Abstract
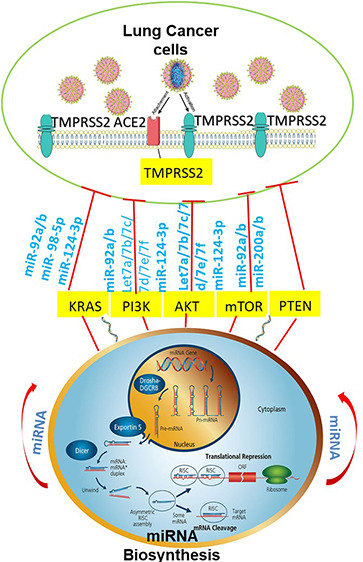
In this study, we investigated the interactions between SARS-CoV-2 and miRNAs associated with lung cancer using bioinformatic approaches. A special focus was placed on TMPRSS2 and lung cancer progression pathways involving AKT/PI3K/PTEN genes.
Keywords: SARS-CoV-2, microRNA, lung cancer
The recent SARS-CoV-2 pandemic brought the world to a halt due to its unforeseen severity and fast spread. SARS-CoV-2 symptoms can cause great damage to the organs of the respiratory system, particularly the lungs. This places individuals with respiratory diseases such as COPD (chronic obstructive pulmonary disease), cystic fibrosis, pulmonary fibrosis, and lung cancer at an increased risk for severe illness from the virus that causes COVID-19.1
Several research groups have established that SARS-CoV-2 enters the host cell through the spike protein (S-protein) binding to the human angiotensin-converting enzyme 2 (hACE2) aided by the host’s proteases, especially transmembrane serine protease 2 (TMPRSS2).2 Alveolar epithelial type II cells, a major source of hACE2 in the adult lung, are dormant under conventional circumstances, but they actively multiply and downregulate this protective enzyme in lung fibrosis.1
TMPRSS2 is a cell-surface protein expressed by several epithelial tissues and known to be overexpressed in prostate cancer. The TMPRSS2 gene is a member of the ETS (E26 transformation-specific or Erythroblast Transformation Specific genes) family of oncogenic transcription factors, most commonly ERG (ETS-related gene), which is subsequently regulated by androgen receptor signaling and upregulating in prostate cancers.3 As mentioned earlier, the internalization of SARS-CoV-2 depends on the host’s proteases, particularly TMPRSS2.4 The contribution of TMPRSS2 to viral infection is not limited to SARS-CoV-2, as this protease facilitates the entry of other viruses such as influenza viruses. Multiple studies have pegged TMPRSS2 as a key player in SARS-CoV-2 infections, and its link to lung cancer pathophysiology is still being investigated.
Lung cancer is one of the most prevalent cancers worldwide and can be broadly categorized into small-cell lung cancer (SCLC) and nonsmall-cell lung cancer (NSCLC). The latter accounts for more than 80% of lung cancer cases and is further divided into adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma.5 Substantial advances have been made in lung cancer molecular biology, which paved the way for developing novel therapeutic approaches based on targeting specific genes and pathways. The major signaling pathways that could be used for such therapy approaches include the growth-stimulating pathways (EGFR/PI3K pathway), the growth suppressing pathways (p53/Rb/P14ARF, STK11), the apoptotic pathways (Bcl-2/Bax/Fas/FasL), and the pathway linked to DNA repair genes.6,7 According to recent research, the altered expression of renin-angiotensin system (RAS) components has been found to correlate with tumor progression. Furthermore, tumor grading and clinical studies suggest that angiotensin II (Ang II), a key effector of the RAS system, is enhanced in the epithelial-mesenchymal transition (EMT) process.8 On the other hand, the tumor suppressor PTEN (phosphatase and tensin homologue) has been established as a critical regulator of growth factors and an inhibitor of phosphoinositide 3-kinase (PI3K) in several cancers, including lung cancer. Moreover, loss of PTEN is frequently observed in cancer, resulting in the deregulation of cancer cells’ survival, growth, and proliferation. Currently, few studies have linked TMPRSS2 to lung cancer; one recent study investigated the expression of TMPRSS2 in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC). Their data revealed that LUAD patients were more vulnerable to the SARS-CoV-2 infection than LUSC patients.9
MicroRNAs (miRNAs) are well-conserved, small (20–22 nt) noncoding RNA molecules that control multiple cellular functions post-transcription by binding to the 3′ untranslated region (UTR) of target mRNA (mRNA) transcripts, thereby promoting translational repression and mRNA degradation. miRNAs act as key effector molecules in the complex interactions between viruses and host cells. Viral miRNAs can evade the human immune system by altering the expression of several host genes meant for controlling cell growth and development. On the other hand, cell-encoded miRNAs can affect viral infection by regulating host factors involved in viral pathogenesis.10 Therefore, studies on host cell-derived miRNAs can enhance the understanding of the mechanisms underlying the interactions between viruses and host cells and provide a framework for discovering novel antiviral agents and strategies.
In this study, we conducted an in silico analysis using different microRNA target prediction software programs to shortlist potential miRNAs capable of regulating TMPRSS2/PI3K/AKT/PTEN individually or as an axis associated with lung cancer.
Methods
The method used to determine potential miRNAs that can directly bind to TMPRSS2, AKT, PI3K, mTOR, and PTEN was detailed in our previous paper.11
Given TMPRSS2’s crucial role in cell infection, the development of novel approaches for regulating the expression of TMPRSS2 is becoming increasingly important within the context of the COVID-19 pandemic.4 Gordanpour et al. showed that miR-221 is downregulated in prostatic tumors bearing TMPRSS2-ERG fusion transcripts.12,13 However, the role of miRNAs targeting TMPRSS2 associated with lung cancer is still unknown. Our analysis revealed that several miRNAs are, directly and/or indirectly, associated with TMPRSS2, enabling the inhibition of its oncogenic properties. Table 1 presents a list of potential miRNA candidates against the TMSRSS2 gene, which were not studied previously.
Table 1. miRNAs with Strong Binding Potential towards TMPRSS2.
| no. | miRNA | sequence (5′ to 3′) |
|---|---|---|
| 1 | hsa-let7a-5p | TGAGGTAGTAGGTTGTATAGTT |
| 2 | hsa-let7b-5p | TGAGGTAGTAGGTTGTGTGGTT |
| 3 | hsa-let7c-5p | TGAGGTAGTAGGTTGTATGGTT |
| 4 | hsa-let7d-5p | AGAGGTAGTAGGTTGCATAGTT |
| 5 | hsa-let7e-5p | TGAGGTAGGAGGTTGTATAGTT |
| 6 | hsa-let7f-5p | TGAGGTAGTAGATTGTATAGTT |
| 7 | hsa-let7g-5p | TGAGGTAGTAGTTTGTACAGTT |
| 8 | hsa-let7i-5p | TGAGGTAGTAGTTTGTGCTGTT |
| 9 | hsa-miR-7-5p | TGGAAGACTAGTGATTTTGTTGTT |
| 10 | hsa-miR-25-3p | CATTGCACTTGTCTCGGTCTGA |
| 11 | hsa-miR-32-5p | TATTGCACATTACTAAGTTGCA |
| 12 | hsa-miR-92a-3p | TATTGCACTTGTCCCGGCCTGT |
| 13 | hsa-miR-92b-3p | TATTGCACTCGTCCCGGCCTCC |
| 14 | hsa-miR-98-5p | CTATACAACTTACTACTTTCCC |
| 15 | hsa-miR-153-5p | TTGCATAGTCACAAAAGTGATC |
| 16 | hsa-miR-182-5p | TTTGGCAATGGTAGAACTCACACT |
| 17 | hsa-miR-183-5p | TATGGCACTGGTAGAATTCACT |
| 18 | hsa-miR-221-3p | ACAGCAGGCACAGACAGGCAGT |
| 19 | hsa-miR-363-3p | AATTGCACGGTATCCATCTGTA |
| 20 | hsa-miR-367-3p | AATTGCACTTTAGCAATGGTGA |
| 21 | hsa-miR-448 | TTGCATATGTAGGATGTCCCAT |
| 22 | hsa-miR-494-3p | TGAAACATACACGGGAAACCTC |
| 23 | hsa-miR-511-3p | AATGTGTAGCAAAAGACAGA |
| 24 | hsa-miR-4458 | AGAGGTAGGTGTGGAAGAA |
| 25 | hsa-miR-4500 | TGAGGTAGTAGTTTCTT |
| 26 | hsa-miR-4778-3p | TCTTCTTCCTTTGCAGAGTTGA |
| 27 | hsa-miR-4796-5p | TGTCTATACTCTGTCACTTTAC |
| 28 | hsa-miR-5197-5p | CAATGGCACAAACTCATTCTTGA |
PI3K/AKT/mTOR play a key role in the pathogenesis of various forms of cancer, including lung cancer.6 Genomic amplification of PI3K was identified in a large number of NSCLC tumors and preinvasive lesions. The PI3K signaling pathway is an important intracellular signal transduction pathway with a significant role in cell proliferation, growth, survival, vesicle trafficking, glucose transport, and cytoskeletal organization.6 PI3Ks are usually activated by receptor tyrosine kinases (RTKs) such as EGFR, IGF1-R, and HER2/neu.14 Moreover, the mammalian target of rapamycin complex 1 (mTORC) contributes to the complete activation of AKT via phosphorylation at serine 473.15 Activated AKT promotes cell growth and survival through various mechanisms. It was found to be mutated in more than 30% of 188 lung adenocarcinomas and is also frequently activated in lung cancer cell lines, especially in ones harboring genetic mutations. There are studies correlating the activation of mTOR with tumor progression and metastatic potential in KRAS-mutated NSCLC models. Furthermore, Anagnostou et al. reported a better outcome for patients with early stage lung adenocarcinoma that overexpressed mTOR.16Table 2 provides a list of potential miRNA candidates against the PI3K/AKT/mTOR pathway.
Table 2. miRNAs with Strong Binding Potential towards the PI3K/AKT/mTOR Pathway.
| no. | miRNA | sequence (5′ to 3′) |
|---|---|---|
| 1 | hsa-let7a-5p | TGAGGTAGTAGGTTGTATAGTT |
| 2 | hsa-let7b-5p | TGAGGTAGTAGGTTGTGTGGTT |
| 3 | hsa-let7c-5p | TGAGGTAGTAGGTTGTATGGTT |
| 4 | hsa-let7d-5p | TCTTCTTCCTTTGCAGAGTTGA |
| 5 | hsa-let7e-5p | AGAGGTAGTAGGTTGCATAGTT |
| 6 | hsa-let7f-5p | TGAGGTAGGAGGTTGTATAGTT |
| 7 | hsa-let7g-5p | TGGAAGACTAGTGATTTTGTTGTT |
| 8 | hsa-miR-92a-3p | TATTGCACTTGTCCCGGCCTGT |
| 9 | hsa-miR-98-5p | CTATACAACTTACTACTTTCCC |
| 10 | hsa-miR-124-3p | TAAGGCACGCGGTGAATGCCAA |
| 11 | hsa-miR-200b-5p | TAATACTGCCTGGTAATGATGA |
| 12 | hsa-miR-200c-5p | TAATACTGCCGGGTAATGATGGA |
| 13 | hsa-miR-4458 | AGAGGTAGGTGTGGAAGAA |
| 14 | hsa-miR-4500 | TGAGGTAGTAGTTTCTT |
| 15 | hsa-miR-200b-3p | TAATACTGCCTGGTAATGATGA |
| 16 | hsa-miR-200c-3p | TAATACTGCCGGGTAATGATGGA |
| 17 | hsa-miR-202-3p | TTCCTATGCATATACTTCTTTG |
| 18 | hsa-miR-429 | TAATACTGTCTGGTAAAACCGT |
The most common genetic alteration of the PI3K pathway observed in human cancer is the deletion or downregulated expression of the tumor suppressor gene PTEN.17 PTEN acting as a direct antagonist of PI3K negatively regulates the PI3K pathway. Homozygous or hemizygous deletions of PTEN and missense mutations may result in the increased activation of the PI3K pathway and are frequently observed in many cancer types but are not very frequent in NSCLC. However, partial or complete loss of PTEN protein expression is frequently observed in lung cancer. Transcriptional repression and epigenetic silencing of PTEN, commonly through promoter hypermethylation, has been described as a mechanism of PTEN inactivation in several studies. Table 3 presents a list of potential miRNA candidates, which were not studied previously, against the PTEN gene.
Table 3. miRNAs with Strong Binding Potential towards PTEN.
| no. | miRNA | sequence (5′ to 3′) |
|---|---|---|
| 1 | hsa-let7a-5p | TGAGGTAGTAGGTTGTATAGTT |
| 2 | hsa-let7b-5p | TGAGGTAGTAGGTTGTGTGGTT |
| 3 | hsa-let7c-5p | TGAGGTAGTAGGTTGTATGGTT |
| 4 | hsa-let7d-5p | AGAGGTAGTAGGTTGCATAGTT |
| 5 | hsa-let7e-5p | TGAGGTAGGAGGTTGTATAGTT |
| 6 | hsa-let7f-5p | TGAGGTAGTAGATTGTATAGTT |
| 7 | hsa-let7g-5p | TGAGGTAGTAGTTTGTACAGTT |
| 8 | hsa-miR-10a-5p | TACCCTGTAGATCCGAATTTGTG |
| 9 | hsa-miR-10b-3p | TACCCTGTAGAACCGAATTTGTG |
| 10 | hsa-miR-92a-3p | TATTGCACTTGTCCCGGCCTGT |
| 11 | hsa-miR-92b-3p | TATTGCACTCGTCCCGGCCTCC |
| 12 | hsa-miR-98-5p | CTATACAACTTACTACTTTCCC |
| 13 | hsa-miR-124-3p | TAAGGCACGCGGTGAATGCCAA |
The COVID-19 pandemic had a devastating impact on the health of millions of people and became a challenge for universal healthcare systems. The high mortality rate associated with this pandemic is generally due to lung failure induced by acute respiratory distress syndrome (ARDS).18 This infection is particularly devastating for patients suffering from lung cancer. The mechanism of host cell infection by SARS-CoV-2 is not completely understood and remains an active research topic. However, it became evident that the key players in the host cell internalization of the virus are hACE2 and TMPRSS2, as the interaction of these membrane proteins with the viral S-protein is crucial for host-membrane fusion and endocytosis.2
In this study, we report on the interaction between SARS-CoV-2 and miRNAs associated with lung cancer. Our analysis focused on TMPRSS2 and lung cancer progression pathways involving AKT/PI3K/mTOR/PTEN genes. The algorithms and bioinformatic approach we adopted are detailed in our previous work.11 Our analysis resulted in a network of miRNA–mRNA interactions illustrated in Tables 1–3.
Genetic abnormalities commonly associated with lung cancer include somatic mutations and gene amplifications in EGFR, P53, KRAS,8 BRAF, Erb-B2 receptor tyrosine kinase 2 (ERBB2), MET, serine/threonine kinase 11, PIK3CA (PI3K), and Parkin RBR E3 ubiquitin protein ligase. Other genetic abnormalities related to lung cancer have been identified in numerous pathways, including the Notch, EGFR, PI3K, phosphatase, and tensin homologue (PTEN)/phospho-AKT/p53 pathway,14 mitogen-activated protein kinase (MAPK), and cell cycle pathways. The PI3K/AKT/mTOR pathway is commonly activated in NSCLC. It plays an important role in promoting oncogenesis in lung cancer and mediating resistance to EGF receptor tyrosine kinase inhibitors. Lately, some studies have revealed that TMPRSS2-ERG positive tumors are also enhanced in PTEN loss and subsequent enrichment of PI3K,17 suggesting a strong collaboration among these genes in the context of prostate tumorigenesis. TMPRSS2-ERG fusions seem to further associate with the PI3K pathway through cooperation with active AKT, the combination resulting in the development of invasive carcinoma.
miRNAs have shown potential in the therapeutic approach against SARS-CoV-2. In 2004, a study by Takamizawa et al. revealed that lower expressions of let-7 microRNA were associated with shorter survival in patients with surgical lung cancer. Several studies revealed that miR-1, miR-21, miR-30a, miR-92a,miR-107, miR-124, miR-425-5p, miR-503, and miR-520a targeting the PI3K/PTEN/AKT signaling axis regulate multiple biological functions in human lung cancer. These miRNAs were also found to bind at 3′UTR of TMPRSS2. However, no studies so far have been able to establish a connection between the TMPRSS2 and AKT/PI3K/PTEN/mTOR axis and explain the possibility of using this connection in developing therapeutics for SARS-CoV-2 associated with lung cancer.
In this paper, we investigated the use of miRNAs as novel targets for the SARS-CoV-2 infection in patients with lung cancer. Our findings revealed a highly immunogenic viral–host interaction as well as some common miRNAs that can regulate both TMPRSS2 and PI3K/AKT/PTEN individually, suggesting a novel relationship among them. The findings of this study highlight the potential of miRNAs in the development of diagnostics, biomarkers, and novel targets for SARS-CoV-2 associated with lung cancer.
Author Contributions
D.M. conceived and designed the study, conducted the analysis, and drafted the manuscript. D.M., N.S., and G.H. revised and edited the manuscript. All authors approved the final version.
The authors declare no competing financial interest.
References
- Gupta I.; Rizeq B.; Elkord E.; Vranic S.; Moustafa A. E. Al. (2020) Sars-Cov-2 Infection and Lung Cancer: Potential Therapeutic Modalities. Cancers 1–21. 10.3390/cancers12082186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N. H.; Nitsche A.; Müller M. A.; Drosten C.; Pöhlmann S. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181 (2), 271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John J.; Powell K.; Katie Conley-LaComb M.; Chinni S. R. (2012) TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. Journal of Cancer Science and Therapy 94–101. 10.4172/1948-5956.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn L. B.; Sharma N.; Elhaik E.; Sekulic A.; Bryce A. H.; Fonseca R. (2020) Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 95 (9), 1989–1999. 10.1016/j.mayocp.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall A. L. (2014) Cancer: Lung. Cambridge Handbook of Psychology, Health and Medicine 605–606. 10.1017/CBO9780511543579.138. [DOI] [Google Scholar]
- Sarris E.; Saif M.; Syrigos K. (2012) The Biological Role of PI3K Pathway in Lung Cancer. Pharmaceuticals 5 (11), 1236–1264. 10.3390/ph5111236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla E.; Gazdar A. (2009) Pathogenesis of Lung Cancer Signalling Pathways: Roadmap for Therapies. Eur. Respir. J. 1485–1497. 10.1183/09031936.00014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaux C.; Iannino N.; Martin B.; Paesmans M.; Berghmans T.; Dusart M.; Haller A.; Lothaire P.; Meert A. P.; Noel S.; Lafitte J. J.; Sculier J. P. (2005) The Role of RAS Oncogene in Survival of Patients with Lung Cancer: A Systematic Review of the Literature with Meta-Analysis. Br. J. Cancer 131–139. 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q.; Xiang Z.; Wu Y.; Gu Y.; Guo J.; Geng F. (2020) Analysis of the Susceptibility of Lung Cancer Patients to SARS-CoV-2 Infection. Mol. Cancer 19 (1), 80. 10.1186/s12943-020-01209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandan K.; Gupta M.; Sarwat M. (2020) Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Frontiers in Immunology 3081. 10.3389/fimmu.2019.03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D.; AlSawaftah N.; Husseini G. A. (2021) Identification of Novel MicroRNAs as Promising Therapeutics for SARS-CoV-2 by Regulating the EGFR-ADAM17 Axis: An In Silico Analysis. ACS Pharmacol. Transl. Sci. 4 (1), 396–399. 10.1021/acsptsci.0c00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordanpour A.; Stanimirovic A.; Nam R. K.; Moreno C. S.; Sherman C.; Sugar L.; Seth A. (2011) MiR-221 Is down-Regulated in TMPRSS2:ERG Fusion-Positive Prostate Cancer. Anticancer Res. 31 (2), 403–410. [PMC free article] [PubMed] [Google Scholar]
- Zoni E.; Karkampouna S.; Thalmann G. N.; Kruithof-de Julio M.; Spahn M. (2018) Emerging Aspects of MicroRNA Interaction with TMPRSS2-ERG and Endocrine Therapy. Mol. Cell. Endocrinol. 462, 9–16. 10.1016/j.mce.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Tan A. C. (2020) Targeting the PI3K/Akt/MTOR Pathway in Non-Small Cell Lung Cancer (NSCLC). Thoracic Cancer 511–518. 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.; Huang S. (2010) The Complexes of Mammalian Target of Rapamycin. Curr. Protein Pept. Sci. 11 (6), 409–424. 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou V. K.; Bepler G.; Syrigos K. N.; Tanoue L.; Gettinger S.; Homer R. J.; Boffa D.; Detterbeck F.; Rimm D. L. (2009) High Expression of Mammalian Target of Rapamycin Is Associated with Better Outcome for Patients with Early Stage Lung Adenocarcinoma. Clin. Cancer Res. 15 (12), 4157–4164. 10.1158/1078-0432.CCR-09-0099. [DOI] [PubMed] [Google Scholar]
- Gkountakos A.; Sartori G.; Falcone I.; Piro G.; Ciuffreda L.; Carbone C.; Tortora G.; Scarpa A.; Bria E.; Milella M.; Rosell R.; Corbo V.; Pilotto S. (2019) PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers (Basel). 11 (8), 1141. 10.3390/cancers11081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Acosta M. A.; Singer B. D. (2020) Pathogenesis of COVID-19-Induced ARDS: Implications for an Ageing Population. Eur. Respir. J. 56 (3), 2002049. 10.1183/13993003.02049-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


