Abstract
Background
COVID-19 has mutation capability, and there are no specific drug therapies that are available to fight or inhibit the proteins of this virus. The present study aims to investigate the binding affinity of the bioactive and synthetic compounds with the main protease (Mpro) enzymes and angiotensin-converting enzyme 2 (ACE 2) by computational approach. PASS prediction, pharmacokinetics, and toxicological properties prediction studies were performed through the Google PASS prediction and Swiss ADME/T website. Besides, molecular docking studies were accomplished by BIOVIA Discovery Studio 2020, UCSF Chimera, and PyRx autodock vina.
Results
The docking scores were inferred and the selected compounds showed results varying from −3.2 to −9.8 (kcal/mol). Theaflavin scored the highest docking score to the 5REB, 6VW1, and 1R42 enzymes and showed the binding affinity as −6.3 kcal/mol, −9.8 kcal/mol, and −8.6 kcal/mol, respectively. Again, kaempferol showed the best binding affinity to the 7BQY (−7.1 kcal/mol) and 6Y2FB (−6.6 kcal/mol) enzymes. All the chemical constituents showed better probability in action in pass prediction analysis. Besides, no ligands (except theaflavin) have any conflict with Lipinski’s rules of five, which authorized the drug probability of these ligands.
Conclusion
Therefore, the selected compounds could be considered a potential herbal treatment source against SARS-CoV-2.
Keywords: SARS-CoV-2, Theaflavin, Kaempferol, Drug targets, Molecular docking
Background
According to the WHO (World Health Organization), SARS-CoV-2 is now a pandemic crisis. It first appeared in the Wuhan province of China on 31 December 2019, and spread rapidly in the different parts of the world. Till 30 April 2021, there are 151,837,341 confirmed cases of 2019-nCoV infection, with 3,188,507 deaths found around the globe. The causative agent was identified from throat swab samples conducted by the Chinese Center for Disease Control and Prevention (CCDC) on 7 January 2020, and was subsequently named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The disease was named COVID-19 by the WHO [1]. Most SARS-CoV-2 infected patients are seen with symptoms such as dry cough, sore throat, and fever. The majority of cases have spontaneously resolved. However, some have developed various fatal complications, including organ failure, septic shock, pulmonary edema, severe pneumonia, and acute respiratory distress syndrome (ARDS) [2]. WHO declared the Chinese outbreak of COVID-19 to be a public health emergency of international concern, posing a high risk to countries with vulnerable health systems on 30 January 2020. The emergency committee has stated that the spread of COVID-19 may be interrupted by early detection, isolation, prompt treatment, and the implementation of a robust system to trace contacts [3].
Coronaviruses belong to the Coronaviridae family in the Nidovirales order. Corona contains crown-like spikes on the outer surface of the virus; thus, it was named coronavirus. Coronaviruses are minute in size (65-125 nm in diameter) and contain a single-stranded RNA as a nucleic material, varying in length from 26 to 32 kbs. The subgroups of the coronaviruses family are alpha (α), beta (β), gamma (γ), and delta (δ) coronavirus [4]. SARS-CoV-2 fits the beta Coronavirus group [5]. COVID-19 (SARS-CoV-2) is composed of a spike protein (S), a membrane glycoprotein (M), a hemagglutinin-esterase dimer (HE), an envelope protein (E), a nucleocapsid protein (N), and an RNA. Spike glycoproteins are composed of two subunits (S1 and S2). Homotrimers of S proteins compose the spikes on the viral surface, guiding the link to host receptors [5, 6]. Nucleocapsid protein (N) binds in vitro to RNA and is heavily phosphorylated. N protein binds with the viral genome in a bead on a string type conformation, and E protein is found in small quantities within the virus, whereas the most abundant structural protein is the M protein. This M protein contains no signal sequence and is present in the virion as a dimer. HE is present in a subset of beta coronaviruses and binds sialic acids on surface glycoproteins [7]. Currently, no specific therapies for SARS-CoV-2 are available and investigations regarding the treatment of SARS-CoV-2 are lacking [8]. The viral genome also encodes nonstructural proteins which can be potential drug targets, including RNA-dependent RNA polymerase (RdRp), CoV main protease (3CLpro), and papain-like protease (PLpro) [9, 10].
SARS-CoV-2 (2019-nCoV) outbreak has become a global pandemic that has raised the concern of the scientific community to design and discover a definitive cure for this deadly virus. Research institutions are accelerating the discovery of vaccines and therapies for the SARS-CoV-2. During the epidemic and pandemic outbreak of new viral pathogens, the conventional method of developing drugs and vaccination is not possible to control as it is a time-consuming process [11]. Consequently, the rapid approach based on in silico informatics has become very popular with recent advances in sequencing many pathogen genomes and protein sequence databases [12]. The steady rise of corona patient’s with high mortality rate reinforces the urgency to produce a safe and efficient vaccine. The pharmacological effect of phytochemicals can be adequately explained by the use of virtual screening [13]. Methods and resources like computer-aided drug discovery (CADD) is also an effective way to design new pharmaceutical products. An effective molecular interaction through molecular docking can activate the native ligand to detect the three-dimensional binding site of the enzyme and link to the relationship to the accompanying chemical compounds [14]. Taking the truth into account, a molecular docking study has been conducted to explain the phytoconstituents binding affinity with the five SARS-CoV-2 receptors. In this work, pass prediction, molecular docking, and pharmacokinetic regarding Lipinski’s rules were checked for twelve ligands. To investigate the binding affinity of the ligands and SARS-CoV-2’s receptors, the ligands were docked with the main protease and human angiotensin-converting enzyme 2 (ACE2) receptors of the SARS-CoV-2 virus owing to the best-characterized target among the SARS-CoV-2. Mainly, the binding interaction of caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, and hydroxychloroquine (Fig. 1) against the proteins of COVID-19 have been screened by implying molecular docking simulations. The main protease (Mpro) (PDB ID: 7BQY, 6Y2F, 5REB) and human angiotensin-converting enzyme 2 (PDB ID: 6VW1, 1R42) were used as the target of the ligand interaction (Fig. 2). It was also previously reported that polyphenols, flavonoids, tannin, and derivate have fighting endeavors against many virions [15–17]. These components were chosen based on their therapeutic properties to bind with the SARS-CoV-2 proteins. Again, the mechanism of action of the established antiviral compounds like favipiravir and hydroxychloroquine is not fully uncovered. These compounds have the probable capacity to bind with the pockets of SARS-CoV-2; the study was encouraged to investigate the potential binding affinity of selected proteins with the previously selected ligands.
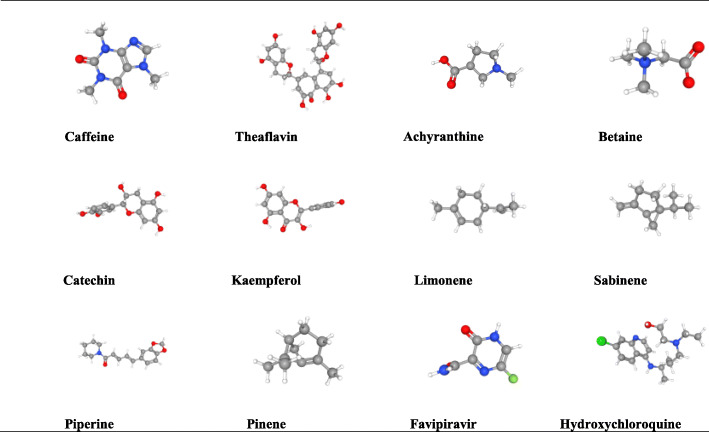
Fig. 1.
3D chemical structure of selected ligands (caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, hydroxychloroquine)
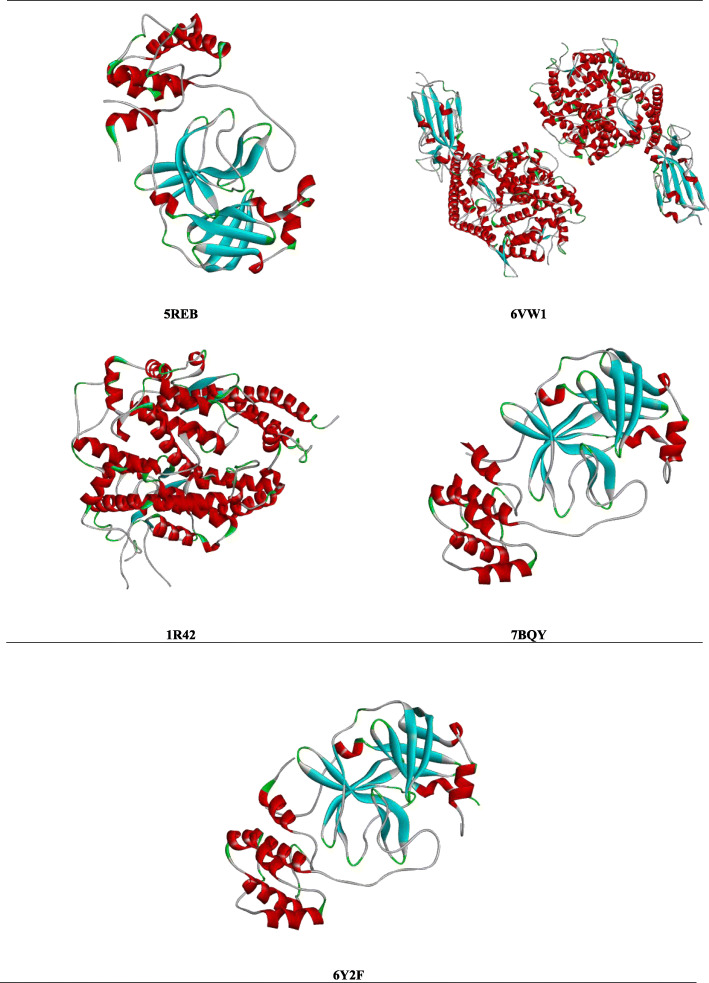
Fig. 2.
3D structure of the pure receptors (5REB, 6VW1, 1R42, 7BQY, and 6Y2F) of SAR-CoV-2
Methods
Bioactive constituents from black tea (Camellia sinensis L.)
Tea (Camellia sinensis L.) is the second most used non-alcoholic beverage in the world after water. It has been consumed traditionally for about 5000 years. Black tea has 3 to 6% thearubigin, theaflavin, phenolics, and catechin. Thearubigins of tea are responsible for the deep red color of the tea, while theaflavins are accountable for the astringency and red/orange color of the tea. In black tea, the highest number of phenolic pigments in black tea is thearubigin. Theaflavins individually claimed to show antiviral potency [18]. Caffeine is one of the most popular natural stimulants which can be found in tea, cacao, and coffee [19]. A cup of black tea may contain 47 to 90 mg of caffeine [20]. Caffeine seems to have a strong behavioral effect which is widely used as CNS stimulants. The phenolic component of tea was reported to have antiviral efficacy over adenovirus [21, 22].
Bioactive constituents from prickly chaff (Achyranthes aspera L.)
The herb is well known owing to have anti-inflammatory, antidot (snake and scorpion bite), carminative, gastric, blood purifier, and diuretic effects. Decoction of the entire plant has been used in ascites, eczema, gastritis, skin rashes, and boils, and juice of the leaves often used to treat toothache [23]. The constituents of this pant are also reported to have antiviral properties [24, 25]. Though the entire plant ash has been used in herbal medicines for asthma, gaseous distention, urinary bladder stones, gastritis, and cough, honey ash can also be used to alleviate cough [26]. Achyranthine and betaine are abundantly found in A. aspera [27]. Betaine (BET) is known to protect the liver from toxicants as an indigenous drug. Betaine inhibits hepatitis B viruses with the advantages of reducing resistance to interferon and lamivudine [28].
Bioactive constituents from catechu (Areca catechu Linn.)
Areca nut (Areca catechu) is frequently used in betel quid, often consumed in betel and lime. Betel quid chewing seems to be extremely common, particularly in many countries in Southeast Asia [19]. Areca catechu is one of 54 recognized alkaloid-containing species in the Areca [29]. Several active ingredients such as arecatannin B1, procyanidins, and seed extracts have shown HIV enzyme inhibitory function. Alkaloids (0.5 %), phenol (31.1%), fiber (10.8%), fat (14.0%), and polysaccharide (18.7%) are key elements of the fruits of Areca catechu L. Alkaloids and polyphenols are the primary components in flower [30]. In Areca catechu, catechin and kaempferol were identified previously [31].
Bioactive constituents from lemon (Citrus limon)
Citrus limon is a flowing plant in the Rutaceae family [32]. The compounds of Citrus plants are the most common essential oil sources used in food products and medicines. Lemon seems to be very abundant in natural substances such as flavonoids, citric acid, minerals, ascorbic acid, and essential oils. Although modern citrus cultivars were primarily designed for fresh use, their naturally drawn constituents, such as the flavonoids and phenolic compound, contributed to their use throughout food technology and pharmacological research [33]. Valuable academic publications concentrate on lemon fruit extract, juice, and essential oil, which are becoming much more comprehensive in pharmacological terms. These reports claim antiviral, antibacterial, anti-cancer, anti-inflammatory, and cardioprotective activities of lemon [34]. Sabinene (15.9%) and limonene (31.5%), are the major constituents of the citrus lemon [35]. Citrus flavonoids exert several pharmacological potentialities, including antiviral action [33].
Bioactive constituents from black pepper (Piper nigrum)
Among the kitchen spices, pepper is one of the most commonly used spices around the globe. Pepper contains piperine and due to the pungent odor of the piperine, it has taken place as a kitchen spice. It is commonly used and widely permissible in various western medicinal systems, such as Unani and Ayurvedic [36]. Many illnesses, including antihypertensives [37, 38], antiplatelets, antioxidant, analgesic, antitumor, antithyroid, anticonvulsant [39], antibacterial, antidiarrheal, antidepressants, antispasmodic, immunomodulatory, anti-inflammatory, hepatoprotective, and antifungal have already been treated by this plant [37]. As a result, studies on its derivative synthesis, SAR modification and biological tests are underway and this has encouraged researchers to keep investigating it [40]. Piperine is also used in both conventional Chinese and Indian therapies. In influenza, nausea, rheumatoid arthritis, fever, and chills, piperine is used extensively [41]. Again, the previous studies reported that pinene has a strong suppression capacity for the herpes simplex virus [42]. It has been used for centuries to manufacture aromas and fragrances and demonstrates fungicidal activity. Various pharmacological activities, including natural insecticide, are attributed to pinenes. The studies showed strong antiviral potency of pinens against infectious bronchitis virus [42].
Synthetic bioactive compounds (favipiravir and hydroxychloroquine)
Favipiravir and hydroxychloroquine are under examination for the treatment of SARS-CoV-2, but the antiviral efficacy of these drugs is not apparent [43]. Favipiravir is a nucleoside precursor approved for the treatment of pandemic influenza in Japan [44]. However, its antiviral effect in patients with COVID-19 needs demanding data to support [45]. Hydroxychloroquine is a safer analog of chloroquine that has fewer concerns about drug-drug interactions [46]. Over one research, patients with combined treatment had lower viral loads in contrast to patients with a similar viral load-receiving hydroxychloroquine alone [47]. The suppression of cytokine release has been accelerated by downregulating the inflammatory effect of the drug [48]. Inhibition of cytokines reduces the elevation of chemotaxin of polymorphonuclear leucocytes in the lungs, which finally decreases the orientation of reactive oxygen [49].
Protein targets
Several approaches have been taken to establish CoV vaccines, most of which aim against spikes (S) or S-proteins, as they are the primary adjuvant to collapse antibodies. The main protease (M-pro) enzyme has been described as one of the primary goals for producing antiviral vaccines or drugs [50]. M-pro is present in the SARS-CoV-2’s polyprotein ORF1ab and is important for virus replication. This protease participates in the degeneration of polyprotein [51]. Except for a residue (Ala285Thr), the association of the M-pro enzyme has been highly identified in the SARS-CoV virus [52]. In addition, two subunits S1 and S2, are found in the S protein molecule. Again, the ACE-2 receptor is linked to the S2 subunit and within this receptor, the S2 subunit forms fusion between the membrane of the host cell and the virus. As a result, the viral RNA can access the cytoplasm of the viral cell and replicate again [53]. Vaccine regarding S protein can also trigger antibodies that obstruct the binds of a viral receptor and even the uncoating of the viral genome in the cytoplasm. The S protein-based vaccine will play a major role in inducing protective immunity from SARS-CoV infection by neutralizing the T cell responses and triggering antibodies [53]. Based on the full-length genome phylogenetic study, SARS-CoV-2 has an almost 89% similarity with the SARS-CoV [54]. This was the foundation for the initial development of SARS-CoV-2 and indicated that the receptor of SARS-CoV-2 may be consistent with the receptor of SARS-CoV (ACE2) [55]. SARS-CoV-2 is reported to use angiotensin-converting enzyme 2 (ACE2) receptors to penetrate the target cells [56]. Therefore, any agent increasing ACE2 production may be anticipated to improve its susceptibility to intense COVID-19 by enhancing viral cellular invasion. However, biochemically, Angiotensin II is converted to angiotensin (1–7), defending lung damage by reducing the ACE2 receptor and vasodilation [57]. The patients with COVID-19 and co-morbidities such as hypertension, cardiovascular disorder, and diabetes that often have angiotensin-converting enzyme inhibitors ACEIs or angiotensin receptor blockers ARBs, there is often contradictory evidence about continuity or discontinuation of medications inhibiting the renin-angiotensin aldosterone system (RAAS), including inhibitors of angiotensin-converting enzyme (ACEIs) and angiotensin receptor blockers (ARBs) [58]. 5REB [59], 7BQY [60], and 6Y2F [61] have been previously denoted as main protease (Mpro) of SARS-CoV-2, and 6VW1 [62] and 1R42 [63] have been denoted as human angiotensin-converting enzyme 2 (ACE2). Taking all these considerations into account, five proteins (5REB, 7BQY, 6Y2F, 6VW1, 1R42) have been selected to comply with the binding interactions between proteins and ligands.
Pass prediction
Caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, and hydroxychloroquine were allowed to predict the antiviral activity by using the PASS online server [64]. PASS online server predicts the activity as probable of activity (Pa) and probable inactivity (Pi).
Molecular analysis: compounds and target proteins
Three-dimensional structures of the main protease in complex (hydrolase receptor) (PDB: 5REB) [65], the structure of SARS-CoV-2 chimeric receptor-binding domain complexed with its human receptor ACE2 (PDB: 6VW1) [66], native human angiotensin-converting enzyme-related carboxypeptidase (ACE2) (PDB: 1R42) [67], the crystal structure of the COVID-19 main protease (PDB: 7BQY) [68], and crystal structure (monoclinic form) of the COVID-19 main protease were downloaded in PDB format. Besides, the chemical compounds have been selected based on the bioactive dietary compounds and synthetic compounds (caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, and hydroxychloroquine) including antiviral properties. The compounds have been derived from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) [69] in SDF format.
Molecular analysis: preparation of target protein and compounds
The ligands have been imported in 2DSDF format; hereafter, the ligands have been minimized and converted to pdbqt format across the PyRx tools to find the best hit in these targets. The protein structure was created with Discovery Studio and UCSF Chimera. Default settings in the PyRx from the MGL tools [70] have been used for the virtual screening. Through the Discovery Studio 2020, all water and the heteroatom were eliminated from proteins. A combination of non-polar hydrogen and the Gasteiger charge was used to assemble proteins. Besides, extra solvents were deleted, selenomethionine (MSE), methionine (MET), bromo UMP to UMP (U), methylselenyl-dUMP (UMS), to UMP (U), methylselenyl-dCMP (CSL) to CMP (C) have been marked to keep only highest occupancy. Again, the incomplete side chains were replaced by Dunbrack 2010 rotomer library. Furthermore, all proteins have been lowered to the least energy level by keeping the residues in AMBER ff14sB and Gasteiger mode in UCSF Chimera [71].
Molecular analysis: molecular docking
For the protein-ligand binding operation of the selected protein-ligand complexes, PyRx Autodock Vina was used [26]. A semi-flexible docking system has been applied to perform the docking research. A semi-flexible docking system has been applied to perform the docking research. The phytochemicals were translated into PDBQT formats with PyRx AutoDock tools. The rigidity of proteins and ligands was retained for this analysis. Ligand molecules had given the freedom for 10 degrees. AutoDock determines the molecules to format pdbqt, box style, grid box formation, etc. The grid box with an active position was built in the middle of the box. BIOVIA Discovery Studio Visualizer 2020 [72] was eventually accelerated to evaluate the docking sites for the possible linking approaches.
Determination of pharmacokinetic parameters by Swiss ADME
Lipinski’s rule evaluates the different descriptors which are important for drug design. According to Lipinski’s rule, an orally active drug should fulfill the following drug-likeness parameters to demonstrate their pharmaceutical fidelity such as (i) molecular mass less than 500 Daltons, (ii) no more than 5 H-bond donors, (iii) no more than 10 H-bond acceptors, and (iv) O/W partition coefficient log P not greater than 5. If the molecule violates more than 3 descriptor parameters, it will not fit into the criteria of drug likeliness, and it is not considered to proceed with drug discovery.
Results
Pass prediction of selected ligands
The results of the PASS prediction have been presented in Table 1. The study revealed that, among the four compounds, favipiravir and theaflavin retained the highest drug probability Pa (0.662 and 0.609).
Table 1.
Pass prediction of caesalpinine A, diffutidin, favipiravir, hydroxychloroquine antiviral activity
| Chemical constituents | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiviral activity | Caffeine | Theaflavin | Achyranthine | Betaine | Catechin | Kaempferol | Limonene | Sabinene | Piperine | Pinene | Favipiravir | Hydroxychloroquine |
| Pa | 0.513 | 0.609 | 0.590 | 0.525 | 0.520 | 0.496 | 0.574 | 0.282 | 0.221 | 0.346 | 0.662 | 0.323 |
| Pi | 0.045 | 0.012 | 0.021 | 0.040 | 0.018 | 0.005 | 0.009 | 0.105 | 0.167 | 0.066 | 0.008 | 0.207 |
Pa probability of activity, Pi probability of inactivity
Molecular docking study for SARS-CoV-2 inhibition
In the case of the antiviral docking study, the selected compounds were docked against the SARS-CoV-2’s main protease (PDB: 5REB, 7BQY, 6Y2F), and angiotensin-converting enzyme 2 (PDB: 6VW1, 1R42) enzymes and displayed docking scores ranging from −3.2 to −9.8 (kcal/mol). The docking score and the best interaction figure have been shown in Table 2 and Fig. 3. From the findings, it was observed that the compound theaflavin exposed the highest score against PDB ID: 5REB, 6VW1, and 1R42, followed by kaempferol attained the highest binding affinity with the pockets of the main protease enzyme (Mpro) (7BQY and 6Y2F). The ranking of docking score for antiviral (SARS-CoV-2) effect against PDB ID: 5REB is as follows: theaflavin > kaempferol > favipiravir > hydroxychloroquine > caffeine > limonene > sabinene > achyranthine > betaine. Docking observed docking score and with the selected components and PDB ID: 6VW1 receptor, the scores were observed: theaflavin (−9.8 kcal/mol), kaempferol (7.0 kcal/mol), piperine (−6.8 kcal/mol), catechin (−6.7 kcal/mol), hydroxychloroquine (5.4 kcal/mol), limonene (4.8 kcal/mol), caffeine (4.8 kcal/mol), favipiravir (−4.7 kcal/mol), sabinene (4.5 kcal/mol), pinene (4.5 kcal/mol), achyranthine (4.1 kcal/mol), and betaine (3.2 kcal/mol) and respectively. Theaflavin yields the highest docking score against the 1R42 receptor. Theaflavin binds to the pocket of the 1R42 receptor through a series of bonds: conventional hydrogen bond (arg518, thr371), Pi-anion (glu406, glu375), and Pi-alkyl (leu370). The order of docking score against PDB ID: 7BQY is as follows: kaempferol > catechin > theaflavin > piperine > hydroxychloroquine > favipiravir > limonene > pinene > achyranthine > sabinene > betaine. Caffeine does not conjugate and neither oriented any docking to this ACE2 receptor. Again, kaempferol also exerted the best docking score as well as the best binding affinity to the main protease enzyme (PDB: 6Y2F). Kaempferol obtained the highest binding score via interaction of the amino acid series (asn158, phe294, pro293, and thr292) of the protein.
Table 2.
Docking score of the selected dietary bioactive and synthetic compounds
| Compounds | Docking score | ||||
|---|---|---|---|---|---|
| 5REB | 6VW1 | 1R42 | 7BQY | 6Y2F | |
| Caffeine | −5.1 | −4.8 | −5.7 | - | −5.0 |
| Theaflavin | −6.3 | −9.8 | −8.6 | −6.7 | −6.4 |
| Achyranthine | −4.4 | −4.1 | −4.0 | −4.2 | −4.7 |
| Betaine | −3.5 | −3.2 | −3.2 | −3.4 | −3.5 |
| Catechin | - | −6.7 | −6.9 | −7.0 | −6.5 |
| Kaempferol | −6.2 | −7.0 | −6.8 | −7.1 | −6.6 |
| Limonene | −4.6 | −4.8 | −4.7 | −4.4 | −5.2 |
| Sabinene | −4.5 | −4.5 | −4.8 | −4.1 | −4.7 |
| Piperine | - | −6.8 | −7.0 | −6.5 | −7.1 |
| Pinene | - | −4.5 | −4.5 | −4.4 | −4.6 |
| Favipiravir | −5.4 | −4.7 | −5.2 | −5.5 | −5.3 |
| Hydroxychloroquine | −5.4 | −5.6 | −5.8 | −5.7 | −6.0 |
Fig. 3.
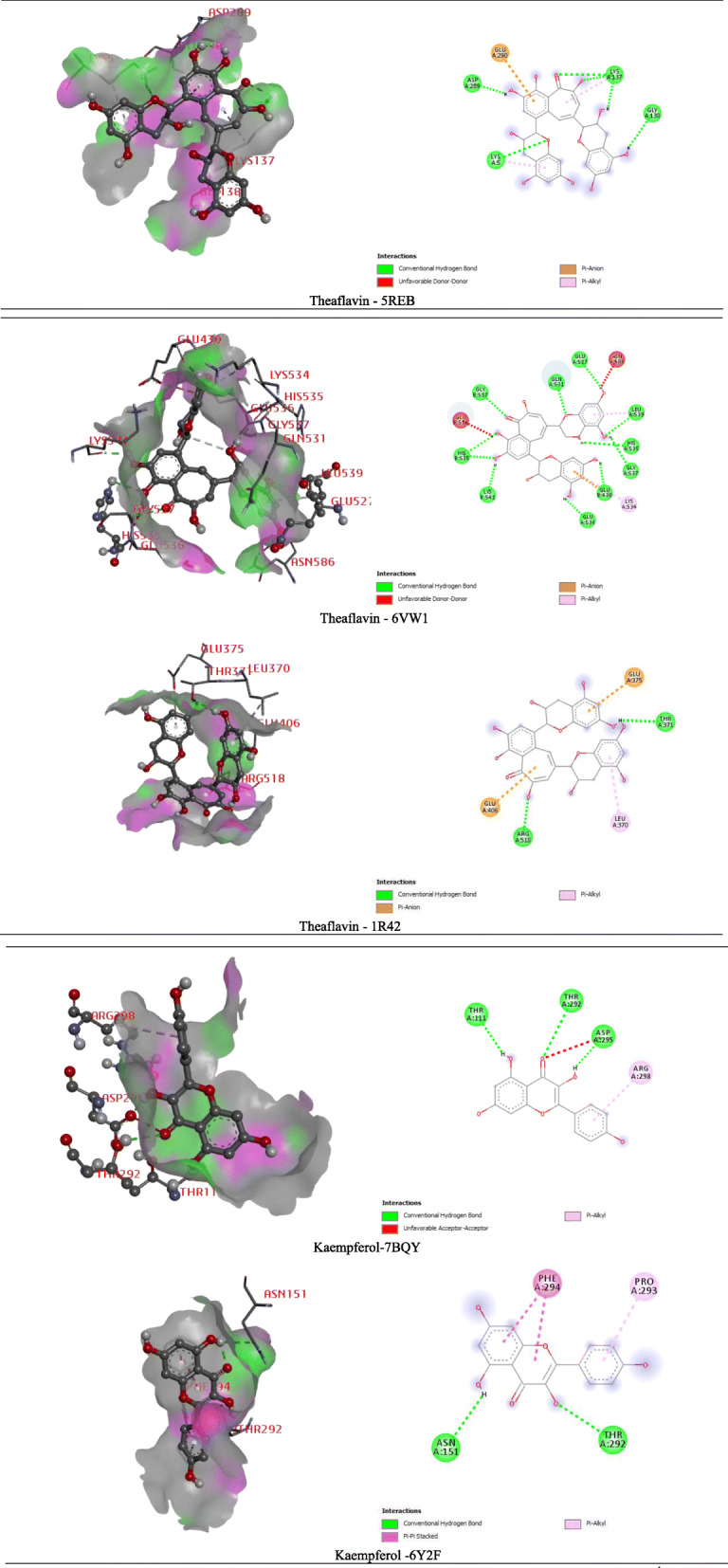
The presentation of the best binding interactions of the selected bioactive dietary components and synthetic compounds with the five SARS-CoV-2 proteins (main protease and angiotensin-converting enzyme 2 receptors)
Pharmacokinetics (ADME) and toxicological properties prediction
The study was performed to check the pharmacokinetics and the toxicological properties of the caffeine, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, and sabinene, piperine, pinene, favipiravir, and hydroxychloroquine. The pharmacokinetics and the toxicological properties of the selected ligands for the antiviral (SAR-CoV-SAR) efficacy have been shown in Table 3. The study revealed no violation of Lipinski’s five rules and the ligands does not contain carcinogens.
Table 3.
Absorption, distribution, metabolism, excretion, and toxicological properties of the selected compounds
| Compounds | Molecular Weight (M.W) (g/mol) | HBD | HBA | log P (o/w) | HIA | Carcinogenicity (binary) | Violation score |
|---|---|---|---|---|---|---|---|
| Caffeine | 194.19 | 0 | 6 | −1.03 | 0.9824 | 0.9429 | 0 |
| Theaflavin | 564.50 | 12 | 9 | 2.21 | 0.9836 | 0.9571 | 3 |
| Achyranthine | 129.16 | 1 | 2 | 0.02 | 0.9021 | 0.9571 | 0 |
| Betaine | 117.15 | 0 | 2 | −1.56 | 0.9333 | 0.7316 | 0 |
| Catechin | 290.27 | 5 | 6 | 1.55 | 0.9887 | 0.9286 | 0 |
| Kaempferol | 286.24 | 4 | 6 | 2.28 | 0.9881 | 0.6985 | 0 |
| Limonene | 136.24 | 0 | 0 | 3.31 | 0.9692 | 0.5856 | 0 |
| Sabinene | 136.24 | 0 | 0 | 3.00 | 0.9828 | 0.7286 | 0 |
| Piperine | 285.34 | 0 | 3 | 3.00 | 0.9639 | 0.9198 | 0 |
| Pinene | 136.24 | 0 | 0 | 3.14 | 0.9677 | 0.7286 | 0 |
| Favipiravir | 157.10 | 2 | 3 | −0.99 | 0.9612 | 0.9286 | 0 |
| Hydroxychloroquine | 335.88 | 2 | 4 | 3.78 | 0.9934 | 0.8429 | 0 |
HBD hydrogen bond donor, HBA hydrogen bond acceptor, LogP lipophilicity, HIA human intestinal absorption
Discussion
Molecular docking and computer-aided drug design (CADD) are vital tools in structural molecular biology. This tool contributes to predicting the binding mode of active compounds against the targeted proteins [73]. Additionally, it is used to comprehend the possible molecular mechanism of actions of various pharmacological activities [74]. The molecular docking was also performed to correlate with our current research outcomes and better understand the molecular mechanism [72]. In this study, twelve bioactive and synthetic compounds were examined against five targeted receptors or enzymes. The ideal antiviral drug candidate should have low toxicity, appropriate pharmacokinetics, and preferably a large spectrum of activity. Many adjuvants failed to be clinically used due to the lack of one or more of these properties [75]. In this study, 10 bioactive dietary constituents have been selected owing to their wide availability with antiviral efficacy. Besides, favipiravir and hydroxychloroquine have been pronounced to antiviral efficacy in previous investigations, though the investigations were not successfully proven [76–79]. The search for effective inhibitors of 5REB, 6Y2F, 6VW1, 1R42, and 7BQY has been the focus of drug discovery efforts and has led to the identification of some promising leads (caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, and hydroxychloroquine) have shown good inhibitory activity on selective enzymes. Theaflavin yielded highest binding score among all the selected compounds. It scored −6.3, −9.8, and −8.6 (kcal/mol) upon it was docked with the 5REB, 6VW1, and 1R42 enzymes. Theaflavin binds to the pocket of 6VW1 receptor through a series of bonds: conventional hydrogen bond (lys541, his535, gly537, gln531, glu527, leu539, gly537, glu430, gly536), Pi-alkyl (lys534), unfavorable donor (glu536, asn526), and resulted best binding affinity among the selected compounds. Besides, kaemperol showed highest binding affinity to the rest of the receptors (Fig. 3 and Table 2).
Favipiravir is one of the antiviral drugs tested against several SARS-CoV-2 envelope proteins [80]. Favipiravir, a pyrazine carboxamide derivative molecule, works as an antiviral molecule that is a prodrug against RNA viruses. This antiviral activity is attenuated by the competitive nature of favipiravir with purine nucleoside rather than pyrimidine nucleosides. Madin Darby Canine Kidney (MDCK) cell treated with favipiravir during in vitro assay provided favipiravir ribofuranosyl-5′-triphosphate (favipiravir-RTP), favipiravir ribofuranose (favipiravir-R), and favipiravir ribofuranosyl-5′-monophosphate (favipiravir-RMP) in HPLC analysis as metabolites among which chemically synthesized favipiravir-RTP showed positive outcome to inhibit the viral RNA polymerase activity in concentrations ranging from nanomolar to micromolar. This assay infers the projection that favipiravir, a prodrug, emits the antiviral property when it is intra-cellularly phosphoribosylated to be an active form, favipiravir-RTP to halt RNA polymerase. When a single molecule of favipiravir-RTP is efficiently incorporated into a nascent RNA strand, a partial inhibition of extension in viral RNA is observed and when double incorporation takes place, complete inhibition results in [81].
Despite not being an elucidated absolute mechanism of action of hydroxychloroquine against coronavirus, several mechanisms to elucidate its antiviral property are already proposed. Being a weekly basic drug, hydroxychloroquine upraises the pH within cells and at the cellular membrane, consequently inhibiting the virus’s ability to fuse onto the cell membrane and enter host cells. Another proposed hydroxychloroquine mechanism includes inhibition of DNA and RNA synthesis along with immunomodulating and anti-inflammatory effects [82]. Hydroxychloroquine also infers the constraint of glycosylation of viral proteins, virus assembly, new virus particle transport, virus release, and other processes to achieve its antiviral effects [83]. Docking studies indicated that the binding interaction of selected bioactive and synthetic constituents with 5REB, 6Y2F, 6VW1, 1R42, and 7BQY had been concluded that the selected constituents might, in part, be responsible for the antiviral activity against SARS-CoV-2 through interactions with these target enzyme or receptor.
Conclusions
Theaflavin and kaempferol exert a high binding affinity to the pockets of SARS-CoV-2 proteins. Interaction of caffeine, theaflavin, achyranthine, betaine, catechin, kaempferol, limonene, sabinene, piperine, pinene, favipiravir, and hydroxychloroquine with main protease and angiotensin-converting enzyme 2 (ACE-2) proteins is ensured by molecular docking, which accredits the assertion of remarkable antiviral properties of these test moieties. Owing to design drugs for anti-COVID-19, this is just a primary investigation and further investigations are suggested to confirm the capacity of the ligands.
Acknowledgements
The authors of this manuscript are thankful to the “The Article” (Road to Drug Discovery) and A.T.M. Mostafa Kamal, Chairman, Department of Pharmacy, International Islamic University for the instrumental support and directions.
Authors’ contributions
NUE: methodology, conceptualization; designing; investigation; formal analysis; software; writing-original draft; review and editing; supervision. MMA: methodology; formal analysis. IA: formal analysis; visualization; writing-original draft. SA: formal analysis; software. AAS: data curation; validation. MI: data curation; validation. MA: investigation. MHC: methodology. AM: writing-original draft. SH: writing-original draft. The authors read and approved the final manuscript.
Funding
This research does not obtain any grant or funding from any individuals or organizations.
Availability of data and materials
Data and material are available upon request.
Declarations
Ethics approval and consent to participate
The ethical consideration for this study is not applicable
Consent for publication
Not consent to publish.
Competing interests
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nazim Uddin Emon, Email: nazim7emon@gmail.com.
Md. Munsur Alam, Email: nurarafat111222@gmail.com.
Irin Akter, Email: irinmaftuha01688@gmail.com.
Saima Akhter, Email: saima.iiuc16@gmail.com.
Anjuman Ara Sneha, Email: anjumansneha2@gmail.com.
Md. Irtiza, Email: irtizairtiza8@gmail.com
Marufa Afroj, Email: marufaafroj72@gmail.com.
Arifa Munni, Email: arifamunni10@gmail.com.
Masruba Hossen Chowdhury, Email: masruba9653s@gmail.com.
Summiya Hossain, Email: summiya8303@gmail.com.
References
- 1.Organization, W. H (2020) Director-General’s remarks at the media briefing on 2019-nCoVon 11 February 2020, Internet. World Health Organization.
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization, W. H (2020). Novel coronavirus (2019-nCoV): situation report, 3.
- 4.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19). In StatPearls Internet., StatPearls Publishing [PubMed]
- 6.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Morales AJ, MacGregor K, Kanagarajah S, Patel D, Schlagenhauf P. Going global–travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Bartlam M, Rao Z. Drug design targeting the main protease, the Achilles’ heel of coronaviruses. Curr Pharm Des. 2006;12(35):4573–4590. doi: 10.2174/138161206779010369. [DOI] [PubMed] [Google Scholar]
- 10.Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 11.Biondi-Zoccai G, Landoni G, Carnevale R, Cavarretta E, Sciarretta S, Frati G. SARS-CoV-2 and COVID-19: facing the pandemic together as citizens and cardiovascular practitioners. Minerva Cardioangiol. 2020;68(2):61–64. doi: 10.23736/S0026-4725.20.05250-0. [DOI] [PubMed] [Google Scholar]
- 12.Kapetanovic I. Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem Biol Interact. 2008;171(2):165–176. doi: 10.1016/j.cbi.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emon NU, Alam MM, Uddin Sawon MS, Rana EH, Afroj M, Hasan Tanvir MM. Biological and computational studies provide insights into Caesalphinia digyna Rottler stems. Biochemistry Biophys Rep. 2021;26:100994. doi: 10.1016/j.bbrep.2021.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emon NU, Alam S, Rudra S, Chowdhury S, Rajbangshi JC, Ganguly A. Advances in traditional medicine. 2020. Evaluation of pharmacological potentials of the aerial part of Achyranthes aspera L.: in vivo, in vitro and in silico approaches. [Google Scholar]
- 15.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51(1):116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tait S, Salvati AL, Desideri N, Fiore L. Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antivir Res. 2006;72(3):252–255. doi: 10.1016/j.antiviral.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Muhamad M, Kee LY, Rahman NA, Yusof R. Antiviral actions of flavanoid-derived compounds on dengue virus type-2. Int J Biol Sci. 2010;6:294. doi: 10.7150/ijbs.6.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zu M, Yang F, Zhou W, Liu A, Du G, Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antivir Res. 2012;94(3):217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Chu N-S. Sympathetic response to betel chewing. J Psychoactive Drugs. 1995;27(2):183–186. doi: 10.1080/02791072.1995.10471690. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Saluja M, Agarwal G, Alam M. Green tea: a boon for periodontal and general health. J Indian Soc Periodontol. 2012;16(2):161–167. doi: 10.4103/0972-124X.99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark K, Grant P, Sarr A, Belakere J, Swaggerty C, Phillips T, Woode G. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet Microbiol. 1998;63(2-4):147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhatre S, et al (2020) Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine p. 153286 [DOI] [PMC free article] [PubMed]
- 23.Akbar S (2020) Achyranthes aspera L.(Amaranthaceae). In: Handbook of 200 medicinal plants. Springer, Cham, pp 69–80
- 24.Goyal BR, Goyal RK, Mehta AA (2007) PHCOG REV.: plant review phyto-pharmacology of Achyranthes aspera: a review. Pharmacogn Rev 1(1)
- 25.Mukherjee H, Ojha D, Bag P, Chandel HS, Bhattacharyya S, Chatterjee TK, Mukherjee PK, Chakraborti S, Chattopadhyay D. Anti-herpes virus activities of Achyranthes aspera: an Indian ethnomedicine, and its triterpene acid. Microbiol Res. 2013;168(4):238–244. doi: 10.1016/j.micres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Emon NU, Alam S, Rudra S, Chowdhury S, Rajbangshi JC, Ganguly A. Advances in traditional medicine. 2020. Evaluation of pharmacological potentials of the aerial part of Achyranthes aspera L.: in vivo, in vitro and in silico approaches; pp. 1–14. [Google Scholar]
- 27.Abhaykumar K. Phytochemical studies on Achyranthes aspera. World Scientific News. 2018;100:16–34. [Google Scholar]
- 28.Zhang M, Wu X, Lai F, Zhang X, Wu H, Min T. Betaine inhibits hepatitis B virus with an advantage of decreasing resistance to lamivudine and interferon α. J Agric Food Chem. 2016;64(20):4068–4077. doi: 10.1021/acs.jafc.6b01180. [DOI] [PubMed] [Google Scholar]
- 29.Evans W. Trease and Evans Pharmacognosy Bailliere Tindall, London. 1989. pp. 216–217. [Google Scholar]
- 30.Peng W, Liu Y-J, Wu N, Sun T, He X-Y, Gao Y-X, Wu C-J. Areca catechu L.(Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164:340–356. doi: 10.1016/j.jep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Shen D, Wu Q, Wang M, Yang Y, Lavoie EJ, Simon JE. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization− mass spectrometry. J Agric Food Chem. 2006;54(9):3219–3224. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 32.Hsouna AB, Halima NB, Smaoui S, Hamdi N. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:1–11. doi: 10.1186/s12944-016-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Rio J, Fuster M, Gómez P, Porras I, Garcıa-Lidón A, Ortuño A. Citrus Limon: a source of flavonoids of pharmaceutical interest. Food Chem. 2004;84:457–461. doi: 10.1016/S0308-8146(03)00272-3. [DOI] [Google Scholar]
- 34.Kim J, Jayaprakasha GK, Uckoo RM, Patil BS. Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem Toxicol. 2012;50(2):423–430. doi: 10.1016/j.fct.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Klimek-Szczykutowicz M, Szopa A, Ekiert H. Citrus Limon (lemon) phenomenon-a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants (Basel) 2020;9(1):119. doi: 10.3390/plants9010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L. (black pepper): a review. Asian Pac J Trop Biomed. 2012;2(3):S1945–S1953. doi: 10.1016/S2221-1691(12)60524-3. [DOI] [Google Scholar]
- 37.Swathy J, Mishra P, Thomas J, Mukherjee A, Chandrasekaran N. Antimicrobial potency of high-energy emulsified black pepper oil nanoemulsion against aquaculture pathogen. Aquaculture. 2018;491:210–220. doi: 10.1016/j.aquaculture.2018.03.045. [DOI] [Google Scholar]
- 38.Vasavirama K, Upender M. Piperine: a valuable alkaloid from piper species. Int J Pharm Pharm Sci. 2014;6:34–38. [Google Scholar]
- 39.Sabina EP, Nasreen A, Vedi M, Rasool M. Analgesic, antipyretic and ulcerogenic effects of piperine: an active ingredient of pepper. J Pharmaceut Sci Res. 2013;5:203. [Google Scholar]
- 40.Park TS, Jeong NS. Piperine derivatives and uses thereof. 2016. [Google Scholar]
- 41.Tiwari A, Mahadik KR, Gabhe SY. Piperine: a comprehensive review of methods of isolation, purification, and biological properties. Med Drug Discov. 2020;7:100027. doi: 10.1016/j.medidd.2020.100027. [DOI] [Google Scholar]
- 42.Silva ACR d, Lopes PM, Azevedo MMB d, Costa DCM, Alviano CS, Alviano DS. Biological activities of a-pinene and β-pinene enantiomers. Molecules (Basel, Switzerland) 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen C, et al (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama 323(16):1582-9 [DOI] [PMC free article] [PubMed]
- 44.Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treatment Options Infect Dis. 2016;8(4):311–328. doi: 10.1007/s40506-016-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan Cooley FPSST. Making a case for policy implications in research: a reflexive contemplation and call to action. 2016. [Google Scholar]
- 46.Ziaie S, Koucheck M, Miri M, Salarian S, Shojaei S, Haghighi M, Sistanizad M. Review of therapeutic agents for the treatment of COVID-19. J Cellular Mol Anesthesia. 2020;5:32–36. [Google Scholar]
- 47.Gudadappanavar AM, Benni J (2020) An evidence-based systematic review on emerging therapeutic and preventive strategies to treat novel coronavirus (SARS-CoV-2) during an outbreak scenario. J Basic Clin Physiol Pharmacol 31(6). 10.1515/jbcpp-2020-0113 [DOI] [PubMed]
- 48.Lane, J. C., Weaves, J., Kostka, K., Alser, O., Prats-Uribe, A., Newby, D., and Prieto-Alhambra, D. (2020) Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study, MedRXiv.
- 49.Gabriels J, Saleh M, Chang D, Epstein LM. Inpatient use of mobile continuous telemetry for COVID-19 patients treated with hydroxychloroquine and azithromycin. HeartRhythm Case Reports. 2020;6(5):241–243. doi: 10.1016/j.hrcr.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gimeno A, Mestres-Truyol J, Ojeda-Montes MJ, Macip G, Saldivar-Espinoza B, Cereto-Massagué A, Pujadas G, Garcia-Vallvé S. Prediction of novel inhibitors of the main protease (M-pro) of SARS-CoV-2 through consensus docking and drug reposition. Int J Mol Sci. 2020;21(11):3793. doi: 10.3390/ijms21113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang S, Du L, Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9(1):275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veljkovic V, et al (2020) Use of the informational spectrum methodology for rapid biological analysis of the novel coronavirus 2019-nCoV: prediction of potential receptor, natural reservoir, tropism and therapeutic/vaccine target. F1000Res 9:52. 10.12688/f1000research.22149.4 [DOI] [PMC free article] [PubMed]
- 56.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen K-Y. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreutz R, Algharably EAE-H, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang J-G. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19: European Society of Hypertension COVID-19 task force review of evidence. Cardiovasc Res. 2020;116(10):1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandes MS, et al (2021) Insights on 3D structures of potential drug-targeting proteins of SARS-CoV-2: application of cavity search and molecular docking. Mol Informat 40(2):2000096 [DOI] [PMC free article] [PubMed]
- 60.Alsafi MA, Hughes DL, Said MA (2020) First COVID-19 molecular docking with a chalcone-based compound: synthesis, single-crystal structure and Hirshfeld surface analysis study. Acta Crystallogr C Struct Chem 76(Pt 12):1043-1050. 10.1107/S2053229620014217 [DOI] [PubMed]
- 61.Wlodawer A, Dauter Z, Shabalin IG, Gilski M, Brzezinski D, Kowiel M, Minor W, Rupp B, Jaskolski M. Ligand-centered assessment of SARS-CoV-2 drug target models in the protein data Bank. FEBS J. 2020;287(17):3703–3718. doi: 10.1111/febs.15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammadi S, Heidarizadeh M, Entesari M, Esmailpour A, Esmailpour M, Moradi R, Sakhaee N, Doustkhah E. In silico investigation on the inhibiting role of nicotine/caffeine by blocking the S protein of SARS-CoV-2 versus ACE2 receptor. Microorganisms. 2020;8(10):1600. doi: 10.3390/microorganisms8101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussain M, Jabeen N, Raza F, Shabbir S, Baig AA, Amanullah A, Aziz B. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J Med Virol. 2020;92(9):1580–1586. doi: 10.1002/jmv.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filimonov D, Lagunin A, Gloriozova T, Rudik A, Druzhilovskii D, Pogodin P, Poroikov V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem Heterocycl Compd. 2014;50(3):444–457. doi: 10.1007/s10593-014-1496-1. [DOI] [Google Scholar]
- 65.Chan H, Pearson S, Green CM, Li Z, Zhang J, Lippard S, Belfort G, Shekhtman A, Li HM, Belfort M (2020) RecA mini intein-Zeise’s salt complex.
- 66.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Zhang S, Li F, Zhou Y, Zhang Y, Wang Z, Zhang R, Zhu J, Ren Y, Tan Y. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48(D1):D1031–D1041. doi: 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herowati R, Widodo GP. Molecular docking studies of chemical constituents of Tinospora cordifolia on glycogen phosphorylase. Procedia Chemistry. 2014;13:63–68. doi: 10.1016/j.proche.2014.12.007. [DOI] [Google Scholar]
- 71.Beglov D, Hall DR, Brenke R, Shapovalov MV, Dunbrack RL, Jr, Kozakov D, Vajda S. Minimal ensembles of side chain conformers for modeling protein–protein interactions. Proteins Structure Function Bioinformatics. 2012;80(2):591–601. doi: 10.1002/prot.23222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emon NU, et al (2021) Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches. Food Sci Nutr 9(2):833-46 [DOI] [PMC free article] [PubMed]
- 73.Khan S, Nazir M, Raiz N, Saleem M, Zengin G, Fazal G, Saleem H, Mukhtar M, Tousif MI, Tareen RB. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): a comprehensive approach. Ind Crop Prod. 2019;131:117–124. doi: 10.1016/j.indcrop.2019.01.044. [DOI] [Google Scholar]
- 74.Alam S, Emon NU, Shahriar S, Richi FT, Haque MR, Islam MN, Sakib SA, Ganguly A. Pharmacological and computer-aided studies provide new insights into Millettia peguensis Ali (Fabaceae) Saudi Pharmaceutical J. 2020;28(12):1777–1790. doi: 10.1016/j.jsps.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Halim H, Abdelhalim A, Abdelmalek S. The search of potential inhibitors of the AcrAB–TolC system of multidrug-resistant Escherichia coli: an in silico approach. Appl Microbiol Biotechnol. 2019;103(15):6309–6318. doi: 10.1007/s00253-019-09954-1. [DOI] [PubMed] [Google Scholar]
- 76.Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:1–15. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75(7):2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan J, et al (2020) Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 79.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valle C, Martin B, Touret F, Shannon A, Canard B, Guillemot JC, Coutard B, Decroly E. Drugs against SARS-CoV-2: what do we know about their mode of action? Rev Med Virol. 2020;30(6):1–10. doi: 10.1002/rmv.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reilly J (2020) Treatment considerations for coronavirus (COVID-19). Hospital Pract 48(3):119-20 [DOI] [PubMed]
- 83.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available upon request.