Abstract
Objectives
Long-term follow-up is important for determining performance characteristics of thyroid fine-needle aspiration (FNA).
Methods
Histologic or 3 or more years of clinical follow-up was used to calculate performance characteristics of thyroid FNA before and after implementation of The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) classification was also investigated.
Results
Follow-up was obtained for 1,277/1,134 and 1,616/1,393 aspirates/patients (median clinical follow-up, 9.9 and 4.4 years, pre- and post-TBSRTC, respectively). Nondiagnostic, suspicious for follicular neoplasm, and suspicious for malignancy (SFM) diagnoses decreased and benign diagnoses increased post-TBSRTC, while atypical rate remained less than 1%. Negative predictive value for benign nodules and positive predictive value (PPV) for SFM increased significantly. Eleven nodules were reclassified as NIFTP, slightly decreasing PPV/risk of malignancy (ROM).
Conclusions
Appropriate ROM for thyroid FNA can be achieved through application of TBSRTC terminology with minimal use of atypical category.
Keywords: Bethesda, Cytopathology, Fine-needle aspiration, Atypia of undetermined significance/follicular lesion of undetermined significance, Risk of malignancy, Follicular neoplasm/suspicious for follicular neoplasm, Papillary thyroid carcinoma, Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), Thyroid, FNA
There have been many recent advances in thyroid fine-needle aspiration (FNA). Utilization of ultrasound guidance has improved the ability to obtain diagnostic material. Improved imaging techniques and increased surveillance for nonthyroid malignancies have increased the detection of incidental nodules.1-4 The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), introduced in 2009 and subsequently widely implemented, has standardized diagnostic terminology.5 Based on the evidence available at the time, TBSRTC provided an estimated risk of malignancy (ROM) for each cytology diagnostic category, and these were used to develop management guidelines for each category.5 In 2015, the American Thyroid Association provided updated recommendations for the management of thyroid nodules and endorsed TBSRTC for this purpose.6 Determining appropriate management for indeterminate nodules (atypia of undetermined significance/follicular lesion of undetermined significance [AUS/FLUS] and suspicious for follicular neoplasm/follicular neoplasm [SFN/FN]) remains challenging. Molecular testing of aspirated material from thyroid nodules has emerged to assist in perioperative decision making.7-9 However, molecular profiling has limitations, and unnecessary surgery and, consequently, morbidity remain important challenges in the treatment of thyroid nodules.
The most recent advance in thyroid pathology is the reclassification of noninvasive encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).10 This nomenclature was proposed in an effort to reduce overtreatment of indolent thyroid nodules.10-12
Long-term clinical follow-up in addition to histology is important for minimizing surgical selection bias when determining performance characteristics of thyroid FNA, but most prior studies include only pathology (histology with or without cytology) follow-up.4,13 To address this void in the literature, we evaluated the performance characteristics of thyroid FNA before and after implementation of TBSRTC terminology at our institution using histologic and long-term clinical follow-up, and we investigated the potential impact of NIFTP nomenclature.
Materials and Methods
Study Cohorts
Following institutional review board approval from Mayo Clinic, Rochester, MN, two cohorts were identified: all patients undergoing initial thyroid FNA by endocrinologists or interventional radiologists at Mayo Clinic (Rochester, Minnesota) during 3-year periods prior to (2001-2003) and following (2010-2013) implementation of TBSRTC. The cytology preparation used for diagnosis during this timeframe was alcohol-fixed Papanicolaou-stained direct aspirate smears. Aspirations from multiple nodules in the same patient were counted separately. All nodules were included, regardless of size. A sensitivity analysis excluding nodules 1 cm or less did not change the findings.
Data were collected using REDCap electronic data capture held at Mayo Clinic, Rochester, MN.14
Cytology Diagnoses
TBSRTC was used to classify all cytology diagnoses and includes the following diagnostic categories: nondiagnostic/unsatisfactory (ND/UNS), benign, AUS/FLUS, SFN/FN, suspicious for malignancy (SFM), and malignant.5 Prior to implementing TBSRTC, the diagnostic criteria and reporting at our institution were similar to those suggested by TBSRTC and included all of the major TBSRTC diagnostic categories other than AUS/FLUS. Instead of a using a distinct diagnostic category of “atypical,” descriptive interpretations were provided for atypical cases.
Follow-up
All available pathology follow-up was extracted from the laboratory information system. Only surgical pathology data for nodules previously aspirated were included. Whenever possible, the site of the surgical pathology findings was correlated with the site of the initial FNA. Incidental histologic findings not involving previously aspirated nodules were excluded. All available endocrinology clinical follow-up, including examination and ultrasound (for nodules without subsequent pathology), was obtained via the electronic medical record with detailed review of subsequent clinical examinations and imaging studies performed at least 3 months following initial aspiration. A minimum of 3 years of clinical follow-up was required for study inclusion. To evaluate thyroid cytology in a cohort of patients for whom longer clinical follow-up was available, performance characteristics were determined for patients with either histologic or a minimum of 10 years of clinical follow-up available. Patients from both pre- and post-TBSRTC with histologic follow-up and patients from the pre-TBSRTC cohort with a minimum of 10 years of clinical follow-up were included in this analysis.
NIFTP Review
All available surgical pathology slides for all EFVPTCs in both cohorts were retrospectively reviewed. Nodules meeting consensus criteria for NIFTP (ie, encapsulated nodules with follicular growth pattern and nuclear features of papillary thyroid carcinoma but no areas of papillary or solid growth or psammoma bodies) were reclassified as such.10 All nodules reclassified as NIFTP underwent microscopic examination of the entire capsule, another criterion for NIFTP classification.
Statistical Analysis
Follow-up data were used to determine the performance characteristics of thyroid FNA in both cohorts, including negative predictive value (NPV) and positive predictive value (PPV)/ROM for each cytology diagnostic category. For the purposes of this study, a “true result” (malignant vs benign) was determined for each nodule as follows: surgical pathology diagnosis for the nodule aspirated was used if surgery was performed following baseline cytology, repeat cytology diagnosis was used if there was a subsequent FNA but no surgery, and most recent clinical assessment including a clinical examination by an endocrinologist and ultrasound was used if no subsequent pathology was available. “Malignant” was defined as any malignant histology, including papillary microcarcinomas, lymphoma, and metastases, or a repeat cytology result of SFM or malignant (if no subsequent surgery) or clinically malignant (if no subsequent surgery or repeat cytology). Benign neoplasms on histology were considered “benign.” A total of six nodules (one in the pre-TBSRTC cohort that was a SFN on initial cytology and five in the post-TBSRTC cohort [one nondiagnostic, one atypical, and three SFNs on initial cytology]) without surgical follow-up but repeat cytology diagnoses of SFN were excluded from the analysis since these remain indeterminate. For nodules without subsequent pathology, clinical documentation of stability with respect to size and sonographic characteristics was used to establish whether an unresected nodule was benign.
NPV and PPV/ROM were calculated separately within each baseline cytology category, based on true benign and malignant percentages within each group, respectively. Prevalence of malignancy was determined by number of malignant nodules divided by all nodules. Performance characteristics of thyroid FNA were also calculated following NIFTP reclassification. Since NIFTP is associated with a very low risk of adverse outcome10 and since the second edition of the TBSRTC (TBSRTC II) provides reduced ROM for categories affected by NIFTP, nodules reclassified as NIFTP were considered “benign” for the purposes of calculating performance characteristics for this study. However, the authors do recognize that NIFTP is currently considered an indolent but not benign neoplasm in clinical practice. Ninety-five percent score confidence intervals (CIs) were reported.
Age at baseline FNA was compared between cohorts with a t test. Sex and selected performance measures, including NPV and PPV within selected cytology diagnoses, were compared between cohorts with χ2 tests. All P values are two-sided, and P values less than .05 were considered statistically significant. Given the low number of statistical tests and the descriptive nature of this analysis, no P values have been adjusted for multiple testing. Analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study Cohorts and Follow-up
In total, 5,175 total aspirates (1,949 and 3,226, pre- and post-TBSRTC, respectively) were performed in 4,502 patients. Follow-up was obtained for 2,893 aspirates: 1,277 (1,134 patients) and 1,616 (1,393 patients), pre- and post-TBSRTC, respectively. Median clinical follow-up among patients without subsequent pathology was 9.9 years (range, 3.0-15.2 years) in the pre-TBSRTC cohort and 4.4 years (range, 3.0-6.3 years) in the post-TBSRTC cohort. The percentages of nodules in the ND/UNS, SFN/FN, and SFM categories decreased, while the percentage of benign diagnoses increased post-TBSRTC. The atypical rate (ie, percentage of AUS/FLUS diagnoses) in both cohorts was less than 1% Table 1 and Image 1. Demographics of patients with follow-up are shown in Table 2.
Table 1.
Performance Characteristics of Thyroid Fine-Needle Aspiration
| Cytology Category | Pre-TBSRTC | Post-TBSRTC | ||||||
|---|---|---|---|---|---|---|---|---|
| Follow-up | Follow-up | |||||||
| All Nodules, No. (%) | No. (%) | NPV, % | PPV, %a | All Nodules, No. (%) | No. (%) | NPV, % | PPV, %b | |
| ND/UNS | 335 (17.2) | 217 (17.0) | 94.0 | 6.0 | 338 (10.5) | 202 (12.5) | 96.5 | 3.5 |
| Benign | 1,102 (56.6) | 607 (47.5) | 97.0 | 3.0 | 2,350 (72.8) | 945 (58.5) | 98.5 | 1.5 |
| AUS/FLUS | 14 (0.7) | 5 (0.4) | 100 | 0 | 25 (0.8) | 20 (1.2) | 85.0 | 15.0 |
| SFN/FN | 237 (12.2) | 208 (16.3) | 91.8 | 8.2 | 177 (5.5) | 147 (9.1) | 88.4 | 11.6 |
| SFM | 87 (4.5) | 78 (6.1) | 26.9 | 73.1 | 77 (2.4) | 70 (4.3) | 12.9 | 87.1 |
| Malignant | 174 (8.9) | 162 (12.7) | 1.9 | 98.1 | 259 (8.0) | 232 (14.3) | 1.7 | 98.3 |
| Total No. | 1,949 | 1,277 | 3,226 | 1,616 | ||||
AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; ND/UNS, nondiagnostic/unsatisfactory; NPV, negative predictive value; PPV, positive predictive value; SFM, suspicious for malignancy; SFN/FN, suspicious for follicular neoplasm/follicular neoplasm; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology.
aPrevalence = 20.7% (264/1,277).
bPrevalence = 20.4% (330/1,616).
Image 1.
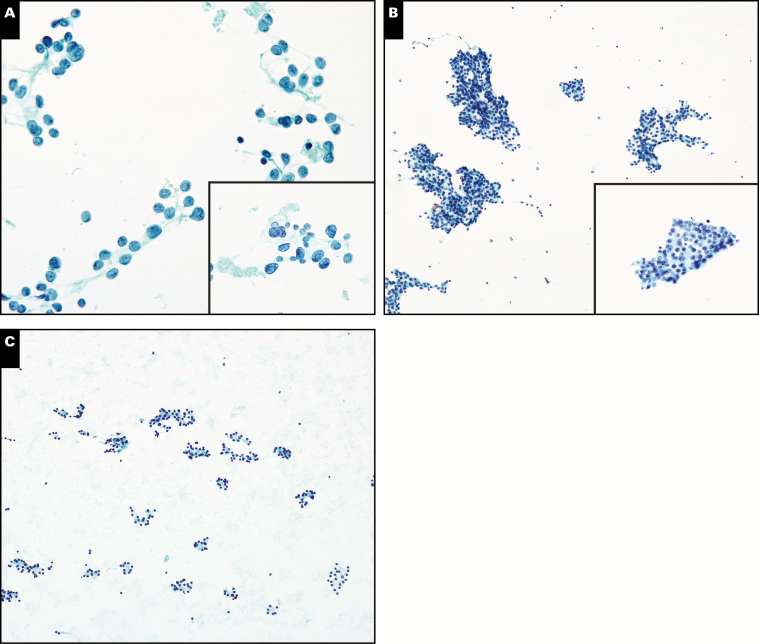
A, Aspirate of a thyroid nodule with rare follicular cells containing atypical features, including nuclear enlargement, micronucleoli, and rare nuclear grooves. Given the scant nature of this specimen, a diagnosis of atypia of undetermined significance (AUS) was rendered (Papanicolaou, ×400 and ×600 [inset]). Follow-up histology showed follicular variant of papillary thyroid carcinoma (not shown). B, Aspirate of a thyroid nodule with sheets of follicular cells with atypical features, including nuclear enlargement, open chromatin, and nuclear grooves (Papanicolaou, ×400 and ×600 [inset]). Follow-up histology showed a benign follicular adenoma in a background of thyroiditis (not shown). C, A hypocellular aspirate of a thyroid nodule with a rare area demonstrating a microfollicular pattern. Due to the scant nature of this aspirate, a diagnosis of AUS was most appropriate Papanicolaou, ×200). Follow-up histology showed a benign follicular adenoma (not shown).
Table 2.
Demographics of Patients With Follow-up
| Characteristic | Pre-TBSRTC | Post-TBSRTC |
|---|---|---|
| Aspirates, No. | 1,277 | 1,616 |
| Patients, No. | 1,134 | 1,393 |
| Sex, % female | 76.3 | 71.6a |
| Age, mean (SD), y | 54.4 (15.1) | 57.3 (14.2)b |
SD, standard deviation; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology.
a P = .008.
b P < .001.
Performance Characteristics of Thyroid FNA Pre- and Post-TBSRTC
Prevalence of malignant thyroid diagnoses was 20.7% (95% CI, 18.5%-23.0%) and 20.4% (95% CI, 18.5%-22.5%) for pre- and post-TBSRTC cohorts, respectively. PPV for malignant diagnoses was over 98% in both cohorts (95% CI, 94.7%-99.4% and 95.7%-99.3% pre- and post-TBSRTC, respectively). While the percentage of SFN/FN nodules decreased, the PPV for SFM increased from 73.1% to 87.1% (P = .03), and NPV for benign cytology increased significantly from 97.0% pre-TBSRTC to 98.5% post-TBSRTC (P = .04; Table 1).
Overall Outcomes in Patients With Surgical or 10 or More Years of Clinical Follow-up
Surgical follow-up or a minimum of 10 years of clinical follow-up was obtained for 1,964 nodules. In this cohort, NPV of combined ND/UNS, benign, and AUS/FLUS cytology diagnoses was 94.9% (95% CI, 93.4%-96.1%) and combined benign and AUS/FLUS diagnoses only was 95.3% (95% CI, 93.6%-96.6%) Table 3. No deaths from thyroid cancer occurred in patients with a minimum of 10 years of clinical follow-up, but there were two deaths due to thyroid cancer (2.7 and 3.6 years following baseline cytology) in the post-TBSTRC cohort. In the cohort with a minimum of 10 years of clinical follow-up, 58 (85.3%) of 68 nodules (from 65 patients) initially classified as benign, AUS/FLUS, or SFN by baseline cytology, which were eventually diagnosed as malignant by surgical pathology, were diagnosed by histology within 3 years of initial FNA.
Table 3.
Performance Characteristics of Thyroid Fine-Needle Aspiration in Patients With Surgical Pathology or Minimum of 10 Years of Clinical Follow-up
| Cytology Category | All Nodules, No. (%) | Follow-up Available | ||
|---|---|---|---|---|
| No. (%) | NPV, % | PPV, % | ||
| ND/UNS | 673 (13.0) | 335 (17.1) | 94.0 | 6.0 |
| Benign | 3,452 (66.7) | 726 (37.0) | 95.6 | 4.4 |
| AUS/FLUS | 39 (0.8) | 22 (1.1) | 86.4 | 13.6 |
| SFN/FN | 414 (8.0) | 343 (17.5) | 90.1 | 9.9 |
| SFM | 164 (3.2) | 147 (7.5) | 20.4 | 79.6 |
| Malignant | 433 (8.4) | 391 (19.9) | 1.5 | 98.5 |
| Total No. of nodules | 5,175 | 1,964 | 1,373 | 591 |
AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; ND/UNS, nondiagnostic/unsatisfactory; NPV, negative predictive value; PPV, positive predictive value; SFM, suspicious for malignancy; SFN/FN, suspicious for follicular neoplasm/follicular neoplasm.
Impact of NIFTP Classification
After rereview of surgical pathology from all nodules in both cohorts originally diagnosed as EFVPTC, a total of 11 nodules were reclassified as NIFTP: seven pre-TBSRTC (one benign, two SFN/FN, and four SFM cases) and four post-TBSRTC (one SFN/FN, one SFM, and two malignant cases) Image 2 and Image 3. PPVs decreased while NPVs increased slightly in the cytology categories affected by NIFTP reclassification. SFM categories in both cohorts were affected the most Table 4.
Image 2.
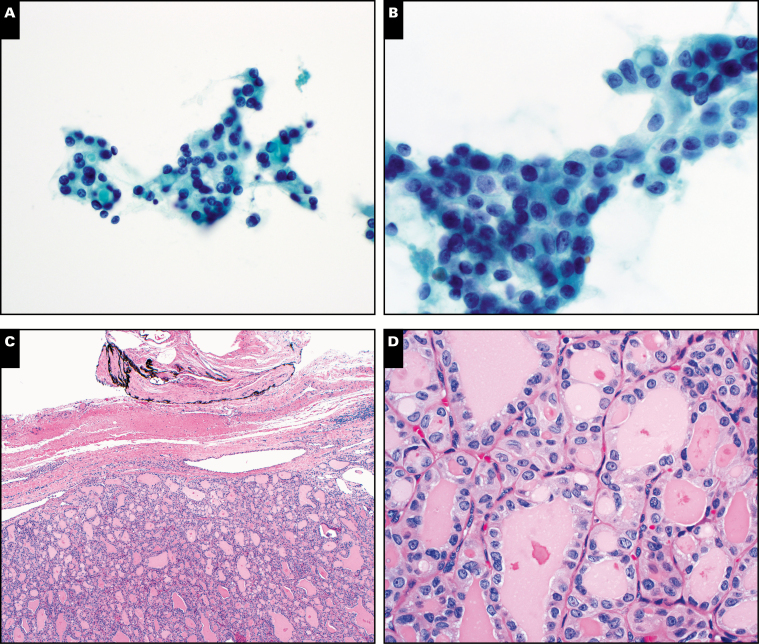
Thyroid nodule with an initial cytology diagnosis of suspicious for papillary thyroid carcinoma (Papanicolaou, ×400 [A] and ×600 [B]). Original histology diagnosis was follicular variant of papillary thyroid carcinoma. This nodule would currently be classified histologically as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (H&E, ×100 [C] and ×400 [D]).
Image 3.
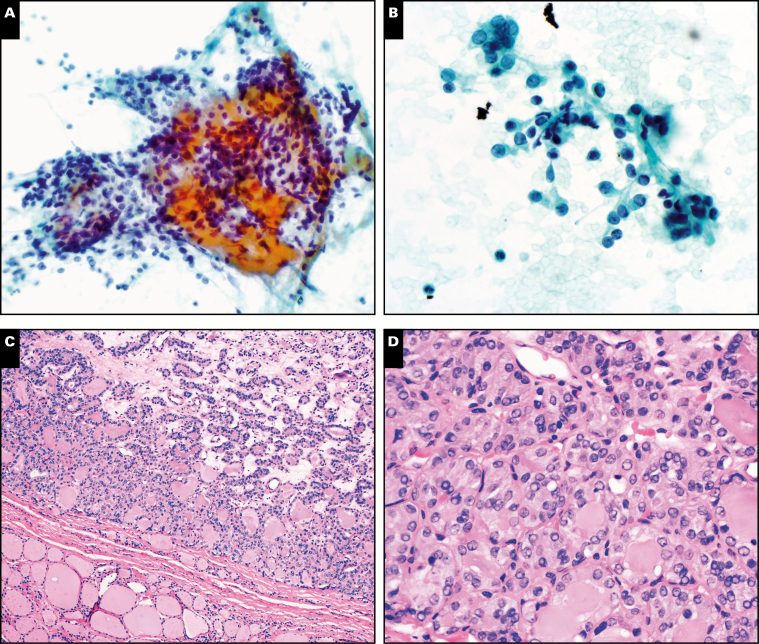
Thyroid nodule with initial cytology diagnosis of benign (Papanicolaou, ×200 [A] and ×600 [B]). Original histology diagnosis was follicular variant of papillary thyroid carcinoma. This nodule would currently be classified histologically as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (H&E, ×100 [C] and ×400 [D]).
Table 4.
Impact of NIFTP Reclassification on Performance Characteristics of Thyroid Fine-Needle Aspiration
| Cytology Categorya | Pre-TBSRTC | Post-TBSRTC | ||||||
|---|---|---|---|---|---|---|---|---|
| NPV, % | PPV, % | NPV, % | PPV, % | |||||
| Original | NIFTP | Original | NIFTP | Original | NIFTP | Original | NIFTP | |
| Benign | 97.0 | 97.2 | 3.0 | 2.8 | ||||
| SFN/FN | 91.8 | 92.8 | 8.2 | 7.2 | 88.4 | 87.3 | 11.6 | 12.7 |
| SFM | 26.9 | 32.1 | 73.1 | 67.9 | 12.9 | 14.3 | 87.1 | 85.7 |
| Malignant | 1.7 | 2.6 | 98.3 | 97.4 | ||||
NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; NPV, negative predictive value; PPV, positive predictive value; SFN/FN, suspicious for follicular neoplasm/follicular neoplasm; SFM, suspicious for malignancy; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology.
aOnly categories affected by reclassification included.
Discussion
We observed an increase in number of thyroid FNAs performed at our institution and an increase in benign nodules post-TBSRTC. The increase in benign diagnoses is likely multifactorial and possibly related to (1) increase in incidentally identified nodules from increased radiologic surveillance for nonthyroid tumors, (2) fewer ND/UNS aspirates with improved ultrasound technique, and (3) shift in diagnoses following TBSRTC. In particular, utilization of more strict criteria for SFN/FN may have shifted some diagnoses to the benign category.
An important strength of this study is the inclusion of long-term clinical follow-up. When only histology is used for follow-up of thyroid FNA, surgical selection bias may contribute to overestimation of ROM.4,13 In the current study, some patients followed clinically were lost to follow-up. Therefore, there may be some overestimation of ROM in the current study due to less follow-up available for nodules without surgical management. Institutional referral bias and publication bias also contribute to overestimated ROMs.13 Although Mayo Clinic is a referral center, it also serves the local community; therefore, the risk of referral bias is somewhat restricted in this study. ROM is also overestimated in studies that include incidental microcarcinomas detected on surgical pathology that were not the nodule targeted for FNA.13 In the current study, the surgical reports were closely reviewed so that, to the best of our ability, the histology follow-up included only nodules that were previously aspirated, whereas incidental microcarcinomas detected histologically but not previously aspirated were excluded in follow-up data.
In the cohort of patients with 10 years of clinical follow-up, most malignancies were detected within 3 years of baseline cytology in patients whose nodules were initially diagnosed as benign or indeterminate by cytology but later diagnosed as malignant by histology. This finding suggests that 3 years of clinical follow-up may be sufficient for studying the performance of thyroid FNA as a screening test for clinically significant thyroid cancer resulting in mortality. Another study that investigated long (ie, >4 years) and short (ie, minimum of 6 months) interval follow-up of thyroid nodules with benign cytology diagnoses found that malignancy and mortality did not differ between the two follow-up intervals.15
The first edition of TBSRTC recommended limiting ND/UNS diagnoses to less than 10% of thyroid FNA diagnoses, but several reports indicate nondiagnostic rates of up to 34% at some institutions.5,6,16-22 Although ultrasound guidance has been used at our institution as early as 2001, the improved nondiagnostic rate post-TBSTRC may partly be related to increased training and experience with ultrasound guidance over time. Like the first edition, TBSRTC II provides predicted ROMs for each cytology category but updated based on additional data published since 2009. The predicted ROM for ND/UNS in TBSRTC II increased to 5% to 10%.23 However, recent studies report ROMs for ND/UNS of up to 9% to 32% in surgically resected nodules.5,18,20,21,24 In the current study, ROMs for ND/UNS categories were 6.0% and 3.5%, pre- and post-TBSRTC, respectively, and much lower than reported by studies without clinical follow-up.
The predicted ROM of 0% to 3% for the benign category did not change in TBSTRC II.23 In the current study, ROMs for the benign category in both cohorts fell within this range. A meta-analysis of studies prior to 2010 from 21 institutions reported ROM of 1% to 10% for benign cytology.25 However, these studies likely overestimate ROM since only histologic follow-up was used. Our study, which uses both clinical and histologic follow-up, shows lower ROMs in the benign categories.
Overutilization of the AUS/FLUS category reduces the power of thyroid FNA as a screening test and may result in unnecessary surgery.26 Although repeat FNA is the recommended clinical management following an AUS/FLUS diagnosis, reported rates of surgery following AUS/FLUS diagnoses have ranged from 19% to 54%.19,20,27-33 TBSRTC II recommends limiting AUS/FLUS to less than 10% of all thyroid FNA diagnoses and cautions that it is “an interpretation of last resort and should be used judiciously.”23 In clinical practice, however, AUS/FLUS rates vary considerably among institutions, and many report rates of well over 10%. A meta-analysis of studies from 51 institutions (2009-2014) found AUS/FLUS rates of 12.5% to 50%.34 One study that did include clinical follow-up (minimum of 6 months) in addition to pathology follow-up reports an AUS/FLUS rate of 6.4% with an overall malignancy rate of 28.5%.35 However, these values are higher than the findings of the current study, which uses a longer clinical follow-up. The low rate of AUS/FLUS at the study institution was achieved by utilization of TBSTRC criteria to place nearly all cases into more definitive categories whenever possible and to avoid the use of AUS/FLUS except in rare cases that could not be placed in more definitive categories.
In addition, there is only moderate reproducibility of AUS/FLUS among pathologists.36 Minimal use of AUS/FLUS did not increase the rate of other indeterminate diagnoses in this study. Rather, the rate of SF/SFN decreased and the rate of benign diagnoses increased post-TBSTRC. While this increase in benign nodules is likely partially related to increased detection of incidental thyroid nodules, it is also likely that many nodules that would be placed in the AUS/FLUS category at other institutions were placed in the benign category. Minimal use of AUS/FLUS with increased use of the benign category at our institution appears to be appropriate since we demonstrate high NPVs and low ROMs for benign diagnoses.
In general, the ROMs for the cytology diagnostic categories in both cohorts of this study fit within the ranges suggested in TBSRTC II. Determining an accurate ROM for the AUS/FLUS category is challenging since conservative management is recommended and surgical resection is rare.6 However, ROM for AUS/FLUS in our post-TBSRTC cohort was 15%, which falls within the range of 10% to 30% in TBSRTC II.23 The ROMs for SFN/FN in both cohorts (8.2% and 11.6%, pre- and post-TBSRTC, respectively) were lower than the predicted ROM of 25% to 40%, while the ROM for the SFM category in our post-TBSRTC was higher (87.1%) than the 50% to 75% predicted. The ROMs in our malignant categories fit within the TBSRTC II predicted range.23
TBSRTC II provides additional predicted ROMs for each category when NIFTP is considered an indolent neoplasm.23 When adjusted for NIFTP, decreases in ROM generally occur in the indeterminate categories (AUS/FLUS, SFN/FN, and SFM).37-42 Since AUS/FLUS is only rarely used at our institution, the SFN/FN and SFM categories were affected the most when nodules were reclassified using NIFTP criteria. However, given the low number of NIFTP diagnoses in this study, no statistically significant changes in the performance characteristics of thyroid FNA were observed. The implications of NIFTP on thyroid FNA diagnoses remain to be studied in large cohorts with long-term follow-up.
An important strength of this study is the use of a minimum of 3 years of clinical follow-up for patients without subsequent pathology for determining the performance characteristics of thyroid FNA. Another strength is the comparison to a subgroup of patients with a minimum of 10 years of clinical follow-up or surgical pathology follow-up. An important limitation is that it is impossible to adequately control for all variables that influence thyroid cytology diagnoses, including operator experience with thyroid FNA, shifting patient populations, and changes in personnel over the study time periods, including turnover in both the cytopathologists interpreting aspirates as well as endocrinologists and interventional radiologists performing ultrasound-guided thyroid FNAs. Another limitation of this study is that the data are from a single institution and the findings may not be applicable to other institutions with differing rates of the various TBSRTC diagnostic categories. However, it is also difficult for multi-institutional studies to control for diagnostic differences among pathologists, in both interpretation of thyroid FNAs as well as surgical pathology diagnoses for histology follow-up. Data from a single institution are more likely to have more diagnostic consistency among pathologists.
Like many prior studies investigating the effectiveness of TBSRTC, the overall findings of this study demonstrate that use of TBSRTC improves cytopathology diagnoses of thyroid FNA. This study is unique and adds to the large body of literature on TBSRTC in two ways. First, long-term clinical follow-up was used in addition to surgical follow-up to obtain more accurate positive and negative predictive values for cytopathology diagnostic categories. Second, unlike other prior studies with higher rates of AUS/FLUS diagnoses, the findings of the current study demonstrate that it is possible to achieve a very low rate of AUS/FLUS diagnoses using TBSRTC criteria while accurately identifying benign and malignant nodules, achieving appropriate ROMs in indeterminate categories (ie, AUS/FLUS and SFN/FN), and maintaining overall excellent performance characteristics of thyroid FNA. These findings are an important addition to the current literature on TBSRTC, as the field is moving toward efforts to prevent overtreatment of thyroid nodules, and pathologists can contribute to this effort by attempting to limit the use of the AUS/FLUS category whenever it is possible to use a more definitive TBSRTC category.
This work was supported by departmental funding from the Division of Anatomic Pathology, Mayo Clinic, Rochester, MN.
Acknowledgments
We thank Allyne Manzo for technical assistance with figure design.
References
- 1. Sinnott JD, Mortimer R, Smith J, et al. The effect of routine radiological reporting of thyroid incidentalomas on rates of thyroid needle biopsy, thyroid surgery and detection of thyroid malignancy. Clin Endocrinol. 2017;87:825-831. [DOI] [PubMed] [Google Scholar]
- 2. Sosa JA, Hanna JW, Robinson KA, et al. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154:1420-1426. [DOI] [PubMed] [Google Scholar]
- 3. Grodski S, Brown T, Sidhu S, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144:1038-1043. [DOI] [PubMed] [Google Scholar]
- 4. Singh Ospina N, Brito JP, Maraka S, et al. Diagnostic accuracy of ultrasound-guided fine needle aspiration biopsy for thyroid malignancy: systematic review and meta-analysis. Endocrine. 2016;53:651-661. [DOI] [PubMed] [Google Scholar]
- 5. Ali SZ, Cibas ES, eds. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. New York, NY: Springer; 2009. [Google Scholar]
- 6. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nikiforova MN, Wald AI, Roy S, et al. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98:E1852-E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexander EK, Schorr M, Klopper J, et al. Multicenter clinical experience with the Afirma gene expression classifier. J Clin Endocrinol Metab. 2014;99:119-125. [DOI] [PubMed] [Google Scholar]
- 9. Ferris RL, Baloch Z, Bernet V, et al. ; American Thyroid Association Surgical Affairs Committee . American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25:760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26:1508-1514. [DOI] [PubMed] [Google Scholar]
- 12. Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336-1340. [DOI] [PubMed] [Google Scholar]
- 13. Iskandar ME, Bonomo G, Avadhani V, et al. Evidence for overestimation of the prevalence of malignancy in indeterminate thyroid nodules classified as Bethesda category III. Surgery. 2015;157:510-517. [DOI] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medici M, Liu X, Kwong N, et al. Long- versus short-interval follow-up of cytologically benign thyroid nodules: a prospective cohort study. BMC Med. 2016;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naïm C, Karam R, Eddé D. Ultrasound-guided fine-needle aspiration biopsy of the thyroid: methods to decrease the rate of unsatisfactory biopsies in the absence of an on-site pathologist. Can Assoc Radiol J. 2013;64:220-225. [DOI] [PubMed] [Google Scholar]
- 17. Sarkis LM, Norlen O, Aniss A, et al. The Australian experience with the Bethesda classification system for thyroid fine needle aspiration biopsies. Pathology. 2014;46:592-595. [DOI] [PubMed] [Google Scholar]
- 18. Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012;56:333-339. [DOI] [PubMed] [Google Scholar]
- 19. Renshaw AA. Should “atypical follicular cells” in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol. 2010;118:186-189. [DOI] [PubMed] [Google Scholar]
- 20. Theoharis CG, Schofield KM, Hammers L, et al. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215-1223. [DOI] [PubMed] [Google Scholar]
- 21. Luu MH, Fischer AH, Pisharodi L, et al. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol. 2011;119:68-73. [DOI] [PubMed] [Google Scholar]
- 22. Kiernan CM, Broome JT, Solórzano CC. The Bethesda System for Reporting Thyroid Cytopathology: a single-center experience over 5 years. Ann Surg Oncol. 2014;21:3522-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali SZ, Cibas ES, eds. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. 2nd ed. New York, NY: Springer; 2018. [Google Scholar]
- 24. Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306-315. [DOI] [PubMed] [Google Scholar]
- 25. Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry M. The potential for overuse of atypical thyroid diagnoses. Cancer Cytopathol. 2012;120:108-110. [DOI] [PubMed] [Google Scholar]
- 27. Rabaglia JL, Kabbani W, Wallace L, et al. Effect of The Bethesda System for Reporting Thyroid Cytopathology on thyroidectomy rates and malignancy risk in cytologically indeterminate lesions. Surgery. 2010;148:1267-1272. [DOI] [PubMed] [Google Scholar]
- 28. Layfield LJ, Morton MJ, Cramer HM, et al. Implications of the proposed thyroid fine-needle aspiration category of “follicular lesion of undetermined significance”: a five-year multi-institutional analysis. Diagn Cytopathol. 2009;37:710-714. [DOI] [PubMed] [Google Scholar]
- 29. Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol. 2010;118:17-23. [DOI] [PubMed] [Google Scholar]
- 30. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117:195-202. [DOI] [PubMed] [Google Scholar]
- 31. VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: should repeated FNA be the preferred initial approach? Am J Clin Pathol. 2011;135:770-775. [DOI] [PubMed] [Google Scholar]
- 32. Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol. 2010;38:731-739. [DOI] [PubMed] [Google Scholar]
- 33. Jo VY, Stelow EB, Dustin SM, et al. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010;134:450-456. [DOI] [PubMed] [Google Scholar]
- 34. Straccia P, Rossi ED, Bizzarro T, et al. A meta-analytic review of The Bethesda System for Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015;123:713-722. [DOI] [PubMed] [Google Scholar]
- 35. Chandra S, Chandra H, Bisht SS. Malignancy rate in thyroid nodules categorized as atypia of undetermined significance or follicular lesion of undetermined significance—an institutional experience. J Cytol. 2017;34:144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cibas ES, Baloch ZW, Fellegara G, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013;159:325-332. [DOI] [PubMed] [Google Scholar]
- 37. Canberk S, Gunes P, Onenerk M, et al. New concept of the encapsulated follicular variant of papillary thyroid carcinoma and its impact on The Bethesda System for Reporting Thyroid Cytopathology: a single-institute experience. Acta Cytol. 2016;60:198-204. [DOI] [PubMed] [Google Scholar]
- 38. Strickland KC, Howitt BE, Marqusee E, et al. The impact of noninvasive follicular variant of papillary thyroid carcinoma on rates of malignancy for fine-needle aspiration diagnostic categories. Thyroid. 2015;25:987-992. [DOI] [PubMed] [Google Scholar]
- 39. Maletta F, Massa F, Torregrossa L, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol. 2016;54:134-142. [DOI] [PubMed] [Google Scholar]
- 40. Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. 2016;124:181-187. [DOI] [PubMed] [Google Scholar]
- 41. Howitt BE, Chang S, Eszlinger M, et al. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2015;144:850-857. [DOI] [PubMed] [Google Scholar]
- 42. Lau RP, Paulsen JD, Brandler TC, et al. Impact of the reclassification of “noninvasive encapsulated follicular variant of papillary thyroid carcinoma” to “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” on the Bethesda System for Reporting Thyroid Cytopathology: a large academic institution’s experience. Am J Clin Pathol. 2017;149:50-54. [DOI] [PubMed] [Google Scholar]