Abstract
This experiment was performed to verify whether dietary heat-killed Lactobacillus rhamnosus (LR) improves growth performance and modulates immune responses of weaned pigs. Ninety-six weaned pigs ([Landrace × Yorkshire] × Duroc; 6.95 ± 0.25 kg body weight [BW]; 28 d old) were randomly allocated to four treatments: 1) a basal diet without heat-killed LR (CON), 2) T1 (CON with 0.1% heat-killed LR), 3) T2 (CON with 0.2% heat-killed LR), and 4) T3 (CON with 0.4% heat-killed LR). Each treatment had six pens with four pigs (6 replicates per treatment) in a randomized completely block design. The heat-killed LR used in this study contained 1 × 109 FU/g of LR in a commercial product. Pigs were fed each treatment for four weeks using a two-phase feeding program to measure growth performance and frequency of diarrhea. During the last week of this study, all diets contained 0.2% chromic oxide as an indigestible marker. Fecal sampling was performed through rectal palpation for the consecutive three days after the four adaptation days to measure apparent total tract digestibility (ATTD) of dry matter, crude protein, and gross energy (GE). Blood sampling was also performed on day 1, 3, 7, and 14 after weaning to measure immune responses such as serum tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), C-reactive protein (CRP), and cortisol. The heat-killed LR increased (p < 0.05) growth rate, feed efficiency, and ATTD of GE for overall experimental period compared with CON, but reduced (p < 0.05) post-weaning diarrhea. In addition, pigs fed diets contained heat-killed had lower concentrations of serum TNF-α (d 7; p < 0.05), TGF-β1 (d 7; p < 0.10), and cortisol (d 3 and 7; p < 0.05) than pigs fed CON. In conclusion, dietary heat-killed LR improved growth rate, modified immune responses of weaned pigs, and alleviated post-weaning diarrhea.
Keywords: Diarrhea, Growth performance, Heat-killed Lactobacillus rhamnosus, Immune responses, Inactivated probiotics, Weaned pigs
INTRODUCTION
The concept of probiotics is widely known as “live micro-organisms that confer a health benefit on the host when administered in adequate amounts” [1]. Probiotics are known to increase growth rate and nutrient utilization and to change immunity when it is used in pig diets [2–6]. However, when probiotics are added to animal feeds, there can be side effects, such as toxicity in the host caused by production of enterotoxins from micro-organisms included in the probiotics [5,7] and a risk of infection due to strong adhesion to the intestinal tract by probiotic micro-organism [8]. In addition, there is a concern to maintain the activity of probiotics and thus stability of probiotics is required in term of viability and metabolic and functional activities during shelf life [9].
Current research suggests that inactivated probiotics can have the same beneficial effects as probiotics [10–12]. Inactivated probiotics not only benefit the host by regulating immunity, suppressing pathogens through the increased intestine adhesion, and providing metabolites secreted from dead cells but also offer easier storage, longer product shelf life, and more convenient transportation [13,14]. Inactivated probiotics can also complement the risk and stability of probiotics [15]. Inactivated probiotics can be obtained by heat treatment methods and thus it is generally called heat-killed probiotics. Heat-killed probiotics can release components such as lipoteichoic acids, peptidoglycan, or exopolysaccharides (EPS) with immuno-regulating effects against pathogens [16].
Lactobacillus rhamnosus (LR) has been shown to increase growth rate of pre-weaning pigs, mitigate diarrhea incidence, and regulate immunity [17,18]. However, previous study reported LR isolated from humans reduced growth performance, increased diarrhea scores, and reduced immunoglobulins of the Escherichia coli challenged pigs [19]. On the other hand, previous studies showed heat-killed LR improved growth rate, feed application, and immunity and reduced stress of fish [20,21]. In addition, heat-killed LR can act as an immune regulator by reducing lipopolysaccharide-induced pro-inflammatory cytokines in the human placenta and increasing anti-inflammatory cytokines in infant rats [22,23]. However, there are limited information about dietary heat-killed LR in animal diets.
Therefore, the objective of present study was to evaluate dietary effects of heat-killed LR on growth performance, nutrient digestibility, and immune responses of weaned pigs.
MATERIALS AND METHODS
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, Daejeon, Korea (approval code: CNU-00832).
Inactivated probiotics
The LR stains in the study were isolated from pigs and cultured in MRS (DeMan-Rogosa-Sharpe) broth (BD DifcoTM, Franklin Lakes, NJ, USA) for 18 hours at 37°C in an anaerobic incubator before use. Heat-killed LR was processed by heating at 80°C for 30 min. The stain is a white flowable powder which contains 1 × 109 FU/g of EPS-cell particle (CJ CheilJedang Biotechnology Research Institute, Seoul, Korea).
Experimental design, animal, and diets
Ninety-six weaned pigs ([Landrace × Yorkshire] × Duroc; 6.95 ± 0.25 kg body weight [BW]; 28 d old) were randomly allotted to four treatments: 1) a basal diet without heat-killed LR (CON), 2) T1 (CON with 0.1% heat-killed LR), 3) T2 (CON with 0.2% heat-killed LR), and 4) T3 (CON with 0.4% heat-killed LR). Each treatment had six pens with four pigs (2 barrows and 2 gilts; 6 replicates per treatment) in a randomized completely block design (blocks: BW and sex). The different inclusion levels of heat-killed LR were deiced based on the results from previous pilot studies and producer’s recommendations. All diets were formulated to meet and exceed the nutrient requirements for weaned pigs [24] and were provided in a two-phase feeding program with each phase lasting two weeks (Table 1). All pigs had ad libitum access to feed and water.
Table 1. Composition of basal diet for weaned pigs (as-fed basis)1).
| Item | Phase Ⅰ | Phase Ⅱ |
|---|---|---|
| Ingredient (%) | ||
| Corn | 48.48 | 66.37 |
| Soybean meal (44%) | 19.10 | 19.50 |
| Whey powder | 15.00 | 5.00 |
| Fish meal | 6.00 | 3.00 |
| Spray-dried plasma protein | 4.00 | 3.00 |
| Lactose | 4.00 | - |
| Soybean oil | 1.00 | - |
| Dicalcium phosphate | 0.60 | 0.80 |
| Limestone | 0.70 | 0.80 |
| Salt | 0.20 | 0.20 |
| Vit-min premix2) | 0.50 | 0.50 |
| L-Lysine-HCl | 0.25 | 0.44 |
| DL-Methionine | 0.12 | 0.15 |
| L-Threonine | 0.05 | 0.14 |
| L-Tryptophan | - | 0.10 |
| Total | 100 | 100 |
| Calculated energy and nutrient contents (%) | ||
| Metabolizable energy (Mcal/kg) | 3.39 | 3.34 |
| Crude protein (%) | 21.66 | 19.63 |
| Calcium (%) | 0.80 | 0.71 |
| Phosphorus (%) | 0.70 | 0.62 |
| Lysine (%) | 1.53 | 1.43 |
Phase Ⅰ, 1 to 2 weeks (14 days); phase Ⅱ , 3 to 4 weeks (14 days).
Provide per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; D-pantothenic acid, 15 mg; nicotinic acid, 40 mg; choline, 400 mg; and vitamin B12, 12 μg, Fe, 90 mg from iron sulfate; Cu, 8.8 mg from copper sulfate; Zn, 100 mg from zinc oxide; Mn, 54 mg from manganese oxide; I, 0.35 mg from potassium iodide; Se, 0.30 mg from sodium selenite.
Data analysis and sample collection
Growth performance (average daily gain [ADG], average daily feed intake [ADFI], and feed efficiency [gain to feed ratio, G:F]) was calculated by measuring the weight of individual pigs and pen feeders on day 1, 14, and 28. Daily visual diarrhea scores of individual pigs (1 = hard and dry normal feces, 2 = well formed, 3 = moist feces, 4 = diarrhea, 5 = watery diarrhea) were recorded for 2 weeks after weaning. The frequency of diarrhea was calculated by counting pens per day with an average diarrhea score over 4 in each pen [25,26]. On day 1, 3, 7, and 14 of the experimental period, one pig was randomly chosen from each pen for blood collection. Whole blood and serum were collected from the jugular vein of pigs using EDTA and non-EDTA tubes, respectively (10 mL; BD Vacutainer Systems, Franklin Lakes, NJ, USA). The blood was kept at room temperature to coagulate and centrifuged to separate the serum at 3,000×g at 4°C for 15 min and stored at −20°C. Dietary treatments contained 0.2% chromic oxide as an indigestible marker for the last week of the experimental period. Fecal samples were collected by rectal palpation from randomly chosen one pig in each pen for the last 3–4 days of experiment after the 4 adaptation days and immediately stored at −20°C.
Sample analyses
Feed and fecal samples were dried in a drying oven for 72 h at 65°C and ground with a cyclone mill (1.0 mm screen; Foss Tecator cyclotec 1093, Foss, Hillerød, Denmark). Samples were analyzed for dry matter (DM), and crude protein (CP; N × 6.25) following the method of the AOAC [27]. Gross energy (GE) was analyzed by automatic bomb calorimeter (PARR 1261 Bomb Calorimeter, PARR Instrument, Moline, IL, USA). Chromium content was determined by the absorption spectrophotometer (Hitachi Z-5000 Absorption Spectrophotometer, Hitachi High-Tech, Tokyo, Japan) based on the method of Williams et al. [28]. Nutrient digestibility (apparent total tract digestibility [ATTD]) was calculated according to the index method formula [29].
The number of white blood cell (WBC) was determined by an eight-parameter hematology analyzer (Scil vet abcTM Animal Blood Counter, Scil Animal Care Company, Altorf, France). Immune responses such as serum tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), C-reactive protein (CRP), and cortisol were measured using ELISA kits according to the manufacturer’s protocol (TNF-α, TGF-β1, and CRP [Genorise Scientific, Berwyn, PA, USA]; cortisol [Cusabio Biotech, Wuhan, China]).
Statistical analyses
Data were subjected using the PROC MIXED procedure of SAS version 9.4 (SAS Institute, Cary, NC, USA) in a randomized completely block design (blocks: BW and sex). Experimental unit was the pen. Statistical model for growth performance, ATTD of nutrients, immune responses included dietary treatment as a fixed effect and blocks as random effects. Contrasts were performed to verify linear and quadratic effects of heat-killed LR. Frequency of diarrhea was analyzed by the chi-square test. Data were given as least square mean ± SEM. Values of p < 0.05 and 0.05 ≤ p ≤ 0.10 were considered as statistical significance and tendency, respectively.
RESULTS AND DISCUSSION
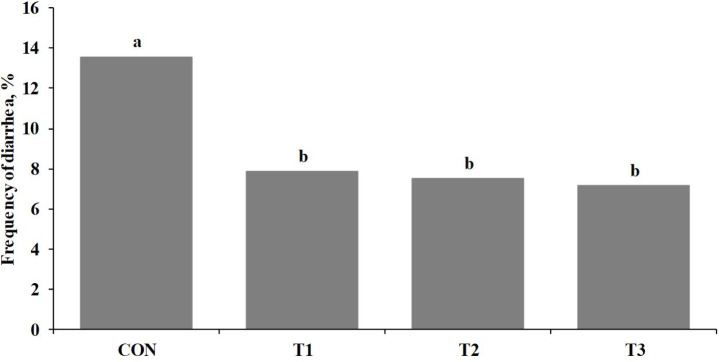
During the first two weeks of this study, the heat-killed LR group increased growth rate (ADG; p < 0.05) and feed efficiency (G:F; p ≤ 0.10) of weaned pigs compared with CON (Table 2). In addition, there were linear improvements (p < 0.05) of growth rate and feed efficiency of weaned pigs as the addition levels of heat-killed LR were increased in the diet (Table 2). Growth rate (ADG; p < 0.05) and feed efficiency (G:F; p < 0.05) of weaned pigs were increased in the heat-killed LR group for overall experimental period compared with those in CON (Table 2). Moreover, growth rate and feed efficiency of weaned pigs during overall experimental period were linearly improved (p < 0.05) when the inclusion levels of heat-killed LR were increased in the diet (Table 2). The heat-killed LR group decreased (p < 0.05) post-weaning diarrhea of weaned pigs compared with CON (Fig. 1). Previous studies also showed live and heat-killed Lactobacillus strains increased weight gain in pigs [30–32], mice [33], and fish [34] and mitigated diarrhea in human and animals [18,35,36]. When pigs are infected by enterotoxigenic E. coli (ETEC) after weaning, it increases cell fluid and electrolytes into the lumen and decreases absorbing them into intestinal cells by ETEC, leading post-weaning diarrhea [37]. In addition, the ETEC infection increases the paracellular permeability and further the penetration of pathogens and toxins into the lumen, thereby increasing the inflammatory immune responses and reducing the growth of animals [37]. The results from present study may be due to the mechanism of live and inactivated probiotics that prevent adherence of pathogens to the intestinal barrier and regulate the immune system of animals [14,16,32,38].
Table 2. Dietary effects of heat-killed LR on growth performance of weaned pigs1).
| Item | Treatments2) | SEM | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Diet | Linear | Quadratic | |||
| Day 1 to 14 | |||||||||
| Initial BW (kg) | 6.95 | 6.95 | 6.95 | 6.95 | 0.26 | 1.000 | 0.991 | 0.991 | |
| Final BW (kg) | 11.52 | 12.53 | 12.63 | 12.95 | 0.45 | 0.160 | 0.052 | 0.308 | |
| ADG (g/d) | 326a | 398b | 406c | 428d | 25.00 | 0.045 | 0.014 | 0.192 | |
| ADFI (g/d) | 522 | 519 | 519 | 520 | 22.00 | 1.000 | 0.945 | 0.937 | |
| G:F (g/g) | 0.625a | 0.768b | 0.782b | 0.824b | 0.045 | 0.069 | 0.027 | 0.204 | |
| Day 15 to 28 | |||||||||
| Initial BW (kg) | 11.52 | 12.53 | 12.63 | 12.95 | 0.45 | 0.160 | 0.052 | 0.308 | |
| Final BW (kg) | 18.96a | 20.13ab | 20.97b | 21.31b | 0.62 | 0.064 | 0.015 | 0.254 | |
| ADG (g/d) | 531 | 543 | 596 | 597 | 26.00 | 0.188 | 0.057 | 0.482 | |
| ADFI (g/d) | 931 | 924 | 930 | 926 | 41.00 | 0.999 | 0.954 | 0.980 | |
| G:F (g/g) | 0.571 | 0.588 | 0.641 | 0.645 | 0.030 | 0.290 | 0.092 | 0.571 | |
| Overall | |||||||||
| Initial BW (kg) | 6.95 | 6.95 | 6.95 | 6.95 | 0.26 | 1.000 | 0.991 | 0.991 | |
| Final BW (kg) | 18.96 | 20.13 | 20.97 | 21.31 | 0.62 | 0.064 | 0.015 | 0.254 | |
| ADG (g/d) | 428a | 471b | 501c | 513d | 16.00 | 0.005 | 0.001 | 0.110 | |
| ADFI (g/d) | 727 | 722 | 725 | 723 | 28.00 | 0.999 | 0.944 | 0.960 | |
| G:F (g/g) | 0.590a | 0.652a | 0.691ab | 0.710b | 0.030 | 0.022 | 0.005 | 0.193 | |
Each value is the mean value of 6 replicates (4 pigs/pen).
CON, control diet based on corn and soybean meal; T1, CON + 0.1% heat-killed LR; T2, CON + 0.2% heat-killed LR; T3, CON + 0.4% heat-killed LR.
Means with different superscripts in the row differ (p ≤ 0.10).
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, gain to feed ratio.
Fig. 1. Frequency of diarrhea of weaned pigs for the first 2 weeks after weaning.
Frequency of diarrhea (%) = (number of diarrhea if the diarrhea score over 4 / number of pen days) × 100. Data was subjected by the chi-square test. Diarrhea scores were 1 = hard and dry normal feces, 2 = well formed, 3 = moist feces, 4 = diarrhea, 5 = watery diarrhea. a,bMeans with different letters within each treatment differ (p < 0.05). CON, control diet based on corn and soybean meal; T1, CON + 0.1% heat-killed LR; T2, CON + 0.2% heat-killed LR; T3, CON + 0.4% heat-killed LR.
The ATTD of GE of weaned pigs was higher (p < 0.05) in the heat-killed LR group than that in CON (Table 3). There was also a linear improvement (p < 0.05) of ATTD of GE of weaned pigs related to the increased inclusion concentrations of heat-killed LR in the diet (Table 3). However, no differences were found on ATTD of DM and CP of weaned pigs among dietary treatments (Table 3). These results are similar to the results from previous studies using live probiotics [39,40]. The EPS produced by Lactobacillus strains exists for a long time in the intestine and promotes colonization of Lactobacillus in the intestinal tract, thereby alleviating lactose intolerance, modulation of immunity against pathogens, and reducing mutagenic enzymes such as β-glucuronidase, nitroreductase and chologlycine hydrolase [41]. In addition, Lactobacillus strains increased nutrient utilization by enzymes such as β-galactosidase that are potentially activated and helps nutrient digestion [40].
Table 3. Dietary effects of heat-killed LR on nutrient digestibility of weaned pigs1).
| Item | Treatments2) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Diet | Linear | Quadratic | ||
| DM (%) | 85.45 | 86.45 | 86.00 | 86.29 | 0.43 | 0.407 | 0.336 | 0.442 |
| CP (%) | 82.48 | 83.26 | 84.00 | 83.84 | 0.73 | 0.466 | 0.202 | 0.361 |
| GE (%) | 85.25a | 86.87ab | 87.29ab | 88.53b | 0.66 | 0.018 | 0.003 | 0.447 |
Each value is the mean value of 6 replicates (4 pigs/pen).
CON, control diet based on corn and soybean meal; T1, CON + 0.1% heat-killed LR; T2, CON + 0.2% heat-killed LR; T3, CON + 0.4% heat-killed LR.
Means with different superscripts in the row differ (p ≤ 0.10).
DM, dry matter; CP, crude protein; GE, gross energy.
Serum TNF-α of weaned pigs was decreased in the heat-killed LR group (d 7; p < 0.05) compared with that in CON (Table 4). In addition, there was a significant linear decrease (p < 0.05) in serum TNF-α of weaned pigs as the inclusion levels of heat-killed LR were increased in the diet (Table 4). The heat-killed LR decreased serum TGF-β1 (d 7; p ≤ 0.10) and cortisol (d 3 and 7; p < 0.05) of weaned pigs compared with CON (TTable 4). There was also a significant linear decrease (d 7; p ≤ 0.05) in serum cortisol of weaned pigs when the inclusion levels of heat-killed LR were increased in the diet (Table 4). However, there were no significant differences on the number of WBC and serum CRP among dietary treatments (Table 4). Previous studies reported the live and heat-killed Lactobacillus stains decreased TNF-α [22,42,43], TGF-β1 [44,45], and cortisol [46,47] in human and mice. Cortisol is a substance released in responses to acute stress. Generally, weaned pigs can face social, physical, and physiological stresses and contribute to increasing intestinal permeability and depressing immune responses [48]. It is known that live LR decreases cortisol by secreting neurotransmitters [48] and that the EPS produced by Lactobacillus strains shields adhesive surface polymers to prevent pathogens from sticking to intestinal barrier and regulates immune responses to inhibit the releases of pro-inflammatory cytokines. [43,49,50]. The CRP is the first acute-phase protein that can be used as an inflammatory response indicator and pro-inflammatory cytokines such as TNF-α and TGF-β1 are important inducers of CRP synthesis [51]. In present study, the CRP of weaned pigs was no significant difference among dietary treatments, but the concentration of CRP was decreased during the experimental period. Therefore, heat-killed LR may be thought to provide anti-inflammatory effects in the immune system [44,45].
Table 4. Dietary effects of heat-killed LR on immune responses of weaned pigs1).
| Item | Treatments2) | SEM | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Diet | Linear | Quadratic | |||
| The number of WBC (×103/µL) | |||||||||
| D1 | 17.93 | 18.12 | 17.79 | 18.13 | 1.31 | 0.997 | 0.944 | 0.932 | |
| D3 | 17.96 | 17.02 | 17.88 | 17.98 | 0.86 | 0.829 | 0.772 | 0.677 | |
| D7 | 16.99 | 15.59 | 16.20 | 16.13 | 0.61 | 0.454 | 0.553 | 0.321 | |
| D14 | 17.38 | 16.13 | 16.49 | 15.80 | 1.05 | 0.739 | 0.370 | 0.751 | |
| TNF-α (pg/mL) | |||||||||
| D1 | 1,135 | 1,189 | 1,095 | 1,052 | 174 | 0.952 | 0.652 | 0.889 | |
| D3 | 988 | 935 | 927 | 910 | 97 | 0.947 | 0.605 | 0.800 | |
| D7 | 849a | 600b | 542b | 523b | 57 | 0.002 | 0.001 | 0.018 | |
| D14 | 548 | 495 | 480 | 485 | 60 | 0.843 | 0.507 | 0.559 | |
| TGF-β1 (pg/mL) | |||||||||
| D1 | 1,404 | 1,397 | 1,473 | 1,469 | 119 | 0.949 | 0.639 | 0.892 | |
| D3 | 1,552 | 1,418 | 1,404 | 1,397 | 195 | 0.934 | 0.626 | 0.700 | |
| D7 | 775a | 602ab | 601ab | 578b | 59 | 0.096 | 0.053 | 0.146 | |
| D14 | 557 | 536 | 536 | 525 | 72 | 0.990 | 0.771 | 0.918 | |
| CRP (pg/mL) | |||||||||
| D1 | 8,436 | 8,363 | 8,468 | 8,248 | 288 | 0.950 | 0.677 | 0.803 | |
| D3 | 7,039 | 6,929 | 6,918 | 6,985 | 404 | 0.996 | 0.954 | 0.824 | |
| D7 | 6,081 | 5,918 | 5,895 | 5,602 | 521 | 0.930 | 0.523 | 0.969 | |
| D14 | 5,616 | 5,423 | 5,430 | 5,098 | 391 | 0.822 | 0.367 | 0.946 | |
| Cortisol (ng/mL) | |||||||||
| D1 | 8.94 | 9.03 | 8.98 | 8.75 | 0.43 | 0.970 | 0.708 | 0.762 | |
| D3 | 7.71a | 5.43b | 5.67b | 5.70b | 0.57 | 0.037 | 0.065 | 0.042 | |
| D7 | 7.25a | 5.58b | 5.56b | 5.44b | 0.43 | 0.023 | 0.020 | 0.052 | |
| D14 | 5.62 | 4.76 | 4.76 | 4.60 | 0.51 | 0.500 | 0.232 | 0.424 | |
Each value is the mean value of 6 replicates (4 pigs/pen).
CON, control diet based on corn and soybean meal; T1, CON + 0.1% heat-killed LR; T2, CON + 0.2% heat-killed LR; T3, CON + 0.4% heat-killed LR.
Means with different superscripts in the row differ (p ≤ 0.10).
WBC, white blood cell; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1; CRP, C-reactive protein.
CONCLUSION
Dietary heat-killed LR improved growth rate, modified immune responses of weaned pigs, and alleviated post-weaning diarrhea. Supplementation of dietary heat-killed LR in weaner diets may reduce post-weaning diarrhea by protecting the intestinal barrier from pathogens and attenuating pro-inflammatory immune responses and post-weaning stress.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Useful Agricultural Life Resources Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (120051-02-2-HD020), and supported by CJ CheilJedang Biotechnology Research Institute, Seoul, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Kang J, Kim SH, Song M.
Data curation: Kang J, Lee JJ, Cho JH.
Formal analysis: Choe J, Kyoung H, Kim SH.
Methodology: Kang J, Lee JJ, Cho JH, Kyoung H.
Software: Kang J, Lee JJ, Kim SH.
Validation: Cho JH, Choe J, Kim HB.
Investigation: Kang J, Cho JH, Kim HB.
Writing - original draft: Kang J, Lee JJ, Song M.
Writing - review & editing: Lee JJ, Cho JH, Song M.
Ethics approval and consent to participate
This experimental protocol for this research was reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, Daejeon, Korea (approval code #CNU-00832).
REFERENCES
- 1.Food and Agriculture Organization of the United Nations [FAO] Probiotics in animal nutrition – production, impact and regulation. Rome: FAO; 2016. FAO Animal Production and Health Paper No. 179. [Google Scholar]
- 2.Kelly D. Probiotics in young and newborn animals. J Anim Feed Sci. 1998;7:15–23. doi: 10.22358/jafs/69952/1998. [DOI] [Google Scholar]
- 3.Cho JH, Zhao PY, Kim IH. Probiotics as a dietary additive for pigs: a review. J Anim Vet Adv. 2011;10:2127–34. doi: 10.3923/javaa.2011.2127.2134. [DOI] [Google Scholar]
- 4.Stein H. Proceedings of the 7th London Swine Conference Proceedings. London, ON: Canada; 2007. Feeding the pigs’ immune system and alternatives to antibiotics; pp. 65–82. p. [Google Scholar]
- 5.Zhu GQ, Musa HH, Wu SL, Zhu CH, Seri HI. The potential benefits of probiotics in animal production and health. J Anim Vet Adv. 2009;8:313–21. [Google Scholar]
- 6.Patil AK, Kumar S, Verma AK, Baghel RPS. Probiotics as feed additives in weaned pigs: a review. Livest Res Int. 2015;3:31–9. [Google Scholar]
- 7.Isa K, Oka K, Beauchamp N, Sato M, Wada K, Ohtani K, et al. Safety assessment of the Clostridium butyricum MIYAIRI 588® probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Hum Exp Toxicol. 2016;35:818–32. doi: 10.1177/0960327115607372. [DOI] [PubMed] [Google Scholar]
- 8.Boyle RJ, Robins-browne RM, Tang MLK. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–64. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 9.Gueimonde M, Sánchez B. Enhancing probiotic stability in industrial processes. Microb Ecol Health Dis. 2012;23:2–6. doi: 10.3402/mehd.v23i0.18562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mottet C, Michetti P. Probiotics: wanted dead or alive. Dig Liver Dis. 2005;37:3–6. doi: 10.1016/j.dld.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Adams CA. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010;23:37–46. doi: 10.1017/S0954422410000090. [DOI] [PubMed] [Google Scholar]
- 12.Lahtinen SJ. Probiotic viability – does it matter? Microb Ecol Health Dis. 2012;23:10–4. doi: 10.3402/mehd.v23i0.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou CC, Lin SL, Tsai JJ, Lin MY. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J Food Sci. 2011;76:M260–7. doi: 10.1111/j.1750-3841.2011.02161.x. [DOI] [PubMed] [Google Scholar]
- 14.de Almada CN, Almada CN, Martinez RCR, Sant’Ana AS. Paraprobiotics: evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci Technol. 2016;58:96–114. doi: 10.1016/j.tifs.2016.09.011. [DOI] [Google Scholar]
- 15.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–74. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (Tyndallized) probiotics: an overview. Int J Mol Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Gong L, Wu YP, Cui ZW, Wang YQ, Huang Y, et al. Oral administration of Lactobacillus rhamnosus GG to newborn piglets augments gut barrier function in pre-weaning piglets. J Zhejiang Univ Sci B. 2019;20:180–92. doi: 10.1631/jzus.B1800022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLOS ONE. 2012;7:e40666. doi: 10.1371/journal.pone.0040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trevisi P, Casini L, Coloretti F, Mazzoni M, Merialdi G, Bosi P. Dietary addition of Lactobacillus rhamnosus GG impairs the health of Escherichia coli F4-challenged piglets. Animal. 2011;5:1354–60. doi: 10.1017/S1751731111000462. [DOI] [PubMed] [Google Scholar]
- 20.Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI. Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol. 2016;57:170–8. doi: 10.1016/j.fsi.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Yokoyama S, Wang WL, et al. Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol Biochem. 2017;43:179–92. doi: 10.1007/s10695-016-0277-4. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Russell WM, Douglas-Escobar M, Hauser N, Lopez M, Neu J. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res. 2009;66:203–7. doi: 10.1203/PDR.0b013e3181aabd4f. [DOI] [PubMed] [Google Scholar]
- 23.Bloise E, Torricelli M, Novembri R, Borges LE, Carrarelli P, Reis FM, et al. Heat-killed Lactobacillus rhamnosus GG modulates urocortin and cytokine release in primary trophoblast cells. Placenta. 2010;31:867–72. doi: 10.1016/j.placenta.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 24.NRC [National Research Council] Nutrient requirement of swine. 11th ed. Washington, DC: National Academy Press; 2012. [Google Scholar]
- 25.Lee JJ, Choi SH, Cho JH, Choe J, Kang J, Kim S, et al. Effects of dietary carbohydrases on productive performance and immune responses of lactating sows and their piglets. J Anim Sci Technol. 2019;61:359–65. doi: 10.5187/jast.2019.61.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Lee JJ, Yang BM, Cho JH, Kim S, Kang J, et al. Dietary protease improves growth performance, nutrient digestibility, and intestinal morphology of weaned pigs. J Anim Sci Technol. 2020;62:21–30. doi: 10.5187/jast.2020.62.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AOAC [Association of Official Analytical Chemists] International. Official methods of analysis of AOAC International. Gaithersburg, MD: AOAC International; 2005. [Google Scholar]
- 28.Williams CH, David DJ, Iismaa O. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agric Sci. 1962;59:381–5. doi: 10.1017/S002185960001546X. [DOI] [Google Scholar]
- 29.Lewis AJ. Digestion and balance techniques in pigs. In: Lewis AJ, Southern LL, editors. Swine nutrition. 2nd ed. Boca Raton, Fl: CRC Press; 2001. pp. 903–16. p. [Google Scholar]
- 30.Busanello M, dos Santos Pozza MS, Pozza PC, Nunes RV, Chambo APS, Eckstein II. Probiotics: viable and inactivated cells on the performance, microflora and blood parameters of piglets. Rev Bras Saúde e Prod Anim. 2015;16:387–96. doi: 10.1590/S1519-99402015000200013. [DOI] [Google Scholar]
- 31.Bocourt R, Savon L, Díaz J, Brizuela MA, Serrano P, Prats A, et al. Effect of the probiotic activity of Lactobacillus rhamnosus on productive and health indicators of piglets. Cuba J Agric Sci. 2004;38:75–9. [Google Scholar]
- 32.di Gioia D, Biavati B. Probiotics and prebiotics in animal health and food safety: conclusive remarks and future perspectives. In: di Gioia D, Biavati B, editors. Probiotics and prebiotics in animal health and food safety. Cham, Switzerland, Swiss: Springer Nature; 2018. pp. 269–73. p. [DOI] [Google Scholar]
- 33.Bernardeau M, Vernoux JP, Gueguen M. Safety and efficacy of probiotic lactobacilli in promoting growth in post-weaning Swiss mice. Int J Food Microbiol. 2002;77:19–27. doi: 10.1016/S0168-1605(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 34.Sewaka M, Trullas C, Chotiko A, Rodkhum C, Chansue N, Boonanuntanasarn S, et al. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.) Fish Shellfish Immunol. 2019;86:260–8. doi: 10.1016/j.fsi.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Basu S, Chatterjee M, Ganguly S, Chandra PK. Effect of Lactobacillus rhamnosus GG in persistent diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol. 2007;41:756–60. doi: 10.1097/01.mcg.0000248009.47526.ea. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Xu YQ, Liu HY, Lai T, Ma JL, Wang JF, et al. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol. 2010;141:142–8. doi: 10.1016/j.vetmic.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Kim JC, Hansen CF, Mullan BP, Pluske JR. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2012;173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022. [DOI] [Google Scholar]
- 38.Tareb R, Bernardeau M, Gueguen M, Vernoux J. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol. 2013;62:637–49. doi: 10.1099/jmm.0.049965-0. [DOI] [PubMed] [Google Scholar]
- 39.Lan R, Koo J, Kim I. Effects of Lactobacillus acidophilus supplementation on growth performance, nutrient digestibility, fecal microbial and noxious gas emission in weaning pigs. J Sci Food Agric. 2017;97:1310–5. doi: 10.1002/jsfa.7866. [DOI] [PubMed] [Google Scholar]
- 40.Zhao PY, Kim IH. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim Feed Sci Technol. 2015;200:86–92. doi: 10.1016/j.anifeedsci.2014.12.010. [DOI] [Google Scholar]
- 41.Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52:3–12. doi: 10.1007/s12088-011-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, et al. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609–15. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh NS, Joung JY, Lee JY, Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLOS ONE. 2018 Feb;13:e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ting WJ, Kuo WW, Hsieh DJY, Yeh YL, Day CH, Chen YH, et al. Heat killed Lactobacillus reuteri GMNL-263 reduces fibrosis effects on the liver and heart in high fat diet-hamsters via TGF-β suppression. Int J Mol Sci. 2015;16:25881–96. doi: 10.3390/ijms161025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchinaka A, Azuma N, Mizumoto H, Nakano S, Minamiya M, Yoneda M, et al. Anti-inflammatory effects of heat-killed Lactobacillus plantarum L-137 on cardiac and adipose tissue in rats with metabolic syndrome. Sci Rep. 2018;8:1–20. doi: 10.1038/s41598-018-26588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida K, Sawada D, Kuwano Y, Tanaka H, Sugawara T, Aoki Y, et al. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J Funct Foods. 2017;36:112–21. doi: 10.1016/j.jff.2017.06.031. [DOI] [Google Scholar]
- 47.Yong SJ, Tong T, Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020;13:1361. doi: 10.3389/fnins.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moeser AJ, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. 2017;3:313–21. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front Microbiol. 2018;9:2426. doi: 10.3389/fmicb.2018.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao K, Cao ST, Jiao LF, Lin FH, Wang L, Hu CH. Anemonin improves intestinal barrier restoration and influences TGF-β1 and EGFR signaling pathways in LPS-challenged piglets. Innate Immun. 2016;22:344–52. doi: 10.1177/1753425916648223. [DOI] [PubMed] [Google Scholar]
- 51.Lee JJ, Kang J, Park S, Cho JH, Oh S, Park DJ, et al. Effects of dietary protease on immune responses of weaned pigs. J Anim Sci Technol. 2020;62:174–9. doi: 10.5187/jast.2020.62.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]