Abstract
Introduction
Published reports of associations between β‐amyloid (Aβ) and cortical integrity conflict. Tau biomarkers may help elucidate the complex relationship between pathology and neurodegeneration in aging.
Methods
We measured cortical thickness using magnetic resonance imaging, Aβ using Pittsburgh compound B positron emission tomography (PiB‐PET), and tau using flortaucipir (FTP)‐PET in 125 cognitively normal older adults. We examined relationships among PET measures, cortical thickness, and cognition.
Results
Cortical thickness was reduced in PiB+/FTP+ participants compared to the PiB+/FTP– and PiB–/FTP– groups. Continuous PiB associations with cortical thickness were weak but positive in FTP– participants and negative in FTP+. FTP strongly negatively predicted thickness regardless of PiB status. FTP was associated with memory and cortical thickness, and mediated the association of PiB with memory.
Discussion
Past findings linking Aβ and cortical thickness are likely weak due to opposing effects of Aβ on cortical thickness relative to tau burden. Tau, in contrast to Aβ, is strongly related to cortical thickness and memory.
Keywords: Alzheimer's disease, atrophy, cortical integrity, flortaucipir, literature review, neurodegeneration, normal aging, PART, Pittsburgh compound B, positron emission tomography, publication bias, structural magnetic resonance imaging
1. BACKGROUND
β‐amyloid (Aβ) accumulation and neurodegeneration are both key features of Alzheimer's disease (AD).1 Models of AD pathogenesis describe Aβ accumulation as an early event with neurodegeneration and corresponding cognitive impairment occurring later in the course of the disease.2 Thus, many studies have examined the relationship between Aβ and neurodegeneration hypothesizing that pathological Aβ accumulation predicts neuronal loss. Cerebrospinal fluid (CSF) and positron emission tomography (PET) biomarkers of Aβ and magnetic resonance (MR) measures of cortical volume or thickness have allowed the in vivo assessment of this model. Findings have been mixed and possibly affected by publication bias such that unexpected relationships (eg, more Aβ predicting greater cortical thickness or volume) are underrepresented in the literature.3, 4, 5
Abnormal, hyperphosphorylated tau, another key pathology in AD, appears to aggregate and spread in a process that is driven in part by Aβ.2, 6 With the more recent addition of tau biomarkers, studies have begun to explore relationships among Aβ, tau, and neurodegeneration hypothesizing that neuronal loss in AD is complex and related to both Aβ and tau.7, 8 Pathological tau shows stronger associations to neurodegeneration and to cognition than Aβ.9 Thus, Aβ and tau effects on neurodegeneration could reflect a primary role of both pathologies, an interaction or mediation of one process relative to another, or unique effects of each protein aggregate on neuronal loss. Finally, while associations between tau and atrophy appear negative and roughly linear, Aβ may have unique effects on cortical integrity that are non‐linear and do not conform with conventional hypotheses.
The main goals of the present study were to understand how PET measures of Aβ and tau relate to cortical thickness in normal aging, especially whether there are non‐linear relationships between Aβ and atrophy, and to interpret our findings in the context of the extensive literature. We also performed analyses relating Aβ and tau to cognitive domain scores, for which we expected to observe stronger relationships with tau compared to Aβ. First, we categorized cognitively healthy older adults (OA) into groups based on Aβ and tau biomarker status. Next, we compared cortical thickness across groups and measured the continuous effect of Aβ and tau on cortical thickness. Finally, we examined how Aβ, tau, and cortical thickness are related to cognition and tested mediation models to better understand the complex relationships. Using a systematic review approach, we compared our findings to those reported in the literature and discuss possible drivers of conflicting results across studies.
2. METHODS
2.1. Participants and study design
We enrolled 125 cognitively healthy OA participants who are part of the Berkeley Aging Cohort Study (BACS), an ongoing longitudinal observational study of normal aging. In the present study, participants were required to be age 55 or older with PET imaging for Aβ and tau, structural magnetic resonance imaging (sMRI), and neuropsychological testing data (see supporting information). Pittsburgh compound B (PiB) and flortaucipir (FTP) were used to quantify Aβ and tau, respectively. We used the FTP‐PET imaging session as the baseline visit and required PiB‐PET, sMRI, and neuropsychological testing within 6 months of baseline. The institutional review boards of participating institutions approved the present study and written, informed consent was obtained from all participants.
Participants were categorized into one of four possible groups based on their PiB‐PET and FTP‐PET scans: PiB–/FTP–, PiB–/FTP+, PiB+/FTP–, or PiB+/FTP+. This grouping strategy follows the principles of the A/T/N framework,10 grouping biomarkers into amyloid (A), tau (T), or neurodegeneration/neuronal injury (N) categories with binary classifications. We were specifically interested in how A/T classification affects cortical thickness, a measure of neurodegeneration (or "N"). Thus, in aggregate we refer to the four groups as “A/T groups” and do not classify based on an N biomarker.
2.2. Image acquisition
Detailed descriptions of FTP and PiB‐PET acquisition are available in previous publications.11, 12 All PET scans were acquired on a Siemens Biograph 6 Truepoint PET/CT scanner in 3D acquisition mode. Prior to PET scans a low‐dose computed tomography (CT) scan was collected for attenuation correction. Beginning at the start of an injection of 15mCi of PiB into an antecubital vein, 90 minutes of dynamic emission data were acquired and subsequently binned into 35 frames (4 × 15 seconds, 8 × 30 seconds, 9 × 60 seconds, 2 × 180 seconds, 10 × 300 seconds, and 2 × 600 seconds). After the PIB‐PET scan, participants were injected with 10 mCi of FTP and data binned as 4 × 5 minute frames starting 80 minutes after injection. PiB and FTP images were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation and smoothed with a 4 mm Gaussian kernel with scatter correction (image resolution 6.5 × 6.5 × 7.25 mm3).
HIGHLIGHTS
Past studies on amyloid beta (Aβ) and atrophy conflict and suggest a weak relationship.
The relationship between Aβ and atrophy is dependent on tau.
Tau accumulation is related to thinner cortex and worse memory in older adults.
Our findings and literature review suggest Aβ drives tau, which then drives atrophy.
RESEARCH IN CONTEXT
Systematic Review: We used defined search terms on PubMed to identify potentially relevant papers. We reviewed the literature broadly and then narrowed our focus to reports that were similar in design to the present study. The studies meeting criteria are summarized in Table 2.
Interpretation: The results of this work support a reframing of the relationship between amyloid beta (Aβ) and atrophy, which we show is not straightforward or linear. The literature shows mixed results. In contrast, tau accumulation is related to neurodegeneration, and to memory, in a consistent and linear manner in the present study and in the literature.
Future directions: Our work suggests a temporal evolution of Alzheimer's disease pathology, even in healthy older adults. To test this model in vivo, we need longitudinal data to confirm causal relationships. Further work is also needed to determine the mechanism by which tau accumulation leads to neurodegeneration.
1.5T T1‐weighted magnetization‐prepared rapid acquisition with gradient echo (MPRAGE) sMRI scans were acquired for each participant on a Siemens Magnetom Avanto with the following parameters: sagittal slice orientation, repetition time (TR) = 2110 ms, echo time (TE) = 3.58 ms, flip angle = 15°, voxel size = 1 mm isotropic.
2.3. Image preprocessing
Distribution volume ratios (DVR) values for PiB‐PET images were generated with Logan graphical analysis on PiB frames corresponding to 35 to 90 minutes post‐injection using a cerebellar gray matter reference region.13, 14 Participants’ global Aβ burden was measured based on a mean cortical DVR (>1.065 used to determine PiB positivity) in FreeSurfer‐derived frontal, temporal, parietal, and posterior cingulate regions of interest (ROIs) as previously described.15, 16
FTP standardized uptake value ratio (SUVR) images were created based on mean tracer uptake 80 to 100 minutes post‐injection normalized by mean inferior cerebellar gray matter uptake.17 SUVR images were partial volume (PV) corrected using the Geometric Transfer Matrix approach18 on FreeSurfer‐derived ROIs.19 An SUVR >1.26 in a region approximating Braak stage III/IV was used to determine FTP positivity.12, 20 We focused on two additional ROIs for FTP measures in the present study: entorhinal cortex (ERC) and a MetaROI comprised of several temporal regions including ERC, amygdala, parahippocampal cortex, fusiform, and inferior and middle temporal gyri.21 For more on FTP cut‐off and ROIs, see supporting information.
T1‐weighted MPRAGE sMRI scans were processed using FreeSurfer version 5.3 (http://freesurfer.net/).22, 23, 24 Cortical thickness estimates for each ROI were obtained in native space. To calculate the MetaROI thickness, we averaged the thickness values for each of the cortical regions (i.e., excluding amygdala): ERC, parahippocampal cortex, fusiform, and inferior and middle temporal gyri.
2.4. Neuropsychological assessment
All BACS participants undergo neuropsychological testing to measure verbal and visual memory, working memory, processing speed, executive function, language, and attention. For the present study, composite z‐scores were calculated as previously described for three cognitive domains: episodic memory, working memory, and processing speed (see supporting information and Harrison et al.25).
2.5. Statistical analyses
Statistical analyses were conducted using R (https://www.R-project.org/), jamovi (https://www.jamovi.org), and FreeSurfer tools (http://freesurfer.net). Analysis of variance (ANOVA) models were used to compare demographic, cognitive scores, and cortical thickness in 34 bilateral Freesurfer cortical ROIs between the four A/T groups. Post hoc t‐tests were used for pairwise group comparisons. Multiple regressions were used to explore continuous relationships between cortical thickness and pathology adjusting for age and sex. Associations with age and cognition were explored using Pearson correlation. Mediation analyses were performed using the “medmod” package in jamovi. Briefly, mediation analyses were used to test whether a mediator accounted for a relationship observed between two variables.26 General linear models implemented in FreeSurfer were used to run vertexwise cortical thickness comparisons between A/T groups. All statistical analyses used a two‐tailed level of 0.05 for defining statistical significance. Omnibus tests were not corrected for multiple comparisons, but whether post hoc tests survived Benjamini–Hochberg correction at a false discovery rate of 0.05 was noted in the main text or figure legends.27
2.6. Systematic literature review
A PubMed search was performed on February 5, 2020 for articles published in English with the keywords “amyloid or Aβ or β‐amyloid or amyloid‐β” and “cortical thickness or gray matter volume or grey matter volume or VBM or MTL atrophy or MTL thickness” and “cognitively normal or normal control or CN or healthy control or older adults or aging or ADNI.” The retrieved articles were reviewed for any relevant studies missed in the database search. The inclusion criteria for this literature review specify that a study must have: (1) a PET‐based or CSF‐based measure of Aβ in the brain, (2) a measure of gray matter thickness or volume in the brain, (3) a sample of at least 30 cognitively normal older adults, (4) a cross‐sectional design and results of a direct comparison between Aβ+ and Aβ– subjects or an association between continuous Aβ and gray matter across subjects. In some cases there were several papers published on overlapping datasets from the same senior author. In these cases, we included the article with the largest sample of cognitively healthy older adults. The included studies were carefully summarized focusing on demographic information, main findings regarding Aβ and cortical integrity associations and, finally, whether the study used a measure of tau.
3. RESULTS
3.1. A/T groups did not differ across demographic or cognitive measures
A total of 125 participants (age 76.6 ± 6.7, 58% female) were categorized into four groups based on PiB and FTP status (Table 1). Our cohort was enriched for Aβ positivity to ensure an adequate distribution of tau burden (see supporting information). With this caveat, it is interesting to note that A/T groups were not evenly distributed with nearly half of participants being PiB–/FTP–. Only 11% of participants were PiB–/FTP+ and these individuals had lower FTP than the PiB+/FTP+ group in the MetaROI (P = .01), but this difference was a trend in ERC (P = .05). There were no significant differences between A/T groups in age; sex; years of education; Mini‐Mental State Examination (MMSE) score; or cognitive domain scores for episodic memory, working memory, and processing speed.
TABLE 1.
Cohort Characteristics
| All subjects (n = 125) | PiB–/FTP–(n = 61) | PiB–/FTP+(n = 14) | PiB+/FTP–(n = 28) | PiB+/FTP+(n = 22) | |
|---|---|---|---|---|---|
| Age (years) | 76.6 (6.70) | 76.1 (7.72) | 78.2 (7.69) | 76.1 (5.72) | 77.7 (3.56) |
| Sex (M/F) | 52/73 | 26/35 | 6/8 | 11/17 | 9/13 |
| APOE ε4 (C/NC) a, b, c, d | 32/90 |
5/54 *missing 2 |
1/13 |
13/14 *missing 1 |
13/9 |
| Avg. years education (years) | 16.9 (1.86) | 17.2 (1.82) | 17.4 (1.99) | 16.5 (1.99) | 16.4 (1.53) |
| Global PiB DVR a , b , c , d , e | 1.14 (0.221) | 1.02 (.0301) | 1.02 (.0212) | 1.26 (.213) | 1.42 (.270) |
| ERC FTP SUVR a , b , c , e , f | 1.28 (0.236) | 1.16 (0.140) | 1.38 (0.165) | 1.26 (0.187) | 1.56 (0.286) |
| MetaROI FTP SUVR a , b , c , d , e , f | 1.26 (0.167) | 1.17 (0.079) | 1.34 (0.052) | 1.23 (0.069) | 1.50 (0.218) |
| MMSE | 28.7 (1.20) | 28.8 (1.12) | 29.0 (.961) | 28.8 (1.09) | 28.1 (1.55) |
| Episodic memory | * | .0956 (.834) | .195 (.837) | –.0144 (.842) | –.371 (.759) |
| Working memory | * | –0.0494 (1.06) | .247 (1.28) | .123 (.793) | –.177 (.883) |
|
Processing speed |
* | .00528 (.684) | .0291 (.393) | .0618 (.559) | –0.0948 (.504) |
Abbreviations: APOE, apolipoprotein E; C, carrier; DVR, distribution volume ratio; ERC, entorhinal cortex; F, female; FTP, flortaucipir; M, male; MMSE, Mini‐Mental State Examination; NC, non‐carrier; PiB, Pittsburgh compound B; ROI, region of interest; SUVR, standardized uptake value ratio; yrs, years.
Significant differences between PiB–/FTP– and PiB+/FTP– groups (P < .05)
Significant differences between PiB–/FTP‐ and PiB+/FTP+ groups (P < .05)
Significant differences between PiB–/FTP+ and PiB+/FTP– groups (P < .05)
Significant differences between PiB–/FTP+ and PiB+/FTP+ groups (P < .05)
Significant differences between PiB+/FTP– and PiB+/FTP+ groups (P < .05)
Significant differences between PiB‐/FTP– and PiB–/FTP+ groups (P < .05)
Approaching 0 due to z‐score generation process.
3.2. A/T groups showed spatially widespread differences in cross‐sectional cortical thickness
Using a standard atlas (Desikan et al.23), we compared average bilateral cortical thickness in anatomical ROIs across A/T groups using ANOVAs. Association cortex in each cerebral lobe showed differences in cortical thickness across A/T groups (Figure 1A). Patterns of post hoc relationships driving significant ANOVAs followed one of two patterns: PiB+/FTP+ was significantly thinner than PiB+/FTP– (e.g., middle temporal gyrus; Figure 1B) or PiB+/FTP+ was significantly thinner than both PiB+/FTP– and PiB–/FTP– (e.g., inferior parietal lobe; Figure 1C).
FIGURE 1.
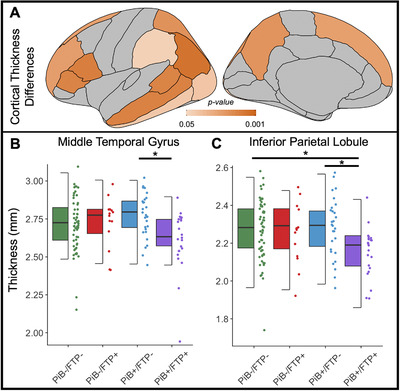
A/T groups showed spatially widespread differences in cross‐sectional cortical thickness. A) Analysis of variance (ANOVA) was used to compare cortical thickness across the four groups: PiB–/FTP– (n = 61), PiB–/FTP+ (n = 14), PiB+/FTP– (n = 28), and PiB+/FTP+ (n = 22). Significant (P < .05) differences in cortical thickness were observed in all illustrated regions of interest. B) Middle temporal thickness was plotted to demonstrate differences between the groups (ANOVA P = .013). Thickness in the PiB+/FTP– group was significantly increased compared to the PiB+/FTP+ group (post hoc t‐test P = .007). C) Inferior parietal thickness showed differences across groups (ANOVA P = .007). Inferior parietal thickness in the PiB+/FTP+ group was significantly lower than both the PiB–/FTP– (post hoc t‐test P = .009) and the PiB+/FTP– group (post hoc t‐test P = .010). Post hoc t‐tests did not survive correction for multiple comparisons. FTP, flortaucipir; PiB, Pittsburgh compound B
Increasing age was associated with thinner cortex across the whole cohort and within PiB–/FTP– and PiB+/FTP+ groups (Figure S1 in supporting information). In all A/T groups, including those that did not reach statistical significance, there was a negative, linear relationship between age and cortical thickness.
3.3. Aβ‐cortical thickness relationships depended on tau but greater tau predicted lower cortical thickness regardless of Aβ
Next, we explored the continuous relationship between cortical thickness and PiB in FTP– and FTP+ participants separately. In all analyses we adjusted for age and sex. We found that in FTP– participants higher global PiB DVR was positively associated with cortical thickness in several regions including insula, inferior frontal gryrus, fusiform, and lingual gyrus (Figure 2A). There were no negative associations between global PiB DVR and cortical thickness in this low tau pathology group. In contrast, in the FTP+ participants higher global PiB DVR was negatively associated with cortical thickness in several temporal regions as well as paracentral and caudal middle frontal ROIs (Figure 2B). In the fusiform gyrus the relationship between cortical thickness and global PiB DVR was positive in FTP– participants and negative in FTP+ participants. We showed a significant interaction effect between global PiB DVR and FTP status on fusiform cortical thickness across the entire cohort (P = .002; Figure 2C).
FIGURE 2.
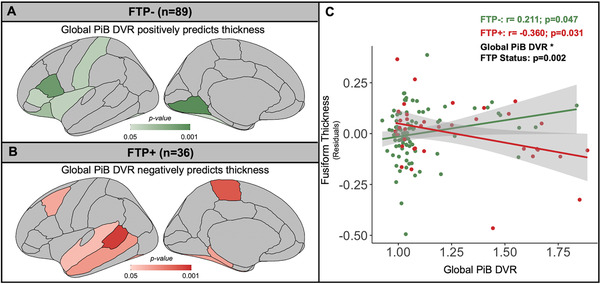
Amyloid beta (Aβ)‐cortical thickness relationships depended on tau. A) Global PiB DVR predicting cortical thickness in FTP– subjects (n = 89), adjusted for age and sex, yielded only positive significant associations in illustrated ROIs. B) Across all regions of interest (ROIs), global PiB DVR negatively predicted cortical thickness in FTP+ subjects (n = 36), adjusted for age and sex, in the illustrated ROIs. C) Generalized linear models of PiB DVR predicting fusiform thickness, adjusted for age and sex , stratified by FTP status (FTP– in green; FTP+ in red; Braak III/IV threshold >1.26 SUVR) showed a significant positive relationship in FTP– subjects (r = 0.211, P = .047) and a significant negative relationship in FTP+ subjects (r = –0.360, P = .031). There was a significant global PiB DVR by FTP status interaction (P = .002) across all subjects. Correlations between global PiB DVR and cortical thickness did not survive correction for multiple comparisons. DVR, distribution volume ratio; FTP, flortaucipir; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio
Continuous relationships between ERC FTP SUVR and cortical thickness were negative regardless of PiB status. In PiB– participants, ERC FTP SUVR was associated with thinner cortex only in the ERC region (Figure 3A, B). In contrast, in PiB+ participants, ERC FTP SUVR predicted lower cortical thickness across most of the cortex, especially in temporal lobe including ERC (Figure 3C and D). We also compared A/T groups pairwise in vertexwise analyses (Figure S2 in supporting information). Results were statistically weak but consistent with ROI findings (see supporting information).
FIGURE 3.
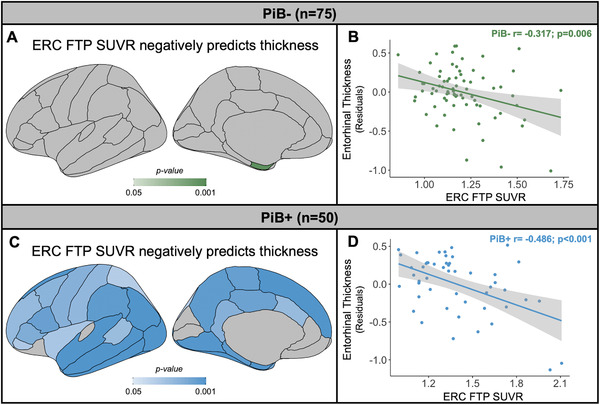
Tau predicted lower cortical thickness regardless of amyloid beta (Aβ). A) In PiB– subjects (n = 75), FTP SUVR was negatively associated with only ERC thickness. B) There was a significant negative relationship between entorhinal cortex (ERC) thickness, adjusted for age and sex, and ERC FTP SUVR in PiB– subjects (r = –0.317, P = .006). C) In PiB+ subjects (n = 50), FTP SUVR was negatively associated with a majority of cortical ROIs. D) In PiB+ subjects ERC FTP SUVR was a significant negative predictor of ERC thickness, adjusted for age and sex (r = –0.486, P < .001). Correlations between ERC FTP SUVR and cortical thickness survived correction for multiple comparisons. FTP, flortaucipir; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio
3.4. Tau and cortical thickness were related to episodic memory
Ultimately, understanding relationships between pathology and neurodegeneration may help uncover mechanisms of cognitive decline and dysfunction in aging and AD. Global PiB DVR predicted episodic memory performance (r = 0.20, P = .02), but this relationship was mediated by ERC FTP SUVR (indirect path P < .001, 89.7%; direct P = .79, 10.3%; total P = .02). In other words, the relationship between global PiB DVR and episodic memory performance was nearly 90% mediated by ERC FTP SUVR via an indirect path modeled as global PiB DVR → ERC FTP SUVR → episodic memory performance. Global PiB DVR was not related to cognition in either the working memory (r = ‐0.06, P = .49) or processing speed (r = –0.02, P = .85) domains. We further explored relationships between cognition and FTP measures or cortical thickness in ERC and the temporal MetaROI. As expected, we observed that higher FTP in ERC or MetaROI were associated with worse episodic memory (Figure 4A and B). There were no significant associations between FTP measures and working memory (ERC: r = –0.02, P = .79; MetaROI: r = –0.03, P = .77) or processing speed (ERC: r = –0.10, P = .28; MetaROI: r = –0.15, P = .10). We also observed positive associations between ERC or MetaROI cortical thickness and episodic memory performance (Figure 4C and D). There were no significant associations between cortical thickness and working memory (ERC: r = –0.06, P = 0.52; MetaROI: r = 0.08, P = .40), but cortical thickness in ERC and MetaROI were both related to processing speed (ERC: r = 0.19, P = .03; MetaROI: r = 0.35, P < .001; ERC finding does not survive multiple comparison correction).
FIGURE 4.
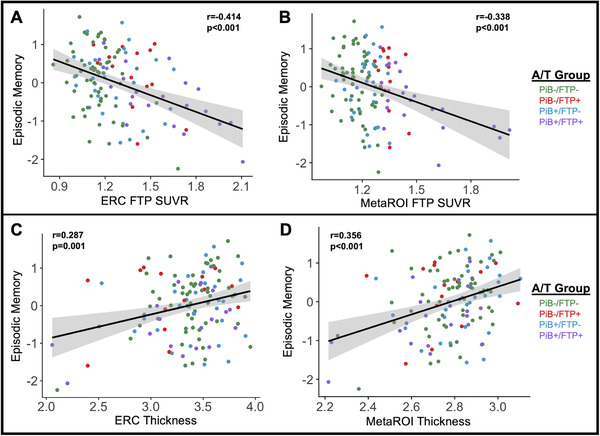
Tau and cortical thickness were related to episodic memory. A) ERC FTP SUVR was negatively associated with episodic memory performance (r = ‐0.414; P < .001) B) MetaROI FTP SUVR negatively predicted episodic memory performance (r = ‐0.338; P < .001). C) ERC thickness was positively related to episodic memory performance (r = 0.287; P = .001). D) MetaROI thickness was positively associated with episodic memory performance (r = 0.356; P < .001). All significant correlations with cognition survived correction for multiple comparisons. ERC, entorhinal cortex; FTP, flortaucipir; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio
Interestingly, the PiB+/FTP– group appeared to show weaker associations than the other three A/T groups (see Figure S3 in supporting information) although there were no significant group interaction effects. Given both FTP measures and cortical thickness are related to memory, we tested mediation models to assess whether cortical thickness, a measure of neurodegeneration, mediates the effect of tau accumulation on memory function. Focusing on the MetaROI across our entire cohort we found evidence of a mediation of MetaROI thickness on the relationship between MetaROI FTP SUVR and memory (indirect path 24.5% mediation; Figure S4A in supporting information). In PiB– participants the mediation was not significant, while in PiB+ participants the mediation was stronger than in the whole cohort (indirect path 26.9% mediation; Figure S4B and C).
3.5. Tau‐dependent relationships between Aβ and cortical thickness may account for inconsistent literature and weak association between cross‐sectional Aβ and cognition
We completed a systematic review of the literature focused on relationships between PET or CSF Aβ biomarkers and cortical integrity. The results of this review included 32 studies, which are presented in Table 2. Among the 22 studies where tau biomarkers were not available, the most common finding was a negative association between Aβ and neurodegeneration such that higher Aβ predicted thinner or lower volume cortex. A substantial proportion reported a null association between Aβ and cortical integrity and several reported positive associations between Aβ and cortex thickness or volume. A subset of ten studies had both Aβ and tau biomarkers available. All but one used CSF analytes to measure Aβ and tau.7 A significant negative association between tau and cortical integrity was reported in eight of ten studies, including the single study that used PET measures. The remaining two reported null associations between tau and neurodegeneration. The relationships between Aβ and cortical integrity in these studies were more variable with five studies reporting null findings, four reporting negative associations, and three reporting positive associations (two reported both positive and negative relationships).
TABLE 2.
Systematic Literature Review of Studies Examining Aβ Biomarkers and Cortical Integrity in Cognitively Normal Older Adults
| Study | Cohort | N | Mean age (SD) | Biomarker modality | GM∼Aβ | GM∼Tau | Description of findings: |
|---|---|---|---|---|---|---|---|
| Desikan et al. PLoS One 2010 | Alzheimer's Disease Neuroimaging Initiative (ADNI) | 208 | 76.0 (4.9) | CSF | Neg | Neg | Aβ+ subjects had decreased GM compared to Aβ– subjects in F. Tau+ subjects had decreased GM compared to tau– subjects in T. |
| Glodzik et al. Neurobiol Aging 2012 | Center for Brain Health and Alzheimer's Disease Center at NYU School of Medicine | 115 | 62.6 (9.5) | CSF | Neg | Neg | Aβ+/tau+ subjects had decreased GM compared to Aβ–/tau– subjects in P, T, L, W. Tau negatively predicted GM in P, T. Tau+ subjects had decreased GM compared to tau– subjects in P, L, T. |
| Stricker et al. Brain Imaging Behav. 2012 | ADNI | 103 | 75.5 (5.21) | CSF | Null | Neg | In all subjects, tau negatively predicted F GM. |
| Fortea et al. Alzheimers Dement 2014 | ADNI | 145 | 73.4 (6.2) | CSF | Pos | Neg | In tau– subjects, Aβ positively predicted P, O, T, GM. In Aβ+ subjects, tau negatively predicted F, P, O, T GM. |
| Wang et al. Neurology 2015 | Knight Alzheimer's Disease Research Center at Washington University | 188 | 73.0 (6.0) | CSF | Null | Neg | In tau+ subjects, tau negatively predicted P, O, T, L GM. |
| Pettigrew et al. Neuroimage Clin 2017 | Biomarkers of Cognitive Decline Among Normal Individuals (BIOCARD) | 207 | 56.9 (10.0) | CSF | Null | Null | |
| Maass et al. J Neurosci 2018 | Berkeley Aging Cohort Study (BACS) | 83 | 77 (6) | PET | Null | Neg | In all subjects, tau negatively predicted T GM. In Aβ+ subjects, tau was negatively related to change in T GM. In Aβ– subjects, tau was negatively related to change in T GM. |
| Montal et al. Alzheimers Dement 2018 | Hospital de Sant Pau, Barcelona, Spain; Hospital Marqués de Valdecilla, Satander, Spain; CITA Alzheimer, San Sebastian, Spain | 254 | 58.6 (7.7) | CSF | Neg, Pos | Neg | Aβ+/tau‐–subjects had increased GM compared to Aβ–/tau– subjects in P, T. Aβ+/T+ subjects had decreased GM compared to Aβ–/tau– subjects in T. |
| Batzu et al. Neurobiol Aging 2019 | ADNI | 122 | 72.3 (5.7) | CSF | Neg, Pos | Neg | Aβ+/tau– subjects had increased GM compared to Aβ–/tau– subjects in F, P, O. Aβ+/tau+ subjects had decreased GM compared to Aβ–/tau– subjects in F. |
| Luo et al. Front Neurosci 2019 | ADNI | 76 | 75.0 (5.5) | CSF | Null | Null | |
| Becker et al. Ann Neurol 2011 | Massachusetts General and Brigham and Women's Hospitals, and referring memory clinics (S.S., G.M., and D.M.) | 87 | 75.0 (8.0) | PET | Neg | N/A | In all subjects, Aβ negatively predicted F, P, T, L GM. Aβ+ subjects had decreased GM compared to Aβ– subjects in F, P, L. |
| Arenaza‐Urquijo et al. J Alzheimers Dis 2013 | Alzheimer's Disease and Cognitive Disorders Unit, Neurology Service, Hospital Clinic, Barcelona, Spain | 33 | 70.3 (7.1) | CSF | Neg | N/A | Aβ+ subjects had decreased GM compared to Aβ– subjects in F, P, T, L. |
| Doré et al. JAMA Neurol 2013 | Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) | 93 | 73.9 (7.4) | PET | Neg | N/A | Aβ+ subjects had decreased GM in P, L compared to Aβ– subjects. In Aβ+ subjects, Aβ negatively predicted P, T, L GM. Aβ+ subjects had an increased rate of GM atrophy than Aβ– subjects in P, T, L. |
| Whitwell et al. Neuroimage Clin 2013 | Mayo Clinic Alzheimer's Disease Research Center and Mayo Clinic Study of Aging (MCSA) | 230 | 80* | PET | Neg, Pos | N/A | Aβ+ subjects had increased GM compared to Aβ– subjects in P; Aβ+ subjects had decreased GM compared to Aβ– subjects in F, O, P, T |
| Kljajevic et al. Neurobiol Aging 2014 | ADNI | 57 | 76.7 (5.8) | PET | Null | N/A | |
| Araque et al. Neurobiol Aging 2015 | ADNI | 40 | 75.2 (6.8) | PET | Neg | N/A | In all subjects, Aβ+ subjects had a higher rate of GM atrophy compared to Aβ– in P, O, L. |
| Doherty et al. Alzheimers Dement (Amst) 2015 | Wisconsin Registry for Alzheimer's Prevention (WRAP) | 109 | 60.7 (5.7) | PET | Neg | N/A | Aβ+ subjects had decreased GM compared to Aβ– subjects in T. |
| Kaffashian et al. Neurobiol Aging 2015 | Three‐City Dijon Study | 1187 | 72.0 (3.98) | CSF | Neg | N/A | Subjects in the highest tertile of Aβ at baseline and follow‐up had faster GM atrophy in W than those in the lowest tertile. |
| Llado‐Saz et al. Neurobiol Aging 2015 | Laboratory of Functional Neuroscience at Pablo de Olavide University | 120 | 68.9 (3.7) | CSF | Neg | N/A | Aβ+ subjects had decreased GM compared to Aβ– subjects in F, T. |
| Mattsson et al. Neurology 2015 | ADNI | 280 | 73.6(6.3) | PET | Null | N/A | |
| Susanto et al. J Alzheimers Dis 2015 | ADNI | 103 | 75.5 (5.2) | CSF | Neg | N/A | Aβ+ subjects had decreased GM compared to Aβ–subjects in P. |
| Hanseeuw et al. Alzheimers Dement 2016 | Harvard Aging Brain Study (HABS) | 250 | 73.8 (6.0) | PET | Null | N/A | |
| Hedden et al. Cereb Cortex 2016 | HABS | 186 | 73.8 (6.0) | PET | Neg | N/A | In all subjects, Aβ negatively predicted T, L GM. |
| Li et al. J Alzheimers Dis 2017 | ADNI | 251 | 75.5 (6.5) | PET | Null | N/A | |
| Sala‐Llonch et al. J Alzheimers Dis 2017 | N/A | 89 | 73.1 (6.0) | CSF | Neg | N/A | In Aβ+ subjects, Aβ negatively predicted F GM. |
| Voevodskaya et al. Neurobiol Aging 2017 | Biomarkers for Identifying Neurodegenerative Disorders Early and Reliably (BioFinder) | 299 | 73.3 (5.0) | CSF | Null | N/A | |
| Wolk et al. Neurobiol Aging 2017 | ADNI | 86 | 74.3 (6.9) | PET | Null | N/A | |
| Knopman et al. Neurobiol Aging 2018 | MCSA | 1164 | 70.0 (10.0) | PET | Neg | N/A | In all subjects, Aβ negatively predicted O, T GM. |
| Ten Kate et al. Alzheimers Res Ther 2018 | European Medical Information Framework for Alzheimer's Disease Multimodal Biomarker Discovery (EMIF‐AD MBD) | 337 | 66.5 (7.2) | PET | Neg | N/A | Aβ+ subjects had decreased GM compared to Aβ– subjects in O, T. |
| Haller et al. Front Neurosci 2019 | University Hospitals of Geneva | 133 | 76.8 (4.0) | PET | Null | N/A | |
| Rabin et al. JAMA Neurol 2019 | HABS | 182 | 73.4 (6.2) | PET | Neg | N/A | In all subjects, Aβ negatively predicted W GM. |
| Rahayel et al. Eur J Nucl Med Mol Imaging 2019 | Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM) | 103 | 73.4 (6.2) | PET | Neg, Pos | N/A | In all subjects, Aβ negatively predicted F and positively predicted F, T GM. |
Abbreviations: Aβ, amyloid beta; F, frontal lobe; GM, gray matter; L, limbic lobe (cingulate gyrus, parahippocampal gyrus, dentate gyrus); Neg, negative association between pathology biomarker and cortical measure; Null, no significant association between pathology biomarker and cortical measure; O, occipital lobe; P, parietal lobe; Pos, positive association between pathology biomarker and cortical measure; SD, standard deviation; T, temporal lobe; W, whole cortex.
Notes: Studies testing cross‐sectional associations between Aβ and cortical integrity (thickness or volume) in cognitively healthy older adults. Citation, cohort, sample size, mean age, and pathology biomarker modality are listed in addition to a brief summary of results. The locations of cortical areas with significant results are noted by the lobe of the brain for brevity. Studies including Aβ and tau biomarkers are listed in chronological order followed by studies with only an Aβ biomarker in chronological order.
*This study reported a median age.
4. DISCUSSION
Our findings in a normal aging cohort grouped according to A/T status indicated that the direction of the relationship between Aβ and cortical thickness was dependent on tau pathology. Moreover, the correlation between early Aβ (before significant tau accumulation) and cortical thickness may be positive, the opposite direction expected from AD pathogenesis models.2 Based on these findings, we posit that measuring associations between Aβ and cortical integrity (thickness or volume) is challenging without a tau biomarker to stage tau burden. Thus, before the relatively recent development of tau biomarkers, the ability to detect a significant relationship between Aβ and cortical integrity was likely complicated by the opposing effects of Aβ on cortical integrity in low‐ and high‐tau individuals.
Other studies have reported positive associations between Aβ biomarkers and cortical integrity.28, 29, 30, 31 One framework, dubbed the biphasic model, attempts to account for complex relationships between early Aβ, tau, and cortical integrity. This framework is consistent with the present study showing that cognitively normal A+/T– participants have thicker cortex compared to a A–/T– group.30 They also report continuous association in tau– participants between their CSF Aβ biomarker and cortical thickness such that more Aβ accumulation is associated with greater cortical thickness in temporal and parietal regions.
The mechanism driving positive associations between Aβ and cortical thickness in tau– participants (Figure 2A) is unknown but there are several possibilities. First, Aβ accumulation could drive a neuroimmune response that causes local fluid increases (swelling) or glial recruitment leading to increases in estimates of cortical thickness or volume.32, 33, 34 Second, the accumulation of Aβ itself could cause estimates of cortical thickness or volume to increase due to the presence of space‐occupying plaques. In support of this possibility, clinical trials of Aβ‐lowering therapies have shown that as Aβ is removed from cortex there is concurrent thinning.35 Last, positive associations between Aβ and cortical integrity could be driven by measurement bias such that individuals with thicker cortex simply have more tissue in which Aβ can accumulate making PiB‐PET signal higher. This, however, would not explain similar results reported with CSF biomarkers for Aβ and tau.36, 37
Our analyses examining continuous relationships between tau and cortical thickness showed that ERC tau predicted cortical thickness in only ERC in Aβ– participants. In contrast, ERC tau negatively predicted cortical thickness across the majority of cortex in Aβ+ participants, nicely illustrating that the presence of Aβ is associated with tau and cortical thickness relationships outside of the medial temporal lobe. This is consistent with the idea that Aβ plays a role in driving tau spread, which then leads to neurodegeneration.6, 38, 39 Across the entire cohort, we found that tau measures in ERC and a temporal MetaROI were related to corresponding cortical thickness in ERC and MetaROI and to episodic memory. Aβ was also related to memory, but this relationship was mediated by tau. Mediation models also revealed that across the whole cohort, but especially in Aβ+ participants, thickness mediated the effect of tau on memory.
The predominant model of AD pathogenesis describes a typical temporal order for the emergence of abnormal biomarkers.2 In this model, the emergence of tau pathology occurs subsequent to Aβ deposition but before neurodegeneration. Based on research criteria, even cognitively normal older adults who have Aβ pathology are on the AD continuum, and those with evidence of both Aβ and tau are defined as having preclinical AD.40 Our and others’ data suggest that it is challenging to link Aβ to neurodegeneration without considering tau which, as the model suggests, likely represents a transition event between the emergence of Aβ and atrophy. Some groups have addressed this challenge by focusing on only high Aβ participants, but this of course has the effect of increasing the tau burden because Aβ and tau are correlated.41, 42 Here, we attempt to reframe interpretations away from the idea that high Aβ is needed to detect atrophy effects, but rather that tau pathology is the likely driver of these associations when Aβ is high. Isolated Aβ may have detrimental effects on processes that affect cortical integrity, but it is increasingly clear that with current in vivo tools we cannot convincingly detect Aβ effects on atrophy that are tau independent. Thus, in cognitively normal individuals with evidence of preclinical AD, pathology‐related neurodegeneration appears to be driven by tau.
A review of the literature revealed that studies exploring the relationship between Aβ and atrophy in cognitively healthy older adults most frequently reported negative associations such that higher Aβ was related to thinner or lower volume cortex. However, of the studies we reviewed, 5 (15%) reported positive associations between Aβ and neurodegeneration and 12 (38%) found no relationship. The higher proportion of negative associations could be the result of publication bias, though this is difficult to prove. In contrast, the relationship between tau and cortical integrity appeared more straightforward with higher levels of tau predicting more severe cortical thinning or volume loss in 8 of 10 reports. The remaining two found no association between a CSF‐based tau biomarker and cortical integrity. This context supports the main findings of the present study and our interpretation that Aβ is not related to atrophy in a simple linear manner but rather tau deposition, which is associated with elevated Aβ, is predictive of cortical thickness.
Our study had several limitations and caveats. First, 125 cognitively normal participants, while larger than the majority of the similar papers included in our literature review, is still a relatively small sample size to study potentially subtle effects. Relatedly, while some of our findings did not survive correction for multiple comparisons, our main focus is not on specific regional results but on the overall patterns of associations (eg, negative or positive) between pathology and cortical thickness. Second, this is a highly educated, relatively healthy volunteer convenience sample and is not reflective of the breadth of older adults. Third, we use cross‐sectional data rather than longitudinal data to attempt to interpret temporal processes. Finally, 1.5T MRI and standard neuropsychological tests may be less sensitive than higher field strengths or specific preclinical AD cognitive composite approaches, respectively.
By presenting novel empirical findings and a systematic review of similar studies we aimed to accomplish two things: first, to highlight the fact that the relationship between Aβ and cortical integrity in normal aging is not straightforward and may be biphasic and second, that measuring tau is critical to determining the effect of early AD pathology on cortical thickness or volume. We showed that Aβ relationships with cortical thickness are relatively weak, are positive in tau– participants, and are negative in tau+ participants. By contrast, we showed strong negative associations between ERC tau and ERC thickness in both Aβ– and Aβ+ participants, with additional strong negative associations to cortical thickness across the rest of the brain in the Aβ+ group. Finally, we demonstrated that tau is related not only to cortical thickness but also to memory, which is further evidence of the importance of tau pathology in driving early atrophy and functional decline in aging.
CONFLICT OF INTEREST
William J. Jagust serves as a consultant to Bioclinica, Biogen, Genentech, CuraSen, and Grifols.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health grants F32‐AG057107 (to T.M.H.) and R01‐AG034570 (to William J. Jagust). Support was also provided by the Tau Consortium (to William J. Jagust) and the Swiss National Science Foundation (to G.K.). Avid Radiopharmaceuticals enabled the use of the [18F] flortaucipir tracer, but did not provide direct funding and were not involved in data analysis or interpretation.
Harrison TM, Du R, Klencklen G, Baker SL, Jagust WJ. Distinct effects of amyloid beta and tau on cortical thickness in cognitively healthy older adults. Alzheimer's Dement. 2021;17:1085–1096. 10.1002/alz.12249
REFERENCES
- 1.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's Dement. 2012;8:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitwell JL, Tosakulwong N, Weigand SD, et al. Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects?. NeuroImage Clin. 2013;2:249‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker JA, Hedden T, Carmasin J, et al. Amyloid‐β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanseeuw Bernard J., Schultz Aaron P., Betensky Rebecca A., Sperling Reisa A., Johnson Keith A. (2016) Decreased hippocampal metabolism in high‐amyloid mild cognitive impairment. Alzheimer's & Dementia, 12 (12), 1288–1296. 10.1016/j.jalz.2016.06.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pooler AM, Polydoro M, Maury EA, et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer's disease. Acta Neuropathol Commun. 2015;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maass A, Lockhart SN, Harrison TM, et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montal V, Vilaplana E, Alcolea D, et al. Cortical microstructural changes along the Alzheimer's disease continuum. Alzheimer's Dement. 2018;14:340‐351. [DOI] [PubMed] [Google Scholar]
- 9.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schöll M, Lockhart SN, Schonhaut DR, et al. PET Imaging of tau deposition in the aging human brain. Neuron. 2016;89:971‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996:834‐840. [DOI] [PubMed] [Google Scholar]
- 14.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound‐B. J Cereb Blood Flow Metab. 2005;25:1528‐1547. [DOI] [PubMed] [Google Scholar]
- 15.Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between β‐amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399‐2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villeneuve S, Rabinovici GD, Cohn‐Sheehy BI, et al. Existing Pittsburgh Compound‐B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker SL, Lockhart SN, Price JC, et al. Reference tissue‐based kinetic evaluation of 18F‐AV‐1451 for tau imaging. J Nucl Med. 2017;58:332‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904‐911. [PubMed] [Google Scholar]
- 19.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting18F‐AV‐1451 tau PET data. Data Br. 2017;15:648‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maass A, Landau S, Baker SL, et al. Comparison of multiple tau‐PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017;157:448‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack Clifford R., Wiste Heather J., Weigand Stephen D., et al. (2017) Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's & Dementia, 13 (3), 205–216. 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050‐11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968‐980. [DOI] [PubMed] [Google Scholar]
- 24.Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. Neuroimage. 1999;9:179‐194. [DOI] [PubMed] [Google Scholar]
- 25.Harrison TM, Maass A, Baker SL, Jagust WJ. Brain morphology, cognition, and β‐amyloid in older adults with superior memory performance. Neurobiol Aging. 2018;67:162‐170. 10.1016/j.neurobiolaging.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach. Guilford Press; 2013. [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate ‐ A practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289‐300. [Google Scholar]
- 28.Oh H, Habeck C, Madison C, Jagust W. Covarying alterations in Aβ deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum Brain Mapp. 2014;35:297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chételat G, Villemagne VL, Pike KE, et al. Larger temporal volume in elderly with high versus low beta‐amyloid deposition. Brain. 2010;133:3349‐3358. [DOI] [PubMed] [Google Scholar]
- 30.Fortea J, Vilaplana E, Alcolea D, et al. Cerebrospinal fluid β‐amyloid and phospho‐tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann Neurol. 2014;76:223‐230. [DOI] [PubMed] [Google Scholar]
- 31.Fortea J, Sala‐Llonch R, Bartrés‐Faz D, et al. Cognitively preserved subjects with transitional cerebrospinal fluid ß‐Amyloid 1‐42 values have thicker cortex in Alzheimer's disease vulnerable areas. Biol Psychiatry. 2011;70:183‐190. [DOI] [PubMed] [Google Scholar]
- 32.Batzu L, Westman E, Pereira JB. Cerebrospinal fluid progranulin is associated with increased cortical thickness in early stages of Alzheimer's disease. Neurobiol Aging. 2020;88:61‐70. [DOI] [PubMed] [Google Scholar]
- 33.Hanzel CE, Pichet‐Binette A, Pimentel LSB, et al. Neuronal driven pre‐plaque inflammation in a transgenic rat model of Alzheimer's disease. Neurobiol Aging. 2014;35:2249‐2262. [DOI] [PubMed] [Google Scholar]
- 34.Grand'Maison M, Zehntner SP, Ho MK, et al. Early cortical thickness changes predict β‐amyloid deposition in a mouse model of Alzheimer's disease. Neurobiol Dis. 2013;54:59‐67. [DOI] [PubMed] [Google Scholar]
- 35.Fox NC, Black RS, Gilman S, et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563‐1572. [DOI] [PubMed] [Google Scholar]
- 36.Fortea J, Vilaplana E, Alcolea D, et al. Cerebrospinal fluid β‐amyloid and phospho‐tau biomarker interactions affecting brain structure in preclinical Alzheimer disease. Ann Neurol. 2014;76:223‐230. [DOI] [PubMed] [Google Scholar]
- 37.Fortea J, Sala‐Llonch R, Bartrés‐Faz D, et al. Cognitively preserved subjects with transitional Cerebrospinal Fluid ß‐Amyloid 1‐42 values have thicker cortex in Alzheimer's disease vulnerable areas. Biol Psychiatry. 2011;70:183‐190. [DOI] [PubMed] [Google Scholar]
- 38.Hurtado DE, Molina‐Porcel L, Iba M, et al. Aβ accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model. Am J Pathol. 2010;177:1977‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid‐β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142:3230‐3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack Clifford R., Bennett David A., Blennow Kaj, et al. (2018) NIA‐AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia, 14 (4), 535–562. 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaffashian S, Tzourio C, Soumaré A, et al. Association of plasma β‐amyloid with MRI markers of structural brain aging the 3‐City Dijon study. Neurobiol Aging. 2015;36:2663‐2670. [DOI] [PubMed] [Google Scholar]
- 42.Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid‐β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142:3230‐3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


