To the Editor,
Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT) (also termed vaccine-induced thrombotic thrombocytopenia or vaccine-induced immune thrombocytopenia or thrombosis with thrombocytopenia syndrome (TTS) by the CDC and FDA) is characterized by (i) venous or arterial thrombosis; (ii) mild to severe thrombocytopenia ; (iii) positive antiplatelet factor 4 (PF4)–polyanion antibodies or anti-PF4-heparin antibodies detected by the HIT (heparin-induced thrombocytopenia) ELISA assay (iv) occurring five to 24 days after ChAdOx1 nCoV-19 or Ad26.COV2.S vaccination [1]. In initial reports, patients were likely young and under 50 years, female (more than two thirds), median platelet counts at diagnosis about 20 to 30 × 109 L−1, no risk factors for thrombosis and faced unusual sites for thrombosis including cerebral sinus venous thrombosis (more than two thirds) or portal vein with high fatality rates. VITT is associated with the detection of anti-PF4 antibodies, unrelated to previous use of heparin therapy. Heparin-independent platelet activation, known as autoimmune heparin-induced thrombocytopenia (aHIT) was previously described in patients with positive antiPF4–polyanion antibodies [2]. Similarly to aHIT, PF4 antibodies are sought to activate platelets via the platelet FcγRIIA receptors, but there is to date no clear data supporting that PF4 is either a bystander component within an immune complex that activates platelets, or directly contribute directly to platelet aggregation [3]. Based on current evidence, the precise mechanisms and molecular pathways triggering the production of anti-PF4 antibodies after adenovirus vectored vaccines remain to be determined. The Ad26.COV2.S and ChAdOx1 nCoV-19 vaccines have different vaccine phenotypes with different host cell receptors and biological effects making unclear which component of the vaccine (adenoviral sequence, spike protein, other component) may be held responsible for the production of anti-PF4 antibodies.
Potential risk factors for VITT may include young age and female sex while estrogen-replacement therapy or oral contraceptives were inconstantly reported among women. Moreover, no evidence to date is sufficient to establish a causal relationship between these events and hormonal therapy. The unusual topography and rare incidence for acute cerebral sinus venous thrombosis (CSVT), have led us to the hypothesis that procoagulant microparticles (MPs) enriched in phosphatidylserine (PS) and tissue factor (TF) may be important cofactors in the pathogenesis of VITT.
Microparticles (MPs) refer to small vesicles, ranging from 0.1 to 2 μm, originating from the plasma membrane of stimulated or apoptotic cells including, platelets, leukocytes and endothelial cells. Mps are constituted of material from their cells of origin and carry or express pro-inflammatory lipids, specific membrane glycoproteins (selectins, adhesion molecules, CDs…), antigens, mRNA etc. Procoagulant MPs (platelet and monocyte-derived) are circulating MPs that express phosphatidylserine (PS) and tissue factor (TF) and contribute to pro-coagulant responses. So what links circulating procoagulant microparticles, VITT and CSVT?
First, acute heparin-induced thrombocytopenia (HIT) is characterized by an increased level of procoagulant circulating MPs and a distinct phenotype of circulating PF4-bearing MPs associated with an increased risk of thrombosis [4]. Nevzorova et al. confirmed that PF4-containing pathogenic immune complexes lead to platelet activation, phosphatidylserine and P-selectin exposure on the outer leaflet of the platelet plasma membrane together with the shedding of procoagulant MPs that express PS [5]. In addition, HIT Ab complexes induced tissue factor expression by monocytes and the release of TF-bearing MPs. Altogether these data advocated the importance of a TF dependent and driven prothrombotic state supported by platelet and leukocyte-derived MPs in HIT [5, 6].
Second, the atypical cerebral venous distribution of thrombi in VITT may be explained by the fact that (i) circulating TF is more likely to play a role in venous thrombosis that is not associated with vessel damage [7] and (ii) TF involvement is crucial in cerebral microvascular thrombogenesis, with endothelial cell-associated TF mediating this response in venules, but not arterioles [8]. This gives echo to the platelet–neutrophil interaction triggered by HIT antibodies known to activate vascular endothelium [9].
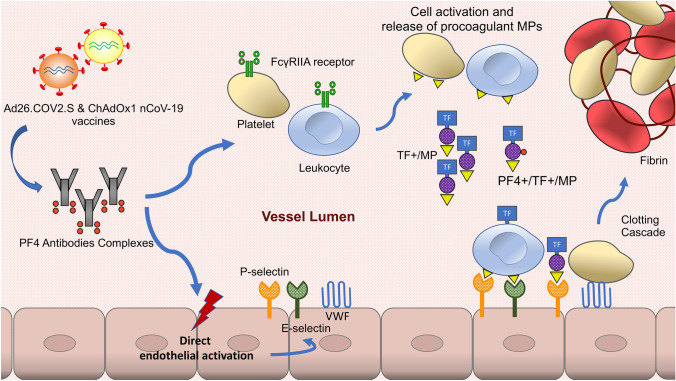
Therefore, we formulate the hypothesis that the pathogenesis of VITT involves (i) a FcγRIIA receptors pathway with circulating PF4 antibodies complexes that bind platelets and monocytic FcγRIIA receptors, causing cell monocytic activation and release of procoagulant MPs (ii) a direct activation of the endothelium by HIT Ab complexes leading to enhanced thrombogenicity through the release of P-selectins, E-selectins, von Willebrand factor and IL-6 [10]. Altogether, this results in enhanced PS and TF expression and subsequent thrombin generation (Fig. 1) more likely to occur in the cerebral venous system due to its specific thrombogenesis process.
Fig. 1.
Proposed mechanisms for cerebral sinus venous thrombosis in vaccine-induced immune thrombocytopenia (VITT). We proposed that venous thrombosis can be divided into two complementary and synergistic pathways in VITT. First, circulating PF4 antibodies complexes bind platelets and monocytic FcγRIIA receptors, causing cell activation and release of procoagulant microparticles (MPs). Second, the endothelium is directly activated by PF4 antibodies complexes and expresses the adhesion proteins E-selectin P-selectin and von Willebrand factor (VWF). Circulating TF+/MPs, PF4+/TF+/MPs, platelets and leukocytes bind to the activated endothelium and lead to the activation of the coagulation cascade. Cerebral sinus venous thrombosis in VITT is likely the consequence of (i) the key role played by circulating TF in venous thrombosis unrelated to vessel damage and (ii) the key role of endothelial cell-associated TF pathway in cerebral microvascular thrombogenesis. MPs microparticles, PF4 platelet factor 4, TF tissue factor, VWF Von Willebrand factor
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CSVT
Cerebral Sinus Venous Thrombosis
- FDA
Food and Drug Administration
- HIT
Heparin-induced thrombocytopenia
- MPs
Microparticles
- PF4
Platelet factor 4
- PS
Phosphatidylserine
- TF
Tissue factor
- VITT
Vaccine-induced immune thrombotic thrombocytopenia
- VWF
Von Willebrand factor
Author contribution
BM, AC, AT, AC, OM: drafting the article. OM: critical revision of the article.
Funding
This work was supported by Fondation Cœur et Recherche.
Declarations
Conflict of interest
All authors have nothing related to this paper to disclose. OM received Institutional Research Grants from Fédération Française de Cardiologie.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makris M, Pavord S, Lester W, Scully M, Hunt BJ. Vaccine-induced immune thrombocytopenia and thrombosis (VITT) Res Pract Thromb Haemost. 2021 doi: 10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 3.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia [published online ahead of print, 2021 Apr 16]. N Engl J Med. 2021. 10.1056/NEJMe2106315 [DOI] [PMC free article] [PubMed]
- 4.Campello E, Radu CM, Duner E, et al. Activated platelet-derived and leukocyte-derived circulating microparticles and the risk of thrombosis in heparin-induced thrombocytopenia: a role for PF4-bearing microparticles? Cytometry B Clin Cytom. 2018;94(2):334–341. doi: 10.1002/cyto.b.21507. [DOI] [PubMed] [Google Scholar]
- 5.Nevzorova TA, Mordakhanova ER, Daminova AG, et al. Platelet factor 4-containing immune complexes induce platelet activation followed by calpain-dependent platelet death. Cell Death Discov. 2019;5:106. doi: 10.1038/s41420-019-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasthuri RS, Glover SL, Jonas W, et al. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcγRI. Blood. 2012;119(22):5285–5293. doi: 10.1182/blood-2011-06-359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M, Yilmaz CE, Kirchhofer D, Esmon CT, Mackman N, Granger DN. Role of coagulation factors in cerebral venous sinus and cerebral microvascular thrombosis. Neurosurgery. 2010;66(3):560–566. doi: 10.1227/01.NEU.0000365745.49583.FD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walenga JM, Jeske WP, Prechel MM, Bakhos M. Newer insights on the mechanism of heparin-induced thrombocytopenia. Semin Thromb Hemost. 2004;30(Suppl 1):57–67. doi: 10.1055/s-2004-823004. [DOI] [PubMed] [Google Scholar]
- 10.Blank M, Shoenfeld Y, Tavor S, et al. Anti-platelet factor 4/heparin antibodies from patients with heparin-induced thrombocytopenia provoke direct activation of microvascular endothelial cells. Int Immunol. 2002;14(2):121–129. doi: 10.1093/intimm/14.2.121. [DOI] [PubMed] [Google Scholar]