoding tyrosine hydroxylase, the rate limiting enzyme in catecholamine biosynthesis, which is activated by chronic morphine in the VTA and regulates dopamine release during morphine CPP 26,31 and (4) Dat, (Slc6a3), which encodes the dopamine transporter that negatively modulates morphine reward 32. Gene expression was determined from the onset of adolescence (PND 28) to adulthood (PND 112). Nr4a2 and Cartpt were both significantly upregulated at PND 84 (Figure 2B, C), while, Dat was downregulated at PND 112 (Figure 2D). There were no significant changes in Th expression across the developmental time points examined (Figure 2E).
Figure 1. Study Design.
Nine tissue sets of VTA were collected at the indicated times and processed within-subject for qChIP and qPCR. To examine adolescent gene expression, we compared dopamine related gene expression at PND 56, 84, and 112 (see Figure 2). To distinguish between epigenetic effects of oxycodone treatment and normal development of gene expression, we normalized gene expression to the naïve adolescent mice following adolescent and adult oxycodone exposure at 1 and 28 days of withdrawal (WD) (see Figure 3 and 4). To determine epigenetic effects of oxycodone exposure at 1 and 28 days of WD, we normalized oxycodone exposed mice to age-matched saline exposed mice and compared adult and adolescent drug exposure (see Figure 5 and 6).
To interrogate the mechanism by which gene expression is altered across postnatal development, we measured the enrichment of the repressive histone modification, H3K27me3, at promoter regions. In agreement with the upregulation of Nr4a2 and Cartpt (Figure 2B, C), H3K27me3 was significantly depleted at these genes at PND 84 (Figure 2F, G). In addition, the H3K27me3 mark was reduced at the Cartpt promoter on PND 56 (Figure 2G). The repression of Dat was associated with enrichment of H3K27me3 at its promoter on PND 112 (Figure 2D, H). There were no significant changes in H3K27me3 modified histones at the Th promoter (Figure 2I) in line with the absence of a corresponding changes in gene expression (Figure 2E). Taken together, these findings indicate that key genes in dopamine transmission are associated with epigenetic mechanisms during postnatal development.
Adolescent oxycodone exposure alters expression of key genes in dopamine transmission
To determine the effects of adolescent oxycodone exposure on gene expression and epigenetic modifications at key genes in the dopamine system, we exposed adolescent mice (PND28–56) to oxycodone and measured gene expression and H3K27me3 enrichment in the VTA after 1- and 28-days of withdrawal (Figure 3A). All comparisons were made relative to the onset of adolescence (PND 28) to detect deviations from normal development 22. Adolescent oxycodone exposure dramatically activated Nr4a2 expression at 1- and 28-days of withdrawal (Figure 3B). There were no changes in Cartpt expression at either 1- or 28-days relative to the naïve adolescent brain (PND28; Figure 3C). Dat was transiently activated at 1-day but not at 28-days of withdrawal (Figure 3D). There were no significant changes in Th expression (Figure 3E).
We next examined the abundance of the H3K27me3 mark at the relevant promoters during withdrawal. We found that although Nr4a2 expression was activated at both 1- and 28-days of withdrawal (Figure 3B), the repressive H3K27me3 was depleted only at 28-days of withdrawal, suggesting that another mechanism must be responsible for its acute regulation at 1-day of withdrawal (Figure 3F). Consistent with the lack of changes in Cartpt mRNA levels following adolescent oxycodone exposure (Figure 3C), we found no changes in H3K27me3 enrichment (Figure 3G). The H3K27me3 mark was increased at the Dat promoter at 28-days of withdrawal (Figure 3H), when gene expression returned to baseline (Figure 3D). In contrast, loss of the H3K27me3 modification at the Th promotor (Figure 3I) corresponded with increased gene expression at 1-day of withdrawal (Figure 3E). These data suggest that adolescent oxycodone exposure disrupts normal developmental gene expression, such that Nr4a2 is prematurely activated (compare to Figure 2B).
Adult oxycodone exposure alters expression of key genes in dopamine transmission differently than adolescent treatment
To determine if oxycodone-induced changes in gene expression and histone modifications were contingent on exposure during adolescence, we treated adult mice (PND56–84) with oxycodone and measured mRNA and H3K27me3 following withdrawal in the VTA (Figure 4A). All comparisons were made relative to the onset of adolescence (PND 28) to detect deviations from normal development. Adult oxycodone exposure activated Nr4a2 expression at 1- and 28-days of withdrawal (Figure 4A, B). In contrast to the findings in adolescent exposure, we found Cartpt expression was activated specifically at 1- and 28-days of withdrawal (Figure 4C). Dat and Th expression did not differ at either withdrawal time point relative to the naïve adolescent brain (PND28; Figure 4D, E).
Next, we measured H3K27me3 enrichment at promotor regions following adult oxycodone exposure. H3K27me3 was depleted at Nr4a2 at 28-days and Cartpt at 1- and 28-days of withdrawal (Figure 4F, G), which corresponded with increased gene expression at these time points (Figure 3B, C). We found no significant change in H3K27me3 enrichment at the Dat promoter when compared to the naïve brain (PND28, Figure 4H). At Th we found a reduction of H3K27me3 at 28-days of withdrawal (Figure 4I). In conclusion, adult oxycodone exposure prolonged the increased gene expression of Nr4a2 and Cartpt, relative to normal development (Figure 2B, C), which is associated with the abundance of the repressive H3K27me3 mark at gene promoters.
Adolescent oxycodone exposure causes gene expression changes at 1-day of withdrawal.
To determine the age-dependent effects of drug exposure in the VTA, we compared adolescent and adult oxycodone exposed mice relative to age-matched, saline treated controls (Figure 5A). Analysis of gene expression found an age x drug effect following 28-days of oxycodone exposure followed by 1-day of withdrawal (Figure 5B, D, E). Specifically, only adolescent oxycodone exposure activated Nr4a2, Dat and Th expression when compared to saline age-matched controls (PND56). There was no change in the expression of these genes following adult oxycodone exposure relative to saline age-matched controls (PND84). Cartpt mRNA levels were unchanged following either exposure paradigm (Figure 5C). Unexpectedly, we did not find any changes in the abundance of H3K27me3 at the promotor regions of Nr4a2, Cartpt, Dat, or Th by any of the treatments (Figure 5F–I), suggesting that alternative mechanisms of regulation are responsible for the observed gene expression patterns in adult mice exposed to oxycodone during adolescence.
Adult oxycodone exposure causes gene expression changes after 28-days of withdrawal.
To interrogate long-term effects of adolescent and adult oxycodone exposure we examined the abundance of H3K27me3 and gene expression in the VTA at 28-days of withdrawal following four weeks of opioid treatment (Figure 6A). We found an age x drug effect following oxycodone exposure at 28-days of withdrawal (Figure 6B–E). Specifically, adult oxycodone exposure activated Nr4a2, Cartpt, Dat, and Th expression when compared to saline age-matched controls (Figure 6B–E). Remarkably, at the same time point no changes in steady-state mRNA levels were observed if oxycodone was given to adolescent mice (Figure 6B–E). We then measured corresponding changes in H3K27me3 abundance and found adult exposure reduced and adolescent exposure increased the repressive mark H3K27me3 at selected genes after 28-days of withdrawal (Figure 6F–I). Specifically, adult oxycodone exposure depleted H3K27me3 modified histones at Nr4a2, Cartpt, Dat, and Th promoters which corresponded to changes in gene expression (see Figure 6B–I). In contrast, adolescent oxycodone increased the H3K27me3 mark at the Cartpt and Dat promoters when compared saline age-matched controls (PND84, Figure 6G, H). These data suggest adult oxycodone exposure induces long-term changes in H3K27me3, whereas, adolescent oxycodone exposure abrogates dopamine related gene expression via the maintenance of H3K27me3.
Discussion
Recent studies find that prescription opioid abuse in adolescence causes heroin use in adulthood 8. To investigate the gene regulatory mechanisms underlying this transition, we exposed either adolescent or adult mice to oxycodone and examined epigenetic modifications at genes associated with dopamine transmission following 1- or 28- days of withdrawal. Using this approach, we identified adolescence-specific gene regulation that may render this developmental phase particularly sensitive to oxycodone exposure. The subtle distinction between naïve mice at PND28 controls and age-matched controls in our study allows one to concluded that oxycodone does not alter normal development of dopamine related gene expression, per se, but rather inhibits abstinence related expression via oxycodone induced epigenetic modifications following adolescent exposure. Specifically, increased gene expression of Nr4a2 and its downstream target gene, Cartpt, was associated with reduced H3K27me3 at PND84. At PND112 the expression of Nr4a2 and Cartpt returned to levels observed at PND28, and Dat expression declined below baseline 33. We examined gene expression during oxycodone exposure relative to PND28 to examine abnormalities in normal development. Both adolescent and adult oxycodone exposure activated Nr4a2 expression during 1- or 28- days of withdrawal, relative to PND28. The expression pattern of Cartpt relative to PND28 was specific to adult exposure.
By comparing oxycodone exposed mice to age-matched, saline exposed mice, we were able to define changes related to both withdrawal and age at initial drug exposure. We discovered epigenetic changes specific to adult oxycodone exposure, such as depleted H3K27me3 and increased expression of Cartpt. Adolescent oxycodone exposure led to increased Th and Dat expression at 1-day of withdrawal relative to age-matched controls, while adult oxycodone exposure resulted in activation of these genes at 28-days of withdrawal relative to age-matched controls. We defined age and length of withdrawal-dependent gene regulation, in which dopamine related gene expression is transient following adolescent oxycodone exposure and delayed following adult oxycodone exposure. We further identified developmental differences in a putative underlying epigenetic mechanism. Adult, but not adolescent, oxycodone exposure led to loss of H3K27me3 at dopamine related genes at 28-days of withdrawal. We hypothesize that the failure of adolescent oxycodone exposure to induce prolonged dopamine related gene expression may underlie developmental differences in the risk of transition to heroin addiction following oxycodone abuse 6. Specifically, Nr4a2 and its downstream target, Cartpt, function to repress drug sensitivity via homeostatic control of dopaminergic signaling 26,34,35, and we find that Cartpt activation is lost specifically during adolescent oxycodone exposure and withdrawal.
Adaptive changes in cellular and synaptic function after prolonged opiate exposure and withdrawal is well documented 6,36. For example, consistent with our studies of the VTA, others have observed increased expression of Nr4a2, Cartpt, Th and Dat during withdrawal 30,37. These changes showed a high degree of co-localization with TH+ neurons in the VTA suggesting that Nr4a2 may be associated with withdrawal induced alterations of dopamine neuron activity 38. Human studies provide neurological evidence that abnormal expression of Nr4a2 in heroin abusers may be exacerbated by age 39. In addition, gene expression changes following adult opioid exposure in mice is correlated with epigenetic changes, including loss of histone H3 lysine 9 acylation (H3K9ac), histone H3 lysine 9 methylation (H3K9me3) and H3K27me3 36,40,41. In the VTA, morphine exposure increases histone deacetylase 2 activity in dopamine neurons and reduces levels of H3K9ac genome-wide 40. These changes are associated with greater disinhibition of dopaminergic neurons which can be restored via increased histone acetylation 40. Systemic administration of HDAC inhibitors (HDACi) facilitates extinction of morphine CPP, blocks priming-induced reinstatement and attenuates sensitization 42,43. Opioid-induced withdrawal is also associated with Ezh2 (enhancer of zester homolog 2) expression, which catalyzes H3K27me3 44. Virally-mediated overexpression of Ezh2 in the VTA represses genes for dopaminergic neuron survival and enhances morphine CPP 21. We propose that prolonged exposure to oxycodone during adolescence increases the sensitivity to morphine later in adulthood via the suppression of adaptive gene expression. Conversely, the net effect of adult oxycodone exposure is reward tolerance measured by decreased dopamine output in response to opiates 26,45.
The relative immaturity of the dopamine system during adolescence leads to enhanced behavioral responses to drug exposure 23,46–48. Innervation of dopamine neurons and striatal dopamine levels gradually increase across adolescence, and then decline throughout adulthood 49,50. Our current study provides further evidence that adolescence is a specific developmental period with respect to epigenetic modifications at genes associated with dopamine transmission 3,22. We defined simultaneous effects of age and drug exposure by examining gene expression following adult and adolescent oxycodone exposure across development, relative to both naïve and age-matched controls. Our work is consistent with reports of declining levels of Nr4a2 51 and Dat 33 during normal aging in the VTA. To our knowledge, we are the first to report age-dependent changes in Cartpt expression. Importantly, we found that at 1-day of withdrawal following adolescent exposure, expression of Nr4a2 and Dat were greater than that in adult at PND56, compared to PND28, although we cannot completely exclude effects of intracranial surgery on dopamine related gene expression. During adolescence, mice have larger pools of releasable dopamine and self-administer less oxycodone than adults, which may be associated with an enhanced response to oxycodone 11,19. This conclusion is further supported by enhanced opioid (oxycodone and morphine) CPP following adolescent self-administration and subcutaneous oxycodone 6,9. Interestingly, adolescent increase in oxycodone CPP relative to adult, has only been observed at lower doses, suggesting a leftward shift in the dose response curve following adolescence exposure 9. We suppose that adolescent oxycodone exposure results in persistence of increased dopamine release and opioid sensitivity that is maintained beyond adolescence 11. In regard to adult oxycodone exposure, the increased expression of Cartpt and Dat may reflect a greater capacity for dopamine uptake 6,35,52. Taken together, we identified a putative epigenetic mechanism by which withdrawal following adolescent oxycodone exposure leads to persistent adolescent dopamine related gene expression into adulthood.
Similar to the enhanced drug-induced behavioral responses observed during adolescence, epigenetic changes in the brain following drug exposure are more salient in adolescents than adults 6. For example, adolescent ethanol self-administration attenuates dopamine D2 receptor (D2) expression and histone H3 acetylation at 1-day of withdrawal in the NAc 53. These changes do not occur after adult exposure until 28-days of withdrawal 46,47. Furthermore, inhibition of histone deacetylation attenuates adolescent specific increases in ethanol self-administration 54. These data suggest that alcohol-induced gene expression is developmentally regulated. Our study was the first to explore gene expression in the VTA across development and withdrawal from oxycodone. We found that H3K27me3 depletion is only associated with increased gene expression at 28-days of withdrawal or adulthood (PND112). This suggests that H3K27me3 plays a key role in delayed gene activation following drug exposure. We focused specifically on how these changes were associated with long term neuroadaptations in response to drug reward. Increased expression of Cartpt following adult oxycodone exposure may be related to its role in homeostatic control of dopamine signaling in adulthood. We found a reduction in H3K27me3, which was associated with increased Cartpt expression at PND84, but both H3K27me3 abundance and Cartpt expression return to baseline at PND112 (Figure 2C). Cartpt was activated in the oxycodone exposed adult brain at 1- and 28-days of withdrawal (PND 84 and 112), when compared to both the naïve brain (PND28; Figure 4C) and age-matched controls (PND112; Figure 6C). However, Cartpt was not activated at 1-day or 28-days of withdrawal following adolescent exposure (Figure 5C). Rather, Cartpt, expression was suppressed, and H3K27me3 enrichment was maintained when compared to both the naïve brain (PND28; Figure 3C) and age-matched controls (PND56; Figure 6C, G). This finding is significant given that Cartpt functions in vivo to reduce levels of dopamine in response to morphine 34,35.
Opioid exposure during adolescence alters normal development of the dopamine reward pathway, and leads to long-term changes in behavior and gene expression 6,10,19. In the context of development and drug exposure, we discovered specific stages of gene expression which are defined by long-lasting changes in the abundance of the repressive chromatin modification, H3K27me3, at key dopamine related genes. This work lays the groundwork to examine the functional relevance of the H3K27me3 using novel strategies of locus specific epigenetic editing with virally expressed CRISPR/Cas9 fusion enzymes. Future work will interrogate additional epigenetic mechanisms of gene activation that underlie the development of heroin addiction following nonmedical use of prescription opioids.
Supplementary Material
Supplementary Table 1. Primers used for qPCR and qChip.
Supplementary Figure 1. Surgery does not affect epigenetic modifications at dopamine related genes across developmental time points in the VTA. Timeline of developmental time points following surgery. B.) Nr4a2 is activated at PND 84 despite the age of surgery (One-way ANOVA F = 4.500, p=0.026, followed by Dunnett’s multiple comparisons test, PND 28 vs. Adolescent 28D saline (A 28D Sal), p=0.044; PND 28 vs. Adult 1D saline (D 1D Sal), p=0.045). C.) Cartpt is activated at PND 84 despite the age of surgery (One-way ANOVA F = 7.040, p=0.007, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.006; PND 28 vs. D 1D Sal, p=0.038). D.) No significant changes in Dat. E.) No significant changes in Th. F.) H3K27me3 is depleted at the Nr4a2 promotor at PND 84 (One-way ANOVA F = 7.040, p=0.007, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.042; PND 28 vs. D 1D Sal, p=0.066). G.) H3K27me3 is depleted at the Cartpt promotor at PND 84 (One-way ANOVA F = 5.061, p=0.022, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.049; PND 28 vs. D 1D Sal, p=0.023). H.) No significant changes in Dat. I.) No significant changes in Th. All data normalized to GAPDH relative to PND28. n = 5 – 7 mice per group. Error bars represent S.E.M. *p<.05 relative to PND28.
Supplementary Figure 2. Two-stage Benjamini, Krieger, & Yekutieli FDR procedure to adjust for multiple comparison testing. Multiple comparison adjustments to account for the seven independent comparisons made relative to the naive PND 28 brain. P values were generated using two-tailed ttest (black dots). q values (red dots, adjusted p value) were generated using the p values and the two-stage Benjamini, Krieger, & Yekutieli FDR procedure. Each of the seven comparisons for gene expression (A-D) and H3K27me3 enrichment (E-H) are represented for Nr4a2, Cartpt, Dat, and Th. A q value was considered significance level of 5% (black dashed line). Adolescent 1D (A-1D), Adolescent 28D (A-28D), Adult 1D (D-1D), Adult 28D (D-28D), Postnatal Day 56 (PND 56), PND 84, and PND 112 are relative to PND 28 and Gapdh.
Acknowledgements
We are grateful to Delaney Fisher for critical reading of the manuscript. Financial support is kindly acknowledged from Charles E. Kaufman Foundation Young Investigator Award (E.A.H.), Whitehall Foundation Grant (E.A.H.), NIH-NIDA Avenir Director’s Pioneer Award (E.A.H., DP1 DA044250) and Research Supplements to Promote Diversity in Health-Related Research (E.A.H., M.D.C., DP1 DA044250-01), T32 Predoctoral Training Grant in Pharmacology (M.D.C., T32GM008076), NIH Drug Abuse Dissertation Research Award (M.D.C., R36 DA050877), NIH-NIDA R21 Award (J.A.B., DA04401). We would also like to thank the NIDA drug supply program for providing the drugs used within this study.
References
- 1.Birnbaum HG et al. Societal Costs of Prescription Opioid Abuse, Dependence, and Misuse in the United States. Pain Med. 12, 657–667 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Manchikanti L & Singh A Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 11, S63–88 (2008). [PubMed] [Google Scholar]
- 3.Spear LP The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Doremus-Fitzwater TL, Varlinskaya EI & Spear LP Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 72, 114–23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark DB, Kirisci L & Tarter RE Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 49, 115–121 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Sanchez V, Carpenter MD, Yohn NL & Blendy JA Long-lasting effects of adolescent oxycodone exposure on reward-related behavior and gene expression in mice. Psychopharmacology (Berl.) 1–12 (2016) doi: 10.1007/s00213-016-4425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Behavioral Health Statistics and Quality, S. A. and M. H. S. A. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. HHS Publ. No. PEP19-, (2019). [Google Scholar]
- 8.Cerdá M, Santaella J, Marshall BDL, Kim JH & Martins SS Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. J. Pediatr. 167, 605–612.e2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y et al. Adolescent oxycodone self administration alters subsequent oxycodone-induced conditioned place preference and anti-nociceptive effect in C57BL/6J mice in adulthood. Neuropharmacology 111, 314–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niikura K, Ho A, Kreek MJ & Zhang Y Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol. Biochem. Behav. 110, 112–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamford JA Development and Ageing of the Rat Nigrostriatal Dopamine System Studied with Fast Cyclic Voltammetry. J. Neurochem. 52, 1582–1589 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Caudle WM et al. Reduced Vesicular Storage of Dopamine Causes Progressive Nigrostriatal Neurodegeneration. J. Neurosci. 27, 8138–8148 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philpot RM, Wecker L & Kirstein CL Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int. J. Dev. Neurosci. 27, 805–815 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Naneix F, Marchand AR, Di Scala G, Pape J-R & Coutureau E Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci. Off. J. Soc. Neurosci. 32, 16223–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison KE, Rodgers AB, Morgan CP & Bale TL Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 17–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naneix F, Marchand AR, Di Scala G, Pape J-R & Coutureau E Parallel Maturation of Goal-Directed Behavior and Dopaminergic Systems during Adolescence. J. Neurosci. 32, 16223–16232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Chiara G & Imperato A Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 244, 1067–80 (1988). [PubMed] [Google Scholar]
- 18.Vander Weele CM et al. Rapid dopamine transmission within the nucleus accumbens: Dramatic difference between morphine and oxycodone delivery. Eur. J. Neurosci. 40, 3041–3054 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y et al. Behavioral and Neurochemical Changes Induced by Oxycodone Differ Between Adolescent and Adult Mice. Neuropsychopharmacology 34, 912–922 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y et al. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl.) 231, 1277–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wever I, von Oerthel L, Wagemans CMRJ & Smidt MP EZH2 Influences mdDA Neuronal Differentiation, Maintenance and Survival. Front. Mol. Neurosci. 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe HHA, Hamidullah S, Jenkins BW & Khokhar JY Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol. Ther. 206, 107431 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Cass DK, Thomases DR, Caballero A & Tseng KY Developmental Disruption of Gamma-Aminobutyric Acid Function in the Medial Prefrontal Cortex by Noncontingent Cocaine Exposure During Early Adolescence. Biol. Psychiatry 74, 490–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe HHA, Hamidullah S, Jenkins BW & Khokhar JY Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol. Ther. 206, 107431 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Jacobs FMJ et al. Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons. Development 136, 2363–2373 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo JW et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat. Neurosci. 18, 415–422 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ & Schmittgen TD Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y & Hochberg Y Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995). [Google Scholar]
- 29.Volakakis N et al. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. 107, 12317–12322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spijker S et al. Morphine exposure and abstinence define specific stages of gene expression in the rat nucleus accumbens. FASEB J. 18, 848–850 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Liang J et al. Dynamic Changes of Tyrosine Hydroxylase and Dopamine Concentrations in the Ventral Tegmental Area-Nucleus Accumbens Projection During the Expression of Morphine-Induced Conditioned Place Preference in Rats. Neurochem. Res. 37, 1482–1489 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Spielewoy C et al. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur. J. Neurosci. 12, 1827–1837 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR & Fleckenstein AE Age-dependent differences in dopamine transporter and vesicular monoamine transporter-2 function and their implications for methamphetamine neurotoxicity. Synapse 63, 147–151 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter MD et al. Nr4a1 suppresses cocaine-induced behavior via epigenetic regulation of homeostatic target genes. Nat. Commun. 11, 504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rakovska A et al. Neurochemical evidence that cocaine- and amphetamine-regulated transcript (CART) 55–102 peptide modulates the dopaminergic reward system by decreasing the dopamine release in the mouse nucleus accumbens. Brain Res. Bull. 134, 246–252 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Koo JW et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat. Neurosci. 18, 415–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Pérez D, Núñez C, Laorden ML & Milanés MV Regulation of dopaminergic markers expression in response to acute and chronic morphine and to morphine withdrawal. Addict. Biol. 21, 374–386 (2016). [DOI] [PubMed] [Google Scholar]
- 38.García-Pérez D, Núñez C, Laorden ML & Milanés MV Regulation of dopaminergic markers expression in response to acute and chronic morphine and to morphine withdrawal. Addict. Biol. 21, 374–386 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Horvath M Cs. et al. Heroin Abuse Is Characterized by Discrete Mesolimbic Dopamine and Opioid Abnormalities and Exaggerated Nuclear Receptor-Related 1 Transcriptional Decline with Age. J. Neurosci. 27, 13371–13375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Authement ME et al. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J. Neurophysiol. 116, 1093–1103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Authement ME et al. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J. Neurophysiol. 116, 1093–1103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R, Zhang Y, Qing H, Liu M & Yang P The extinction of morphine-induced conditioned place preference by histone deacetylase inhibition. Neurosci. Lett. 483, 137–142 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Jing L et al. Effect of the histone deacetylase inhibitors on behavioural sensitization to a single morphine exposure in mice. Neurosci. Lett. 494, 169–173 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Wu X et al. A computational strategy for finding novel targets and therapeutic compounds for opioid dependence. PLOS ONE 13, e0207027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazei-Robison MS & Nestler EJ Opiate-Induced Molecular and Cellular Plasticity of Ventral Tegmental Area and Locus Coeruleus Catecholamine Neurons. Cold Spring Harb. Perspect. Med. 2, a012070–a012070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmisano M & Pandey SC Epigenetic mechanisms of alcoholism and stress-related disorders. Alcohol 60, 7–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feltmann K et al. Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats. Alcohol. Clin. Exp. Res. 42, 338–351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Natividad LA, Buczynski MW, Parsons LH, Torres OV & O’Dell LE Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. J. Neurochem. 123, 578–588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willing J, Cortes LR, Brodsky JM, Kim T & Juraska JM Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev. Psychobiol. 59, 583–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothmond DA, Weickert CS & Webster MJ Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neurosci. 13, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwapis JL et al. HDAC3-Mediated Repression of the Nr4a Family Contributes to Age-Related Impairments in Long-Term Memory. J. Neurosci. 39, 4999–5009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shieh KR Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology 44, 940–948 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Pascual M, Boix J, Felipo V & Guerri C Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 108, 920–931 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Jeanblanc J, Gonzalez Marin MDC, Lebourgeois S, Legastelois R & Naassila M Efficacy of HDAC1 inhibitors in a new model of binge drinking in rats. Alcohol 60, 205 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Primers used for qPCR and qChip.
Supplementary Figure 1. Surgery does not affect epigenetic modifications at dopamine related genes across developmental time points in the VTA. Timeline of developmental time points following surgery. B.) Nr4a2 is activated at PND 84 despite the age of surgery (One-way ANOVA F = 4.500, p=0.026, followed by Dunnett’s multiple comparisons test, PND 28 vs. Adolescent 28D saline (A 28D Sal), p=0.044; PND 28 vs. Adult 1D saline (D 1D Sal), p=0.045). C.) Cartpt is activated at PND 84 despite the age of surgery (One-way ANOVA F = 7.040, p=0.007, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.006; PND 28 vs. D 1D Sal, p=0.038). D.) No significant changes in Dat. E.) No significant changes in Th. F.) H3K27me3 is depleted at the Nr4a2 promotor at PND 84 (One-way ANOVA F = 7.040, p=0.007, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.042; PND 28 vs. D 1D Sal, p=0.066). G.) H3K27me3 is depleted at the Cartpt promotor at PND 84 (One-way ANOVA F = 5.061, p=0.022, followed by Dunnett’s multiple comparisons test, PND 28 vs. A 28D Sal, p=0.049; PND 28 vs. D 1D Sal, p=0.023). H.) No significant changes in Dat. I.) No significant changes in Th. All data normalized to GAPDH relative to PND28. n = 5 – 7 mice per group. Error bars represent S.E.M. *p<.05 relative to PND28.
Supplementary Figure 2. Two-stage Benjamini, Krieger, & Yekutieli FDR procedure to adjust for multiple comparison testing. Multiple comparison adjustments to account for the seven independent comparisons made relative to the naive PND 28 brain. P values were generated using two-tailed ttest (black dots). q values (red dots, adjusted p value) were generated using the p values and the two-stage Benjamini, Krieger, & Yekutieli FDR procedure. Each of the seven comparisons for gene expression (A-D) and H3K27me3 enrichment (E-H) are represented for Nr4a2, Cartpt, Dat, and Th. A q value was considered significance level of 5% (black dashed line). Adolescent 1D (A-1D), Adolescent 28D (A-28D), Adult 1D (D-1D), Adult 28D (D-28D), Postnatal Day 56 (PND 56), PND 84, and PND 112 are relative to PND 28 and Gapdh.