Abstract
The brain endocannabinoid system plays a crucial role in reward processes by mediating appetitive learning and encoding the reinforcing properties of substances. Evidence also suggests that endocannabinoids are an important constituent of neuronal substrates involved in emotional responses to stress. Thus, it is critical to understand how the endocannabinoid system and stress may affect reward processes given their importance in substance use disorders. We examined the relationship between factors that regulate endocannabinoid system signaling (i.e., cannabinoid receptor genes and prolonged cannabis exposure) and stress on fMRI BOLD response to reward cues using a multivariate statistical technique. We found that proxies for endocannabinoid system signaling (i.e., endocannabinoid genes and chronic exposure to cannabis) and stress have differential effects on neural response to cannabis cues. Specifically, a single nucleotide polymorphism (SNP) variant in the cannabinoid receptor 1 (CNR1) gene, early life stress, and current perceived stress modulated reward responsivity in long-term, heavy cannabis users, while a variant in the fatty acid amide hydrolase (FAAH) gene and current perceived stress modulated cue-elicited response in non-using controls. These associations were related to distinct neural responses to cannabis-related cues compared to natural reward cues. Understanding the contributions of endocannabinoid system factors and stress that lead to downstream effects on neural mechanisms underlying sensitivity to rewards, such as cannabis, will contribute towards a better understanding of endocannabinoid-targeted therapies as well as individuals risks for cannabis use disorder.
1. Introduction
The current literature suggests that the endocannabinoid system interacts with the dopaminergic system to signal motivation for natural rewards and modulates the rewarding effects of addictive substances [1]. Endocannabinoids mediate retrograde signaling in neuronal tissues and are involved in the regulation of synaptic transmission to modulate neurotransmitter release by presynaptic cannabinoid receptors. A reduction in the catabolic enzyme fatty acid amide hydrolase (FAAH) that metabolizes anandamide (N-arachidonoyl-ethanolamine) - an endogenous agonist for cannabinoid 1 receptors (CB1Rs) [2] – has been associated with increased dopamine D3 receptors, suggesting a link between the endocannabinoid and dopaminergic systems that may underlie risk for substance use disorders [3]. Potential mechanisms by which cannabinoids modulate dopamine transmission include (1) location of CB1Rs in the ventral tegmental area, where mesocorticolimbic dopaminergic efferent projections originate, (2) CB1R expression in dopamine neuronal targets, and (3) location of CB1Rs on pre-synaptic GABAergic and glutaminergic neurons that directly interact with dopaminergic neurons [4, 5] [1]. Thus, activation of CB1Rs can, to some degree, modulate dopaminergic function in regions related to reward processing. Taken together, prolonged activation of CB1Rs by agonists, via chronic exposure to delta (9)-tetrahydrocannabinol (THC), may therefore stimulate dopaminergic neurotransmission associated with the rewarding effects of substances.
The modulatory action of endocannabinoids on synaptic transmission also has significant functional implications on synaptic plasticity in stress response. During acute stress, FAAH acts to degrade anandamide, which then leads to increased amygdala excitability [6]. Preclinical models have suggested that targeting the endocannabinoid system by increasing deficient levels of anandamide through FAAH inhibition can alleviate effects of stress [7]. Gray and colleagues (2016) indicated that increased corticotropin-releasing hormone in mice is common following increased stress-induced changes in endocannabinoid signaling, where increased FAAH led to reductions in anandamide in the amygdala and prefrontal cortex [8]. Rossi et al. (2008) reported that social defeat stress altered neural transmission in the CB1Rs within the striatum and that this effect was due to corticosteroids as the alterations were prevented by blockade of glucocorticoid receptors [9].
Genes that modulate endocannabinoid signaling are therefore likely to have downstream effects on reward processing and stress response, and impact risk for substance use disorders. Variability in the cannabinoid receptor 1 (CNR1) single nucleotide polymorphism (SNP) rs2023239 that codes for CB1 protein causes alternative splicing of CNR1 [10] and has been associated with craving and withdrawal [11] [12] as well as proclivity toward cannabis use disorder [13]. The C allele of the CNR1 rs2023239 SNP has been indicated to have enhanced cue-elicited activation in mesocorticolimbic areas associated with increased reward sensitivity [1]. Similarly, the FAAH gene regulates the expression of anandamide, and has been associated with anti-stress effects (e.g., anxiolytic effects during high stress conditions) [14]. In clinical models, a specific SNP, FAAH C385A, increases reward-related neural response in the ventral striatum in healthy controls, suggesting a role of FAAH in susceptibility to substance abuse [15] [16]. A recent study in female mice by Burgdorf et al., [17] found that this FAAH SNP variant leads to densely populated CB1Rs on GABAergic interneurons associated with dopamine increases in the ventral tegmental area [11, 17]. The degradation of endocannabinoid signaling in the nucleus accumbens after chronic stress in mice [18] has also been translated in human studies demonstrating modulated subcortical response associated with CNR1 gene variability in those with depression and anxiety [19]. Interestingly, stress-induced synaptic deficiencies were relieved by natural rewards, such as access to a running wheel or sucrose, in addition to a cocaine injection, suggesting a potential mechanism by which the interaction between the endocannabinoid system and stress contributes to the development of reward dysfunction [10]. Preclinical studies on alcohol dependence have shown that the interaction between CNR1 variants and stress was associated with increased alcohol consumption in CNR1+/+ mice, but not CNR1−/− mice [20].
Taken together, dysregulation within the endocannabinoid system through genetic [11] and environmental (e.g., prolonged cannabis exposure, stress) [21] [22] factors appear to disrupt response to rewards [23]. Despite clear links between stress and endocannabinoid signaling, including apparent vulnerability for cannabis use disorder, the neurobiological mechanisms that underlie this link have not yet been directly examined in humans. The goal of this study was to determine the contribution of endocannabinoid system factors (endocannabinoid genes, prolonged exposure to cannabis) and stress (early trauma, current perceived stress) in neural response to reward. We hypothesized that there would be contributions of CNR1 and FAAH SNP variants and stress in cannabis users that would be associated with neural response to cannabis cues. Based on the existing literature, we expected that the presence of CNR1 rs2023239 G and FAAH rs324420 C alleles, perceived and early life stress and greater neural response to cannabis cues (vs. control cues) will be associated in cannabis users but not in non-using controls.
2. Materials and Methods
The Institutional Review Board of the University of Texas at Dallas and University of Texas Southwestern Medical Center approved these study procedures. All experiments were conducted according to the principles expressed in the Declaration of Helsinki.
2.1. Participants
One hundred and thirty-seven participants were recruited for this study aimed to examine the contributions of genes and neurobiological mechanisms in cannabis use disorder [11, 21, 24]. Participants were included if they were: right-handed, spoke English as their primary language, and had no current or history of psychosis, traumatic brain injury, and MRI contraindications (e.g., pregnancy, non-removal metallic implants, claustrophobia). All participants were screened via urinalysis for drugs of abuse and were excluded if drugs were detected (except cannabis for the cannabis group). Participants were excluded for regular tobacco use (i.e., more than one pack of cigarettes per month) or current alcohol dependence based on the Structured Clinical Interview for DSM-IV (SCID) [25]. We defined participants as regular cannabis users based on self-reported history of cannabis use with a minimum of 5,000 lifetime occasions and daily use over the preceding 60 days. Cannabis use was verified via quantification of THC metabolites as ng/ml (over creatinine) via gas chromatography/mass spectroscopy (GC/MS). We defined participants as non-using controls based on the absence of daily cannabis use at any period in their lifetime and no current illicit drug use in the past 60 days.
Of the 137 participants who provided consent, five were excluded for excessive motion during the fMRI scans (>3 mm or >3 degrees) and four had incomplete data. Of the resulting 128 participants with complete imaging, genetic, and behavioral data, 72 were non-using controls [CON; mean (SD) age = 30.5 (10.4) years] and 56 were prolonged heavy cannabis users [MJ; 20 qualified as dependent; mean (SD) age = 29.7 (8.1) years]. See Table 1 for demographic and group information.
Table 1:
Demographic, substance use, stress, and genetics measures of participants across the control (CON) and cannabis (MJ) groups. Permutation was performed for χ2, with 5000 permutations. Welch’s two-sample t-test was used to test for group differences, which adjusts degrees of freedom when variance is unequal.
| CON (N = 72) Mean (SD) | MJ (N = 56) Mean (SD) | Statistic | |
|---|---|---|---|
| Age (years) | 30.5 (10.4) | 29.7 (8.1) | t(126) = 0.47, p = 0.64 |
| Sex (M/F) | 39/33 | 36/20 | χ2 = 1.3, pperm = 0.29 |
| IQ | 111.2 (12.5) | 107.6 (10.9) | t(124.2) = 1.7, p = 0.087 |
| Years of education | 16.5 (2.6) | 13.4 (2.6) | t(119.5) = 6.8, p < 0.001 |
| Ethnicity | χ2 = 0.0008, pperm = 1 | ||
| Hispanic/Latino | 13 | 10 | |
| Non-Hispanic/Latino | 59 | 46 | |
| Race | |||
| Caucasian/White | 32 | 35 | |
| African American/Black | 15 | 11 | |
| Asian | 21 | 1 | |
| Other | 4 | 9 | |
| Substance use variables | |||
| Age of first cannabis use (years) | n/a | 15 (3.2) | |
| Duration of regular cannabis use (years) | n/a | 11.4 (8.1) | |
| # Cannabis use / last 90 days | n/a | 58.4 (8.2) | |
| # Cigarette smoking / last 90 days | 0.3 (2.6) | 0.9 (2.7) | t(117.1) = −1.1, p = 0.28 |
| # Alcohol drinking / last 90 days | 7.9 (14.1) | 11.6 (14.6) | t(116.5) = −1.4, p = 0.15 |
| Stress measures | |||
| Perceived Stress Scale | 35.7 (7.2) | 36.4 (9.1) | t(102.4) = −0.42, p = 0.68 |
| Early Trauma Inventory | 4.1 (3.3) | 6.3 (5.2) | t(88) = −2.9, p = 0.0053 |
| Genetics | |||
| FAAH (rs324420) | χ2 = 0.29, pperm = 0.72 | ||
| CC | 42 | 30 | |
| AC/AA | 30 | 26 | |
| CNR1 (rs2023239) | χ2 = 5.4, pperm = 0.027 | ||
| TT | 54 | 31 | |
| CT/CC | 18 | 25 | |
2.2. Behavioral Measures
We measured stress with the Perceived Stress Scale [26] and the Early Trauma Inventory [27]. The perceived stress scale measures current perception of stress and the early trauma inventory measures early (i.e., developmental) physical, emotional, and sexual abuse traumas. See Table 1 for summary scores for the perceived stress scale and early trauma inventory. There was no difference in perceived stress scale (t (102.4) = −0.42, p = 0.68), but there was a significant difference in early trauma inventory (t (88) = −2.9, p = 0.0053) between the cannabis users and non-using controls.
We assessed cannabis use behaviors as age of first cannabis use, number of years of cannabis use, self-reported craving via the Marijuana Craving Questionnaire [28] before and after the cannabis cue-exposure task, and withdrawal via the Marijuana Withdrawal Checklist [29].
2.3. DNA Collection and Genotyping of Candidate Loci
Two SNPs, rs2023239 (CNR1) and rs324420 (FAAH), have previously been reported to be associated with neural response to cannabis cues [11] were included in the analysis. See Supplemental Methods for details of genetic data acquisition and processing. Table 2 provides a distribution of genotypes across race and ethnicity. While the minor allele frequency for both SNPs was suitable (> 5%), there were two clear distributional effects: (1) the minor homozygotes for both genes were relatively infrequent (FAAH = 11%, CNR1 = 7%), where in the “other” group the minor homozygote was non-existent and (2) there was an overrepresentation of the heterozygote in those that identified as Hispanic or African American. We used the dominant model (i.e., AA vs.{Aa + aa}) for both SNPs. There was no distributional effect between groups and genotypes in FAAH (χ2 = 0.29, pperm = 0.72), but there was a distributional effect between groups and genotypes for CNR1 (χ2 = 5.4, pperm = 0.027). See Section 2.6 Data Analyses below for additional preprocessing details.
Table 2:
Distribution of genotypes by ethnicity and race.
| FAAH (rs324420) | CNR1 (rs2023239) | |||||
|---|---|---|---|---|---|---|
| CC (N= 72) | AC (N = 42) | AA (N = 14) | TT (N= 85) | CT (N = 39) | CC (N = 9) | |
| Hispanic/Latino (N = 23) | 9 | 11 | 3 | 17 | 5 | 1 |
| Non-Hispanic/Latino (N = 105) | 63 | 31 | 11 | 68 | 29 | 8 |
| Caucasian/White (N = 67) | 37 | 23 | 7 | 47 | 16 | 4 |
| African American/Black (N = 26) | 8 | 13 | 5 | 10 | 12 | 4 |
| Asian (N = 22) | 18 | 2 | 2 | 18 | 3 | 1 |
| Other (N = 13) | 9 | 4 | 0 | 10 | 3 | 0 |
2.4. MRI Procedure
Similar to our published studies [22, 24], MJ were scanned following a 72-hour abstinence from cannabis use to capture peak craving following last cannabis use. According to Budney et al. (2001), craving for marijuana is significantly increased within 72 hours of abstinence from ad libitum use among heavy users [30]. We measured THC metabolites as ng/ml (over creatinine) (via GC/MS) from the participants before and after the ~72-hour period to detect reductions in THC metabolites in addition to self-report. All participants were asked to abstain from alcohol for 24 hours and from caffeine and cigarettes for the 2 hours before their scheduled scan. Breath alcohol level was collected to confirm blood alcohol content of 0.0 at the beginning of the scan. All participants were asked to eat a meal before their scan appointment to reduce confounding effects of hunger.
MRI scan acquisition took place in the Advanced Imaging Research Center (AIRC) at the University of Texas Southwestern Medical Center (UTSW). MRI images were collected using a 3T Philips whole body scanner equipped with Quasar gradient subsystem (40 mT/m amplitude, a slew rate of 220 mT/m/ms). Structural MRI scans were collected with a MPRAGE sequence with the following parameters: TR/TE/TI = 8.2/3.70/1100 ms, flip angle = 12°, FOV = 256×256 mm, slab thickness = 160 mm (along left-right direction), voxel size =1×1×1 mm, total scan time = 3 min 57 sec. fMRI scans were collected using a gradient echo, echo-planar sequence with the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 29ms, flip angle: 75 degrees, matrix size: 64 × 64, 39 slices, voxel size: 3.44 × 3.44 × 3.5 mm3).
2.5. fMRI cue-reactivity task
Participants completed a previously described cannabis cue-reactivity task designed to examine BOLD response to cannabis, neutral, and appetitive cues to measure cue-reactivity [24]. This task was presented in two separate EPI runs of 18 pseudorandom tactile presentations of cannabis paraphernalia (MJ cue × 6 trials), a pencil (neutral cue × 6 trials), or the participants’ preferred fruit (appetitive cue × 6 trials). Each trial started with a cue-exposure period when participants were presented with both tactile exposure (cue placed in the participant’s left hand) and visual (images of themselves holding the) cues. Participants then had a 5-second urge rating period in which they were asked to “Please rate your level of urge to use marijuana right now” on a scale of 1 (no urge) to 10 (high urge) via button presses. There was a washout period with a fixation cross in between each trial. The number of repetitions per run was 405 with a task duration of 27 minutes for the entire experiment. The task was presented using a back-projection to a mirror system mounted on the head coil. Stimulus presentation was delivered using E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA) and was synchronized with trigger pulses from the scanner to ensure precise temporal integration of stimulus presentation and fMRI data acquisition.
2.6. Data analyses
2.6.1. First-level analyses of imaging data
First-level analyses of the imaging data were the same as those described in Filbey et al., 2016 [24] and outlined here briefly. Time series for each participant were analyzed with FSL’s FILM. Regressors were generated using FEAT by convolving the regressors (Cue exposure, Craving Rating, Washout) for each cue type (MJ, neutral, and appetitive) with a double gamma hemodynamic response function. Activation maps were then registered to their own MRI T1 weighted MPRAGE structural images and co-registered to the MNI 152 template space with FLIRT. Individual level estimates for MJ cue > appetitive (fruit) cue were then extracted for use in our primary statistical analysis (partial least squares correlation; PLSC). Estimates were extracted from a common mask and then vectorized. Each vector was normalized so that results are not simply because of very large magnitudes of activation in one or a few individuals [31]. Although there are many possible contrasts for the experimental design, the focus in this study was the MJ cue > fruit cue contrast to investigate neural response to a cannabis-related cue beyond a response to a natural reward cue.
2.6.2. Partial least squares correlation analysis
Partial least squares correlation (PLSC) is a multivariate statistical technique that identifies orthogonal components (i.e., latent variables; LVs) that explain maximal variance from the cross-covariance between two data sets [32]. The two data sets here were: 1) fMRI scores - BOLD response during the cannabis cue-exposure task and 2) explanatory variables - genetics, group, and behavioral data. LVs are rank-ordered by explained variance. Bootstrap resampling was used to generate confidence intervals and “bootstrap ratios” (BSRs; are akin to t- or Z-like tests) for the voxels and explanatory variables. We used a lower threshold for multiple comparison corrections (https://neuroimage.usc.edu/brainstorm/Tutorials/PLS). For more detailed explanations see [33–35]. PLSC and resampling were performed in R with the TExPosition 2.6.10 package [36] and additional custom code.
Group and genetic measures were coded as presence (i.e., 1) vs. absence (i.e., 0) for their respective variables. FAAH and CNR1 were each coded under a dominant model: major homozygote vs. {heterozygote + minor homozygote} because minor homozygotes were rare. The Early Trauma Inventory and Perceived Stress Scale scores were included as continuous data. The explanatory variables of group, gene, and stress measures were then combined, and each variable was normalized to a sum of squares equal to 1. Explanatory variables were corrected for effects of race and ethnicity.
Bootstrap resampling was performed 1,000 times in order to identify explanatory variables and voxels that stably contributed to each component. A large BSR means that an item has a high loading with small variance. BSRs at a cut-off magnitude > ±3 (α ≈ 0.001) identified which explanatory variables and voxels stably contributed to the components [37]. We used Multi-Image Analysis GUI (Mango 4.0.1; http://ric.uthscsa.edu/mango/) with no minimum cluster-size and no cluster-wise correction to identify voxel clusters; all voxels within a cluster had a BSR > ±3. Finally, LV scores were correlated with cannabis use measures and cue-related responses within the cannabis group.
3. Results
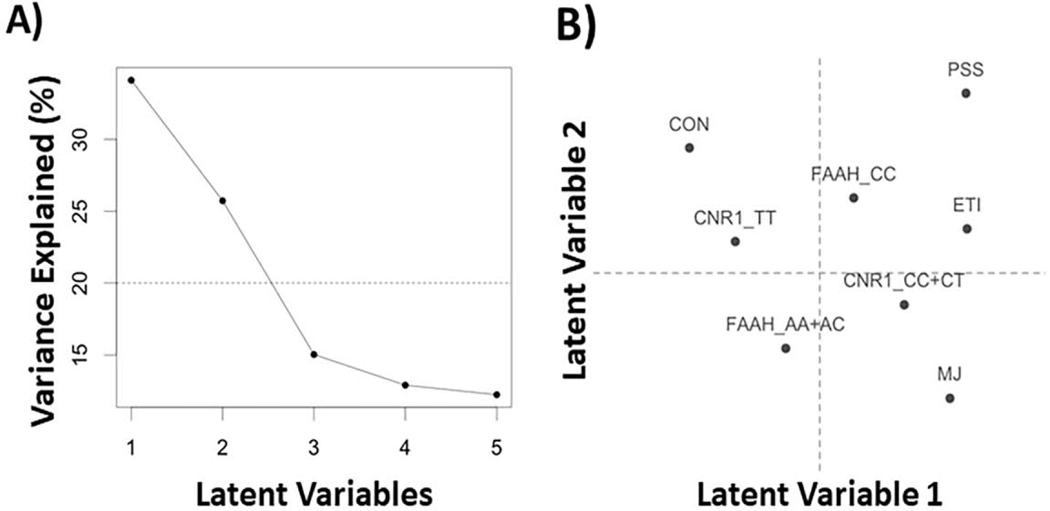
The first two LVs were included in the interpretation because they explained the majority of the variance (i.e., 59.8%; Figure 1A). The expected variance component scores for the first two LVs are shown in Figure 1B. For the explanatory variables, both BSRs are provided and their corresponding confidence intervals for each LV are shown (see Figure 2).
Figure 1:
Scree plot and latent variable (LV) scores for the explanatory variables (i.e., groups, genes, and stress). (A) The scree plot shows the explained variance per LV with a horizontal line as a cutoff for average (expected) variance. (B) The LV scores plot show how each explanatory variable contributes to the first two LVs. ETI=early trauma inventory; PSS=perceived stress scale; MJ=cannabis users; CON=controls
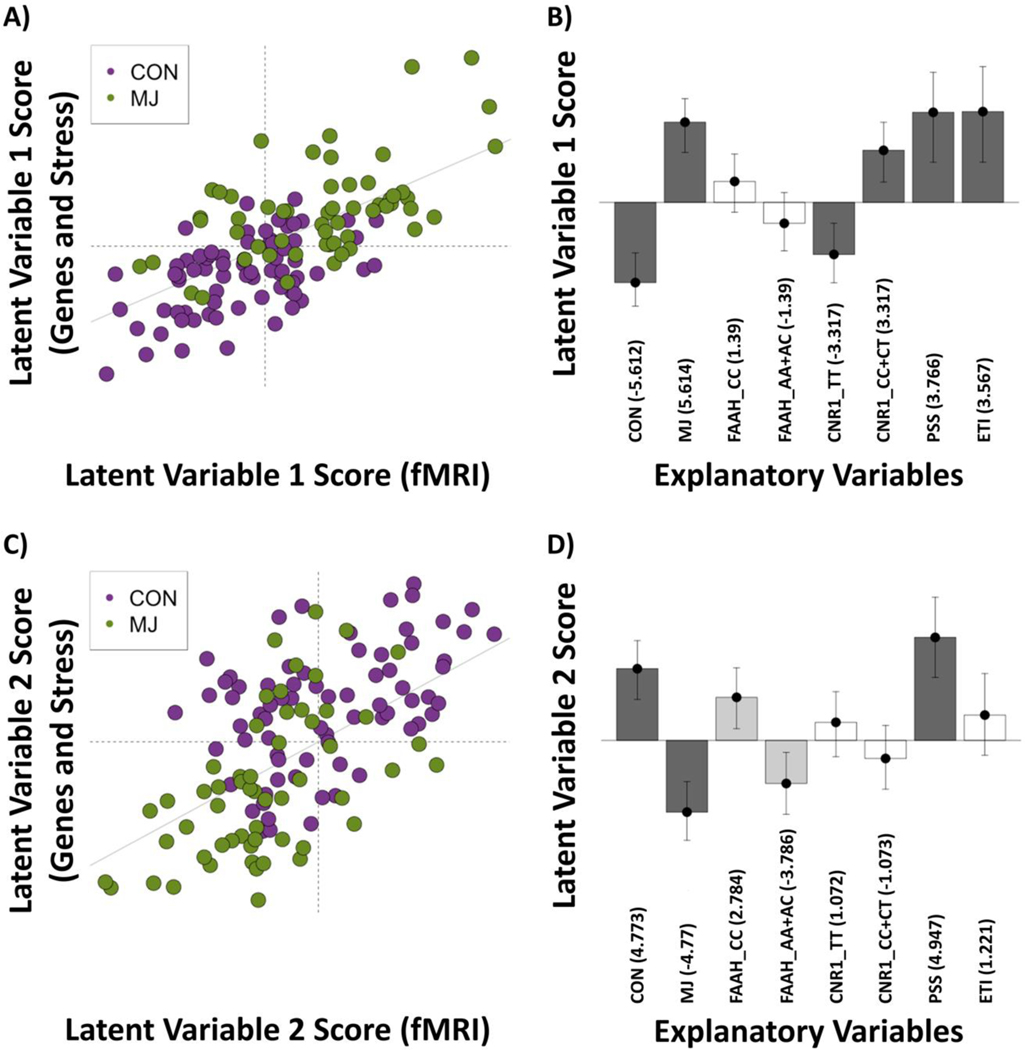
Figure 2:
Latent variable 1 (LV1) and 2 (LV2) scores and loadings. (A) LV1: fMRI scores vs. [38] scores and (B) Loadings for expected variance with bootstrap ratios (BSRs) denoted in parentheses; 95% confidence intervals around loadings shown. The LV plot shows that in general there is a separation of cannabis users (MJ) from controls (CON). The BSRs for the explanatory variables show that the effect is driven by [39] vs. {MJ CNR1 CC/CT, Early Trauma Inventory (ETI), Perceived Stress Scale (PSS)}. (C) LV2: fMRI scores vs. [38] scores and (D) Loadings for explanatory variables with BSRs denoted in parentheses; 95% confidence intervals around loadings shown. The LV plot shows that in general there is a separation of MJ from CON. The BSRs for the explanatory variables show that the effect is driven by {Controls (CON), PSS} vs. [40], with a likely (albeit not significant at BSR > 3) effect of FAAH.
Note: Explanatory variables that load in the same direction are positively correlated.
3.1. Latent variable 1 (LV1)
LV1 explained 34.1% of the variance. Figures 2A and 2B show the LV1 scores for participants and how each participant contributed to the explanatory variables and fMRI scores. Group, CNR1, and both stress measures (i.e., Early Trauma Inventory and Perceived Stress Scale) significantly contributed to LV1 (Figure 2B). LV1 separated the data by group and CNR1 genotype, showing that CON and CNR1 TT (major homozygote) were associated, while MJ, CNR1 CC+CT (presence of minor allele), and increasing scores on the perceived stress scale and early trauma inventory were associated.
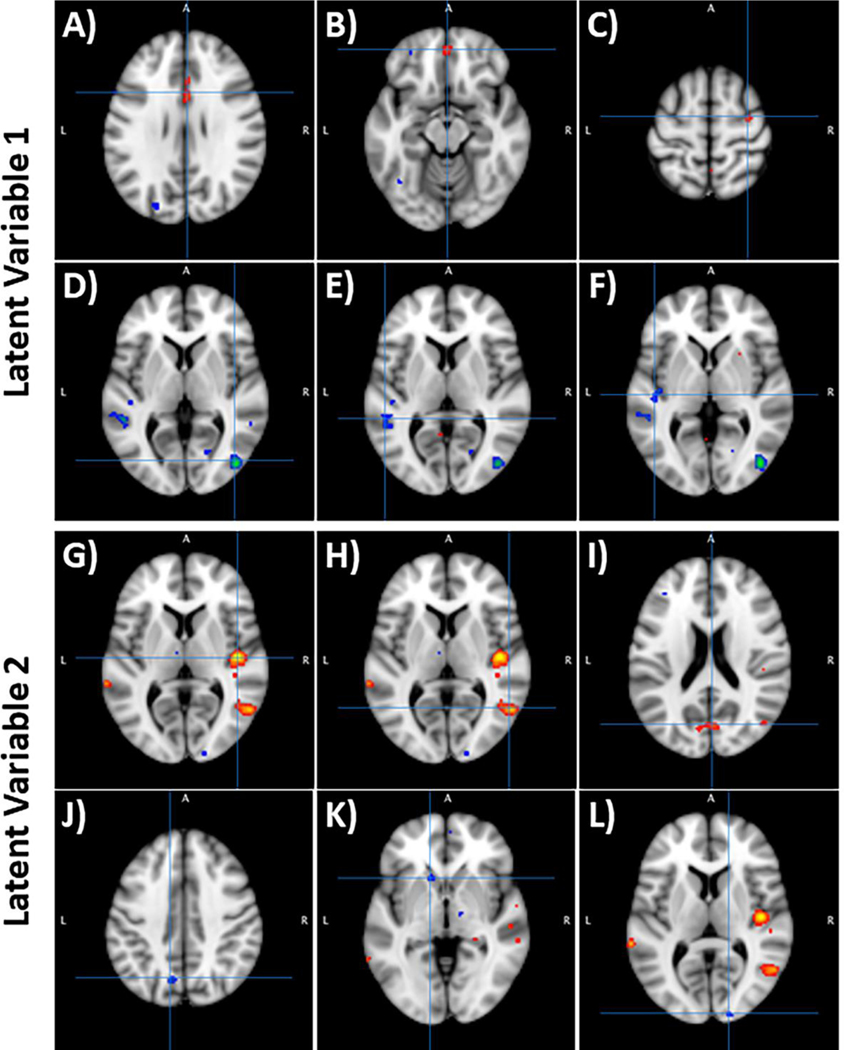
We described the three largest brain activation clusters in response to {MJ > Fruit} that were related to LV1 in Figure 3A-F and Table 3A-F (see Supplemental Tables 1 and 2 for all clusters). The top clusters associated with {CON and CNR1 TT} were found in the middle and inferior occipital gyri, superior and middle temporal gyri, and superior temporal gyrus and insula. Thus, the CON group and CNR1 TT are positively correlated with {MJ > Fruit} responses in these brain regions. The top three clusters associated with {MJ, CNR1 CC+CT}, increasing scores on the Perceived Stress Scale and Early Trauma Inventory} were located in the cingulate gyrus, medial frontal gyrus, and precentral gyrus. These results suggest a negative correlation between these variables such that MJ group, CNR1 CC+CT, and increasing stress scores are related to reduced response to {MJ > Fruit} in these brain regions.
Figure 3:
Clusters associated with latent variables 1 (LV1) and 2 (LV2). Hot colors reflect positive correlations (+ BSR scores), whereas cool colors reflect negative correlations (- BSR scores). A–C (top row) – Activation clusters of LV1 that were associated with the {cannabis users, CNR1 CC/CT, early trauma inventory, perceived stress scale} effects. The top three clusters were in (A) Cingulate Gyrus, (B) Medial Frontal Gyrus, and (C) Precentral Gyrus. D–F (second row) – Activation clusters of LV1 that were associated with the {controls, CNR1 TT} effects. The top three clusters were in (D) Middle and Inferior Occipital Gyrus, (E) Middle and Superior Temporal Gyrus, and (F) Superior Temporal Gyrus and Insula. G–I (third row) - The clusters of LV2 that were associated with {controls, perceived stress scale} effects. The top three clusters were in (G) Superior Temporal Gyrus and Insula, (H) Middle Temporal Gyrus, and (I) Cuneus. J–L (bottom row) - The clusters of LV2 that were associated with the {cannabis users} effects. The top three clusters were in (J) Precuneus and cuneus, (K) Caudate, and (L) Cuneus.
Table 3:
Three largest clusters associated with latent variable 1 and latent variable 2. (A-F) Largest clusters for LV1 associations, respectively and (G-L) Largest for LV2. BSR = bootstrap ratio. LV = latent variable.
| BSR | x | y | z | Region | Brodmann Area | |
|---|---|---|---|---|---|---|
| (A) LV1{MJ, CNR1 CC+CT, increasing scores on the Perceived Stress Scale and Early Trauma Inventory}, Cluster 1 (Voxels = 118) | ||||||
| Max | 3.6 | 0 | 18 | 22 | Cingulate Gyrus | BA24 |
| Min | 3.0 | −2 | 18 | 26 | Cingulate Gyrus | BA24 |
| Centroid | 3.1 | 2 | 14 | 28 | Cingulate Gyrus | BA24 |
| (B) LV1{MJ, CNR1 CC+CT, increasing scores on the Perceived Stress Scale and Early Trauma Inventory}, Cluster 2 (Voxels = 34) | ||||||
| Max | 3.4 | 0 | 50 | −14 | Medial Frontal Gyrus | BA10 |
| Min | 3.0 | −2 | 52 | −14 | Medial Frontal Gyrus | BA10 |
| Centroid | 3.4 | 0 | 50 | −14 | Medial Frontal Gyrus | BA10 |
| (C) LV1{MJ, CNR1 CC+CT, increasing scores on the Perceived Stress Scale and Early Trauma Inventory}, Cluster 3 (Voxels = 22) | ||||||
| Max | 3.6 | 34 | −8 | 58 | Precentral Gyrus | BA6 |
| Min | 3.0 | 34 | −6 | 56 | Precentral Gyrus | BA6 |
| Centroid | 2.9 | 32 | −6 | 60 | Precentral Gyrus | BA6 |
| (D) LV1{CON and CNR1 TT}, Cluster 1 (Voxels = 158) | ||||||
| Max | −3.0 | 40 | −78 | 10 | Medial Occipital Gyrus | BA19 |
| Min | −4.4 | 44 | −78 | 2 | Inferior Occipital Gyrus | BA19 |
| Centroid | −4.0 | 42 | −76 | 4 | Medial Occipital Gyrus | BA19 |
| (E) LV1{CON and CNR1 TT}, Cluster 2 (Voxels = 82) | ||||||
| Max | −3.0 | −46 | −48 | 6 | Superior Temporal Gyrus | BA39 |
| Min | −3.9 | −50 | −42 | 4 | Medial Temporal Gyrus | BA22 |
| Centroid | −3.1 | −52 | −40 | 6 | Medial Temporal Gyrus | BA22 |
| (F) LV1{CON and CNR1 TT}, Cluster 3 (Voxels = 40) | ||||||
| Max | −3.0 | −46 | −22 | 2 | Superior Temporal Gyrus | BA22 |
| Min | −3.6 | −42 | −16 | 0 | Insula | BA13 |
| Centroid | −3.1 | −46 | −20 | 2 | Superior Temporal Gyrus | BA22 |
| (G) LV2{CON, increasing scores on the Perceived Stress Scale, and FAAH CC}, Cluster 1 (Voxels = 276) | ||||||
| Max | 4.5 | 44 | −16 | 6 | Insula | BA13 |
| Min | 3.0 | 52 | −20 | 2 | Superior Temporal Gyrus | BA22 |
| Centroid | 4.5 | 44 | −16 | 6 | Insula | BA13 |
| (H) LV2{CON, increasing scores on the Perceived Stress Scale, and FAAH CC}, Cluster 2 (Voxels = 141) | ||||||
| Max | 4.1 | 54 | −60 | 6 | Medial Temporal Gyrus | BA37 |
| Min | 3.0 | 44 | −58 | 8 | Medial Temporal Gyrus | BA39 |
| Centroid | 3.8 | 52 | −58 | 6 | Medial Temporal Gyrus | BA37 |
| (I) LV2{CON, increasing scores on the Perceived Stress Scale, and FAAH CC}, Cluster 3 (Voxels = 83) | ||||||
| Max | 3.7 | 4 | −72 | 18 | Cuneus | BA18 |
| Min | 3.0 | 8 | −74 | 18 | Cuneus | BA18 |
| Centroid | 3.2 | 2 | −72 | 20 | Cuneus | BA18 |
| (J) LV2 {MJ and FAAH AA+AC}, Cluster 1 (Voxels = 23) | ||||||
| Max | −3.0 | −14 | −68 | 38 | Precuneus | BA7 |
| Min | −3.5 | −10 | −68 | 38 | Cuneus | BA7 |
| Centroid | −2.9 | −12 | −66 | 40 | Precuneus | BA7 |
| (K) LV2 {MJ and FAAH AA+AC}, Cluster 2 (Voxels = 19) | ||||||
| Max | −3.0 | −16 | 20 | −6 | Caudate | Caudate Head |
| Min | −3.4 | −12 | 16 | −4 | Caudate | Caudate Head |
| Centroid | −3.1 | −14 | 18 | −2 | Caudate | Caudate Head |
| (L) LV2{MJ and FAAH AA+AC}, Cluster 3 (Voxels = 10) | ||||||
| Max | −3.0 | 20 | −98 | 10 | Cuneus | BA17 |
| Min | −3.3 | 18 | −98 | 8 | Cuneus | BA17 |
| Centroid | −3.0 | 16 | −96 | 8 | Cuneus | BA17 |
3.2. Latent variable 2 (LV2)
LV2 explained 25.7% of the variance. Figures 2C and 2D show the LV2 scores for the participants and how each participant contributed to the explanatory variables and fMRI scores. Group and increasing scores on the Perceived Stress Scale significantly contributed to LV2 whereas FAAH had a moderately stable contribution (BSR ≈ ± 2.78; Figure 2D). Specifically, LV2 showed the following: (1) CON and increasing scores on the Perceived Stress Scale were associated [with a lesser contribution by FAAH CC (major homozygote)], and (2) MJ, and to a lesser extent FAAH AA+AC (presence of minor allele) were associated.
As with LV1, we focused on the three largest clusters associated with LV2 (shown in Figure 3G-L and Table 3G-L; see Supplemental Tables 3 and 4 for all clusters). The top three clusters associated with {CON, increasing scores on the Perceived Stress Scale, and FAAH CC} were positively correlated with {MJ>fruit} response located in the superior temporal gyrus, insula, middle temporal gyrus, and cuneus. Those associated with the {MJ and FAAH AA+AC} were generally negatively correlated with {MJ>fruit} response located in the cuneus and precuneus, caudate, and cuneus.
3.3. Post-hoc Correlations
The correlation analyses between fMRI LV scores and cannabis use measures showed a significant positive correlation between scores on the Marijuana Withdrawal Scale and both LVs. This suggests that the greater the withdrawal symptoms in the cannabis users, the greater the strength of associations between regions active during response to cues. There were no significant correlations between the fMRI LV scores and other cannabis use variables.
4. Discussion
The aim of this study was to determine how endocannabinoid system factors, specifically, endocannabinoid genes and chronic exposure to cannabis, interact with stress to influence neural response to salient reward cues. We predicted that the presence of CNR1 rs2023239 G and FAAH rs324420 C alleles, perceived and early life stress, and neural response to cannabis cues (vs. control cues) will be associated in cannabis users but not in non-using controls. Contrary to our predictions, PLSC analyses showed that these variables contributed differentially between the groups: current perceived stress, early life stress, and presence of the CNR1 C allele were associated with increased cue-elicited response in heavy cannabis users, while current perceived stress and the FAAH CC was associated with greater cue-elicited response in controls. Additionally, cannabis withdrawal symptoms were associated with the strength of the association among the regions active during response to cues.
In general, the explanatory variables (group, genes, stress) were associated with neural response to cues in widespread brain areas. Cue-elicited response in the anterior cingulate gyrus [41] [42] [43] has been reported frequently in cannabis users, especially as they relate to problems related to cannabis use [24] [44]. A study recently demonstrated that altered activation and connectivity of the anterior cingulate may underlie the impact of early life stress on the reward network. The dysfunctional connectivity between the anterior cingulate gyrus regions, implicated in decision-making, suggest aberrant regulatory mechanisms conferring risk for affective disorders [45].
The cannabis group exhibited patterns of responsivity predominantly in the precuneus, cuneus, and caudate; each of which also play important roles in the reward circuit. While the caudate has been shown to play a role in responsivity in chronic cannabis use, van Hell and colleagues (2010) reported that caudate activity decreased with respect to reward anticipation [46]. The cuneus was a region that exhibited activation in both the control and cannabis groups; however, LV2 revealed that the cuneus responded in opposite ways in the cue-exposure task. In the control group the cuneus exhibited greater activity in response to the fruit cue whereas in the cannabis group the cuneus exhibited greater activity in response to the cannabis cue. Increased activation in the cuneus in cannabis users compared to controls has been consistently found [24, 46]; however, the differential brain responsivity to cannabis and fruit cues in these groups suggests an effect of exogenous cannabis beyond that of a natural reward. Similar differential responsivity in reward regions has been previously observed in a number of tasks and populations including substance using adolescents [47] and unmedicated major depressive disorder [48].
These regions span across several brain networks including the salience network, the cognitive control network, and to a lesser degree, midline regions often associated with the default mode network. Recent work has shown that these networks, specifically the “canonical regions” of these networks, are not necessarily distinct in that they share structural and functional connections [49] and tend to be dynamic across a wide range of cognitive processes [50], including reward processing and visual attention [51]. For example, the posterior medial frontal gyrus, cingulate gyrus, caudate, and anterior insula have been postulated to play a role in the visual processing as well as the encoding of reward [52]. THC administration has been found to alter functional connectivity in these networks [53] that has been associated with impairment in reward-based decision making [54]. Overall, these networks, through neuromodulatory inputs from the dopaminergic system, contribute towards maintaining homeostasis through the integration of autonomic signals with environmental demands [55]. Aberrant connections and activation in these three brain networks have been associated with multiple psychiatric disorders and affective states, referred to as the “triple network model of psychopathology” [56], including substance use disorder [57]. Notably, while the preclinical literature demonstrates that CB1R modulation of the dopamine system involves the amygdala, we did not find associations with cue response in this region. Because it has been suggested that coupling between the amygdala and anterior cingulate cortex more effectively enhances emotional control [58], it is possible that an absence of association between the explanatory variables (group, genes, stress) and amygdala response to cues reflects a dysfunction in connectivity in regions underlying control and affective processing.
4.1. CNR1 and both current perceived and early life stress are associated with reward sensitivity in chronic, heavy cannabis users
Our findings indicated that the CNR1 G allele and higher levels of early life stress and current perceived stress were present in cannabis users. These contributions were associated with response to cannabis cues in midline areas and extends our previous findings of cue-responsivity in cannabis users with CNR1 G allele [24] to demonstrate positive associations with stress. This increased neural response to cues may be attributed to the impact of stress on regulatory and affective networks that influence reward response. For instance, studies have shown disruptions in amygdala-cingulate inhibitory circuitry during failure to regulate emotional conflict [59]. This disruption has been associated with attenuated reward sensitivity that has been related to risk for psychopathology, such as substance use disorders. Alternatively, exposure to cannabis particularly during adolescent development may exacerbate response to stress. Animal models demonstrate that adolescent rats exposed to CB1R agonists had higher levels of corticosterone and increased stress responsivity [60] and that these effects may be long-term on multiple neurotransmitter systems including those in mesolimbic brain structures. Notably, despite low stress scores in both groups, scores on the perceived stress scale and early trauma inventory were more related to the cannabis group but not the control group. This could be interpreted as potentiating effects of CNR1 CC+CT on reward system signaling such that even relatively low levels of stress have an effect in cannabis users.
4.2. Current perceived stress and FAAH are associated with reward sensitivity in controls
Latent variable 2 showed contributions of FAAH and stress that were orthogonal to that of CNR1 and stress (i.e., latent variable 1 discussed above). Specifically, we found associations between perceived stress scores and a variant in the FAAH SNP in the non-using controls, which was associated with greater response to the appetitive (fruit) cues in temporal regions and insula. Response in the insula in the control group supports previous reports of the role of the insula in interoception and response to natural rewards (i.e., the appetitive cue in this context) [61–64]. The interaction between FAAH and stress is well established in the literature and describes how chronic stress exacerbates disruptions of FAAH (and anandamide) as in the case of FAAH CC carriers. For example, rodents exposed to chronic stress show long-term reductions in amygdala anandamide levels resulting in dendritic branching and heightened intrinsic excitability related to enhanced anxiety. Inversely, FAAH inhibition led to recovery of signaling and amygdala function [7].
Latent variable 2 also showed that the cannabis users were associated with FAAH A allele, which supports recent findings suggesting that reduced FAAH is associated with increased D3 receptor density in the amygdala that demonstrates how the endocannabinoid system modulates the dopaminergic system to increase risk for substance use disorders [3]. It is interesting to note that while this allele was associated with cannabis users, it was not associated with elevated self-reported stress. This is consistent with findings demonstrating reduced stress-induced negative affect in FAAH A allele carriers along with attenuated stress-induced anandamide levels reduction [65].
4.3. Differential genes-stress interaction on prolonged cannabis exposure
Taken together, the differentiation between the effect of CNR1 and FAAH variants on cannabis users and controls poses an interesting question regarding how these variants interact with stress differently in the presence of prolonged cannabis exposure. These significant group effects on both latent variables underlines the large group effect contributing to higher overall variance than any of the other measures. Results from the current study indicate that the presence of CNR1 C allele (CC+CT) and stress resulted in a greater neural response to cannabis cues in cannabis users, while FAAH CC resulted in a greater neural response to natural reward cues in non-using controls. This suggests that while both CNR1 and FAAH SNP variants have been considered risk factors for cannabis use disorder, particularly as it relates to increasing response cue-elicited craving [11, 12] [66], the mechanisms by which these two genetic factors influence response to cues differ. Additionally, while previous studies have linked both CNR1 and FAAH variants to cannabis use [11, 12], they are limited in that they only included cannabis users. Our findings suggest that CNR1 and stress contribute to this relationship and are associated with greater neural response to cannabis cues whereas FAAH does not interact with stress factors related to cannabis cue response. The differential effect in cannabis users and non-using controls found in the current study advances our understanding and suggests a more nuanced interaction with both exogenous cannabis and stress.
Previous studies have reported differential effects of CNR1 and FAAH variants on affective states. For example, Palmer and colleagues (2019) reported an interaction between acute THC exposure and CNR1 such that those with the CNR1 rs2023239 C allele had higher anger-hostility scores (from Profile of Mood States questionnaire) following THC administration relative to placebo. This supports our finding of greater stress in CNR1 C allele carriers that is associated with greater neural response to cues. Lazary et al’s (2009) study reported that CNR1 rs2023239 T allele carriers have significantly greater CB1R binding site density in the prefrontal cortex, which likely mediate the hypothalamic-pituitary-adrenal axis’ negative feedback response to stress [67]. The same study by Palmer and colleagues (2019) also found that FAAH A allele carriers reported lower fatigue-inertia scores following exposure to placebo (vs. THC) [68]. This finding also overlaps with the absence of association between stress factors and FAAH A allele in cannabis users in the current study. Dincheva et al. (2015) reported that there was selectively enhanced fronto-amygdala connectivity in FAAH A allele carriers that may explain the control of stress response observed in mice and humans [69] [70]. An anti-stress effect has previously been demonstrated in preclinical studies where acute stress via foot-shock enhanced THC and a FAAH inhibitor’s (URB597) anti-stress effects but did not affect reward response (via conditioned place preference paradigm) [71]. This is consistent with preclinical studies reporting that inhibiting FAAH increases anandamide levels in the basolateral amygdala and alleviated effects of stress [7]. N-Arachidonoylserotonin (AA-5-HT), a dual blocker at FAAH, also generates anti-stress effects in preclinical models [14], supporting the role of the endocannabinoid pathway as a potential target for anti-stress therapeutics. It is also worth noting the possibility of opposing effects of the two endocannabinoids in mediating the stress response. Specifically, stress-related increases in 2-Arachidonoylglycerol or 2-AG in the amygdala has been thought to have restorative effects that counteract the effects of stress-induced anandamide depletion. For example, chronic administration of 2-AG inhibitors appear to prevent stress-related increases in anxiety and amygdala impairments [7]. In this context, it is possible that stress-related 2-AG related to the presence of THC may have a role in off-setting the expected correlations between FAAH CC and stress in cannabis users.
4.4. Limitations
One of the challenges of our complex study design is the feasibility of acquiring a sample size sufficiently large for typical genetic studies, despite being comparatively large for neuroimaging studies. Despite this limitation, our findings replicate and extend previous work and put forth a testable framework on the interactions between endocannabinoid system, genetic and environmental factors on cue-reactivity.
4.5. Conclusions
This study applied a multivariate statistical approach to integrate data from various modalities and indicated that both endocannabinoid system factors and stress (i.e., prolonged cannabis use, current stress, and early life stress) underlie differential neural mechanisms of reward cue responsivity. Specifically, PLSC identified two latent variables that revealed that CNR1, early stress, and current perceived stress play an important role in reward responsivity to cannabis cues in cannabis users and that current perceived stress, and to a lesser degree FAAH, play an important role in reward responsivity to natural reward cues in non-using controls. These results suggest that, together, endocannabinoid genes and stress contribute to a differential reward cue response in multiple regulatory networks.
Supplementary Material
Table 4:
Post-hoc correlations between the fMRI LV scores and cannabis use measures. LV = latent variable
| Measure | Statistic | |
|---|---|---|
| LV1 | LV2 | |
| Age of onset of cannabis use | r(54) = −0.047, p = 0.73 | r(54) = −0.18, p = 0.18 |
| Duration of regular cannabis use | r(54) = −0.018, p = 0.90 | r(54) = −0.18, p = 0.19 |
| Marijuana craving score (pre-scan minus post-scan) | r(52) = −0.15, p = 0.28 | r(52) = −0.08, p = 0.57 |
| Average marijuana cue rating | r(53) = 0.26, p = 0.056 | r(53) = 0.22, p = 0.11 |
| Average fruit cue rating | r(53) = 0.090, p = 0.52 | r(53) = 0.22, p = 0.11 |
| Marijuana Withdrawal Scale score | r(53) = 0.37, p = 0.006* | r(53) = 0.30, p = 0.025* |
Highlights.
The interaction between cannabinoid receptor 1 (CNR1) CC+CT, early life stress, and current perceived stress modulate the brain’s reward responsivity in long-term, heavy cannabis users
Long-term cannabis using adults with CNR1 CC+CT genotype, perceived current stress and early life stress had brain response to cannabis cues in midline areas such as post- and precentral gyrus and medial frontal regions with the largest effects in the cingulate gyrus.
The interaction between fatty acid amide hydrolase gene (FAAH) CC and current perceived stress modulate the brain’s reward response in healthy controls.
Healthy controls with CNR1 TT genotype had largest brain response to cannabis cues in the middle and inferior occipital gyrus.
Acknowledgements
We are grateful to Hao Nguyen for assisting with the revisions of this paper.
This research was funded by NIH R01 DA030344 (Filbey). During this work DB was supported via training grant by the National Institute on Drug Abuse (F31DA035039).
Footnotes
Ethical Statement
The Institutional Review Board of the University of Texas at Dallas and University of Texas Southwestern Medical Center approved these study procedures. All experiments were conducted according to the principles expressed in the Declaration of Helsinki.
Financial Disclosures
The authors report no biomedical financial interests or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parsons LH and Hurd YL, Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci, 2015. 16(10): p. 579–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cravatt BF and Lichtman AH, Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol, 2003. 7(4): p. 469–75. [DOI] [PubMed] [Google Scholar]
- 3.Mansouri E, et al. , D3 dopamine receptors and a missense mutation of fatty acid amide hydrolase linked in mouse and men: implication for addiction. Neuropsychopharmacology, 2020. 45(5): p. 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald ML, Shobin E, and Pickel VM, Cannabinoid modulation of the dopaminergic circuitry: implications for limbic and striatal output. Prog Neuropsychopharmacol Biol Psychiatry, 2012. 38(1): p. 21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupica CR, Riegel AC, and Hoffman AF, Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol, 2004. 143(2): p. 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S, et al. , Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology, 2009. 34(13): p. 2699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunduz-Cinar O, et al. , Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci, 2013. 34(11): p. 637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray JM, et al. , Sustained glucocorticoid exposure recruits cortico-limbic CRH signaling to modulate endocannabinoid function. Psychoneuroendocrinology, 2016. 66: p. 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi S, et al. , Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci, 2008. 28(29): p. 7284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchison KE, et al. , The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry, 2008. 65(7): p. 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filbey FM, et al. , Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology, 2010. 35(4): p. 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haughey HM, et al. , Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction, 2008. 103(10): p. 1678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal A, et al. , Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am J Med Genet B Neuropsychiatr Genet, 2009. 150B(5): p. 736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griebel G, et al. , The selective reversible FAAH inhibitor, SSR411298, restores the development of maladaptive behaviors to acute and chronic stress in rodents. Sci Rep, 2018. 8(1): p. 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri AR, et al. , Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry, 2009. 66(1): p. 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel MM, et al. , FAAH variant Pro129Thr modulates subjective effects produced by cocaine administration. Am J Addict, 2018. 27(7): p. 567–573. [DOI] [PubMed] [Google Scholar]
- 17.Burgdorf CE, et al. , Endocannabinoid genetic variation enhances vulnerability to THC reward in adolescent female mice. Sci Adv, 2020. 6(7): p. eaay1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, et al. , Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology, 2010. 35(11): p. 2249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domschke K, et al. , Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol, 2008. 18(10): p. 751–9. [DOI] [PubMed] [Google Scholar]
- 20.Racz I, et al. , A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci, 2003. 23(6): p. 2453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filbey FM, et al. , Marijuana craving in the brain. Proc Natl Acad Sci U S A, 2009. 106(31): p. 13016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filbey FM, et al. , Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A, 2014. 111(47): p. 16913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koob GF, Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry, 2013. 4: p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filbey FM, et al. , fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users. Hum Brain Mapp, 2016. 37(10): p. 3431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, S R, Gibbon M, Williams JBW, Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). New York, Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 26.Cohen S, Kamarck T, and Mermelstein R, A global measure of perceived stress. J Health Soc Behav, 1983. 24(4): p. 385–96. [PubMed] [Google Scholar]
- 27.Bremner JD,V E, Mazure C, The Early Trauma Inventory: Development, reliability, validity. Depress Anxiety 2001: p. 12:1–12. [DOI] [PubMed] [Google Scholar]
- 28.Heishman SJ, et al. , Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend, 2009. 102(1–3): p. 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budney AJ, Novy PL, and Hughes JR, Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction, 1999. 94(9): p. 1311–22. [DOI] [PubMed] [Google Scholar]
- 30.Budney AJ, et al. , Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry, 2001. 58(10): p. 917–24. [DOI] [PubMed] [Google Scholar]
- 31.Abdi H, et al. , Analysis of regional cerebral blood flow data to discriminate among Alzheimer’s disease, frontotemporal dementia, and elderly controls: a multi-block barycentric discriminant analysis (MUBADA) methodology. J Alzheimers Dis, 2012. 31 Suppl 3: p. S189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdi HW, Lynne, Partial least squares regression and projection on latent structure regression (PLS regression). Encycl. Res. Des Thousand Oaks., 2010. 23: p. 655–660. [Google Scholar]
- 33.Beaton D, et al. , Partial least squares correspondence analysis: A framework to simultaneously analyze behavioral and genetic data. Psychol Methods, 2016. 21(4): p. 621–651. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan A, et al. , Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage, 2011. 56(2): p. 455–75. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh AR and Lobaugh NJ, Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage, 2004. 23 Suppl 1: p. S250–63. [DOI] [PubMed] [Google Scholar]
- 36.Beaton D CFC, Abdi H, An ExPosition of multivariate analysis with the singular value decomposition in R. Comput Stat Data Anal 2014: p. 72:176–189. [Google Scholar]
- 37.T H, Bootstrap. Wiley Interdiscip Rev Comput Stat; 2011: p. 3:497–526. [Google Scholar]
- 38.Lazary J, et al. , Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. Eur Neuropsychopharmacol, 2016. 26(6): p. 1020–8. [DOI] [PubMed] [Google Scholar]
- 39.Arias F, et al. , Lack of association between polymorphisms in cannabinoid receptor gene (CNR1) and fatty acid amide hydroxylase gene (FAAH) and eating disorders in a preliminary study. Psychiatr Genet, 2009. 19(6): p. 336. [DOI] [PubMed] [Google Scholar]
- 40.Filbey FM, et al. , Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci, 2015. 16: p. 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush G, et al. , Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A, 2002. 99(1): p. 523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vingerhoets WA, et al. , Cue-induced striatal activity in frequent cannabis users independently predicts cannabis problem severity three years later. J Psychopharmacol, 2016. 30(2): p. 152–8. [DOI] [PubMed] [Google Scholar]
- 43.Yankouskaya A, et al. , An anterior-posterior axis within the ventromedial prefrontal cortex separates self and reward. Soc Cogn Affect Neurosci, 2017. 12(12): p. 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wetherill RR, et al. , Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl), 2014. 231(7): p. 1397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckstrand KL, et al. , Trauma-associated anterior cingulate connectivity during reward learning predicts affective and anxiety states in young adults. Psychol Med, 2019. 49(11): p. 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hell HH, et al. , Chronic effects of cannabis use on the human reward system: an fMRI study. Eur Neuropsychopharmacol, 2010. 20(3): p. 153–63. [DOI] [PubMed] [Google Scholar]
- 47.Stice E, Yokum S, and Burger KS, Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry, 2013. 73(9): p. 869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizzagalli DA, et al. , Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry, 2009. 166(6): p. 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham SI, Tomasi D, and Volkow ND, Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp, 2017. 38(2): p. 938–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellana B, et al. , Similarities and differences in the default mode network across rest, retrieval, and future imagining. Hum Brain Mapp, 2017. 38(3): p. 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Small DM, et al. , Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex, 2005. 15(12): p. 1855–65. [DOI] [PubMed] [Google Scholar]
- 52.Engelmann JB, et al. , Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci, 2009. 3: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall MB, et al. , Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol, 2019. 33(7): p. 822–830. [DOI] [PubMed] [Google Scholar]
- 54.Imperatori C, et al. , Increased Resting State Triple Network Functional Connectivity in Undergraduate Problematic Cannabis Users: A Preliminary EEG Coherence Study. Brain Sci, 2020. 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeley WW, The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J Neurosci, 2019. 39(50): p. 9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menon V, Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci, 2011. 15(10): p. 483–506. [DOI] [PubMed] [Google Scholar]
- 57.Reese ED, et al. , Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. J Clin Med, 2019. 8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kienast T, et al. , Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci, 2008. 11(12): p. 1381–2. [DOI] [PubMed] [Google Scholar]
- 59.Marusak HA, et al. , Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 2015. 40(5): p. 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee TT, et al. , Sex, drugs, and adult neurogenesis: sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity, and amphetamine sensitization. Hippocampus, 2014. 24(3): p. 280–92. [DOI] [PubMed] [Google Scholar]
- 61.Garavan H, Insula and drug cravings. Brain Struct Funct, 2010. 214(5–6): p. 593–601. [DOI] [PubMed] [Google Scholar]
- 62.Naqvi NH and Bechara A, The hidden island of addiction: the insula. Trends Neurosci, 2009. 32(1): p. 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelchat ML, et al. , Images of desire: food-craving activation during fMRI. Neuroimage, 2004. 23(4): p. 1486–93. [DOI] [PubMed] [Google Scholar]
- 64.Wang GJ, et al. , Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage, 2004. 21(4): p. 1790–7. [DOI] [PubMed] [Google Scholar]
- 65.Mayo LM, et al. , Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol Psychiatry, 2020. 25(5): p. 993–1005. [DOI] [PubMed] [Google Scholar]
- 66.Bidwell LC, et al. , Impulsivity, variation in the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes, and marijuana-related problems. J Stud Alcohol Drugs, 2013. 74(6): p. 867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazary J, et al. , Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. 2009. 150B(8): p. 1118–27. [DOI] [PubMed] [Google Scholar]
- 68.Palmer RHC, et al. , CNR1 and FAAH variation and affective states induced by marijuana smoking. Am J Drug Alcohol Abuse, 2019. 45(5): p. 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dincheva I, et al. , FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun, 2015. 6: p. 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gartner A, et al. , Impact of FAAH genetic variation on fronto-amygdala function during emotional processing. Eur Arch Psychiatry Clin Neurosci, 2019. 269(2): p. 209–221. 71. [DOI] [PubMed] [Google Scholar]
- 71.DeVuono MV, et al. , Effect of footshock stress on place conditioning produced by Delta(9)-tetrahydrocannabinol and the fatty acid amide hydrolase (FAAH) inhibitor, URB597, in Sprague-Dawley rats. Psychopharmacology (Berl), 2017. 234(21): p. 3229–3240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.