Abstract
Human endometrial stem cell/stromal cells (hEnSCs) are isolated from endometrium or menstrual blood and are recognized as a valuable cell type in tissue engineering and cell therapy. Furthermore, hEnSCs, which have CD90 (a mesenchymal marker), CD105 (endoglin), CD44, CD146 (endometrial stem cell markers) and lack CD31 (Endothelial marker), CD34 (hematopoietic marker) and CD133 on the cell surface, are a new source of mesenchymal stem/stromal cells. Additionally, these cells can be encapsulated into self-assembling peptide nanofibers as a 3D scaffold for applications in the treatment of neurodegenerative diseases. Here, we describe a protocol to isolate hEnSCs from endometrium or menstrual blood.
Keywords: Endometrial stem cell, Endometrial stromal cell, Menstrual blood, Cell isolation, Neural differentiation, Self-assembling peptide nanofiber, 3D cell culture
Background
Cell replacement therapy is being studied as a new strategy to manage neurodegenerative diseases such as Alzheimer’ s disease, Stroke, and spinal cord injury ( Tavakol et al., 2014b ; 2015; 2016a and 2016c). Human endometrial stem cell/stromal cells (hEnSCs) can be isolated from menstrual blood ( Azedi et al., 2014 and 2017) and endometrium. These cells have adipogenic ( Khanmohammadi et al., 2014 ), osteogenic ( Darzi et al., 2012 ), and chondrogenic ( Kazemnejad et al., 2012 ) differentiation potential. Furthermore, these cells may be used in combination with scaffolds as an ingredient of smart cell-scaffolds in tissue engineering applications ( Tavakol et al., 2014a ; 2016b and 2017). It is worth noting that cell compartments such as the exosome, the microvesicle and other components are considered safer than therapies that use the whole cell. hEnSCs are attractive candidates for cell therapy because they are immunosuppressive and have high clonogenicity (1.25%) potential. They also have advantages over other cell types. hEnSCs are better suited for cell therapy than embryonic stem cells because hESCs do not develop teratomas. Unlike mesenchymal stem cells, hEnSCs do not decrease proliferative potency in elderly people (Ebrahimi- Barough et al., 2013 ). Furthermore, another advantageous feature of hEnSCs is that they can be encapsulated into self-assembling peptide nanofibers and differentiated into neuronal cells ( Tavakol et al., 2014c ; 2016a; 2016c and 2017). This protocol describes a simple strategy to obtain higher yields of hEnSC from both menstrual blood and endometrium for in-vitro and in-vivo which can be used in numerous studies.
Materials and Reagents
Pipette tips: crystalline, yellow and blue (Gilson, catalog numbers: JHA004, JHA005 and JHA007)
SPL cell culture dish, 90 x 15 mm (SPL Life Sciences, catalog number: 11090)
Falcon tubes 15 ml (SPL Life Sciences, catalog number: 50115)
PluriStrainer® 70 µm (Pluriselect, catalog number: 43-50070)
Falcon cell strainer 40 µm (Corning, Falcon®, catalog number: 352340)
T25 culture flasks (SPL Life Sciences, catalog number: 70125)
T75 culture flasks (SPL Life Sciences, catalog number: 70175)
Diva cups (Diva International, Lunette, Finland)
Falcon tubes 50 ml (SPL Life Sciences, catalog number: 50050)
Endometrium
Menstrual blood
Ficoll-Hypaque (GE Healthcare, Amersham, catalog number: 17-5442-02)
FITC-conjugated anti CD105 (Abcam, catalog number: ab18278)
Allophycocyanin-conjugated anti-CD44 antibody (Abcam, catalog number: ab81424)
Anti-CD34 antibody [EP373Y] (Abcam, catalog number: ab81289)
Anti-CD133 antibody (Abcam, catalog number: ab19898)
Penicillin/Streptomycin (Pen/Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
Amphotrypsin B solution (Sigma-Aldrich, catalog number: A2942)
Collagenase I (Thermo Fisher Scientific, GibcoTM, catalog number: 17100017)
Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: 806552)
DMEM-F12 (Thermo Fisher Scientific, GibcoTM, catalog number: 11330057)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number:10270106)
Trypsin-EDTA 0.25% (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
Glutaraldehyde, 25% Aqueous Solution (Merck, catalog number: 354400)
Ethanol (Merck, catalog number: 818760)
Fungizone (Thermo Fisher Scientific, GibcoTM, catalog number: 15290018)
Ethylenediaminetetraacetic acid tetrasodium salt dehydrate (EDTA) (Sigma-Aldrich, catalog number: E6511)
Non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number:11140035)
L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081)
Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 24020117)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2058)
Isolation media for endometrium (see Recipes)
Plating media (see Recipes)
Basal media for the rest of passage except first passage (see Recipes)
Isolation media for menstrual blood (see Recipes)
Collagenase I (see Recipes)
Equipment
Scalpel handle with scalpel (Fine Science Tools, catalog numbers: 10011-00, 10003-12)
Standard sterile forceps (BYDAND, catalog number: BSU101)
Sterile pipette (SPL LIFE SICENCES, catalog number: 91005)
37 °C, 5% CO2 cell culture incubator (New Brunswic Scientific, model: CO-150)
Centrifuge (Eppendorf, model: 5810 R)
Inverted microscope (Olympus, model: IX51)
Refrigerator (Pars, model: 1300)
Software
FlowJo 7.6.1 software (FlowJo, LLC; https://www.flowjo.com)
Procedure
Note: Biopsy of the endometrium must be performed by an expert surgeon in accordance with local ethics policies and guidelines.
-
To isolate hEnSCs from the endometrium, the following steps are performed
-
Endometrium specimen biopsy
The biopsy should be taken from:
Healthy females
Age: between 20-35 years of age
-
Time: Endometrium biopsy and menstrual blood are isolated on menstrual cycle days 19-24 and 2-4, respectively.
The biopsy should not be taken from:
Subjects with non-endometrial benign pathological conditions such as polyps, hyperplasia, or cancer.
Subjects that have taken exogenous hormones such as GnRH, progesterone and other hormones for several months prior to the date of the biopsy.
Subjects with endometriosis or any pathological condition in the uterus.
Subjects that have had a device during the past 3 months.
-
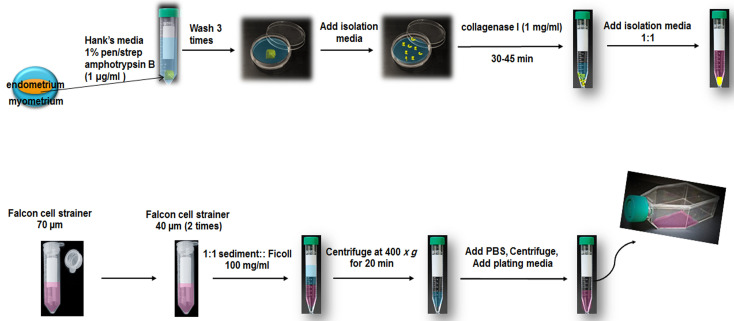
It is recommended that the size of the endometrium specimen be larger than 0.5 x 1 x 1 cm3. The endometrium specimen should be placed in pre-warmed (37 °C) Hank’s media containing 1% Pen/Strep and 1 μg/ml amphotrypsin B for transport. We recommend that the biopsy should be delivered to the laboratory within 2 h.
Note: Use pre-warmed media (37 °C) for all steps.
Transfer the specimen to a sterile 10 cm Petri dish using standard sterile forceps.
Add fresh, pre-warmed Hank’s media containing 1% Pen/Strep and 1 μg/ml amphotrypsin B.
Dissect myometrium from the endometrium and discard blood and mucus.
Wash 3 times with Hank’s media (add 3-4 ml media and carefully wash tissue and discard media with a sterile pipette).
-
Transfer the specimen to another sterile Petri dish with sterile forceps. Add isolation media (avoid letting the specimen dry out) and chop it with a sharp scalpel. Cut the specimens into 1-2 mm pieces.
Notes:
To avoid cell damage, make vertical cuts with the scalpel.
Do not let the specimen dry.
Transfer the cut specimen into sterile 15 ml Falcon tubes with standard sterile forceps. Add collagenase I (1 mg/ml) and incubate at 37 °C (cell incubator or water bath) for 30-45 min. Invert the falcon tube every 5 min.
When cells are disassociated from the specimen (it maybe takes 2 h based on the size of the specimen (1 x 1 x 0.5 cm3), add pre-warmed (37 °C) sterile isolation media (1:2 collagenase I:isolation media) to neutralized collagenase I.
Pass it through a 70 µm Falcon cell strainer once. Pass it through a 40 µm Falcon cell strainer twice to remove glandular epithelial components. Centrifuge the cells that pass through the strainer at 300 × g for 10 min. However, the centrifugation step may be performed or omitted.
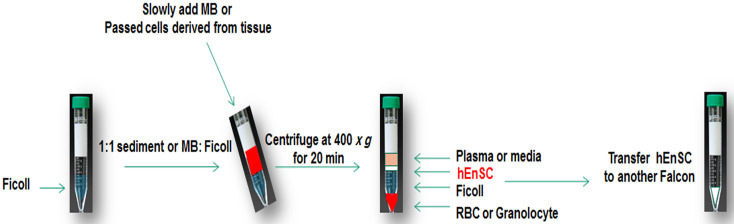
Slowly add the passed cells (1:1 sediment:Ficoll [100 mg/ml]) on Ficoll in a 15 ml Falcon tube at room temperature (Figure 1).
Centrifuge at 400 × g for 20 min.
Discard sediment containing red blood cells and take the turbid interface phase of Ficoll and the cell culture media.
Add 4 ml pre-warmed PBS to the interface phase and centrifuge at 100 × g for 10 min.
Discard the supernatant, add 1 ml plating media (see Recipes) into the sediment and transfer them to T25 culture flasks. Add an additional 2.5 ml of plating media containing DMEM-F12, 15% FBS, 1% Pen/Strep. Incubate for 24 h in the cell incubator. A total volume of 3.5 ml cell culture media in T25 culture flasks for the first day can increase the frequency of cells adhering to the flask. Note that larger volumes may result in fewer cells adhering to the flask.
The next day, slowly add 3 ml more of plating media and keep it in the cell incubator for 1 week. Do not shake it or disturb it each day.
When cells are 90% confluent, wash them with pre-warmed PBS, discard PBS, then add 0.25% trypsin-EDTA (1 ml). Incubate in the cell incubator for 5 min. After the cells dissociate from the flask, transfer the cells to a 15 ml Falcon and add 1 ml DMEM-F12 supplanted with 10% FBS, 1% Pen/Strep. Centrifuge at 300 × g for 10 min.
Add cell sediment to another T25 culture flasks (100,000 cells/ml) or T75 culture flasks (300,000 cells/ml) and for the rest of cell passages use 5 and 9 ml DMEM-F12 supplemented with 10% FBS, 1% Pene/Strep, respectively (Figure 2).
After 3 passages you may scan cells by scanning electron microscopy (SEM) (Figure 3).
Cell preparation for SEM:
Cells are rinsed with PBS and then fixed in 2% glutaraldehyde for 2 h.
Rinse with PBS.
Incubate in 50% ethanol for 10 min.
Incubate in 70% ethanol for 10 min.
Incubate in 80% ethanol for 10 min.
Incubate in 95% ethanol for 10 min (twice).
Incubate in 100% ethanol for 10 min (twice)
-
Freeze dry cells for 3 h.
Note: You may sort cells as a CD146 by cell sorter (MAGS and etc…) to be sure that cell population is purified as endometrial stem cell.
-
-
To isolate hEnSCs from menstrual blood, the following steps are performed (Figure 4)
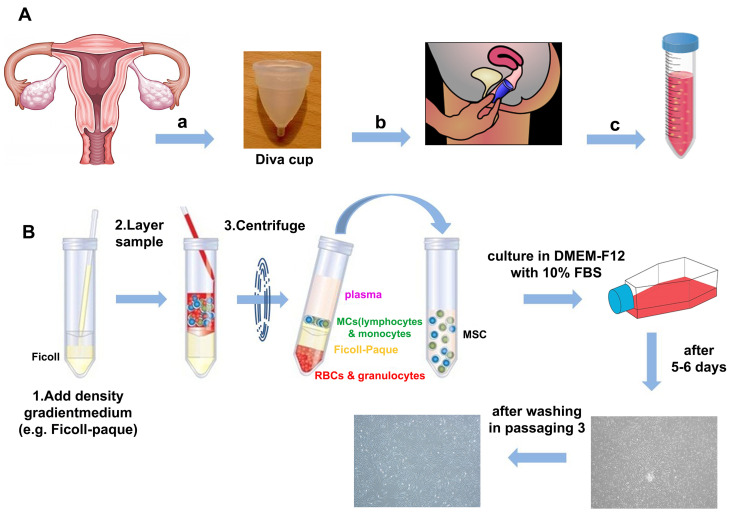
Inclusion criteria for menstrual blood (MB) are healthy females without vaginal discharge or infection, negative for HBV and HCV infection, and age ranges between 20 and 35 years old.
Collect 5 ml MB with sterile Diva cups and decant into the isolation buffer 1:4 (see Recipes) in a 50 ml Falcon tube containing 2.5 µg/ml fungizone, 1% Pen/Strep and 0.5 mM EDTA in 20 ml phosphate buffered saline (PBS).
Slowly add 1:1 blood sample (overlaid) to the Ficoll and centrifuge for 20 min at 400 × g.
Discard sediment (pellet) containing the red blood cells and take turbid interface phase of Ficoll and cell culture media (Figure 1).
Add 3 ml PBS to the interface phase and centrifuge at 100 × g for 10 min.
The cell pellet is suspended in 1 ml DMEM-F12 supplemented with 10% FBS, 0.1 mM non-essential amino acids and 2 mM L-glutamine.
Transfer the cell suspension to T25 culture flasks and add extra 2.5 ml of plating media. Incubate for 24 h at 37 °C in a humidified 5% CO2.
-
Slowly add 3 ml plating media to the flask after 24 h.
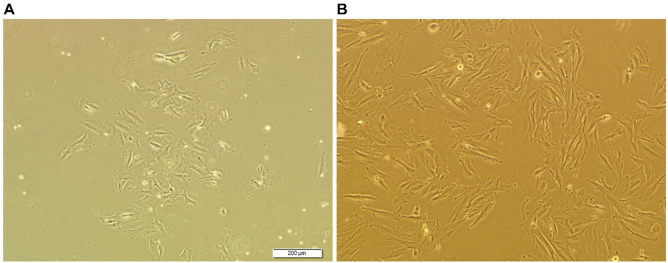
After 2 days incubation, non-adherent cells are washed away (the cell culture media is removed from the flask and 3 ml PBS is added to the flask and then 5 ml media is added to the flask). The adherent cell population (Figure 5A) and the media are replaced with media containing DMEM-F12, 1% Pen/Strep and 10% FBS.
The culture media is refreshed every 3-4 days.
When cells reach 90% confluence, they are passaged using trypsin/EDTA (0.25%) (Figure 5B).
Figure 1. hEnSC separation by Ficoll.
Figure 2. Schematic of isolation of hEnSCs from endometrium.
Figure 3. SEM image of endometrial stem cell derived from endometrium.
Figure 4. Isolation of hEnSCs from menstrual blood.
A. Sample collection. Sample collection of menstrual blood during menstrual period in day 2 and 3 with Diva cup. This device can be inserted in vaginal canal by donors and then, blood is transported into Falcon. B. Ficoll separation.
Figure 5. Microscopic image of MenSCs.
A. 5 days after isolation; B. After passage 1.
Data analysis
To confirm that endometrial stem cells are isolated after the third passage, cells can be characterized by flow cytometry for surface markers: CD90 (mesenchymal marker), CD105 (endoglin), CD44, CD146 (endometrial stem cell markers), CD31 (Endothelial marker), CD34 (hematopoietic marker). They should be negative for these surface markers: CD31, CD34 and CD133 while are positive for these surface markers: CD90, CD105, CD44 and CD146 (Figure 1: Ebrahimi- Barough et al., 2013 and Figure 2: Mobarakeh et al., 2012 ).
Wash cells with PBS, discard PBS, add 0.25% trypsin-EDTA, centrifuge floating cells.
Wash with HBSS + 2% BSA twice and incubate with CD90, CD44, CD146, CD34, CD133 antibodies at defined concentrations recommended by their respective suppliers.
Incubate for 20 min in the dark at room temperature.
Asses by flow cytometry. If the stain is green, it may measure in FL1 channel and if it is a red stain, it may measure in FL2 channel. Besides, vertical column may select as a histogram. The gating strategy is based on isotype.
Analyze flow cytometry data with FlowJo software.
Notes
Cells may be destroyed if cutting with the scalpel is un-carefully performed or not cut vertically. However, movement of the flask every day may decrease cell density and not allow them to completely adhere. Data analysis can be found at cited references. To isolate hEnSC from menstrual blood, you must be careful to collect the sample in a sterile manner if you do not have Diva cups. During the time you collect and transfer specimen to the laboratory, it may be kept at 4 °C in a refrigerator for 24 h. However, it is recommended that the sample should be immediately transferred to the laboratory to increase cell survival.
Recipes
-
Isolation media for endometrium
Pre-warmed Hank’s media supplemented with:
5% FBS
1% Pene/Strep
1 μg/ml amphotrypsin B
-
Plating media
Pre-warmed DMEM-F12
15% FBS
1% Pen/Strep
1% glutamine
1 μg/ml amphotrypsin B
-
Basal media for the rest of passage except first passage
Pre-warmed DMEM-F12
10% FBS
1% Pene/Strep
1% glutamine
-
Isolation media for menstrual blood
2.5 µg/ml fungizone
1% Pen/Strep
0.5 mM EDTA
Phosphate buffered saline (PBS)
-
Collagenase I
1 mg collagenase is dissolved in 1 ml Hank’s media
Acknowledgments
We are very thankful to Dr. Somayeh Ebrahimi borough and Dr. Roya Karimi for their kindness help in cell isolation and characterization. Our previous works the presented protocol adapted from were funded by ‘grant numbers: 92-03-61-21215 and 92-02-61-22861’. Autours declare that there was no conflicts of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Azedi F., Kazemnejad S., Zarnani A. H., Behzadi G., Vasei M., Khanmohammadi M., Khanjani S., Edalatkhah H. and Lakpour N.(2014). Differentiation potential of menstrual blood- versus bone marrow-stem cells into glial-like cells. Cell Biol Int 38(5): 615-624. [DOI] [PubMed] [Google Scholar]
- 2. Azedi F., Kazemnejad S., Zarnani A. H., Soleimani M., Shojaei A. and Arasteh S.(2017). Comparative capability of menstrual blood versus bone marrow derived stem cells in neural differentiation. Mol Biol Rep 44(1): 169-182. [DOI] [PubMed] [Google Scholar]
- 3. Darzi S., Zarnani A. H., Jeddi-Tehrani M., Entezami K., Mirzadegan E., Akhondi M. M., Talebi S., Khanmohammadi M. and Kazemnejad S.(2012). Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng Part A 18(15-16): 1720-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebrahimi-Barough S., Kouchesfehani H. M., Ai J., Mahmoodinia M., Tavakol S. and Massumi M.(2013). Programming of human endometrial-derived stromal cells(EnSCs) into pre-oligodendrocyte cells by overexpression of miR-219. Neurosci Lett 537: 65-70. [DOI] [PubMed] [Google Scholar]
- 5. Kazemnejad S., Akhondi M. M., Soleimani M., Zarnani A. H., Khanmohammadi M., Darzi S. and Alimoghadam K.(2012). Characterization and chondrogenic differentiation of menstrual blood-derived stem cells on a nanofibrous scaffold. Int J Artif Organs 35(1): 55-66. [DOI] [PubMed] [Google Scholar]
- 6. Khanmohammadi M., Khanjani S., Edalatkhah H., Zarnani A. H., Heidari-Vala H., Soleimani M., Alimoghaddam K. and Kazemnejad S.(2014). Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif 47(6): 615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mobarakeh Z. T., Ai J., Yazdani F., Sorkhabadi S. M., Ghanbari Z., Javidan A. N., Mortazavi-Tabatabaei S. A., Massumi M. and Barough S. E.(2012). Human endometrial stem cells as a new source for programming to neural cells. Cell Biol Int Rep(2010) 19(1): e00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tavakol S., Aligholi H., Eshaghabadi A., Mousavi M. M., Ai J. and Rezayat M.(2014). Investigation on the motor recovery effect of a self-assembling nonofiber in the spinal cord injury model in rat. Shefaye Khatam 2: 41-46. [Google Scholar]
- 9. Tavakol S., Aligholi H., Gorji A., Eshaghabadi A., Hoveizi E., Tavakol B., Rezayat S. M. and Ai J.(2014). Thermogel nanofiber induces human endometrial-derived stromal cells to neural differentiation: In vitro and in vivo studies in rat . J Biomed Mater Res A 102(12): 4590-4597. [DOI] [PubMed] [Google Scholar]
- 10. Tavakol S., Modarres Mousavi S. M., Massumi M., Amani A., Rezayat S. M. and Ai J.(2015). The effect of Noggin supplementation in Matrigel nanofiber-based cell culture system for derivation of neural-like cells from human endometrial-derived stromal cells. J Biomed Mater Res A 103(1): 1-7. [DOI] [PubMed] [Google Scholar]
- 11. Tavakol S., Mousavi S. M. M., Tavakol B., Hoveizi E., Ai J. and Sorkhabadi S. M. R.(2017). Mechano-transduction signals derived from self-assembling peptide nanofibers containing long motif of laminin influence neurogenesis in in-vitro and in-vivo . Mol Neurobiol 54(4): 2483-2496. [DOI] [PubMed] [Google Scholar]
- 12. Tavakol S., Musavi S. M., Tavakol B., Hoveizi E., Ai J. and Rezayat S. M.(2016). Noggin along with a self-assembling peptide nanofiber containing long motif of laminin induces tyrosine hydroxylase gene expression. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- 13. Tavakol S., Saber R., Hoveizi E., Aligholi H., Ai J. and Rezayat S. M.(2014). W6: Self-assembling peptide nanofiber containing biologic motif induces neural differentiation, tubulin polymerization and neurogenesis in-vitro, ex-vivo and in-vivo studies . Shefaye Khatam 2: 49-49. [DOI] [PubMed] [Google Scholar]
- 14. Tavakol S., Saber R., Hoveizi E., Aligholi H., Ai J. and Rezayat S. M.(2016). Chimeric self-assembling nanofiber containing bone marrow homing peptide's motif induces motor neuron recovery in animal model of chronic spinal cord injury; an in vitro and in vivo investigation . Mol Neurobiol 53(5): 3298-3308. [DOI] [PubMed] [Google Scholar]
- 15. Tavakol S., Saber R., Hoveizi E., Tavakol B., Aligholi H., Ai J. and Rezayat S. M.(2016). Self-assembling peptide nanofiber containing long motif of laminin induces neural differentiation, tubulin polymerization, and neurogenesis: in vitro, ex vivo, and in vivo studies . Mol Neurobiol 53(8): 5288-5299. [DOI] [PubMed] [Google Scholar]