Key Points
Question
What is the association of direct to angiography (DTA) vs repeated imaging treatment paradigms with stroke management workflow and outcomes in patients transferred to endovascular thrombectomy centers?
Findings
In a multicenter cohort of 1140 patients with large vessel occlusion, DTA was associated with faster times from arrival to groin puncture (34 vs 60 minutes) and better functional and safety outcomes, overall and in both early and late windows. Furthermore, no meaningful difference in workflow parameters or functional and safety outcomes were found in patients arriving during regular vs on-call hours.
Meaning
These findings suggest that DTA may be associated with faster treatment and better functional outcomes during all hours and treatment windows; repeated imaging may be reasonable with prolonged transfer times.
Abstract
Importance
A direct to angiography (DTA) treatment paradigm without repeated imaging for transferred patients with large vessel occlusion (LVO) may reduce time to endovascular thrombectomy (EVT). Whether DTA is safe and associated with better outcomes in the late (>6 hours) window is unknown. Also, DTA feasibility and effectiveness in reducing time to EVT during on-call vs regular-work hours and the association of interfacility transfer times with DTA outcomes have not been established.
Objective
To evaluate the functional and safety outcomes of DTA vs repeated imaging in the different treatment windows and on-call hours vs regular hours.
Design, Setting, and Participants
This pooled retrospective cohort study at 6 US and European comprehensive stroke centers enrolled adults (aged ≥18 years) with anterior circulation LVO (internal cerebral artery or middle cerebral artery subdivisions M1/M2) and transferred for EVT within 24 hours of the last-known-well time from January 1, 2014, to February 29, 2020.
Exposures
Repeated imaging (computed tomography with or without computed tomographic angiography or computed tomography perfusion) before EVT vs DTA.
Main Outcomes and Measures
Functional independence (90-day modified Rankin Scale score, 0-2) was the primary outcome. Symptomatic intracerebral hemorrhage, mortality, and time metrics were also compared between the DTA and repeated imaging groups.
Results
A total of 1140 patients with LVO received EVT after transfer, including 327 (28.7%) in the DTA group and 813 (71.3%) in the repeated imaging group. The median age was 69 (interquartile range [IQR], 59-78) years; 529 were female (46.4%) and 609 (53.4%) were male. Patients undergoing DTA had greater use of intravenous alteplase (200 of 327 [61.2%] vs 412 of 808 [51.0%]; P = .002), but otherwise groups were similar. Median time from EVT center arrival to groin puncture was faster with DTA (34 [IQR, 20-62] vs 60 [IQR, 37-95] minutes; P < .001), overall and in both regular and on-call hours. Three-month functional independence was higher with DTA overall (164 of 312 [52.6%] vs 282 of 763 [37.0%]; adjusted odds ratio [aOR], 1.85 [95% CI, 1.33-2.57]; P < .001) and during regular (77 of 143 [53.8%] vs 118 of 292 [40.4%]; P = .008) and on-call (87 of 169 [51.5%] vs 164 of 471 [34.8%]; P < .001) hours. The results did not vary by time window (0-6 vs >6 to 24 hours; P = .88 for interaction). Three-month mortality was lower with DTA (53 of 312 [17.0%] vs 186 of 763 [24.4%]; P = .008). A 10-minute increase in EVT-center arrival to groin puncture in the repeated imaging group correlated with 5% reduction in the functional independence odds (aOR, 0.95 [95% CI, 0.91-0.99]; P = .01). The rates of modified Rankin Scale score of 0 to 2 decreased with interfacility transfer times of greater than 3 hours in the DTA group (96 of 161 [59.6%] vs 15 of 42 [35.7%]; P = .006), but not in the repeated imaging group (75 of 208 [36.1%] vs 71 of 192 [37.0%]; P = .85).
Conclusions and Relevance
The DTA approach may be associated with faster treatment and better functional outcomes during all hours and treatment windows, and repeated imaging may be reasonable with prolonged transfer times. Optimal EVT workflow in transfers may be associated with faster, safe reperfusion with improved outcomes.
This pooled cohort study evaluates the functional and safety outcomes of the direct to angiography vs repeated imaging approaches in the different treatment windows and on-call vs regular hours in patients with large vessel occlusion undergoing transfer for endovascular thrombectomy.
Introduction
Endovascular thrombectomy (EVT) has been shown to improve outcomes in patients with acute ischemic stroke due to anterior circulation large vessel occlusion (LVO) with severe deficit and favorable imaging profiles, both in the early (0-6 hours)1,2 and late (>6 to 24 hours) windows.3,4 Endovascular thrombectomy outcomes are also inversely related to time from initial imaging to reperfusion, especially in patients presenting in the early window, emphasizing the need for optimal workflow to expedite the patient’s arrival to the endovascular suite.5
Many patients present initially to non-EVT centers and require transfer to EVT centers, resulting in significant delays during interfacility transfer.6 Efforts to streamline arrivals of patients with LVO to EVT-capable centers continue to evolve, including prehospital triaging directly to EVT-capable centers7 and optimal methods for transporting patients to and from non-EVT to EVT centers.8,9 Meanwhile, delays may occur at EVT centers owing to processes and treatment protocols adopted by these hospitals. Minimizing the time needed from arrival to the EVT center into the angiography suite will continue to play a vital role in maximizing EVT benefit.
One way to save time during this transfer process is to reduce unnecessary imaging. Usually at least 1 non–contrast-enhanced computed tomographic (CT) scan is obtained at the initial non-EVT center, and uncertainty remains about whether more precise patient selection by repeated imaging after EVT center arrival is needed and worth the additional time, or whether outcomes would be better if patients were transferred directly to the angiography suite (DTA approach). Current guidelines do not clearly outline the utility of repeated imaging at EVT centers after interhospital transfers.
On the contrary, it is unknown whether DTA is safe, given potential stroke evolution during interfacility transfer. It is also unknown whether the value of repeated imaging in transferred patients with LVO should differ between those presenting in the early (0-6 hours) vs late (>6 to 24 hours) treatment window or based on the duration of time elapsed from non-EVT center imaging to arrival at the EVT center. Also, the efficacy and safety of DTA might be affected by whether the patient arrives at the EVT-capable center within working hours or during on-call hours, given the differences in logistics and personnel, including the interventional team and stroke team on-site availability.10,11
We sought to evaluate the time savings, efficacy, and safety of DTA in patients who were transported to EVT-capable centers in the overall study population and a propensity-matched sample. Furthermore, we assessed whether those findings differ between early and late treatment windows and between work and on-call hours. Finally, we assessed the association of the time elapsed during transfer (from arrival at referral center to arrival at the EVT center) as well as time from imaging acquisition at the outside facility to arrival to EVT centers with the safety and outcomes of EVT in patients undergoing DTA and whether repeated imaging would be indicated in certain subgroups of patients as related to transfer times to EVT centers.
Methods
Study Design, Settings, and Participants
The study population consisted of a multicenter, retrospective cohort of 6 comprehensive stroke centers across the US and Europe from January 1, 2014, to February 29, 2020. All patients with acute ischemic stroke 18 years or older with anterior circulation LVO who initially presented to a non-EVT center and then were transferred to an EVT-capable center and received EVT up to 24 hours from the last-known-well time (LKW) were included. We defined LVO as an occlusion in the anterior circulation (internal cerebral artery or middle cerebral artery with subdivisions M1 or M2) identified on coronal CT angiography, anterior-posterior projection of the magnetic resonance angiography, or digital subtraction angiography as read locally by the participating centers. Patients were triaged at the referral centers based on their non–contrast-enhanced CT, CT angiography when acquired, or stroke severity in cases where baseline vessel imaging was not performed. The study was approved by the institutional review board at the coordinating center (The University of Texas McGovern Medical School, Houston), which waived the requirement of informed consent because of the retrospective nature of the study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Treatments, Exposures, and Intervention
All patients transferred from non-EVT centers for EVT evaluation were reviewed; patients who did not receive EVT and the reasons for their exclusion were identified. Patients receiving EVT were divided based on whether they underwent repeated imaging (CT with or without CT angiography or CT perfusion) and those who bypassed imaging and underwent DTA. Triage protocols for the DTA vs repeated imaging approaches at the EVT centers are listed in eTable 1 in the Supplement. Endovascular treatment included EVT by means of primary aspiration, retrievable stents with or without adjunctive aspiration techniques, and in some cases adjunctive intra-arterial thrombolytics. Medical management was based on current American Heart Association and European guidelines, including use of intravenous alteplase in patients meeting guideline criteria.2,12 We further stratified the cohort into an early-window group (defined as time from LKW to arrival at the EVT-capable center of ≤6 hours) and a late-window group (defined as time from LKW to arrival at the EVT-capable center of >6 to 24 hours). In addition, patients were stratified based on arrival time at the EVT center during regular hours (Monday to Friday, 8 am-5 pm) vs on-call hours.
Imaging Analysis
Early ischemic changes on CT were measured by the Alberta Stroke Program Early Computed Tomographic Score (ASPECTS; range, 0-10, with lower scores indicating more ASPECTS areas demonstrating early ischemic changes on non–contrast-enhanced CT).13 Recanalization was defined by the modified Thrombolysis in Ischemic Stroke score14 with successful reperfusion defined as a score of at least 2b at the end of the procedure as read locally by the participating centers.
Study Outcomes
The primary outcome was 90-day functional independence defined as modified Rankin Scale (mRS) scores of 0 to 2, obtained using a standardized questionnaire by trained evaluators through a telephone or an in-person assessment as a part of ongoing registries. Safety outcomes between the 2 groups were defined as the rate of symptomatic intracranial hemorrhage (sICH) as defined by European Cooperative Acute Stroke Study III criteria15 and mortality.
Statistical Analysis
Demographics, baseline characteristics, and clinical outcomes in patients undergoing repeated imaging and EVT after transfer were compared with those who bypassed repeated imaging (DTA group). Univariate comparisons were made using the χ2 test or the Fisher exact test for categorical variables and the unpaired t test or Wilcoxon rank sum test for continuous variables, as appropriate. Multivariable logistic regression analysis was conducted to evaluate the differences in outcome between the 2 groups after adjustment for prespecified variables, including age, admission National Institutes of Health Stroke Scale (NIHSS) score, admission serum glucose levels, time from LKW to the procedure, intravenous alteplase use, thrombus location, and successful reperfusion. Adjusted odds ratios (aORs) with their 95% CIs were reported. To address the unmeasured confounding, an instrumental variable analysis was also attempted using 2-stage residual inclusion method. A 2-stage probit model was developed using functional independence at 90 days as the dependent variable; repeated imaging as the endogenous variable; age, admission NIHSS score, admission serum glucose levels, time from LKW to the procedure, intravenous alteplase use, thrombus location, and successful reperfusion as exogenous variables; and proportion of patients receiving repeated imaging (number of patients receiving repeated imaging divided by the total number of patients) before a given patient at each site as the instrumental variable. Results of instrumental variable analysis were presented using coefficients (β) and asymptotically corrected standard errors (SE) with corresponding P values from the 2-stage probit model as well as illustrations of predicted probabilities from the naive model and the 2-stage probit model. Further details regarding instrumental variable analysis are provided in the eMethods in the Supplement.
Furthermore, important demographic and clinical characteristics that can affect the outcome as well as variables that demonstrated significant differences at baseline were used to create propensity scores. Using age, NIHSS score at presentation, admission glucose level, intravenous alteplase administration, thrombus location, ASPECTS at the referral center at non–contrast-enhanced CT, and time from LKW to the procedure, a propensity score was calculated using a logit model, and patients in the DTA and repeated imaging groups were matched using the nearest neighbor method in a 1:1 ratio to create a matched-pair population. The analyses were repeated in the matched-pair population.
Analyses were repeated in patients presenting within early vs late windows and in patients arriving during regular vs on-call hours. In addition, we assessed the association of the time elapsed during interfacility transfer (from arrival at the referral center to arrival at the EVT center) and the time from imaging acquisition at the referral center to arrival at the EVT center with clinical outcomes. Furthermore, in a sensitivity analysis, the effect of the ASPECTS score at referral hospital before transfer was evaluated by limiting the analysis to patients with available outside ASPECTS. An instrumental variable analysis was also repeated in the population with available ASPECTS at the referral center.
All analyses were performed using Stata, version 15 (StataCorp LLC). All hypothesis testing was conducted using 2-sided statistical tests, and P < .05 was considered statistically significant.
Results
Study Population
Of 2533 transferred patients, 1393 were excluded from thrombectomy after arrival at the EVT center, whereas 1140 received EVT (529 female [46.4%] and 609 male [53.4%], with 2 missing data; median age, 69 [interquartile range (IQR), 59-78 years). Of those who received EVT, 327 (28.7%) were managed with the DTA approach, whereas 813 (71.3%) had repeated imaging before EVT. The distribution of patients undergoing EVT with the DTA and repeated imaging approaches at each participating center is provided in eTable 2 in the Supplement.
Of the 1393 patients who did not receive EVT, 648 (46.5%) were excluded owing to clinical reasons, whereas 745 (53.5%) were not treated owing to their imaging findings. Clinical improvement (n = 265) and low NIHSS score (n = 65) were the 2 main clinical exclusion reasons, whereas recanalization on repeated imaging (n = 307), low ASPECTS with a large ischemic core volume (n = 213), and distal occlusions (n = 151) were the main imaging exclusions. eFigure 1 in the Supplement illustrates the flowchart of the study population. Imaging triage methods at the referral hospital are summarized in eTable 3 in the Supplement.
Baseline Patient Characteristics
The characteristics of patients undergoing EVT and stratified into DTA and repeated imaging groups are listed in Table 1. The median age (68 [IQR, 59-78] years for DTA vs 69 [IQR, 59-78] years for repeated imaging groups; P = .57) and proportion of female patients (152 of 326 [46.6%] for DTA vs 377 of 812 [46.4%] for repeated imaging groups; P = .95) were similar between the 2 groups. Patients in the DTA group had lower median serum glucose levels (118 [IQR, 104-148] vs 129 [IQR, 109-164] mg/dL), received alteplase more frequently (200 of 327 [61.2%] vs 412 of 808 [51.0%]; P = .002), and had a lower median NIHSS score (17 [IQR, 12-20] vs 17 [IQR, 13-21]; P = .07). Conscious sedation was the preferred anesthesia technique, with no difference observed between groups.
Table 1. Baseline Characteristics Based on DTA vs Repeated Imaging.
| Characteristic | Patient groupa | |
|---|---|---|
| DTA (n = 327) | Repeated imaging (n = 813) | |
| Age, median (IQR), y | 68 (59-78) | 69 (59-78) |
| Sex | ||
| Female | 152/326 (46.6) | 377/812 (46.4) |
| Male | 174/326 (53.4) | 435/812 (53.6) |
| Side of occlusion | ||
| Right | 161/326 (49.4) | 377/812 (46.4) |
| Left | 165/326 (50.6) | 434/812 (53.4) |
| Bilateral | 0 | 1/812 (0.1) |
| Presentation mRS score, median (IQR)b | 0 (0-1) | 0 (0-1) |
| Presentation NIHSS score, median (IQR)c | 17 (12-20) | 17 (13-21) |
| Presentation glucose level, median (IQR), mg/dL | 118 (104-148) | 129 (109-164) |
| Onset type witnessed | 188/318 (59.1) | 262/686 (38.2) |
| Alteplase administered | 200/327 (61.2) | 412/808 (51.0) |
| If imaging repeated, was NCCT repeated? | NA | 517/811 (63.7) |
| If imaging repeated, was CTA repeated? | NA | 494/811 (60.9) |
| If imaging repeated, was CTP repeated? | NA | 419/687 (61.0) |
| Clot location | ||
| ICA | ||
| Cervical | 16/327 (4.9) | 13/813 (1.6) |
| Intracranial | 83/327 (25.4) | 224/813 (27.6) |
| MCA M1 | 172/327 (52.6) | 426/813 (52.4) |
| MCA M2 | 56/327 (17.1) | 150/813 (18.5) |
| Anesthesia | ||
| Conscious sedation | 169/314 (53.8) | 367/686 (53.5) |
| General | 145/314 (46.2) | 319/686 (46.5) |
| ASPECTS, median (IQR)d | ||
| EVT center | NA | 8.0 (7.0-9.0) |
| Transferring center | 9.0 (9.0-10.0) | 8.0 (7.0-9.0) |
| Time from LKW to arrival at EVT hospital, median (IQR), min | 269 (190-430) | 280 (190-518) |
| Time from LKW to intravenous alteplase bolus, median (IQR), min | 150 (100-240) | 120 (82-176) |
| Time from LKW to procedure, median (IQR), min | 320 (226-495) | 351 (260-587) |
| Time from arrival at EVT center to groin puncture, median (IQR), min | 34 (20-62) | 60 (37-95) |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomographic Score; CTA, computed tomographic (CT) angiogram; CTP, CT perfusion; DTA, direct to angiography; EVT, endovascular thrombectomy; ICA, internal cerebral artery; IQR, interquartile range; LKW, last-known-well time; MCA, middle cerebral artery; mRS, modified Rankin Scale; NA, not applicable; NCCT, non–contrast-enhanced CT; NIHSS, National Institutes of Health Stroke Scale.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Unless otherwise indicated, data are expressed as No./total No. (%) of patients. Owing to missing data, numbers may not total column headings.
Scores range from 0 to 6, with higher scores indicating worse function.
Scores range from 0 to 42, with higher scores indicating higher stroke severity.
Scores range from 0 to 10, with lower scores indicating more ASPECTS areas demonstrating early ischemic changes on NCCT.
Of all patients, 623 (54.6%) arrived at the EVT center in the early window (202 [32.4%] in the DTA group and 421 [67.6%] in the repeated imaging group), whereas 517 (45.4%) arrived in the late window (125 [24.2%] in the DTA group and 392 [75.8%] in the repeated imaging group). eTables 4 and 5 in the Supplement compare the baseline characteristics between the DTA and repeated imaging groups stratified by the time of the patient’s arrival at the EVT center. Within 6 hours of LKW, patients in the DTA group had lower median NIHSS scores at presentation (17 [IQR, 12-20] vs 18 [IQR, 14-21]; P = .006) and were more likely to have experienced a witnessed stroke (109 of 198 [55.1%] vs 124 of 335 [37.0%]; P < .001). Other baseline characteristics were largely similar between the 2 groups (eTable 4 in the Supplement). For patients presenting beyond 6 hours of LKW, the DTA group also had a higher rate of witnessed strokes (79 of 120 [65.8%] vs 138 of 351 [39.3%]) and more frequent pretreatment with alteplase (45 of 125 [36.0%] vs 106 of 388 [27.3%]) (eTable 5 in the Supplement).
Time Metrics Comparison Between DTA and Repeated Imaging Groups
Both groups had a similar median time from LKW to arrival at EVT centers (269 [IQR, 190-430] minutes in the DTA group vs 280 [IQR, 190-518] minutes in the repeated imaging group; P = .11). The median time from EVT center arrival to groin puncture was 26 minutes shorter in patients treated with DTA (34 [IQR, 20-62] vs 60 [IQR, 37-95] minutes; P < .001).
A shorter median time from EVT center arrival to groin puncture with DTA was seen in both the early (29 [IQR, 19-50] vs 52 [IQR, 33-85] minutes; P < .001) and late (45 [IQR, 25-96] vs 69 [IQR, 43-111] minutes; P < .001) treatment windows. eFigure 2 in the Supplement illustrates the time metrics in the DTA and repeated imaging groups in the overall cohort and the early and late treatment windows.
We observed an improvement in both DTA and repeated imaging time metrics between the first (2014-2016) and second (2017-2020) halves of the study period. The median time from DTA arrival to groin puncture was shorter in both periods (50 [IQR, 21-95] vs 67 [IQR, 47-102] minutes in 2014-2016; 30 [IQR, 20-50] vs 55 [IQR, 33-92] minutes in 2017-2020) as illustrated in eFigure 3 in the Supplement.
Outcomes Comparison Between DTA and Repeated Imaging Groups
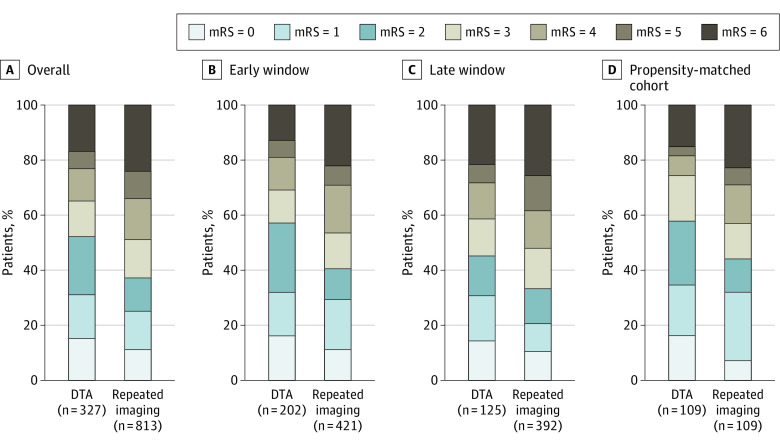
The rate of 3-month functional independence was higher with DTA (164 of 312 [52.6%] vs 282 of 563 [37.0%]; aOR, 1.85 [95% CI, 1.33-2.57]; P < .001) (Table 2). The rates were also significantly higher with DTA, both in the early window (109 of 191 [57.1%] vs 159 of 391 [40.7%]; aOR, 1.63 [95% CI, 1.07-2.48]; P = .02) and late window (55 of 121 [45.5%] vs 123 of 372 [33.1%]; aOR, 2.18 [95% CI, 1.28-3.37]; P = .004). Figure 1A-C illustrates the distribution of 3-month mRS scores in the DTA and repeated imaging groups in the overall cohort and early and late treatment windows. There was no interaction between outcomes and time window (0-6 vs >6 to 24 hours; P = .88 for interaction). An instrumental variable analysis also demonstrated a significantly higher functional independence in patients treated with DTA approach compared with patients who received repeated imaging (probit β, 0.52 [asymptotically corrected SE, 0.20]; P = .009). The effect size was larger than that demonstrated by the naive model, as illustrated by the predicted probabilities of functional independence with increasing age, NIHSS score, and serum glucose level in patients receiving DTA and repeated imaging approaches for EVT (eFigures 4-6 in the Supplement). In patients with repeated imaging, a 10-minute increase from arrival at the EVT center to groin puncture was associated with a 5% reduction in the odds of 3-month functional independence (aOR, 0.95 [95% CI, 0.91-0.99]; P = .01).
Table 2. Functional and Safety Outcomes of DTA vs Repeat Imaging.
| Factor | Patient groupa | P value | Test | |
|---|---|---|---|---|
| DTA (n = 312) | Repeated imaging (n = 763) | |||
| 90-d mRS score, including LOCF | ||||
| 0 | 47/312 (15.1) | 82/763 (10.7) | <.001 | Univariate ordinal logistic regression |
| 1 | 51/312 (16.3) | 109/763 (14.3) | ||
| 2 | 66/312 (21.2) | 91/763 (11.9) | ||
| 3 | 39/312 (12.5) | 106/763 (13.9) | ||
| 4 | 37/312 (11.9) | 114/763 (14.9) | ||
| 5 | 19/312 (6.1) | 75/763 (9.8) | ||
| 6 | 53/312 (17.0) | 186/763 (24.4) | ||
| Outcome at 90 d | ||||
| Good (mRS score 0-2) | 164/312 (52.6) | 282/763 (37.0) | <.001 | Pearson χ2 |
| Excellent (mRS score 0-1) | 98/312 (31.4) | 191/763 (25.0) | .03 | |
| Moderate (mRS score 0-3) | 203/312 (65.1) | 388/763 (50.9) | <.001 | |
| Disability at 90 d | ||||
| Severe (mRS score 4-6) | 109/312 (34.9) | 375/763 (49.1) | <.001 | Pearson χ2 |
| Profound disability (mRS score 5-6) | 72/312 (23.1) | 261/763 (34.2) | <.001 | |
| Symptomatic ICH | 28/324 (8.6) | 82/802 (10.2) | .42 | Pearson χ2 |
| Mortality (mRS score 6) at 90 d | 53/312 (17.0) | 186/763 (24.4) | .008 | Pearson χ2 |
Abbreviations: DTA, direct to angiography; ICH, intracerebral hemorrhage; LOCF, last observation carried forward; mRS, modified Rankin Scale.
Data are expressed as No./total No. (%) of patients.
Figure 1. Distribution of the 90-Day Modified Rankin Scale Score (mRS) in the Different Treatment Approach Groups at the Endovascular Thrombectomy (EVT) Center.
Patients are compared by repeated imaging vs direct to angiography (DTA) approaches. The early window indicates within 6 hours or less; the late window, within 6 to 24 hours. Patients who were treated with DTA achieved higher functional independence and lower mortality rates compared with the repeated imaging group, which was consistent across time windows. Furthermore, these results were confirmed in a propensity-matched analysis of 109 pairs.
Three-month mortality trended lower with DTA (53 of 312 [17.0%]) vs repeated imaging (186 of 763 [24.4%]; aOR, 0.66 [95% CI, 0.37-1.20]; P = .17) and was significantly lower in the early time window (25 of 191 [13.1%] vs 85 of 391 [21.7%]; P = .01). Rates of sICH were similar overall (28 of 324 [8.6%] in the DTA group vs 82 of 802 [10.2%] in the repeated imaging group; P = .42) and in the early window (16 of 200 [8.0%] vs 40 of 417 [9.6%], respectively; P = .52) and late window (12 of 124 [9.7%] vs 42 of 385 [10.9%], respectively; P = .70). Table 2 details the clinical and imaging outcomes by DTA and repeated imaging in the overall cohort, whereas eTables 6 and 7 in the Supplement describe the clinical and imaging outcomes for patients in the early and late windows, respectively.
In propensity-matched 109 pairs, the DTA group had a shorter time from arrival at the EVT center to groin puncture (34 [IQR, 20-64] vs 53 [IQR, 34-102] minutes; P < .001) and a higher rate of 3-month functional independence (64 of 109 [58.7%] vs 48 of 109 [44.0%]; aOR, 1.94 [95% CI, 1.03-3.65]; P = .04) (Figure 1D). In addition, 3-month mortality was numerically lower with DTA (17 of 109 [15.6%] vs 25 of 109 [22.9%]; P = .17), whereas sICH rates (10 of 109 [9.2%] vs 15 of 109 [13.8%]; P = .30) were similar.
No significant trends in the functional and safety outcomes in DTA and repeated imaging groups were observed during the study years (eFigures 7 and 8 in the Supplement). Furthermore, a sensitivity analysis excluding centers with a limited proportion of patients (<10%) also demonstrated a significant improvement in functional independence with the DTA approach (aOR, 1.65 [95% CI, 1.16-2.34]; P = .005) (eTable 8 in the Supplement).
Outcomes Comparison Between DTA and Repeated Imaging Adjusted to Referral Hospital Baseline Imaging
Data on referral center ASPECTS were available for 652 patients (270 in the DTA group and 382 in the repeated imaging group). Most of the patients (n = 649) in both groups had good ASPECTS (range, 6-10) at baseline before being transferred. Those in the DTA group had higher median ASPECTS at the referral center compared with patients who underwent repeated imaging (9 [IQR, 9-10] vs 9 [IQR, 8-10]; P = .03) (eTable 9 in the Supplement). In patients with available ASPECTS at referral centers, the DTA group had higher rates of functional independence (136 of 256 [53.1%] vs 138 of 371 [37.2%]; P < .001); this association also remained statistically significant in multivariable analysis after adjusting for potential confounders (aOR, 1.66 [95% CI, 1.10-2.51]; P = .02). Mortality rate was lower with DTA (42 of 256 [16.4%] vs 102 of 371 [27.5%]; P = .001), with a smaller difference for sICH (23 of 269 [8.6%] vs 51 of 382 [13.4%]; P = .06), as detailed in eTable 10 in the Supplement. Using an instrumental variable approach, the effect of DTA approach compared with repeating imaging was significant (probit β, 0.55 [asymptotically corrected SE, 0.26]; P = .03), with effect in the same direction and with a larger magnitude. eFigures 9 to 11 in the Supplement demonstrate the predicted probabilities of functional independence with increasing age, NIHSS score, and serum glucose level as demonstrated in the naive model and the 2-stage probit model for the instrumental variable analysis in patients with available CT ASPECTS at the transferring center.
Association of Arrival Time at EVT Center During Regular vs On-Call Hours With Outcomes in DTA vs Repeated Imaging
Of all patients, 458 (40.2%) arrived at the EVT center during regular work hours (DTA, 149 [32.5%]; repeated imaging, 309 [67.5%]), whereas 682 (59.8%) arrived during on-call hours (DTA, 178 [26.1%]; repeated imaging, 504 [73.9%]). eTables 11 and 12 in the Supplement compare the baseline characteristics between the DTA and repeated imaging groups arriving at the EVT center during regular work hours vs on-call hours. Overall, the main baseline characteristics were similar except for a higher proportion of patients who had a witnessed onset in the DTA group during both regular (82 of 146 [56.2%] vs 93 of 260 [35.8%]) and on-call (106 of 172 [61.6%] vs 169 of 426 [39.7%]) hours and higher intravenous alteplase pretreatment rates in the DTA group during regular hours (83 of 149 [55.7%] vs 149 of 308 [48.4%]).
The DTA approach was associated with a shorter median time from arrival to groin puncture, both during regular work hours (34 [IQR, 19-62] vs 57 [IQR, 38-89] minutes; P < .001) and on-call hours (34 [IQR, 21-62] vs 60 [IQR, 36-99] minutes; P < .001) (eFigure 12 in the Supplement). Three-month functional independence rates were higher for patients in the DTA group during regular work hours (77 of 143 [53.8%] vs 118 of 292 [40.4%]; P = .008), which retained statistical significance even after adjustment (aOR, 1.66 [95% CI, 1.01-2.72]; P = .04). During on-call hours, DTA was also associated with higher rates of 3-month functional independence (87 of 169 [51.5%] vs 164 of 471 [34.8%]; aOR, 2.01 [95% CI, 1.28-3.16]; P = .003). Mortality was lower with DTA during regular hours (22 of 143 [15.4%] vs 60 of 292 [20.5%]; P = .20) and on-call hours (31 of 169 [18.3%] vs 126 of 471 [26.8%]; P = .03) (eFigure 13 in the Supplement).
Rates of sICH did not differ between the DTA and repeated imaging groups both during regular (DTA, 13 of 147 [8.8%]; repeated imaging, 27 of 304 [8.9%]; P = .99) and on-call hours (DTA, 15 of 177 [8.5%]; repeated imaging, 55 of 498 [11.0%]; P = .29). eTables 13 and 14 in the Supplement detail the clinical and imaging outcomes in the DTA and repeated imaging groups based on their arrival time to the EVT center (during regular work and on-call hours).
Association of Transfer Time With Clinical Outcomes in DTA vs Repeated Imaging
Transfer times (from arrival at referral center to arrival at the EVT center) were available for 636 patients (DTA group, 204 [32.1%]; repeated imaging group, 432 [67.9%]). The median transfer times were significantly shorter for patients treated with DTA approach by approximately 46 minutes (2.13 [IQR, 1.55-2.83] hours) compared with patients who received repeated imaging at the EVT-capable center (2.90 [IQR, 2.07-4.52] hours; P < .001). We adjusted for this interval in the multivariate analysis with maintained statistical significance (aOR, 1.82 [95% CI, 1.20-2.76]; P = .005).
In addition, times from imaging acquisition at non-EVT centers to arrival to EVT centers were available for 561 patients (DTA group, 249 [44.4%]; repeated imaging group, 312 [55.6%]), and were longer in the repeated imaging group by approximately 24 minutes (105 [IQR, 77-139] vs 129 [IQR, 92-176] minutes; P < .001). Similarly, the results were maintained after adjustment to these time intervals in the multivariate analysis (aOR, 2.18 [95% CI, 1.38-3.42]; P = .001).
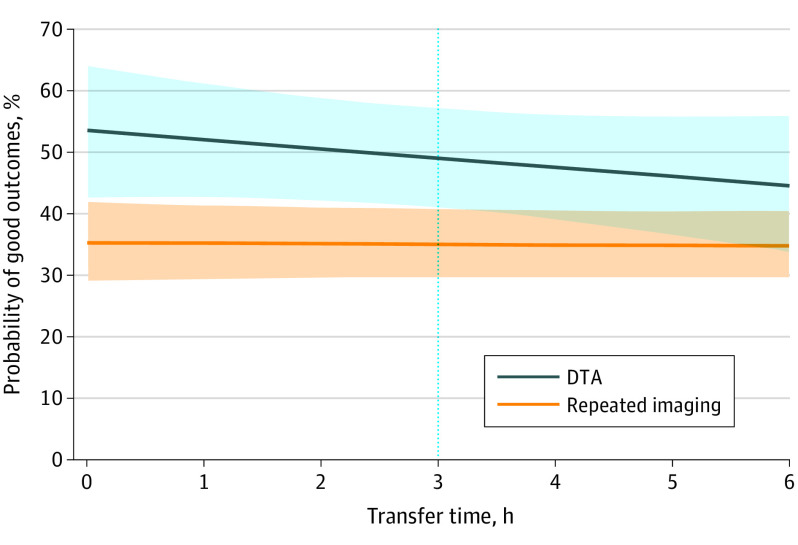
The rates of 3-month functional independence with DTA decreased as transfer (door in to door in) times increased (<3 hours, 96 of 161 [59.6%]; ≥3 hours, 15 of 42 [35.7%]; P = .006), whereas these rates did not change in patients with repeated imaging (<3 hours, 75 of 208 [36.1%]; ≥3 hours, 71 of 192 [37.0%]; P = .85). Similarly, mortality rates were higher in patients with transfer times of longer than 3 hours in DTA (<3 hours, 18 of 161 [11.2%]; ≥3 hours, 14 of 42 [33.3%]; P < .001), whereas no difference was observed in patients with repeated imaging (<3 hours, 53 of 208 [25.5%]; ≥3 hours, 52 of 192 [27.1%]; P = .72). Rates of sICH were also numerically higher with DTA as transfer times increased (<3 hours, 15 of 158 [9.5%]; ≥3 hours, 6 of 42 [14.3%]; P = .37), whereas they did not differ between patients with repeated imaging and increasing transfer times (<3 hours, 18 of 220 [8.2%]; ≥3 hours, 20 of 203 [9.9%]; P = .55). Figure 2 illustrates how the likelihood of 3-month functional independence changed with increment in transfer times in patients treated with the DTA and repeated imaging approaches. The likelihood decreased with increasing transfer times in patients in the DTA group, which began to overlap with patients receiving repeated imaging at 3 hours. Similar trends in functional independence, mortality, and sICH comparison between the DTA and repeated imaging groups were seen based on time from imaging acquisition at the referral center to arrival at the EVT center (<3 vs ≥3 hours), as shown in eTable 15 in the Supplement.
Figure 2. Probability of 3-Month Functional Independence After Endovascular Thrombectomy .
Data are stratified by direct to angiography (DTA) and repeated imaging approaches, as correlated with the transfer time. The probability decreases with increasing transfer times in the DTA group, beginning to overlap with patients receiving repeated imaging at 3 hours. Shaded areas indicate 95% CI.
Outcomes in Patients Excluded From Thrombectomy
The outcomes at 90-day follow-up were available for 228 patients excluded from EVT. The functional independence rate varied from 13 of 15 (86.7%) in those who had clinical improvement during transfer to 4 of 97 (4.1%) in patients who had large ischemic core volume/low ASPECTS on repeated imaging. eTable 16 in the Supplement summarizes the functional independence, excellent outcomes, and mortality rates of the patients who were excluded from EVT after repeated imaging. The overall functional independence rates in the available patients (47 of 228 [20.6%]) were not superior to those receiving EVT after repeated imaging.
Discussion
Although approximately three-quarters of patients with LVO transferred for EVT in our cohort received repeated imaging, our findings show that bypassing repeated imaging and moving directly to EVT resulted in faster time from EVT center arrival to groin puncture (by 26 minutes overall) and better clinical outcomes. These results were consistent in patients arriving in both the early (0-6 hours) and late (>6 to 24 hours) windows and during both regular work hours and on-call hours, which supports implementation of the DTA approach at any time. Faster treatment times may have contributed to the roughly 15% absolute increase in the rate of 3-month functional independence in patients undergoing DTA seen in early and late treatment windows and during regular and on-call hours. In addition, DTA was associated with a good safety profile, because sICH rates did not differ between DTA and repeated imaging at all times and settings—and if anything, mortality was lower with DTA. These same results at EVT arrival to groin puncture and clinical outcomes were observed in a propensity-matched sample of patients undergoing DTA and repeated imaging. Finally, similar potential treatment effect was found with the DTA approach in an instrumental variable analysis that accounted for the unmeasured confounding without evidence of endogeneity.
Overall, we found that every 10-minute increase in the time from arrival at the EVT center to EVT initiation in the repeated imaging group resulted in a 5% reduction in the likelihood of achieving functional independence. However, the trend to worsening mortality and other safety outcomes in patients in the DTA group with transfer times of 3 or more hours with similar functional independence rates compared with repeated imaging suggests that it is probably reasonable to repeat imaging in patients with prolonged transfer times to improve the safety profile.
The decline in the functional independence rates with increasing transfer times in the DTA approach can be explained by the prolonged transfer times resulting in further stroke evolution and subsequently worse outcomes, negating the potential benefit from faster treatment times at the EVT center. In the repeated imaging group, outcomes were mostly stable across the time epochs studied. It is plausible that transfer time did not affect the outcomes as much in the repeated imaging group, because the same imaging criteria for EVT were applied regardless of transfer times. Thus, repeated imaging probably resulted in excluding patients with significant stroke evolution during interfacility transfers. On the other hand, because imaging criteria for EVT may have differed among the treating centers, some patients with unfavorable imaging profiles after repeated imaging may have still received EVT. This may explain why previous randomized clinical trials, all of which all used strict criteria for EVT based on repeated imaging, demonstrated generally higher functional independence rates than those in our analysis.16
Faster times to treatment with DTA have been reported previously.17,18 One prior study17 reported similar outcomes between DTA and repeated imaging, whereas another18 reported better outcomes in patients arriving at EVT centers within 3 hours from LKW, with no difference beyond 3 hours. However, both studies were single-center studies without a significant number of patients beyond 6 hours. In addition, a recent single-center randomized trial19 of 60 patients showed a shorter time from imaging to treatment when patients underwent imaging in the angiography suite. However, the time saving was outweighed by longer arrival to imaging times that did not translate into a shorter time from arrival to reperfusion.
The times from arrival to groin puncture were similar in the repeated imaging group in both early and late windows. In contrast, we observed almost 1.5-fold longer times from door to procedure for patients treated with the DTA approach in late window. Because the study period overlapped with the ongoing clinical trials evaluating functional and safety outcomes of EVT in patients, it is plausible that the decision-making for these patients would be systematically different compared with patients presenting in the early window, when EVT was an established treatment. Furthermore, if patients who presented in the late window had good imaging at the referral center and short transfer times, they probably were considered slow progressors20 and there may be a sense of less urgency in treating them.
Prior estimates on the effect of time on thrombectomy outcomes reported a lower effect size estimate of 1% effect on ordinal mRS for every 4-minute delay in HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) data.5 Our results may be explained by a few plausible reasons. The estimates from HERMES represent the effect of the time from LKW to reperfusion compared with arrival to procedure time in our report and included patients only from the early window. Our report describes the effect of arrival to procedure time and has included patients from both early and late windows. Furthermore, our cohort represents all transferred patients, compared with only 32% transfers in the HERMES cohort. Another plausible explanation may be the difference in the infarct growth rates between the 2 cohorts and the breakdown of patient with slow vs fast progression20 in each approach (DTA vs repeated imaging).
Direct access to EVT centers remains limited,6 and several optimization methods are being initiated to improve this access, including improving LVO recognition and triage in the field,7,21 improving interventionalist access to patients,22,23 improving access through mobile stroke units,24 and establishing expedited transfer protocols.8,9 Meanwhile, our data show that optimizing the workflow within the EVT center should not be neglected and may substantially save time and improve outcomes. We found that the shorter times to treatment were persistent during work and on-call hours. This finding may be related to the fact that prehospital notification of the interventional team plausibly resulted in being on-site at the time of the patient’s arrival. However, our study shows that bypassing repeated imaging and transferring the patient directly to the angiography suite is feasible at all times and is not limited to work hours.
Recently, the RACECAT (Transfer to the Local Stroke Center vs Direct Transfer to Endovascular Center of Acute Stroke Patients With Suspected Large Vessel Occlusion in the Catalan Territory) randomized trial assessed the effect of in-field triaging of patients with suspected LVO directly to EVT centers compared with triage to the nearest stroke center with secondary transfer to the EVT center if necessary (the “drip and ship” model). The trial showed no difference between these approaches, suggesting that the drip and ship model will be maintained for now. This emphasizes the need for optimal protocols for smooth interfacility transfers and expedited workflow to the angiography suite after arrival to EVT centers.
We also describe the patients who were excluded from receiving EVT and the 90-day available outcomes. Although a good proportion of patients were excluded from EVT for recanalization and clinical improvement during transfer, another high proportion were excluded owing to large ischemic core volume/low ASPECTS. The overall functional independence rates in the patients with available 90-day outcomes were not superior to those receiving EVT after repeated imaging. In addition, a similar proportion of patients may have been included in the DTA group. Nevertheless, repeated imaging may be valuable in some cases, because it could result in excluding some patients who may not need or would not benefit from EVT.
The degree of improved outcomes with the DTA approach is consistent with the expected improvement associated with a time saving of 30 to 40 minutes, and was supported by propensity matched analysis. The magnitude of DTA effect in our results may have been affected by selection bias based on differences in baseline characteristics between patients in the DTA and repeated imaging groups. For instance, the DTA group had higher rates of alteplase administration and less severe strokes, as reflected in lower baseline NIHSS scores. We also observed higher frequency of unwitnessed strokes in patients with repeat imaging. The uncertainty of onset time associated with stroke of unknown onset in these patients may have contributed to more patients being treated conservatively by reacquiring additional qualifying images before proceeding with thrombectomy, given the uncertainty of how much time elapsed from LKW to arrival at EVT centers. Furthermore, the DTA group had more frequent witnessed onset and therefore may have been more likely to forego repeated imaging than those whose time of onset was unclear.
As with any retrospective cohort comparison, there were also undoubtedly other selection biases in the management of our patients that resulted in a tendency to bypass repeated imaging and choose DTA and which may have contributed to the group differences we observed. These selection biases and the true effect of DTA vs repeated imaging can only be evaluated in a prospective randomized study of the 2 approaches.
One potential selection bias could have been introduced by the fact that we did not have all the results of baseline imaging at the referral hospital before transfer. Although most patients in our cohort had good baseline ASPECTS scores (6-10) before transfer, those in the DTA group had higher median and distribution ASPECTS, reflecting less severe tissue damage. Patients with DTA had higher rates of functional independence on both univariate and multivariate analysis. However, our positive results in favor of DTA could be due in part to selection of more patients with slow progression20 who had less stroke evolution than those in the repeated imaging group.
Recently, the ANGIOCAT (Evaluation of Direct Transfer to Angiography Suite vs Computed Tomography Suite in Endovascular Treatment) trial25 demonstrated the feasibility and potential efficacy with reasonable safety of a DTA approach in a small, single-center randomized clinical trial in transferred patients as well as patients arriving directly at the EVT-capable center within 6 hours of LKW. The study results, however, are limited to the early (0-6 hours) window, and EVT was only performed when the angiography team was on site with a population mixed between direct and transferred patients. Our study cohort was larger and consisted of multicenter data of all transfer patients, for whom the DTA vs repeated imaging approach is much more of a significant impact question, because initial imaging or physician assessment has already excluded stroke mimics, indicated LVOs, and provided some inference into the status of the affected brain tissue on initial imaging. In addition, ANGIOCAT restricted the enrollment to the early window, whereas our cohort represents patients presenting in both early and late windows, with a significant proportion of patients presenting in the late window. Our study incorporated a pragmatic, real-world approach with patients presenting in both regular and on-call hours, whereas ANGIOCAT represented the patients enrolled in optimal scenario, when the angiography team is available. That said, the effect size of DTA vs repeated imaging approach in our study is very similar to what was shown in ANGIOCAT.
For validation of the findings and better generalizability, the ANGIOCAT investigators are planning an international multicenter trial (WE-TRUST [Workflow Optimization to Reduce Time to Endovascular Reperfusion for Ultra-fast Stroke Treatment]26). The DIRECTANGIO (Effect of Direct Transfer to Angio Suite on Functional Outcome in Severe Acute Stroke) trial27 is also being developed.
Limitations
As mentioned, the main limitation of our study is its nonrandomized nature and possibility of selection bias. Although we accounted for observed differences between groups by adjusting for these variables in the analyses provided and also by analyzing the question in a propensity-matched subgroup, the potential for unobserved confounding cannot be dismissed. Using the proportion of patients receiving repeated imaging at each site as the instrumental variable, we repeated the analysis and found the effect to be in a similar direction (ie, higher functional independence with the DTA approach) and with a larger magnitude. Even with robust analyses, however, unmeasured confounding variables may only be accounted for in a randomized trial setting. Measuring the true effect of the DTA approach vs repeated imaging would only be achievable via a randomized study—and even then, accounting for factors such as infarct progression, clot migration, and hemorrhagic transformation precluding EVT would be difficult.
Although we investigated the reasons for EVT exclusion after repeated imaging and attempted to evaluate the outcomes in these patients, we could not synthesize the results of the excluded patients’ outcomes with those of patients who received EVT after repeated imaging. In addition, these outcomes were available for only one-fourth of the excluded patients; thus, the effect of the excluded cases could not be fully evaluated. However, the overall functional independence rates in the available patients who were excluded from EVT after repeated imaging were not superior to those in repeated imaging EVT. Thus, the treatment effect direction would probably be the same when factoring these patients in the comparison between DTA and repeated imaging in transferred patients for the purpose of EVT.
The transfer times were shorter in DTA approach, but the results maintained their significance after adjustment for those differences. In addition, we acquired consistent results both when using interfacility transfer times as well as the time between imaging acquisition at the referral facility and arrival at the EVT center.
The patients in our cohort had different triage protocols at the referral hospitals based on vessel imaging or stroke severity, because baseline vessel images were not acquired in all patients before transfer. Our data, although from multiple centers, could be specific to these centers’ practices, which may affect the generalizability of the results. However, we obtained consistent results for both the early and late windows and for regular and on-call hours. These results indicate that the DTA approach is feasible and may result in better outcomes.
Conclusions
Our pooled, nonrandomized cohort study suggests that in transferred patients with LVO, bypassing repeated imaging and pursuing direct EVT could result in faster reperfusion and better functional outcomes during all hours and treatment windows. The potential efficacy and safety of the DTA approach decreased as transfer time increased. Therefore, repeated imaging may be reasonable in patients with prolonged transfer times. Optimizing EVT workflow in transferred patients may result in faster, safe reperfusion with higher chances of achieving functional independence.
eMethods. Instrumental Variable Approach
eReferences
eTable 1. Triage Protocols for DTA vs RI Approach at Different EVT Centers Included in the Study
eTable 2. Proportion of Patients Receiving Direct-to-Angio Approach and Repeat Imaging Approaches in Participating Centers
eTable 3. Type of Imaging Received at Referral Centers in Patients Receiving EVT After Transfer, Stratified Based on Treatment Approach
eTable 4. Baseline Clinical, Imaging, and Time Characteristics in Patient Presenting in Early Time Window
eTable 5. Baseline Clinical, Imaging, and Time Characteristics in Patient Presenting in Late Time Window
eTable 6. Functional and Safety Outcomes in Patient Presenting in Early Time Window
eTable 7. Functional and Safety Outcomes in Patient Presenting in Late Time Window
eTable 8. Results of Additional Sensitivity Analyses Performed
eTable 9. Baseline Clinical, Imaging, and Time Characteristics in Patients With Known Outside ASPECTS
eTable 10. Functional and Safety Outcomes in Patients With Known Outside ASPECTS
eTable 11. Baseline Clinical, Imaging, and Time Characteristics in Patients Presenting in Regular Hours
eTable 12. Baseline Clinical, Imaging, and Time Characteristics in Patients Presenting During On-Call Hours
eTable 13. Functional and Safety Outcomes in Patients Presenting in Regular Hours
eTable 14. Functional and Safety Outcomes in Patients Presenting During On-Call Hours
eTable 15. Functional and Safety Outcomes Stratified By Time From Imaging Acquisition at the Referral Center to Arrival at EVT-Capable Center
eTable 16. Reasons for Not Receiving EVT After Repeated Imaging and Outcomes at 90-d Follow-up in Medical Management Patients With Available 90-d Follow-up
eFigure 1. Flow Diagram of the Study Population
eFigure 2. Time Metrics From Last Known Well to Arrival to EVT Center and Time From Arrival to EVT Center to Procedure
eFigure 3. Distribution of Time From Arrival to Groin Puncture Stratified Over the Study Time Periods in Patients Treated With Direct-to-Angio and Repeat Imaging Approaches
eFigure 4. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing Age
eFigure 5. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing NIHSS at Presentation
eFigure 6. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing Serum Glucose
eFigure 7. Distribution of Functional Outcomes as Measured by Modified Rankin Scale Scores Over the Study Time Periods
eFigure 8. Trends of Functional Independence, Mortality, and Symptomatic ICH Over Study Time Period
eFigure 9. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Model and Instrumental Variable Model With Increasing Age in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 10. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model With Increasing NIHSS at Presentation in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 11. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model With Increasing Serum Glucose Level at Presentation in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 12. Time Metrics (Time From Last Known Well to Arrival at EVT Center and Time From Arrival at EVT Center to Procedure
eFigure 13. Distribution of the 90-d mRS Scores Stratified Based on DTA and Repeated Imaging Approaches
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 6.Sarraj A, Savitz S, Pujara D, et al. Endovascular thrombectomy for acute ischemic strokes: current US access paradigms and optimization methodology. Stroke. 2020;51(4):1207-1217. doi: 10.1161/STROKEAHA.120.028850 [DOI] [PubMed] [Google Scholar]
- 7.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 8.Sablot D, Farouil G, Laverdure A, Arquizan C, Bonafe A. Shortening time to reperfusion after transfer from a primary to a comprehensive stroke center. Neurol Clin Pract. 2019;9(5):417-423. doi: 10.1212/CPJ.0000000000000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst M, Psychogios M-N, Schlemm E, et al. Modeling the optimal transportation for acute stroke treatment: impact of diurnal variations in traffic rate. Clin Neuroradiol. 2020. Published online July 16, 2020. doi: 10.1007/s00062-020-00933-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulouis G, Lauer A, Siddiqui AK, et al. Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol. 2017;74(11):1361-1367. doi: 10.1001/jamaneurol.2017.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Requena M, Olivé-Gadea M, Boned S, et al. Clinical and neuroimaging criteria to improve the workflow in transfers for endovascular treatment evaluation. Int J Stroke. 2020;15(9):988-994. doi: 10.1177/1747493019874725 [DOI] [PubMed] [Google Scholar]
- 12.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group; Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 14.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 16.Sarraj A, Mlynash M, Savitz SI, et al. Outcomes of thrombectomy in transferred patients with ischemic stroke in the late window: a subanalysis from the DEFUSE 3 trial. JAMA Neurol. 2019;76(6):682-689. doi: 10.1001/jamaneurol.2019.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke. 2017;48(7):1884-1889. doi: 10.1161/STROKEAHA.117.016946 [DOI] [PubMed] [Google Scholar]
- 18.Requena M, Olivé M, García-Tornel Á, et al. Time matters: adjusted analysis of the influence of direct transfer to angiography-suite protocol in functional outcome. Stroke. 2020;51(6):1766-1771. doi: 10.1161/STROKEAHA.119.028586 [DOI] [PubMed] [Google Scholar]
- 19.Pfaff JAR, Schönenberger S, Herweh C, et al. Direct transfer to angio-suite versus computed tomography-transit in patients receiving mechanical thrombectomy: a randomized trial. Stroke. 2020;51(9):2630-2638. doi: 10.1161/STROKEAHA.120.029905 [DOI] [PubMed] [Google Scholar]
- 20.Sarraj A, Hassan AE, Grotta J, et al. ; SELECT Investigators . Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the SELECT Study. Stroke. 2021;52(1):57-69. doi: 10.1161/STROKEAHA.120.030912 [DOI] [PubMed] [Google Scholar]
- 21.Abilleira S, Pérez de la Ossa N, Jiménez X, et al. Transfer to the local stroke center versus direct transfer to endovascular center of acute stroke patients with suspected large vessel occlusion in the catalan territory (RACECAT): study protocol of a cluster randomized within a cohort trial. Int J Stroke. 2019;14(7):734-744. doi: 10.1177/1747493019852176 [DOI] [PubMed] [Google Scholar]
- 22.Wei D, Oxley TJ, Nistal DA, et al. Mobile interventional stroke teams lead to faster treatment times for thrombectomy in large vessel occlusion. Stroke. 2017;48(12):3295-3300. doi: 10.1161/STROKEAHA.117.018149 [DOI] [PubMed] [Google Scholar]
- 23.Brekenfeld C, Goebell E, Schmidt H, et al. “Drip-and-drive”: shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg. 2018;10(10):932-936. doi: 10.1136/neurintsurg-2017-013634 [DOI] [PubMed] [Google Scholar]
- 24.Parker SA, Bowry R, Wu T-C, et al. Establishing the first mobile stroke unit in the United States. Stroke. 2015;46(5):1384-1391. doi: 10.1161/STROKEAHA.114.007993 [DOI] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov. Evaluation of Direct Transfer to Angiography Suite vs Computed Tomography Suite in Endovascular Treatment: Randomized Clinical Trial (ANGIOCAT). NCT04001738. Accessed March 29, 2021. https://clinicaltrials.gov/ct2/show/NCT04001738
- 26.ClinicalTrials.gov. WE-TRUST (Workflow Optimization to Reduce Time to Endovascular Reperfusion for Ultra-fast Stroke Treatment). NCT04701684. Accessed March 24, 2021. https://clinicaltrials.gov/ct2/show/NCT04701684
- 27.ClinicalTrials.gov. Effect of Direct Transfer to Angiosuite on Functional Outcome in Severe Acute Stroke (DIRECTANGIO). NCT03969511. Accessed March 29, 2021. https://clinicaltrials.gov/ct2/show/NCT03969511
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Instrumental Variable Approach
eReferences
eTable 1. Triage Protocols for DTA vs RI Approach at Different EVT Centers Included in the Study
eTable 2. Proportion of Patients Receiving Direct-to-Angio Approach and Repeat Imaging Approaches in Participating Centers
eTable 3. Type of Imaging Received at Referral Centers in Patients Receiving EVT After Transfer, Stratified Based on Treatment Approach
eTable 4. Baseline Clinical, Imaging, and Time Characteristics in Patient Presenting in Early Time Window
eTable 5. Baseline Clinical, Imaging, and Time Characteristics in Patient Presenting in Late Time Window
eTable 6. Functional and Safety Outcomes in Patient Presenting in Early Time Window
eTable 7. Functional and Safety Outcomes in Patient Presenting in Late Time Window
eTable 8. Results of Additional Sensitivity Analyses Performed
eTable 9. Baseline Clinical, Imaging, and Time Characteristics in Patients With Known Outside ASPECTS
eTable 10. Functional and Safety Outcomes in Patients With Known Outside ASPECTS
eTable 11. Baseline Clinical, Imaging, and Time Characteristics in Patients Presenting in Regular Hours
eTable 12. Baseline Clinical, Imaging, and Time Characteristics in Patients Presenting During On-Call Hours
eTable 13. Functional and Safety Outcomes in Patients Presenting in Regular Hours
eTable 14. Functional and Safety Outcomes in Patients Presenting During On-Call Hours
eTable 15. Functional and Safety Outcomes Stratified By Time From Imaging Acquisition at the Referral Center to Arrival at EVT-Capable Center
eTable 16. Reasons for Not Receiving EVT After Repeated Imaging and Outcomes at 90-d Follow-up in Medical Management Patients With Available 90-d Follow-up
eFigure 1. Flow Diagram of the Study Population
eFigure 2. Time Metrics From Last Known Well to Arrival to EVT Center and Time From Arrival to EVT Center to Procedure
eFigure 3. Distribution of Time From Arrival to Groin Puncture Stratified Over the Study Time Periods in Patients Treated With Direct-to-Angio and Repeat Imaging Approaches
eFigure 4. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing Age
eFigure 5. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing NIHSS at Presentation
eFigure 6. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model in DTA and RI Approaches With Increasing Serum Glucose
eFigure 7. Distribution of Functional Outcomes as Measured by Modified Rankin Scale Scores Over the Study Time Periods
eFigure 8. Trends of Functional Independence, Mortality, and Symptomatic ICH Over Study Time Period
eFigure 9. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Model and Instrumental Variable Model With Increasing Age in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 10. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model With Increasing NIHSS at Presentation in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 11. Assessment of Unmeasured Bias Using Predicted Probabilities of Functional Independence Derived From Naive Overall Model and Instrumental Variable Model With Increasing Serum Glucose Level at Presentation in Patients With Available ASPECTS Scores at Transferring Centers
eFigure 12. Time Metrics (Time From Last Known Well to Arrival at EVT Center and Time From Arrival at EVT Center to Procedure
eFigure 13. Distribution of the 90-d mRS Scores Stratified Based on DTA and Repeated Imaging Approaches