Abstract
Objective
Acyl-ghrelin regulates eating, body weight, blood glucose, and GH secretion upon binding to its receptor GHSR (growth hormone secretagogue receptor; ghrelin receptor). GHSR is distributed in several brain regions and some peripheral cell-types including pituitary somatotrophs. The objective of the current study was to determine the functional significance of acyl-ghrelin's action on GHSR-expressing somatotrophs in mediating GH secretion and several of acyl-ghrelin's metabolic actions.
Methods
GH-IRES-Cre mice and loxP-flanked (floxed) GHSR mice were newly developed and then crossed to one another to generate mice that lacked GHSR selectively from somatotrophs. Following validation of mice with somatotroph-selective GHSR deletion, metabolic responses of these mice and control littermates were assessed following both acute and chronic acyl-ghrelin administration, a 24-h fast, and a prolonged 60% chronic caloric restriction protocol modeling starvation.
Results
In mice with somatotroph-selective GHSR deletion, a single peripheral injection of acyl-ghrelin failed to induce GH secretion or increase food intake, unlike wild-type and other littermate control groups. However, the usual acute blood glucose increase in response to the acyl-ghrelin bolus was preserved. Similarly, chronic s.c. acyl-ghrelin administration to mice with somatotroph-selective GHSR deletion failed to increase plasma GH, food intake, or body weight. Physiologically elevating plasma acyl-ghrelin via a 24-h fast also failed to raise plasma GH and resulted in a limited hyperphagic response upon food reintroduction in mice with somatotroph-selective GHSR deletion, although those mice nonetheless did not exhibit an exaggerated reduction in blood glucose. Physiologically elevating plasma acyl-ghrelin via a 15-day caloric restriction protocol which provided only 40% of usual daily calories failed to raise plasma GH in mice with somatotroph-selective GHSR deletion, although those mice did not exhibit life-threatening hypoglycemia.
Conclusions
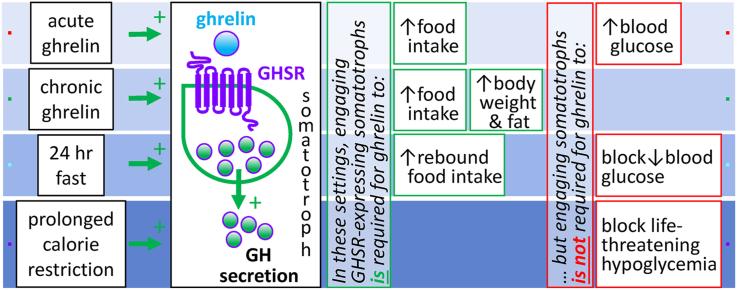
These results reveal that direct engagement of GHSR-expressing somatotrophs is required for a peripheral ghrelin bolus to acutely stimulate GH secretion and the actions of chronic acyl-ghrelin delivery and physiological plasma acyl-ghrelin elevations to increase plasma GH. These results also suggest that actions of acyl-ghrelin to increase food intake and body weight are reliant on direct activation of GHSRs expressed on somatotrophs. Furthermore, these results suggest that the glucoregulatory actions of acyl-ghrelin – in particular, its actions to raise blood glucose when acutely administered, prevent small blood glucose drops following a 24-h fast, and avert life-threatening hypoglycemia during an acute-on-chronic caloric restriction protocol – do not depend on GHSR expression by somatotrophs.
Keywords: Ghrelin, GHSR, Growth hormone, Pituitary, Somatotroph
Graphical abstract
Highlights
-
•
Mice with pituitary somatotroph-selective GHSR deletion were generated.
-
•
Somatotroph-expressed GHSRs mediate GH secretion and food intake after acute ghrelin.
-
•
Body weight effects of chronic ghrelin infusion require somatotroph-expressed GHSRs.
-
•
Somatotroph-expressed GHSRs enable GH to increase upon chronic caloric restriction.
-
•
Mice lacking somatotroph GHSRs maintain euglycemia upon chronic caloric restriction.
1. Introduction
Acyl-ghrelin is a hormone that serves as a key regulator of eating, body weight, adiposity, blood glucose, and growth hormone (GH) secretion upon binding to its receptor, the growth hormone secretagogue receptor (GHSR) [1,2]. In humans and rodents, plasma acyl-ghrelin increases during fasting and declines in individuals with obesity [[3], [4], [5]]. Plasma acyl-ghrelin levels also are dynamically affected by feeding status, with levels rising pre-prandially and falling after a meal [6,7]. In mice, acyl-ghrelin administration increases food intake, body weight gain, and blood glucose [1,2,5]. Administering acyl-ghrelin or GHSR agonists lowers energy expenditure, upregulates gene expression of fat storage-promoting enzymes in adipose tissue, and engages hedonic eating behaviors [2,8,9]. In contrast, neutralizing bioavailable acyl-ghrelin or administering GHSR antagonists lowers body weight and/or food intake [[10], [11], [12], [13]]. Although neither ghrelin nor GHSR loss-of-function (knock-out) mouse models exhibit marked reductions in food intake or body weight in normal laboratory conditions with ad libitum feeding, upon exposure to a high-fat diet early in life, these mice eat less, gain less weight, and develop less adiposity than wild-type controls [14,15]. Furthermore, in the setting of acute-on-chronic caloric restriction, in particular, a starvation-like state-simulating protocol in which mice are provided access to only 40% of their usual daily calories for several successive days, resulting in severe fat store depletion, with superimposed acute starvation for at least 20 h, ghrelin system loss-of-function models develop marked hypoglycemia just prior to food access and increased mortality [4,[16], [17], [18]]. Given these prominent effects of acyl-ghrelin on feeding, body weight, adiposity, and blood glucose, it is crucial to gain a better understanding of the tissues and factors mediating acyl-ghrelin's metabolic actions.
Compared to other central and peripheral sites of GHSR expression, less attention has been paid to the role of GH-secreting pituitary somatotrophs in acyl-ghrelin action, particularly as it relates to acyl-ghrelin's metabolic actions. This is despite the following findings: GHSR was first identified in the pituitary; acyl-ghrelin was first defined by its ability to engage GHSRs to potently stimulate GH secretion; GC tumor cell-induced GH overexpression lowers pituitary GHSR mRNA expression; pituitary GH mRNA, GH immunoreactivity, and Pit-1 (pituitary-specific transcription factor) mRNA levels and pituitary weight are reduced in GHSR knock-out mice; GH is a key mediator of acyl-ghrelin's anti-hypoglycemic actions during the previously described acute-on-chronic caloric restriction protocol and during the counterregulatory response to insulin-induced hypoglycemia; and GH modulates body composition [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. These data not only demonstrate that pituitary somatotrophs are a direct target of ghrelin, but also suggest that GH's action contributes to at least some of acyl-ghrelin's metabolic effects. In contrast, other findings oppositely suggest that GH does not impact some of acyl-ghrelin's most well-known metabolic actions. For instance, food intake and/or body weight increases resulting from administered acyl-ghrelin have been observed in rats with GH deficiency, suggesting that acyl-ghrelin's effects on metabolism might be GH-independent [5,20]. Given these discrepancies in the literature, our overall goal herein was to test the hypothesis that activation of GHSRs expressed by pituitary somatotrophs is required for acyl-ghrelin's actions to increase GH secretion, food intake, body weight, adiposity, and blood glucose.
2. Methods and materials
2.1. Animals
All the experiments performed in this study were approved by the UT Southwestern Medical Center Institutional Animal Care and Use Committee. The mice were housed under standard laboratory conditions and provided ad libitum access to standard chow (Harlan Teklad 2016, with an energy density of 3 kcal/g, of which 12% of kcal were derived from fat) and water. Percentage body fat mass and percentage body lean mass were calculated by dividing the fat mass or lean mass as determined by an EchoMRI-100 (EchoMRI, Houston, TX, USA) by body weight as measured by a scale and multiplying the result by 100.
2.2. Generation and validation of GH-IRES-Cre mice
A novel GH-IRES (internal ribosome entry site)-Cre knock-in mouse line was created by insertion of an IRES-Cre cassette [27] 65 bp after the GH stop codon using CRISPR-Cas9 technology [28]. A targeting construct was produced using gBlocks Gene Fragment construction (Integrated DNA Technologies, Coralville, IA, USA). The gene fragment contained IRES-Cre flanked on each side by a 500-bp homologous arm sequence surrounding the Cas9 cut site. NB.BbvC1 restriction enzyme sites were included on each end. This entire fragment was cloned using an NEB PCR cloning kit (cat.# E1202S, New England Biolabs, Ipswich, MA, USA) vector backbone. The construct was digested with NB.BbvC1 (which cut only one of the DNA strands) and run on a formamide gel to separate the single-stranded fragments. This band was extracted and purified with a PCR clean-up kit (cat.# BS353, BioBasic EX-10 Spin Column DNA Gel Extraction kit) twice to insure the purity of the single-strand DNA. Following resuspension of the purified single-strand DNA fragment in pronuclear injection buffer, 5 μg of the single-strand DNA fragment together with the guide RNA and transactivating CRISPR RNA (5′-rUrUrU rArUrU rArGrG rArCrA rArArG rCrGrC rArGrG rUrUrU rUrArG rArGrC rUrArU rGrCrU-3′) (GH sequence targeted 5′-TTTATTAGGACAAAGΔCGCAGG-3’; the Cas9 cut site is indicated by Δ) were microinjected into C57BL/6N zygotes by the UT Southwestern Transgenic Technology Core Facility. Guides, trcRNA, and Cas9 protein were supplied by Integrated DNA Technologies. Correctly targeted mice containing the IRES-Cre construct-modified GH gene were identified by performing PCR on genomic DNA, and a founder line was chosen after sequencing the entire GH-IRES-Cre gene to confirm the lack of any inadvertently introduced mutations. The founder was crossed to C57BL/6N mice and resulting heterozygous progeny were crossed to each other to generate homozygotes, heterozygotes, and wild-type mice in the expected Mendelian genetic ratios.
To validate the somatotroph-selective Cre activity of the newly developed GH-IRES-Cre line, we performed quantitative reverse transcriptase-PCR on pituitaries and various other tissues from GH-IRES-Cre heterozygotes. We assessed GH and Cre recombinase mRNA levels in these tissues (see below). Also as validation, female GH-IRES-Cre mice were crossed with male Rosa26-lox-STOP-lox-tdTomato reporter mice (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, The Jackson Laboratory, Bar Harbor, ME, USA). (Notably, unlike when female GH-IRES-Cre mice were used as breeders, when male GH-IRES-Cre mice were crossed with female Rosa26-lox-STOP-lox-tdTomato reporter mice, tdTomato expression was observed in all organs examined, including the skin; thus female GH-IRES-Cre breeders were used to generate all the mice used throughout the study). Progeny containing one copy of the tdTomato transgene +/− one copy of the GH-IRES-Cre allele were deeply anesthetized with an i.p. injection of chloral hydrate (500 mg/kg) and perfused transcardially with diethylpyrocarbonate-treated 0.9% PBS at a pH of 7.0 followed by 10% neutral-buffered formalin at 8 weeks of age, after which their pituitaries and brains were collected and processed using a published protocol [29]. Briefly, tissues were sectioned (pituitaries, longitudinally at 8 μM thick using a cryostat; brains, coronally at 25 μM thick [5 equal series] using a sliding microtome), mounted on glass slides, and then processed for GH immunoreactivity using a published immunohistochemistry protocol [30] and the following anti-sera: the primary antibody (rabbit anti-serum for GH, cat.# AFP5672099, National Hormone and Peptide Program, Torrance, CA, USA) was diluted at 1:1000. The secondary antibody (donkey anti-rabbit Alexa fluor 488, cat.# A21206, Invitrogen, Pittsburgh, PA, USA) was diluted at 1:500. The slides were cover-slipped with Vectashield DAPI (Vector Laboratories, Burlingame, CA, USA) mounting medium. For pituitaries, co-expression of GH immunoreactivity and tdTomato fluorescence formally was assessed in fluorescent digital images taken using a 40x objective of an Olympus BX41 microscope (Shinjuku, Tokyo, Japan) with a defined sampling field of 350 × 350 μm. Five fluorescent digital images from each of two pituitary sections separated by at least 110 μm were examined from each of four separate tdTomato mice with GH-IRES-Cre and from each of two tdTomato littermates without GH-IRES-Cre mice. NIH ImageJ software (http://rsbweb.nih.gov/ij/) was used to adjust the laser intensity, offset, and gain to improve the signal-to-background ratio of the photomicrographs. For each image, channels representing GH immunoreactivity (green) and tdTomato fluorescence (red) were overlapped using Imaris software to obtain a grid overlay. To determine the degree of overlap between cells with tdTomato fluorescence and GH immunoreactivity, all of the cells with either tdTomato fluorescence alone, GH immunoreactivity alone, or both were counted. Criteria used to determine whether a tdTomato-expressing cell co-expressed GH immunoreactivity included visualization of co-localized fluorescent signals, whereby red fluorescence overlying green fluorescence appeared as yellow fluorescence. Immunoreactive cells were only counted when the nuclei were clearly visible by DAPI staining.
For the brain, co-expression of GH immunoreactivity and endogenous tdTomato fluorescence was assessed using a combination of 10x, 20x, and 40x objectives of an Olympus BX41 microscope. One brain series extending from the caudal olfactory lobe to a region just caudal to the dorsal vagal complex from each of four separate mice carrying both the tdTomato and GH-IRES-Cre transgenes and from each of two separate littermates carrying only the tdTomato transgene were examined. No GH immunoreactivity or tdTomato fluorescence was observed in any brain region. Representative images of the hippocampus and arcuate nucleus are presented.
Genotyping primers used to validate the presence of the GH-IRES-Cre allele and tdTomato transgene were as follows: GH-IRES-Cre: M804, 5′-CTGCTCTTCCATCAGCTCACA-3’; M813, 5′- CGTAACCTGGATAGTGAAACAG-3’ (which amplified a 198-bp PCR fragment if the GH-IRES-Cre gene was present) and tdTomato: M452, 5′-AAGGGAGCTGCAGTGGAGTA-3’; M453, 5′-CCGAAAATCTGTGGGAAGTC-3’; M454, 5′-CTGTTCCTGTACGGCATGG-3′, M455, 5′-GGCATTAAAGCAGCGTATCC-3’ (which amplified 297-bp and 196-bp PCR fragments if the genomic DNA and tdTomato transgene, respectively, were present).
2.3. Generation of floxed GHSR (GHSRfl/fl) mice
A new mouse line (GHSRfl/fl) containing a recombinant GHSR gene flanked by loxP sites (or rather, a “floxed” GHSR allele) was generated using previously described methods [4,31,32]. Of note, this line was different than another previously reported floxed GHSR mouse line [[33], [34], [35], [36]]. To accomplish this, a GHSR-containing BAC clone (RPCI.22 494 N7; BACPAC Resources Center at Children's Hospital Oakland Research Institute) containing the entire GHSR gene was transformed into EL350 cells. Using BAC recombineering techniques, a 10,143-bp fragment containing the complete GHSR sequence and homology arms was cloned into a commercially available pGEM vector that had been altered to contain the thymidine kinase gene used for negative selection. A loxP-neomycin resistance gene-loxP (loxP-Neo-loxP) cassette from pL452 [37] was inserted 256-bp upstream of the GHSR start codon using homologous recombination followed by removal of Neo-loxP by arabinose induction of Cre recombinase. A loxP-frt-Neo-frt cassette from pL451 with a BamH1 site located at its 5′-end was inserted 230-bp downstream of the GHSR stop codon. The resulting targeting construct was prepared and used for electroporation into 129/SvEvTac (SM-1 cells) at the UT Southwestern Transgenic Technology Core Facility. Correctly targeted ES cell clones were identified by Southern blotting and PCR analyses. Three ES cell clones were selected for blastocyst injection, and germline transmission was established for one clone via PCR analysis of genomic DNA obtained by tail snips. To remove the Neo-frt sequence, the resulting lines were then crossed to “Flp1 recombinase” mice [B6.Cg-Tg(ACTFLPe)9205Dym/J, The Jackson Laboratory] that had been backcrossed for more than 10 generations onto the C57BL/6N background in our laboratory. The mice were validated by PCR analysis of genomic DNA obtained by tail snips, and one of the lines was chosen for subsequent breeding to generate experimental mice. To demonstrate germline transmission of the loxP-flanked GHSR allele and correct targeting of the endogenous GHSR gene, heterozygous GHSRWT/fl mice were crossed with each other, yielding the predicted numbers of mice with 2 copies of the loxP-flanked GHSR allele, mice with 2 copies of the WT GHSR allele, and heterozygotes as determined using a Southern blotting strategy. The Southern blotting strategy involved BamH1 restriction endonuclease-mediated digestion of tail DNA, electrophoresis, and detection on the resulting blot of restriction fragments using a 5′ probe to detect insertion of the 5′ end of the targeting construct and a 3′ probe to detect insertion of the 3′ end of the targeting construct. The functionality of the loxP sites was confirmed by crossing the GHSRfl/fl line to zona pellucida 3 (ZP3)-Cre mice [B6.FVB-Tg(Zp3-Cre)3Mrt/J, The Jackson Laboratory] to generate mice with Zp3-Cre-mediated GHSR deletion from all cells. Those mice lacked the usual orexigenic response to administered rat acyl-ghrelin (SP-GHRL-1, Innovagen, Lund, Sweden; 2 mg/kg body weight s.c.) at 0.5, 1, and 2 h post-injection, which was otherwise observed in age-matched wild-type control mice on the same genetic background (n = 5–7; data not shown).
Primers used to validate the presence of the floxed GHSR allele, the WT GHSR allele, or a deleted (“delta”) GHSR allele were as follows: M496, 5′-TCCGTTCCTCCTCTCCCATAACTTC-3’; M318, 5′-CCGATAGAGTGACAGGTAAGTGA-3’ (which amplified a 160-bp PCR fragment if the floxed GHSR allele was present) and M308, 5-TCCGTTCCTCCTCTCCCCTCCCT-3’; M640, 5′-GGAGTTTAGTGTTACACACCAAGA-3’; and M318, 5′-CCGATAGAGTGACAGGTAAGTGA-3’ [which amplified a 130-bp fragment if the WT allele was present and a 311-bp PCR fragment if the deleted (“delta”) GHSR allele was present]. Primers used to generate the probes for Southern blotting were as follows: 5′-probe: M373, 5′-CTGGAGTCAGCTACAGGCAT-3′ and M374, 5′-TACATGTGTACCTATGTGTGCA-‘3’; 3′ probe: M271, 5′-CTTAGTGATCTCACTATCACCTCT-3′ and M272, and 5′-GGCAGGCATGCATGTTTAAAGTGA-3’.
2.4. Generation of mice with somatotroph-selective GHSR deletion
Female GH-IRES-Cre mice (already on a C57BL/6N background due to the use of C57BL/6N zygotes during their generation) were crossed with (heterozygous) GHSRWT/fl mice (that had first been backcrossed > 10 generations to C57Bl/6N mice) to generate female breeders harboring one copy of the floxed GHSR allele and one copy of the GH-IRES-Cre allele. These mice were bred with male GHSRWT/fl mice to yield the 4 groups used throughout the study. Validation of the desired site-selective GHSR deletion was achieved by measuring GHSR expression as determined using qPCR within pituitaries and hypothalami from representative mice in each of the 4 study groups.
2.5. Quantitative reverse-transcriptase PCR
The mice were euthanized by cervical dislocation. Pituitaries, hypothalami, and/or various other organs were immediately collected into RNAlater (Invitrogen, Carlsbad, CA, USA) and stored at 4 °C overnight or up to one week, after which the RNAlater was removed and the tissues either immediately processed to isolate RNA or frozen at −20 °C until processing at a later date. Total RNA was isolated using the guanidium thiocyanate-phenol-chloroform extraction method by adding RNA STAT-60 (AMSBIO, Cambridge, MA, USA). The isolated RNA was quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Total RNA (83 ng per gene) was treated with ribonuclease-free deoxyribonuclease (Roche, Indianapolis, IN, USA), and complementary DNA was synthesized by reverse transcription using SuperScript III (Invitrogen, Carlsbad, CA, USA). A novel TaqMan gene expression assay for GHSR to specifically detect Cre-mediated GHSR deletion between the loxP sites was designed using Primer Express Software (PerkinElmer Life Sciences, Boston, MA, USA). This assay included the following primers: GHSR TM(504)F: 5′-GCCTTCTCCGATCTGCTCATC-3′, GHSR TM(581)R: 5′-GAAGTTCCAGGGCCGATACTG-3′, and a GHSR TM probe (541): 5′-TGGACCTCGTCCGCC-3’. Primers used to detect Cre recombinase using SYBR green chemistry were as follows: Cre F: 5′-GCGGTCTGGCAGTAAAAACTATC-3′ and Cre R: 5′-GTGAAACAGCATTGCTGTCACTT-3’.
A TaqMan Gene Expression Assay (Mm00433590_g1) was used to detect GH. Quantitative PCR was performed using a QuantStudio 5 Real-Time PCR System (Applied Biosystems by Thermo Fisher Scientific, Foster City, CA, USA). The mRNA levels were represented relative to the invariant control gene 18s (TaqMan Gene Expression Assay Mm04277571_s1) and calculated using the comparative threshold cycle (ΔΔCt) method. Data are presented as a percentage of the expression of the gene in wild-type control mice or as indicated.
2.6. Acute acyl-ghrelin injections
Mice aged 8–10 weeks were individually housed and handled daily for 5 days. On the sixth day, food was removed from their cages and the mice were injected with 0.25 mg/mL of rat acyl-ghrelin (SP-GHRL-1, Innovagen, Lund, Sweden) to achieve a final concentration of 1 mg/kg. In the first cohort, 15 min prior to the acyl-ghrelin injection, male mice first were anesthetized with pentobarbital (50 mg/kg body weight, i.p.). For this first cohort, acyl-ghrelin was administered i.p. Blood was collected from tail veins at 0, 5, and 15 min after acyl-ghrelin injection into ice-cold EDTA-coated microfuge tubes and stored at −80 °C prior to processing for plasma GH determination. In a second cohort, acyl-ghrelin (at a concentration of 0.25 mg/mL to achieve a final dose of 1.0 mg/kg body weight) was administered s.c. to unanesthetized male mice. Blood was obtained following tail snips at 0 and 30 min after acyl-ghrelin injection to determine blood glucose (using a Bayer Contour blood glucose monitoring system, Mishawaka, IN, USA). Another sample of blood was collected from the tail snips into ice-cold EDTA-coated Eppendorf tubes followed by the addition of P-hydroxymercuribenzoic acid (at a final concentration of 1 mmol/L, Sigma–Aldrich, St. Louis, MO, USA), centrifuged at 4 °C at 1,500 g × 15 min to separate plasma, addition of 1 N of HCl to achieve a final concentration of 0.1 N, and stored at −80 °C prior to processing for plasma acyl-ghrelin. In a third cohort of 8- to 10-week-old unanesthetized male and female mice (for which access to food was restricted beginning 2 h prior to acyl-ghrelin [1.0 mg/kg body weight s.c.] injection), food was reintroduced immediately after acyl-ghrelin injection. Food intake was determined at 45 min, 90 min, 4 h, and 24 h after acyl-ghrelin treatment. The food intake study was repeated in the third cohort 2 weeks later following saline administration in place of acyl-ghrelin administration. Of note, as the same food intake trends were observed regardless of sex (when the data for females [n = 3–8] and males [n = 7–10] were analyzed separately), the data presented herein represent the combined food intake data from both sexes.
2.7. Twenty-four-h fast
Male mice aged 8–10 weeks were individually housed and handled daily for 6 days. On the seventh day, the mice were again handled and food was removed from their cages at 10 am. Twenty-four hours later, food was reintroduced and food intake was determined after 1, 2, 4, and 24 h. In a second cohort of 8- to 10-week-old male mice, blood first was obtained in the ad libitum-fed state following tail snips and processed for blood glucose, plasma GH, and plasma acyl-ghrelin as previously described. Two weeks later, the mice in this second cohort were fasted for 24 h, at which time blood was again obtained following tail snips and processed for blood glucose, plasma GH, and plasma acyl-ghrelin.
2.8. Chronic acyl-ghrelin administration
Male mice 8 weeks of age were anesthetized with 2% isoflurane and implanted with a pre-filled Alzet osmotic pump (Model 1002, Alzet Osmotic Pumps, Cupertino, CA, USA) to deliver continuous s.c. infusions of rat acyl-ghrelin at a rate of 0.25 μL/h to achieve a cumulative daily dose of 4 mg/kg of body weight as previously described [4,38]. The mice were given carprofen analgesia (6.25 mg/kg, s.c.) daily for 2 days following osmotic pump implantation. Food intake, body weight, and blood glucose (from tail nicks) were measured daily for 12 days following pump implantation. Notably, the body weight measures reported include the weights of the implanted pumps. On the penultimate day of the study, body composition was determined as previously described and is presented as g instead of % body weight (because of the implanted pumps). On the final day of the study, trunk blood was collected following decapitation and processed for blood glucose, plasma GH, and acyl-ghrelin determination as previously described and IGF-1. Pituitaries were removed and processed for GH mRNA determination by quantitative reverse-transcriptase PCR as previously described.
2.9. Acute-on-chronic caloric restriction protocol
Eight-week-old male mice were provided access to 40% of their usual daily calories in the form of their usual diet as previously described [16] for 15 d. All of the mice were individually housed during the study and acclimatized for 1 week before starting the caloric restriction protocol. During the acclimatization period, daily food intake was measured for 5 days to determine the mean usual daily caloric intake for each mouse. Percentage body fat mass was determined just prior to beginning the caloric restriction protocol and again on the last day of the study. Food (40% of their usual daily diet) was provided daily at 5:30 pm (30 min prior to lights-off). Body weights were measured daily and blood glucose was measured every other day just prior to delivery of their food ration. On the final day of the study just prior to the usual food ration delivery time, trunk blood was collected following decapitation and processed for plasma GH and acyl-ghrelin determination as previously described, corticosterone (using the same samples that were processed for plasma GH determination), and glucagon (using samples collected into ice-cold EDTA-coated microfuge tubes containing aprotinin and stored at −80 °C). As the mice under this protocol quickly consumed the food provided, by the time their blood was collected, they had undergone an acute fast of approximately ∼22–22.5 h on top of the prolonged caloric restriction (“acute-on-chronic” caloric restriction). A separate ad libitum-fed group was similarly treated except unlimited food was provided.
2.10. Determination of plasma hormone levels
ELISA kits were used for acyl-ghrelin (Millipore-Merck, Burlington, MA, USA), GH (cat.# EZRMGH-45K, EMD Millipore Corporation, St. Charles, MO, USA), corticosterone (Enzo Life Sciences, Farmingdale, NY, USA), glucagon (Mercodia AB, Uppsala, Sweden), and IGF-1 (cat.# EMI1001-1, Assaypro, St. Charles, MO, USA). Calorimetric assays were performed using a BioTek PowerWave XS Microplate spectrophotometer (Winooski, VT, USA) and BioTek KC4 junior software.
2.11. Statistics
All the data are expressed as the mean ± SEM. Two-tailed statistical analysis and graph preparations were performed using GraphPad Prism 9.0.1 (GraphPad Software, San Diego, CA, USA). Student's t-test, one-way ANOVA, two-way ANOVA, or repeated-measures two-way ANOVA followed by post hoc comparison tests were used to test for significant differences among the test groups as indicated in the figure legends. Data were log transformed where appropriate if variances among the groups were statistically significant. Outliers were detected by Grubb's test. P values less than 0.05 were considered statistically significant, and P values greater than or equal to 0.05 and less than 0.1 were considered evidence of statistical trends.
3. Results
3.1. Generation of mice with somatotroph-selective GHSR deletion
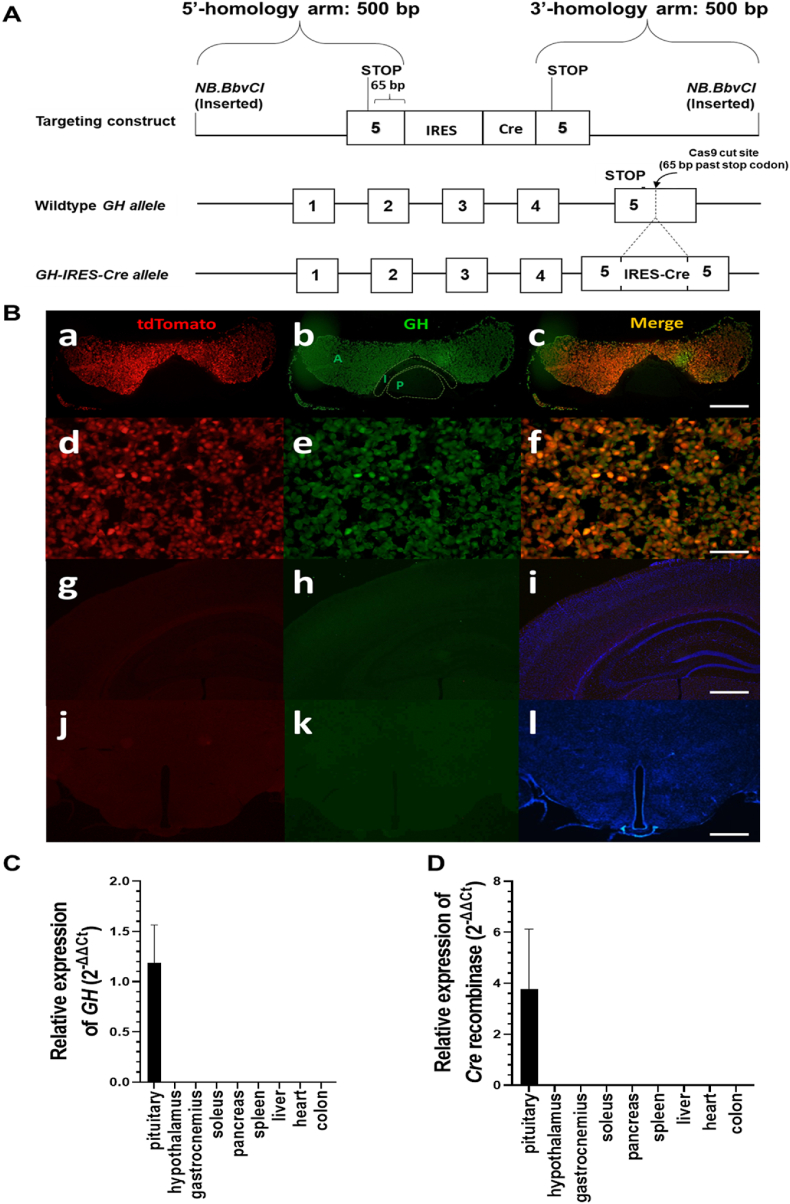
To generate mice with somatotroph-selective GHSR deletion (GHSRfl/fl/GHWT/iCre), a novel GH-IRES-Cre knock-in mouse line was created by inserting an IRES-Cre cassette into the endogenous GH gene downstream of the GH stop codon (Figure 1A). This allows for expression of Cre recombinase selectively in GH-expressing cells. To assess this selectivity of Cre recombinase activity, GH-IRES-Cre mice were crossed to ROSA26-tdTomato reporter mice, resulting in mice in which Cre-mediated removal of a loxP-flanked stop cassette in the ROSA26-tdTomato transgene enabled tdTomato expression. Pituitaries of tdTomato reporter mice harboring the GH-IRES-Cre allele demonstrated tdTomato fluorescence (red) throughout the bilobed pars anterior (Figure 1B.a and d), which overlapped (yellow) nearly completely with GH immunoreactivity (green) (Figure 1B.b, c, e, and f; Table 1). The overlapping Cre activity and GH immunoreactivity within the anterior pituitary was as expected [39]. Neither tdTomato fluorescence nor GH immunoreactivity was observed in brains of tdTomato reporter mice harboring the GH-IRES-Cre allele (Figure 1B.g-l). Quantitative reverse transcriptase PCR on various organs from GH-IRES-Cre heterozygotes demonstrated restricted expression of both GH and Cre to the pituitary as opposed to undetectable expression in the hypothalamus or a panel of other peripheral organs (Figure 1C,D).
Figure 1.
Generation and validation of a novel GH-IRES-Cre mouse line. (A) Schematic of the derivation of the GH-IRES-Cre mice using CRISPER-Cas9 genome editing to insert an IRES-Cre cassette into exon 5 of the GH gene (insertion site marked by an arrow). (B) Validation of somatotroph-specific Cre activity in the GH-IRES-Cre mouse line as performed by observing overlapping GH immunoreactivity and tdTomato fluorescence in pituitaries but not brains of mice harboring a GH-IRES-Cre allele and a Rosa26-lox-STOP-lox-tdTomato transgene. Representative photomicrographs of a pituitary [at 10x magnification (a–c) and 40x magnification (d–f)] and brain [at 10x magnification as a negative control)] (g–l). tdTomato fluorescence (red) (a, d, g, and j); GH immunoreactivity (green) in the same sections (b, e, h, and k); co-localized tdTomato and GH immunoreactivity (yellow) in the same sections (c, f, i, and l); and DAPI nuclear stain (blue) (I and l). Note: In panel B.b, the labels A, I, and P indicate A: anterior lobe; I: intermediate lobe; and P: posterior lobe of the pituitary. n = 4. Scale bars = 100 μm. Expression of (C) GH mRNA and (D) Cre in various tissues. Data are 2-ΔΔCt values relative to the expression of GH or Cre in the pituitary, n = 5.
Table 1.
Percentage of cells with GH immunoreactivity co-expressing tdTomato fluorescence in GH-IRES-Cre x tdTomato reporter mice.
| % of cells with GH immunoreactivity co-expressing tdTomato fluorescence | % of cells with tdTomato fluorescence co-expressing GH immunoreactivity |
|---|---|
| 97.4 ± 0.0 | 96.8 ± 0.0 |
The data are reported as the mean percentage ± SEM for 4 mice at 2 different levels of longitudinally sectioned pituitary (each section containing the bilobed pars anterior) for each animal, each separated by at least 110 μm.
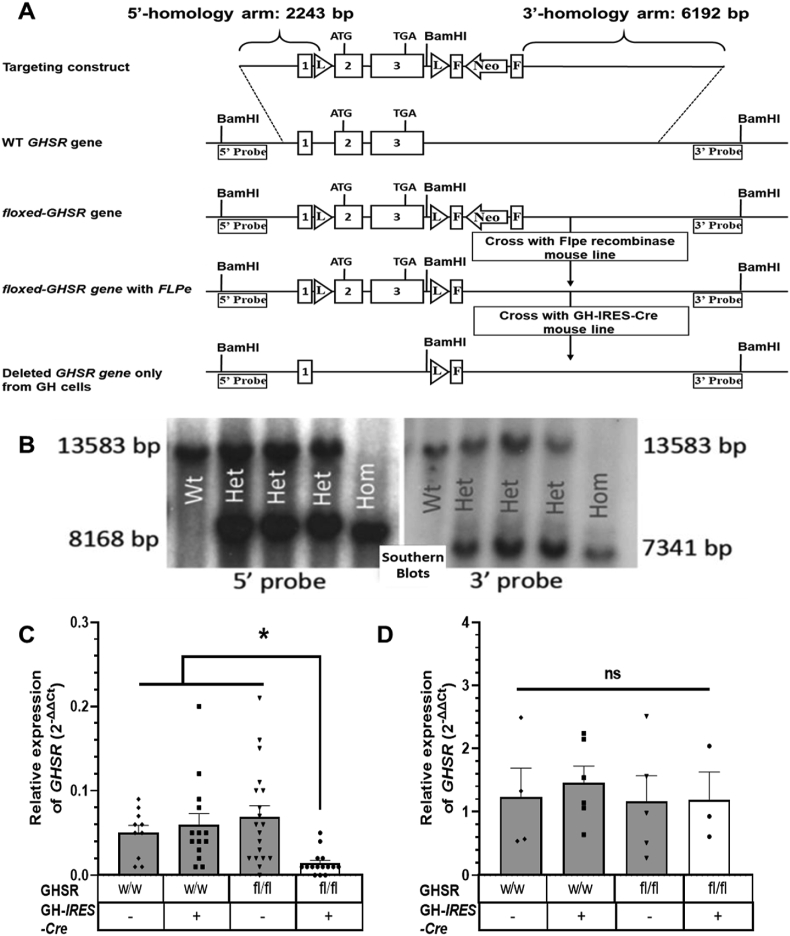
The GH-IRES-Cre line was then crossed with a novel conditional GHSR knock-out line. This “floxed” GHSR line (GHSRfl/fl) was developed by flanking the endogenous single-exon GHSR gene with inserted loxP sites (Figure 2A). Germline transmission of the recombinant loxP-flanked GHSR gene was validated by Southern blotting analysis of genomic DNA obtained by tail snips (Figure 2B). Four experimental groups were generated. These included mice with somatotroph-selective GHSR deletion (GHSRfl/fl/GHWT/iCre) and 3 littermate control groups: floxed GHSR mice (GHSRfl/fl/GHWT/WT), GHSR-IRES-Cre mice (GHSRWT/WT/GHWT/iCre), and wild-type mice (GHSRWT/WT/GHWT/WT). Expected Cre-mediated deletion of the GHSR gene selectively in pituitaries from the GHSRfl/fl/GHWT/iCre mice vs retained GHSR gene expression in pituitaries from the three control genotypes and in hypothalami from all 4 genotypes was confirmed by qPCR (Figure 2C,D).
Figure 2.
Generation and validation of a novel floxed GHSR mouse line and mice with somatotroph-selective GHSR deletion. (A) Schematic diagrams showing the homologous recombination targeting strategy to flank the GHSR-coding region with loxP sites, creating floxed GHSR mice. Binding sites for the 5′ and 3′ Southern blotting probes used to detect correctly targeted genomic DNA (after restriction digestion with BamH1) are depicted. (B) Southern blotting of genomic DNA of representative pups derived from crosses of mice heterozygous for the loxP-flanked GHSR allele, including pups homozygous for the GHSRfl allele (GHSRfl/fl; “Hom”), mice with two copies of the wild-type GHSR allele (GHSRWT/WT; “Wt”), and heterozygotes (GHSRWT/fl; “Het”). Expression pattern of GHSR mRNA in pituitaries (C) and hypothalami (D) of mice generated by crossing GH-IRES-Cre mice to floxed GHSR mice. Data are relative 2−ΔΔCt values and were analyzed by one-way ANOVA followed by Sidak's multiple comparisons test. n = 10–19 (pituitary) and n = 3–6 (hypothalamus). All of the values are expressed as mean ± SEM. ∗P < 0.05 and ns = not significant. w/w, mice with 2 copies of the Wt GHSR gene; fl/fl, mice homozygous for the floxed GHSR gene; –, absence or +, presence of the GH-IRES-Cre allele.
3.2. Somatotroph-selective GHSR deletion blunts GH secretory and orexigenic actions but not blood glucose-raising actions of acute acyl-ghrelin administration
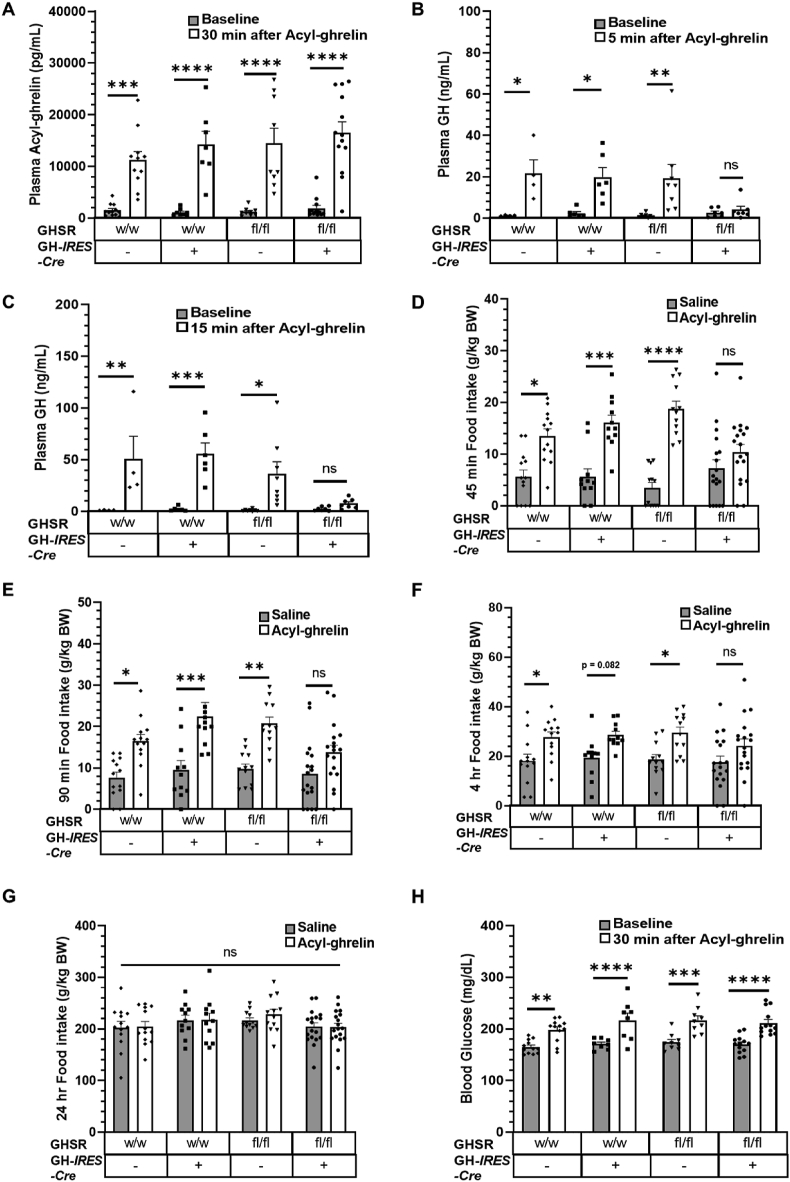
To study the physiological impact of somatotroph-selective GHSR deletion, we first injected mice with acyl-ghrelin (1 mg/kg BW) in the form of a single bolus s.c. or i.p. (see Methods). Plasma acyl-ghrelin levels increased in all of the experimental groups when checked 30 min after the acyl-ghrelin bolus (Figure 3A). No statistically significant increase in plasma GH was observed acutely (at either 5 min or 15 min) following ghrelin administration in pentobarbital-anesthetized GHSRfl/fl/GHWT/iCre mice, unlike in the control groups that each exhibited a marked increase (on average, a 1,173% increase at 5 min and a 2,884% increase at 15 min) in plasma GH (Figure 3B,C). Of note, while within this 15-min time period following the acyl-ghrelin bolus, no earlier peak in GH secretion was observed in mice with somatotroph-selective GHSR deletion, the absence of GH levels from time points later than 15 min may have masked revealing a potential delay in pulsatile GH secretion induced by administered acyl-ghrelin in those mice. That said, peaks in GH secretion are most often reported within the first 15 min of an acute peripheral bolus of acyl-ghrelin or GH secretagogue to rats or mice [25,[40], [41], [42]].
Figure 3.
Effects of somatotroph-selective GHSR deletion on acyl-ghrelin bolus-induced GH secretion, food intake, and blood glucose. (A) Plasma acyl-ghrelin measured at baseline and 30 min post-ghrelin (1 mg/kg BW, i.p.) administration. n = 13–18. Plasma GH measured at baseline (B) and 5 min after and (C) 15 min after acyl-ghrelin treatment. n = 4–8. Food intake measured at (D) 45 min, (E) 90 min, (F) 4 h, and (G) 24 h after acyl-ghrelin (1 mg/kg BW, s.c.) injection. n = 11–18 (including n = 3 female + 10 male GHSRWT/WT/GHWT/WT mice; n = 4 female + 7 male GHSRWT/WT/GHWT/iCre mice; n = 5 female + 7 male GHSRfl/fl/GHWT/WT mice; n = 8 female + 10 male GHSRfl/fl/GHWT/iCre mice). (H) Blood glucose measured after 30 min following acyl-ghrelin (1 mg/kg BW, s.c.) treatment. n = 13–18. Data in A-C and H were analyzed by repeated-measures two-way ANOVA followed by Sidak's multiple comparisons test. Data in D-G were analyzed by two-way ANOVA followed by Sidak's multiple comparisons test. All of the values are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 for significant changes or ns = no significant changes.
To investigate the effects of this blunted GH secretory response, we repeated the single bolus acyl-ghrelin injections in two additional cohorts, in one, examining food intake, and in the other, examining blood glucose. Unlike the three control genotypes in which statistically significant increases in food intake were observed at 45 min (on average, a 258% increase), 90 min (on average, a 120% increase), and 4 h (on average, a 52% increase) after acyl-ghrelin treatment (compared to saline treatment), no statistically significant differences in food intake were observed in the GHSRfl/fl/GHWT/iCre mice at those same time points (on average, a 44% increase at 45 min; on average, a 60% increase at 90 min; on average, a 38% increase at 4 h; see Figure 3D–F). The effect of the ghrelin bolus on food intake was short-lived as differences between ghrelin-induced and saline-induced food intake disappeared in the control groups by 24 h following ghrelin administration and as the differential orexigenic responses of the GHSRfl/fl/GHWT/iCre mice vs the control groups were no longer apparent by the 24-h time point (Figure 3G). In contrast to the blunted orexigenic response to administered ghrelin in the GHSRfl/fl/GHWT/iCre mice, their blood glucose response (a 24% increase) was similar to that observed in the control genotypes (on average, a 24% increase; see Figure 3H).
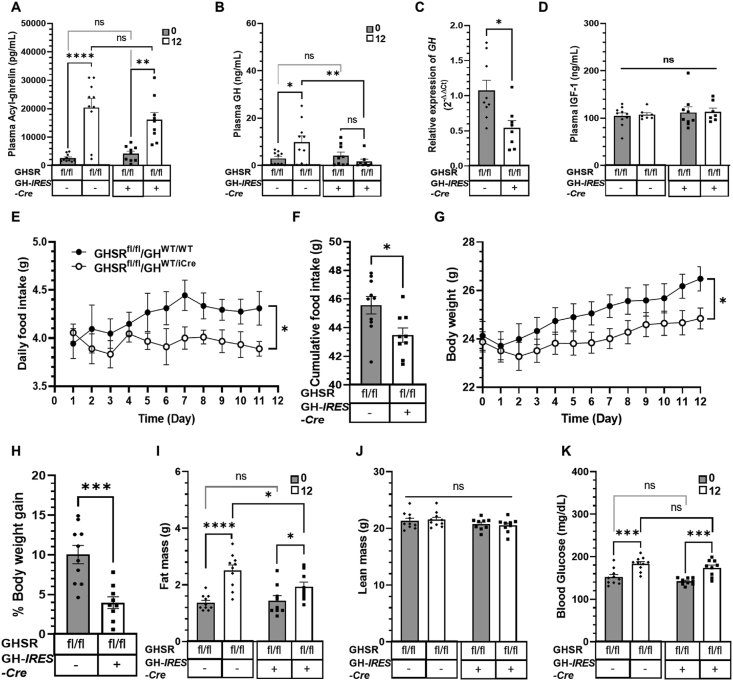
3.3. Somatotroph-selective GHSR deletion blunts the increase in plasma GH, pituitary GH mRNA levels, body weight gain, food intake, and fat accumulation associated with chronic acyl-ghrelin administration
We then administered acyl-ghrelin chronically to mice via s.c. osmotic minipumps using a previously reported paradigm [38]. Ghrelin infusion increased plasma acyl-ghrelin equivalently in the floxed GHSR control and GHSRfl/fl/GHWT/iCre mice (Figure 4A); these levels were similar to those attained physiologically during acute-on-chronic caloric restriction (see Section 3.5). The increase in plasma acyl-ghrelin was accompanied by a 226% increase in plasma GH in the floxed GHSR control mice but no increase in the GHSRfl/fl/GHWT/iCre mice (Figure 4B). Similarly, chronic acyl-ghrelin delivery was associated with a mean level of GH mRNA in pituitaries of the GHSRfl/fl/GHWT/iCre mice, which was 51% of the mean level of GH mRNA in pituitaries of the floxed GHSR control mice (Figure 4C). Of note, it was previously shown that a 15-day regimen of twice-daily GHRP-6 (a GHSR agonist) increased both plasma GH and pituitary GH mRNA levels in 3-month-old and 24-month-old rats as well as pituitary GH content in the 24-month-old rats [43], suggesting that increased levels of plasma GH resulting from chronic GHSR agonist delivery go hand-in-hand with increased pituitary GH mRNA. Neither ghrelin administration nor the presence or absence of somatotroph GHSR expression affected plasma IGF-1 levels (Figure 4D). The increased food intake and body weight gain noted in the floxed GHSR control mice over the course of the 12-day acyl-ghrelin infusion were blunted in the GHSRfl/fl/GHWT/iCre mice (Figure 4E–H). Similarly, although fat mass increased in the acyl-ghrelin-administered floxed GHSR control mice and acyl-ghrelin-administered GHSRfl/fl/GHWT/iCre mice, these increases were of statistically significant different magnitudes (an 83% increase in the floxed GHSR control mice vs a 34% increase in the GHSRfl/fl/GHWT/iCre mice; see Figure 4I). No genotype- or treatment-associated differences in lean mass were noted (Figure 4J). Blood glucose increased equivalently in both groups as a result of chronic acyl-ghrelin administration (Figure 4K).
Figure 4.
Effects of somatotroph-selective GHSR deletion and chronic acyl-ghrelin administration on GH secretion, GH mRNA levels, plasma IGF-1, food intake, body weight, and body composition. (A) Plasma acyl-ghrelin and (B) plasma GH measured before (indicated as “0”) and after 12 days (indicated as “12”) of chronic s.c. acyl-ghrelin infusion via osmotic minipumps. (C) Pituitary GH mRNA levels after 12 days of chronic s.c. acyl-ghrelin infusion. (D) Plasma IGF-1 levels before and after 12 days of chronic s.c. acyl-ghrelin infusion. (E) Daily food intake, (F) cumulative food intake, (G) body weight, and (H) % body weight gain over the 12-day course of ghrelin administration. (I) Fat mass, (J) lean mass, and (K) blood glucose measured on the last day of ghrelin administration. Data in A, B, D, E, G, and I–K were analyzed by repeated-measures two-way ANOVA followed by Sidak's multiple comparisons test. Data in C, F, and H were measured by unpaired Student t-test. n = 9–10 (for A-B and E-K), n = 8–9 (for C), n = 7–9 (for D). All the values are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001 for statistically significant changes or ns = no significant changes. fl/fl, mice homozygous for the loxP-flanked GHSR gene; –, absence or +, presence of GH-IRES-Cre.
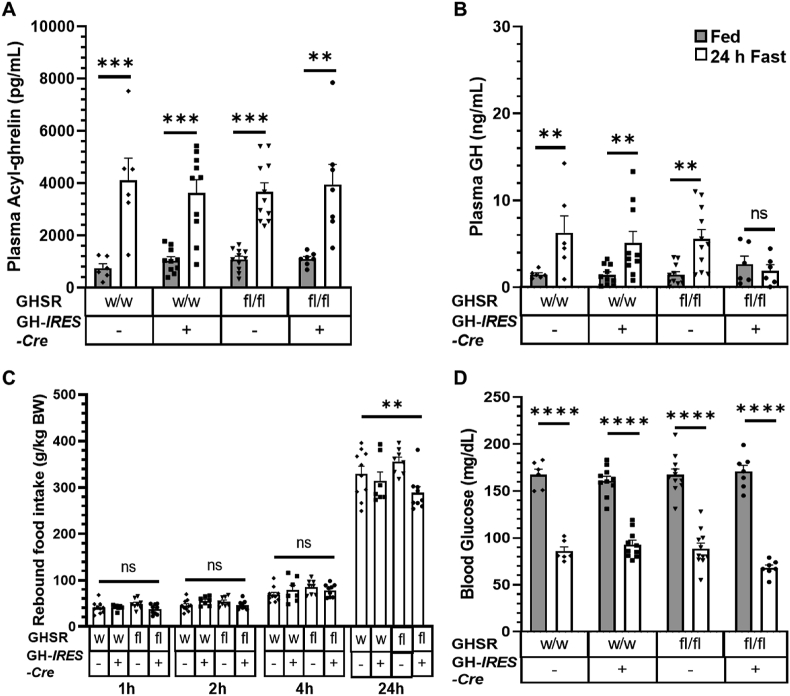
3.4. Somatotroph-selective GHSR deletion blunts the increase in plasma GH and the magnitude of the rebound feeding response to a 24-h fast but has no impact on blood glucose
To determine how mice with somatotroph-selective GHSR deletion respond to physiological elevations in acyl-ghrelin, we first fasted mice for 24 h. Plasma acyl-ghrelin rose equivalently upon fasting compared to the ad libitum-fed state in the GHSRfl/fl/GHWT/iCre mice and 3 control genotypes (Figure 5A). Although there was no statistically significant effect of genotype on plasma GH levels in ad libitum-fed mice, the plasma GH of the GHSRfl/fl/GHWT/iCre mice did not increase upon fasting, unlike in the control groups in which plasma GH rose on average by 289% (Figure 5B). As pharmacologic GHSR blockade has been shown to reduce rebound hyperphagia after an acute fast [44], we measured food intake upon reintroduction of food following the fast. Although no statistically significant genotype-dependent differences in rebound feeding were observed at 1, 2, or 4 h of food access, rebound food intake at 24 h was significantly lower in the GHSRfl/fl/GHWT/iCre mice compared to the GH-IRES-Cre control mice (Figure 5C). This reduction at 24 h but not at earlier time points was similar to the effect of ghrelin cell-selective β1-adrenergic receptor deletion, which markedly reduces plasma acyl-ghrelin levels [4]. Also, as mice with complete GHSR deletion exhibit exaggerated reductions in blood glucose following overnight and 24-h fasts [4,38], we assessed blood glucose in a separate cohort of 24 h-fasted mice. Blood glucose levels in the GHSRfl/fl/GHWT/iCre mice were comparable to those in control groups in both the ad libitum-fed condition and after the 24-h fast (Figure 5D).
Figure 5.
Effect of somatotroph-selective GHSR deletion on plasma acyl-ghrelin, GH secretion, rebound hyperphagia, and blood glucose following a 24-h fast. (A) Plasma acyl-ghrelin and (B) plasma GH in fed mice and after a 24-h fast. (C) Rebound food intake at different time points following a 24-h fast. (D) Blood glucose in fed mice and after a 24-h fast. All the data were analyzed using repeated-measures two-way ANOVA followed by Sidak's multiple comparisons test. All the values are expressed as mean ± SEM. n = 6–11 (for A, B, and D) and n = 7–10 (for C).∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 for significant changes or ns = no significant changes.
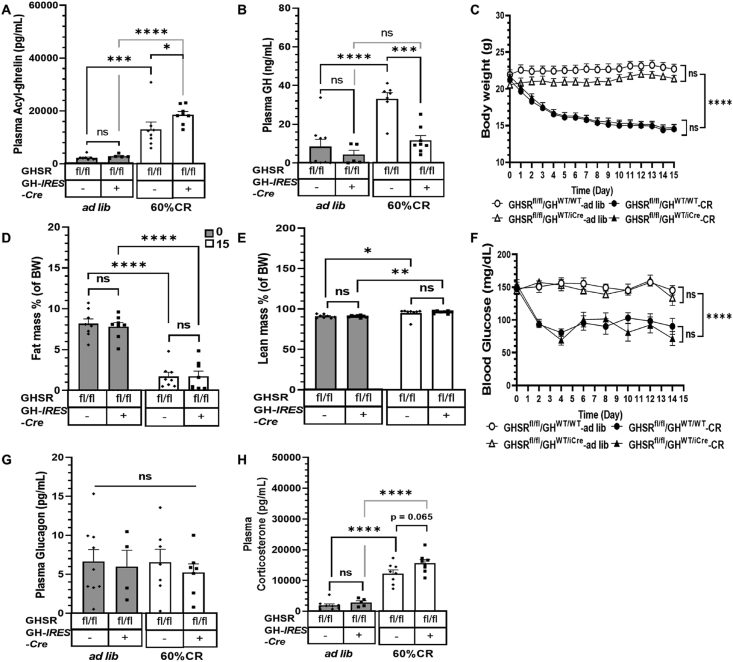
3.5. Somatotroph-selective GHSR deletion of GHSR reduces the increase in plasma GH in response to prolonged calorie restriction but does not lead to frank hypoglycemia
We also physiologically increased plasma acyl-ghrelin by subjecting mice to the previously described acute-on-chronic caloric restriction protocol. As expected [[17], [18], [19]], this protocol increased plasma acyl-ghrelin and GH in the floxed GHSR control mice compared to ad libitum-fed mice of the same genotype (Figure 6A–B). Interestingly, the plasma acyl-ghrelin levels induced by the acute-on-chronic caloric restriction protocol were 42% higher in the GHSRfl/fl/GHWT/iCre mice than in the floxed GHSR control mice (Figure 6A). Just as observed in the other protocols, the GHSRfl/fl/GHWT/iCre mice failed to significantly increase plasma GH in response to the acute-on-chronic caloric restriction protocol (Figure 6B). The GHSRfl/fl/GHWT/iCre and floxed GHSR control mice lost equivalent amounts of body weight and % fat mass and increased % lean mass equivalently over the 15-day protocol (Figure 6C–E). Despite their severe depletion of fat mass to <2%, which is required for the development of life-threatening hypoglycemia in other ghrelin system loss-of-function mouse models submitted to the acute-on-chronic caloric restriction protocol [[17], [18], [19]], herein, the GHSRfl/fl/GHWT/iCre mice resisted the development of frank hypoglycemia (Figure 6F) and did not exhibit increased mortality (data not shown) just as the floxed GHSR control mice. While end-of-study plasma glucagon levels were genotype- and treatment-independent (Figure 6G), end-of-study plasma corticosterone levels were 27% higher (P = 0.065) in the GHSRfl/fl/GHWT/iCre mice than those in the floxed GHSR control mice (Figure 6H).
Figure 6.
Effect of somatotroph-selective GHSR deletion on metabolic parameters and plasma GH during an acute-on-chronic calorie restriction protocol. (A) Plasma acyl-ghrelin and (B) plasma GH measured on day 15 of the caloric restriction protocol 23.5 h after the delivery of their last food ration (40% of their usual daily calories). Data for ad libitum-fed controls are also shown. (C) Daily body weights taken at the same time of day over the course of the study. (D) Percentage fat mass and (E) percentage lean mass, measured at the start and end of the caloric restriction. (F) Blood glucose levels measured every other day over the course of the study just prior to delivery of the daily food ration. (G) Plasma glucagon and (H) Plasma corticosterone measured on day 15 of the caloric restriction protocol 23.5 h after the delivery of their last food ration. Data in A, B, G, and H were analyzed by two-way ANOVA followed by Sidak's multiple comparisons test. Data in C, D, E, and F were measured by repeated-measures two-way ANOVA followed by Sidak's multiple comparisons test. n = 5–9 (for A-C, F, and H), n = 8 (for D and E), and n = 4–9 (for G). All of the values are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 for significant changes or ns = no significant changes. fl/fl, mice homozygous for the loxP-flanked GHSR gene; –, absence or +, presence of the GH-IRES-Cre allele.
4. Discussion
In this study, we report for the first time key roles of pituitary somatotroph-expressed GHSRs in mediating acyl-ghrelin's orexigenic and body weight-raising effects. In particular, newly generated mice with somatotroph-selective GHSR deletion were used to demonstrate that somatotroph-expressed GHSRs are required for the usual acute orexigenic effects of acyl-ghrelin delivered by s.c. bolus and the usual orexigenic and obesogenic effects of chronic s.c. acyl-ghrelin administration. These data demonstrate an essential role of somatotroph-expressed GHSRs in mediating the acute GH secretagogue actions of acyl-ghrelin delivered by s.c. bolus as well as in mediating the increase in plasma GH levels usually associated with chronic s.c. acyl-ghrelin administration, a 24-h fast, and an acute-on-chronic caloric restriction protocol that severely depleted fat stores. However, both the blood glucose-raising effects of administered ghrelin and the effects of elevated endogenous ghrelin resulting from a 24-h fast or acute-on-chronic caloric restriction to prevent exaggerated decreases in blood glucose were independent of somatotroph-expressed GHSRs.
An unexpected finding of the current study was the dichotomy between the requirement of somatotroph-expressed GHSRs for ghrelin's orexigenic and body weight effects vs their apparent superfluousness as it relates to ghrelin's glucoregulatory actions, at least in the paradigms assessed herein. Indeed, the literature suggests that just the opposite would have been observed. While on the one hand, GH has a well-established yet complex effect on body composition [45,46], the first two descriptions of ghrelin-induced actions to increase food intake and body weight specifically reported those actions to be GH-independent. In particular, a continuous i.c.v. infusion of ghrelin increased food intake in both wild-type Wistar rats and GH-deficient SDR rats (spontaneous dwarf rats), prompting the authors of that study to conclude that “the stimulatory effect of ghrelin on feeding does not depend on the stimulation of growth hormone” [20]. In a separate study, daily s.c. ghrelin administration similarly increased body weight gain in GH-deficient dwarf (dw/dw) rats, while a single s.c. ghrelin injection increased the respiratory quotient [5]. Those findings, in addition to differential effects of single s.c. injections of ghrelin vs GH on the respiratory quotient, energy expenditure, and fat accumulation (fat gain with ghrelin vs fat loss with GH) in 129SV mice prompted the authors of that study to conclude that “induction of a positive energy balance by ghrelin appears unlikely to be caused by its ability to stimulate GH secretion” [5]. In contrast, GH has been reported to be a key mediator of ghrelin's actions to prevent life-threatening hypoglycemia in the acute-on-chronic caloric restriction protocol used herein. Specifically, when mice are chronically given once-daily access to only 40% of their usual calories (such that body fat drops to < 2% of their body weight), those deficient in acyl-ghrelin [due to either ghrelin O-acyltransferase (GOAT) or ghrelin deficiency], develop frank hypoglycemia during the period just prior to delivery of their food ration [[17], [18], [19]]. Compared to wild-type mice that avoid this outcome and exhibit progressive elevations in circulating acyl-ghrelin and GH, GOAT-KO and ghrelin-KO mice exhibit a markedly blunted increase in GH (∼one-third the levels occurring in wild-type mice) [17,18]. Not only does continuous ghrelin infusion during the caloric restriction protocol normalize plasma GH levels in GOAT-KO mice, but also continuous ghrelin infusion and continuous GH infusion correct caloric restriction-associated hypoglycemia [18]. Continuous GH infusion also rescues exaggerated hypoglycemia and markedly improves survival during the acute-on-chronic caloric restriction protocol when performed in mice overexpressing the GHSR antagonist/inverse agonist liver enriched antimicrobial peptide 2 (LEAP2) [16]. This effect of GH in fasted fat-depleted mice has been attributed to GH stimulation of hepatic autophagy and gluconeogenesis via hepatocyte-expressed GH receptors [17,19,47].
Although explanations for the discrepant findings regarding the requirement of an intact ghrelin-GH axis between the current study and those mentioned will require future exploration as it relates to regulation of blood glucose during the acute-on-chronic caloric restriction protocol, it is highly plausible that the body would not rely solely on acyl-ghrelin-stimulated GH secretion via direct engagement of GHSR-expressing somatotrophs. Indeed, when performed such that the acute-on-chronic caloric restriction protocol results in near total fat depletion, mice with decreased GHSR signaling from either ghrelin gene deletion, GOAT gene deletion, disrupted ghrelin secretion (secondary to ghrelin cell-selective deletion of β1-adrenergic receptors), or viral overexpression of LEAP2 have been reported as being moribund or exhibiting increased mortality [4,[16], [17], [18]]. Thus, we suggest that the persistence of GHSRs in regions other than the pituitary in our newly described mouse model enable acyl-ghrelin to recruit other downstream targets during caloric restriction to avert life-threatening hypoglycemia. Importantly, ghrelin is known to influence blood glucose through several different effectors and processes depending on the scenario. As reviewed in [48], acyl-ghrelin's glucoregulatory actions in post-prandial settings involve inhibition of insulin secretion, reduction in insulin sensitivity, and priming of enteroendocrine L cells to stimulate GLP-1 release; during acute fasting conditions, acyl-ghrelin stimulates glucagon secretion and enhances hepatic glucose production. Adding further complexity, acyl-ghrelin has been shown to decrease insulin secretion both directly via GHSR-expressing pancreatic β cells and indirectly via stimulating somatostatin release from GHSR-expressing pancreatic δ cells [[49], [50], [51], [52], [53]]. Similarly, acyl-ghrelin has been shown to increase glucagon secretion both directly via GHSR-expressing pancreatic α cells and indirectly via pancreatic δ cells or by GHSR-expressing arcuate hypothalamic AgRP neurons [48,49,54]. Acyl-ghrelin's glucoregulatory actions in some scenarios also likely include its known actions to stimulate eating and/or increase glucocorticoid levels (such as corticosterone) [48,55]. For example, during hyperinsulinemic-hypoglycemic clamps, ghrelin knock-out mice not only require a markedly higher glucose infusion rate to maintain the goal glycemic level, but also exhibit less robust GH and corticosterone responses, all of which are rescued upon GHSR agonist administration [21]. Elevations in plasma corticosterone also are attenuated in GHSR-null mice following exposure to a 10-day chronic social defeat stress protocol [56]. Notably, in the current study, we observed two major differences besides plasma GH levels between the mice with somatotroph-selective GHSR deletion and floxed GHSR control littermates at the end of the acute-on-chronic caloric restriction protocol. The mice with somatotroph-selective GHSR deletion exhibited higher plasma levels of both acyl-ghrelin and corticosterone. Further studies are needed to determine how increased acyl-ghrelin and corticosterone might compensate for the disrupted ghrelin-GH axis resulting from somatotroph-selective GHSR deletion and if any other effectors downstream of acyl-ghrelin might be involved.
Just as there are numerous potential downstream effectors available to mediate acyl-ghrelin's glucoregulatory effects, there is also a distributed set of cell types that mediate acyl-ghrelin's effects on food intake, body weight, and adiposity. The current study reveals key actions for GHSR-expressing somatotrophs in those effects, and by extension, especially given the singular key role of pituitary somatotrophs as a producer of GH as well as the lower-than-usual plasma GH observed in the mice with somatotroph-selective GHSR deletion observed herein upon acyl-ghrelin administration, a 24-h fast, and acute-on-chronic caloric restriction, suggests key actions of acyl-ghrelin-induced GH release. Acyl-ghrelin's effects to increase food intake, body weight, and adiposity are also mediated by several neuronal populations in which GHSRs are expressed [15,29,57]. To date, acyl-ghrelin action at several of these sites has been investigated by its direct microinjection into different brain sites. For instance, acyl-ghrelin microinjection into the hypothalamic arcuate nucleus, the dorsal vagal complex in the caudal brainstem, and the ventral hippocampus induce food intake [58,59]. GHSR antagonist microinjection into the ventral tegmental area in the midbrain blocks the orexigenic effect of peripherally administered ghrelin [60]. Using RNA interference methodology, vagal afferent neuron-selective GHSR knock-down increases meal frequency without effects on cumulative food intake or refeeding following an unanticipated fast [61]. Interestingly, separate studies have alternatively demonstrated that peri-vagal capsaicin treatment and/or surgical subdiaphragmatic vagotomy block food intake [62] vs do not affect the actions of administered acyl-ghrelin to increase food intake [63]. Using a different floxed GHSR model than that described here, neuronal deletion of GHSR (as achieved via a cross to Syn1-Cre mice) was described as almost “completely abolish(ing) diet-induced obesity” [35]. Arcuate hypothalamic AgRP/NPY neuron-selective GHSR deletion was shown to exaggerate reductions in body weight, fat mass, and blood glucose in response to a mild caloric restriction protocol [34], while AgRP/NPY neuron-selective GHSR expression was shown to partially restore the orexigenic response to administered acyl-ghrelin otherwise lacking in mice with total GHSR deletion [64]. A similar finding was observed using mice with selective GHSR expression in catecholaminergic neurons, although mice with Phox2b neuron-selective GHSR expression did not exhibit an orexigenic response to acyl-ghrelin [56,65]. While derivation of mice with total GHSR deletion was not possible from the GHSRWT/fl/GHWT/iCre x GHSRWT/fl crosses performed herein (preventing comparison of the food intake, body weight, and body composition phenotypes of the mice with somatotroph-selective GHSR deletion to potential littermates with total GHSR deletion), the lack of statistically significant differences in food intake acutely between acyl-ghrelin-treated and saline-treated GHSRfl/fl/GHWT/iCre mice nevertheless suggests a fairly robust effect of somatotroph-expressed GHSRs in that particular setting. Future studies that include GHSR knock-out control littermates would facilitate objective comparisons of the effects of somatotroph-selective GHSR deletion vs total GHSR deletion.
It is also important to mention that the full extent to which GH secretion would be affected by somatotroph-selective GHSR deletion could not necessarily have been predicted from the available literature. While the pituitary was the site from which GHSR was first cloned and the validation of ghrelin as an endogenous GH secretagogue was first conducted using primary cultured pituitary cells [25,66], two other major mediators of GH secretion exist. These include growth hormone-releasing hormone (GHRH) and somatostatin. Acyl-ghrelin interacts with both GHRH and somatostatin. Indeed, a tripartite model has been proposed to describe how acyl-ghrelin, GHRH, and somatostatin cooperate to regulate GH secretion [[67], [68], [69]]. Complexity within such a system stems at least in part from data demonstrating that acyl-ghrelin and/or synthetic GHSR agonists can stimulate GH release not only directly at the level of the GHSR-expressing somatotroph but also indirectly via effects to stimulate GHRH release from the hypothalamus, inhibit hypothalamic somatostatin release, and oppose inhibition of GHRH and GH secretion by somatostatin [[67], [68], [69], [70], [71], [72]]. Our evaluation of GH secretion following acute administration of an i.p. acyl-ghrelin bolus was performed in pentobarbital-anesthetized mice. Notably, pentobarbital anesthetization is associated with a relative (but not absolute) reduction in hypothalamus-derived somatostatin secretion into hypophyseal portal blood, which increases plasma GH levels, whereas other anesthetics have the opposite effect on somatostatin release and GH [73]. For that reason, pentobarbital anesthetization has become a common tool to investigate pulsatile GH secretion, including following bolus administration of GH secretagogues and acyl-ghrelin [25,41,42,74,75]. Nevertheless, GH secretion in response to bolus delivery of acyl-ghrelin has also been observed even without use of pentobarbital in freely moving animals [40]. Even further complexity within this tripartite model of the regulation of GH secretion stems from known effects of NPY released from arcuate NPY/AgRP neurons, which represent a major site of GHSR expression, to influence pulsatile GH secretion via indirect effects on hypothalamic somatostatin neurons and/or GHRH neurons (especially in the fasted state) as well as from expression of GH receptors by those same NPY/AgRP neurons [[76], [77], [78], [79]].
Regarding GHRH as a downstream mediator of acyl-ghrelin action, GHSR immunoreactivity has been localized to hypothalamic GHRH neurons, and peripherally administered GHSR agonist induces c-fos in those neurons [71,80]. Mice (lit/lit) and human subjects carrying two copies of loss-of-function GHRH gene mutations exhibit limited GH responses to administered GHSR agonist [81,82]. Administration of anti-GHRH antibodies abolishes acyl-ghrelin-induced GH secretion [83]. Selective transgenic GHSR overexpression within GHRH neurons not only causes increased hypothalamic GHRH expression and pituitary GH content, but also increased post-weaning body weight gain and reduced adiposity later in adulthood [84]. Selective transgenic expression of an antisense GHSR transcript within tyrosine hydroxylase-containing cells, which include hypothalamic GHRH neurons, also reduces GH levels [85]. Furthermore, in rats, blockade of vagal afferent signaling via gastric branch vagotomy and peri-vagal capsaicin application blocks c-fos induction in hypothalamic GHRH neurons and limits GH secretion in response to peripheral acyl-ghrelin administration, although in human subjects, peripherally administered acyl-ghrelin stimulates GH secretion equivalently in vagotomized patients with gastrectomy and normal subjects [62,86]. Altogether, these data suggest partial dependence of acyl-ghrelin-induced GH secretion on intact GHRH signaling and presumably direct and/or indirect interaction of acyl-ghrelin with GHRH neurons. It is as yet unclear why selective GHSR deletion from somatotrophs, as accomplished herein, and the various methods to block GHRH mediation of acyl-ghrelin action previously described have such profound effects on acyl-ghrelin-induced GH secretion. This may suggest a coordinated regulatory process in which GHRH receptors and GHSRs +/− somatostatin receptors work in conjunction at the level of the somatotroph, perhaps via heterodimerization, which has been demonstrated between GHSRs and other G protein-coupled receptors [87].
As a final point of discussion, with the exception of the i.p. acyl-ghrelin bolus study in which plasma samples to assess GH levels were taken at 0, 5, and 15 min allowing us to assess the effects of somatotroph-selective GHSR deletion on acyl-ghrelin-induced GH secretion, the other paradigms used herein in which plasma GH levels were determined mostly involved only single point measurements or measurements only at the start and end of the manipulation. Given the well-known ultradian pulsatile pattern of GH in the circulation defined by several peaks and troughs in GH secretion and influenced by clearance of GH [69], the absence of frequent plasma sampling in those other paradigms prevents us from specifically commenting on GH secretion per se or on whether the measured plasma GH levels simply reflect either a peak or trough in GH's usual pulsatile secretory pattern vs a true change in basal plasma GH levels. Nonetheless, the reported absent increase in plasma GH in mice with somatotroph-selective GHSR deletion following chronic acyl-ghrelin administration is supported by data showing ∼50% lower pituitary GH mRNA levels in those mice compared to floxed GHSR control mice. Similarly, there are a few studies in the literature in which a progressive increase in plasma GH was reported in wild-type mice over the course of the acute-on-chronic chronic caloric restriction protocol used herein, whereas mice lacking acyl-ghrelin and mice carrying a mutation that ablates constitutive (acyl-ghrelin-independent) GHSR activity, similar to the mice with somatotroph-selective GHSR deletion studied herein, lacked this usual marked plasma GH elevation [18,74].
5. Conclusions
These data reveal that GHSRs expressed by somatotrophs are required for the acute GH secretory responses of acyl-ghrelin delivered as an i.p. bolus and increased plasma GH levels usually associated with peripherally administered acyl-ghrelin, a 24-h fast, and an acute-on-chronic caloric restriction protocol modeling starvation. The usual orexigenic, body weight-raising, and fat accumulation responses to administered acyl-ghrelin are also dependent on somatotroph-expressed GHSRs. However, the glucoregulatory actions induced by administered acyl-ghrelin or mediated by acyl-ghrelin during acute fasting and acute-on-chronic caloric restriction occur even without somatotroph-expressed GHSRs. The absence of life-threatening hypoglycemia and the observed elevated plasma acyl-ghrelin and corticosterone in mice with somatotroph-selective GHSR deletion upon exposure to the acute-on-chronic caloric restriction protocol suggest the existence of a distributed network of potential downstream effectors through which acyl-ghrelin might act in that setting to regulate blood glucose. Future studies such as those in which mice with somatotroph-selective GHSR deletion are compared to mice with selective deletion of GHSRs from hypothalamic GHRH neurons or from somatostatin-expressing neurons, those in which possible functional coordination between GHSRs and GHRH receptors is explored, and those in which mice with somatotroph-selective GHSR deletion are treated with GH replacement might help reveal additional mechanistic details regarding acyl-ghrelin regulation of GH secretion and GH involvement in acyl-ghrelin's action.
Acknowledgments
The authors thank the UT Southwestern Metabolic Core Facility for access to the EchoMRI and the UT Southwestern Transgenic Core Facility for their assistance in generating the GH-IRES-Cre and floxed GHSR mouse lines. The authors thank Drs. Ralf Nass and Michael Thorner for helpful discussions.
Contribution statement
DG designed the study, collected the data, performed the statistical analyses, and helped write the manuscript. AMP contributed to the validation of the GH-IRES-Cre mouse model. ALB and SCW helped produce the GH-IRES-Cre mouse model under the supervision of JKE. SOL helped produce the GH-IRES-Cre and floxed GHSR mouse models. SV, KS, and OS helped with the data collection. NPM and CPR organized the breeding schedule and all aspects of animal husbandry required for the study. JMZ oversaw all aspects of the study, helped write the manuscript, and secured funding. All the authors approved the final version.
Funding
This study was supported by the Diana and Richard C. Strauss Professorship in Biomedical Research, the Mr. and Mrs. Bruce G. Brookshire Professorship in Medicine, the Kent and Jodi Foster Distinguished Chair in Endocrinology, in Honor of Daniel Foster, MD, and the NIH (R01DK103884 and R01DK119341 to JMZ and P01DK119130 to JKE and JMZ).
Conflict of interest
The authors declare that no conflicts of interest exist.
References
- 1.Mani B.K., Zigman J.M. Ghrelin as a survival hormone. Trends in Endocrinology and Metabolism. 2017;28(12):843–854. doi: 10.1016/j.tem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J. Ghrelin. Molecular Metabolism. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariyasu H., Takaya K., Tagami T., Ogawa Y., Hosoda K., Akamizu T. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. Journal Clinical Endocrinology Metabolism. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Mani B.K., Osborne-Lawrence S., Vijayaraghavan P., Hepler C., Zigman J.M. beta1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. Journal of Clinical Investigation. 2016;126(9):3467–3478. doi: 10.1172/JCI86270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 6.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Prudom C.E., Nass R., Pezzoli S.S., Oliveri M.C., Johnson M.L. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. Journal Clinical Endocrinology Metabolism. 2008;93(5):1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120(2):337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 9.Theander-Carrillo C., Wiedmer P., Cettour-Rose P., Nogueiras R., Perez-Tilve D., Pfluger P. Ghrelin action in the brain controls adipocyte metabolism. Journal of Clinical Investigation. 2006;116(7):1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett B.P., Hwang Y., Taylor M.S., Kirchner H., Pfluger P.T., Bernard V. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330(6011):1689–1692. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esler W.P., Rudolph J., Claus T.H., Tang W., Barucci N., Brown S.E. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 12.Shearman L.P., Wang S.P., Helmling S., Stribling D.S., Mazur P., Ge L. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology. 2006;147(3):1517–1526. doi: 10.1210/en.2005-0993. [DOI] [PubMed] [Google Scholar]
- 13.Zorrilla E.P., Iwasaki S., Moss J.A., Chang J., Otsuji J., Inoue K. Vaccination against weight gain. Proceedings of the National Academy of Sciences of the U S A. 2006;103(35):13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y., Ahmed S., Smith R.G. Deletion of ghrelin impairs neither growth nor appetite. Molecular and Cellular Biology. 2003;23(22):7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zigman J.M., Nakano Y., Coppari R., Balthasar N., Marcus J.N., Lee C.E. Mice lacking ghrelin receptors resist the development of diet-induced obesity. Journal of Clinical Investigation. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X., Yang H., Bednarek M.A., Galon-Tilleman H., Chen P., Chen M. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metabolism. 2018;27(2):461–469. doi: 10.1016/j.cmet.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Li R.L., Sherbet D.P., Elsbernd B.L., Goldstein J.L., Brown M.S., Zhao T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. Journal of Biological Chemistry. 2012;287(22):17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao T.J., Liang G., Li R.L., Xie X., Sleeman M.W., Murphy A.J. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the U S A. 2010;107(16):7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Fang F., Goldstein J.L., Brown M.S., Zhao T.J. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proceedings of the National Academy of Sciences of the U S A. 2015;112(4):1226–1231. doi: 10.1073/pnas.1423643112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 21.Shankar K., Gupta D., Mani B.K., Findley B.G., Lord C.C., Osborne-Lawrence S. Acyl-ghrelin is permissive for the normal counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2020;69(2):228–237. doi: 10.2337/db19-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway L., Butterfield G., Hintz R.L., Gesundheit N., Marcus R. Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women. Journal Clinical Endocrinology Metabolism. 1994;79(2):470–479. doi: 10.1210/jcem.79.2.7519191. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman A.R., Kuntze J.E., Baptista J., Baum H.B., Baumann G.P., Biller B.M. Growth hormone (GH) replacement therapy in adult-onset gh deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. Journal Clinical Endocrinology Metabolism. 2004;89(5):2048–2056. doi: 10.1210/jc.2003-030346. [DOI] [PubMed] [Google Scholar]
- 24.Yang H., Dixit V.D., Patel K., Vandanmagsar B., Collins G., Sun Y. Reduction in hypophyseal growth hormone and prolactin expression due to deficiency in ghrelin receptor signaling is associated with Pit-1 suppression: relevance to the immune system. Brain, Behavior, and Immunity. 2008;22(8):1138–1145. doi: 10.1016/j.bbi.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 26.Nass R., Gilrain J., Anderson S., Gaylinn B., Dalkin A., Day R. High plasma growth hormone (GH) levels inhibit expression of GH secretagogue receptor messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 2000;141(6):2084–2089. doi: 10.1210/endo.141.6.7503. [DOI] [PubMed] [Google Scholar]
- 27.Tong Q., Ye C.P., Jones J.E., Elmquist J.K., Lowell B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature Neuroscience. 2008;11(9):998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani B.K., Walker A.K., Lopez Soto E.J., Raingo J., Lee C.E., Perello M. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. The Journal of Comparative Neurology. 2014;522(16):3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez J.A., Bruggeman E.C., Mani B.K., Osborne-Lawrence S., Lord C.C., Roseman H.F. Ghrelin receptor agonist rescues excess neonatal mortality in a Prader-Willi Syndrome mouse model. Endocrinology. 2018;159(12):4006–4022. doi: 10.1210/en.2018-00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muyrers J.P., Zhang Y., Stewart A.F. Techniques: recombinogenic engineering--new options for cloning and manipulating DNA. Trends in Biochemical Sciences. 2001;26(5):325–331. doi: 10.1016/s0968-0004(00)01757-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee E.C., Yu D., Martinez de Velasco J., Tessarollo L., Swing D.A., Court D.L. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73(1):56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 33.Wu C.S., Bongmba O.Y.N., Yue J., Lee J.H., Lin L., Saito K. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. International Journal of Molecular Sciences. 2017;18(4):832. doi: 10.3390/ijms18040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C.S., Bongmba O.Y.N., Lee J.H., Tuchaai E., Zhou Y., Li D.P. Ghrelin receptor in agouti-related peptide neurones regulates metabolic adaptation to calorie restriction. Journal of Neuroendocrinology. 2019;31(7) doi: 10.1111/jne.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.H., Lin L., Xu P., Saito K., Wei Q., Meadows A.G. Neuronal Deletion of ghrelin receptor almost completely prevents diet-induced obesity. Diabetes. 2016;65(8):2169–2178. doi: 10.2337/db15-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L., Lee J.H., Wang R., Wang R., Sheikh-Hamad D., Zang Q.S. aP2-Cre mediated ablation of GHS-R attenuates adiposity and improves insulin sensitivity during aging. International Journal of Molecular Sciences. 2018;19(10):3002–3018. doi: 10.3390/ijms19103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P., Jenkins N.A., Copeland N.G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Research. 2003;13(3):476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang J.C., Sakata I., Kohno D., Perello M., Osborne-Lawrence S., Repa J.J. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Molecular Endocrinology. 2011;25(9):1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitrofanova L.B., Konovalov P.V., Krylova J.S., Polyakova V.O., Kvetnoy I.M. Plurihormonal cells of normal anterior pituitary: facts and conclusions. Oncotarget. 2017;8(17):29282–29299. doi: 10.18632/oncotarget.16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seoane L.M., Tovar S., Baldelli R., Arvat E., Ghigo E., Casanueva F.F. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. European Journal of Endocrinology. 2000;143(5):R7–R9. doi: 10.1530/eje.0.143r007. [DOI] [PubMed] [Google Scholar]
- 41.Mallo F., Alvarez C.V., Benitez L., Burguera B., Coya R., Casanueva F.F. Regulation of His-dTrp-Ala-Trp-dPhe-Lys-NH2 (GHRP-6)-induced GH secretion in the rat. Neuroendocrinology. 1993;57(2):247–256. doi: 10.1159/000126366. [DOI] [PubMed] [Google Scholar]
- 42.Micic D., Mallo F., Peino R., Cordido F., Leal-Cerro A., Garcia-Mayor R.V. Regulation of growth hormone secretion by the growth hormone releasing hexapeptide (GHRP-6) Journal of Pediatric Endocrinology. 1993;6(3–4):283–289. doi: 10.1515/jpem.1993.6.3-4.283. [DOI] [PubMed] [Google Scholar]
- 43.Frutos M.G., Cacicedo L., Fernandez C., Vicent D., Velasco B., Zapatero H. Insights into a role of GH secretagogues in reversing the age-related decline in the GH/IGF-I axis. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(5):E1140–E1152. doi: 10.1152/ajpendo.00236.2007. [DOI] [PubMed] [Google Scholar]
- 44.Perello M., Sakata I., Birnbaum S., Chuang J.C., Osborne-Lawrence S., Rovinsky S.A. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biological Psychiatry. 2010;67(9):880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies J.S., Gevers E.F., Stevenson A.E., Coschigano K.T., El-Kasti M.M., Bull M.J. Adiposity profile in the dwarf rat: an unusually lean model of profound growth hormone deficiency. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(5):E1483–E1494. doi: 10.1152/ajpendo.00417.2006. [DOI] [PubMed] [Google Scholar]
- 46.Nass R., Johannsson G., Christiansen J.S., Kopchick J.J., Thorner M.O. The aging population--is there a role for endocrine interventions? Growth Hormone & IGF Research. 2009;19(2):89–100. doi: 10.1016/j.ghir.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Fang F., Shi X., Brown M.S., Goldstein J.L., Liang G. Growth hormone acts on liver to stimulate autophagy, support glucose production, and preserve blood glucose in chronically starved mice. Proceedings of the National Academy of Sciences of the U S A. 2019;116(15):7449–7454. doi: 10.1073/pnas.1901867116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mani B.K., Shankar K., Zigman J.M. Ghrelin's relationship to blood glucose. Endocrinology. 2019;160(5):1247–1261. doi: 10.1210/en.2019-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adriaenssens A.E., Svendsen B., Lam B.Y., Yeo G.S., Holst J.J., Reimann F. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiGruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-Zitron C. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Molecular Metabolism. 2016;5(7):449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurashina T., Dezaki K., Yoshida M., Sukma Rita R., Ito K., Taguchi M. The beta-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Scientific Reports. 2015;5:14041. doi: 10.1038/srep14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 53.Kaiser J., Krippeit-Drews P., Drews G. Acyl-ghrelin influences pancreatic beta-cell function by interference with KATP channels. Diabetes. 2021;70(2):423–435. doi: 10.2337/db20-0231. [DOI] [PubMed] [Google Scholar]
- 54.Wierup N., Yang S., McEvilly R.J., Mulder H., Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. Journal of Histochemistry and Cytochemistry. 2004;52(3):301–310. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 55.Spencer S.J., Xu L., Clarke M.A., Lemus M., Reichenbach A., Geenen B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biological Psychiatry. 2012;72(6):457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Chuang J.C., Perello M., Sakata I., Osborne-Lawrence S., Savitt J.M., Lutter M. Ghrelin mediates stress-induced food-reward behavior in mice. Journal of Clinical Investigation. 2011;121(7):2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mani B.K., Osborne-Lawrence S., Mequinion M., Lawrence S., Gautron L., Andrews Z.B. The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Molecular Metabolism. 2017;6(8):882–896. doi: 10.1016/j.molmet.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faulconbridge L.F., Cummings D.E., Kaplan J.M., Grill H.J. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52(9):2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 59.Suarez A.N., Liu C.M., Cortella A.M., Noble E.E., Kanoski S.E. Ghrelin and orexin interact to increase meal size through a descending hippocampus to hindbrain signaling pathway. Biological Psychiatry. 2020;87(11):1001–1011. doi: 10.1016/j.biopsych.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abizaid A., Liu Z.W., Andrews Z.B., Shanabrough M., Borok E., Elsworth J.D. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. Journal of Clinical Investigation. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis E.A., Wald H.S., Suarez A.N., Zubcevic J., Liu C.M., Cortella A.M. Ghrelin signaling affects feeding behavior, metabolism, and memory through the vagus nerve. Current Biology. 2020;30(22):4510–4518. doi: 10.1016/j.cub.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Date Y., Murakami N., Toshinai K., Matsukura S., Niijima A., Matsuo H. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 63.Arnold M., Mura A., Langhans W., Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. Journal of Neuroscience. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Q., Liu C., Uchida A., Chuang J.C., Walker A., Liu T. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Molecular Metabolism. 2014;3(1):64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott M.M., Perello M., Chuang J.C., Sakata I., Gautron L., Lee C.E. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PloS One. 2012;7(8) doi: 10.1371/journal.pone.0044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howard A.D., Feighner S.D., Cully D.F., Arena J.P., Liberator P.A., Rosenblum C.I. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 67.Farhy L.S., Veldhuis J.D. Deterministic construct of amplifying actions of ghrelin on pulsatile growth hormone secretion. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288(6):R1649–R1663. doi: 10.1152/ajpregu.00451.2004. [DOI] [PubMed] [Google Scholar]
- 68.Veldhuis J.D. A tripeptidyl ensemble perspective of interactive control of growth hormone secretion. Hormone Research. 2003;60(Suppl 1):86–101. doi: 10.1159/000071232. [DOI] [PubMed] [Google Scholar]
- 69.Steyn F.J., Tolle V., Chen C., Epelbaum J. Neuroendocrine regulation of growth hormone secretion. Comparative Physiology. 2016;6(2):687–735. doi: 10.1002/cphy.c150002. [DOI] [PubMed] [Google Scholar]
- 70.Guillaume V., Magnan E., Cataldi M., Dutour A., Sauze N., Renard M. Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology. 1994;135(3):1073–1076. doi: 10.1210/endo.135.3.7915227. [DOI] [PubMed] [Google Scholar]
- 71.Dickson S.L., Luckman S.M. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138(2):771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 72.Tolle V., Zizzari P., Tomasetto C., Rio M.C., Epelbaum J., Bluet-Pajot M.T. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73(1):54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 73.Chihara K., Arimura A., Schally A.V. Immunoreactive somatostatin in rat hypophyseal portal blood: effects of anesthetics. Endocrinology. 1979;104(5):1434–1441. doi: 10.1210/endo-104-5-1434. [DOI] [PubMed] [Google Scholar]
- 74.Torz L.J., Osborne-Lawrence S., Rodriguez J., He Z., Cornejo M.P., Mustafa E.R. Metabolic insights from a GHSR-A203E mutant mouse model. Molecular Metabolism. 2020;39:101004. doi: 10.1016/j.molmet.2020.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steyn F.J., Huang L., Ngo S.T., Leong J.W., Tan H.Y., Xie T.Y. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165–3171. doi: 10.1210/en.2011-0253. [DOI] [PubMed] [Google Scholar]
- 76.Chan Y.Y., Clifton D.K., Steiner R.A. Role of NPY neurones in GH-dependent feedback signalling to the brain. Hormone Research. 1996;45(Suppl 1):12–14. doi: 10.1159/000184820. [DOI] [PubMed] [Google Scholar]
- 77.Minami S., Kamegai J., Sugihara H., Suzuki N., Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocrine Journal. 1998;45(Suppl):S19–S26. doi: 10.1507/endocrj.45.suppl_s19. [DOI] [PubMed] [Google Scholar]
- 78.Foradori C.D., Whitlock B.K., Daniel J.A., Zimmerman A.D., Jones M.A., Read C.C. Kisspeptin stimulates growth hormone release by utilizing neuropeptide y pathways and is dependent on the presence of ghrelin in the Ewe. Endocrinology. 2017;158(10):3526–3539. doi: 10.1210/en.2017-00303. [DOI] [PubMed] [Google Scholar]
- 79.Huang L., Tan H.Y., Fogarty M.J., Andrews Z.B., Veldhuis J.D., Herzog H. Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state. Journal of Neuroscience. 2014;34(49):16309–16319. doi: 10.1523/JNEUROSCI.4622-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tannenbaum G.S., Lapointe M., Beaudet A., Howard A.D. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology. 1998;139(10):4420–4423. doi: 10.1210/endo.139.10.6330. [DOI] [PubMed] [Google Scholar]
- 81.Peroni C.N., Hayashida C.Y., Nascimento N., Longuini V.C., Toledo R.A., Bartolini P. Growth hormone response to growth hormone-releasing peptide-2 in growth hormone-deficient little mice. Clinics (Sao Paulo) 2012;67(3):265–272. doi: 10.6061/clinics/2012(03)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gondo R.G., Aguiar-Oliveira M.H., Hayashida C.Y., Toledo S.P., Abelin N., Levine M.A. Growth hormone-releasing peptide-2 stimulates GH secretion in GH-deficient patients with mutated GH-releasing hormone receptor. Journal Clinical Endocrinology Metabolism. 2001;86(7):3279–3283. doi: 10.1210/jcem.86.7.7694. [DOI] [PubMed] [Google Scholar]
- 83.Tannenbaum G.S., Epelbaum J., Bowers C.Y. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology. 2003;144(3):967–974. doi: 10.1210/en.2002-220852. [DOI] [PubMed] [Google Scholar]
- 84.Lall S., Balthasar N., Carmignac D., Magoulas C., Sesay A., Houston P. Physiological studies of transgenic mice overexpressing growth hormone (GH) secretagogue receptor 1A in GH-releasing hormone neurons. Endocrinology. 2004;145(4):1602–1611. doi: 10.1210/en.2003-1509. [DOI] [PubMed] [Google Scholar]
- 85.Shuto Y., Shibasaki T., Otagiri A., Kuriyama H., Ohata H., Tamura H. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. Journal of Clinical Investigation. 2002;109(11):1429–1436. doi: 10.1172/JCI13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeno R., Okimura Y., Iguchi G., Kishimoto M., Kudo T., Takahashi K. Intravenous administration of ghrelin stimulates growth hormone secretion in vagotomized patients as well as normal subjects. European Journal of Endocrinology. 2004;151(4):447–450. doi: 10.1530/eje.0.1510447. [DOI] [PubMed] [Google Scholar]
- 87.Edwards A., Abizaid A. Clarifying the ghrelin system's ability to regulate feeding behaviours despite enigmatic spatial separation of the ghsr and its endogenous ligand. International Journal of Molecular Sciences. 2017;18(4):859. doi: 10.3390/ijms18040859. [DOI] [PMC free article] [PubMed] [Google Scholar]