Abstract
Diffuse pollution in urban receiving waters often adversely impacts both humans and ecosystems. Identifying such pollution sources is challenging and limits the effectiveness of management actions intended to reduce risk. Here, we evaluated the use of nontarget analysis via high-resolution mass spectrometry (HRMS) to develop chemical fingerprints/signatures for source tracking. Specifically, we applied nontarget HRMS to characterize and differentiate two urban chemical sources: roadway runoff and wastewater influent. We isolated 112 and 598 nontarget compounds (both known and unidentified chemicals) that co-occurred in all roadway runoff and wastewater influent samples, respectively, and were unique relative to other sampled sources. For example, methamphetamine, often considered wastewater derived, was detected in all samples, implying that individual wastewater indicators may lack sufficient specificity in urban receiving waters impacted by multiple sources. Hierarchical cluster analysis differentiated source types, and normalized abundance profiling prioritized nontarget compounds with consistent relative abundance patterns across field sites for a given source. Hexa(methoxymethyl)melamine, 1,3-diphenylguanidine, and polyethylene glycols co-occurred in roadway runoff across geographic areas and traffic intensities, supporting continued development of a universal roadway runoff fingerprint based on ubiquitous compounds. This study provides a proof-of-concept for isolating nontarget source fingerprints to track diffuse contamination in urban receiving waters.
Graphical Abstract
INTRODUCTION
Urban receiving waters, such as rivers, small streams, and nearshore marine waters, are often impacted by poorly characterized diffuse pollution sources that degrade water quality and adversely impact human and ecosystem health.1,2 For example, urban runoff contributes to elevated fecal levels that often prompt beach closures and cause infectious diseases in beachgoers.3 Effective water quality management requires accurate identification of potential contaminant sources.4
To date, most efforts to identify and track chemical sources have primarily relied on small (<20) suites of known indicator compounds that were previously identified or are related to source type.5,6 However, these efforts may fail when targeted indicators lack source specificity or are insufficiently abundant or when the source is poorly characterized. In contrast, nontarget analyses (NTA) use liquid or gas chromatography coupled to high-resolution mass spectrometry (LC-HRMS, GC-HRMS)7,8 to detect hundreds to thousands of chemicals easily (both known and unidentified)2 and can establish unique chemical fingerprints that provide more statistical power for source characterization without relying upon pre-existing knowledge or assumptions about chemical composition.9,10 Accordingly, HRMS fingerprints may be especially well suited to differentiate and resolve the contributions of anthropogenic contaminant sources to receiving waters.9,11–14
Many environmental applications of NTA7,8 have focused on prioritizing and identifying unknown and novel compounds15–19 and improving hazard assessments.20–25 Other efforts combine the richness of nontarget HRMS data with advanced data mining techniques10,26,27 and statistical analyses to correlate chemical data with effect-based monitoring results,2,28,29 assess the performance of treatment technologies,30–32 or isolate specific chemical markers.13 Recent NTA applications even demonstrate that bulk HRMS data can aid environmental management even without extensive contaminant identification.7,9,10,33,34 Notably, HRMS chromatographic fingerprints have been successfully applied to validate sample origin in food and medicine fields.35,36 However, environmental HRMS fingerprinting approaches are relatively less developed, and uncertainties remain in our understanding of their specificity and variability.
Here, we hypothesized that HRMS fingerprints of representative urban sources could be consistently associated with source type, and their occurrence and/or relative abundance pattern would remain sufficiently unique (i.e., source specific) to support source tracking and apportionment. While identification of individual contaminants may be helpful to explain specific anthropogenic influences, such efforts can be labor intensive and may not actually be necessary to support source differentiation and apportionment. A previous laboratory-based study demonstrated that unidentified nontarget HRMS data could be used to confidently distinguish and quantify source contributions to a complex water sample,9 but this approach has not yet been extended to the field. Here, to demonstrate the use of nontarget HRMS data to differentiate key urban contaminant sources and specifically to support resolution of ongoing pollution issues where untreated wastewater contributions are suspected, we applied LC-HRMS to differentiate the chemical fingerprints of municipal wastewater influent, roadway runoff, and baseflow in urban storm drains. This study provides a proof-of-concept for using nontarget HRMS fingerprints to differentiate urban contaminant sources and addresses challenges and limitations in extending these concepts to field applications.
MATERIALS AND METHODS
Chemicals.
A list of chemicals and solvents are provided in the Supporting Information.
Sample Collection, Processing, and Analysis.
Grab samples (4 L precleaned amber glass bottles) collected in Southern California during winter–spring 2019 (Table S1) were transported on ice to the SCCWRP laboratory and extracted within 24 h, as described previously.9,29 Samples included municipal wastewater influent (n = 5, collected in sewer pipes, representing commercial and residential sources) and dry weather storm drains (n = 3, representing baseflow from upstream surface water, groundwater infiltration, and/or watering of residential lawns) from San Diego County, California, USA. Roadway runoff (n = 4) was sampled from two high-traffic arterials (toward the end of first flush) and two local roads (during the first flush) in Orange County, California, USA. Unfiltered samples (1 L) were extracted in triplicate using Infinity solid phase extraction (SPE) cartridges (3 mL, 100 mg, Osorb media, ABS Materials, Wooster, OH, USA). For geographic comparison, roadway runoff extracts (n = 2, collected from Seattle metro area highways, extracted by identical methods) were also analyzed (Table S1). Extracts were analyzed (full scan MS1, 100–1700 m/z; reinjected for data-dependent MS/MS to confirm identifications, 50–1700 m/z) using an Agilent 1290 Infinity UHPLC coupled to an Agilent 6530 quadrupole time-of-flight mass spectrometer (Santa Clara, CA, USA) with positive electrospray ionization. A check-tune including mass calibration was performed before each run; mass accuracy was continuously corrected via infusion of purine and HP-921. To monitor analytical performance, solvent blanks and internal standard controls (Table S2) were analyzed every 12 samples (no carry-over observed in blanks), and triplicate method blanks (deionized water) were extracted/analyzed alongside samples. Mass error and retention time variation for internal standard controls were <5 ppm and <0.2 min, respectively. Relative standard deviations (RSD) of internal standard peak areas across all samples were 39%–118%.
Data Analysis.
We used Agilent MassHunter Profinder (B.08.00) and Mass Profiler Professional (MPP; B.13.00) to extract and align compounds (exact mass-retention time pairs), group isotopes/adducts into nontarget compounds, filter detections, and perform statistical analyses (detailed settings in Table S3). Compounds detected in 100% (3/3) of extraction replicates, with peak areas >5000 and ≥5-fold peak areas relative to all blanks, were retained for subsequent analysis. Hierarchical cluster analysis (HCA) was performed in MPP to compare chemical profiles, with Euclidean distances calculated from log-normalized peak areas and Ward’s method used to cluster samples and compounds. To isolate compounds that exhibited consistent relative concentration patterns, compound peak areas were normalized to the sum peak area of all compounds in a given sample,23 and RSDs of normalized peak areas were calculated across samples from a given source type. An in-house database with molecular formula and retention time for ~1100 contaminants (pharmaceuticals, personal care products, human tracers, vehicle/roadway related chemicals)15 was used to identify prioritized compounds. Matches with mass errors <5 ppm, isotope pattern scores >85, and retention time differences <0.3 min were recognized as S1 identifications with reference standard verification of MS/MS fragmentation, S2a with match to MS/MS in open-source/commercial mass spectral libraries, or S2b with expert assessment of MS/MS fragmentation.37
RESULTS AND DISCUSSION
Source Signature Complexity and Specificity.
To evaluate if HRMS fingerprints could consistently identify and differentiate a given source type, we compared the chemical fingerprints of three types of urban waters (n = 3–5 field sites each): baseflow in storm drains, roadway runoff, and wastewater influent. The storm drain baseflow samples represented an urban “chemical background” signature for winter–spring, while roadway runoff and wastewater influent represented more intense and distinct contaminant sources. Although wastewater effluent would likely provide a more persistent set of chemical markers, wastewater influent was specifically selected for evaluation here based on a common source tracking challenge (fecal contamination in urban receiving waters and the potential role of sewer line exfiltration). We first applied HCA to compare the entire nontarget chemical profiles of individual samples (n = 10,034 nontarget compounds in total; 685–3482 detections per site; Figure S1). Samples clustered by source and visual inspection of the HCA revealed clusters of overlapping detections at similar abundances within each source type. This initial inspection supported development of HRMS fingerprints both to differentiate these sources and to identify source contributions in receiving waters with multiple chemically similar inputs. The high degree of overlap between source types also highlighted the importance of holistic approaches when characterizing the chemical composition of urban sources, as it is obvious that urban waters typically share many chemical detections, including both natural products and anthropogenic contaminants. The only real distinction between such chemical detections is their relative concentration across different source types, locations, and time periods. Therefore, source tracing, identification, or fingerprinting efforts can plausibly exploit not only unique chemicals (commonly relied upon) but also the implicit metadata inherent to relative concentration patterns among the many multisource contaminants to generate metrics of resolution and sensitivity for identification and apportionment efforts.
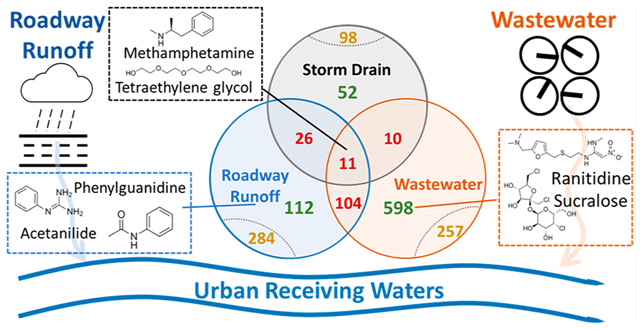
To isolate source-specific HRMS fingerprints, we selected for compounds that had 100% co-occurrence in all samples of each source type (i.e., detected in each of the 3–5 field sites per source type).2 This relatively stringent screening criteria reduced the wastewater (3919 ± 410 detections/field site), roadway runoff (1603 ± 226 detections/site), and storm drain baseflow (812 ± 147 detections/site) chemical signatures to 980 (detected in n = 5/5 field sites), 537 (n = 4/4), and 197 (n = 3/3) compounds, respectively. We then evaluated the specificity of each source signature by examining their qualitative overlap with other samples (Figure 1).9 We also identified several compounds in the wastewater and roadway runoff signatures to understand if the unique chemicals driving the clustering seemed reasonable and appropriate as source indicators.
Figure 1.
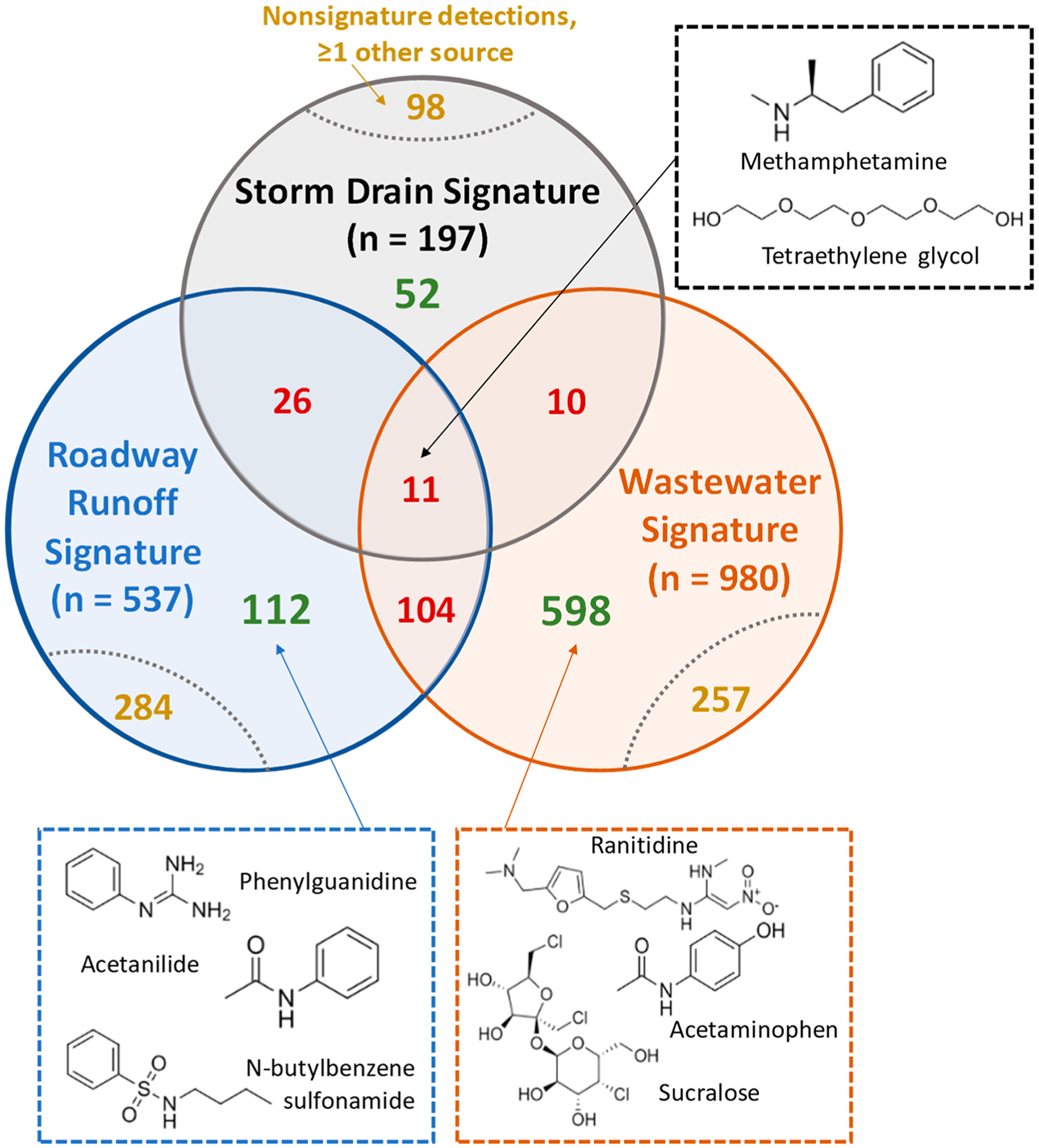
Venn diagrams showing the overlap of HRMS signatures (i.e., compounds detected in 100% of samples for a source type) for roadway runoff (blue, n = 537), wastewater (orange, n = 980), and storm drain baseflow (gray, n = 197) with each other and with nonsignature detections (i.e., compounds detected in <100% of samples for other source types). Compounds are classified by level of source specificity: good/unique (green; only present in a single source signature), moderate (yellow; overlapped with nonsignature detections), and poor (red; co-occurred in another source signature). Square panels show representative detections: Methamphetamine (S1) and tetraethylene glycol (S1) co-occurred in all samples of all source types. Phenylguanidine (S2b), acetanilide (S1), and N-butylbenzenesulfonamide (S1) were unique to the roadway runoff signature. Ranitidine (S1), acetaminophen (S1), and sucralose (S1) were unique to the wastewater signature.
Each roadway runoff and wastewater influent, 21% (n = 112) and 61% (n = 598), respectively, were completely unique to each source type (Figure 1, Figure S1, Table S4). Although sampling of additional sites beyond those included here is needed for refinement of this list, these unique detections represent indicator compounds with sufficient specificity to identify source contributions to mixed samples. Unique wastewater signature compounds included sucralose, acetaminophen, and the antihistamine/antacid ranitidine (all S1), agreeing with previously reported wastewater indicators.38,39 Likewise, compounds unique to the roadway runoff signature included phenylguanidine (a transformation product of the tire rubber vulcanization accelerator 1,3-diphenylguanidine; S2b),15,40 acetanilide (an intermediate used in rubber and dye production; S1),29 and N-butylbenzenesulfonamide (a plasticizer and antifungal; S1).15 Relative to compounds preselected prior to analysis, these compounds are more specific to the sources of interest, and could complement existing targeted approaches to source tracking.
In contrast, 26% of the roadway runoff signature compounds (n = 141; red in Figure 1) were also detected in 100% of samples from at least one of the other two source types (i.e., wastewater and/or storm drain baseflow signatures). Likewise, 13% of the wastewater influent signature compounds (n = 125, red in Figure 1) co-occurred in one or both of the other two source signatures. Only 11 compounds co-occurred in all three HRMS source signatures (12 of 12 samples), including methamphetamine (S1) and tetraethylene glycol (S1).37 Tetraethylene glycol is used in a wide variety of applications (e.g., personal care products, vehicle antifreeze, dispersants) and has been widely detected in the environment.41 However, co-occurrence of methamphetamine, a compound previously suggested as a wastewater indicator compound,42 in all targeted urban waters indicated that simple assessments of presence or absence of typical representative chemical indicator(s)5,6,43 may lack sufficient resolution for tracking wastewater contributions. This observation reflected previous reports that contaminants traditionally considered “wastewater derived”, such as prescription/recreational drugs, have been frequently detected in urban receiving waters that are disconnected from known sewage sources, with elevated levels especially evident during storm events.40,44
The remaining portion of each source signature (roadway runoff: 53%, n = 284; wastewater influent: 26%, n = 257) was detected sporadically in other source types (<100% of samples), indicating only moderate uniqueness. For example, 1,3-diphenylguanidine (DPG, S1), HMMM (S1),18,45 and dicyclohexylamine (S2a) co-occurred in the roadway runoff signature and in 20%, 40%, and 80% of wastewater samples, respectively, although at lower relative abundance (Figure S2). Although these chemicals are primarily tire rubber derived, their occurrence in wastewater may reflect their use in other products (e.g., HMMM in plastic coatings45 or fabrics46) or contributions of “roadway-like” sources (e.g., garages, industrial floors) to wastewater. These overlapping detections again indicated that advance selection of source indicator compounds can introduce false positives or source bias when tracking source contributions to receiving waters. For compounds subject to multiple sources (e.g., roadway runoff, municipal wastewater), it is critical either to recognize and exclude these co-occurring compounds or to consider relative concentration patterns more explicitly.
Abundant Compounds in Wastewater and Roadway Runoff.
Polymeric surfactants were the most abundant identifications in both sources (top 25 average peak area, Tables S5 and S6; all identified compounds, Tables S7 and S8), with the ubiquitous polyethylene glycols (PEGs, C2n−2H4n−2On) as the most abundant co-occurring group47. PEG monodecyl ethers (C2n+8H4n+18On), which have long saturated aliphatic chains and are used in high volumes in household detergents, were more abundant in wastewater (6 of top 25) than in roadway runoff (0 of top 25). The most abundant nonpolymeric compounds in wastewater were drugs (methamphetamine, caffeine, and ranitidine; S1), while the most abundant nonpolymeric compounds in roadway runoff were DPG (S1), HMMM (S1), dicyclohexylamine (S2a), and hexylamine (S2a).2
Wastewater Signature.
Among the five wastewater samples, three represented primarily commercial wastewater (WW 1–3, >50% commercial), while two were primarily residential (WW 4–5, <50% commercial). Residential- and commercial-dominated wastewaters uniquely contained 1047 (co-occurring in 2/2 samples) and 732 (co-occurring in 3/3 samples) compounds, respectively. This uniqueness could aid source tracking in situations where both the type of source (i.e., wastewater vs roadway runoff) and the specific source (i.e., one sewer pipe vs another, a sewer pipe vs an encampment) require elucidation. Although additional sampling may be required to ensure that the selected compounds exhibit consistent occurrence and relative abundance patterns across space and time, laboratory experiments that examine the dilution/fate behavior of these compounds9 should enable selection of “fit-for-purpose” stable marker compounds specific to both the source type and location.
We also used the wastewater chemical signature to further investigate approaches that rely on the relative abundance profiles of co-occurring detections. To isolate compounds that exhibited consistent relative concentration patterns across the five wastewater influents, we evaluated the RSDs of normalized peak areas for the 980 signature compounds across the five wastewater samples and selected those within the first quartile of the RSD distribution (RSD < 47%) (Figure 2a, inset). This analysis limited the signature to 245 compounds (Figure 2a, b). Notably, PEGs (C2n−2H4n−2On; n = 4–15, 19–20) were among the highest relative abundance compounds in this reduced signature. However, PEGs co-occurred in roadway runoff and exhibited an abundance pattern similar to that in wastewater, indicating that PEGs are a consistent marker of human impacts but have limited source specificity. However, caffeine was retained within the reduced wastewater signature and was detected in only 1 of 4 roadway runoff samples and 1 of 3 storm drain samples, indicating its relative wastewater specificity in this data set. This reduced signature could be used more broadly to assess wastewater contributions, whether from sewer pipe exfiltration, septic systems, or homeless encampments. However, the number of sampling events/sites (both of the source of interest and potential confounding sources) and sampling methods (e.g., grab vs composite, time weighted vs flow weighted) needed to isolate such a “universal signature” is not yet clear, and it will require additional investigation to establish best practices. Furthermore, we anticipate that the relative abundance of the signature compounds will change over space and time as environmental fate processes (e.g., sorption, degradation) occur. Thus, while this approach provides a starting point to develop consistent source-specific HRMS fingerprints and systematic evaluation of these variables, improved understanding of the chemical characteristics of source fingerprints (e.g., polarity, halogenation) is necessary to support practical application.
Figure 2.
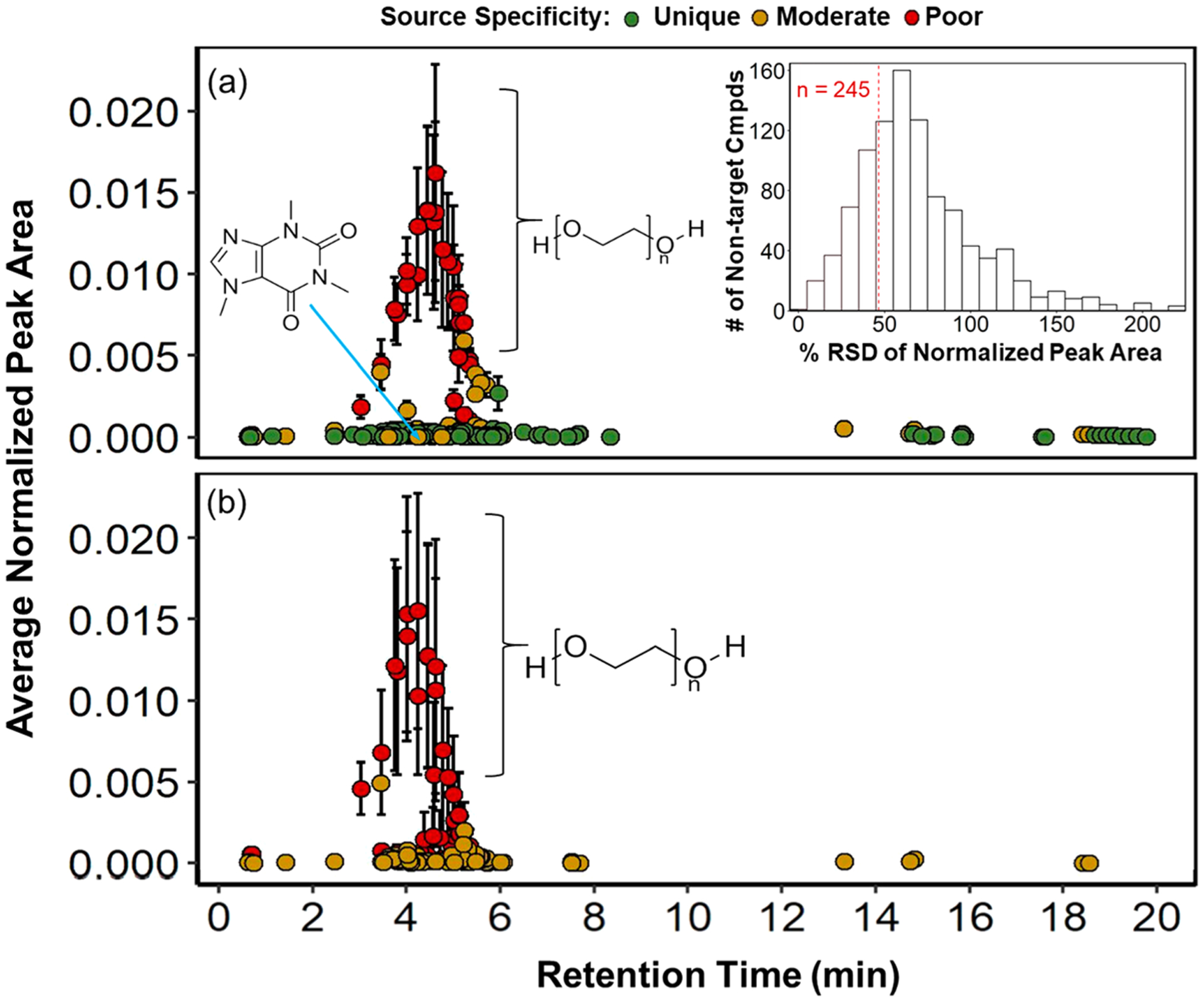
Average normalized peak area in (a) wastewater samples and (b) roadway runoff plotted against retention time for 245 compounds from the wastewater chemical signature (n = 980 compounds total) that exhibited relatively low variability in their abundance pattern across the five wastewater samples. The variability threshold was set to the first quartile of the RSD distribution (RSD < 46%, see inset), where compounds highlighted in pink are included in the main plot. As in Figure 1, compounds are classified based on good/unique (green), moderate (yellow), and poor (red) source specificity based on the extent of co-occurrence with other source signatures and samples.
Characterizing Roadway Runoff.
To evaluate the fidelity of the roadway runoff source signature across geographic areas, we first assessed the degree of overlap between the roadway runoff signature (developed using Orange County runoff) to that of roadway runoff collected from two Seattle metro area highways. Of the 537 roadway runoff signature compounds, 36% (n = 191) were also detected in both Seattle roadway runoff samples (Figure S3). Among these, 7 of the 10 most abundant (highest peak area from either metro area) were detected in both metro areas, including the vulcanization accelerator DPG and two PEG monomethyl ether surfactants: tetraethylene and pentaethylene glycol monomethyl ether (S1; S2b). Phenylguanidine and the corrosion inhibitor 5-methyl-1H-benzotriazole (S1) also co-occurred in roadway runoff from both geographic areas.
Using the 191 geographically co-occurring compounds, we applied HCA to evaluate clustering within the four Orange County and two Seattle metro area samples (Figure 3).9 The HCA produced two main clusters based on geographic location, with the Orange County samples further subclustered by traffic intensity (highway vs local roads). Visual inspection also revealed two groups of compounds (Figure 3, left side of dendrogram) present in consistently high abundance (box A) or consistently low abundance (box B) in all roadway runoff samples, representing a group of conservative compounds (i.e., detected ubiquitously and at similar relative abundance) that could contribute to more universal roadway runoff chemical fingerprints. For example, DPG, HMMM, and dicyclohexylamine, which are derived from vehicle tires and are commonly detected in roadway runoff,2,18 were all identified within box A. To further evaluate the relative abundance profiles, we used a similar analysis as described above for the wastewater signature (Figure S4). This analysis revealed 48 compounds with consistent normalized abundance across all six roadway runoff samples (first quartile of RSD distribution, RSD < 49%). Notably, this group included 1,3-diphenylguanidine, which has been previously recognized as a ubiquitous roadway runoff contaminant.2,18
Figure 3.
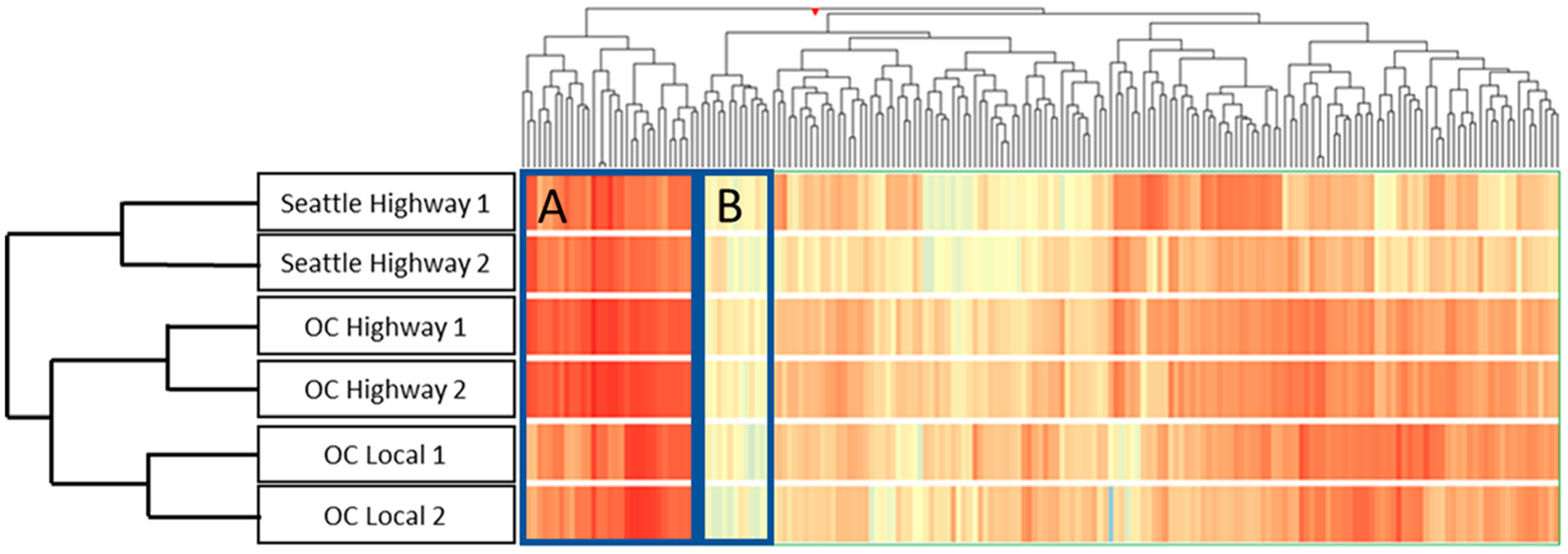
Hierarchical cluster analysis of roadway runoff samples from Seattle and Orange County (OC) constrained to the 191 geographically co-occurring compounds. Each row represents an average of three replicate samples, and each vertical line represents an individual compound with color representing peak area (absent = dark blue, increasing to yellow, light red, and dark red with increasing peak area). Boxes A and B highlight groups of indicator compounds that were present at consistently high or consistently low abundance, respectively, and thus represent the most conservative, universal indicators for roadway runoff.
Implications.
We used nontarget HRMS analyses to develop source fingerprints, demonstrating the potential of structured NTA to isolate and sort detected nontarget peaks for applications of source differentiation and tracking. Although NTA-based approaches require advanced statistical analyses to prioritize indicator compounds and evaluate the specificity of source fingerprints, these analyses can ultimately improve the efficacy of receiving water management efforts. Our previous lab-based study demonstrated the potential of using unidentified chemical fingerprints to estimate source contributions quantitatively,9 suggesting that chemical identity is not strictly necessary for source tracking. Here, we extended that foundation by evaluating the potential to develop HRMS fingerprints from disparate samples of specific source types and use the approach to resolve and (sometimes) identify those compounds most amenable to source tracking. Isolated source signatures still had enough compounds to be statistically powerful (e.g., 100s), and we observed consistent relative abundance profiles across geographic space (roadway runoff) and development type (wastewater). This study represents a key initial validation of HRMS fingerprint development in field samples and indicates the potential for development of broader, more universal source fingerprints.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Joshua Steele and other SCCWRP staff for collecting wastewater and storm drain samples and Dr. Jian Peng and Michael Thompson (Orange County Public Works) for sampling assistance. This work was supported by funding from the Southern California Coastal Water Research Project Authority. This work was performed while the author Katherine Peter held a National Research Council Research Associateship award at NIST. Certain commercial equipment, instruments, or materials may be identified in this research to adequately specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.0c00749.
Information as mentioned in the text (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.estlett.0c00749
Contributor Information
Bowen Du, Southern California Coastal Water Research Project Authority, Costa Mesa, California 92626, United States.
Zhenyu Tian, Interdisciplinary Arts and Sciences, University of Washington Tacoma, Tacoma, Washington 98421, United States; Center for Urban Waters, Tacoma, Washington 98421, United States.
Katherine T. Peter, National Institute of Standards and Technology, Charleston, South Carolina 29412, United States.
Edward P. Kolodziej, Interdisciplinary Arts and Sciences, University of Washington Tacoma, Tacoma, Washington 98421, United States; Center for Urban Waters, Tacoma, Washington 98421, United States; Department of Civil and Environmental Engineering, University of Washington, Seattle, Washington 98195, United States.
Charles S. Wong, Southern California Coastal Water Research Project Authority, Costa Mesa, California 92626, United States.
REFERENCES
- (1).Walsh CJ; Roy AH; Feminella JW; Cottingham PD; Groffman PM; Morgan RP The urban stream syndrome: current knowledge and the search for a cure. J. North Am. Benthological Soc 2005, 24 (3), 706–723. [Google Scholar]
- (2).Peter KT; Tian Z; Wu C; Lin P; White S; Du B; McIntyre JK; Scholz NL; Kolodziej EP Using high-resolution mass spectrometry to identify organic contaminants linked to urban stormwater mortality syndrome in coho salmon. Environ. Sci. Technol 2018, 52 (18), 10317–10327. [DOI] [PubMed] [Google Scholar]
- (3).Steele JA; Blackwood AD; Griffith JF; Noble RT; Schiff KC Quantification of pathogens and markers of fecal contamination during storm events along popular surfing beaches in San Diego. Water Res. 2018, 136, 137–149. [DOI] [PubMed] [Google Scholar]
- (4).Brack W; Hollender J; de Alda ML; Müller C; Schulze T; Schymanski E; Slobodnik J; Krauss M High-resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environ. Sci. Eur 2019, 31 (1), 62. [Google Scholar]
- (5).Glassmeyer ST; Furlong ET; Kolpin DW; Cahill JD; Zaugg SD; Werner SL; Meyer MT; Kryak DD Transport of Chemical and Microbial Compounds from Known Wastewater Discharges: Potential for Use as Indicators of Human Fecal Contamination. Environ. Sci. Technol 2005, 39 (14), 5157–5169. [DOI] [PubMed] [Google Scholar]
- (6).Dickenson ERV; Snyder SA; Sedlak DL; Drewes JE Indicator compounds for assessment of wastewater effluent contributions to flow and water quality. Water Res. 2011, 45 (3), 1199–1212. [DOI] [PubMed] [Google Scholar]
- (7).Hollender J; Schymanski EL; Singer HP; Ferguson PL Nontarget screening with high resolution mass spectrometry in the environment: Ready to go? Environ. Sci. Technol 2017, 51 (20), 11505–11512. [DOI] [PubMed] [Google Scholar]
- (8).Farré MJ; Jaén-Gil A; Hawkes J; Petrovic M; Catalán, N. Orbitrap molecular fingerprint of dissolved organic matter in natural waters and its relationship with NDMA formation potential. Sci. Total Environ 2019, 670, 1019–1027. [DOI] [PubMed] [Google Scholar]
- (9).Peter KT; Wu C; Tian Z; Kolodziej EP Application of Nontarget High Resolution Mass Spectrometry Data to Quantitative Source Apportionment. Environ. Sci. Technol 2019, 53 (21), 12257–12268. [DOI] [PubMed] [Google Scholar]
- (10).Samanipour S; Kaserzon S; Vijayasarathy S; Jiang H; Choi P; Reid MJ; Mueller JF; Thomas KV Machine learning combined with non-targeted LC-HRMS analysis for a risk warning system of chemical hazards in drinking water: A proof of concept. Talanta 2019, 195, 426–432. [DOI] [PubMed] [Google Scholar]
- (11).Peter KT; Herzog S; Tian Z; Wu C; McCray JE; Lynch K; Kolodziej EP Evaluating emerging organic contaminant removal in an engineered hyporheic zone using high resolution mass spectrometry. Water Res. 2019, 150, 140–152. [DOI] [PubMed] [Google Scholar]
- (12).Carpenter CMG; Helbling DE Widespread Micro-pollutant Monitoring in the Hudson River Estuary Reveals Spatiotemporal Micropollutant Clusters and Their Sources. Environ. Sci. Technol 2018, 52 (11), 6187–6196. [DOI] [PubMed] [Google Scholar]
- (13).Soulier C; Coureau C; Togola A Environmental forensics in groundwater coupling passive sampling and high resolution mass spectrometry for screening. Sci. Total Environ 2016, 563–564, 845–854. [DOI] [PubMed] [Google Scholar]
- (14).Köppe T; Jewell KS; Dietrich C; Wick A; Ternes TA Application of a non-target workflow for the identification of specific contaminants using the example of the Nidda river basin. Water Res. 2020, 178, 115703. [DOI] [PubMed] [Google Scholar]
- (15).Tian Z; Peter KT; Gipe AD; Zhao H; Hou F; Wark DA; Khangaonkar T; Kolodziej EP; James CA Suspect and Nontarget Screening for Contaminants of Emerging Concern in an Urban Estuary. Environ. Sci. Technol 2020, 54 (2), 889–901. [DOI] [PubMed] [Google Scholar]
- (16).Wang M; Helbling DE A non-target approach to identify disinfection byproducts of structurally similar sulfonamide antibiotics. Water Res. 2016, 102, 241–251. [DOI] [PubMed] [Google Scholar]
- (17).Schollée JE; Bourgin M; von Gunten U; McArdell CS; Hollender J Non-target screening to trace ozonation transformation products in a wastewater treatment train including different post-treatments. Water Res. 2018, 142, 267–278. [DOI] [PubMed] [Google Scholar]
- (18).Seiwert B; Klöckner P; Wagner S; Reemtsma T Source-related smart suspect screening in the aqueous environment: search for tire-derived persistent and mobile trace organic contaminants in surface waters. Anal. Bioanal. Chem 2020, 412, 4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pochodylo AL; Helbling DE Emerging investigators series: prioritization of suspect hits in a sensitive suspect screening workflow for comprehensive micropollutant characterization in environmental samples. Environmental Science: Water Research & Technology 2017, 3 (1), 54–65. [Google Scholar]
- (20).Escher BI; Stapleton HM; Schymanski EL Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367 (6476), 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ulrich EM; Sobus JR; Grulke CM; Richard AM; Newton SR; Strynar MJ; Mansouri K; Williams AJ EPA’s non-targeted analysis collaborative trial (ENTACT): genesis, design, and initial findings. Anal. Bioanal. Chem 2019, 411 (4), 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Petras D; Minich JJ; Kunselman E; Wang M; White ME; Allen EE; Aluwihare LI; Dorrestein PC Non-target tandem mass spectrometry enables the prioritization of anthropogenic pollutants in seawater along the northern San Diego coast. ChemRxiv, 2019. DOI: 10.26434/chemrxiv.9817133.v2. [DOI] [Google Scholar]
- (23).Chiaia-Hernández AC; Günthardt BF; Frey MP; Hollender J Unravelling Contaminants in the Anthropocene Using Statistical Analysis of Liquid Chromatography–High-Resolution Mass Spectrometry Nontarget Screening Data Recorded in Lake Sediments. Environ. Sci. Technol 2017, 51 (21), 12547–12556. [DOI] [PubMed] [Google Scholar]
- (24).Schymanski EL; Singer HP; Longrée P; Loos M; Ruff M; Stravs MA; Ripolleś Vidal C; Hollender J Strategies to characterize polar organic contamination in wastewater: Exploring the capability of high resolution mass spectrometry. Environ. Sci. Technol 2014, 48 (3), 1811–1818. [DOI] [PubMed] [Google Scholar]
- (25).Black GP; Anumol T; Young TM Analyzing a broader spectrum of endocrine active organic contaminants in sewage sludge with high resolution LC-QTOF-MS suspect screening and QSAR toxicity prediction. Environ. Sci.: Processes Impacts 2019, 21 (7), 1099–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ruff M; Mueller MS; Loos M; Singer HP Quantitative target and systematic non-target analysis of polar organic micro-pollutants along the river Rhine using high-resolution mass-spectrometry – Identification of unknown sources and compounds. Water Res. 2015, 87, 145–154. [DOI] [PubMed] [Google Scholar]
- (27).Karpuzcu ME; Fairbairn D; Arnold WA; Barber BL; Kaufenberg E; Koskinen WC; Novak PJ; Rice PJ; Swackhamer DL Identifying sources of emerging organic contaminants in a mixed use watershed using principal components analysis. Environmental Science: Processes & Impacts 2014, 16 (10), 2390–2399. [DOI] [PubMed] [Google Scholar]
- (28).Sobus JR; Wambaugh JF; Isaacs KK; Williams AJ; McEachran AD; Richard AM; Grulke CM; Ulrich EM; Rager JE; Strynar MJ; Newton SR Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. J. Exposure Sci. Environ. Epidemiol 2018, 28 (5), 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Du B; Lofton JM; Peter KT; Gipe AD; James CA; McIntyre JK; Scholz NL; Baker JE; Kolodziej EP Development of suspect and non-target screening methods for detection of organic contaminants in highway runoff and fish tissue with high-resolution time-of-flight mass spectrometry. Environmental Science: Processes & Impacts 2017, 19 (9), 1185–1196. [DOI] [PubMed] [Google Scholar]
- (30).Parry E; Young TM Comparing targeted and non-targeted high-resolution mass spectrometric approaches for assessing advanced oxidation reactor performance. Water Res. 2016, 104, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Blum KM; Andersson PL; Renman G; Ahrens L; Gros M; Wiberg K; Haglund P Non-target screening and prioritization of potentially persistent, bioaccumulating and toxic domestic wastewater contaminants and their removal in on-site and large-scale sewage treatment plants. Sci. Total Environ 2017, 575, 265–275. [DOI] [PubMed] [Google Scholar]
- (32).Nürenberg G; Schulz M; Kunkel U; Ternes TA Development and validation of a generic nontarget method based on liquid chromatography – high resolution mass spectrometry analysis for the evaluation of different wastewater treatment options. Journal of Chromatography A 2015, 1426, 77–90. [DOI] [PubMed] [Google Scholar]
- (33).Krauss M; Hug C; Bloch R; Schulze T; Brack W Prioritising site-specific micropollutants in surface water from LC-HRMS non-target screening data using a rarity score. Environ. Sci. Eur 2019, 31 (1), 45. [Google Scholar]
- (34).Anliker S; Loos M; Comte R; Ruff M; Fenner K; Singer H Assessing Emissions from Pharmaceutical Manufacturing Based on Temporal High-Resolution Mass Spectrometry Data. Environ. Sci. Technol 2020, 54 (7), 4110–4120. [DOI] [PubMed] [Google Scholar]
- (35).Gao W; Yang H; Qi L-W; Liu EH; Ren M-T; Yan Y-T; Chen J; Li P Unbiased metabolite profiling by liquid chromatography–quadrupole time-of-flight mass spectrometry and multivariate data analysis for herbal authentication: Classification of seven Lonicera species flower buds. Journal of Chromatography A 2012, 1245, 109–116. [DOI] [PubMed] [Google Scholar]
- (36).Esslinger S; Riedl J; Fauhl-Hassek C Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res. Int 2014, 60, 189–204. [Google Scholar]
- (37).Schymanski EL; Jeon J; Gulde R; Fenner K; Ruff M; Singer HP; Hollender J Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol 2014, 48 (4), 2097–2098. [DOI] [PubMed] [Google Scholar]
- (38).Radjenovic J; Petrovic M; Barceló, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem 2007, 387 (4), 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Subedi B; Kannan K Fate of Artificial Sweeteners in Wastewater Treatment Plants in New York State, U.S.A. Environ. Sci. Technol 2014, 48 (23), 13668–13674. [DOI] [PubMed] [Google Scholar]
- (40).Peter KT; Hou F; Tian Z; Wu C; Goehring M; Liu F; Kolodziej EP More Than a First Flush: Urban Creek Storm Hydrographs Demonstrate Broad Contaminant Pollutographs. Environ. Sci. Technol 2020, 54 (10), 6152–6165. [DOI] [PubMed] [Google Scholar]
- (41).Ferguson PL; Iden CR; Brownawell BJ Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environ. Sci. Technol 2001, 35 (12), 2428–2435. [DOI] [PubMed] [Google Scholar]
- (42).Goulding N; Hickman M; Reid M; Amundsen EJ; Baz-Lomba JA; O’Brien JW; Tscharke BJ; de Voogt P; Emke E; Kuijpers W; Hall W; Jones HE A comparison of trends in wastewater-based data and traditional epidemiological indicators of stimulant consumption in three locations. Addiction 2020, 115 (3), 462–472. [DOI] [PubMed] [Google Scholar]
- (43).Gasser G; Rona M; Voloshenko A; Shelkov R; Tal N; Pankratov I; Elhanany S; Lev O Quantitative Evaluation of Tracers for Quantification of Wastewater Contamination of Potable Water Sources. Environ. Sci. Technol 2010, 44 (10), 3919–3925. [DOI] [PubMed] [Google Scholar]
- (44).Arnold BF; Schiff KC; Ercumen A; Benjamin-Chung J; Steele JA; Griffith JF; Steinberg SJ; Smith P; McGee CD; Wilson R; Nelsen C; Weisberg SB; Colford JM Jr. Acute Illness Among Surfers After Exposure to Seawater in Dry- and Wet-Weather Conditions. Am. J. Epidemiol 2017, 186 (7), 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Hug C; Ulrich N; Schulze T; Brack W; Krauss M Identification of novel micropollutants in wastewater by a combination of suspect and nontarget screening. Environ. Pollut 2014, 184, 25–32. [DOI] [PubMed] [Google Scholar]
- (46).Zheng G; Salamova A Are Melamine and Its Derivatives the Alternatives for Per- and Polyfluoroalkyl Substance (PFAS) Fabric Treatments in Infant Clothes? Environ. Sci. Technol 2020, 54 (16), 10207–10216. [DOI] [PubMed] [Google Scholar]
- (47).Lee SS; Paspalof AM; Snow DD; Richmond EK; Rosi-Marshall EJ; Kelly JJ Occurrence and Potential Biological Effects of Amphetamine on Stream Communities. Environ. Sci. Technol 2016, 50 (17), 9727–9735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


