Abstract
In dialysis patients, cholesterol‐lowering therapy with statins is less effective than in other high‐risk patients. This may be explained by a shift from cholesterol synthesis toward cholesterol absorption. In line, markers of cholesterol absorption—such as campesterol—better predict atherosclerotic cardiovascular events than markers of cholesterol synthesis—such as lathosterol—in dialysis patients. To test the association between markers of cholesterol absorption such as campesterol—and markers of cholesterol synthesis—such as lathosterol—against cardiovascular events in non‐dialysis CKD patients. Altogether 251 patients those not on lipid‐lowering agents were followed annually for the composite endpoint atherosclerotic cardiovascular disease (ASCVD) and all‐cause death. During follow‐up of 5.2 ± 2.1 years, 61 participants reached the primary endpoint atherosclerotic cardiovascular disease/all‐cause death [ASCVD/D], 47 participants suffered from ASCVD, and 46 participants died. In univariate Cox regression analysis, campesterol/lathosterol ratio did not significantly predict ASCVD/D (HR 0.643; 0.358–1.155; 3rd vs. 1st tertile), all‐cause death (HR 1.309; 0.604–2.838; 3rd vs. 1st tertile) nor ASCVD (HR 0.589; 0.311–1.118; 3rd vs. 1st tertile). We did not observe a shift from cholesterol synthesis to cholesterol absorption across the spectrum of non‐dialysis CKD. Campesterol/lathosterol ratio did not predict future ASCVD or all‐cause death in non‐dialysis CKD.
Keywords: cardiovascular disease, cholesterol absorption, cholesterol synthesis, renal impairment
This study tested the association between markers of cholesterol metabolism (campesterol‐to‐lathosterol) against atherosclerotic cardiovascular disease and all‐cause death in non‐dialysis CKD patients.

Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- CKD
chronic kidney disease
- CKD
chronic kidney disease
- LDL‐C
LDL cholesterol
1. INTRODUCTION
Despite improved treatments of chronic kidney disease (CKD), patients are still affected by an inappropriately high cardiovascular morbidity and mortality. 1 Dyslipidemias—especially hypercholesterinemia—is a leading cardiovascular risk factor in all populations except heart failure 2 or advanced CKD. 3 In the latter, two randomized controlled trials in patients on hemodialysis have shown, 3 , 4 that LDL cholesterol (LDL‐C) lowering with statins did not reduce cardiovascular events while LDL‐C lowering with statins reduced major cardiovascular events in non‐dialysis patients. 5 In patients with advanced CKD, comprising both non‐dialysis CKD and hemodialysis patients, a reduction of cardiovascular events was observed in SHARP 6 by combining statins with ezetimibe, which additionally inhibits intestinal cholesterol absorption.
Both hepatic cholesterol synthesis and intestinal absorption contribute to cholesterol homeostasis. In individuals without cholesterol lowering treatment, about two thirds of plasma cholesterol is derived from hepatic cholesterol synthesis. However, strong interindividual variations exist, and inhibition of cholesterol synthesis by statin will eventually induce a compensatory increase in cholesterol absorption. 7
In hemodialysis patients, a shift from cholesterol synthesis to cholesterol absorption occurs, which may render their cholesterol levels more sensitive to ezetimibe but less responsive to statin treatment. 8 , 9 Interestingly, patients characterized as “high absorbers” have worse clinical outcome than “high synthesizers.” 9 At which stage across the spectrum of CKD such a shift toward increased cholesterol absorption occurs first is not known. Identification of phenotypes of cholesterol hemostasis could provide guidance to improve cholesterol lowering therapy possibly for CKD patients at particular high risk.
We hypothesized that cholesterol absorption may contribute more, and cholesterol synthesis may contribute less to plasma cholesterol levels in patients across a wide spectrum of CKD. We investigated whether campesterol/lathosterol ratios predict outcome in patients with non‐dialysis CKD.
2. MATERIALS AND METHODS
2.1. Study participants
The present study utilizes the database of the CARE FOR HOMe (Cardiovascular and Renal Outcome in CKD 2–4 Patients—The Fourth Homburg evaluation) study. From 2008 to 2017, the study recruited 599 patients with CKD KDIGO GFR categories G2–G4 (estimated GFR between 15 and 89 ml/min/1.73 m² according to the MDRD equation, patients with CKD stage 2 had to show one or more markers of kidney damage, including albuminuria and/or plasma creatinine/cystatin C above reference values). The study was approved by the local ethics committee (April 2004) and was conducted in concordance with the Helsinki Declaration; all participants provided their written informed consent.
Exclusion criteria were intake of systemic immune suppressive medication, HIV infection, acute infectious disease (C‐reactive protein levels >50 mg/L and/or need for requiring systemic antibiotic therapy), active cancer disease, renal replacement therapy, acute kidney injury (increase of plasma creatinine >50% within 4 weeks), pregnancy, and age <18 years old.
About 555 participants had baseline samples available for analyses of lathosterol and campesterol. We excluded all participants treated with statin and/or other lipid lowering drugs at baseline, leaving 251 participants for this analysis.
All participants were invited annually for follow‐up visits. Participants not able or not willing to attend these follow‐up visits were contacted for a telephone interview. All reported events were verified by medical records from the treating physicians. Two physicians blinded to non‐cholesterol sterol levels judged all events. In case of disagreement, a third investigator was involved to make a final decision.
2.2. Outcome
The primary endpoint was the composite of major atherosclerotic cardiovascular events [ASCVD] defined as non‐fatal acute myocardial infarction, non‐fatal ischemic stroke, cerebrovascular/peripheral arterial or coronary revascularization, major amputation above the ankle and all‐cause death [ASCVD/D]. Secondary endpoints were ASCVD and all‐cause death. Moreover, we investigated whether a shift toward cholesterol absorption occurs in non‐dialysis chronic kidney disease (CKD), and whether the campesterol/lathosterol ratio predicts outcomes in non‐dialysis CKD patients.
2.3. Laboratory analysis
At baseline, fasting blood samples were drawn under standardized conditions after 5 min rest. Cholesterol and non‐cholesterol sterols were quantified in serum samples. After alkaline hydrolysis, the free sterols were extracted with chloroform/methanol (2:1; v/v) and the trimethyl silylated (TMSi) sterol ethers were separated by gas chromatography (GC). Cholesterol‐TMSi ethers were detected by less sensitive but specific flame‐ionization detection (FID) (5α‐cholestane, internal standard, ISTD) and the non‐cholesterol sterol‐TMSi ethers (epicoprostanol‐TMSi ether, ISTD) by highly specific and highly sensitive mass spectrometry in the selected ion monitoring mode (MS‐SIM) as described in detail previously. 10 , 11 Additionally, analysis of total cholesterol and lipoprotein profile was performed by routine methods in our central laboratory. All other laboratory parameters were measured using standard methods. 12
2.4. Statistical analysis
Data management and statistical analysis were performed with IBM SPSS statistics software (IBM, SPSS Statistics 25). Categorical variables are presented as percentage of participants and were compared by Fishers exact test or chi‐squared test, as appropriate. Continuous data are expressed as mean ± SD and were compared using one‐way ANOVA test. In case of skewed distribution, variables are presented as median (interquartile range) and were compared using Kruskal‐Wallis test. Correlations were analyzed by Spearman's rank correlation coefficient.
As primary hypothesis, we tested whether the plasma campesterol/lathosterol ratio is an independent predictor of ASCVD/D, as defined above, after pre‐defined adjustment for confounders. If this hypothesis was confirmed, we would test the following secondary hypotheses in prespecified hierarchy: (1) the campesterol/cholesterol ratio is an independent predictor of ASCVD after pre‐defined adjustment for confounders; (2) the lathosterol/cholesterol ratio is independent predictor of all‐cause death after pre‐defined adjustment for confounders.
Secondary end‐points were only analyzed exploratorily.
To test those hypotheses, we stratified participants into tertiles by their plasma campesterol/lathosterol ratio, their plasma lathosterol/cholesterol levels and their plasma campesterol/cholesterol levels, respectively. We performed Kaplan–Meier analysis with subsequent log‐rank testing for event‐free survival and univariate (model 1) and multivariate (model 2 ‐ model 4) Cox regression analyses. The models were defined as follows: Model 2: adjustment for age and gender, Model 3: further adjustment for eGFR and log‐transformed albuminuria, and Model 4: further adjustment for prevalent cardiovascular disease, current smoking, diabetes mellitus, systolic blood pressure, and body mass index. Campesterol/lathosterol ratio, as well as campesterol/cholesterol ratio and lathosterol/cholesterol ratio were considered in Cox regression models as continuous linear variables and then categorized into tertiles. A two‐sided p value <.05 was considered as statistically significant.
3. RESULTS
As we excluded all participants treated with statins or other lipid lowering drugs, 251 patients’ data were available for the following analysis. Mean age was 63.0 ± 14.0 years and mean eGFR 47.5 ± 16.9 ml/min/1.73 m². 42.6% were women, 12% smoker, 32.7% suffered from diabetes mellitus, and 14.7% had prevalent cardiovascular disease. Mean campesterol/cholesterol levels were 1.68 µg/mg [1.11; 2.34], mean lathosterol/cholesterol levels were 0.30 µg/mg [0.22; 0.40], and mean campesterol/lathosterol ratio was 1.07 µg/mg [0.63; 1.89]. Participants with the lowest campesterol/lathosterol ratio (1st tertile) were older [66.6 ± 12.4 years.; p = 0.004], had a higher BMI [31.1 ± 5.9 kg/m2; p < .001], a higher diastolic blood pressure value [84 ± 11 mmHg; p = .016], and lower HDL levels [45 mg/dl [38; 58]; p = .015] compared with those participants with the highest campesterol/lathosterol ratio (3rd tertile). Further baseline characteristics are presented in Table 1.
TABLE 1.
Baseline characteristics
|
Campesterol/lathosterol ratio (1st tertile) n = 84 <0.8 |
Campesterol/lathosterol ratio (2nd tertile) n = 83 0.81–1.52 |
Campesterol/lathosterol ratio (3rd tertile) n = 84 1.53–11.58 |
Total cohort n = 251 |
p‐value | |
|---|---|---|---|---|---|
| Gender [female] | 34 [40.5%] | 40 [48.2%] | 33 [39.3%] | 107 [42.6%] | .451 |
| Age [years] | 66.6 ± 12.4 | 62.1 ± 12.2 | 59.8 ± 15.5 | 63.0 ± 14.0 | .004 |
| BMI [kg/m²] | 31.1 ± 5.9 | 30.8 ± 5.5 | 27.7 ± 4.5 | 30.0 ± 5.6 | <.001 |
| Current smoker [yes] | 10 [12%] | 10 [12%] | 10 [12%] | 30 [12%] | .999 |
| eGFR [ml/min/1.73 m²] | 45.9 ± 16.1 | 51.3 ± 16.6 | 45.6 ± 17.6 | 47.5 ± 16.9 | .034 |
| DM [yes] | 36 [42.9%] | 22 [26.5%] | 24 [28.6%] | 82 [32.7%] | .049 |
| Albuminuria [mg/g creatinine] | 27 [7; 153] | 25 [6; 124] | 68 [11; 350] | 34 [7; 202] | .369 |
| BP sys [mmHg] | 151 ± 23 | 153 ± 20 | 153 ± 24 | 152 ± 22 | .872 |
| BP dia [mmHg] | 84 ± 11 | 62 ± 12 | 60 ± 16 | 87 ± 12 | .016 |
| Prevalent CVD [yes] | 17 [20.2%] | 9 [10.8%] | 11 [13.1%] | 37 [14.7%] | .201 |
| CRP [mg/L] | 3.2 [1.3; 5.9] | 2.5 [1.3; 5.0] | 2.2 [1.1; 4.4] | 2.7 [1.3; 5.2] | .078 |
| Cholesterol [mg/dL] | 196 [175; 220] | 222 [190; 245] | 202 [182; 229] | 202 [183; 234} | .006 |
| Triglyceride [mg/dl] | 150 [108; 200] | 128 [91; 205] | 133 [91; 175] | 135 [93; 190] | .160 |
| LDL‐C [mg/dL] | 121 [103; 138] | 131 [114; 155] | 124 [104; 142] | 124 [105; 147] | .037 |
| HDL‐C [mg/dL] | 45 [38; 58] | 54 [44; 64] | 52 [40; 68] | 49 [40; 64] | .015 |
| Cholestanol [mg/dL] | 0.21 [0.17; 0.24] | 0.24 [0.21; 0.29] | 0.30 [0.25; 0.36] | 0.24 [0.20; 0.31] | <.001 |
| Cholestanol/cholesterol [µg/mg] | 1.04 [0.85; 1.24] | 1.13 [0.98; 1.35] | 1.47 [1.20; 1.72] | 1.19 [1.00; 1.50] | <.001 |
| Desmosterol [mg/dL] | 0.16 [0.12; 0.22] | 0.14 [0.12; 0.18] | 0.11 [0.08; 0.14] | 0.13 [0.10; 0.18] | <.001 |
| Desmosterol/cholesterol [µg/mg] | 0.77 [0.55; 1.00] | 0.68 [0.53; 0.77] | 0.54 [0.40; 0.69] | 0.65 [0.50; 0.81] | <.001 |
| Lathosterol [mg/dL] | 0.40 [0.32; 0.58] | 0.33 [0.26; 0.39] | 0.21 [0.14; 0.26] | 0.30 [0.22; 0.40] | <.001 |
| Lathosterol/cholesterol [µg/mg] | 2.06 [1.63; 2.79] | 1.49 [1.24; 1.76] | 1.03 [0.72; 1.24] | 1.47 [1.09; 1.88] | <.001 |
| Campesterol [mg/dL] | 0.18 [0.16; 0.26] | 0.35 [0.29; 0.42] | 0.53 [0.45; 0.65] | 0.34 [0.23; 0.50] | <.001 |
| Campesterol/cholesterol [µg/mg] | 0.96 [0.77; 1.28] | 1.66 [1.39; 1.89] | 2.66 [2.03; 3.30] | 1.68 [1.11; 2.34] | <.001 |
| Campesterol/lathosterol ratio | 0.51 [0.36; 0.64] | 1.07 [0.93; 1.29] | 2.41 [1.89; 3.87] | 1.07 [0.63; 1.89] | <.001 |
| Stigmasterol [µg/dL] | 3.92 [2.80; 5.44] | 5.19 [4.05; 7.48] | 7.77 [5.79; 10.81] | 5.48 [3.64; 8.49] | <.001 |
| Stigmasterol/cholesterol [µg/mg] | 0.018 [0.015; 0.027] | 0.025 [0.018; 0.034] | 0.040 [0.029; 0.051] | 0.027 [0.017; 0.041] | <.001 |
| Lanosterol [µg/dL] | 34.34 [28.85; 44.51] | 31.34 [26.33; 37.25] | 25.25 [20.97; 30.19] | 30.16 [24.93; 37.30] | <.001 |
| Lanosterol/cholesterol [µg/mg] | 0.17 [0.14; 0.22] | 0.15 [0.12; 0.17] | 0.13 [0.11; 0.15] | 0.15 [0.12; 0.18] | <.001 |
| Sitosterol [mg/dL] | 0.15 [0.11; 0.19] | 0.25 [0.20; 0.31] | 0.38 [0.30; 0.51] | 0.25 [0.16; 0.35] | <.001 |
| Sitosterol/cholesterol [µg/mg] | 0.75 [0.54; 0.96] | 1.14 [0.96; 1.37] | 1.89 [1.41; 2.35] | 0.79 [1.14; 1.76] | <.001 |
Bold values indicate p < .05.
Categorical variables are presented as percentage of participants and were compared by Fisher's exact test or chi‐squared test, as appropriate. Continuous data are expressed as mean ± SD and were compared using one‐way ANOVA test. In case of skewed distribution, variables are presented as median (interquartile range) and were compared using Kruskal–Wallis test.
Abbreviations: BMI, body mass index; BP, blood pressure; CRP, C‐reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Across eGFR categories, the campesterol/lathosterol ratio did not change significantly (p = .438); (Figure 1).
FIGURE 1.
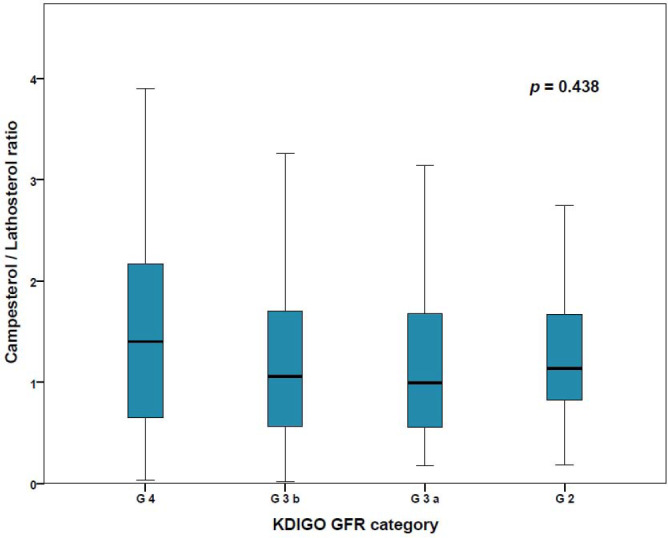
Box plots of campesterol/lathosterol ratio in CKD KDIGO GFR categories G4 (eGFR 15–30 ml/min/1.73 m2), G3b (eGFR 30–45 ml/min/1.72 m2), G3a (eGFR 45–60 ml/min/1.73 m2), and G2 (eGFR 60–90 ml/min/1.73 m2). Mean campesterol/lathosterol ratio did not change significantly between the different categories of CKD
Similarly, neither campesterol/cholesterol nor lathosterol/cholesterol correlated significantly with eGFR (Table S1a/b). Campesterol/cholesterol and the campesterol/lathosterol ratio correlated negatively with BMI (r = −.268, p < .001 and r = −.179; p = .004), and lathosterol/cholesterol correlated weakly with BMI (r = .240; p < .001). Campesterol/cholesterol and campesterol/lathosterol ratio were negatively associated with age (r = −.256; p < .001 and r = −.166; p = .008, respectively). Correlations between cholesterol measured by the routinely used enzymatic method and cholesterol measured by FID were high (r = .965; r < .001); (Table S1a/b).
During the follow‐up of 5.2 ± 2.1 years, 61 participants reached the primary endpoint [ASCVD/D]: 46 participants died and 47 participants suffered from ASCVD.
In univariate Kaplan–Meier analyses, campesterol/lathosterol ratio was not significantly associated with the primary endpoint [MACE/D] (p = .145) (Figure 2). According to our study protocol, the findings for MACE (Figure S1) and all‐cause death (Figure S2) were not tested for statistical significance.
FIGURE 2.
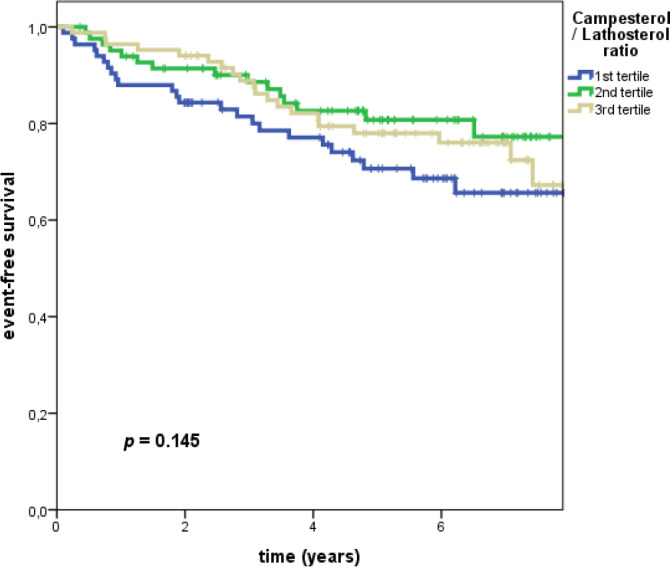
Kaplan–Meier analysis with subsequent log rank test (primary endpoint ASCVD + all‐cause death): Event‐free survival in patients with CKD stratified by tertiles of campesterol/lathosterol ratio. Campesterol/lathosterol ratio was not significantly associated with the primary endpoint
In univariate Cox regression analysis, the campesterol/lathosterol ratio (considered either as continuous or as categorized variable) was neither significantly associated with the primary endpoint [ASCVD/D], nor with all‐cause death, nor with ASCVD (Table 2, Tables S2 and S3). Full adjustment for confounders did not change these results (Table 2, Tables S2 and S3).
TABLE 2.
Cox regression models (primary endpoint: ASCVD +all‐cause death [ASCVD/D])
| Exposure variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Categorized predictors | ||||||||
| log Campesterol/lathosterol ratio | ||||||||
| First tertile (0.02–0.80, n = 84) | Reference | Reference | Reference | Reference | ||||
| Second tertile α (0.81–1.52, n = 83) | 0.579 (0.306;1.095) | .093 | 0.721 (0.380;1.368) | .317 | 1.058 (0.545;2.052) | .868 | 1.201 (0.604;2.390) | .601 |
| Third tertile α (1.52–11.58, n = 84) | 0.643 (0.358;1.155) | .140 | .802 (0.446;1.442) | .461 | .833 (0.461;1.507) | .546 | 1.024 (0.539;1.946) | .942 |
| log Lathosterol/cholesterol | ||||||||
| First tertile (0.229–1.206, n = 84) | Reference | Reference | Reference | Reference | ||||
| Second tertile α (1.219–1.738, n = 83) | 0.818 (0.453;1.477) | 0.641 (0.352;1.170) | 1.152 (0.621;2.134) | 1.039 (0.551;1.959) | ||||
| Third tertile α (1.741–18.529, n = 84) | 0.596 (0.318;1.117) | 0.557 (0.297;1.045) | 0.582 (0.309;1.094) | 0.378 (0.185;0.772) | ||||
| log Campesterol/cholesterol | ||||||||
| First tertile (0.133–1.311, n = 84) | Reference | Reference | Reference | Reference | ||||
| Second tertile α (1.313–2.001, n = 83) | 2.391 (1.266;4.518) | 1.815 (0.956;3.448) | 2.009 (1.046;3.858) | 1.960 (1.001;3.838) | ||||
| Third tertile α (2.005–7.079, n = 84) | 1.507 (0.756;3.003) | 1.225 (0.629;2.503) | 1.095 (0.537;2.235) | 0.946 (0.441;2.032) | ||||
| Continuous predictors | ||||||||
| log Campesterol/lathosterol ratio | 0.682 (0.355;1.196) | .167 | 0.810 (0.427;1.538) | 0.519 | 0.856 (0.489;1.499) | 0.587 | 0.956 (0.525;1.744) | .885 |
| log Lathosterol/cholesterol | 0.401 (0.133;1.216) | 0.331 (0.107;1.028) | 0.463 (0.180;1.188) | 0.299 (0.106;0.844) | ||||
| log Campesterol/cholesterol | 0.161 (0.065; 0.401) | 0.200 (0.076;0.553) | 0.211 (0.080;0.557) | 0.200 (0.070;0.571) | ||||
Model 1 is the univariate analysis. Model 2 is adjusted for age and gender, Model 3 is additionally adjusted for eGFR and log‐transformed albuminuria, Model 4 is additionally adjusted for body mass index, active smoking, diabetes mellitus, systolic blood pressure and prevalent cardiovascular disease. α Reference is the first tertile.
After rejecting our primary hypothesis, we analyzed campesterol/cholesterol ratio and lathosterol/cholesterol ratio exploratorily, following our study protocol, and did not test for statistical significance (Table 2, Tables S2 and S3). Interestingly, high ratios of campesterol/cholesterol and lathosterol/cholesterol both tended to indicate low ASCVD risk.
4. DISCUSSION
The present study does not confirm a significant relationship between markers of cholesterol metabolism, in particular, campesterol/lathosterol ratio and cardiovascular outcomes and all‐cause mortality in non‐dialysis CKD (KDIGO GFR categories G2–G4). Moreover, markers of cholesterol metabolism did not correlate with eGFR. This is neither the case for campesterol/lathosterol ratio, nor for campesterol/cholesterol and lathosterol/cholesterol. Moreover, markers of cholesterol metabolism were not associated with ASCVD. Taken together, in patients with different degrees of CKD—but not on dialysis—cardiovascular events are not associated with markers of cholesterol metabolism.
The current 2019 ESC/EAS guidelines for the management of dyslipidemias categorize patients with moderately reduced CKD (eGFR 30–59 mL/min/1.73 m²) as “high risk.” Moreover, patients with severely impaired CKD defined as eGFR <30 mL/min/1.73 m² are classified in the highest risk category. Therefore, the association of cholesterol metabolism and lipid‐lowering therapy with cardiovascular risk in these patient populations is of particular importance. 13
A large number of randomized trials prove that targeting LDL‐C reduces cardiovascular events both in primary and secondary prevention. 14 However, these findings cannot be transferred to patients with advanced CKD on dialysis. The international KDIGO guidelines recommend to refrain from initiating lipid‐lowering therapy in patients on dialysis and to keep incident dialysis patients on their preexisting lipid lowering regimen. 15 These recommendations are based on two large prospective: placebo‐controlled statin trails which investigated atorvastatin 20 mg (4 D) 4 and rosuvastatin 10 mg (AURORA) 3 in patients on dialysis. Both trials failed to reduce cardiovascular outcomes, even though LDL‐C reduction in AURORA and in 4 D was 41% and 42%, respectively. According to the cholesterol treatment trialists’ (CTT) analysis LDL‐C lowering by more than 40% should have yielded a dramatic reduction of cardiovascular events in this high‐risk patient population. It has been argued that the lack of benefit of statin therapy in these two trials might be due to differences in the pathophysiology of underlying cardiovascular diseases. 3 However, previous findings have suggested alternative explanations 8 : In a pilot study of 113 patients on dialysis and 229 healthy controls with normal kidney function, we observed that hemodialysis patients were characterized by significantly higher cholesterol absorption and lower cholesterol synthesis compared with controls. These findings were confirmed in two Japanese cross‐sectional studies in patients with impaired renal function, 16 , 17 an only recently published study on patients on dialysis in Taiwan, 18 and later in a post hoc analysis of the 4 D study, whose participants were characterized by higher markers of cholesterol absorption than those reported in general population cohort studies and interventions trials. 9 Findings in our study revealed that high cholesterol absorption was associated with a two‐fold increase in all‐cause mortality. 8 These findings are in line with those of Silbernagel and colleagues who reported in their post hoc analysis of the German 4 D study that only patients with low cholesterol absorption (and consecutively high cholesterol synthesis) benefit from statins in terms of reductions of cardiovascular events. 9 In contrast to the two large statin only trials, SHARP compared a combined lipid‐lowering therapy with simvastatin and ezetimibe versus placebo and reduced cardiovascular event rates, even though LDL‐C reduction was less pronounced than in the previously published statin trails. 6
These findings are in agreement with the concept of “individualized lipid‐lowering therapy,” proposed earlier with the determination of markers of cholesterol metabolism prior to starting lipid‐lowering therapy. 19 , 20 This concept is based on findings in “sitosterolemia,” 21 on genetic association studies in the general population, 22 , 23 epidemiological observation studies 24 , 25 and, most importantly, results from prospective randomized trials. 26 , 27 Findings in “4S” revealed that patients who were characterized by high cholesterol absorption did not benefit from statin treatment. 26 On the other hand, HIJ‐PROPER demonstrated that only patients with high markers of cholesterol absorption benefit from ezetimibe treatment. 27 Only recently our group found in patients undergoing coronary angiography that increased plasma levels of 7‐α‐hydroxy‐campesterol (a marker for cholesterol absorption) at baseline were associated with cardiovascular events. 28
On the basis of these findings, we hypothesized that in patients with impaired renal function (CKD KDIGO GFR categories G2–G4)—but not on dialysis, in particular, increased cholesterol absorption might contribute to cardiovascular risk. Surprisingly, in this study we did not observe a significant association of markers of cholesterol metabolism and renal impairment. However, we found a strong impact of age category on cholesterol metabolism. As depicted in Table 1 patients in the lowest tertile of cholesterol absorption were significantly older than patients in the highest tertile. Even though we adjusted our results to age, we cannot exclude that these tremendous effects of changes in cholesterol metabolism between the age groups of 59 and 66 years affected our results. Moreover, these results are not in line with recently published trials. Bach et al. 29 as well as Ouchi et al. 30 reported in patients over 75 years that ezetimibe treatment resulted in cardiovascular risk reduction, which could not be explained by mere LDL‐C reductions. This is of particular interest, since Strandberg et al. reported in patients over 75 years increased cholesterol absorption rates and suggested in this age group combined lipid‐lowering with a statin and cholesterol absorption inhibition. 31 , 32
One of the limitations of this analysis is the exclusion of statin‐treated patients leaving only those patients for analysis that were not considered at high cardiovascular risk and consequently not on lipid‐lowering therapy. Moreover, the average LDL‐C of 124 mg/dl in patients without lipid lowering therapy is comparatively low and, especially older patients with higher cardiovascular risk and increased cholesterol synthesis in tertile 1 could have benefited rather from a statin than from a cholesterol absorption inhibitor.
Taken together, in patients with renal impairment (CKD KDIGO GFR categories G2–G4), we did not find an association of eGFR and markers of cholesterol metabolism. Moreover, in this patient population we could not reveal the expected association of an increased risk for cardiovascular event rates with higher levels of markers of cholesterol absorption. Further research is required to elucidate the association of markers of cholesterol metabolism and cardiovascular risk in this subset of CKD patients not on dialysis.
DISCLOSURE
Dr. Emrich received advisory board and speaker honoraria from Pharmacosmos. Dr. Weingärtner received advisory board and speaker honoraria from AMGEN, SANOFI, Hexal, Novartis, and Berlin‐Chemie Menarini. Dr. Böhm is supported by the Deutsche Forschungsgemeinschaft (DFG, TTR 219, S‐01, M‐03, M‐05) and reports support from Abbott, Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Medtronic, Novartis, ReCor, Servier and Vifor. Dr. Wagenpfeil is supported by the Deutsche Forschungsgemeinschaft (DGF, TTR 219, PI: S‐01) and reports support from Servier. Dr Fliser is supported by the Deutsche Forschungsgemeinschaft (DGF, TTR 219, S‐01, M‐03, M‐05) and reports support from Abbott, Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, FMC, Medtronic, Novartis, ReCor, Servier, and Vifor.
AUTHORS CONTRIBUTIONS
I.E.E., O.W., G.H.H. and D.L. designed research; O.W., K.S.R., D.L., D.F., G.H.H., M.B. and I.E.E. conducted research; I.E.E., M.B., G.H.H., and S.W. analyzed data and performed statistical analysis; I.E.E., G.H.H., P.C.S. and O.W. wrote the paper. All authors read and approved the final manuscript.
ETHICS STATEMENT
All participants gave their written informed consent at baseline. The study was carried out in accordance with the Declaration of Helsinki and approved by the local Ethics Committee.
Supporting information
TableS1‐S3‐FigS1‐S2
ACKNOWLEDGMENTS
Intramural research funding (“Forschungspool”) was granted to Dr. Weingärtner by the School of Medicine and Health Sciences, Carl von Ossietzky University Oldenburg.
The results presented in this paper have not been published previously in whole or part, except in the abstract form.
We thank Fabio Lizzi, Saarland University Medical Center for helpful discussion and advice.
Emrich IE, Heine GH, Schulze PC, et al. Markers of cholesterol synthesis to cholesterol absorption across the spectrum of non-dialysis CKD: An observational study. Pharmacol Res Perspect. 2021;9:e00801. 10.1002/prp2.801
Dieter Lütjohann and Oliver Weingärtner are contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Ballew SH, Matsushita K. Cardiovascular risk prediction in CKD. Semin Nephrol. 2018;38:208‐216. 10.1016/j.semnephrol.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 2. Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e535–e578. 10.1161/CIR.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 3. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395‐1407. 10.1056/NEJMoa0810177 [DOI] [PubMed] [Google Scholar]
- 4. Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238‐248. 10.1056/NEJMoa043545 [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267‐1278. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 6. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet (London, England). 2011;377:2181‐2192. 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mashnafi S, Plat J, Mensink RP, Baumgartner S. Non‐cholesterol sterol concentrations as biomarkers for cholesterol absorption and synthesis in different metabolic disorders: a systematic review. Nutrients. 2019;11:124. 10.3390/nu11010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rogacev KS, Pinsdorf T, Weingärtner O, et al. Cholesterol synthesis, cholesterol absorption, and mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:943‐948. 10.2215/CJN.05170511 [DOI] [PubMed] [Google Scholar]
- 9. Silbernagel G, Fauler G, Genser B, et al. Intestinal cholesterol absorption, treatment with atorvastatin, and cardiovascular risk in hemodialysis patients. J Am Coll Cardiol. 2015;65:2291‐2298. 10.1016/j.jacc.2015.03.551 [DOI] [PubMed] [Google Scholar]
- 10. Mackay DS, Jones PJH, Myrie SB, Plat J, Lütjohann D. Methodological considerations for the harmonization of non‐cholesterol sterol bio‐analysis. J Chromatogr B. 2014;957:116‐122. 10.1016/j.jchromb.2014.02.052 [DOI] [PubMed] [Google Scholar]
- 11. Šošić‐Jurjević B, Lütjohann D, Renko K, et al. The isoflavones genistein and daidzein increase hepatic concentration of thyroid hormones and affect cholesterol metabolism in middle‐aged male rats. J Steroid Biochem Mol Biol. 2019;190:1‐10. 10.1016/j.jsbmb.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 12. Saarland University Medical Centre n.d. http://www.uniklinikumsaarland.de/de/einrichtungen/kliniken_institute/zentrallabor/analysenspektrum_und_referenzwerte/. Accessed June 2, 2021.
- 13. Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111‐188 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 14. Cholesterol Treatment Trialists’ (CTT) Collaboration , Herrington W, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin‐based regimens: a meta‐analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016;4:829‐839. 10.1016/S2213-8587(16)30156-5 [DOI] [PubMed] [Google Scholar]
- 15. Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303‐1309. 10.1038/ki.2014.31 [DOI] [PubMed] [Google Scholar]
- 16. Fukushima M, Miura S, Mitsutake R, Fukushima T, Fukushima K, Saku K. Cholesterol metabolism in patients with hemodialysis in the presence or absence of coronary artery disease. Circ J. 2012;76:1980‐1986. 10.1253/circj.cj-11-1302 [DOI] [PubMed] [Google Scholar]
- 17. Sonoda M, Shoji T, Kimoto E, et al. Kidney function, cholesterol absorption and remnant lipoprotein accumulation in patients with diabetes mellitus. J Atheroscler Thromb. 2014;21:346‐354. 10.5551/jat.20594 [DOI] [PubMed] [Google Scholar]
- 18. Lee WC, Kuo WH, Moi SH, Chiu B, Chen JB, Yang CH. Associations between circulating markers of cholesterol homeostasis and macrovascular events among patients undergoing hemodialysis. Nutrients. 2021;13:1‐11. 10.3390/nu13031014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weingärtner O, Lütjohann D, Böhm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis. 2010;210:362‐365. 10.1016/j.atherosclerosis.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 20. Lütjohann D, Stellaard F, Mulder MT, Sijbrands EJG, Weingärtner O. The emerging concept of “individualized cholesterol‐lowering therapy”: a change in paradigm. Pharmacol Ther. 2019;199:111‐116. 10.1016/j.pharmthera.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharyya AK, Connor WE. β‐Sitosterolemia and xanthomatosis. J Clin Invest. 1974;53:1033‐1043. 10.1172/JCI107640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teupser D, Baber R, Ceglarek U, et al. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet. 2010;3:331‐339. 10.1161/CIRCGENETICS.109.907873 [DOI] [PubMed] [Google Scholar]
- 23. Myocardial Infarction Genetics Consortium Investigators , Stitziel NO, Won H‐H, Morrison AC, et al. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371:2072‐2082. 10.1056/NEJMoa1405386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weingärtner O, Weingärtner N, Scheller B, et al. Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron Artery Dis. 2009;20:376‐382. 10.1097/MCA.0b013e32832fa947 [DOI] [PubMed] [Google Scholar]
- 25. Matthan NR, Pencina M, LaRocque JM, et al. Alterations in cholesterol absorption/synthesis markers characterize Framingham Offspring Study participants with CHD. J Lipid Res. 2009;50:1927‐1935. 10.1194/jlr.P900039-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miettinen TA, Gylling H, Strandberg T, Sarna S. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. BMJ. 1998;316:1127‐1130. 10.1136/bmj.316.7138.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi J, Kawada‐Watanabe E, Koyanagi R, et al. Baseline serum sitosterol level as predictor of adverse clinical events in acute coronary syndrome patients with dyslipidaemia: a sub‐analysis of HIJ‐PROPER. Atherosclerosis. 2018;274:139‐145. 10.1016/j.atherosclerosis.2018.04.036 [DOI] [PubMed] [Google Scholar]
- 28. Fuhrmann A, Weingärtner O, Meyer S, et al. Plasma levels of the oxyphytosterol 7α‐hydroxycampesterol are associated with cardiovascular events. Atherosclerosis. 2018;279:17‐22. 10.1016/j.atherosclerosis.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 29. Bach RG, Cannon CP, Giugliano RP, et al. Effect of simvastatin‐ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older. JAMA Cardiol. 2019;4:846. 10.1001/jamacardio.2019.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid‐lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75). Circulation. 2019;140:992‐1003. 10.1161/CIRCULATIONAHA.118.039415 [DOI] [PubMed] [Google Scholar]
- 31. Strandberg TE, Tilvis RS, Pitkala KH, Miettinen TA. Cholesterol and glucose metabolism and recurrent cardiovascular events among the elderly. J Am Coll Cardiol. 2006;48:708‐714. 10.1016/j.jacc.2006.04.081 [DOI] [PubMed] [Google Scholar]
- 32. Sittiwet C, Simonen P, Gylling H, Strandberg TE. Mortality and cholesterol metabolism in subjects aged 75 years and older: the Helsinki Businessmen study. J Am Geriatr Soc. 2020;68(2):281‐287. 10.1111/jgs.16305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1‐S3‐FigS1‐S2
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


