Abstract
Permanent occlusion of bilateral common carotid arteries (2VO) in rat is considered as a suitable animal model to mimic chronic brain hypoperfusion status, which is proved to be a risk factor to precede the Alzheimer’s disease or/and vascular dementia. In this protocol, we describe how to successfully ligate the bilateral common carotid arteries covered by anterior cervical muscle group, and provide the details for understanding the surgical procedures of 2VO.
Keywords: Permanent occlusion of bilateral common carotid arteries, Chronic brain hypoperfusion, Rat
Background
Currently, chronic brain hypoperfusion (CBH) is considered as a preclinical condition of mild cognitive impairment, which is thought to precede dementia ( Ruitenberg et al., 2005 ; Gorelick et al., 2011 ). However, how CBH produces dementia is largely unknown.
The method of permanent occlusion of bilateral common carotid arteries (2VO) in rat was first established in the 1970s (Eklof and Siesjo, 1972). Since 2VO in rats provokes CBH without motor dysfunction, it is considered as a suitable animal model to elicit CBH status ( Tanaka et al., 1996 ). After the 2VO procedures, three phases are theoretically determined by the degree of cerebral blood flow (CBF) and metabolic changes of rats ( Farkas et al., 2007 ). After initiation of 2VO procedures, the first phase of acute ischemia lasts for about 2-3 days ( Ohta et al., 1997 ; Otori et al., 2003 ; Tomimoto et al., 2003 ). During this period, the CBF drops dramatically and remains at a significantly low level in the following 4 weeks with reduced glucose utilization ( Otori et al., 2003 ), sudden depletion of ATP and phosphocreatine (Plaschke, 2005). The second phase lasts for 8 to 12 weeks and corresponds well with the CBH in aging and dementia, and a slight reduction of the CBF with restored ATP as well as remaining low level of phosphocreatine. However, in the final phase, the CBF recovers to the baseline and the metabolic changes gradually cease after 6 months of 2VO ( Ohta et al., 1997 ; Otori et al., 2003 ).
We also noted that numerous studies using 2VO rat model also reveal lots of pathological characteristics, including impaired learning and memory evaluated by both Morris water maze or eight-arm radial maze, where the 2VO rats display longer escape latencies to find hiding platform in Morris water maze ( Liu et al., 2005 ) and commit more errors to enter a never-baited arm than the rats from sham group in the eight-arm radial maze (Sopala and Danysz, 2001); the neuronal cell death in hippocampus ( Farkas et al., 2004 ); reduced dendritic arborizations ( Chen et al., 2017 ); astrocytic reactions (Panickar and Norenberg, 2005) and microglial activation (Abraham and Lazar, 2000), etc. All these phenomena provide the sounded evidence that 2VO rat model is a valuable animal model to study the molecular mechanism of CBH associated diseases. In this protocol, we describe the detailed procedures of how to successfully establish a rodent model of CBH by permanently ligating the bilateral common carotid arteries of rat. And this protocol was based on and modified from the previously published paper: Kumaran et al., 2008 .
Materials and Reagents
75% alcohol cotton ball (Bettering, catalog number: BY ACB)
22 mm (3/8 circle) surgical needle with suture (Foosin Medical Supplies, Weigao, model: 300 series) (Figure 1B)
1 ml syringe for anesthetizing (Xi’an Sentansha Medical Investment Management, catalog number: STS-SY003-#0037) (Figure 1H)
3-0 silk suture (Jinhuan, model: 3-0) (Figure 1A)
Medical absorbent cotton (Zhushi Parmaceutical Group, model: ZS-HM019)
-
Animals
Male Sprague-Dawley rats (weight 280-300 g, usually 4-5 months old, obtained from the Animal Center of the Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China) were housed at 23 ± 1 °C with 55 ± 5% of humidity and maintained on 12 h dark/light artificial cycle (lights on at 7:00 AM) with food and water available ad libitum. All procedures regarding the animal experiments were approved by the Institutional Animal Care and Use Committee at Harbin Medical University (No. HMUIRB-2008-06) and the Institute of Laboratory Animal Science of China (A5655-01)
0.9% sodium chloride (Qingdao Fraken International Trading, model: High Quality 0.9% Compound Sodium Chloride Injection)
Chloral hydrate (Aladdin, catalog number: C104202)
Gentamycin sulfate, 50 mg/ml solution, sterile (Sangon Biotech, catalog number: B540724)
10% chloral hydrate solution (see Recipes)
20 mg/ml gentamycin sulfate solution (see Recipes)
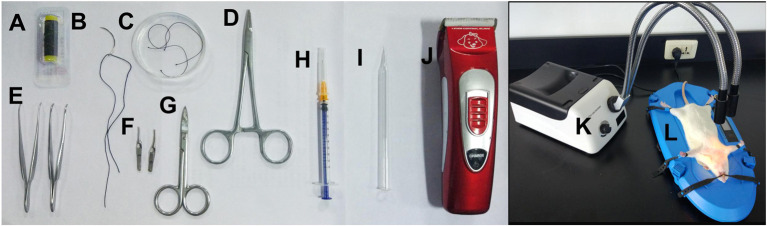
Figure 1. The materials and equipment.
A. 3-0 silk suture; B. Surgical needle with suture; C. Glass dish with the sterile 0.9% sodium chloride; D. Needle holder; E. Forceps; F. Artery clips; G. Scissors; H. 1 ml syringe for anesthetizing; I. Glass hook; J. Electric animal shaver; K. Fiber optic illuminator; L. Electric heating pad.
Equipment
Glass dish (Canfort Laboratory and Education Supplies, catalog number: LG075) (Figure 1C)
Needle holder (Sklar Surgery Instrument, model: 5-1/4”, catalog number: 21-8001) (Figure 1D)
Forceps (Fine Science Tools, catalog number: 11009-13) (Figure 1E)
Artery clips (Nut Link International, model: Approximator Clamps) (Figure 1F)
Scissors (Fine Science Tools, catalog number: 14068-12) (Figure 1G)
Glass hook (Figure 1I)
Electric animal shaver (Yiwu Kemei Electric Appliances, model: KM-970) (Figure 1J)
Fiber optic illuminator (Mineralogical Research, catalog number: MA312101) (Figure 1K)
-
Electric heating pad for pet (Dongxiyi, catalog number: wi95919) (Figure 1L)
Notes: Before the surgery, all the metal instruments should be sterilized at high temperature, 200 °C.
Procedure
-
Anesthesia
After fasting 12 h, the rat is anesthetized with 10% chloral hydrate (300 mg/kg, see Recipes) by intraperitoneal injection and the rat should reach surgical anesthesia within 5-10 min.
Note: Depth of anesthesia is assessed by corneal blink and tail-pinch reflexes. Surgery should not be operated until the rat has reached full anesthesia. Anesthesia should be maintained at least for 1 h to complete all surgical procedures.
-
Expose the common carotid artery of rat (Video 1).
Note: To maintain body temperature, it is recommended to place the rat on a heating pad (37 °C) during procedures.
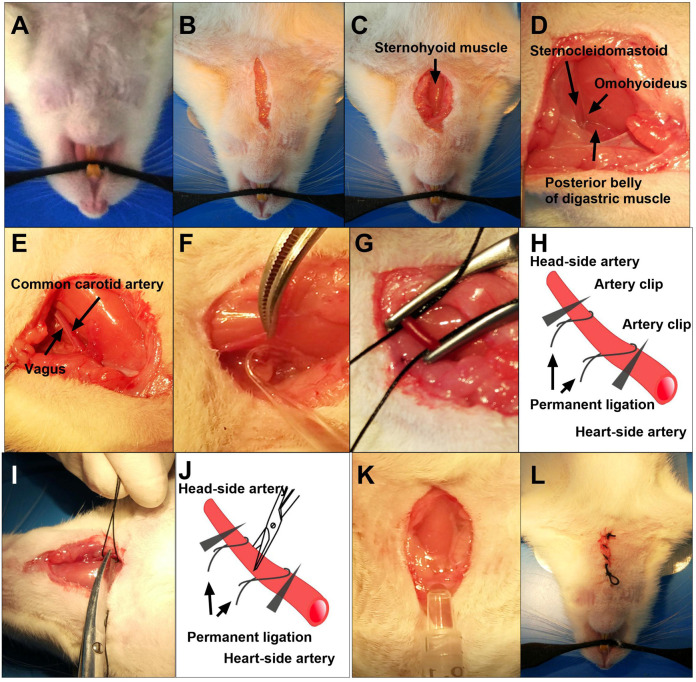
Mount the rat onto an electric heating pad and clean the fur around neck using an electric shaver. (Figure 2A)
Sterilize neck skin using 75% alcohol cotton ball and then cut a 2-cm incision above the manubrium along the anterior midline of the neck. (Figure 2B)
Carefully blunted dissect hypoderm and expose the sternohyoid muscle. (Figure 2C)
-
Carefully blunted dissect the fascia in order to expose the carotid triangle muscle structure that is consist of posterior belly of digastric muscle, omohyoideus and sternocleidomastoid, and then vertically separate omohyoideus to finally expose the sheath for crucial vessels and nerves. (Figure 2D)
Note: Within the exposed triangle area, two important vessels and nerve trunks could be identified, including internal vein and common carotid artery with their tributaries, and Vagus and aortic depressor nerve (50-80 μm varying upon the age and gender) that commonly located between carotid artery and Vagus. The dark red pulse-free internal vein mostly covered by sternocleidomastoid muscle is located outside the red pulsing common carotid artery. So they can be easily distinguished by the color and pulse. Importantly, Careful attention would be necessary for successful surgery during the blunted dissection to avoid damaging both nerves.
-
Separate the common carotid artery from the Vagus and aortic depressor nerve. (Video 2)
Carefully peel off the sheath around the common carotid artery. (Figure 2E)
-
Slightly lift up the common carotid artery with one forceps and gently separate vagal nerve from the common carotid artery using the glass hook. (Figure 2F)
Note: More attention should be paid to avoid the damage of the Vagus as well as aortic depressor never, as the damage can induce vegetative nerve functional disturbance and increase mortality rate.
-
Permanently ligate or cut off the common carotid artery. (Video 3)
Clamp the common carotid artery using two artery clips at the heart and head side each to avoid bleeding. (Figure 2G)
Two 3-0 silk sutures (immersed in the 0.9% sterile sodium chloride) are placed beneath the common carotid artery with the help of forceps and right in between the clips (Figures 2G and 2H). For the permanent ligation of the common carotid artery, silk sutures are tightened up and then the common carotid artery is cut off in between the two ligated silk sutures (Figures 2I and 2J).
Gently take off the artery clips.
During the surgical procedures, the medical absorbent cotton would be necessary to remove any potential bleeding and exudates to reduce the chance of postoperative infection maximally.
Perform the same procedures on the contralateral common carotid artery. (Video 4)
-
Postoperative caring
After the surgical procedures, all the anterior cervical muscles should be back to their original location.
To avoid potential postoperative infection, the wounds should be washed before closing by 20 mg/ml gentamycin sulfate solution and the redundant solution has to be removed completely. (Figure 2K) (Video 5)
Close the wounds. (Figure 2L)
Place the rat back to the cage, and use a warm illuminant to keep a stable temperature until the rat recovers from anesthesia that may take at least 1-2 h after the surgery.
Video 1. Expose the common carotid artery of rat.
This video shows the exact surgical procedures of how to expose common carotid artery covered by omohyoideus.
Figure 2. The Procedures 2-6 of 2VO rat model.
A. Mount the rat to the heating pad; B. Cut a 2.0 cm incision above the manubrium; C. Expose the sternohyoid muscle; D. Expose the carotid triangle muscle structures that are consist of posterior belly of digastric muscle, omohyoideus and sternocleidomastoid; E. Carefully peel off the carotid sheath; F. Separate the Vagus from the common carotid artery; G. Permanently ligate the bilateral common carotid artery; H. Schematic diagram of permanent ligation of bilateral common carotid artery; I. Cut off the common carotid artery in between the two ligated silk sutures; J. Schematic diagram of cutting off the common carotid artery in between the two ligated silk sutures; K. Use 20 mg/ml gentamycin sulfate solution to avoid potential postoperative infection; L. Close the wounds.
Video 2. Separate the common carotid artery from the Vagus and aortic depressor nerve.
Video 3. Permanently ligate or cut off the common carotid artery.
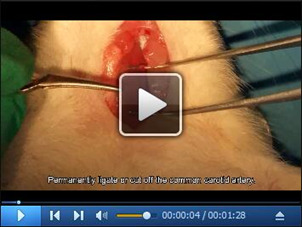
Video 4. Permanently ligate the contralateral carotid artery.
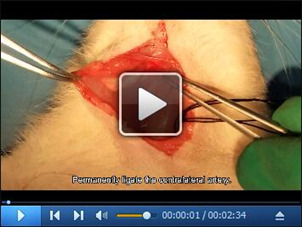
Video 5. Protect the wounds from infection.
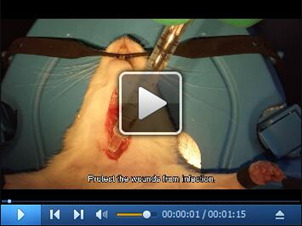
Notes
Timing: All the surgical procedures need to be finished within 5 min after the rat is completely relaxed, which will significantly reduce the chance of infection.
Selection of animals: Rats are considered to be the suitable rodent species for the 2VO operation because rats can survive from the severe ischemia by their complete circle of Willis, which provides constant reduction of blood flow after the 2VO. In other words, the Willis’ circle in gerbils or most strains of mice are under developed. It is worth mentioning that, in our group, SD rather than Wistar rats have been selected to establish the 2VO animal model simply because of a higher mortality while using Wistar compared with SD rats that have relatively large circle of Willis. Additionally, the body weight of rat is a significant factor. In our experience, an ideal body weight is between 280-300 g, and it will definitely increase the risk of mortality during and after procedures if the body weight is lower than 280 g or higher than 300 g.
Selection of silk suture size: The most suitable size of suture is 3-0 to 5-0, because the diameter of silk suture less than 3-0 may damage the artery while ligation resulting in bleeding and the diameter of silk suture larger than 5-0 may cause incompletely ligation or re-perfusion due largely to the loosen of ligated sutures and finally result in the failure to induce a CBH status.
Protection of Vagus and aortic depressor nerve: The damage of vagal and aortic depressor nerve during operation will induce vegetative nerve functional disturbance of rats and increase the possibility of death.
Gentle performance: Gentle and quick performance is essential here. A rough operation can easily induce severe bleeding and result in the death of rats.
Recipes
-
10% chloral hydrate solution
5 g chloral hydrate is dissolved in 50 ml physiological saline solution
Store at 37 °C
-
20 mg/ml gentamycin sulfate solution
4 ml gentamycin sulfate (50 mg/ml) is diluted with sterile sodium chloride (0.9%) up to 10 ml
Store at -20 °C
Acknowledgments
Thanks for the support of the Natural Science Foundation of China (81271207, 81070882, 81471115, 81671052). This protocol was based on and modified from the previously published paper: Kumaran et al. (2008, Neuroscience, 155: 626-639). We want to thank Shuai Zhang for shooting the videos and Biddyut Das for words editing.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Abraham H. and Lazar G.(2000). Early microglial reaction following mild forebrain ischemia induced by common carotid artery occlusion in rats. Brain Res 862(1-2): 63-73. [DOI] [PubMed] [Google Scholar]
- 2. Chen X., Jiang X. M., Zhao L. J., Sun L. L., Yan M. L., Tian Y., Zhang S., Duan M. J., Zhao H. M., Li W. R., Hao Y. Y., Wang L. B., Xiong Q. J. and Ai J.(2017). MicroRNA-195 prevents dendritic degeneration and neuron death in rats following chronic brain hypoperfusion. Cell Death Dis 8(6): e2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eklof B. and Siesjo B. K.(1972). The effect of bilateral carotid artery ligation upon the blood flow and the energy state of the rat brain. Acta Physiol Scand 86(2): 155-165. [DOI] [PubMed] [Google Scholar]
- 4. Farkas E., Institoris A., Domoki F., Mihaly A., Luiten P. G. and Bari F.(2004). Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusion-related learning dysfunction and brain damage after carotid artery occlusion. Brain Res 1008(2): 252-260. [DOI] [PubMed] [Google Scholar]
- 5. Farkas E., Luiten P. G. and Bari F.(2007). Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54(1): 162-180. [DOI] [PubMed] [Google Scholar]
- 6. Gorelick P. B., Scuteri A., Black S. E., Decarli C., Greenberg S. M., Iadecola C., Launer L. J., Laurent S., Lopez O. L., Nyenhuis D., Petersen R. C., Schneider J. A., Tzourio C., Arnett D. K., Bennett D. A., Chui H. C., Higashida R. T., Lindquist R., Nilsson P. M. and Roman G. C., et al.(2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42(9): 2672-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumaran D., Udayabanu M., Kumar M., Aneja R. and Katyal A.(2008). Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience 155(3): 626-639. [DOI] [PubMed] [Google Scholar]
- 8. Liu H. X., Zhang J. J., Zheng P. and Zhang Y.(2005). Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139(1): 169-177. [DOI] [PubMed] [Google Scholar]
- 9. Ohta H., Nishikawa H., Kimura H., Anayama H. and Miyamoto M.(1997). Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rats. Neuroscience 79(4): 1039-1050. [DOI] [PubMed] [Google Scholar]
- 10. Otori T., Katsumata T., Muramatsu H., Kashiwagi F., Katayama Y. and Terashi A.(2003). Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol 30(4): 266-272. [DOI] [PubMed] [Google Scholar]
- 11. Panickar K.S. and Norenberg M. D.(2005). Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 50(4): 287-98. [DOI] [PubMed] [Google Scholar]
- 12. Plaschke K.(2005). Aspects of ageing in chronic cerebral oligaemia. Mechanisms of degeneration and compensation in rat models. J Neural Transm(Vienna) 112(3): 393-413. [DOI] [PubMed] [Google Scholar]
- 13. Ruitenberg A., den Heijer T., Bakker S. L., van Swieten J. C., Koudstaal P. J., Hofman A. and Breteler M. M.(2005). Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57(6): 789-794. [DOI] [PubMed] [Google Scholar]
- 14. Sopala M. and Danysz W.(2001). Chronic cerebral hypoperfusion in the rat enhances age-related deficits in spatial memory. J Neural Transm(Vienna) 108(12): 1445-56. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka K., Ogawa N., Asanuma M., Kondo Y. and Nomura M.(1996). Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res 729(1): 55-65. [PubMed] [Google Scholar]
- 16. Tomimoto H., Ihara M., Wakita H., Ohtani R., Lin J. X., Akiguchi I., Kinoshita M. and Shibasaki H.(2003). Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol 106(6): 527-534. [DOI] [PubMed] [Google Scholar]