Abstract
Maintaining physical mobility is important for healthy aging and for preventing age-related comorbidities in older adults. Aerobic exercise training has been shown to prevent mobility loss in older people, but exercise alone does not always induce the expected improvements in physical and cardiopulmonary function among older adults. Recent preclinical evidence suggests that one reason for the variability in responsiveness to exercise training may be the age-related dysregulation of the nicotinamide adenine dinucleotide (NAD+) metabolome. NAD+ is an essential enzymatic cofactor in energetic and signaling pathways. NAD+ insufficiency, leading to reduced energy metabolism, could result in an inadequate exercise response. Recently, the NAD+ precursor nicotinamide riboside (NR), a vitamin B3 derivate, showed an ability to improve NAD+ metabolome homeostasis of fuel utilization and over cellular function in various organs in animals. NR has also been tested in older humans and is considered safe, but the effects of NR supplementation alone on physical performance are equivocal. The purpose of this review, therefore, is to outline preclinical and clinical evidence on the potential benefits of NR supplementation strategies alone and in combination with physical activity on skeletal muscle, mobility, and cardiovascular (CV) function.
Introduction
Regular exercise training has been recognized as a key component in successful aging promoting independence in older people (1). The current 2018 Physical Activity Guidelines emphasize the importance of regular exercise as a medical therapy in older adults (2). Benefits of exercise training in elders has been demonstrated in a wide range of disease conditions including hypertension, type 2 diabetes, obesity, dyslipidemia, osteoporosis, cancer, cognitive impairment, depression, anxiety, sleep disorders (2). The main goals of exercise interventions remain the preservation of skeletal muscle function to prevent physical disability (3), and improvement of CV function to reduce CV risk factors and the incidence of CV events (4, 5). Well-functioning skeletal muscle and CV systems are important components for an effective response to acute exercise, but also to adapt to chronic exercise training (6, 7). Despite the largely recognized benefits of exercise training in preserving healthy aging, it is becoming clear that the human responsiveness to exercise training varies, and exercise alone may not always induce the expected improvements in physical function among older adults (8). In particular, even when participants engage in carefully controlled exercise training regimens, the nature of the training response is heterogeneous (9). It is also difficult to predict whether skeletal muscle and CV functions, which are highly related to the mobility disability and mortality of older adults, will improve with exercise training (10). For example, evidence from the HERITAGE Family Study showed that genetics may explain 30–60% of the interindividual differences in response to 20-week exercise training in terms of maximal oxygen uptake (VO2max), endurance performance and cardiometabolic risk factors (11–14). Sometimes exercise can even have adverse effects on cardiometabolic parameters (e.g. resting systolic blood pressure, triglycerides, insulin) (9). These findings highlight there might be a complex of genetical, biological, behavioral, environmental factors which may explain interindividual variability of exercise response.
Recent preclinical evidence suggests that one reason for the variability in the responsiveness to exercise training may be the age-related dyshomeostasis of NAD+ metabolome (15). NAD+ is an essential enzymatic cofactor involved in energy and signal transduction processes (16). Reduced NAD+ levels may represent a major contributor to the development of many age-associated diseases and physical disability (17). The poor-exercise-response phenomenon could be related to the observed NAD+ decline that occurs during aging and cellular senescence (18–20). In rodents, it has been described a stepwise reduction of intracellular NAD+ going from 3- to 24-month old mice, in different tissues and in particular in the heart (18). There is also evidence of strong negative correlation between NAD+ levels and age in human tissues (21). Restoring NAD+ homeostasis has therefore been proposed as one of the most promising strategies to counteract this functional decline (22).
Nicotinamide riboside (NR) is an emerging compound that has been shown to increase NAD+ biosynthesis in a variety of tissues (23). NR is a form of vitamin B3 and a precursor molecule in the biosynthesis of NAD+ (23), which has demonstrated excelent tolerabilty and safety in clinical studies receiving the GRAS (Generally Recognised as Safe) status by the FDA (24). Notably, NR and other NAD+ precursors appear more effective in preserving muscular functions in aged animals and humans than in young ones (15, 25, 26). For example, it has been shown that NR treatment improved maximal running time and distance, and limb grip strength only in aged (~24 months), and not in young (~3 months) mice (25). Similar effects have been observed also in healthy human males who were not previously involved in regular exercise training (26). After a single dose of NR 500 mg, the subgroup of older individuals (mean age 71.5 years old) showed a significant improvement of isometric strength in lower limbs and higher resistance to fatigue, but not the subgroup of young subjects (mean age 22.9 years old) (26). This suggests that age-associated impairment of NAD+ homeostasis could be improved efficiently by NR supplementation. The combination of NAD+ precursors and exercise training in older adults may be important to enhancing exercise therapy and helping to prevent physical disability and other age-related comorbidities, but the evidence is unclear. In this paper, preclinical and clinical studies describing the effects of NR on skeletal muscle and CV function are reviewed. The potential for NR to optimize effects of exercise training is discussed.
Literature search
A systematic literature search was conducted through PubMed, from database inception until January 2020, using combinations of the following words: “nicotinamide riboside,” AND (“exercise,” OR “training,” OR “physical activity,” OR “muscle,” OR “cardiovascular”). The main focus of this article is to find relevant clinical and preclinical studies exploring the effects of the NAD+ precursor, NR, alone or in combination with exercise training, on systems mainly involved in physical performance (skeletal muscle and CV system). As there is limited evidence in older animals and humans, we applied no age criterion in the selection process. Overall, we found 58 potentially eligible articles. We excluded studies on people or animal models with ischemic heart disease, heart failure, stroke, neurodegenerative diseases, muscular dystrophy, and mitochondrial diseases, all conditions that could influence per se the results on outcomes of interest. We included 14 studies, 9 preclinical and 5 clinical studies, for a qualitative synthesis (25–38).
NAD+ metabolome during aging
NAD+ and its reduced form nicotinamide adenine dinucleotide hydride (NADH) are co-factors to multiple enzymes involved in glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), and other redox reactions in cells (16, 39). NAD+ is a precursor for nicotinamide adenine dinucleotide phosphate (NADP) co-enzyme synthesis. The reduced form of NADP i.e. NADPH is required in anabolic pathways such as such as lipid synthesis, cholesterol synthesis, and fatty acid chain elongation. Also, NADPH is the donor of reductive potential to glutathione and thioredoxins which are used by glutaredoxins, peroxiredoxins and glutathione peroxidases for hydrogen peroxide (H2O2) detoxification (40). NAD+ is required as a substrate by poly-adenosine diphosphate (ADP) ribose polymerases (PARPs), NAD-dependent deacetylases (sirtuins), and cyclic ADP ribose synthetases (cADPRSs). cADPRSs are transmembrane proteins which includes CD38 and hydrolyze NAD+ to NAM and ADP-ribose. Growing evidences suggests that NAD+ levels steadily decline during chronological aging in multiple tissues including liver, skeletal muscle, adipose tissue, heart, brain, kidney, spleen, and is responsible for age-related metabolic decline in these tissues (19, 20, 23, 29, 41–43).
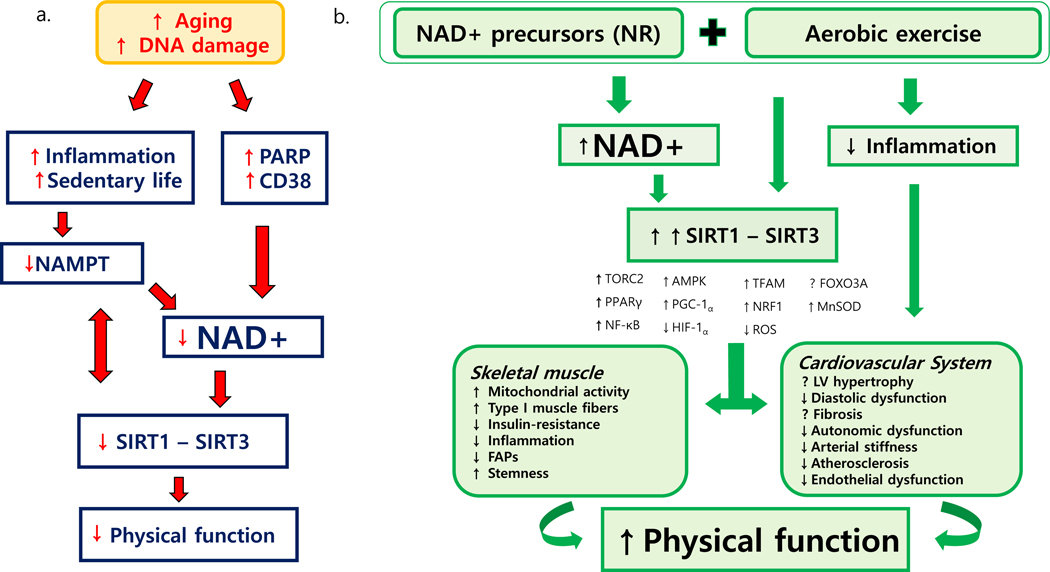
The level of NAD+ in a cell is governed by a balance between its synthesis and utilization. NAD+ biosynthesis is regulated by a rate-limiting enzyme, nicotinamide phosphoribosyl transferase (NAMPT). With aging, expression of NAMPT decreases in association with inflammation (40). Physical activity has shown to increase both skeletal muscle mitochondrial biogenesis and NAMPT expression (44). Decline in NAD+ levels with aging is attributed to overconsumption by two major proteins: PARP1 and CD38 (45). PARP1 is an enzyme involved in various cellular activities, including single-stranded DNA damage repair, post-translational modification, chromatin remodeling, cell-death regulation, DNA replication, and telomere maintenance (46). In aging, in response to DNA damage, PARP1 over-activation which consumes more NAD causing NAD+ deficit conditions, is responsible for impairing cellular metabolic processes (47). Recent evidence has shown that CD38, an enzyme which was first identified to be involved in immune responses and present on the surface of many immune cells (CD4+, CD8+, B lymphocytes and natural killer cells) but also expressed in muscle, liver and brain tissues. CD38 expression increases with chronological aging and is shown as a major NAD+ consumer in muscle, liver and brain tissues (19). Higher CD38 expression and activity in liver tissue from mice were associated with NAD+ depletion, decreased oxygen consumption coupled to ATP synthesis, and decreased mitochondrial membrane potential (19). Conversely, CD38 knockout mice have shown elevated NAD+ levels accompanied with increased mitochondrial function. SIRT3 knockout in CD38 knockout mice, however, abolished increased mitochondrial function, suggesting that SIRT3 may be acting downstream to CD38 in regulating mitochondrial function. NAD+ is an obligate co-substrate for mammalian sirtuins, a class of histone deacetylases that regulate several cellular functions including the control of energy metabolism, circadian rhythms, tissue regeneration, inflammation, neuronal signaling, DNA repair, and cell survival (48). Since their discovery in yeasts, sirtuins have been proposed as potential regulators of longevity (49); and cellular levels of NAD+ are critical for sirtuins activity. Over-activation of PARP1, for example, increases cellular NAD+ deficit conditions, which can in turn decrease NAD+ dependent SIRT1 activity. However, lower SIRT1 activity has been observed only in specific tissues including skeletal muscle and the myocardium of aged animals suggesting that ultimately regulating SIRT1 activity through NR intervention could be potential target for therapeutic intervention to restore skeletal muscle and myocardium function (50, 51). Inhibiting PARP1 increases NAD+ levels to an extent that induces SIRT1 activity (52). In turn, activated SIRT1 deacetylates and activates transcriptional factors (PGC-1α and FOXO1, P53) that are central to mitochondrial biogenesis; results in increased expression of mitochondrial genes and enhanced mitophagy; and maintains mitonuclear protein balance (39, 53). Taken together, decline in cellular NAD+ levels from increased PARPs activation and CD38 expression in aging reduces sirtuins activity and thus contributes to metabolic decline and reduced physical function (Figure 1a).
Figure 1.
Relationship between impaired NAD+ metabolome and reduced physical function during aging (a) and potential effects of combination of exercise training with NR supplementation on muscle and CV functions during aging (b).
In younger people, biosynthetic pathways relying on dietary sources of tryptophan and NAD+ precursor vitamins counterbalance the consumption of NAD+ (54). Diet, however, may become insufficient for maintaining the NAD+ metabolome in aging, and therefore may require supplementation of NAD+ precursors such as NR.
Exercise and NAD+ metabolism
Exercise requires a higher energy expenditure than a resting state, and therefore a higher turnover of adenosine triphosphate (ATP), which depends on the redox balance maintained by NAD+. It has been shown that exercise, stimulating the NAMPT, the rate-limiting enzyme of NAD+ biosynthesis, increases NAD+ levels and enhances SIRT1 and SIRT3 activity (55, 56). Although these findings were demonstrated in aged animals and humans (55, 57), the effects on muscular and CV functions are often markedly heterogeneous (10). The impaired redox status typical of senescent cells with higher ROS generation and the concomitant reduced NAD+ bioavailability may inhibit SIRT1 and SIRT3 activity and stimulate the SIRT-competing enzyme PARP that further reduces the NAD+ pool for SIRT1 (51).
To test the effect of impaired NAD+ metabolism on skeletal muscle function, Frederick et al. created a mouse model carrying specific deletion of Nampt gene in skeletal muscle cells (29). In this model, 3-month old transgenic mice exhibited 85% reduction of intramuscular and mitochondrial NAD+ content and more than halved ATP levels compared to the littermate controls (29). This biochemical signature was associated to smaller fiber size in the hindlimbs, increased fiber stiffness and infiltration by immune cells, and increased progressive degeneration of muscular cells (29). Only from 7 months of age, there was clinical evidence of hindlimb muscle weakness and earlier exhaustion during treadmill exercise, demonstrating that young animals are able to effectively cope with reduced NAD+ bioavailability, but not the older ones (29). Interestingly, the knockout of the Nampt gene in adipocytes (58) and in projection neurons (59) caused a progressive limitation of motor performance, suggesting that reduced NAD+ pool also in other cell types may contribute to reduced physical function. Recently, Das et al. showed that in older mice, treatment with the NAD+ precursor (nicotinamide mononucleotide (NMN)) resulted in increased capillary density, improved blood flow, and increased endurance; and these positive effects were further enhanced in response to exercise (15). Deleting the SIRT1 gene in endothelial cells, however, even with NMN treatment, resulted in reduced muscle density and number of capillaries, and was associated with premature exhaustion in response to a high-intensity endurance test, suggesting that the positive effects of NMN and exercise were mediated by SIRT1 (15). Collectively, this evidence shows that restoration of disrupted NAD+ metabolome by NAD+ intervention together with exercise intervention, through sirtuins, could potentially improve physical function during aging.
Evidence of NR effects in animal studies
Among the 14 identified studies, nine were preclinical exploring the effects of NR on skeletal muscle and CV system (25, 27–32, 37, 38). Table 1 presents current evidence from these studies. The NR has been administered at a dose of 300 (28, 37) or 400 mg/kg/day (25, 27, 29, 30, 32, 38) via chow (25, 27, 30, 32, 38) and oral gavage (28, 29, 37), or supplemented in a 12-mM drinking water solution ad libitum (31). Treatments lasted from 1 week (30) to 6 months (31). Animal studies using administration of study products via drinking water should be treated with caution and may be limited due to variable consumption by an animal, and thus potential insufficiency or high concentrations of the consumed study product (60).
Table 1.
Findings from pre-clinical studies investigating effects of NR on skeletal muscle and CV systems
| Study (Year) | Model (age) | Dose | Length | Key findings related to intervention |
|---|---|---|---|---|
| Cantò et al.(2012) (30) | C57BL/6J mice fed with CD or HFD (10 weeks) | 400 mg/kg/day via food admix | 1 week | ↑ endurance performance; ↑ oxidative profile in muscles; ↑ NAD+ in muscle; ↑ direct NAD+ synthesis; no effect on PARP-1 levels; ↑ sirtuin activity in the skeletal muscle; ↑ expression of nuclear genes encoding transcriptional regulators of oxidative metabolism (Sirt1, PGC-1α, Tfam) and mitochondrial proteins in quadriceps muscles; ↑ mtDNA in muscle |
| Cerutti et al. (2014) (27) | wild type mice (2 months) | 400mg/kg/day via food admix | 4 weeks | No effect on motor performance; ↑ NAD+/NADH ratio; ↑ sirtuin activity; no effect on genes related to fatty acids oxidation and OXPHOS; no effect on mitochondrial respiratory chain activity |
| Zhang et al. (2016) (25) | C57BL/6J mice (22–24 months) | 400 mg/kg/day via food admix | 6 weeks | No effect on muscle mass; ↑ maximal running times and distances; ↑ limb grip strength; after cardiotoxin-induced muscle damage ↑ muscle regeneration and ↓ accumulation of non-myogenic FAPs and macrophage infiltration; |
| Effect on MuSCs: ↑ MuSCs number; ↑ NAD+; ↑ the stemness; ↓ nuclear DNA damage; ↓ expression of cell senescence and apoptosis markers; ↓ pro-inflammatory proteins; ↑ expression of cell cycle genes; ↑ expression of genes encoding TCA cycle and OXPHOS proteins; ↑ mitochondrial activity; knocking out SIRT1 in MuSCs blocked the beneficial effects of NR on MuSC activation and senescence 7 days after regeneration | ||||
|
MuSCs transplanted into a mouse model of muscular dystrophy: ↑ muscle regeneration and MuSCs transplantation efficiency o effect on muscle mass; ↑ maximal running times and distances; ↑ limb grip strength); after cardiotoxin-induced muscle damage ↑ muscle regenerationand ↓ accumulation of nonmyogenic FAPs and macrophage infiltration |
||||
| Kourtzidis et al. (2016) (28) | male Wistar rats (4 months) | 300 mg/kg/day via oral gavage | 3 weeks | ↓ physical performance (swimming performance test) by 35% at the final 10% load |
| Frederick et al. (2016)(29) | muscle-specific Nampt knockout (mNKO) mice (5.5 months) | 400 mg/kg/day via oral gavage | 6 weeks | Prevention of the development of exercise intolerance; ↓ progressive muscle dysfunction; ↓ lactic acidosis at the point of exhaustion; ↑ muscle fiber remodeling; ↑ fiber diameter; ↑ mitochondrial activity; complete restoration of exercise capacity after 1 week, ↑ total and mitochondrial NAD+ |
| Hou et al. (2018)(31) | wild type mice (16–18 months) | 12 mM in drinking water | 6 months | ↑ grip strength |
| Diguet et al. (2018)(32) | Sf/Sf mice(9 months) | 400 mg/kg/day via food admix | 45 days | No effect on heart rate and LV mass index; ↑ the LV thickness-to-radius ratio; no effect on vascular reactivity; ↑ myocardial NAD+ metabolites; no effect on cardiac levels of other markers of NAD+ abundance; ↑ metabolism of citrate; no effect on mitochondrial activity |
| Kourtzidis et al. (2018)(37) | male Wistar rats (4 months) | 300 mg/kg/day via oral gavage | 3 weeks | No effect on NADPH in muscle; ↑pro-oxidative environment; ↓ glucose in plasma; no effect on muscle glycogen content |
| Crisol et al. (2019)(38) | C57BL/6J mice (8 weeks) | 400 mg/kg/day via food admix | 5 weeks | ↑ NAD+ in the skeletal muscle; no effect on exercise performance |
NR: nicotinamide riboside; CD: chow diet; HFD: high-fat diet; NAD: nicotinamide adenine dinucleotide; PARP: poly (ADP-ribose) polymerases; NAMPT: nicotinamide phosphoribosyltransferase; OXPHOS: oxidative phosphorylation; MuSCs: muscle stem cells; COX: cytochrome c oxidase; SIRT: sirtuin; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator-1α; Tfam: mitochondrial transcription factor A; TCA: tricarboxylic acid; FAP: fibro/adipogenic precursors; LV: left ventricular;
Zhang et al. examined the effect of NR 400 mg/kg/day, administered for 6 weeks, on muscle structure and function (25). They found that NR in older animals (24 months old) was able to reverse some biochemical aspects of aging muscle (e.g., insulin resistance, inflammation), switch muscle fiber from glycolytic to a more oxidative fiber type, increase number of muscle stem cells, and promote regeneration of muscle fibers (25). In particular, in skeletal muscle cells and stem cells, NR reduced nuclear and mitochondrial DNA damage, expression of senescence and apoptosis markers and conversely enhanced expression of cell cycle genes (25). Furthermore, NR increased the expression of proteins involved in fatty acid oxidation, TCA cycle and OXPHOS reactions, and increased the activity of mitochondrial respiratory chain, producing more ATP (25). In young and old mice, NR increased the concentration and function of SIRT1 and its downstream target (forkhead transcription factor (FOXO1)), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and superoxide dismutase 2 (SOD2)) (27, 30); and the knockout of the gene encoding the SIRT1 blunted the NR effects (25). In old wild-type mice, NR did not increase muscle mass (25), but improved muscle function, improving endurance capacity by 56–80% (25) and hindlimb grip strength after at least 6-week supplementation (25, 31).
Frederick et al. tested the effect of treatment with NR 400 mg/kg/day for 6 weeks in 5.5 months old mice with NAD+ deficiency, such as those carrying muscle-specific Nampt knockout (29). At this age, the animals already showed initial signs of reduced muscular mass and endurance capacity. NR treatment was able to restore exercise capacity just after one week from the intervention onset, prevent exercise intolerance during a treadmill test, reduce lactates accumulation at the point of exercise exhaustion, and increase force generated by isolated extensor digitorum longus muscle (29). Interestingly, in this model of NAD+ deficiency, NR normalized muscle fiber diameter and hindlimbs muscle mass, but not had any significant effect in same age littermate controls (29).
A potential mechanism explaining the improvement of physical performance could be an increase of muscle stem cells number (25). Zhang et al. found that after inducing muscle damage, mice treated with NR showed faster regeneration of muscle fibers (25). Notably, muscle stem cells isolated from NR-treated mice stimulated myogenesis when transplanted in a disease-specific model of Duchenne muscular dystrophy (25). Moreover, NAD+ also increased insulin-sensitivity, facilitating better utilization of blood glucose for energy requests (30). Finally, the treatment reduced the accumulation of non-myogenic fibro/adipogenic progenitors (FAPs) and inflammatory cells, reducing expression of pro-inflammatory genes (25).
Considering that treatment with NAD+ precursors favors aerobic metabolism, as shown by higher soluble oxygen (sO2) levels and O2 consumption and reduced post-exercise blood lactates (15, 20, 29, 43), Das et al. demonstrated that these effects could be mediated by better muscle perfusion and neo-angiogenesis (15). Indeed, it has been found that NAD+ repletion strategies not only improved vasoreactivity, as shown by restored endothelium-dependent dilation (EDD), nitric oxide-mediated EDD, and reduced vessel-wall stiffness (61), but could also favor better myocardial contractility as measured by left ventricular wall thickness to cavity size ratio (H/R ratio), an index of adaptive hypertrophic remodeling of the heart following exercise training (32).
By contrast, evidence about the efficacy of NAD+ repletion strategies on motor performance from young rodents are conflicting (27, 28, 30). For example, NR significantly increased NAD+ content and sirtuin activity in muscle (27, 30, 38) and nicotinic acid adenine dinucleotide (NAAD) levels and NAD+ metabolites in myocardial cells (32). These changes were not associated with improvement in OXPHOS reactions, mitochondrial function (27, 32), or physical functioning (27, 28, 30), except in young mice that received a high-fat diet (30). In particular, no improvement was found in the aerobic performance during a treadmill test after 4-week supplementation with NR 400 mg/kg/day (27), but interestingly Cantò et al. showed a significant improvement in the same test after 1 week in young mice fed a high-fat diet (30). In contrast, Kourtzidis et al. found faster time to exhaustion in a forced swim test in response to a 3-week supplementation with NR 300 mg/kg/day, compared to rats receiving no treatment (28). These authors argued that in young and healthy animals, NR altered the redox homeostasis toward a pro-oxidant environment, which may explain the poorer functional performance following NR intervention (37). Outcomes of a forced swim for exercise testing in animals should be treated with caution due to other factors such as respiratory reactions and stress caused by contact with water (62).
Overall, these findings support the notion that supplementation of NR could be more effective in particular subgroups at risk of NAD+ deficiency (e.g., older animals) or with higher NAD+ dyshomeostasis (e.g., overweight/obese animals).
Limited evidence on the effect of NR in human studies
Five articles have been published on the effects of NR on muscular and CV functions in humans (26, 33–36) (Table 2). All the studies are randomized, placebo-controlled clinical trials (RCTs), three with a crossover design (26, 34, 35) and one with a parallel design (33, 36). Overall these RCTs were characterized by small sample size (maximum 24 participants per group) with age of participants ranging from 20 to 80 years old. NR treatment varies across the RCTs for dose (from 500 (26) to 2,000 mg/day (33, 36)) and duration (from one day (26) to 12 weeks (33, 36)), but without any adverse reaction even under maximum dosage (33). Compared to placebo, the NR intervention significantly increased circulating NAD+ (more than twofold) (35), NADH (up to 60%) (26, 34), NMN (1.4-fold) (35), and NAAD (4.5-fold) (35) with excellent safety and tolerability (34, 35). NR increased NAAD and products of NAD+ metabolism in muscle (35), but not other markers of NAD+ abundance (NAD+, NADH, NADP+, and NADPH) (35, 36). Also, in one trial, NR reduced NAMPT levels in the muscle, suggesting the presence of a negative-feedback mechanism, which limits the NAD+ synthesis after exposure to exogenous NAD+ precursor supplementation (36). Such discrepancies on NR bioavailability at skeletal muscle level might depend on heterogeneity of the studied population (i.e., age, comorbidities).
Table 2.
Findings from clinical studies investigating effects of NR on skeletal muscle and CV systems
| Study (Year) | Population and age | Dose | Length | Key findings related to intervention | Limitations |
|---|---|---|---|---|---|
| Dollerup et al. (2019)(36) | 40 (20: NR treated) Caucasian males, insulin-resistant, obese (58±2 years old) | 2000 mg/day | 12 weeks | No change of NAD+ metabolites in skeletal muscle; ↓ NAMPT; no change in SIRT3 levels and activity; no effect on glucose metabolism in skeletal muscle; no effect on mitochondrial abundance and activity in skeletal muscle; no effect on lipid deposition in skeletal muscle | - Small sample size - Only white male subjects - Low generalizability - No measure of physical performance |
| Elhassan et al. (2019)(35) | 12 old males (median age: 75 years old) | 1000 mg/day | 21 days | Well tolerability; no adverse events; ↑ NAD+ metabolites in skeletal muscle; ↓ expression of genes involved in energy metabolism in skeletal muscle; no effect on mitochondrial abundance and activity in muscle; no effect on hand-grip strength; no effect on forearm muscle blood flow; no effect on muscle glucose uptake and lactate production; no changes in systemic cardiometabolic parameters (blood pressure, lipid profile, fasting glucose, insulin, and HOMA-IR); ↓ circulating inflammatory markers (IL-6, IL-5, IL-2 and TNF-α) | - Small sample size - Only male subjects - No measure of physical performance at baseline |
| Dolopikou et al. (2019)(26) | 12 old males (71.5±1 years old) and 12 young males (22.9±1 years old) | 500 mg/ day | 1 day | ↑ NAD+ metabolites in erythrocytes; ↓ F2 isoprostanes in urine by 18%; ↑ isometric peak torque by 8% and fatigue index by 15% in old male | - Small sample size - Only male subjects - No measure of NAD+ metabolites in muscle - No safety data |
| Martens et al. (2018)(34) | 24 subjects, (65±7 years old) | 1000 mg/day | 6 weeks | No serious adverse effects; ↑ NAD+ metabolites in PBMCs; ↓ systolic blood pressure in individuals with elevated/stage I hypertension; no other significant change of indicators of CV health; no improvement on aerobic exercise capacity or motor function | - Small sample size - No measure of NAD+ metabolites in muscle |
| Dollerup et al. (2018)(33) | 40 (20: NR treated) Caucasian males, insulin-resistant, obese (58±1.6 years old) | 2000 mg/day | 12 weeks | No serious adverse effects; Vs. Placebo: ↑ NAD+ metabolites in urine; Vs. Baseline: no differences in body composition; not improve insulin sensitivity and other metabolic parameters |
- Small sample size - Only white male subjects - Low generalizability |
NR: nicotinamide riboside; NAD: nicotinamide adenine dinucleotide; HOMA-IR: homeostatic model assessment of insulin resistance; IL: interleukin; TNF-α: tumor necrosis factor alpha; SIRT: sirtuin; NAMPT: nicotinamide phosphoribosyltransferase; PBMCs: peripheral blood mononuclear cells
Nevertheless, findings on NR efficacy in improving physical and CV functions are limited. In 24 healthy middle-aged participants (mean age: 65 years old), for example, Martens et al. showed that administrating NR 1000 mg/day for 6 weeks reduced systolic and diastolic blood pressure in pre-hypertensive subjects and reduced aortic stiffness in individuals with higher baseline systolic blood pressure (34). Other indicators of CV function (i.e., carotid artery compliance, flow-mediated dilation), physical function (i.e., walking distance, muscle strength in upper and lower limbs, dynamic balance, fatigue), and metabolic parameters (i.e., metabolic rate, respiratory exchange ratio (RER), insulin sensitivity), however, did not change (34). Dolopikou et al., in a subgroup of 12 older men (mean age: 71.5 years old), two hours after a single supplementation with 500 mg of NR, found a significant improvement of isometric knee extensor strength (by 8%) and reduction of fatigue (by 15%) as measured by percentage decrease of torque between the first five and the last five repetitions of 30 maximal voluntary concentric leg contractions at 180°/s angular velocity (26).
Elhassan et al. tested the effects of oral NR supplementation with 1000 mg/day for 3 weeks on NAD+ metabolome in skeletal muscle of 12 male older adults (70 to 80 years old) (35). They showed that intervention significantly up-regulated the expression of genes involved in cell adhesion, and motility, but reduced expression of genes involved in OXPHOS metabolism, and mitochondrial function in skeletal muscle (35). NR did not produce any improvement on mitochondrial activity and biosynthesis (i.e., mitochondrial respiration, mitochondrial copy number, levels of proteins involved in the electron transport chain), neither on muscle glucose uptake and lactate production nor on metabolic (i.e., lipid profile, fasting glucose, insulin resistance, RER) and CV (i.e., blood pressure, forearm blood flow) variables compared to placebo. It reduced circulating levels of some pro-inflammatory cytokines such as interleukin (IL)-6, IL-5, IL-2, and tumor necrosis factor alpha (TNF-α) (35). Moreover, SIRT1 activity remained unchanged, and no effect on hand-grip strength was reported (35). Similarly, in a population of 40 middle-aged (40 to 70 years old) obese and sedentary men, chronic treatment with NR 2000 mg/day for 12 weeks did not alter gene expression and protein levels of NAD+ biosynthesis enzymes, did not improve insulin sensitivity, and had no effect on GLUT4, hexokinase II, mammalian target of rapamycin (mTOR), and glycogen synthase (GS) in skeletal muscle (33, 36). Moreover, no change was found in the resting energy expenditure, mitochondrial function, and oxidative capacity, and the network organization of mitochondria were unchanged in skeletal muscle (33, 36). Additionally, NR did not alter body composition as assessed by dual-energy X-ray absorptiometry (DXA) and did not affect intra- and extra-myocellular lipid deposition in skeletal muscle as measured by 1H-MR-spectroscopy (33, 36).
Overall, results derived from these studies could be affected by high likelihood of type II error due to small sample size. This, probably, was large enough to detect change in NAD+ metabolites, but not in functional parameters. Nevertheless based on these preliminary findings, NR supplementation alone might not be sufficient to produce an improvement in physical performance. Considering that the positive effects of regular exercise training may be limited in older adults by the dysregulated NAD+ metabolome, it is conceivable that the combination of exercise training with NR supplementation could optimize exercise therapy in older adults (Figure 1b). In contrast to other studies in combinations of compounds and exercise targeting biological pathways in a synergistic way (63), NR could normalize the NAD+ metabolome to improve fuel utilization and cellular function to optimize the adaptive process to exercise training.
NAD+ homeostasis as an enhancer of exercise training
Two animal studies have explored the effect of combined treatment with exercise training and NR (37, 38), and one more study has tested the effect of another NAD+ precursor, NMN, in combination with exercise (15). The results of the NAD+ precursors combined with exercise interventions are conflicting (Table 3). Crisol et al., for example, demonstrated that young mice undergoing combined intervention with NR (400 mg/kg/day) and a treadmill test (60 min/day for 5 days a week) after 5 weeks had better physical performance (i.e., running distance and maximum power, but not muscle strength) compared to those treated with the single treatment (38). Such improvement in exercise capacities was associated with greater activity of the mitochondrial isoform of NMN adenylyl transferases (NMNAT3) and higher levels of gene activity of the respiratory chain complexes IV and V, which may explain the increased content of type I-oxidative skeletal muscle fibers observed in the group under combined treatment (38).
Table 3.
Findings from studies investigating combined effects of NAD+ precursors and physical exercise
| Study (Year) | Model (age) | Groups | Dose | Length | Key findings |
|---|---|---|---|---|---|
| Crisol et al. (2019)(38) | C57BL/6J mice (8 weeks) | CON, EX, NR, NR+EX | EX: treadmill test for 60 min each session, 5 days a week NR: 400 mg/kg/day NR + EX: NR 400 mg/kg/day + treadmill test for 60 min each session, 5 days a week | 5 weeks | EX: ↓ total body weight and fat depots; ↑ running distance (31%) and maximum power (18%) NR+EX vs. EX: no difference in total body weight and fat depots; ↑ running distance (33%) and maximum power (14%); not increase strength; no effect on NMNAT1 content in the skeletal muscle; ↑ content of NMNAT3; ↑ mitochondrial proteins; ↑ number of type I fibers NR+EX vs. NR: ↑ running distance (89%) and maximum power (39%) |
| Kourtzidis et al.(2018)(37) | male Wistar rats (4 months) | CON, EX, NR, NR+EX | CON: saline solution EX: saline solution + preloaded incremental swimming test (time to exhaustion) NR: 300 mg/kg/day NR+EX: NR 300 mg/kg/day + preloaded incremental swimming test | 3 weeks |
EX: ↓ NADPH in liver; ↑ NADPH in muscle; ↑ F2-isoprostanes in plasma; ↓ glutathione peroxidase in muscle; ↓ catalase in muscle; ↓ glucose in plasma; no effect on both liver and muscle glycogen content NR+EX vs. EX: ↑ liver glycogen content (163%); no effect on muscle glycogen content; trend towards lower maximal lactate accumulation levels after exercise; ↑ F2-isoprostanes in plasma (vs. CON); ↓ glutathione peroxidase in muscle (vs. CON); ↓ catalase in muscle (vs. NR and CON) |
| Das et al.(2018)(15) | C57BL/6J mice (10 months) | CON, NMN, EX, NMN+EX | NMN:500 mg/kg/day EX: treadmill exercise (30 min/day at 15 m/min) | 30 days |
NMN+EX vs. NMN: 70% more capillaries than sedentary mice, more than twice the effect of NMN alone NMN+EX vs. EX: ↑ Capillary/myofiber ratio in quadriceps |
CON: control; EX: exercise; NR: nicotinamide riboside; MN: nicotinamide mononucleotide; NMNAT: NMN adenylyltransferases
In the 4-month old rats, Kourtzidis et al. showed that 11 days of swimming activity associated with administration of NR 300 mg/kg/day increased the liver glycogen, but not the skeletal muscle glycogen contents, and tended to reduce lactate levels after exercise, compared to exercise alone (37). However, they also observed an increase of systemic oxidative stress, marked by F2-isoprostanes in plasma, with concomitant reduction of antioxidant enzymes (37). In particular, they found a significant interaction between NR and exercise intervention in reducing catalase levels in the skeletal muscle cells (37). These findings could explain why in another study by Kourtzidis, the NR blunted the effect of exercise on physical performance in young rats (28). Das et al. treated old mice (10 months) with 500 mg/kg/day of NMN and 30 minutes of treadmill exercise/day for 4 weeks, and found that a combined therapy compared to NMN or exercise alone significantly increased the number of capillaries in the quadriceps muscle (by 70% compared to sedentary mice treated with NMN alone) (15).
As stated above, these studies on the combination of NR with exercise training have reported mixed results. Considering the fact that NR was safe and produced better results in older populations with impaired baseline health status, more studies are warranted to test the combination of NR and exercise in improving the homeostasis of the NAD+ metabolome in older and moderately functioning older populations.
Conclusions and future directions
Beneficial effects of the NR supplementation, mostly in preclinical models, including improvements in skeletal muscle, CV function, and physical performance were mostly observed in conditions characterized by impaired NAD+ metabolome. The results in humans are equivocal regarding effects, optimal dosages and duration of the treatment, and effective NR bioavailability at muscular level. Such studies are likely underpowered to assess change in functional outcomes. Human studies are warranted to confirm efficacy of NAD+ precursors in combination with exercise training. The only preclinical evidence on the combination of NR and exercise, derived mainly from young rodents, is scarce and mixed.
Subsequent well-designed studies on the NAD+ precursors such as NR could further test it as a potential missing piece of the puzzle in optimizing exercise therapy in older adults. Future trials should specifically target subjects who need exercise for preventive and therapeutic reasons and who are also at higher risk of NAD+ deficiency (potentially more responsive to supplementation), such as older adults with cardiometabolic comorbidities.
Clinical implications.
Appropriate exercise prescriptions for the elderly are a very important tool to support a rapidly aging society. Exercise interventions are very difficult to implement, especially when the older adults suffer from various diseases and have poor physical function. Effective physical training can help to prevent age-related diseases, and demonstration that nutritional optimization of the biological response may improve benefit of exercise training, would aid geriatric providers and help prevent age-related comorbidities.
Acknowledgments
This work was supported by the National Institute on Aging grants AG028740, AG064282 (RTM, SDA, and CL) and AHA Career Development Award 18CDA34080001 (RTM). The funding bodies had no role in the design of the study; collection, analysis, and interpretation of data; or the writing of the manuscript.
References
- 1.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, et al. Successful aging: Advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24(Pt B):304–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochrane SK, Chen SH, Fitzgerald JD, Dodson JA, Fielding RA, King AC, et al. Association of Accelerometry-Measured Physical Activity and Cardiovascular Events in Mobility-Limited Older Adults: The LIFE (Lifestyle Interventions and Independence for Elders) Study. J Am Heart Assoc. 2017;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorostegi-Anduaga I, Maldonado-Martin S, MartinezAguirre-Betolaza A, Corres P, Romaratezabala E, Whittaker AC, et al. Effects on Cardiovascular Risk Scores and Vascular Age After Aerobic Exercise and Nutritional Intervention in Sedentary and Overweight/Obese Adults with Primary Hypertension: The EXERDIET-HTA Randomized Trial Study. High Blood Press Cardiovasc Prev. 2018;25(4):361–8. [DOI] [PubMed] [Google Scholar]
- 6.Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation. 2003;107(1):e2–5. [DOI] [PubMed] [Google Scholar]
- 7.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23(6):1034–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buford TW, Miller ME, Church TS, Gill TM, Henderson R, Hsu FC, et al. Antihypertensive Use and the Effect of a Physical Activity Intervention in the Prevention of Major Mobility Disability Among Older Adults: The LIFE Study. J Gerontol A Biol Sci Med Sci. 2016;71(7):974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, et al. Precision exercise medicine: understanding exercise response variability. Br J Sports Med. 2019;53(18):1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). 1999;87(3):1003–8. [DOI] [PubMed] [Google Scholar]
- 12.An P, Rice T, Gagnon J, Leon AS, Skinner JS, Bouchard C, et al. Familial aggregation of stroke volume and cardiac output during submaximal exercise: the HERITAGE Family Study. Int J Sports Med. 2000;21(8):566–72. [DOI] [PubMed] [Google Scholar]
- 13.Perusse L, Gagnon J, Province MA, Rao DC, Wilmore JH, Leon AS, et al. Familial aggregation of submaximal aerobic performance in the HERITAGE Family study. Med Sci Sports Exerc. 2001;33(4):597–604. [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, Rice T, Gagnon J, Perusse L, Province M, Bouchard C, et al. Familiality of triglyceride and LPL response to exercise training: the HERITAGE study. Med Sci Sports Exerc. 2000;32(8):1438–44. [DOI] [PubMed] [Google Scholar]
- 15.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, et al. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173(1):74–89 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015;50(4):284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, et al. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med. 2017;23(10):899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6(4):e19194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23(6):1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, Jayasena T, et al. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid Redox Signal. 2019;30(2):251–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshino J, Baur JA, Imai SI. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018;27(3):513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poljsak B, Milisav I. Vitamin B3 forms as precursors to NAD+: are they safe? Trends in Food Science & Technology. 2018;79:198–203. [Google Scholar]
- 25.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–43. [DOI] [PubMed] [Google Scholar]
- 26.Dolopikou CF, Kourtzidis IA, Margaritelis NV, Vrabas IS, Koidou I, Kyparos A, et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur J Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19(6):1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kourtzidis IA, Stoupas AT, Gioris IS, Veskoukis AS, Margaritelis NV, Tsantarliotou M, et al. The NAD(+) precursor nicotinamide riboside decreases exercise performance in rats. J Int Soc Sports Nutr. 2016;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frederick DW, Loro E, Liu L, Davila A Jr., Chellappa K, Silverman IM, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016;24(2):269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115(8):E1876–E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation. 2018;137(21):2256–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–53. [DOI] [PubMed] [Google Scholar]
- 34.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28(7):1717–28 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dollerup OL, Chubanava S, Agerholm M, Sondergard SD, Altintas A, Moller AB, et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin resistant men. J Physiol. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Kourtzidis IA, Dolopikou CF, Tsiftsis AN, Margaritelis NV, Theodorou AA, Zervos IA, et al. Nicotinamide riboside supplementation dysregulates redox and energy metabolism in rats: Implications for exercise performance. Exp Physiol. 2018;103(10):1357–66. [DOI] [PubMed] [Google Scholar]
- 38.Crisol BM, Veiga CB, Braga RR, Lenhare L, Baptista IL, Gaspar RC, et al. NAD(+) precursor increases aerobic performance in mice. Eur J Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 39.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–13. [DOI] [PubMed] [Google Scholar]
- 41.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345(4):1386–92. [DOI] [PubMed] [Google Scholar]
- 42.Yaku K, Okabe K, Nakagawa T. NAD metabolism: Implications in aging and longevity. Ageing Res Rev. 2018;47:1–17. [DOI] [PubMed] [Google Scholar]
- 43.Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24(6):795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298(1):E117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz MB, Sinclair DA. Why NAD(+) Declines during Aging: It’s Destroyed. Cell Metab. 2016;23(6):965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol Cell. 2015;58(6):947–58. [DOI] [PubMed] [Google Scholar]
- 47.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19(17):1951–67. [DOI] [PubMed] [Google Scholar]
- 48.Suwa M, Sakuma K. The potential role of sirtuins regarding the effects of exercise on aging- related diseases. Curr Aging Sci. 2013;6(2):178–88. [DOI] [PubMed] [Google Scholar]
- 49.Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 Axis in Aging. Rejuvenation Res. 2017;20(3):244–7. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139–50. [DOI] [PubMed] [Google Scholar]
- 51.Mohamed JS, Wilson JC, Myers MJ, Sisson KJ, Alway SE. Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging (Albany NY). 2014;6(10):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13(4):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157(4):882–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolle C, Skoge RH, Vanlinden MR, Ziegler M. NAD biosynthesis in humans--enzymes, metabolites and therapeutic aspects. Curr Top Med Chem. 2013;13(23):2907–17. [DOI] [PubMed] [Google Scholar]
- 55.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. International journal of molecular sciences. 2019;20(11):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson ML, Irving BA, Lanza IR, Vendelbo MH, Konopka AR, Robinson MM, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;70(11):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, et al. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015;21(5):706–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, et al. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Rep. 2017;20(9):2184–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marx JO, Vudathala D, Murphy L, Rankin S, Hankenson FC. Antibiotic administration in the drinking water of mice. J Am Assoc Lab Anim Sci. 2014;53(3):301–6. [PMC free article] [PubMed] [Google Scholar]
- 61.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dos Reis IGM, Martins LEB, de Araujo GG, Gobatto CA. Forced Swim Reliability for Exercise Testing in Rats by a Tethered Swimming Apparatus. Front Physiol. 2018;9:1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mankowski RT, Anton SD, Buford TW, Leeuwenburgh C. Dietary Antioxidants as Modifiers of Physiologic Adaptations to Exercise. Med Sci Sports Exerc. 2015;47(9):1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]